Abstract
Objective Clinical decision support (CDS) alerts built into the electronic health record (EHR) have the potential to reduce the risk of drug-induced long QT syndrome (diLQTS) in susceptible patients. However, the degree to which providers incorporate this information into prescription behavior and the impact on patient outcomes is often unknown.
Methods We examined provider response data over a period from October 8, 2016 until November 8, 2018 for a CDS alert deployed within the EHR from a 13-hospital integrated health care system that fires when a patient with a QTc ≥ 500 ms within the past 14 days is prescribed a known QT-prolonging medication. We used multivariate generalized estimating equations to analyze the impact of therapeutic alternatives, relative risk of diLQTS for specific medications, and patient characteristics on provider response to the CDS and overall patient mortality.
Results The CDS alert fired 15,002 times for 7,510 patients for which the most common response (51.0%) was to override the alert and order the culprit medication. In multivariate models, we found that patient age, relative risk of diLQTS, and presence of alternative agents were significant predictors of adherence to the CDS alerts and that nonadherence itself was a predictor of mortality. Risk of diLQTS and presence of an alternative agent are major factors in provider adherence to a CDS to prevent diLQTS; however, provider nonadherence was associated with a decreased risk of mortality.
Conclusion Surrogate endpoints, such as provider adherence, can be useful measures of CDS value but attention to hard outcomes, such as mortality, is likely needed.
Keywords: clinical decision support, electronic health records, drug-induced QT prolongation
Background and Significance
Since its initial description in the 1960s with quinidine use, 1 drug-induced long QT syndrome (diLQTS) and the subsequent potentially fatal arrhythmia torsades de pointes (TdP) have been major factors in prescription of medications for both cardiac 2 and noncardiac indications. 3 4 Despite its relative rarity with noncardiovascular drugs, diLQTS leading to TdP can have devastating effects when it occurs with medications used for benign conditions, such as diphenhydramine for allergic rhinitis. 5 In these cases, there are alternatives that could be selected if an individual was identified as having an increased risk of diLQTS 6 ; however, often ordering providers are unaware of either risks of diLQTS in a given patient, or that there may be safer alternatives in high-risk patients. In this setting, clinical decision support (CDS) tools can provide guidance.
The move toward widespread adoption of electronic health records (EHRs) was mandated under the American Recovery and Reinvestment Act, 7 and many modern payers including Medicare require compliance with EHR meaningful use guidelines. 8 With this move toward fully electronic data collection and electronic prescriptions comes the potential for full integration of clinical information into routine clinical care decisions, and development of CDS tools. Nonetheless, this potential for end-to-end use of data in the care continuum remains largely underutilized across health care systems. 9 10
Several years ago, our team deployed several CDS tools designed to mitigate risks of diLQTS by alerting providers of potential risk of diLQTS with prescription of several high-risk medications for patients with QT prolongation (QTc ≥ 500 ms). 11 12 These CDS tools decreased use of QT-prolonging medications broadly within the population. However, further work is needed to understand the strengths and weaknesses of this alert at the scale in which it is being applied and to identify measures to optimize its effectiveness.
Objectives
In this investigation, we examined factors that influence provider adherence to a CDS tool for diLQTS and its potential impact on patient outcomes.
Methods
This is a retrospective, observational, cross-sectional study evaluating CDS tools to mitigate diLQTS. The goals of this project are to (1) examine the impact of an available therapeutic alternative on provider response to the CDS, (2) identify the relative risk of diLQTS for specific medications, (3) examine the effect of different patient characteristics (e.g., risk factors and relative risk of diLQTS) on providers' response to the CDS, and (4) explore the impact of these factors on overall patient mortality.
Study Population
Instances of the CDS tools alerting between October 8, 2016 and November 8, 2018 were evaluated. Patients evaluated had a QTc ≥ 500 ms on an electrocardiography (ECG) in the past 14 days and had an order placed for 1 of 10 known high-risk QT prolonging medications during a hospital encounter (see below). This evaluation was conducted at a 13-hospital integrated health care system spanning the greater Colorado, United States, front range region, representing academic, community, rural, and suburban settings. The health care system has used a single instance of its integrated EHR (Epic Systems, Verona, Wisconsin, United States) since 2011.
