Abstract
Takotsubo cardiomyopathy (TC) is associated with significant short-term morbidity and mortality. Several risk factors for poor outcomes have been identified; however, the prognostic implications of pre-existing comorbidity in TC are poorly delineated. We sought to assess the association of aggregate pre-existing comorbidity with short-term outcomes in TC. We performed a retrospective observational study of adult subjects diagnosed with TC at two academic tertiary care hospitals between 2005 and 2018. Overall burden of medical comorbidity was estimated using the Charlson comorbidity index (CCI). Multivariable logistic regression was used to test for independent association of CCI with 30-day mortality and severe shock at index presentation. Multivariable poisson regression was performed to assess the association of CCI with duration of hospitalization. Five-hundred and thirty-eight subjects were diagnosed with TC during the study period. The median CCI score of all subjects was 2 (IQR 1–4). Among subjects with physical triggers of TC, the median CCI score was 2 (IQR 1–4) compared to a median CCI score of 1 (IQR 0–1) in subjects with non-physical triggers of TC (P < 0.001). Seventy-six (14%) subjects died within 30 days of index diagnosis and 185 (34%) subjects experienced severe shock. The median duration of hospitalization was 7 days (IQR 3–14 days). In multivariable logistic regression, CCI was not associated with 30-day mortality or severe shock. In multivariable Poisson regression, CCI (IRR 1.17, 95% CI 1.16–1.18, P < 0.001) was associated with duration of hospitalization. Increased burden of pre-existing medical comorbidity was not independently associated with 30-day mortality or severe shock at index presentation, but was associated with increased duration of hospitalization after diagnosis of TC.
Keywords: Takotsubo, Stress cardiomyopathy, Comorbidity, Charlson comorbidity index, Mortality, Shock
Introduction
Takotsubo cardiomyopathy (TC), also known as stress(induced) cardiomyopathy, broken heart syndrome, and apical ballooning syndrome, was first described in the medical literature in in 1990 [1]. TC is commonly recognized as transient left ventricular dysfunction that may mimic acute coronary syndrome (ACS), but without angiographic evidence of obstructive coronary artery disease (CAD). [2] Additional features of the disorder include onset often following an acute emotional or physical stressor, predomi-nance of presentation in women, and high rates of psychiatric and neurologic comorbidity [3, 4].
The typically transient nature of TC has at times led to its depiction as a relatively benign cardiomyopathy variant. However, more recent reports demonstrate that TC has short-term morbidity and mortality comparable to that of ACS [3]. Approximately, 10% of TC patients are in cardiogenic shock at the time of presentation and 4% do not survive to hospital discharge [3]. Following index discharge, rates of major adverse cardiac and cerebrovascular events and all-cause mortality are estimated at 10% and 6% per patient year, respectively [3].
In recent years, a number of demographic and clinical features have shown prognostic significance in TC. For example, increased age, physical stressor as trigger of TC, reduced left ventricular ejection fraction (LVEF) at diagnosis, typical TC pattern of wall motion abnormality (WMA), and serum troponin level have been consistently identified as predictors of short-term morbidity and mortality [3, 5–8]. The impact of pre-existing medical comorbidity on short-term outcomes in TC is less clear. Here we sought to assess the association of an aggregate measure of pre-existing comorbidity with short-term outcomes in patients with TC.
Methods
Population and study design
After obtaining IRB approval, we performed a retrospective observational study of adult subjects diagnosed with TC at two academic tertiary care hospitals between 2005 and 2018. TC was defined using the Mayo Clinic Criteria: transient abnormality in left ventricle wall motion beyond the perfusion territory of a single epicardial coronary artery, absence of obstructive CAD or angiographic evidence of acute plaque rupture, presence of new electrocardiographic (ECG) abnormalities or elevation in cardiac biomarkers, and the absence of pheochromocytoma or myocarditis [2].
