Abstract
BACKGROUND
Conventional culture frequently fails to identify the dominant pathogen in polymicrobial foot infections, in which Staphylococcus aureus is the most common infecting pathogen. Previous work has shown that species-specific immunoassays may be able to identify the main pathogen in musculoskeletal infections. We sought to investigate the clinical applicability of a S. aureus immunoassay to accurately identify the infecting pathogen and monitor its infectivity longitudinally in foot infection. We hypothesized that this species-specific immunoassay can 1) aid diagnosis of S. aureus and 2) track the therapeutic response in foot infections.
METHODS
From July 2015 to July 2019, 83 infected foot ulcer patients undergoing surgical intervention (debridement or amputation) were recruited and blood was drawn at 0, 4, 8 and 12 weeks. Whole blood was analyzed for S. aureus-specific serum antibodies (mix of historic and new antibodies) and plasmablasts were isolated and cultured to quantify titers of newly synthesized antibodies (NSA). Anti-S. aureus antibody titers were compared to culture results to assess their concordance in identifying S. aureus as the pathogen. The NSA titer changes at follow-ups were compared to wound healing status to evaluate concordance between evolving host immune response versus clinically resolving or relapsing infection.
RESULTS
Analysis of serum for anti-S. aureus antibodies showed significantly increased titers of 3 different anti-S. aureus antibodies, IsdH (p = 0.037), ClfB (p = 0.025), and SCIN (p = 0.005) in S. aureus culture positive patients compared to culture negative patients. Comparative analysis of combining antigens for S. aureus infection diagnosis increased the concordance further. During follow-ups, changes of NSA titers against a single or combination of S. aureus antigens significantly correlated with clinically resolving or recurring infection represented by wound healing statuses. In summary, this S. aureus immunoassay was able to track treatment response and detect recurrent infection.
CONCLUSIONS
In management of foot infection, the use of S. aureus-specific immunoassay may aid in diagnosis of the dominant pathogen and monitor the host immune response against a specific pathogen in response to treatment. Importantly, this immunoassay can detect recurrent foot infection which may guide a surgeon’s decision to intervene.
Level II:
Diagnostic Study
Keywords: Diabetic foot ulcer, S. aureus, Immunoassay, Antibody secreting cell, Newly synthesized antibody
INTRODUCTION
Background
Infected diabetic foot ulcer (DFU) is one of the most common disorders necessitating hospital admission and its incidence continues to rise.2 Two-thirds of lower extremity amputations are associated with infected DFU.15, 22, 28 Most patients with diabetic foot infections present with poor glycemic control and multiple comorbidities. Additionally, these patients exhibit high rates of post-operative complications, such as surgical wound dehiscence (50%) and recurrent ulcer (40-43%), which often lead to revision surgeries (40%), including amputation.1,2,5,21,29 DFU often present as complex polymicrobial infections,7,8,27 where Staphylococcus aureus is the most common offending agent (46-68%), followed by coagulase-negative Staphylococci, Streptococcus agalactiae, Staphylococcus epidermidis, and Enterococcus.7–9,12,16,17,20,22,23,25 The conventional diagnostic microbial culture is subject to sampling error, which may yield a false-positive or false-negative growth, especially if the patient has been on antibiotics prior to obtaining sample.2,6,11,17,35–37 Moreover, culture cannot always differentiate dominant pathogens from benign commensals or bystanders, which may complicate antibiotic treatment after surgery.12,35–37 Previously studies investigating prognostic markers for monitoring infection of DFUs have been inconsistent.3,4,38,39 Most importantly, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are not pathogen specific.20,34 Marmor et al. previously reported successful utilization of a commercially available serum based species-specific immunoassay for identification of dominant pathogens in prosthetic joint infections.19 Yet, currently, there are no sensitive and specific tools for diagnosing and monitoring a pathogen’s ongoing infectivity.24,31
Given the above challenges, we sought to investigate the applicability of a species-specific immunoassay to supplement standard culture in identification of dominant pathogens and tracking therapeutic response. Specifically, we compared titers of serum antibodies versus newly synthesized antibodies (NSA) produced by stimulated plasmabasts or antibody secreting cells (ASC) that emerge into blood only during active infection.9,13,14,20,22,24 Serum antibody titers include all circulating, pre-existing antibodies potentially made in response to historical exposure to pathogens, whereas NSA titers represent antibodies that are newly generated in response to a recent infection (< 2-3 weeks). Therefore, NSA immunoassay may able to accurately differentiate the dominant pathogen perceived by the host’s immune system among various polymicrobial organisms identified by a tissue culture.24 Given that our immunologic response is not site-specific, we aimed to utilize this information to supplement bone culture obtained from an infected foot. In our pilot study of 26 patients with infected DFU, we found 70% concordance rate between the S. aureus immunoassay and standard culture in identification of S. aureus infection.24 Moreover, the NSA immunoassay showed promise for monitoring treatment response and detecting recurrent S. aureus infection.24 Most notably, in some patients who were on a 6-week course of postoperative antibiotics treatment, the 8-week follow-up (2 weeks after cessation of antibiotics treatment) immunoassay revealed persistently elevated or resurging immune response against S. aureus, which concurred with clinical recurrent infection that necessitated unexpected readmission and reoperation.24 We noted that the immunoassay was more sensitive and specific than traditional inflammatory markers (ESR, CRP) in reflecting recurrent infection at an earlier phase of recurrent infection.24 Given the high rate of infection recurrence in DFU and often ambiguous clinical presentation of indolent or recurrent infection, accurate assessment of evolving pathogenicity may improve DFU patients management. NSA immunoassay may provide surgeons with additional information to accurately assess treatment progression and prospect for a limb salvage.