Description of the Clinical Decision Support Tools
The CDS tools we studied in this investigation ( Fig. 1 ) 11 12 are triggered when a patient has had a prior ECG with a QTc value logged in the EHR that is ≥ 500 ms within the past 14 days and is prescribed 1 of 10 medications with potential to cause diLQTS: azithromycin, chlorpromazine, citalopram, clarithromycin, erythromycin, escitalopram, haloperidol, methadone, moxifloxacin, or parenteral ondansetron. These medications were selected because they are the most frequently prescribed QT-prolonging medications in the health care system and are commonly prescribed across service lines and specialties. The decision to have a 14-day look back period for QTc values was determined by the health system to increase clinical relevance of the alert and minimize alert fatigue. The alert interrupts the ordering provider's workflow when an order for 1 of the 10 medications is entered and recommends discontinuation of the medication order. If providers select “Keep,” the order remains queued up for the clinician to sign. Providers who select “Keep” are also given the opportunity to provide a reason they wish to continue with their original medication order, with several structured responses provided, as well as a free-text option. Preliminary work suggested that reasons were inconsistently applied, thus formal examination was beyond the scope of this investigation. 11 12 If providers select “Remove,” then the order for the QT-prolonging medication is canceled. There is also an option to select “Cancel” which allows providers to dismiss the alert and continue with their intended medication order. Selecting “Cancel” is the easiest way to bypass the alert with the fewest “clicks.”
Fig. 1.
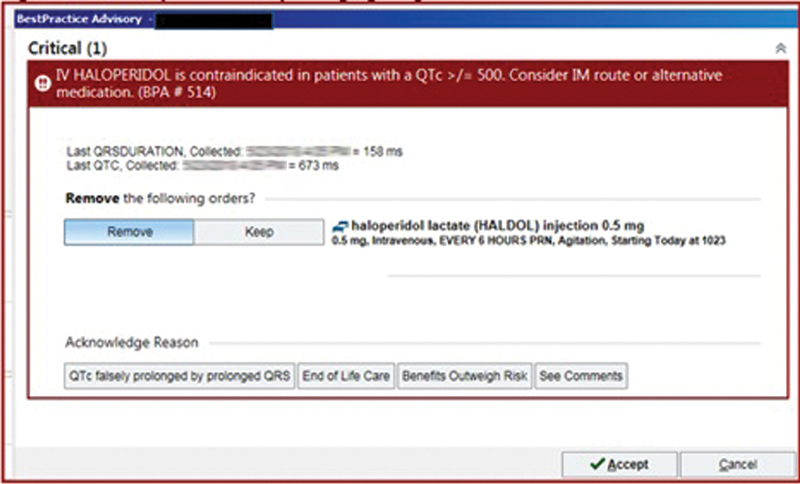
Example of the clinical decision support tools, which trigger upon a provider entering an order for a known QT-prolonging medication for a patient with an ECG in the system with a QTc interval of ≥ 500 ms. ECG, electrocardiography.
Data Collection
All data were collected from a secondary EHR virtual data warehouse or directly from the EHR using a CDS analytic report. The CDS analytic report was used to identify instances of the CDS tools alerting, the identity of the patient and provider, location of the alert, and provider stated action via the response options (e.g., “Keep,” “Cancel”). The secondary virtual data warehouse was used to collect patient characteristics, evaluate actual (versus stated) provider response via medication orders, and instances of death.
Outcome Measures
Outcomes of interest included (1) relative risk of diLQTS for specific medications, (2) whether a QT-prolonging medication was ordered after the alert (irrespective of stated response), (3) whether a provider stated they would remove the QT-prolonging medication order but actually ordered it (nonadherence to the CDS), and (4) mortality.
Separate from the alert data, we determined the rate of diLQTS for each of the 10 QT-prolonging medications and an additional three drugs across our health system as follows: (1) amiodarone, (2) sotalol, and (3) dofetilide. These three drugs were selected based on frequency of use and perceived high propensity to cause diLQTS. A diLQTS event (cases) was defined as an ECG within 24 hours of being administered a known QT-prolonging medication where the QTc measured by the computer was over 500 ms, and the QRS duration was <120 ms. 13 A diLQTS nonevent (controls) was done if an ECG performed within 24 hours after a culprit medication had a QTc of <500 ms. Reason for death was not available, thus any reason for death was included.
We evaluated presence of a QT-prolonging medication order after the date/time of the CDS alert instance during the same encounter and within 30 days of the initial alert. Some clinician responses to the alerts could suppress future instances of the alert firing for that clinician during a given encounter; thus, a 30-day window was chosen to account for instances in which an alert was ignored by a given clinician early in a hospital stay and then ordered multiple days later. Since some medications were ordered multiple times after the initial alert, we only examined the first order of each medication after the alert. Provider stated action was compared with actual medication orders. When a provider stated they would abort prescribing the QT-prolonging medication (selected “Cancel” or “Remove”) but the medication was actually ordered, this was classified as “nonadherence to the CDS.” Mortality was defined as having died during the encounter at which the CDS alert fired.
