Abstract
Background:
Asthma is among the most common chronic diseases of children in the United States (US). Mold exposures have been linked to asthma development and exacerbation. In homes, mold exposures have been quantified using the Environmental Relative Moldiness Index (ERMI) and higher home ERMI values have been linked to occupant asthma.
Objective:
In this analysis of the School Inner-City Asthma Study (SICAS), we aimed to evaluate the ERMI’s applicability to measuring mold in schools compared with home mold levels, and to examine the prevalence of asthma in relationship to students’ demographics and the physical characteristics of school-buildings.
Methods:
Northeastern US schools (n=32) and homes (n=33) were selected and the 36-ERMI molds were quantified in a dust sample from each classroom (n=114) or home. School building characteristics were collected from SICAS. Asthma prevalence and student demographics data were obtained from government websites.
Results:
Levels of outdoor Group 2 molds were significantly (p<0.01) greater in schools compared with homes. The presence of air-conditioning in school buildings correlated significantly (P =0.02) with lower asthma prevalence.
Conclusion:
The prevalence of asthma in student bodies is associated with many factors in schools and homes.
Keywords: asthma, ERMI, mold, Northeast, schools, homes
INTRODUCTION
Asthma is among the most common chronic diseases of children in the United States, with the highest prevalence in African American /Blacks and Hispanic children as well as those of low socio-economic status.1 Children with asthma are more likely to require emergency department visits and hospitalizations compared to children without asthma.2 Although the causes of asthma are unknown, indoor mold exposures have been linked to asthma development and exacerbations.3,4
Mold exposures in children’s homes have been a major focus in asthma epidemiological studies.5–8 However, there is a growing recognition that environments outside the home, particularly the school, are also an important source of mold exposures.9–20 According to, the Health Effects of School Environment study21: “Schools should be routinely tested for global indoor molds’ evaluation”. Subsequently, the School Inner-City Asthma Study (SICAS 1) was designed to understand the effects of indoor allergen exposure, including molds, in school and asthma prevalence.15,22
The SICAS 1 study demonstrated that students’ classroom-specific exposures were associated with worsening asthma symptoms and declines in lung function.23–26 Therefore, an intervention study (SICAS 2) using HEPA filtration in the classrooms in schools was undertaken in order to reduce particle and allergen exposures.14,15 The focus of this analysis was the quantification of the mold at baseline (in years 1 to 4 of the study) in the classrooms of the schools selected for the study.
In the SICAS 1 study, 38 schools were assessed in years 2008 to 2013 and 100% of the 438 classrooms tested were positive for mold in the air collected with Burkard Indoor Recording Air Samplers.23 For this analysis of the SICAS 2 study, a mold quantification metric developed for homes, called the Environmental Relative Moldiness Index (ERMI),27 was evaluated for its usefulness in measuring mold in schools.
The ERMI is based on measurement of 36 molds, using quantitative PCR (qPCR) assays in the analysis of dust samples from a representative national sampling of US homes (n=1096) from the American Healthy Homes Survey (AHHS), conducted through the US Department of Housing and Urban Development (HUD).27 Of the 36 molds quantified, 26 Group 1 molds were selected as indicators of water-damage in the home. The remaining ten Group 2 molds were chosen because they enter homes from the outside air, independent of water damage.27 Because the ERMI was created to estimate mold for homes, it is unclear if the ERMI metric is broadly applicable to schools.
The ERMI metric was applied previously in one small study of asthma prevalence in schools.28 In that study, the average ERMI value for classrooms in one school with a history of water damage was compared to the average ERMI values for five schools without histories of water damage. Although this was only a small study, the results suggested that the ERMI metric might be a useful measure of mold contamination resulting from water damage in schools.
For this study, there were three goals: to evaluate the ERMI’s applicability to measuring mold in schools compared to homes, to assess the relationship between the prevalence of asthma in schools and mold levels, and to examine the prevalence of asthma in relationship to students’ demographics and the physical characteristics of school-buildings.
