Abstract
Background
Coronavirus disease 2019 (COVID-19) has spread worldwide determining dramatic impacts on healthcare systems. Early identification of high-risk parameters is required in order to provide the best therapeutic approach. Coronary, thoracic aorta and aortic valve calcium can be measured from a non-gated chest computer tomography (CT) and are validated predictors of cardiovascular events and all-cause mortality. However, their prognostic role in acute systemic inflammatory diseases, such as COVID-19, has not been investigated.
Objectives
The aim was to evaluate the association of coronary artery calcium and total thoracic calcium on in-hospital mortality in COVID-19 patients.
Methods
1093 consecutive patients from 16 Italian hospitals with a positive swab for COVID-19 and an admission chest CT for pneumonia severity assessment were included. At CT, coronary, aortic valve and thoracic aorta calcium were qualitatively and quantitatively evaluated separately and combined together (total thoracic calcium) by a central Core-lab blinded to patients’ outcomes.
Results
Non-survivors compared to survivors had higher coronary artery [Agatston (467.76 ± 570.92 vs 206.80 ± 424.13 mm2, p < 0.001); Volume (487.79 ± 565.34 vs 207.77 ± 406.81, p < 0.001)], aortic valve [Volume (322.45 ± 390.90 vs 98.27 ± 250.74 mm2, p < 0.001; Agatston 337.38 ± 414.97 vs 111.70 ± 282.15, p < 0.001)] and thoracic aorta [Volume (3786.71 ± 4225.57 vs 1487.63 ± 2973.19 mm2, p < 0.001); Agatston (4688.82 ± 5363.72 vs 1834.90 ± 3761.25, p < 0.001)] calcium values. Coronary artery calcium (HR 1.308; 95% CI, 1.046–1.637, p = 0.019) and total thoracic calcium (HR 1.975; 95% CI, 1.200–3.251, p = 0.007) resulted to be independent predictors of in-hospital mortality.
Conclusion
Coronary, aortic valve and thoracic aortic calcium assessment on admission non-gated CT permits to stratify the COVID-19 patients in-hospital mortality risk.
Keywords: COVID-19, Coronary artery, Aortic valve, Thoracic aorta, Calcification, Calcium score, In-hospital mortality
1. Introduction
Since the first cases observed in Wuhan, China in December 2019, the Coronavirus disease 2019 (COVID-19) has spread worldwide with consequent dramatic impacts on healthcare systems, global economy and social behavior.1
The high rate of COVID-19-related mortality and the uncertainty of the pandemic duration demand for the early identification of high-risk subsets of patients beyond traditional clinical parameters. To date, advanced age and male gender have been identified as independent predictors of mortality in COVID-19 patients. Elevated inflammatory markers and severity of lung involvement also correlate with adverse prognosis.2 Moreover, several data suggest a relevant role of cardiovascular comorbidities in determining COVID-19 patients’ outcome3, 4, 5, 6 Chest computed tomography (CT), already widely utilized for COVID-19 pneumonia diagnosis, might provide additional information beyond lung disease, such as coronary arteries calcium evaluation.7, 8, 9 Coronary, thoracic aorta and aortic valve calcium burden can be quantitatively measured from a non-gated chest CT and are validated long-term predictors of cardiovascular events and all-cause mortality.10, 11, 12 However, their prognostic role in the context of an acute and systemic inflammatory disease, such as.
COVID-19, has not been investigated.
The aim of our study was to evaluate the potential value of coronary artery calcium (CAC) and of total thoracic calcium burden (coronary artery calcium, thoracic aorta and aortic valve calcium) in prediction of in-hospital mortality of COVID-19 patients.
2. Methods
2.1. Study population
Data were derived from the multicenter, retrospective and observational sCORE COVID-19 (calcium score for COVID-19 Risk Evaluation) registry, consisting of 16 participating hospitals located in five Italian regions heavily affected by the pandemic (Lombardy, Piedmont, Emilia-Romagna, Marche and Lazio) (see Methods in the supplement - Participating Centers). All consecutive patients with a positive qualitative polymerase-chain-reaction assay for SARS-CoV-2 and a non-contrast chest CT at admission for pneumonia severity assessment during the study period (March 1st – April 20th, 2020) were included. CT had been performed in those patients with the clinical suspicion of pneumonia.
All institutions were second and third level centers (public or private accredited to the National Health System) directly involved in the COVID-19 emergency. The study was approved by the local ethical committees of each institution.
2.2. Clinical, laboratory and radiological data
Clinical and laboratory data as well as radiological images were collected by each center. Radiological images were sent to the central core-lab for image analysis (Experimental Imaging center, IRCCS, Ospedale San Raffaele, Milano). Clinical and Radiological databases were integrated and analyzed by the coordinating center (Maria Cecilia Hospital, GVM Care & Research, Cotignola). Demographic characteristics (age, gender and body mass index), cardiovascular risk factors (hypertension, diabetes) and comorbidities (chronic lung disease, chronic kidney disease, active malignancy, peripheral artery disease), as well as history of coronary artery disease including previous percutaneous and/or surgical revascularization across the study population were collected.
Laboratory data included baseline admission values of hemoglobin, white blood cells count, creatinine, high sensitivity troponin I (HS-TnI), lactate dehydrogenase (LDH), C-reactive protein (CRP). Interleukin-6 and D-dimer at peak were also assessed. Major adverse cardiac and cerebrovascular events (MACCE) were recorded as well as time frames between hospital admission, CT and in-hospital outcome (death or discharge) were calculated.
Chest CT scans were analyzed by three expert cardio-thoracic radiologists of the core-lab blinded to patients’ clinical data. CT scans were acquired with a standard non-gated chest CT protocol, always on multidetector scanners with at least 16 detector rows.9
For lung parenchyma evaluation, CT were reconstructed at each site with sharp kernel and visualized at the core lab using a standard lung window (width 1400 HU; center −450 HU).
Analyzed lung parameters included: 1) well aerated lung volume (V-WAL) in cm,3 automatically quantified using a certified commercial software (IntelliSpace v 8.0, Philips, The Netherlands) as the sum of voxels with densities between −950 HU and −740 HU, for the exclusion of emphysema (density below −950 HU) and pneumonia (density above −740 HU), respectively13; 2) semi-quantitative pneumonia scoring [no pneumonia (0%); minimal pneumonia (1–25%); mild pneumonia (26–50%); moderate pneumonia (51–75%); severe pneumonia (76–100%).14
For calcium quantification, CT were reconstructed at each site with a soft kernel, transferred to the core lab and then reformatted at a standard slice thickness of 2.5 mm without overlap nor gap, and visualized using a standard mediastinal window (width 350 HU; center 40 HU).
Patients with coronary stenting were excluded for the quantitative assessment of coronary calcium, for impaired measurement.
Coronary artery calcifications were visually assessed (presence/absence and number of involved vessels) and quantitatively computed. Quantification of coronary artery (CAC), thoracic aorta (TAC) and aortic valve calcium (AVC) was performed both with Agatston calcium scoring (CS)15 and calcium volume (CV) methodology,16 semi-automatically, on a commercial software (IntelliSpace v 8.0, Philips, The Netherlands), as follow: vascular calcification were automatically detected as a group of adjacent pixels with an area ≥1 mm2 and a density above 130 HU. Then, an experienced cardio-thoracic radiologist labeled every calcification as belonging either to coronary arteries (left main, left anterior descending, left circumflex, or right coronary artery), thoracic aorta or aortic valve. The total thoracic calcium (TTC) volume was also calculated as the sum of CAC, TAC and AVC volumes.