In this study, we expand on previous work and investigate the clinical applicability of species-specific immunoassay to supplement culture in identification of S. aureus infection and monitor its infectivity in response to surgical treatment. We hypothesized that this species-specific immunoassay can 1) aid in diagnosis of S. aureus and 2) track treatment response in foot infection.
PATIENTS/METHODS
Patient Enrollment
From July 2015 to July 2019, we enrolled 83 patients who displayed clinical symptoms and signs of infected DFU7,8 which necessitated hospitalization and surgical interventions, such as irrigation and debridement or amputation, followed by a course of IV or oral antibiotic treatment. Patients presenting with severe ischemia (ankle-brachial index < 0.45), venous stasis ulcers, pregnancy, malnutrition (albumin < 3g/dl), immune deficiency, or inability to make decision (unconscious, nonconsentable) were excluded from the study. Demographic information is summarized in Table 1.
Table 1:
Patients demographic and surgical procedures performed.
| Characteristic | Values |
|---|---|
| Male:Female | 60 (72.3%): 23 (27.7%) |
| Age (Yrs) | 56.5 ± 11.7 |
| BMI (kg/m2) | 35 ± 7.4 |
| WBC (x 103 /μl) | 11.439 ± 4.441 |
| ESR (mm/hr) | 61 ± 38.8 |
| CRP (mg/L) | 112 ± 105 |
| A1c Levels | 9.3 ± 2.8 |
| Wound Healing (% healed) | 53% |
| Surgeries Performed: | |
| Irrigation & Debridement (%) | 25 (30%) |
| Toe Amputation | 16 (19%) |
| Metatarsal/Ray Amputation | 22 (27%) |
| Midfoot Amputation | 3 (4%) |
| Calcanectomy | 10 (12%) |
| Syme Amputation | 1 (1%) |
| Below the Knee Amputation | 6 (7%) |
Operative Procedures and Postoperative Antibiotics Therapy
When possible, infected DFUs were initially managed by wound debridement followed by daily wet-to-dry dressing change with normal saline solution. For those who presented with extensive soft tissue and bone necrosis, amputation of the infected part was necessary. Biomechanical derangement such as equinus or isolated gastrocnemius contracture was addressed by performing concomitant achilles tendon lengthening or gastrocnemius recession as indicated. In all cases, bone samples were obtained from the base of the infected foot ulcer at the time of surgery. Pathologic evaluation of the bone resection margin was performed for all patients who underwent amputation or bony resection (i. e. calcanectomy) procedure. If clean margins were obtained, patients were placed on oral antibiotics for an additional 2 weeks to eradicate residual soft tissue infection. If the resection margins were contaminated, a targeted long-term (6 weeks) IV or oral antibiotics were instituted as recommended by the consulting infectious disease specialist. The end point of the study for each subject was at the 12-weeks post-surgical intervention or at the time of reoperation as a consequence of treatment failure, whichever occurred first. Healing wound was defined as completely healed or decreased size and depth of the wound and absence of 12 secondary signs and symptoms of infection (pain, erythema, edema, heat, purulent exudate, serous exudate with concurrent inflammation, delayed healing, dislocation of granulation tissue, friable granulation tissue, pocketing at the baes of the wound, foul odor and wound breakdown) as previously described.7,8,24 A non-healing wound is defined as 1) unimproved or 2) increased in size and depth of the wound with persistent 12 signs and symptoms of infection.7,8,24 During the postoperative period, various biomechanical offloading strategies such as total contact cast, pneumatic boot, plastazote insoles or soft cushioned post op shoe were utilized to offload the operated foot as deemed appropriate by the treating surgeon.