METHODS
Recruitment and Data Collection
The School Inner-City Asthma Intervention Study (SICAS) is a randomized, controlled clinical trial using environmental interventions modeled from successful home-based interventions. The SICAS study was approved by the Institutional Review Board of Boston Children’s Hospital. The description of SICAS study methods and data collection details have been previously published.14,15
This arm of the SICAS study was limited to the investigation of 32 schools in school years 2014 through 2018, for grades K-8 (ages 4 to 15 years old). Validated surveys assessed demographic characteristics of the students enrolled in each school and the physical characteristics of the school buildings, as previously described.14,15 Low income was defined as a family income <$20K/year. The data for the prevalence of asthma in each of the schools was obtained from government websites. We compared the ERMI metric from baseline assessments in SICAS schools (n=32) with an independent group of northeastern homes (n=33) sampled in HUD’s second AHHS, conducted in 2017-2019.27
Dust Sample Collection, qPCR Analysis and ERMI calculations
In each of the schools, dust samples were collected from between 1 to 11 classrooms (median 3 classrooms per school) based on the number of students recruited for the study. In each classroom (n=114), settled dust was collected with a Swiffer™ dust cloth (Proctor and Gamble, Cincinnati, OH, US) from above floor surface (e.g., tops of light fixtures, bookshelves, doors etcetera), as previously described.28 The technician, using disposable gloves, wiped each sample site with a single Swiffer™ cloth until the cloth became gray to black. The cloth was then placed in a sealable bag and returned to the laboratory for mold analysis. In each home, dust was collected by vacuuming two m2 in the bedroom and living room, as previously described.27 In side-by-side testing, vacuumed collected dust and Swiffer collected dust provided comparable ERMI analysis results.29
The vacuumed dust from the AHHS 2 samples was recovered from the samplers, as previously described.27 The dust was recovered from the Swiffer cloths, as previously described. 29 The mold analysis was performed on 5.0 ± 0.1 mg of sieved dust (300μ pore) from each sample by a commercial laboratory (Mycometrics LLC, Monmouth Junction, NJ). After the concentrations of each of the 26 Group 1 molds and ten Group 2 molds were determined, the ERMI values were calculated, as shown in the equation below. The summed logs of the concentrations of the Group 2 molds (s2) was subtracted from the summed logs of the concentrations of Group 1 molds (s1) to produce the ERMI value.27
Geospatial Mapping of the Schools and the Enrolled Students’ Prevalence of Asthma
School addresses were geocoded using geographic information systems (GIS) Arc-GIS 10.2.2 (Environmental Systems Research Institute, Redlands, CA). The average prevalence of asthma for each school was shown by proportionately sized spots on the map at the school locations (Figure 1). To assess whether participating school asthma prevalence rates were significantly clustered versus occurring in a random spatial distribution, we used univariate Moran’s I statistic, a global measure of spatial autocorrelation. A