Demographic and clinical data were collected by manual review of the electronic health record (EHR). Pre-existing psychiatric illness was defined as any pre-existing mood, anxiety, or schizophrenia spectrum disorder at the time of TC diagnosis and verified per DSM-IV criteria. The DSM-IV criteria were chosen given that a large portion of the TC cases were diagnosed prior to the DSM-V update in order to ensure consistency throughout the study. The clinical context behind each case of TC was reviewed to differentiate between physical stressors (including critical acute medical illness, acute neurological insult, recent major surgery, etc.) and emotional stressors as likely trigger of TC. Peak serum levels of troponin I and B-type natriuretic peptide were recorded for each patient and are reported in ng/mL and pg/mL, respectively. The initial diagnostic echocardiogram for each case, performed within 24 h of suspicion of ACS or TC, was reviewed to assess the LVEF and the pattern of WMA. Typical WMA was defined as the apical ballooning variant of TC, while atypical WMA was defined as the mid-ventricular or basal variants of TC [3].
The overall burden of medical comorbidity was estimated using the Charlson comorbidity index (CCI). The CCI is a summed score comprising severity-weighted points for a select number of medical disorders, Appendix A [9]. The CCI has been validated as a short-term and long-term prognostic indicator in several disease states, including ACS and heart failure [10, 11]. To facilitate the interpretation of our findings, we also sought to use the CCI score to present a categorical measure of low versus high pre-existing comorbidity. A variety of arbitrary cutoffs in CCI scores have been proposed and used to categorize burden of pre-existing illness in prior reports [12, 13]. In this study, we defined low comorbidity as a CCI score at or below the median of the study cohort. Similarly, a subject with a CCI above the cohort median was deemed to have high comorbidity.
The primary outcome of this study was 30-day mortality after TC diagnosis. Mortality was assessed by review of the EHR and Social Security Death Index (SSDI). Severe shock at index diagnosis, defined as need for pressor use or mechanical circulatory support, and duration of index hospitalization were assessed as secondary outcomes.
Statistical analysis
Statistical analysis was performed using Stata statistical software: release 16 (College Station, TX, USA) [14]. Descriptive statistics are expressed as median with interquartile ranges (IQR) for continuous variables and frequencies (percentages) for categorical variables. Univariate analyses were performed using the Wilcoxon rank sum test for differences in CCI based on the type of stressor triggering TC. Multivariable logistic regression was used to test for independent association of CCI with 30-day mortality and severe shock while adjusting for covariates previously associated with short-term morbidity and mortality in TC: age, physical stressor as trigger of TC, LVEF, atypical WMA, and peak serum troponin I level [3, 5–8]. Results are expressed as odds ratios (OR) with 95% confidence intervals (CI). Similarly, multivariable Poisson regression was performed to assess the association of CCI with duration of hospitalization while controlling for the same covariates. The incidence rate ratio for each variable and 95% CI are shown. For all multivariable models, separate analyses were performed with either the CCI score or categorical measure of pre-existing comorbidity (high versus low comorbidity) included alongside the other covariates. The estimated margins for low versus high comorbidity in association with duration of hospitalization are also shown. All tests were two-tailed and a p value of less than or equal to 0.05 was considered statistically significant.
Results
Five-hundred and thirty-eight subjects were diagnosed with TC during the study period and included in the analysis. Baseline demographic and clinical features are summarized in Table 1. The median CCI score of all subjects was 2 (IQR 1–4). Three-hundred and twenty-one (60%) subjects had low comorbidity with a median CCI score of 1 (IQR 0–2). Two-hundred and seventeen (40%) subjects had high comorbidity with a median CCI score of 4 (IQR 3–6).
Table 1.