Whole Blood Processing
Three tubes (2 green-topped sodium heparin tubes and 1 red-topped serum tube) of whole blood were drawn from the study subjects at the recruitment (t = 0), 4-, 8-, and 12 weeks afterwards. The green-topped tubes were immediately processed to isolate peripheral blood mononuclear cells (PBMCs). The isolated PBMCs were subsequently cultured to populate recently activated circulating plasmablasts (or ASC) specific for S. aureus.14,20,22,24 Serum, which contains mix of pre-existing and newly made antibodies, was collected from the red-topped tube following centrifugation. The secreted products (NSA) from cultured plasmablasts and the collected sera were stored at −80 °C until immunoassay (Figure 1A). As part of standard clinical care, blood serum samples were collected for ESR, CRP, White Blood Counts (WBC), and non-fasting glucose levels (Table 1).
Figure 1.
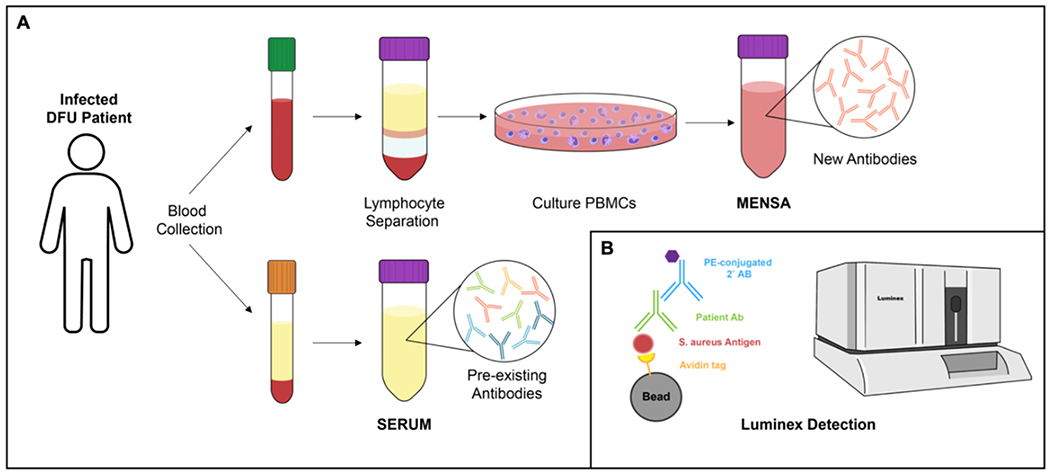
NSA versus Serum Immunoassay. (A) Illustration of newly synthesized antibody (NSA) medium and serum preparations for immunoassay. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood (green top) and cultured to propagate recently activated plasmablasts or antibody secreting cells, which generates newly-synthesized antibodies. Serum is isolated from whole blood (red top) to harvest mix of pre-existing and newly-made antibodies. (B) Illustration of multiplex Luminex immunoassay. Avidin-coated magnetic bead is coupled to a recombinant S. aureus antigen. We utilized 8 different beads with unique spectral signatures to couple with 8 different S. aureus specific antigens. After incubating with serum or NSA medium, human antibody against a S. aureus specific antigen forms a complex. Subsequently, the secondary phycoerythrin-conjugated goat anti-human IgG binds to the complex and run on flow cytometer to measure fluorescence intensity of the beads (to discern different beads couple with unique antigens) and phycoerythrin for quantitative measure.