univariate Moran’s I statistic calculates the degree to which a value at each unit (in this case, school) are associated with the values of its 3 neighboring schools and calculates a global statistic for the study region. This statistic is interpreted similarly to a correlation coefficient with values ranging from −1 indicating negative spatial autocorrelation, schools tend to have neighbors with very different prevalence rates, to 1 indicating positive spatial autocorrelation, schools with high or low prevalence rates tend to be clustered near one another. For confidentiality purposes, masking geographic techniques were utilized for Figure 1 but the spatial relationship between schools was preserved.30
FIGURE 1.
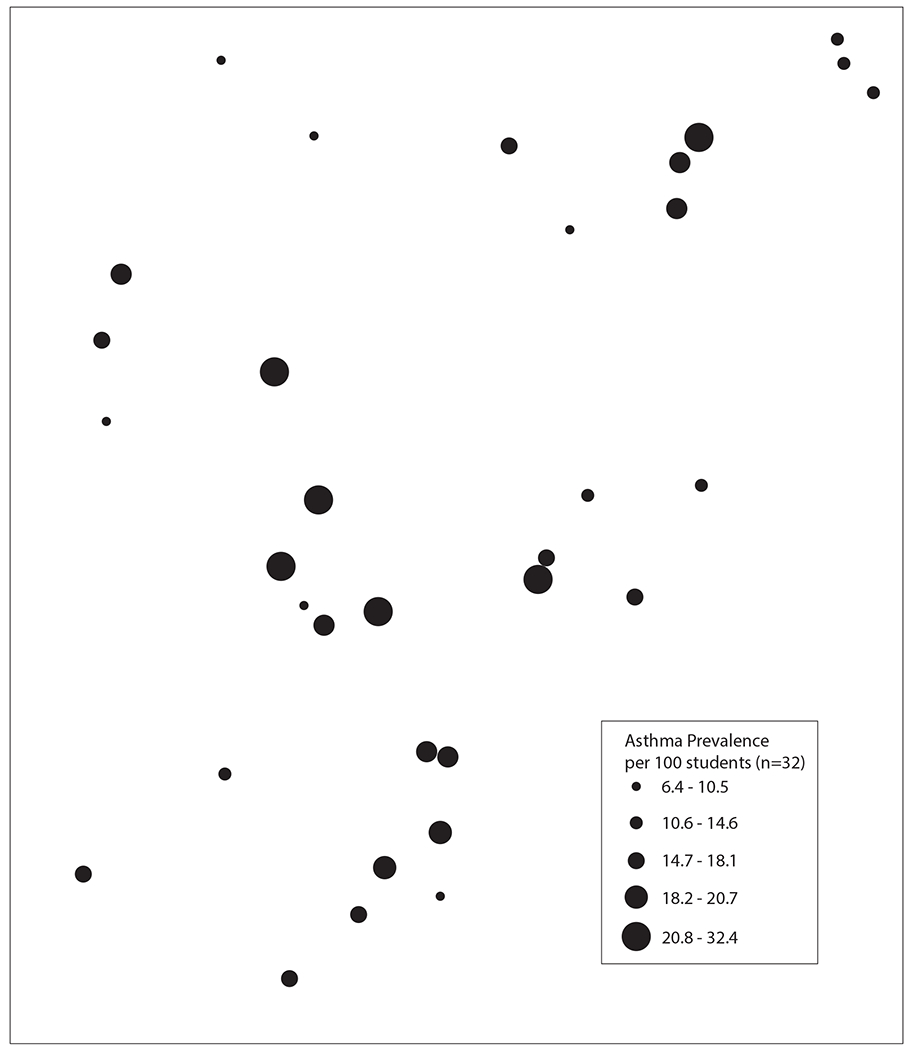
Geospatial map of schools in the study and the relative prevalence of asthma.
Statistical Analysis
The differences in concentrations of each of the 36 ERMI molds in the schools versus homes were evaluated by the Wilcoxon rank sum test, corrected for multiple comparisons using the Holms–Bonferroni test. The average summed logs of the Group 1 and Group 2 molds and the ERMI values for the schools were compared using the Student T-test.
Asthma prevalence was based on the total student population (n=13,027) for all 32 schools using the latest school year for which asthma prevalence data were available, 2016-2017 school year, except for two of the schools. For these two schools, the latest data for asthma prevalence were from the 2009-2010 or 2013-2014 school years, respectively.
For the statistical analyses, some of the physical characteristics of the schools were grouped into categories as follows: the number of rooms in the school were grouped: 1=11-20, 2=21-30, 3=31-40, 4=41-50, and 5=>50. The number of classrooms were grouped as follows: 1=<10, 2=11-20, 3=21-30, 4=31-40, and 5=>40. The school-building age was based on the year the school was built and years were grouped as follows: 1= before 1900, 2=1900-1949, 3=1950-1969, and 4=≥ 1970.
Linear mixed models, with a random intercept at the school level (114 classroom measurements in 32 schools), were used to account for clustering by classrooms in schools. These models were used to test the association between asthma prevalence (independent variable) and ERMI components (summed logs Group1, 2 or ERMI itself) values (dependent variable) (n=31). Covariates were chosen through univariate analysis. Any building or student characteristic that had an association (p<0.05 using linear regression) with asthma prevalence was added to the linear mixed model. All p-values were two-sided (unless specifically stated otherwise) and considered statistically significant with p<0.05. All analyses were performed in STATA 16.0 (StataCorp, College Station TX).
RESULTS
Table 1 presents the geometric means for each of 36 ERMI-molds in the schools (n=32) compared to homes (n=33). In schools, 11.5% (3/26) of the Group 1 molds were significantly higher than in homes, while in homes, 15.4% (4/26) of the Group 1 molds were significantly higher than in schools. However, 60.0% (6/10) of the Group 2 molds in schools were significantly higher than in homes, but only 10.0% (1/10) of the Group 2 molds in homes were significantly higher than in schools.
TABLE 1.