Baseline demographic and clinical features
| Characteristic | N | Overall | Low comorbidity | High comorbidity |
|---|---|---|---|---|
| Age (years) | 538 | 66 (55–75) | 65 (54–73) | 69 (58–78) |
| Female (%) | 538 | 405 (75%) | 260 (81%) | 145 (67%) |
| Race (% black) | 537 | 42 (8%) | 21 (7%) | 21 (10%) |
| Active tobacco use (%) | 531 | 96 (18%) | 74 (23%) | 22 (10%) |
| Active alcohol abuse (%) | 531 | 29 (5%) | 23 (7%) | 6 (3%) |
| Pre-existing psychiatric illness (%) | 535 | 186 (35%) | 114 (36%) | 72 (33%) |
| Hypertension (%) | 535 | 302 (56%) | 182 (57%) | 120 (56%) |
| Hyperlipidemia (%) | 535 | 181 (34%) | 107 (34%) | 74 (34%) |
| Diabetes mellitus (%) | 536 | 97 (18%) | 37 (12%) | 60 (28%) |
| History of malignancy (%) | 535 | 138 (26%) | 29 (9%) | 108 (50%) |
| Charlson Comorbidity index | 534 | 2 (1–4) | 1 (0–2) | 4 (3–6) |
| Physical stressor as trigger of Takotsubo Cardiomyopathy (%) | 538 | 364 (68%) | 189 (59%) | 175 (81%) |
| Troponin I, peak (ng/mL) | 485 | 2.4 (0.7–6.2) | 2.7 (0.8–6.2) | 2.2 (0.6–6.2) |
| B-type natriuretic peptide, peak (pg/mL) | 245 | 703 (192–1610) | 554 (131–1130) | 810 (257–2477) |
| Left ventricle ejection fraction (%) | 538 | 33 (25–43) | 37 (27–45) | 33 (25–43) |
| Atypical wall motion abnormality (%) | 538 | 71 (13%) | 45 (14%) | 26 (12%) |
Data are presented as median (IQR) for continuous variables and number (percentage) of subjects for categorical variables. N represents the number of subjects with non-missing values