Multiplex Luminex Immunoassay
Anti-S. aureus antibodies levels in the DFU patients were determined using a custom multiplex immunoassay.24 Briefly, avidin-coated magnetic LumAvidin™ microspheres (Luminex, Austin, TX) with unique spectral signatures were coupled to individual recombinant S. aureus antigen.24 S. aureus antigens included 1) Iron acquisition proteins – iron-regulated surface determinant proteins (IsdB, IsdH), 2) Cell division proteins – glucosaminidase (Gmd), amidase (Amd), 3) Staphylococcal inhibitory protein (SCIN), 4) Cell attachment proteins – clumping factor B (ClfB), and Panton-Valentine leukocidin subunits (LukS-PV, LukF-PV).24 For detecting species-specific antigen-reactive IgG, 1,000 magnetic microspheres per analyte per well were mixed and incubated with 1) 100 μl of diluted serum (1:10,000) or 2) 100 μl of NSA medium for 2 hours. After washing, the secondary phycoerythrin-conjugated goat anti-human IgG (Southern Biotech, Birmingham, AL) was added and incubated for 1 hour. All serum and NSA samples were run on a flow cytometer (Bio-Plex 200; Bio-Rad, Hercules, CA). The fluorescence intensity of the beads and phycoerythrin (100 beads per analyte per well) were acquired for analysis (Figure 1B).
Statistical Data Analysis
S. aureus specific antibodies measurements obtained from serum and NSA medium at the recruitment (0 week) were compared to intra-operatively obtained bone culture for identification of S. aureus. The concordance of the two was assessed using receiver operating characteristic (ROC) curve analysis, with overall accuracy summarized by the area under the ROC curve (AUC). The subsequent changes of NSA titers at follow-up visits (4-, 8-, and 12 weeks) were comparatively analyzed to the clinical wound healing status. We expected to observe decreasing NSA against S. aureus (less stimulation of ASC with decreased pathogenicity) associated with clinically subsiding infection represented by healing foot ulcer or surgical incision. In contrast, persistently elevated or rebounding NSA against S. aureus antigens was expected in persistent or recurrent infection represented by nonhealing ulcer. Relative changes of baseline to follow-up antibody titers were analyzed against clinical healing versus non-healing wound status. ROC curves were analyzed for each individual and combinations of antibodies against 8 different S. aureus antigens. Combinations were formed using best subsets selection with multivariate logistic regression models to identify groups of antibodies that best diagnosed and tracked infection and healing response. The estimated linear predictor from each of these logistic models combined multiple antibodies into a single numerical score for analysis. Nonparametric estimates and 95% confidence intervals for the AUC were computed for each predictor. P-values for testing the significance of each AUC, and for comparing AUCs across predictors, were also calculated. Analyses were conducted using SAS version 9.4, and R version 3.5.1. A p-value less than 0.05 was considered significant.
RESULTS
Patient Demographics
Eighty-three infected DFU patients formed the basis of this study (Table 1). There were 23 female and 60 male participants with mean age of 56.5 (range, 26 to 82) years-old. Twenty five patients (30%) underwent wound debridement and 58 patients (70%) underwent amputations of the infected part (16 toe amputations (19%), 22 metatarsal amputation (27%), 3 midfoot amputation (4%), 10 calcanectomy (12%), 1 Syme amputation (1%) and 6 below the knee amputation (BKA) (7%)). Forty-five participants (54%) grew S. aureus in their standard culture (SA+) whereas 38 (46%) patients did not grow S. aureus (SA−). Four participants passed away prior to their final 12-week follow up due to other medical complications. At the final follow-up, 56% of SA+ participants and 46% of SA− participants healed the DFU or surgical incision without sign of recurrent infection.
Concordance of Culture versus Immunoassay
Among the 83 patients, serum immunoassay analysis was performed on 75 patients and NSA was successfully cultured and assayed for 79 patients. The serum immunoassay detected S. aureus infection in 48 cases (64%), whereas the NSA immunoassay identified S. aureus infection in 50 cases (63.3%). The concordance rate between standard culture and immunoassay for diagnosis of S. aureus were 66% for serum and 61% for NSA medium (Table 2). Among the 45 culture SA+ patients, both serum and NSA immunoassays detected active host immune response against S. aureus infection in 32 cases (71.1%). In 13 (16%) culture SA+ patients, both the serum and NSA immunoassay failed to detect host immune response against S. aureus; suggestive of suspicious false-positive culture or S. aureus not being the dominant infecting pathogen in these cases.