2.3. Statistical analysis
Descriptive statistics were performed on the overall population. Continuous variables are presented as mean ± standard deviation or median [interquartile range]. Categorical variables as counts and proportions (%). For continuous variables, the differences were compared between groups using the T-test and the one-way analysis of variance or the Wilcoxon rank sum test and the Kruskal Wallis test, for parametric and non-parametric data, respectively. Only variables with missing values below 10% of the total number of observations have been retained for downstream analysis in the analysis dataset. Multivariate imputation by chained equations has been carried out on these variables in order to reduce missing data bias. Continuous variables have been standardized and outliers removed. In this study, exposition to risk factor was considered to be CAC and TTC. In order to assess their role as risk factors, Cox regression analysis was performed for CAC and TTC as regressors considering death as the outcome. The follow-up time was 30 days from hospitalization.
Results were reported as hazard ratios (HR) with associated 95% confidence intervals (CIs). In order to identify the confounders, firstly a linear regression was performed considering CAC and TCC as dependent and all the patients’ clinical features, demographics, laboratory data collected and all remaining CT parameters, as predictor variables. Secondly, all clinical data, demographics, laboratory data and CT parameters was regressed toward the outcome (death). The results were reported as standardized beta-values and associated p-values (Supplementary Table 2). All baseline significative variables (p < 0.05) were included in the adjusted full multivariate Cox regression model (age, gender, white blood cell, creatinine, well aerated lung volume and arterial hypertension, as reported in Table 2, Table 3 ). The variance enrolment correlation among the centers was considered. The multicollinearity was examined using the variance inflation factor (VIF) and variables with VIF > 3 were excluded by the same multivariable model. For CAC and TTC, a full model was built without including variables testing the same clinical set. Variable selection was performed by a backward stepwise algorithm based on Akaike’s information criterion minimization. Cross-validation was performed to validate the models obtained. The p-value related to the likelihood ratio was calculated, along with Harrel’s C-index. The “optimal” cut-off value of CAC and TTC for predicting death was calculated maximizing the odds ratio or the sum of sensitivity and specificity respectively, using receiver-operating characteristic (ROC) curve analysis. Two binary variables derived from the calculated cut-off were employed in multivariable Cox regression model with robust variance. The results were reported as hazard ratios with associated 95% confidence intervals (CIs). Survival curves were plotted as cumulative hazard stratifying by risk factor reporting the number of patients at risk at each time point and the p-value of the log-rank test for the differences in the survival proportions. Predictive model capability was assessed by using time dependent AUC receiver operating characteristic analysis (AUC-tdROC) and sensibility and specificity estimation. Moreover, we evaluated the model discrimination capability together with Bayesian information criterion (BIC) variation. In order to comparing the mortality rate in absences of MACCE, a grouped by MACCE univariable analysis of patients clinical features together with inflammatory markers and anamnestic and laboratory data was carried out.
Table 2.
Independent predictors of mortality at multivariate analysis - the impact coronary artery calcium volume on in-hospital death.
| UNIVARIBLE | HR | 95 CI | p-value | MULTIVARIABLE | HR | 95 CI | p-value |
|---|---|---|---|---|---|---|---|
| Age | 4.169 | 3.281–5.299 | <0.001 | Age | 3.261 | 2.538–4.189 | <0.001 |
| Gender, male | 1.381 | 1.009–1.891 | 0.044 | Gender, male | 1.738 | 1.126–2.682 | 0.013 |
| White blood cells/mm3 | 1.371 | 1.167–1.61 | <0.001 | White blood cells/mm3 | 1.134 | 1.013–1.269 | 0.028 |
| Creatinine mg/dl | 1.814 | 1.63–2.018 | <0.001 | Creatinine mg/dl | 1.419 | 1.288–1.564 | <0.001 |
| Well areaed lung volume cm3 | 0.451 | 0.359–0.567 | <0.001 | Well areaed lung volume cm3 | 0.417 | 0.368–0.473 | <0.001 |
| CAC mm3 | 1.25 | 1.184–1.318 | <0.001 | CAC mm3 | 1.308 | 1.046–1.637 | 0.019 |
| Arterial hypertension | 2.199 | 1.623–2.98 | 0.001 | Arterial hypertension | 1.203 | 1.05–1.378 | 0.008 |
| Active malignancy | 1.587 | 0.906–2.779 | 0.107 | – | – | – | – |
| CPR mg/L | 1.092 | 0.945–1.261 | 0.232 | – | – | – | – |
| Pericardial effusion | 1.14 | 0.664–1.958 | 0.635 | – | – | – | – |
| Orotracheal intubation | 1.199 | 0.837–1.718 | 0.322 | – | – | – | – |
HR Hazard ratio; CI confidence interval; CAC coronary artery calcium.; AUC area under the ROC curve, CPR = c-protein reactive.
The CAC variable in the model is a categorical variable derived from the ROC curve of coronary artery calcium volume values for the outcome mortality.
Table 3.
Independent predictors of mortality at multivariate analysis - the impact of total thoracic calcium on in-hospital death.
| UNIVARIBLE | HR | 95 CI | p-value | MULTIVARIABLE | HR | 95 CI | p-value |
|---|---|---|---|---|---|---|---|
| Age | 4.169 | 3.281–5.299 | <0.001 | Age | 2.783 | 2.127–3.643 | <0.001 |
| Gender, male | 1.381 | 1.009–1.891 | 0.044 | Gender, male | 1.804 | 1.174–2.771 | 0.007 |
| White blood cells/mm3 | 1.371 | 1.167–1.61 | <0.001 | White blood cells/mm3 | 1.155 | 1.046–1.274 | 0.004 |
| Creatinine (mg/dl) | 1.814 | 1.63–2.018 | <0.001 | Creatinine (mg/dl) | 1.411 | 1.294–1.539 | <0.001 |
| Well areaed lung volume cm3 | 0.451 | 0.359–0.567 | <0.001 | Well areaed lung volume cm3 | 0.395 | 0.347–0.449 | <0.001 |
| TTC mm3 | 1.397 | 1.304–1.496 | 0.001 | TTC mm3 | 1.975 | 1.200–3.251 | 0.007 |
| Arterial hypertension | 2.199 | 1.623–2.98 | 0.001 | – | |||
| Active malignancy | 1.587 | 0.906–2.779 | 0.107 | – | |||
| CPR | 1.092 | 0.945–1.261 | 0.232 | – | |||
| Pericardial effusion | 1.14 | 0.664–1.958 | 0.635 | – | |||
| Orotracheal intubation | 1.199 | 0.837–1.718 | 0.322 | – |
HR Hazard ratio; CI confidence interval; TTC; Total thoracic calcium, AUC area under the ROC curve, CPR = c-protein reactive.
The TTC variable in the model is a categorical variable derived from the ROC curve of total thoracic calcium volume values for the outcome mortality.