Three-hundred and sixty-four (68%) subjects were deemed to have had a physical stressor as trigger of TC. Among these subjects, the median CCI score was 2 (IQR 1–4) compared to a median CCI score of 1 (IQR 0–1) in subjects with non-physical triggers of TC (P < 0.001), Fig. 1.
Fig. 1.
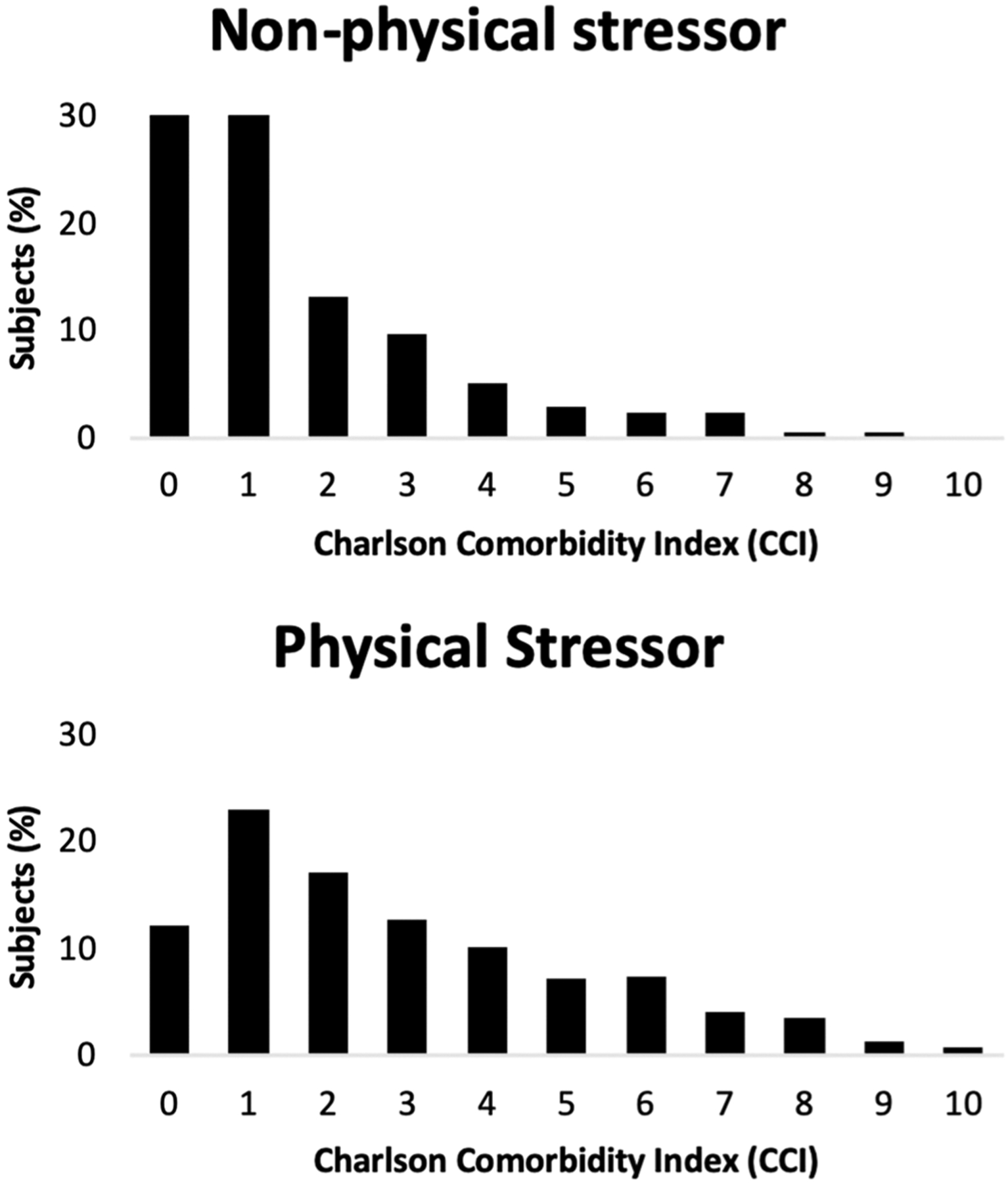
Charlson comorbidity index (CCI) distributions based on the trigger of cardiomyopathy
Seventy-six (14%) subjects died within 30 days of index diagnosis. One-hundred and eighty-five (34%) subjects experienced severe shock, of which 21 (12%) required mechanical circulatory support. Histograms showing the distribution of CCI stratified by 30-day mortality and severe shock are shown Fig. 2. The median duration of hospitalization was 7 days (IQR 3–14 days).
Fig. 2.
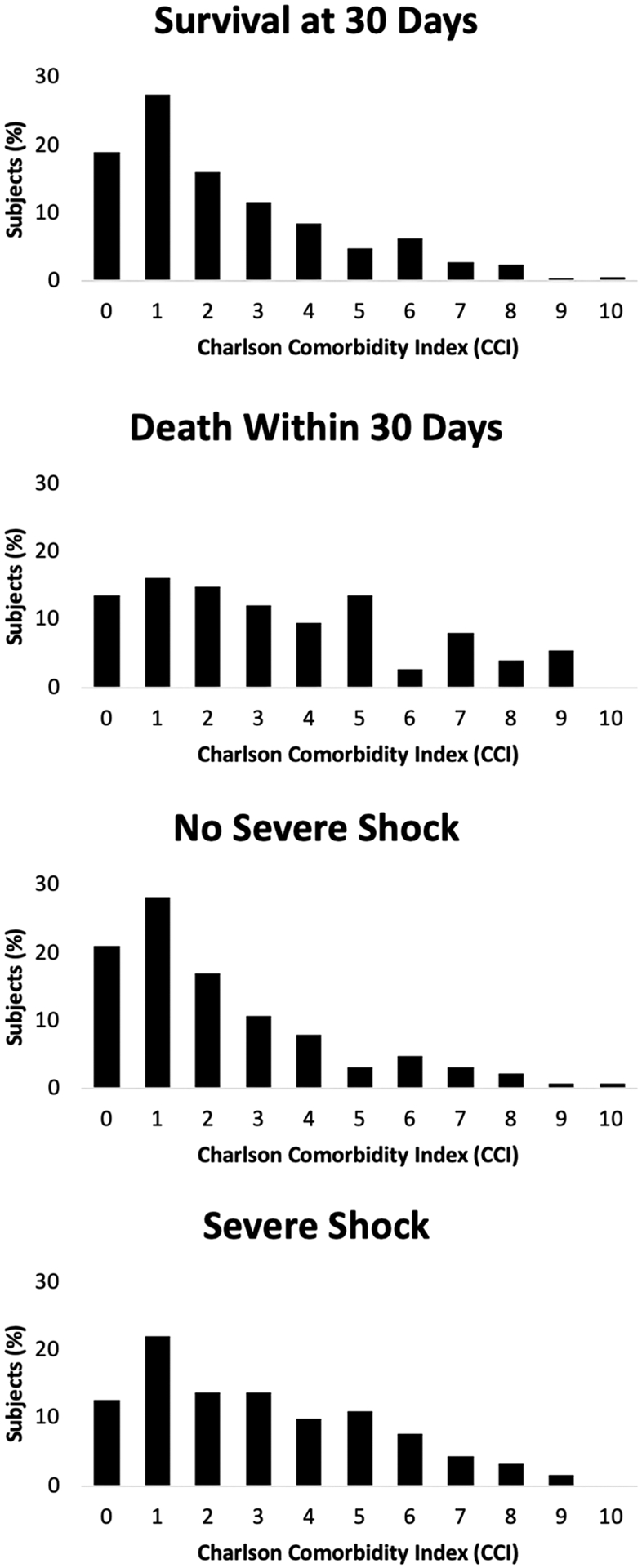
Charlson comorbidity index (CCI) distributions versus 30-day mortality and severe shock
In multivariable logistic regression, CCI score was not associated with 30-day mortality (OR 1.09, 95% CI 0.98–1.22, P 0.110) or severe shock (OR 1.08, 95% CI 0.99–1.18, P 0.083). Similarly, high comorbidity (defined as CCI score > 2) was not associated with 30-day mortality (OR 1.57, 95% CI 0.91–2.72, P 0.104) or severe shock (OR 1.25, 95% CI 0.86–1.48, P 0.150), Table 2. In multivariable Poisson regression, CCI scores (IRR 1.17, 95% CI 1.16–1.18, P < 0.001) and high comorbidity (IRR 2.02, 95% CI 1.92–2.13, P < 0.001) were associated with duration of hospitalization, Table 3. In margin analysis, low comorbidity was associated with a mean hospitalization duration of 5.1 days (95% CI 4.7–5.4 days) compared to a mean duration of 12.3 days (95% CI 11.7–12.9 days) in subjects with high comorbidity.