Table 2:
Concordance of immunoassay (Serum and MENSA) versus culture presented in % and number of patients. Gray box represents culture and immunoassay concordant patients and white box represent discordant patients. Abbreviations: MENSA, medium enriched in newly synthesized antibody.
| Immunoassay | Culture | Percentage | Number | |
|---|---|---|---|---|
| Serum | Positive | Positive | 43% | 32 |
| Negative | Negative | 23% | 17 | |
| Positive | Negative | 21% | 16 | |
| Negative | Positive | 13% | 10 | |
| MENSA | Positive | Positive | 41% | 32 |
| Negative | Negative | 20% | 16 | |
| Positive | Negative | 23% | 18 | |
| Negative | Positive | 16% | 13 | |
Immunoassay for Diagnosis of S. aureus Foot Infection
Analysis of serum immunoassay showed significantly higher S. aureus specific antibody titers in the culture SA+ group compared to the culture SA− group; particularly in response to S. aureus antigens SCIN (AUC = 0.68; p = 0.005), ClfB (AUC = 0.64; p =0.02), and IsdH (AUC = 0.63; p = 0.03) (Figure 2A). Although NSA immunoassay showed slightly increased antibodies titers for IsdH (AUC = 0.56; p = 0.36) and LukS-PV (AUC = 0.58; p = 0.22) in culture SA+ group, it did not reach statistical significance compared to culture SA− patients (Figure 2B). Comparative analysis of combined antibodies and culture showed improved concordance in detecting S. aureus infections. In particular, anti-IsdB, ClfB, SCIN (AUC = 0.71, p = 0.0006) and Anti-IsdB, IsdH, SCIN (AUC = 0.71, p = 0.0006) were the best antigen combinations for serum immunoassay (Figure 2C). Whereas, anti-IsdH, LukF-PV, LukS-PV (AUC = 0.66, p = 0.017) and anti-Amd, IsdH, LukF-PV (AUC = 0.63, p = 0.046) were the best three antigen combinations for NSA immunoassay (Figure 2D).
Figure 2.

Correlation of culture versus serum and NSA immunoassay for detection of S. aureus infection using individual and combinations of antigens. Receiver operating characteristic (ROC) curve analysis, summarized by area under the ROC curve (AUC), was used to identify the best individual and combinations of antigens that correlated with S. aureus positive culture. (A) Single antigen analysis of the serum antibodies against SCIN (AUC = 0.68; p = 0.005), ClfB (AUC = 0.64; p =0.02) and IsdH (AUC = 0.63; p = 0.03) showed statistically significant AUCs. (B) Single antigen analysis for NSA medium failed to show an antigen which significantly correlated with culture identification of S. aureus. (C) The AUC levels increased further by combing 3 different antigens for the serum and (D) NSA medium. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC curve; MENSA, medium enriched in newly synthesized antibody.
Immunoassay for Tracking Evolving Infectivity of S. aureus Foot Infection
NSA assay was suggested to have clinical potential for tracking treatment response by accurately evaluating the host’s evolving immune response against S. aureus infection.24 In the case of S. aureus infection resolution with surgical intervention and antibiotics treatment, less stimulation of plasmablast and subsequent decrease in anti-S. aureus antibodies generation is expected in association with clinically healing foot ulcer or surgical incision (Figure 3A). In contrast, persistently elevated NSA against S. aureus antigens was expected in persistent or recurrent infection of the wound (Figure 3B). To test this, the relative changes of NSA titers during postoperative follow-ups compared to that at the initial recruitment time-point was compared to the clinical wound healing status. Participants were categorized by 1) S. aureus infection status as determined by culture (SA+ versus SA−), then 2) wound healing status (healed versus not-healed). Total 45 culture SA+ patients formed the basis of this analysis. Among them, 25 (56%) patients were classified as healed wound and 20 (44%) as not-healed wound.7,8,24 NSA immunoassay showed significantly greater differences in changes of antibody titers between healed versus not-healed cohorts (Figure 4A). Especially, NSA against IsdH (AUC = 0.74; p = 0.011), LukFPV (AUC = 0.73; p = 0.011), and IsdB (AUC = 0.72; p = 0.014) antigens showed significant correlation with clinical wound healing status (Figure 4B). Combining NSA against IsdH, ClfB, and LukF-PV antigens (AUC = 0.75; p = 0.007) provided the highest correlation with wound healing status (Figure 4C).
Figure 3.

Examples of NSA immunoassays for healed versus not-healed wounds. Each line represents antibodies titers against 8 different S. aureus-specific antigens. (A) NSA immunoassay of S. aureus infected DFU patient who healed the wound. NSA immunoassay showed relatively high titers at 0 weeks, which decreased at 8 weeks, and remained low at the final 12 weeks follow-up. (B) Immunoassay of a S. aureus infected DFU patient who failed to heal. NSA immunoassay showed decreased titers up to 8 weeks, then elevated at 12 weeks as recurrent infection occurred. Abbreviations: DFU, diabetic foot ulcer; MFI, mean fluorescence intensity; MENSA, medium enriched in newly synthesized antibody.