Population of each mold compared in schools and homes.
| Schools | HUD Homes | Wilcoxon | |
|---|---|---|---|
| Group 1 | GM | GM | p-value |
| Aspergillus flavus | 1.3 | 1.0 | NS |
| Aspergillus fumigatus | 6.6 | 1.7 | NS |
| Aspergillus niger | 49.5 | 7.0 | NS |
| Aspergillus ochraceus | 1.9 | 3.1 | NS |
| Aspergillus penicillioides | 37.2 | 116.9 | <0.001 |
| Aspergillus restrictus | 3.4 | 8.8 | <0.001 |
| Aspergillus sclerotiorum | 1.2 | 2.3 | NS |
| Aspergillus sydowii | 5.3 | 3.2 | NS |
| Aspergillus unguis | 1.3 | 1.1 | NS |
| Aspergillus versicolor | 7.1 | 26.4 | <0.001 |
| Aureobasidium pullulans | 483.1 | 215.9 | <0.001 |
| Chaetomium globosum | 2.3 | 1.6 | NS |
| Cladosporium sphaerospermum | 20.9 | 37.4 | NS |
| Eurotium amstelodami | 97.4 | 55.3 | <0.001 |
| Paecilomyces variotii | 5.5 | 1.5 | NS |
| Penicillium brevicompactum | 5.0 | 7.2 | NS |
| Penicillium corylophilum | 3.2 | 5.0 | NS |
| Penicillium crustosum | 10.9 | 7.9 | <0.001 |
| Penicillium purpurogenum | 1.1 | 1.0 | NS |
| Penicillium spinulosum | 1.0 | 1.0 | NS |
| Penicillium variabile | 3.7 | 5.4 | NS |
| Scopulariopsis brevicaulis | 1.8 | 1.4 | NS |
| Scopulariopsis chartarum | 1.3 | 2.7 | NS |
| Stachybotrys chartarum | 1.2 | 1.2 | NS |
| Trichoderma viride | 2.0 | 3.7 | NS |
| Wallemia sebi | 58.2 | 267.5 | <0.001 |
| Group 2 | |||
| Acremonium strictum | 2.8 | 9.6 | <0.001 |
| Alternaria alternata | 94.3 | 28.1 | <0.001 |
| Aspergillus ustus | 2.9 | 1.1 | NS |
| Cladosporium cladosporioides 1 | 493.8 | 487.5 | <0.001 |
| Cladosporium cladosporioides 2 | 45.1 | 21.9 | <0.001 |
| Cladosporium herbarum | 212.2 | 190.5 | <0.001 |
| Epicoccum nigrum | 608.3 | 15.6 | <0.001 |
| Mucor group | 7.9 | 8.4 | NS |
| Penicillium chrysogenum | 46.3 | 22.8 | <0.001 |
| Rhizopus stolonifer | 4.8 | 1.7 | NS |
The geometric means (GM) as cell equivalents per mg dust (CE/mg dust) for each of the 36 molds in the schools and homes and the Wilcoxon test p-values. P-values were adjusted for multiple comparisons using the Holms-Bonferroni test. Significant differences are bolded.
Table 2 shows the mean and standard deviations for the summed logs of the Group 1 molds, summed logs of the Group 2 molds and ERMI values for the schools and homes. The summed logs of the Group 1 molds in both the schools and homes were not significantly different (Table 2). The summed logs of the Group 2 molds were significantly greater in schools compared to homes, but the ERMI values were significantly greater in homes than schools.
TABLE 2.
Comparison of the ERMI metric components in schools and homes.
| Schools | Homes | T-test | |||
|---|---|---|---|---|---|
| Metric | Mean | SD | Mean | SD | p-value |
| Group 1 | 19.2 | 6.2 | 19.9 | 5.9 | 0.59 |
| Group 2 | 15.6 | 3.2 | 12.5 | 3 | <0.001 |
| ERMI | 3.6 | 4.8 | 7.4 | 5.1 | <0.001 |
The mean and standard deviations (STD) for the summed logs Group 1 molds (Group 1), summed logs Group 2 molds (Group 2) and ERMI values for northeastern schools and homes.
Table 3 shows the results of the linear mixed models of asthma prevalence (independent variable) and school ERMI metric component values (dependent variable) (n=31). A 5% significant (p<0.01) difference in asthma prevalence was associated with a 0.90-unit greater Group 2 mold value, i.e., about a 1/3 standard deviation in Group 2 values. Asthma prevalence was not significantly associated with ERMI values (p=0.06) or Group 1 mold levels (p=0.89).
TABLE 3.