Table 2.
Adjusted odds ratios for 30-day mortality and severe shock at index diagnosis
| Characteristic | 30-day mortality | Severe shock | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (years) | 1.00 | 0.98–1.02 | 0.637 | 0.99 | 0.98–1.01 | 0.465 |
| Charlson comorbidity index | 1.09 | 0.98–1.22 | 0.110 | 1.08 | 0.99–1.18 | 0.083 |
| Physical stressor as trigger of Takotsubo Cardiomyopathy | 3.93 | 1.71–9.05 | < 0.001 | 4.25 | 2.50–7.21 | < 0.001 |
| Troponin I, peak (ng/mL) | 1.00 | 0.99–1.01 | 0.891 | 1.00 | 0.99–1.01 | 0.982 |
| Left ventricle ejection fraction (%) | 0.95 | 0.93–0.98 | < 0.001 | 0.95 | 0.94–0.97 | < 0.001 |
| Atypical wall motion abnormality | 0.56 | 0.21–1.50 | 0.251 | 0.66 | 0.34–1.25 | 0.199 |
| Age (years) | 1.00 | 0.99–1.02 | 0.565 | 0.99 | 0.98–1.01 | 0.342 |
| High comorbidity | 1.57 | 0.91–2.72 | 0.104 | 1.25 | 0.86–1.48 | 0.150 |
| Physical stressor as trigger of Takotsubo Cardiomyopathy | 4.04 | 1.76–9.28 | < 0.001 | 4.21 | 2.49–7.13 | < 0.001 |
| Troponin I, peak (ng/mL) | 1.00 | 0.99–1.01 | 0.872 | 1.00 | 0.99–1.01 | 0.992 |
| Left ventricle ejection fraction (%) | 0.95 | 0.93–0.98 | < 0.001 | 0.95 | 0.93–0.97 | < 0.001 |
| Atypical wall motion abnormality | 0.52 | 0.19–1.40 | 0.196 | 0.66 | 0.35–1.26 | 0.208 |
CI: confidence interval, HR: hazard ratio, OR: odds ratio
Table 3.