Figure 4.

Correlation of wound healing status versus evolving antibody titers using individual and combinations of antigens. S. aureus positive patients, identified by culture, were divided as Healed versus Not-healed groups at the final follow-up. (A) The anti-S. aureus antibody titers changes (initial to final follow-up) for 8 different antigens were calculated for serum (Serum SA+) and NSA (MENSA SA+). Then the ratios of antibody titers changes in Not-healed/Healed groups were calculated. The difference in titers of Not-healed/Healed is much greater for NSA medium (MENSA SA+) compared to serum. This indicates NSA titer changes showed stronger correlation with wound healing status. (B) The single antigen ROC curves were calculated to investigate correlation between NSA antibody titers changes versus clinical wound healing status. IsdH (0.74; p = 0.011), LukF-PV(0.73; p = 0.011) and IsdB (0.72; p = 0.014) were significantly correlated with healing status. (C): AUCs analysis using combinations of 3 antigens revealed multiple combinations which could accurately track wound healing status. The IsdH, ClfB, and LukF-PV antigens combination showed the highest AUC value of 0.75 (p = 0.007). * indicates p < 0.05. Abbreviations: SA+, S. aureus positive culture; SA−, S. aureus negative culture; ROC, receiver operating characteristic; AUC, area under the ROC curve; MENSA, medium enriched in newly synthesized antibody.
DISCUSSION
There is a great demand for accurate identification of the dominant pathogen in polymicrobial infections to deliver an effective post-operative antibiotic treatment. Standard microbial culture identification of pathogens has been reported to be inaccurate and its semiquantitative measure does not accurately reflect pathogenicity.7,24,35–37 This inaccuracy and lack of specificity further complicates effective treatment of the main pathogen in polymicrobial foot infection. In this work, we aimed to investigate the use of a S. aureus specific immunoassay in identifying the dominant pathogen among multiple microorganisms identified by culture. We demonstrated that this S. aureus-specific immunoassay can supplement standard microbial culture to identify the main pathogen and monitor host’s immune response, using both serum and NSA, which reflects evolving infectivity during the postoperative period. This assay may be capable of identifying false-positive or false-negative culture growth and detect a persistent or recurrent infection. Since S. aureus is the most common pathogen (46-68%) in DFU, 7,8,12,16,17,22–25 we focused our current investigation to this species. Future work will expand on this study by investigating other species commonly infecting the foot.
Here we show that the concordance rate between bone culture and immunoassay ranged from 61% (NSA) to 66% (Serum). Although serum and NSA assay showed higher detection rates of S. aureus infection compared to standard culture, we are unable to infer that our immunoassays were more sensitive than culture without a tertiary diagnostic method. However, it raises a suspicion for false-negative growth in clinically infected patients. This finding suggests that immunoassays may detect culture-negative S. aureus infections that warrant further investigation with molecular diagnostics, such as 16S rRNA sequencing analysis or Staphylococcal Protein A (spa) genotyping to confirm S. aureus infections.10,24,35–37 A subset (26 patients) of the current study cohort in which spa genotyping was possible revealed a 85% concordance rate with the immunoassay.24 Without a tertiary method of infective species identification for all patients, the exact sensitivity of our immunoassays cannot be concluded. Some cultures may grow a contaminant or commensal and yields false-positive growth, whereas some patients may have been on antibiotics prior to surgery, resulting in false-negative culture. Discrepancy in culture versus immunoassay may be clarified further by comparing with a tertiary molecular diagnostic method, such as next generation sequencing (NGS).35–37 Another notably attribute of diagnostic immunoassay may relate to cost-effectiveness. Its cost is estimated to be $200, in contrast to $60 per sample for culture.
In this study, the serum immunoassay was superior to NSA in initial diagnosis of S. aureus infection (Figure 2A & B). This result may be due to analytic sensitivity or efficient capture of plasmablasts prior to sensitization. Combining three antigens substantially improved the concordance rates between the culture and both serum and NSA immunoassays in detecting S. aureus infections (Figure 2C & D). The application of serum immunoassay for species-specific diagnosis of orthopaedic infections has been reported and commercialized. Marmor et al utilized a serum multiplex immunoassay to successfully diagnose S. aureus, S. epidermidis, S. lugdunensis, S. agalactiae and Propionibacterium acnes in hip, knee and shoulder prosthetic joint infections.19 However, since their immunoassay utilized only the serum the application was limited to identification of pathogens without the ability to track evolving pathogenicity.