Each medication was also categorized by presence of a safe therapeutic alternative. Safe therapeutic alternatives were defined as presence of an alternative medication for all possible indications that was not associated with diLQTS. Presence of a therapeutic alternative was adjudicated by a team of three physicians and clinical pharmacists with expertise in diLQTS. The team determined that escitalopram, citalopram, and methadone had no safe alternative, and there was a safer alternative for the others (note, the current alert does not provide suggestions or recommendations about safer alternatives).
Analysis
Multivariate analysis was performed using generalized estimating equations (GEE) to examine predictors of medication ordering after alert (irrespective of stated action), mortality, and nonadherence to alerts. Unless otherwise specified, continuous variables are presented as mean ± standard deviation and categorical as percentages. GEE with exchangeable covariance matrix clustered at the level of the patient, and binomial family was used to model, that is, medication orders after an alert, nonadherence, and mortality. The rate of diLQTS for each of the medications was evaluated to identify a specific risk of diLQTS for each medication which was then examined as a predictor of providers' responses to the CDS tool, as well as overall mortality. Queries in the virtual data warehouse were conducted on the Google Cloud Platform, using Google Big Query with SQL language. A two-sided p -value of 0.05 was considered statistically significant. Analyses were primarily conducted using R (version 3.4.1) and R Studio (version 1.0.153), with specific packages “summarytools,” “dplyr,” and “gee,” downloaded from a link ( https://cran.r-project.org ). Graphs were created using Stata IC, version 15 (StataCorp, Inc., College Station, Texas, United States). The study was approved by the Colorado Multiple Institutional Review Board.
Results
From October 8, 2016 through November 8, 2018, the CDS alert fired 15,002 times for 7,510 patients. Of these, 7,406 patients had demographic data available for analysis ( Table 1 ). The mean age across the population was 64.4 ± 17.5 years, with 51.3% male patients, 76.9% Caucasian, 9.2% African American, and 1.4% Asian. By far, the most common medication ordered that triggered an alert was parenteral ondansetron, followed by azithromycin and haloperidol. The most common user response was to “Keep” the culprit medication in 60.0%, followed by “Remove” to cancel the culprit order in 37.8% ( Table 2 ). The most common location for alerts firing was inpatient (96.6%). Of patients who had an alert fire for them, 5.5% died during the encounter.
Table 1. Alert and patient characteristics.
| Drug | Triggers (no. of patients) | CDS response n (%) |
Location n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancel Rx | Override (order) | Close CDS | Unknown | Inpatient | Emergency | Outpatient | Unknown | ||
| Azithromycin | 1,929 (924) | 1,079 (55.9) | 837 (43.4) | 0 (0.0) | 13 (0.7) | 1,836 (95.2) | 15 (0.8) | 16 (0.8) | 62 (3.2) |
| Chlorpromazine | 39 (14) | 14 (35.9) | 25 (64.1) | 0 (0.0) | 0 (0.0) | 39 (100) | 0 (0) | 0 (0) | 0 (0) |
| Citalopram | 608 (243) | 97 (16.0) | 509 (83.7) | 0 (0.0) | 2 (0.3) | 576 (94.7) | 0 (0) | 2 (0.3) | 30 (4.9) |
| Clarithromycin | 81 (36) | 38 (46.9) | 43 (53.1 | 0 (0.0) | 0 (0.0) | 61 (75.3) | 0 (0) | 0 (0) | 20 (24.7) |
| Erythromycin | 117 (45) | 56 (47.9) | 60 (51.3) | 0 (0.0) | 1 (0.9) | 116 (99.2) | 0 (0) | 0 (0) | 1 (0.8) |
| Escitalopram | 580 (249) | 107 (18.4) | 470 (81.0) | 0 (0.0) | 3 (0.5) | 514 (88.6) | 0 (0) | 9 (1.6) | 57 (9.8) |
| Haloperidol | 1,754 (886) | 868 (49.5) | 593 (33.8) | 287 (16.4) | 6 (0.3) | 1,751 (99.8) | 0 (0) | 3 (0.2) | 0 (0) |
| Methadone | 200 (57) | 70 (35.0) | 128 (64.0) | 0 (0.0) | 2 (1.0) | 189 (94.5) | 0 (0) | 2 (1.5) | 8 (4.0) |
| Moxifloxacin | 23 (9) | 8 (34.8) | 14 (60.9) | 0 (0.0) | 1 (4.3) | 19 (82.6) | 0 (0) | 1 (4.4) | 3 (13.0) |
| Ondansetron | 9,671 (4,943) | 4,650 (48.1) | 4,969 (51.4) | 0 (0.0) | 52 (0.5) | 9,395 (97.2) | 26 (0.3) | 228 (2.4) | 22 (0.2) |
| Total | 15,002 (7,406) | 6,987 (46.6) | 7,648 (51.0) | 287 (1.9) | 80 (0.5) | 14,496 (96.6) | 41 (0.3) | 262 (1.8) | 203 (1.4) |
Abbreviation: CDS, clinical decision support; Rx, prescription.