The analysis was performed by (MM) with R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
The study population included 1093 hospitalized patients with confirmed COVID-19 infection, admission chest CT and no history of coronary stenting. Table 1 shows the demographic characteristics and comorbidities of the two groups (survivors and non-survivors) and the overall population. Globally, the median age was 68 years (IQR 58–76) and 742 patients (68.3%) were male. Regarding cardiovascular risk factors, 590 patients (54.8%) had arterial hypertension and 178 (16.5%) diabetes mellitus. Preexisting cardiovascular disease included coronary artery disease (44, 6.6%) with a history of previous surgical revascularization in 2.3%. Other comorbidities included chronic lung disease (107, 10%), chronic kidney disease (49, 7.0%) and active malignancy (51, 4.7%). Orotracheal intubation was required in 155 patients (14.2%). In-hospital death occurred in 211 patients (19.3%). Median times from hospital admission to discharge or to in-hospital death were 14 (IQR 8–21) and 7 days (IQR 4–14), respectively. Seventy-eight patients with coronary stents were excluded from the analyses. Their in-hospital mortality rate was higher than that of patients without coronary stents (41.0% vs 19.3%, p < 0.001), while the rate of orotracheal intubation was not different between the two groups (data shown in Supplementary Table 1).
Table 1.
Comparison between survivors and non-survivors.
| Characteristics | Survivors (N = 882) | Non-survivors (N = 211) | Overall (N = 1093) | p-value |
|---|---|---|---|---|
| Age - median [IQR] | 64 [57, 74] | 77 [70, 82] | 68[58, 76] | <0.001 |
| Gender, male (%) | 584 (66.7) | 158 (74.9) | 742 (68.3) | 0.021 |
| BMI - median [IQR] | 26.85 [24.00, 29.38] | 26.84 [25.59, 29.83] | 26.85 [24.11, 29.39] | 0.240 |
| Arterial hypertension (%) | 441 (50.7) | 149 (72.0) | 590 (54.8) | <0.001 |
| Diabetes (%) | 129 (14.8) | 49 (23.7) | 178 (16.5) | 0.003 |
| Chronic kidney disease (%) | 34 (5.6) | 15 (16.9) | 49 (7.0) | <0.001 |
| Coronary artery disease (%) | 28 (4.9) | 16 (17.4) | 44 (6.6) | <0.001 |
| Previous CABG (%) | 10 (1.8) | 5 (5.7) | 15 (2.3) | 0.038 |
| Peripheral artery disease (%) | 34 (3.9) | 21 (10.1) | 55 (5.1) | 0.001 |
| Chronic lung disease (%) | 71 (8.2) | 36 (17.4) | 107 (10.0) | <0.001 |
| Active malignancy (%) | 37 (4.3) | 14 (6.8) | 51 (4.7) | 0.144 |
| Laboratory data at admission | ||||
| Hemoglobin g/dl - median [IQR] | 13.9 [12.5, 14.9] | 13.2 [11.9, 14.4] | 13.8 [12.4, 14.8] | <0.001 |
| White blood cells/mm3 - median [IQR] | 6580.00 [4900.00, 9290.00] | 7250.00 [5457.50, 10965.00] | 6730.00 [5000.00, 9667.50] | 0.001 |
| Creatinine mg/dL - median [IQR] | 0.95 [0.80, 1.17] | 1.23 [0.97, 1.74] | 0.99 [0.81, 1.24] | <0.001 |
| HS-TnI ng/L - median [IQR] | 10.00 [5.30, 18.30] | 31.15 [9.65, 175.97] | 11.20 [6.00, 31.65 | 0.001 |
| LDH mg/dl - median [IQR] | 354.00 [261.25, 460.50] | 460.50 [323.00, 603.50] | 369.00 [272.25, 491.00] | <0.001 |
| CRP mg/L - median [IQR] | 10.80 [4.90, 19.60] | 14.30 [8.37, 21.40] | 11.55 [5.40, 20.11] | 0.001 |
| Laboratory data during the hospitalization | ||||
| IL-6 pg/ml - median [IQR] | 53.70 [23.27, 211.25] | 141.00 [68.20, 368.70] | 60.20 [24.80, 233.00] 11.55 [5.40, 20.11] |
0.004 |
| D-dimer ng/ml - median [IQR] | 1.74 [0.72, 4.82] | 3.06 [1.16, 20.00] | 1.85 [0.77, 5.33] | 0.005 |
| Radiological findings | ||||
| Coronary Artery Calcium (CAC) | ||||
| CAC presence (%) | 554 (62.8) | 180 (85.3) | 734 (67.2) | <0.001 |
| n. of calcified coronary arteries - median [IQR] | 1.00 [0.00, 2.00] | 2.00 [1.00, 3.00] | 1.00 [0.00, 3.00] | <0.001 |
| CAC Agatston | ||||
| Total CAC - mean (SD) | 206.80 (424.13) | 467.76 (570.92) | 257.18 (467.37) | <0.001 |
| Left main - mean (SD) | 25.30 (51.77) | 56.00 (65.00) | 31.23 (55.87) | <0.001 |
| Left anterior descending - mean (SD) | 101.90 (199.99) | 208.72 (260.74) | 122.52 (217.07) | <0.001 |
| Left circumflex - mean (SD) | 47.49 (101.85) | 94.43 (128.70) | 56.52 (109.05) | <0.001 |
| Right coronary artery - mean (SD) | 58.69 (134.16) | 131.22 (177.12) | 56.52 (109.05) | <0.001 |
| CAC volume | ||||
| Total CAC mm3 - mean (SD) | 207.77 (406.81) | 487.79 (565.34) | 261.93 (455.28) | <0.001 |
| Left main mm3 - mean (SD) | 23.63 (47.96) | 52.83 (60.09) | 29.27 (51.80) | <0.001 |
| Left anterior descending mm3 - mean (SD) | 92.85 (172.18) | 196.66 (233.23) | 112.89 (189.89) | <0.001 |
| Left circumflex mm3 - mean (SD) | 53.58 (115.26) | 109.81 (147.91) | 112.89 (189.89) | <0.001 |
| Right coronary artery mm3 - mean (SD) | 72.94 (170.08) | 168.33 (229.32) | 91.30 (186.69) | <0.001 |
| Thoracic Aorta Calcium (TAC) | ||||
| TAC Agatston - mean (SD) | 1834.90 (3761.25) | 4688.82 (5363.72) | 2379.38 (4262.79) | <0.001 |
| TAC volume mm3 - mean (SD) | 1487.63 (2973.19) | 3786.71 (4225.57) | 1926.25 (3370.72) | <0.001 |
| Aortic Valve Calcium (AVC) | ||||
| AVC Agatston - mean (SD) | 111.70 (282.15) | 337.38 (414.97) | 155.50 (324.67) | <0.001 |
| AVC volume mm3 - mean (SD) | 98.27 (250.74) | 322.45 (390.90) | 141.78 (296.76) | <0.001 |
| Lung involvement | ||||
| Well aerated lung volume cm3 - mean (SD) | 2528.42 (1371.50) | 1790.26 (1109.39) | 2386.34 (1356.24) | <0.001 |
| Pneumonia scoring <0.001 | ||||
| Absent (%) | 16 (1.8) | 0 (0.0) | 16 (1.5) | |
| (<25%) (%) | 288 (32.7) | 31 (14.7) | 319 (29.2) | |
| (25–50%) (%) | 399 (45.3) | 106 (50.2) | 505 (46.2) | |
| (50–75%) (%) | 151 (17.1) | 64 (30.3) | 215 (19.7) | |
| (>75%) (%) | 27 (3.1) | 10 (4.7) | 37 (3.4) | |
| Left pleural effusion mm - mean (SD) | 1.33 (3.78) | 1.31 (3.75) | 1.33 (3.77) | 0.926 |
| Right pleural effusion mm - mean (SD) | 1.67 (4.90) | 1.59 (4.80) | 1.66 (4.88) | 0.830 |
| Pericardial effusion mm - mean (SD) | 0.23 (0.93) | 0.28 (1.03) | 0.24 (0.95) | 0.430 |
| Complications during the hospitalization | ||||
| MACCE 0.001 | ||||
| None (%) | 341 (86.1) | 52 (77.6) | 393 (84.9) | |
| Cerebral stroke (%) | 9 (2.3) | 3 (4.5) | 12 (2.6) | |
| Acute coronary syndrome (%) | 1 (0.3) | 5 (7.5) | 6 (1.3) | |
| Pulmonary embolism (%) | 36 (9.1) | 7 (10.4) | 43 (9.3) | |
| Peripheral embolization (%) | 9 (2.3) | 0 (0.0) | 9 (1.9) | |
| Orotracheal intubation (%) | 119 (13.5) | 36 (17.1) | 155 (14.2) | 0.188 |
IQR = interquartile range, SD = standard deviation, BMI=Body mass index, LDH = Lactate dehydrogenase, CPR=C-reactive protein, MACCE = major adverse cardiac and cerebrovascular events; HS-TnI = high-sensitivity troponin I, NIV = noninvasive ventilation.