Multivariable Poisson regression of duration of hospitalization (measured in days) on Charlson Comorbidity Index and covariates
| IRR | 95% CI | P value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (years) | 0.99 | 0.98 | 0.99 | < 0.001 |
| Charlson comorbidity index | 1.17 | 1.16 | 1.18 | < 0.001 |
| Physical stressor as trigger of Takotsubo Cardiomyopathy | 2.51 | 2.32 | 2.71 | < 0.001 |
| Troponin I, peak (ng/mL) | 0.99 | 0.98 | 0.99 | < 0.001 |
| Left ventricle ejection fraction (%) | 0.99 | 0.98 | 0.99 | < 0.001 |
| Atypical wall motion abnormality | 1.17 | 1.09 | 1.26 | < 0.001 |
| IRR | 95% CI | P value | ||
| Lower | Upper | |||
| Age (years) | 0.99 | 0.98 | 0.99 | < 0.001 |
| High comorbidity | 2.02 | 1.92 | 2.13 | < 0.001 |
| Physical stressor as trigger of Takotsubo Cardiomyopathy | 2.62 | 2.43 | 2.84 | < 0.001 |
| Troponin I, peak (ng/mL) | 0.99 | 0.98 | 0.99 | < 0.001 |
| Left ventricle ejection fraction (%) | 0.99 | 0.98 | 0.99 | < 0.001 |
| Atypical wall motion abnormality | 1.08 | 1.01 | 1.16 | 0.034 |
CI: confidence interval, IRR: incidence rate ratio
Discussion
The primary finding of this study is that increased burden of pre-existing medical comorbidity, as assessed by CCI, was not independently associated with 30-day mortality or severe shock at index presentation. Increased comorbidity was independently associated with increased duration of hospitalization.
As shown in Fig. 1 and detailed above, patients with physical triggers of TC had on average higher CCI scores than those with non-physical triggers of TC. As in prior studies, we found that physical trigger of TC has the strongest association with poor short-term outcomes, including severe shock and mortality [3, 6]. Hence, while we may conclude with some confidence that increased comorbidity as assessed by CCI scores is indeed associated with physical trigger of TC which has known poor prognostic value, when accounting for the mode of trigger, CCI may not predict different risk of severe shock or short-term mortality.
There is limited literature evaluating the prognostic value of pre-existing comorbidity in TC. To date, three prior studies have reported the association CCI scores with short-term and long-term outcomes in TC. In a study of TC with right ventricular involvement, Citro et al. reported higher rates of cardiogenic shock and death in patients with increased CCI scores [15]. In a study examining the association of TC with CAD, Parodi et al. reported increased long-term mortality in patients with elevated CCI scores [16]. In a follow-up study with an expanded cohort, Parodi et al. again reported the association of CCI scores and long-term mortality [17]. Of note, none of these studies controlled for the potential confounding effect of stressor type triggering TC in the multivariable analysis. Therefore, to the best of our knowledge, the current study is the first of its kind to assess for the prognostic importance of pre-existing comorbidity in patients with TC, while controlling for the mode of trigger. Here we found that increased comorbidity was associated with higher rates of physical triggers of TC, but not independently associated with short-term mortality and severe shock.
Prognostication of short-term outcomes in TC is of significant clinical importance. As previously mentioned, the short-term morbidity and mortality in TC is not negligible and comparable to ACS [3]. A substantial proportion of TC cases are complicated by cardiogenic shock requiring pharmacological and/or mechanical circulatory support. Increased burden of pre-existing comorbidity may be expected to suggest poor prognoses and thus be used to guide risk versus benefit discussions of aggressive inter-ventions in the acute setting [18]. In this study of patients with TC, however, increasing baseline comorbidity as assessed by CCI scores was not independently associated with short-term mortality. Hence, our findings would argue against any notion of not offering aggressive short-term management strategies, including the use of pharmacological pressor support and MCS as indicated, in TC patients with increased baseline comorbidity.
While we did not associate comorbidity with short-term mortality and severe shock, we were able to show that increased CCI scores were associated with increased duration of hospitalization. Using a categorical distinction of CCI, patients with high comorbidity (defined CCI scores above the cohort median) were shown to have hospitalization durations roughly 7 days longer than those with low comorbidity. To our knowledge, no prior study has identified an association with hospitalization duration in TC and that this is the first study to identify increased comorbidity and physical triggers of TC as predictors of hospitalization duration. As CCI has been previously linked to the duration of hospitalization in other disease states, its association with lengthier hospitalization in TC is not unexpected [19]. Increasing burden of pre-existing comorbidity has also been shown to predict increased risk of rehospitalization [20]. Rehospitalization was not formally evaluated in this study as an outcome measure and there remains a paucity of data regarding pre-existing comorbidity and rehospitalization in TC. Given the increased concern related to the relatively high frequency and potential prognostic implications of rehospitalization in TC, future studies should evaluate the association of CCI with rehospitalization [21, 22].