Note: Escitalopram, citalopram, and methadone were categorized as having no safe alternative.
Table 2. Provider response by medication ordering.
| Ordered after alert | Alert response | ||||
|---|---|---|---|---|---|
| “Remove” Rx | “Keep” Rx | “Cancel” alert | Unknown/other | Total | |
| Yes | 1,276 | 2,506 | 45 | 31 | 3,858 |
| No | 1,995 | 2,735 | 56 | 16 | 4,802 |
| Total | 3,271 | 5,241 | 101 | 47 | 8,660 |
Abbreviation: Rx, prescription.
Specific Response by Medication
As shown in Table 3 , on review of the order entry within the medical record, we found that providers proceeded to order the culprit medication 44.6% of the time during the same encounter, with 16.3% of medications ordered after selecting “Remove” or “cancel” within the alert (defined as nonadherence to the alert). The most common medication ordered after the alert was methadone (75.3%), the least common was azithromycin (33.5%), and the most common medication for which providers did not adhere to the alert was ondansetron (20.5%).
Table 3. Provider prescription behavior by medication.
| Drug | Ordered n (%) |
Time until order after alert (h) | Nonadherent n (%) |
|---|---|---|---|
| Azithromycin | 346 (33.5) | 58.2 ± 142.4 | 73 (7.1) |
| Chlorpromazine | 0 (0) | NA | 0 (0 |
| Citalopram | 293 (74.7) | 60.3 ± 143.4 | 8 (2.0) |
| Clarithromycin | 23 (54.8) | 58.1 ± 140.2 | 6 (14.3) |
| Erythromycin | 27 (55.1) | 62.6 ± 146.8 | 4 (8.2) |
| Escitalopram | 278 (74.3) | 58.1 ± 142.2 | 17 (4.6) |
| Haloperidol | 198 (24.2) | 59.6 ± 142.3 | 86 (10.5) |
| Methadone | 58 (75.3) | 59.3 ± 141.7 | 12 (15.6) |
| Moxifloxacin | 12 (70.6) | 60.8 ± 145.3 | 2 (11.8) |
| Ondansetron | 2,623 (44.9) | 62.1 ± 145.9 | 1,199 (20.5) |
| Total | 3,858 (44.6) | 72.0 ± 154.2 | 1,407 (16.3) |
Abbreviations: CDS, clinical decision support; NA, not available.
Note: Nonadherent refers to situations in which the ordering provider entered “Cancel” or “Remove” in the CDS and then proceeded to order the medication afterwards.
Risk of Drug-Induced Long QT Syndrome by Medication
To examine the association between risk and response to the alerts, we first examined the relative risk of diLQTS for each of the 10 medications within our center by medication ( Table 4 ). Medications associated with the highest rate of diLQTS were dofetilide (45%) followed by amiodarone (36%), sotalol (29%), and clarithromycin (18%). There was a linear association between risk of diLQTS and frequency of ordering the culprit medication ( Fig. 2A ) and an inverse association between risk and frequency of nonadherence to the alert ( Fig. 2B ).