3.1. Comparison between survivors and non-survivors groups
Non-survivors were older [median age 77 (IQR 70–82) years vs 64 (IQR 57–74)] and had a higher prevalence of hypertension (72% vs 50.7%, p < 0.001), diabetes (23.7% vs 14.8%, p = 0.003), chronic lung disease (17.4% vs 8.2%, p < 0.001), and peripheral artery disease (10.1% vs 3.9%, p = 0.001). Coronary artery disease was also more prevalent among non-survivors (17.4% vs 4.9%, p < 0.001), including previous history of surgical revascularization (5.7% vs 1.8%, p = 0.038).
Compared to survivors, non-survivors had higher admission creatinine levels [1.23 (0.97,1.74) vs 0.95 (0.8, 1.17) mg/dl, p < 0.001], LDH [460.50 (323.00, 603.50) vs 354.00 (261.25, 460.50) mg/dl, p < 0.001], CRP [14.30 (8.37, 21.40) vs 10.80 (4.90, 19.60) mg/L, p = 0.001] and HS-TnI [31.15 (9.65, 175.97) vs 10.00 (5.30, 18.30) ng/L, p = 0.001], but lower hemoglobin levels [13.2 (11.9, 14.4) vs 13.9 (12.5, 14.9) g/dl, p < 0.001]. Peak D-dimer [3.06 (1.16, 20.00) vs 1.74 (0.72, 4.82) ng/ml, p = 0.005] and Interleukin-6 [141.00 (68.20, 368.70) vs 53.70 (23.27, 211.25) pg/ml, p = 0.004] values were higher in non-survivors compared to survivors.
Non-survivors had a higher pneumonia extension and lower well aerated lung volume (1790.26 ± 1109.39 cm3 vs 2528.42 ± 1371.50 cm3, p < 0.001). They also experienced a higher rate of MACCE (p = 0.001). In detail, the onset of stroke determined a slight by not significant increase of mortality compared to patients not experiencing MACCE (25% vs 19%, p = 0.45), while patients experienced acute myocardial infarction had higher mortality (83% vs 19%, p < 0.001), despite non-significant differences in age, gender, clinical risk factor and respiratory lung reserve. Pulmonary and peripheral thromboembolic complication were not associated to increased mortality rate (p = 0.64 and p = 0.14 respectively).
The rate of orotracheal intubation was not significantly different between non survivors and survivors (17.1%. vs 13.5%, p = 0.188).
3.2. Impact of coronary artery calcium on in hospital mortality
Compared to survivors, non-survivors were more likely to have coronary calcification (85.3% vs 62.8%, p < 0.001), in more coronary arteries [2 (1, 3) vs 1 (0, 2), p < 0.001] with a higher CAC [mean values (±SD)] calculated with both Agatston score and volume [Agatston (467.76 ± 570.92 vs 206.80 ± 424.13, p < 0.001); Volume (487.79 ± 565.34 vs 207.77 ± 406.81 mm3, p < 0.001)]. Pearson’s product-moment correlation between Agatston score and volume was 0.976 (95% C.I. = 0.974–0.979, p < 0.001). Given that calcium volume had a higher hazard ratio compared to Agatston score at univariate analysis [HR1.25; (95% CI 1.184–1.318, p = 0.0001) vs HR 1.192 (95% CI 1.137–1.25, p = 0.001)], coronary volume was used for further analyses. Univariate analysis is reported in Supplementary Table 3.
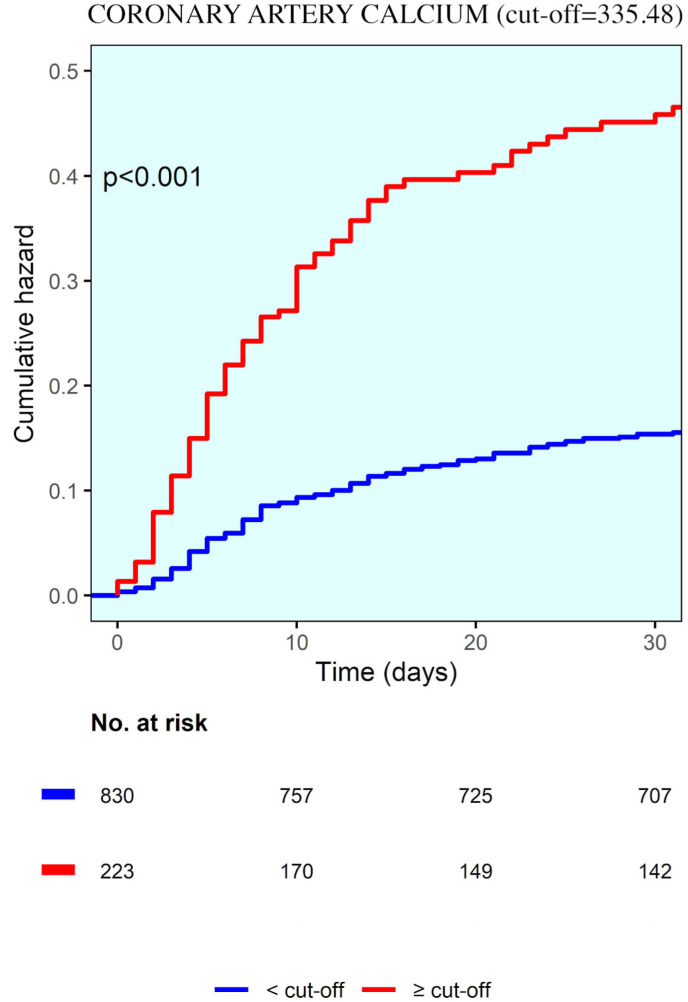
Receiver-operator characteristic curve analysis showed fair discrimination between survivors and non-survivors at a CAC volume of 335.48 mm3 (C-statistic of 0.803) (Supplementary Figure 1). At this value, the sensitivity and specificity for predicting in-hospital death were 96% and 33%, respectively. A total of 225 (20.5%) patients had CAC volume higher than the defined cut-off (CAC > 335.48 mm3) and 868 (79.4%) less or equal than the cut-off value (CAC ≤ 335.48 mm3). The mortality rate was 38.2% and 14.4% respectively (p < 0.001). Table 4 shows a comparison between the two groups.
Table 4.