Beyond this, there remain a number of other knowledge gaps regarding prognosticators in TC. Larger-scale studies may be able to simultaneously evaluate previously identified prognostic indicators in TC with the goal of identifying the true drivers of morbidity and mortality and to potentially derive a clinically applicable risk calculator. Here we recommend careful statistical control for the trigger of TC, i.e., physical vs non-physical triggers, as this has been the most consistently associated with poor outcomes in TC and shown to have the largest effect size [3, 6]. Whether to treat the stressor type as a mediator of other potential drivers of morbidity or mortality or not will likely largely determine whether or not a number of demographic and clinical risk factors, such as CCI scores, will have an independent association with the outcome measures.
This study should be interpreted in the context of its limitations. The retrospective cohort design only allows for identification of association and cannot infer causation. Moreover, the size of our cohort limits statistical power to detect associations. However, we believe that the size of this cohort was sufficiently large to allow for the inclusion of the previously identified predictors of mortality or shock in the multivariable analyses. The limitations of our dataset did not allow for identification of cause of death in all cases, and therefore is not presented in this study. Finally, long-term outcomes including mortality and recurrence of TC are not evaluated in this study.
Conclusions
Increased burden of pre-existing medical comorbidity, as assessed by CCI score, was not independently associated with 30-day mortality or severe shock at index presentation. Patients with physical triggers of TC had on average higher CCI scores than those with non-physical triggers of TC; physical trigger of TC was associated with mortality and severe shock in this study. Increased comorbidity was independently associated with increased duration of hospitalization. Larger-scale studies are needed to further delineate prognostic indicators in TC and to derive clinically relevant risk calculators in this disorder.
Acknowledgements
The authors acknowledge the Vanderbilt Translational and Clinical Cardiovascular Research Center (V-TRACC) and The Vanderbilt System for EHR-based Research in Cardiovascular Health (V-SERCH).
The project is supported by NIH 5R01HL140074, AHA 13FTF16810038, CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences.
Gregg Fonarow reports consulting for Abbott, Amgen, Astra Zeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
Appendix A. Charlson comorbidity index: comorbidity list and disease weights.
See Table 4.
Table 4.
Charlson comorbidity index: comorbidity list and disease weights
| Score | Pre-existing comorbidity |
|---|---|
| 1 | Myocardial infarct |
| Congestive heart failure | |
| Peripheral vascular disease | |
| Cerebrovascular disease | |
| Dementia | |
| Chronic pulmonary disease | |
| Connective tissue disease | |
| Ulcer disease | |
| Mild liver disease | |
| Diabetes | |
| 2 | Hemiplegia |
| Moderate or severe renal disease | |
| Diabetes with end organ damage | |
| Any tumor | |
| Leukemia | |
| Lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid tumor |
| AIDS |
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standards This study has been approved by the institutional review boards of Vanderbilt University Medical Center and University of California, Los Angeles, and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Appropriate steps to ensure the privacy of the subjects included were taken in accordance with institutional review board guidelines.