Table 4. Risk of drug-induced long QT syndrome by drug.
| Drug | Number | Cases n (%) |
Controls n (%) |
|---|---|---|---|
| Haloperidol | 3,963 | 544 (13.7) | 3,419 (86.3) |
| Ondansetron | 12,857 | 2,498 (11.0) | 20,284 (89.0) |
| Citalopram | 2,080 | 304 (14.6) | 1,776 (85.4) |
| Escitalopram | 900 | 129 (14.3) | 771 (85.7) |
| Azithromycin | 1,765 | 256 (14.5) | 1,509 (85.5) |
| Clarithromycin | 68 | 12 (17.6) | 56 (82.4) |
| Erythromycin | 701 | 105 (15.0) | 596 (85.0) |
| Methadone | 492 | 83 (16.9) | 409 (83.1) |
| Moxifloxacin | 254 | 39 (15.4) | 215 (84.6) |
| Sotalol | 340 | 100 (29.4) | 240 (70.6) |
| Dofetilide | 378 | 170 (45.0) | 208 (55.0) |
| Amiodarone | 1,928 | 700 (36.3) | 1,228 (63.7) |
Note: Total cases (QTc > 500 ms after exposure) 3,558; controls (QTc < 500 ms after exposure) 31,081.
Fig. 2.
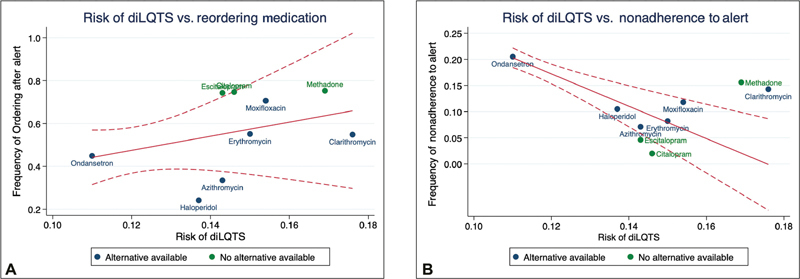
( A ) Graph of medication ordering versus risk of drug-induced long QT syndrome. ( B ). Graph of nonadherence to the CDS versus risk of drug-induced long QT syndrome. CDS, clinical decision support; diLQTS, drug- induced long QT syndrome.
Factors Associated with following the CDS Recommendation and Mortality
After adjustment for sex and location, we found that presence of a therapeutic alternative (odds ratio [OR] = 0.24, 95% confidence interval [CI]: 0.18–0.32, p < 0.00001), older age (OR per 10 years = 1.05, 95% CI: 1.00–1.10, p = 0.032), and higher risk of diLQTS (OR per percentile increase in risk = 0.78, 95% CI: 0.74–0.82, p < 0.00001) were significant predictors of ordering the medication after the alert had fired (irrespective of the stated response). Similarly, after adjustment for sex and location, presence of a therapeutic alternative (OR = 4.0, 95% CI: 2.77–5.75, p < 0.00001), older age (OR per 10 years = 0.88, 95% CI: 0.85–0.91, p < 0.00001), and higher risk of diLQTS (OR per percentile = 0.76, 95% CI: 0.72–0.80, p < 0.00001) were associated with adherence to the CDS (initial stated response aligned with actual action).
After adjustment for presence of an alternative, male sex (OR = 1.47, 95% CI: 1.09–1.98, p = 0.013), older age (OR per 10 years = 1.23, 95% CI: 1.12–1.36, p = 1.26e-5), and nonadherence to the CDS (OR = 0.64, 95% CI: 0.43–0.95, p = 0.028) were associated with mortality.
Discussion
In this single-center EHR-based examination of a CDS tool to prevent diLQTS, we found that small differences in risk of diLQTS within our institution, as well as presence of a safe alternative agent, were significantly associated with provider prescription patterns in response to the alert. When a safe therapeutic alternative was available, providers were more likely to adhere to the CDS. However, we found that risk of diLQTS was positively associated with ordering the culprit medication and inversely associated with nonadherence to the CDS, indicating that providers were more likely to ignore the alerts for medications with a relatively higher risk of diLQTS. In addition, nonadherence to the CDS was inversely associated with mortality for inpatients, which raises the notion that providers may know “better” which patients need a given medication, instead of this CDS intervention that is based on a single variable (prior QTc).
The ability of CDS systems to improve patient safety and reduce medical errors is well documented. In a systematic review and meta-analysis of commercial computerized provider order entry (CPOE) and CDS systems in intensive care units (ICUs), Prgomet et al found that CPOE use resulted in an 85% reduction in medication error rates, and a 12% reduction in ICU mortality. 14 Randomized controlled trials of CDS have been conducted, finding reductions in inappropriate prescriptions, 15 as well as appropriate dose changes based on renal function 16 in the implementation group. However, in a broad meta-analysis and systematic review of 69 articles describing CPOE and CDS systems, Légat et al noted that systems have a 90% override rate and that adverse drug events from overridden drug allergy alerts do not occur frequently, raising concerns about alert fatigue due to excessive false positives in many applications. 17 These studies highlight the potential of CDS to improve patient safety, but also the importance of studies on real-world issues such as provider adherence and impact on outcomes.