Population description according to the identified coronary artery calcium volume cut-off value (= 335.48).
| CAC ≤ cut-off (N = 868) | CAC > cut-off (N = 225) | Overall (N = 1093) | p-value | |
|---|---|---|---|---|
| Age - median [IQR] | 64.00 [56.00, 73.00] | 77.00 [71.00, 83.00] | 68.00 [58.00, 76.00] | <0.001 |
| Gender, male (%) | 569 (66.0) | 173 (76.9) | 742 (68.3) | 0.002 |
| BMI (median [IQR]) | 26.85 [24.13, 29.40] | 26.74 [23.96, 29.38] | 26.85 [24.11, 29.39] | 0.825 |
| Arterial hypertension (%) | 429 (50.2) | 161 (72.5) | 590 (54.8) | <0.001 |
| Diabetes (%) | 119 (13.9) | 59 (26.6) | 178 (16.5) | <0.001 |
| Chronic kidney disease (%) | 31 (5.4) | 18 (14.2) | 49 (7.0) | 0.002 |
| Chronic coronary artery disease (%) | 19 (3.6) | 25 (18.7) | 44 (6.6) | <0.001 |
| Previous CABG (%) | 4 (0.8) | 11 (8.6) | 15 (2.3) | <0.001 |
| Peripheral artery disease (%) | 27 (3.2) | 28 (12.6) | 55 (5.1) | <0.001 |
| Chronic lung disease (%) | 70 (8.2) | 37 (16.7) | 107 (10.0) | <0.001 |
| Active malignancy (%) | 34 (4.0) | 17 (7.7) | 51 (4.7) | 0.032 |
| Laboratory data at admission | ||||
| Hemoglobin (mg/dl) - median [IQR] | 13.90 [12.50, 14.90] | 13.30 [12.00, 14.40] | 13.80 [12.40, 14.80] | <0.001 |
| White blood cells/mm3 - median [IQR] | 6620.00 [4900.00, 9290.00] | 7250.00 [5427.50, 11337.50] | 6730.00 [5000.00, 9667.50] | 0.002 |
| Creatinine mg/dl - median [IQR] | 0.96 [0.79, 1.19] | 1.13 [0.92, 1.54] | 0.99 [0.81, 1.24] | <0.001 |
| TnI HS ng/L- median [IQR] | 10.00 [5.30, 28.50] | 15.00 [9.80, 48.00] | 11.20 [6.00, 31.65] | 0.022 |
| LDH mg/dl- median [IQR] | 369.00 [272.75, 497.00] | 368.00 [272.50, 472.75] | 369.00 [272.25, 491.00] | 0.693 |
| CPR mg/L- median [IQR] | 11.66 [5.43, 20.16] | 10.90 [5.29, 19.86] | 11.55 [5.40, 20.11] | 0.615 |
| Laboratory during the hospitalization | ||||
| IL-6 pg/ml - median [IQR] | 55.50 [22.30, 236.00] | 82.05 [39.03, 186.53] | 60.20 [24.80, 233.00] | 0.203 |
| D-dimer ng/ml - median [IQR] | 1.76 [0.73, 5.39] | 2.18 [0.97, 4.24] | 1.85 [0.77, 5.33] | 0.376 |
| Radiological findings | ||||
| Calcifications present (%) | 509 (58.6) | 225 (100.0) | 734 (67.2) | <0.001 |
| Number of calcified coronary artery - median [IQR] | 1.00 [0.00, 2.00] | 3.00 [3.00, 3.00] | 1.00 [0.00, 3.00] | <0.001 |
| TAC Agatston - mean (SD) | 1287.34 (2957.15) | 6648.70 (5677.91) | 2379.38 (4262.79) | <0.001 |
| TAC calcium volume (mm3)- mean (SD) | 1042.83 (2306.75) | 5380.02 (4479.02) | 1926.25 (3370.72) | <0.001 |
| AVC Agatston - mean (SD) | 86.26 (247.78) | 429.94 (431.50) | 155.50 (324.67) | <0.001 |
| AVC Volume (mm3) - mean (SD) | 76.59 (217.55) | 400.14 (408.06) | 141.78 (296.76) | <0.001 |
| TTC - median [IQR] | 224.38 [21.20, 1051.87] | 5146.77 [2726.08, 12412.07] | 503.77 [44.58, 2684.59] | <0.001 |
| Lung involvement | ||||
| Well aerated lung volume (cm3)- mean (SD) | 2387.35 (1377.13) | 2382.45 (1275.55) | 2386.34 (1356.24) | 0.962 |
| Pneumonia scoring (%) | 0.963 | |||
| Absent | 13 (1.5) | 3 (1.3) | 16 (1.5) | |
| <25% | 252 (29.1) | 67 (29.8) | 319 (29.2) | |
| 25–50% | 404 (46.6) | 101 (44.9) | 505 (46.2) | |
| 50–75% | 170 (19.6) | 45 (20.0) | 215 (19.7) | |
| >75% | 28 (3.2) | 9 (4.0) | 37 (3.4) | |
| Left pleural effusion (mm)- mean (SD) | 1.13 (3.51) | 2.08 (4.55) | 1.33 (3.77) | 0.001 |
| Right pleural effusion (mm) - mean (SD) | 1.37 (4.48) | 2.77 (6.07) | 1.66 (4.88) | <0.001 |
| Pericardial effusion (mm)- mean (SD) | 0.20 (0.88) | 0.37 (1.17) | 0.24 (0.95) | 0.016 |
| In-hospital complications | ||||
| MACCE (%) | 0.386 | |||
| None | 321 (84.9) | 72 (84.7) | 393 (84.9) | |
| Cerebral stroke | 10 (2.6) | 2 (2.4) | 12 (2.6) | |
| Acute coronary syndrome | 3 (0.8) | 3 (3.5) | 6 (1.3) | |
| Pulmonary Embolism | 36 (9.5) | 7 (8.2) | 43 (9.3) | |
| Peripheral embolism | 8 (2.1) | 1 (1.2) | 9 (1.9) | |
| In-hospital outcomes | ||||
| Hospitalization <24 h (%) | 25 (2.9) | 0 (0.0) | 25 (2.3) | 0.005 |
| Hospitalization without oxygen support (%) | 58 (6.7) | 8 (3.6) | 66 (6.0) | 0.085 |
| Hospitalization with oxygen support (%) | 249 (28.7) | 63 (28.0) | 312 (28.5) | 0.869 |
| Hospitalization with NIV (%) | 162 (18.7) | 32 (14.2) | 194 (17.7) | 0.142 |
| Orotracheal intubation (%) | 136 (15.7) | 19 (8.4) | 155 (14.2) | 0.005 |
| In-hospital death (%) | 125 (14.4) | 86 (38.2) | 211 (19.3) | <0.001 |
| Death after orotracheal intubation (%) | 27 (3.1) | 9 (4.0) | 36 (3.3) | 0.529 |
IQR = interquartile range, SD = standard deviation, LDH = Lactate dehydrogenase, CPR = c-protein reactive, MACCE = major adverse cardiac and cerebrovascular events; Tni-HS = high-sensitivity troponin I, NIV = noninvasive ventilation, CAC = coronary artery calcium, TAC = thoracic aorta calcium, AVC = Aortic Valve Calcium.
Table 2 shows the Cox proportional-hazards models for factors associated with in-hospital death. the adjusted time-to-event analyses, CAC volume predicts in-hospital mortality together to older age, male gender, arterial hypertension, well aerated lung volume, elevated levels of white blood cells and creatinine (time dependent AUC = 0.805). In particular, CAC volume >335.48 mm3 predicted in-hospital death with hazard ratio equal to 1.308 895% CI, 1.046–1.637, p = 0.019) (Fig. 1 ).