Associations between asthma prevalence and school variables related to mold quantification, building characteristics and student demographics
| Variable | Beta* | p-value | 95% Confidence Interval |
|---|---|---|---|
| Mold | |||
| Group 1 | 0.09 | 0.89 | −1.14, 1.32 |
| Group 2 | 0.9 | 0.005 | 0.27, 1.53 |
| ERMI | −0.82 | 0.06 | −1.69, 0.04 |
| Standardized Beta | |||
| Building | |||
| # Rooms | 0.16 | 0.37 | −0.20, 0.53 |
| Classroom # | 0.26 | 0.15 | −0.10, 0.62 |
| # Floors | −0.12 | 0.5 | −0.49, 0.25 |
| Air-condition | −0.41 | 0.02 | −0.07, −0.75 |
| Basement | −0.16 | 0.39 | −0.52, 0.21 |
| Age | 0.13 | 0.48 | −0.24, 0.50 |
| Enrollment (#s) | −0.15 | 0.4 | −0.52, 0.21 |
| Low income % | 0.24 | 0.18 | −0.12, 0.61 |
| Race | |||
| Black % | 0.14 | 0.45 | −0.23, 0.51 |
| White % | −0.37 | 0.04 | −0.72, −0.03 |
| Hispanic % | 0.24 | 0.18 | −0.20, 0.61 |
| Other % | −0.37 | 0.04 | −0.71, −0.02 |
Beta values for mold outcomes represent association for each 5% increase in asthma prevalence. Standardized Betas are reported for building and student characteristics and 95% confidence intervals are given for each variable tested. Significant associations are bolded.
Table 3 also shows the results of the linear regression analyses for asthma prevalence and the school building and student demographic characteristics. Of the building characteristics considered in this study, only air-conditioning (AC) was significantly associated with asthma prevalence, i.e., a lower prevalence (7.3% with AC vs. 17.6% without AC, p=0.02).
The percentage enrollment of the different, self-identified racial groups (African American/Black, White, Hispanic or Other), was the only demographic characteristic significantly associated with asthma prevalence (Table 3). Asthma prevalence was negatively correlated with %White (−0.37, p=0.03) and %Other (−0.37, p=0.04). The schools with predominantly African American/Black or Hispanic student bodies were associated with a higher prevalence of asthma but these associations were not statistically significant. However, if African American/Black plus Hispanic percentages are combined, then the correlation between higher prevalence of asthma and student bodies predominantly African American/Black plus Hispanic was significantly associated with increased asthma prevalence (0.41 [0.09,0.78] p=0.02).
As a result of the univariate analysis, AC and racial groupings were added to the linear mixed model to adjust for any changes in the significance of the association of Group 2 mold levels and the higher asthma prevalence. These covariates did not improve but rather reduced the significance of the association of the Group 2 molds with the higher prevalence of asthma (0.72 [−0.09,1.52], p=0.08).
Figure 1 shows the geospatial relationship between the school locations and the relative prevalence of asthma, i.e., proportionally sized spots on the map. The Moran’s I of 0.06 (p=0.22) indicates that there was no significant spatial autocorrelation of asthma prevalence within the participating schools.
DISCUSSION
The ERMI metric was previously used in a study of one school in the northeast US, in Springfield, MA.28 This school was the subject of a Health Impact Assessment conducted by the EPA because the school had a long history of water problems. Visible mold growth was observed on the first level and wet carpeting was detected on the second and third levels of this school. High ERMI values, as well as high Group 1 and Group 2 mold levels were measured in many of this school’s classrooms. About 21% of the students were reported to have asthma. Although this was only one school, the results suggested that the ERMI metric might be usefully applied in other schools.
The ERMI metric was developed as a weighted score to assess potentially harmful indoor home mold exposures. Here, we assessed variations of the ERMI between homes and schools and the utility of the school based ERMI measure in studies of asthma prevalence in school-age children. The schools and homes showed similar average Group 1 mold levels, but the homes had significantly lower Group 2 levels than the schools.
The geospatial analysis (Figure 1) showed that schools with higher prevalence of asthma were not clustered suggesting a widely distributed source of exposure, e.g., from outside air. Those schools with higher Group 2 mold levels had significantly greater asthma prevalence among their students compared to schools with lower levels of Group 2 molds. Therefore, the differences in the prevalence of asthma was not indicative of any significant differences in mold growth resulting from water-damage indoors but rather factors associated with the increased levels of molds from outside entering and accumulating in the schools.