QT duration itself is a well-described risk factor for sudden death, 18 and investigators have found that within certain populations, such as psychiatric patients, who are exposed to several known QT-prolonging medications, screening by QT interval alone provides a cost-effective method to reduce mortality from sudden death. 19 Haugaa et al examined a CDS at the Mayo clinic that alerts providers when any ECG for a given patient has a QTc of ≥500 ms and noted that patients in whom the alert fired had increased mortality, after adjustment for age. 20 This idea has prompted several centers to implement CDS systems to guide providers. In addition to the CDS program, here at the University of Colorado, 11 12 other investigators 21 22 23 have examined automated CDS at their respective institutions for impact on prescription patterns and outcomes. Bertsche et al examined a computerized CDS for drug-drug interactions in the ICU, and found that the CDS tool reduced incidence of QT prolongation by 64%. 23 Sorita et al implemented a CDS integrated with the CPOE at the Mayo clinic and noted a 13.9% reduction in administration of QT-prolonging medications in patients with a QTc of ≥500 ms. 24 Sharma et al examined prescribing behavior of the Mayo clinic CDS and found that, compared with the preimplementation phase, providers were more likely to perform ECG monitoring after receiving the alert, but that there was no significant difference in the actual prescription of QT-prolonging medications. 21 Tisdale et al compared prescription patterns before and after implementation of a CDS system focused on the addition of intensive monitoring for patients prescribed known QT-prolonging medications at one hospital system, and noted a reduction in the prescription of noncardiac medications, including fluoroquinolones and haloperidol (OR = 0.79, 95% CI: 0.63–0.91) but did not examine an effect on mortality. 22 In summary, while most studies have identified some degree of change in provider behavior after CDS, to our knowledge, none have identified a change in mortality or sudden death as a result. Within this context, our finding that mortality was actually lower when providers ignored the CDS recommendations and went ahead and ordered the culprit medication holds major significance and highlights the need for more direct testing, via randomized controlled trials, of CDS tools to prevent diLQTS. It may be that clinicians used their clinical judgment to ignore the alert selectively for patients who were healthier or at lower risk. Further studies should evaluate the impact of incorporating additional indices of “patient risk” into the algorithms that trigger CDS tools and how this effects clinician adherence and patient mortality.
Limitations
There are several limitations in this investigation which highlight the challenges in both CDS design and analysis. First, like most CDS, the triggers for our system were based on very simple criteria, namely, only an ECG with QTc measurement within the past 14 days and stored electronically in structured data fields with a duration of ≥500 ms. This alert was naïve to QRS duration, heart rhythm (i.e., sinus vs. atrial fibrillation), or other perturbations that could have impacted the accuracy of the QT measurement as would reflect abnormal repolarization and increased risk of TdP. 25 In addition, because it was limited to only those QTc intervals measured within the past 14 days, it tended to only fire for inpatient visits, and likely missed patients with an ECG performed outside this time window that might have been prolonged—this limitation was reflected in our finding that over 95% of CDS alerts occurred within the inpatient setting. Further, in our analysis on the backend, we had no other information available about heart rhythm, QRS duration, cardiac function (left ventricular ejection fraction [LVEF]), or laboratory values, all of which could impact the QTc and relative risk of TdP and mortality. CDS tools triggered based on more advanced criteria that more comprehensively account for patient characteristics that influence a provider's decision-making (e.g., QRS duration and heart rhythm) are needed. In addition to indicating that further work is needed on developing analytical approaches to assess accuracy of the CDS, it also provides a potential explanation for providers choosing to ignore alerts; it is not unlikely that many providers simply clicked whichever options were necessary to close the alert, and then went ahead and ordered whichever medication they determined was needed at the time. Our mortality data suggest that providers were correct in doing so, although further investigation is needed into the underlying cause of mortality in these patients. Because we were unable to ascertain reason for death, our measure of mortality includes reasons unrelated to diLQTS and therefore is an overestimate, requiring further evaluation. Second, the size of the data we analyzed, while providing statistical power for determination of effect, also limited the granularity within which we could examine each individual case of the CDS alert. Manual chart review would in theory improve this assessment but chart review of all approximately 9,000 alerts is not practical, and the process of selecting which charts to review raises concerns about selection bias. Further, we are unable to ascertain reasons providers made the decisions they did. A qualitative evaluation of providers would prove useful to understand drivers for their decisions and to uncover further means to optimize the design of the CDS tool. Clinician informational needs and preferences for CDS design is important to optimize acceptance and adherence to CDS recommendations. 26 27 28 29 30 Finally, the relative risk calculations for diLQTS in this investigation were conducted broadly at the drug level across all patients, ignoring the specific patient-level information that may have placed a given patient at greater or lesser risk of diLQTS. Accurate risk prediction of diLQTS is an active area of research in our group, and we anticipate that with improved prediction modeling, a more accurate risk assessment could both improve accuracy of CDS alerts, as well as provide more meaningful guidance for providers at the time of prescription.