References
- 1.Sato H (1990) Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. Clinical aspects of myocardial injury: from ischemia to heart failure 56–64 [Google Scholar]
- 2.Prasad A, Lerman A, Rihal CS (2008) Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 155(3):408–417 [DOI] [PubMed] [Google Scholar]
- 3.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B (2015) Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 373(10):929–938 [DOI] [PubMed] [Google Scholar]
- 4.Nayeri A, Rafla-Yuan E, Farber-Eger E, Blair M, Ziaeian B, Cadeiras M, McPherson JA, Wells QS (2017) Pre-existing psychiatric illness is associated with increased risk of recurrent Takotsubo cardiomyopathy. Psychosomatics 58(5):527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, Lerman, (2018) Natural history and predictors of mortality of patients with Takotsubo syndrome. Int J Cardiol 267:22–27 [DOI] [PubMed] [Google Scholar]
- 6.Konstantinos G, El-Battrawy I, Schramm K, Uzair A, Hoffmann U, Martin B, Ibrahim A (2017) Comparison and outcome analysis of patients with Takotsubo cardiomyopathy triggered by emotional stress or physical stress. Front Psychol 8:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G, Provenza G, Bellino M, Prota C, Silverio A, Antonini-Canterin F (2019) Long-term outcome in patients with Takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Fail 21(6):781–789 [DOI] [PubMed] [Google Scholar]
- 8.Ghadri G JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, (2016) Differences in the clinical profile and outcomes of typical and atypical Takotsubo syndrome: data from the inter-national Takotsubo registry. JAMA Cardiol 1(3):335–340 [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 10.Radovanovic D, Seifert B, Urban P, Eberli FR, Rickli H, Bertel O, Puhan MA, Erne P, Plus Investigators AMIS (2014) Validity of Charlson comorbidity index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 100(4):288–294. 10.1136/heartjnl-2013-304588 [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Laiglesia FJ, Sánchez-Marteles M, Pérez-Calvo JI, Formiga F, Bartolomé-Satué JA, Armengou-Arxé A, Lopez-Quiros R, Perez-Silvestre J, Serrado-Iglesias A, Montero-Pérez-Barquero M (2014) Comorbidity in heart failure results of the Spanish RICA registry. QJM 107(12):989–994. 10.1093/qjmed/hcu127 [DOI] [PubMed] [Google Scholar]
- 12.Huang YQ, Gou R, Diao YS, Yin QH, Fan WX, Liang YP, Chen Y, Wu M, Zang L, Li L, Zang J (2014) Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B 15(1):58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita K, Watanabe M, Mine S, Fukudome I, Okamura A, Yuda M, Hayami M, Imamura Y (2018) The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today 48(6):632–639 [DOI] [PubMed] [Google Scholar]
- 14.StataCorp. 2019. Stata Statistical Software: Release 16. College Station TSL. [Google Scholar]
- 15.Citro R, Bossone E, Parodi G, Carerj S, Ciampi Q, Provenza G, Zito C, Prota C, Silverio A, Vriz O, D’Andrea A (2016) Clinical profile and in-hospital outcome of Caucasian patients with takotsubo syndrome and right ventricular involvement. Int J Cardiol 219:455–461 [DOI] [PubMed] [Google Scholar]
- 16.Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Vel-luzzi S, Carrabba N, Gensini GF, Antoniucci D (2011) Natural history of tako-tsubo cardiomyopathy. Chest 139(4):887–892 [DOI] [PubMed] [Google Scholar]
- 17.Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Vel-luzzi S, Carrabba N, Gensini GF, Antoniucci D (2013) Tako-tsubo cardiomyopathy and coronary artery disease: a possible association. Coron Artery Dis 24(6):527–533 [DOI] [PubMed] [Google Scholar]
- 18.Tseng LJ, Yu HY, Wang CH, Chi NH, Huang SC, Chou HW, Shih HC, Chou NK, Chen YS (2018) Impact of age-adjusted Charlson comorbidity on hospital survival and short-term outcome of patients with extracorporeal cardiopulmonary resuscitation. J Clin Med 7(10):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakomkin N, Kothari P, Dodd AC, VanHouten JP, Yarlagadda M, Collinge CA, Obremskey WT, Sethi MK (2017) Higher Charlson comorbidity index scores are associated with increased hospital length of stay after lower extremity orthopaedic trauma. J Orthop Trauma 31(1):21–26 [DOI] [PubMed] [Google Scholar]
- 20.Bahrmann A, Benner L, Christ M, Bertsch T, Sieber CC, Katus H, Bahrmann P (2019) The Charlson comorbidity and Barthel index predict length of hospital stay, mortality, cardiovascular mortality and rehospitalization in unselected older patients admitted to the emergency department. Aging Clin Exp Res 31(9):1233–1242 [DOI] [PubMed] [Google Scholar]
- 21.Smilowitz NR, Hausvater A, Reynolds HR (2019) Hospital read-mission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes 5(2):114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nayeri A, Bhatia N, Xu M, Farber-Eger E, Blair M, McPherson J, Wang T, Wells Q (2017) Prognostic significance of early rehospitalization after Takotsubo cardiomyopathy. Am J Cardiol 119(10):1572–1575 [DOI] [PubMed] [Google Scholar]