The ten Group 2 molds live primarily outdoors, where they are common in soil, on leaf surfaces, etcetera and many of these molds are the source of known allergens.31 One of the Group 2 molds that was detected in significantly higher concentrations in the schools was Alternaria alternata (Table 1). Baxi et al.23 showed that children with asthma who were sensitized to and exposed to high levels of Alternaria alternata in their classrooms had significantly more asthma symptom days than those who were exposed to lower levels or not sensitized.
In homes, the Group 2 mold levels can vary due to factors like cleaning frequency, window ventilation, etc.32 Additional factors in schools that can affect Group 2 mold levels or growth include the presence of carpeting vs. solid-surface flooring, frequency of vacuuming or floor cleaning by the janitorial staff, etc. These flooring and maintenance differences can be especially critical if excess moisture is not controlled in the school, which could allow molds to grow. Another potentially important factor is the presence or absence of AC.
Most of these schools did not have AC (n=30/32). The lack of AC means that the schools and classrooms are more likely to be open to the outside air. Schools with AC had a significantly lower prevalence of asthma but in our adjusted model, AC was not significantly associated with lower Group 2 mold levels (−1.2 [−4.4,2.0], p=0.48). However, there are two limitations to this interpretation. First, there were only two schools with AC, which limits the power of the test. Second, it is reasonable that the presence of AC will reduce outdoor air from entering a school. Therefore, it may not make sense to adjust the model for AC, since, by necessity, AC will limit the flow of outside air into the schools. Therefore, we tested the AC’s presence with any association with Group 2 mold levels using a linear mixed model with random intercept at the school level with only AC as predictor.
The presence of AC reduced Group 2 mold levels by a factor of 2.6 (p=0.10). Since we are only interested in the negative effect of AC on Group 2 levels, a one-sided test demonstrated that there was a significant negative effect (p=0.05) of the presence of AC on Group 2 mold levels. The larger SICAS study will help to clarify any possible role of AC. In addition to AC, the frequency of window and door opening could also affect the levels of Group 2 molds. Cleaning frequency and thoroughness could also affect the build-up of Group 2 molds inside schools.
Schools with predominantly African American/Black plus Hispanic students were more likely to have a higher prevalence of asthma. This finding is consistent with other studies that showed that the prevalence of asthma is greater in these communities.33,34 There have been many factors that have been identified as contributing to higher levels of asthma in these populations, including genetic differences, diet, exposure to higher levels of air pollution, and so on.35,36
A limitation in this baseline study is the small number of schools and homes evaluated. However, despite this small number, statistically significant differences were detected. Also, asthma prevalence data was only based on the 2016-2017 school year (with the two exceptions noted earlier). However, the prevalence of asthma in each individual school tends to be relatively stable over time. We chose the most recent data to be most temporally associated with our mold sampling and to minimize any potential misclassification of the outcome. Another possible limitation in the study was that only 36 molds were quantified and there are certainly many more molds in these schools and homes. High-throughput sequencing technologies have been used in recent studies to reveal many of these molds that were not quantified by the ERMI.37,38 These sequencing approaches have added greatly to our understanding of the complexity of indoor fungal communities. However, the36-ERMI molds have been found to be relevant to asthma health outcomes in many geographic areas in the US.39
Another possible limitation in this study was that only a few of the possible building and student body characteristics were included in the analysis. However, these characteristics provided important insights into their association with mold levels and/or with the prevalence of asthma.
The ERMI characterization of indoor molds in homes has been successfully used to predict asthma-related health effects of mold exposure, harnessing a weighted scoring system of specific water-damage related molds and molds from outdoor sources.39 Here we assessed the differences between ERMI values in homes and schools in the northeast US and found that values for water-damage related molds were similar; however, the values for outdoor-sourced molds in the school were significantly higher. These findings suggest that intervention studies like SICAS 2 and studies of other methods to improve school indoor air quality may be important to reducing asthma.
Highlight Box.
What is already known about this topic?
Mold exposures have been linked to asthma development and exacerbation.
What does this article add to our knowledge?
The Environmental Relative Moldiness Index (ERMI) metric can be used to objectively quantify mold exposures in schools as well as in homes.
How does this study impact current management guidelines?
Mold contamination should be reduced in schools; air-conditioning may help.
Acknowledgements
We would like to thank the schools and families that participated in this study.