Conclusion
In conclusion, we found that presence of a therapeutic alternative agent was highly important in provider adherence to CDS alerts, which suggests that efforts to include this information in the CDS trigger logic could improve adherence. However, we also found that mortality was lower when providers ignored alerts and/or ordered the culprit medication after closing the alert, indicating that more work is needed to understand methods to bring CDS alert activity in line with provider behavior for the benefit of patient safety and outcomes.
Clinical Relevance Statement
A clinical decision support (CDS) tool for drug-induced long QT system (diLQTS) may be associated with lower overall mortality. When designing a CDS for diLQTS, developers should consider including suggestions for safe therapeutic alternatives when they exist.
Multiple Choice Questions
-
Which of the following are important indicators of CDS effectiveness that are infrequently evaluated?
Changes in hard outcomes.
Changes in provider behaviors.
Provider adherence rates.
Provider override rates.
Correct Answer: The correct answer is option a. CDS often evaluate clinician responses and process outcomes such as changes in behavior. Less often evaluated is the impact of CDS on hard outcomes such as mortality. Although difficult to establish causality of CDS effects on outcomes such as mortality or hospitalization, there is a need to evaluate such relationships.
-
In this study, which of the following factors was associated with an increase in provider adherence to the CDS for diLQTS?
The patient's sex was male.
The patient's sex was female.
Presence of a therapeutic alternative.
None of the above.
Correct Answer: The correct answer is option c. When a therapeutic alternative was available, providers were more likely to adhere to the CDS. Although the CDS tools did not suggest therapeutic alternatives that exist, such inclusion may further improve provider adherence. Patient sex did not influence provider adherence. Other factors that influenced provider adherence were age, relative risk of diLQTS with a given drug.
Funding Statement
Funding Data from the electronic health record was obtained through participation of the Health Data Compass data repository, in the Colorado Center for Personalized Medicine, on the University of Colorado Anschutz Medical Campus. CDS analysis was made possible through cooperation of the Epic CDS Governance Committee, directed by Dr. CT Lin. Funding for this investigation was provided by the National Institutes of Health, National Heart Lung and Blood Institute (MAR: K23 HL127296, R01 HL146824; KET: K12 HL137862).
Conflict of Interest None declared.
Protection of Human and Animal Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, and was reviewed by the Colorado Multiple Institutional Review Board.
References
- 1.Selzer A, Wray H W. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation. 1964;30:17–26. doi: 10.1161/01.cir.30.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Dofetilide U K. Dofetilide. UK 68, UK 68798, tikosyn, xelide. Drugs R D. 1999;1(04):304–311. doi: 10.2165/00126839-199901040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Straus S MJM, Sturkenboom M CJM, Bleumink G S. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26(19):2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 4.Darpo B, Nebout T, Sager P T. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guideline. J Clin Pharmacol. 2006;46(05):498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- 5.Poluzzi E, Raschi E, Godman B. Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe. PLoS One. 2015;10(03):e0119551. doi: 10.1371/journal.pone.0119551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milberg P, Eckardt L, Bruns H J. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002;303(01):218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- 7.Kocher R, Emanuel E J, DeParle N AM. The Affordable Care Act and the future of clinical medicine: the opportunities and challenges. Ann Intern Med. 2010;153(08):536–539. doi: 10.7326/0003-4819-153-8-201010190-00274. [DOI] [PubMed] [Google Scholar]
- 8.Abdolrasulnia M, Menachemi N, Shewchuk R M, Ginter P M, Duncan W J, Brooks R G. Market effects on electronic health record adoption by physicians. Health Care Manage Rev. 2008;33(03):243–252. doi: 10.1097/01.HMR.0000324904.19272.c2. [DOI] [PubMed] [Google Scholar]