Fig. 1.
Kaplan-Maier curve showing cumulative hazard correlated with coronary artery calcium.
3.3. Impact of total calcium thoracic on in-hospital mortality
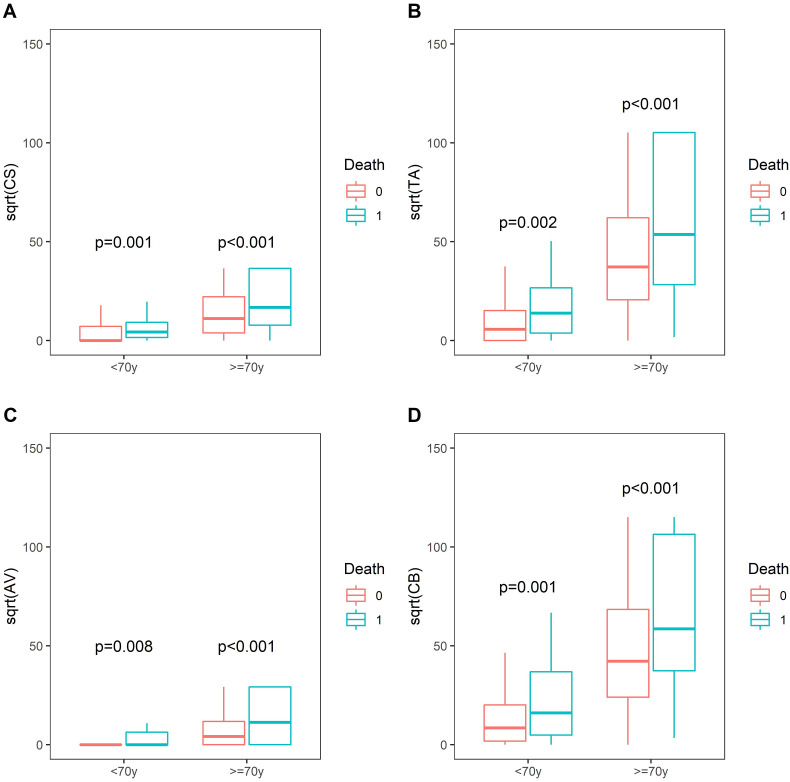
Non-survivors group showed higher calcium volume and Agatston mean values (±SD) of aortic valve [Volume (322.45 ± 390.90 vs 98.27 ± 250.74 mm3, p < 0.001; Agatston 337.38 ± 414.97 vs 111.70 ± 282.15, p < 0.001)] and thoracic aorta [Volume (3786.71 ± 4225.57 vs 1487.63 ± 2973.19 mm3, p < 0.001); Agatston (4688.82 ± 5363.72 vs 1834.90 ± 3761.25, p < 0.001)]. Mortality rates related to CAC, AVC and TAC were higher even after stratification for age <70 years-old and ≥70 years-old (Fig. 2 ).
Fig. 2.
Mortality differences stratified for age (≤/>70 years) according to coronary (A), thoracic aorta (B), aortic valve (C) calcium and total thoracic calcium(D).
Total thoracic calcium, defined as the total of CAC + AVC + TAC volumes, was calculated. The total thoracic calcium mean values (±SD) was significantly higher in non-survivors [4564.20 (±4711.22) vs 1772.81 (±3316.01) mm3, p < 0.001].
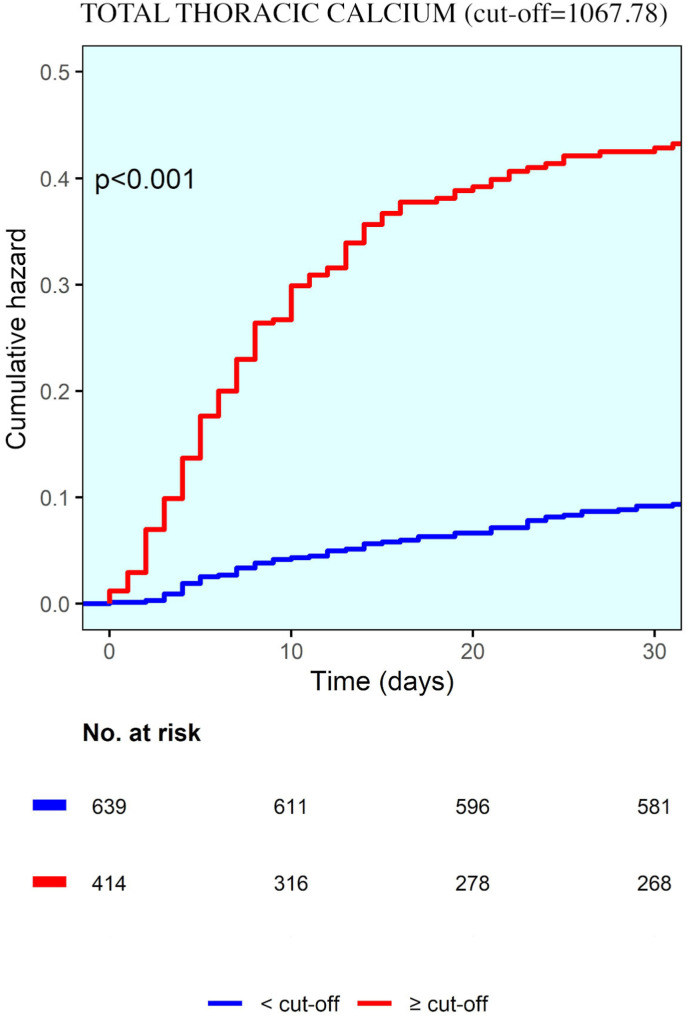
Receiver-operator characteristic curve analysis showed fair discrimination between survivors and non-survivors (C-statistic of 0.817) at a TTC of 1067.78 (Supplementary Figure 2). At this value, the sensitivity and specificity for predicting in-hospital death were 96% and 36%, respectively. The derived TTC optimal cut-off divided the population into two subgroups: a total of 675 patients had a higher TTC (>cut-off) while 418 patients presented a lower TTC (≤cut-off). Mortality rate was significantly higher in patient with higher TTC (35.4% vs 9.3%, p-value. <0.001) (for details see Table 5 ).
Table 5.
Population description according to the identified total thoracic calcium volume cut-off values (=1067.78).