Funding: This work was supported by the National Institutes of Health; grant number (awardee); K23 AI123517 (Permaul), K23 AI143962 (Bartnikas), K23 AI104780 (Sheehan), R01 ES030100 (Gaffin), R01 AI144119 (Lai), R01 AI073964, R01 AI073964-02S1, K24 AI106822, U10 HL098102, U01 AI110397, R01 HL137192, U19AR06952 (Phipatanakul)
Additional funding: This work was also supported by the cooperative agreement award number 1 NU61TS000296-01-00 from the Agency for Toxic Substances and Disease Registry (ATSDR) (Hauptman). The U.S. Environmental Protection Agency (EPA) supports the Pediatric Environmental Health Specialty Unit (PEHSU) by providing partial funding to ATSDR under Inter-Agency Agreement number DW-75-95877701.
Conflict of Interest: Phipatanakul - consulting/advisory Genentech/Novartis, Sanofi, Regeneron, Astra Zeneca. Phipatanakul -trial support Genentech, Novartis, Sanofi, Regeneron, Merck, Circassia Alk Abello, Lincoln Diagnostics, Monaghen, Thermo Fisher
Abbreviations Used
- AHHS II
American Healthy Homes Survey II
- AC
air-conditioning
- ERMI
Environmental Relative Moldiness Index
- GIS
Geographic Information System
- HEPA
High efficiency particulate air
- HUD
US Department of Housing and Urban Development
- qPCR
Quantitative polymerase chain reaction
- SICAS
School Inner-City Asthma Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development collaborated in the research described here. Although this work was reviewed by EPA and approved for publication it may not necessarily reflect official EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. Also, ATSDR does not endorse the purchase of any commercial products or services mentioned in PEHSU publications.
REFERENCES
- 1.CDC. National Health Interview Survey (NHIS) data: 2015 Current Asthma. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. [updated 2016; cited 2019 October 8]; Available from: https://www.cdc.gov/asthma/nhis/2015/data.htm [Google Scholar]
- 2.Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. The national cost of asthma among school-aged children in the United States. Ann Allergy Asthma Immunol 2017;119: 246–52. [DOI] [PubMed] [Google Scholar]
- 3.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119: 748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS ONE 2012;7: e47526. doi: 10.1371/journal.pone.0047526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, et al. High Environmental Relative Moldiness Index during infancy as a predictor of age seven asthma. Ann Allergy Asthma Immunol 2011;107: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reponen T, Lockey J, Berstein DI, Vesper SJ, Levin L, Zheng S, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol 2012;130: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017;139: 501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oluwole O, Kirychuk SP, Lawson JA, Karunanayake C, Cockcroft DW, Willson PJ, et al. Indoor mold levels and current asthma among school-aged children in Saskatchewan, Canada. Indoor Air 2017;27: 311–319. [DOI] [PubMed] [Google Scholar]
- 9.Amr S, Bollinger ME, Myers M, Hamilton RG, Weiss SR, Rossman M, et al. Environmental allergens and asthma in urban elementary schools. Ann Allergy Asthma Immunol 2003;90: 34–40. [DOI] [PubMed] [Google Scholar]
- 10.Abramson SL, Turner-Henson A, Anderson L, Hemstreet MP, Bartholomew LK, Joseph CL, et al. Allergens in school settings: results of environmental assessments in 3 city school systems. J Sch Health 2006;76: 246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salo PM, Sever ML, Zeldin DC. Indoor allergens in school and day care environments. J Allergy Clin Immunol 2009;124: 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan WJ, Rangsithienchai PA, Muilenberg ML, Rogers CA, Lane JP, Ghaemghami J, et al. Mouse allergens in urban elementary schools and homes of children with asthma. Ann Allergy Asthma Immunol 2009;102: 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma 2011;48: 1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Permaul P, Phipatanakul W. School environmental intervention programs. J Allergy Clin Immunol Pract 2018;6: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipatanakul W, Koutrakis P, Coull BA, Kang C- M, Wolfson JM, Ferguson ST, et al. The School Inner-City Asthma Intervention Study: Design, rationale, methods, and lessons learned. Contemporary Clinical Trial 2017:60: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banda E, Persky V, Chisum G, Damitz M, Williams R, Turyk M. Exposure to home and school environmental triggers and asthma morbidity in Chicago inner-city children. Pediatr Allergy Immunol 2013; 24: 734–41. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Chao HJ, Chan CC, Chen BY, Guo YL. Current asthma in schoolchildren is related to fungal spores in classrooms. Chest 2014;146: 123–34. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs J, Borras-Santos A, Krop E, Taubel M, Leppanen H, Haverinen-Shaughnessy U, et al. Dampness, bacterial and fungal components in dust in primary schools and respiratory health in schoolchildren across Europe. Occup Environ Med 2014;71: 704–12. [DOI] [PubMed] [Google Scholar]