- 9.Vis C, Mol M, Kleiboer A. Improving implementation of emental health for mood disorders in routine practice: systematic review of barriers and facilitating factors. JMIR Ment Health. 2018;5(01):e20. doi: 10.2196/mental.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dexheimer J W, Talbot T R, Sanders D L, Rosenbloom S T, Aronsky D. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inform Assoc. 2008;15(03):311–320. doi: 10.1197/jamia.M2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pell J M, Cheung D, Jones M A, Cumbler E. Don't fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc. 2014;21(06):1109–1112. doi: 10.1136/amiajnl-2014-002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung D, Cumbler E, Hale G, Pell J. Reining in the QTc: reducing the risk of torsades de pointes across a major health system. J Am Med Inform Assoc. 2018;25(09):1202–1205. doi: 10.1093/jamia/ocy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology ; Council on Cardiovascular Nursing ; American College of Cardiology Foundation . Drew B J, Ackerman M J, Funk M. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(09):934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prgomet M, Li L, Niazkhani Z, Georgiou A, Westbrook J I. Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J Am Med Inform Assoc. 2017;24(02):413–422. doi: 10.1093/jamia/ocw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terrell K M, Perkins A J, Dexter P R, Hui S L, Callahan C M, Miller D K. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009;57(08):1388–1394. doi: 10.1111/j.1532-5415.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- 16.Terrell K M, Perkins A J, Hui S L, Callahan C M, Dexter P R, Miller D K. Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med. 2010;56(06):623–629. doi: 10.1016/j.annemergmed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Légat L, Van Laere S, Nyssen M, Steurbaut S, Dupont A G, Cornu P. Clinical decision support systems for drug allergy checking: systematic review. J Med Internet Res. 2018;20(09):e258. doi: 10.2196/jmir.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noseworthy P A, Peloso G M, Hwang S J. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17(04):340–348. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poncet A, Gencer B, Blondon M. Electrocardiographic screening for prolonged QT interval to reduce sudden cardiac death in psychiatric patients: a cost-effectiveness analysis. PLoS One. 2015;10(06):e0127213. doi: 10.1371/journal.pone.0127213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugaa K H, Bos J M, Tarrell R F, Morlan B W, Caraballo P J, Ackerman M J. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(04):315–325. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Martijn Bos J, Tarrell R F. Providers' response to clinical decision support for QT prolonging drugs. J Med Syst. 2017;41(10):161. doi: 10.1007/s10916-017-0803-7. [DOI] [PubMed] [Google Scholar]
- 22.Tisdale J E, Jaynes H A, Kingery J R. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7(03):381–390. doi: 10.1161/CIRCOUTCOMES.113.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertsche T, Pfaff J, Schiller P. Prevention of adverse drug reactions in intensive care patients by personal intervention based on an electronic clinical decision support system. Intensive Care Med. 2010;36(04):665–672. doi: 10.1007/s00134-010-1778-8. [DOI] [PubMed] [Google Scholar]
- 24.Sorita A, Bos J M, Morlan B W, Tarrell R F, Ackerman M J, Caraballo P J.Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes J Am Med Inform Assoc 201522(e1):e21–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden D M. Acquired long QT syndromes and the risk of proarrhythmia. J Cardiovasc Electrophysiol. 2000;11(08):938–940. doi: 10.1111/j.1540-8167.2000.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 26.McCullagh L J, Sofianou A, Kannry J, Mann D M, McGinn T G. User centered clinical decision support tools: adoption across clinician training level. Appl Clin Inform. 2014;5(04):1015–1025. doi: 10.4338/ACI-2014-05-RA-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey K E, Mirica M, Phansalkar S, Ozonoff A, Harper M B. Clinician perceptions of timing and presentation of drug-drug interaction alerts. Appl Clin Inform. 2020;11(03):487–496. doi: 10.1055/s-0040-1714276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Rahman S M, Gill H, Carpenter S L. Design and usability of an electronic health record-integrated, point-of-care, clinical decision support tool for modeling and simulation of antihemophilic factors. Appl Clin Inform. 2020;11(02):253–264. doi: 10.1055/s-0040-1708050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinkley K E, Kahn M G, Bennett T D. Integrating the practical robust implementation and sustainability model with best practices in clinical decision support design: implementation science approach. J Med Internet Res. 2020;22(10):e19676. doi: 10.2196/19676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhat S, Derington C G, Trinkley K E. Clinicians' values and preferences for medication adherence and cost clinical decision support in primary care: a qualitative study. Appl Clin Inform. 2020;11(03):405–414. doi: 10.1055/s-0040-1712467. [DOI] [PMC free article] [PubMed] [Google Scholar]