| TTC ≤ cut-off (N = 675) | TTC > cut-off (N = 418) | Overall (N = 1093) | p-value | |
|---|---|---|---|---|
| Age - median [IQR] | 60.50 [54.00, 69.00] | 77.00 [70.00, 82.00] | 68.00 [58.00, 76.00] | <0.001 |
| Gender, male - (%) | 455 (67.9) | 287 (68.8) | 742 (68.3) | 0.789 |
| Arterial hypertension - (%) | 283 (42.8) | 307 (74.0) | 590 (54.8) | <0.001 |
| Diabetes - (%) | 76 (11.5) | 102 (24.6) | 178 (16.5) | <0.001 |
| Chronic kidney disease - (%) | 23 (4.9) | 26 (11.2) | 49 (7.0) | 0.004 |
| Chronic coroanry artery disease - (%) | 13 (3.0) | 31 (13.1) | 44 (6.6) | <0.001 |
| Peripheral artery disease - (%) | 16 (2.4) | 39 (9.4) | 55 (5.1) | <0.001 |
| Previous CABG - (%) | 4 (0.9) | 11 (4.8) | 15 (2.3) | 0.004 |
| Chronic lung disease - (%) | 38 (5.8) | 69 (16.6) | 107 (10.0) | <0.001 |
| Active malignancy - (%) | 25 (3.8) | 26 (6.3) | 51 (4.7) | 0.076 |
| Laboratory data | ||||
| Hemoglobin (mg/dl) - (median [IQR]) | 14.00 [12.70, 15.00] | 13.20 [12.00, 14.40] | 13.80 [12.40, 14.80] | <0.001 |
| White blood cells/mm3 - (median [IQR]) | 6610.00 [4900.00, 9570.00] | 6910.00 [5190.00, 9765.00] | 6730.00 [5000.00, 9667.50] | 0.130 |
| Creatinine mg/dl - median [IQR] | 0.95 [0.79, 1.14] | 1.08 [0.88, 1.42] | 0.99 [0.81, 1.24] | <0.001 |
| TnI HS ng/ml - median [IQR] | 8.00 [4.53, 18.00] | 14.80 [9.00, 49.00] | 11.20 [6.00, 31.65] | 0.001 |
| LDH mg/dl - median [IQR] | 374.00 [272.00, 498.00] | 359.00 [274.50, 486.00] | 369.00 [272.25, 491.00] | 0.432 |
| CRP mg/L - median [IQR] | 11.70 [5.31, 21.09] | 11.23 [5.50, 18.77] | 11.55 [5.40, 20.11] | 0.499 |
| Laboratory data during the hospitalization | ||||
| IL-6 pg/ml- median [IQR] | 54.85 [21.57, 244.25] | 73.00 [34.40, 157.55] | 60.20 [24.80, 233.00] | 0.372 |
| D-dimer ng/ml- median [IQR] | 1.81 [0.68, 5.66] | 1.95 [1.00, 4.05] | 1.85 [0.77, 5.33] | 0.394 |
| Radiological findings | ||||
| Calcification | ||||
| Coronary calcification | 346 (51.3) | 388 (92.8) | 734 (67.2) | <0.001 |
| Number of calcified coronary vessels | 1.00 [0.00, 2.00] | 3.00 [2.00, 3.00] | 1.00 [0.00, 3.00] | <0.001 |
| CAC Agatston - mean (SD) | 53.37 (180.37) | 586.29 (586.18) | 257.18 (467.37) | <0.001 |
| CAC Volume (mm3)- mean (SD) | 56.70 (156.85) | 592.34 (569.86) | 261.93 (455.28) | <0.001 |
| TAC Agatston - mean (SD) | 253.85 (865.11) | 5771.07 (5223.41) | 2379.38 (4262.79) | <0.001 |
| TAC volume (mm3) - mean (SD) | 219.48 (697.46) | 4649.75 (4082.09) | 1926.25 (3370.72) | <0.001 |
| AVC Agatston- mean (SD) | 27.83 (139.33) | 358.31 (418.58) | 155.50 (324.67) | <0.001 |
| AVC Volume (mm3) - mean (SD) | 21.83 (102.07) | 332.32 (390.43) | 141.78 (296.76) | <0.001 |
| Lung involment | ||||
| Well aerated lung volume - mean (SD) | 2417.84 (1413.00) | 2335.41 (1259.09) | 2386.34 (1356.24) | 0.330 |
| Left pleural effusion (mm) - mean (SD) | 1.12 (3.49) | 1.67 (4.16) | 1.33 (3.77) | 0.019 |
| Right pleural effusion (mm) - mean (SD) | 1.26 (4.31) | 2.31 (5.63) | 1.66 (4.88) | 0.001 |
| Pericardial effusion (mm)- mean (SD) | 0.16 (0.78) | 0.36 (1.15) | 0.24 (0.95) | 0.001 |
| Pneumonia - (%) | 0.549 | |||
| 0 | 12 (1.8) | 4 (1.0) | 16 (1.5) | |
| <25% | 189 (28.0) | 130 (31.2) | 319 (29.2) | |
| 25–50% | 312 (46.2) | 193 (46.3) | 505 (46.2) | |
| 50–75% | 140 (20.7) | 75 (18.0) | 215 (19.7) | |
| >75% | 22 (3.3) | 15 (3.6) | 37 (3.4) | |
| Pericardial effusion - mean (SD) | 0.16 (0.78) | 0.36 (1.15) | 0.24 (0.95) | 0.001 |
| Left pleural effusion - mean (SD) | 1.12 (3.49) | 1.67 (4.16) | 1.33 (3.77) | 0.019 |
| Right pleural effusion - mean (SD) | 1.26 (4.31) | 2.31 (5.63) | 1.66 (4.88) | 0.001 |
| In-hospital complications | ||||
| MACCE (%) | 0.143 | |||
| None | 256 (85.3) | 137 (84.0) | 393 (84.9) | |
| Cerebral stroke | 4 (1.3) | 8 (4.9) | 12 (2.6) | |
| Acute coronary syndrome | 3 (1.0) | 3 (1.8) | 6 (1.3) | |
| Pulmonary Embolism | 31 (10.3) | 12 (7.4) | 43 (9.3) | |
| Peripheral embolism | 6 (2.0) | 3 (1.8) | 9 (1.9) | |
| In-hospital outcomes | ||||
| Hospitalization < 24 h (%) | 24 (3.6) | 1 (0.2) | 25 (2.3) | <0.001 |
| Hospitalization without oxygen support (%) | 48 (7.1) | 18 (4.3) | 66 (6.0) | 0.067 |
| Hospitalization with oxygen support (%) | 191 (28.3) | 121 (28.9) | 312 (28.5) | 0.836 |
| Hospitalization with NIV (%) | 134 (19.9) | 60 (14.4) | 194 (17.7) | 0.022 |
| Orotracheal intubation (%) | 113 (16.7) | 42 (10.0) | 155 (14.2) | 0.002 |
| In-hospital death (%) | 63 (9.3) | 148 (35.4) | 211 (19.3) | <0.001 |
| Death after orotracheal intubation (%) | 17 (2.5) | 19 (4.5) | 36 (3.3) | 0.081 |
IQR = interquartile range, SD = standard deviation, LDH = Lactate dehydrogenase, CPR = c-protein reactive, MACCE = major adverse cardiac and cerebrovascular events; Tni-HS = high-sensitivity troponin I, NIV = noninvasive ventilation, TTC total thoracic calcium, CAC = coronary artery calcium, TAC = thoracic aorta calcium, AVC = Aortic Valve Calcium.
Table 3 shows the Cox proportional-hazards models for factors associated with in-hospital death. In the adjusted time-to-event analyses, variables that were independently associated with higher in-hospital mortality were older age, male gender, total thoracic calcium, well aerated lung volume, elevated levels of white blood cells and creatinine (time dependent AUC = 0.82) with an hazard ratio of 1.975 (95% CI, 1.200–3.251, p = 0.007) for TTC. Fig. 3 .
Fig. 3.
Kaplan-Maier curve showing cumulative hazard correlated with total thoracic calcium.
4. Discussion
The major findings of our study are: (1) CAC score quantified with non-gated chest CT is an predictor of mortality among hospitalized COVID-19 patients; (2) coronary calcium volume and total thoracic calcium are associated to higher mortality rate; (3) coronary calcium volume and total thoracic calcium predict in-hospital mortality together to older age, male gender, arterial hypertension, specific laboratory parameters (creatinine and white blood cells) and respiratory lung reserve at CT.