- 19.Kanchongkittiphon W, Sheehan WJ, Friedlander J, Chapman MD, King EM, Martirosyan K, et al. Allergens on desktop surfaces in preschools and elementary schools of urban children with asthma. Allergy 2014;69: 960–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esty B, Phipatanakul W. School exposure and asthma. Ann Allergy Asthma Immunol 2018;120: 482–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoni M, Cai GH, Norback D, Annesi-Maesano I, Lavaud F, Sigsgaard T, et al. Total viable molds and fungal DNA in classrooms and association with respiratory health and pulmonary function of European school children. Pediatr Allergy Immunol 2011:22: 843–52. [DOI] [PubMed] [Google Scholar]
- 22.Huffaker M, Phipatanakul W. Introducing an environmental assessment and intervention program in inner-city schools. J Allergy Clin Immunol 2014;134: 1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxi SN, Sheehan WJ, Sordillo JE, Muilenberg ML, Rogers CA, Gaffin JM, et al. Association between fungal spore exposure in inner-city schools and asthma morbidity. Ann Allergy Asthma Immunol 2019;122: 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaffin JM, Hauptman M, Petty C, Sheehan WJ, Lai PS, Wolfson JM, et al. Nitrogen dioxide exposure in school classrooms of inner-city children with asthma. J Allergy Clin Immunol 2018;141: 2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect on an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: A randomized clinical trial. JAMA 2017;317: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan WJ. Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, Lai PS, Gold DR, Phipatanakul W. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr 2017;171: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vesper SJ, McKinstry C, Haugland RA, Wymer L, Ashley P, Cox D, et al. Development of an environmental relative moldiness index for homes in the U.S. J Occup Environ Med 2007;49: 829–833. [DOI] [PubMed] [Google Scholar]
- 28.Vesper S, Prill R, Wymer L, Adkins L, Williams R, Fulk F. Mold contamination in schools with either high or low prevalence of asthma. Pediatr Allergy Immunol 2015;26: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox J, Indugula R, Vesper S, Zhu Z, Jandarov R, Reponen T. Comparative assessment of indoor air sampling and dust collection methods for fungal exposure assessment using quantitative PCR. Environ Sci: Process Impacts 2017. doi: 10.1039/c7em00257b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zandbergen PA. Ensuring confidentiality of geocoded health data: Assessing geographic masking strategies for individual-level data. Adv Med 2014;2014: 567049. doi: 10.1155/2014/567049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levetin E, Horner WE, James A Scott JA. Environmental Allergens Workgroup. Taxonomy of allergenic fungi. J Allergy Clin Immunol Pract 2016;4: 375–385. [DOI] [PubMed] [Google Scholar]
- 32.Vesper S Traditional mould analysis compared to a DNA-based method of mould analysis. Crit Rev Micro 2011;37: 15–24. [DOI] [PubMed] [Google Scholar]
- 33.Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg 2016:24: 245–9. [DOI] [PubMed] [Google Scholar]
- 34.Urquhart A, Clarke P. US racial/ethnic disparities in childhood asthma emergent health care use: National Health Interview Survey, 2013-2015. J Asthma 2020;57: 510–520. [DOI] [PubMed] [Google Scholar]
- 35.Kingsley SL, Eliot MN, Carlson L, Finn J, MacIntosh DL, Suh HH, et al. Proximity of US schools to major roadways: a nationwide assessment. J Expo Sci Environ Epidemiol 2014;24: 253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ Int 2017;100: 1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air 2016;26: 179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylvain IA, Adams RI, Taylor JW. A different suite: The assemblage of distinct fungal communities in water-damaged units of a poorly maintained public housing building. PLoS One 2019;18: 14(3):e0213355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vesper S, Wymer L. The relationship between Environmental Relative Moldiness Index values and asthma. Inter J Hyg Environ Health 2016;219: 233–8. [DOI] [PubMed] [Google Scholar]