In our study 98.5% of the study population had a radiological diagnosis of pneumonia, which extension was at least moderate (>25% lung volume) in 69.3% of patients.14 , 17 The rates of orotracheal intubation and in-hospital mortality (14.2% and 20%, respectively) were in line with previous experiences.18 , 19
To our knowledge, this is the first large study which demonstrates that CAC volumes is a predictor of in-hospital mortality in the setting of a systemic acute inflammatory disease, such as COVID-19 (HR 1.308, p = 0.019). CAC is a well-established long-term imaging biomarker used to identify individuals at higher risk for cardiovascular events.20 , 21 The assessment of CAC on non-gated chest CT allows to quantify the coronary artery atherosclerosis with an objective and reproducible score, providing evidence of coronary artery disease also if previously clinically unknown. Although the study population had a low prevalence of coronary artery disease (6.6%), the presence of CAC was detected in a significant proportion of patients (67.2%), suggesting a subclinical disease.22
Observational studies have reported the role of cardiovascular involvement (defined as endothelitis, micro- and macro-vascular thrombosis, pulmonary embolism and coronary acute events) in patients with COVID-19,23, 24, 25, 26, 27 which was associated to increased mortality, as also suggested by our data (83% of in-hospital mortality in case of acute myocardial infarction). Being associated to coronary artery disease, CAC behaves as a specific organ function biomarker associated to a higher mortality. In this regard, coronary calcifications have been related to myocardial ischemia, where COVID-19 disease may exacerbate the underlying condition.28 , 29
Aortic valve and thoracic aorta calcifications were qualitatively assessed: both were strongly related to higher in-hospital mortality regardless of age and then subsequently included in the second multivariate analysis of mortality prediction. The extent of aortic valvular calcification directly represents degenerative aortic valve stenosis. A dysfunctional valve could increase the susceptibility to systemic hypoxia, which is the typical clinical scenario of COVID-19 and respiratory distress.30 On the other hand, thoracic aortic calcification reflects systemic atherosclerosis and is related to cardiovascular morbidity and mortality risks. Total thoracic calcium, defined as the combined CAC, AVC and TAC volumes emerged as an independent predictor of mortality with a hazard ratio of 1.9, even stronger than coronary CAC volume.
In our experience, as an element of novelty, calcium scores appear to be suitable outcome predictors of mortality in an infective disease. Patients with high CAC or TTC (> cut-off) experienced a higher rate of in-hospital death with respect to those with lower values (Table 4 and Table 5). Moreover, it is noteworthy to point out that patients with CAC > cut-off had higher incidence of acute myocardial infarction (3.5% vs 0.8%), and patients with high TCT (>cut-off) had higher incidence of stroke (4.9% vs 1.3%) as of acute myocardial infarction (1.8% vs 1%), both associated to higher in-hospital mortality rate (25% and 83% respectively vs 19% in patients without MACE).
Indeed, cardiovascular calcifications may represent a bystander of an impaired vascular reserve, both microvascular and endothelial, but also a sign of vascular senescence. Therefore, it can be considered an index of biological frailty, likely more accurate than age.
Coronary artery calcium, as well as TAC, ACV and TTC can be quantified on non-gated chest CT performed in COVID-19 patients for lung assessment using commercial and widely available software. CAC and TTC may represent additional imaging biomarkers for risk stratification in COVID-19 patients, not requiring further biological costs and healthcare resources. The prognostic role of CAC and TTC could be also investigated in other acute inflammatory systemic syndromes.
Specific limitations in addition to those inherent to a retrospective study should be taken into consideration when interpreting our findings.
Our study population included only COVID-19 patients who underwent chest CT for lung assessment. Therefore, these findings are potentially not reflective of all COVID-19 patients. However, it is relevant to underline that all consecutive patients by each participating center were enrolled. Moreover, clinical, laboratory and outcome features of our study population were consistent to those previously reported in literature for COVID-19.18 Unfortunately, a risk stratification for patients requiring more intensive care is not addressed for selection bias and different protocols adopted in the overwhelmed participating hospitals.
Finally, inter-hospital variability was considered in multivariate analyses.
5. Conclusions
In conclusion, CAC and TTC, both obtained from non-gated chest CT performed for lung evaluation in COVID-19 patients, could be a powerful tool for risk stratification in COVID-19 patients. TTC, as the sum of CAC, TAC and AVC, is a radiological CT biomarker of systemic atherosclerosis and biologic frailty and resulted as a stronger predictor of mortality than CAC alone, in COVID-19 patients.
Footnotes
The authors have no conflict of interest to declare. No funding.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcct.2021.03.003.
Appendix A. Supplementary data
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel Coronavirus originating in wuhan, China: challenges for global Health governance. J Am Med Assoc. 2020;323(8):709. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. Published online May 2020:S1201971220303623. doi:10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed]
- 3.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online March 27, 2020. doi:10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed]
- 4.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, Health care workers, and Health systems during the Coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. Published online March 2020:S0735109720346374. doi:10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed]
- 5.Esposito A., Palmisano A., Toselli M. Chest CT-derived pulmonary artery enlargement at the admission predicts overall survival in COVID-19 patients: insight from 1461 consecutive patients in Italy. Eur Radiol. Published online December. 2020;23 doi: 10.1007/s00330-020-07622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loffi M., Regazzoni V., Toselli M. Incidence and characterization of acute pulmonary embolism in patients with SARS-CoV-2 pneumonia: a multicenter Italian experience. PloS One. 2021;16(1) doi: 10.1371/journal.pone.0245565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmisano A., Scotti G.M., Ippolito D. Chest CT in the emergency department for suspected COVID-19 pneumonia. Radiol Med. 2020 doi: 10.1007/s11547-020-01302-y. Published online November 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in Coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. Published online February 26, 2020:200642. doi:10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 9.Hecht H.S., Cronin P., Blaha M.J. SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. Journal of Cardiovascular Computed Tomography. 2016;11(1):74–84. doi: 10.1016/j.jcct.2016.11.003. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Yano Y., O’Donnell C.J., Kuller L. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2(9):986. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs P.C., Prokop M., van der Graaf Y. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209(2):455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Walsh C.R., Larson M.G., Kupka M.J. Association of aortic valve calcium detected by electron beam computed tomography with echocardiographic aortic valve disease and with calcium deposits in the coronary arteries and thoracic aorta. Am J Cardiol. 2004;93(4):421–425. doi: 10.1016/j.amjcard.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Colombi D., Bodini F.C., Petrini M. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020201433. Published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernheim A., Mei X., Huang M. Chest CT findings in Coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 16.McCollough C.H., Ulzheimer S., Halliburton S.S., Shanneik K., White R.D., Kalender W.A. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007;243(2):527–538. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 17.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. American Journal of Roentgenology. Published online March. 2020;14:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 18.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020;323(16):1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faust J.S., del Rio C. Assessment of deaths from COVID-19 and from seasonal influenza. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2306. Published online May 14. [DOI] [PubMed] [Google Scholar]
- 20.Miedema M.D., Dardari Z.A., Nasir K. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Budoff M.J., Nasir K. Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2017;257:1–8. doi: 10.1016/j.atherosclerosis.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19) doi: 10.1093/eurheartj/ehaa254. 1858-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of Coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. Published online March. 2020;27 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 27.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30145-9. Published online May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenker M.P., Dorbala S., Hong E.C.T. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117(13):1693–1700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw L.J., Raggi P., Berman D.S., Callister T.Q. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188(1):112–119. doi: 10.1016/j.atherosclerosis.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Vincent J.-L., Taccone F.S. Understanding pathways to death in patients with COVID-19. The Lancet Respiratory Medicine. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.