Commensal S. aureus in human skin has reported to elicit both innate and adaptive immunity, which leads to high-titers of anti-S. aureus antibodies for years in even healthy individuals.40 Therefore, this characteristic precludes serum-based assay from being a promising tool for tracking treatment response despite its clinical utility to diagnose new S. aureus infections in DFUs. Since ASCs are only secreted during an ongoing, active infection, NSA immunoassay is a promising method for tracking antibiotic treatment response and detecting recurrent infections.9,13,14,20,22,24 Our results showed that the NSA titers changes during postoperative period against various S. aureus antigens were significantly correlated with wound healing status (Figure 4). The conventional inflammatory markers, such as ESR and CRP, are not specific to a pathogen, disease, or site of infection and may not accurately represent evolving infection. In our pilot study of 26 infected DFU patients, 10 were confirmed to have S. aureus infections. Among the 10 patients, 6 patients healed the DFU, whereas 4 did not. We noted remarkable changes in NSA immunoassay over time significantly reflected the healing status. In some who failed to heal, we noted borderline inflammatory markers (CRP or ESR) value, but resurging anti-S. aureus antibody levels at their follow-ups.24 Combination of borderline inflammatory markers value with ambiguous clinical picture of indolent or recurrent infection may present a diagnostic dilemma to surgeons. In such case, this novel immunoassay may provide further diagnostic and prognostic insight to guide further treatment.
There are a number of limitations in this study. First, we only investigated S. aureus infection in polymicrobial DFU. This novel diagnostic method can be expanded to include other species, such as S. agalactiae, S. epidermidis, and pseudomonas aeruginosa, which commonly infects DFU.2,7,8,16,17 Second, most diabetic patients have various other medical comorbidities and biomechanical derangements, which may have affected their wound healing process. To mitigate this effect, we excluded patients with vascular insufficiency and evidence of immune deficiency or malnutrition. Whenever possible, we tried to address biomechanical derangement of the foot through surgical or conservative means as described. However, it is challenging to account for various other medical and biomechanical factors that could have negatively affected wound healing process. Third, patients were referred to the surgeon’s office at various stages of infection, which may have affected their elicited immune response. Some of the participants were on oral antibiotics prior to recruitment, which may have confounded the culture or immunoassay results. Finally, the immunoassay was compared only against standard culture for assessing its ability to diagnose S. aureus infection. Since standard culture is prone to error, a tertiary diagnostic test with higher sensitivity and resolution, such as emerging molecular diagnostics like NGS may have served as a better counterpart to compare against.18,35–37 Despite the above limitations, the current study suggests a potential for clinical application of immunoassay for species-specific diagnosis and management of foot infection.
Conclusion
This species-specific immunoassay may supplement standard culture for identification of S. aureus infection in a polymicrobial foot infection. It was also able to identify 21-23% potentially false-negative cultures in DFU patients that subsequently needed therapy for S. aureus infections. Moreover, NSA immunoassay can track evolving pathogenicity in response to treatment and detect recurrent infection. Overall, this novel method can help orthopaedic surgeons identify the infecting pathogen, detect recurrent infection, and promptly guide treatment intervention.
Acknowledgement
This work was supported by a grant from the American Orthopaedic Foot & Ankle Society with funding from the Orthopaedic Foot & Ankle Outreach & Education Fund (OEF) and the Orthopaedic Research and Education Foundation (OREF) with designation to the OEF. Also supported by National Institute of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant number R21AR07457 awarded to Irvin Oh, M.D. and NIH/NIAMS grant number P50AR072000 awarded to Edward M. Schwarz, Ph.D. We thank Ms. Samantha Hoffman for assistance with sample collection and documentation.
REFERENCES
- 1.Aragón-Sánchez J, Lázaro-Martínez JL, Hernández-Herrero C, et al. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabet. Med. J. Br. Diabet. Assoc 2012;29:813–818. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N. Engl. J. Med 2017;376:2367–2375. [DOI] [PubMed] [Google Scholar]
- 3.Biteker FS, Özlek B, Özlek E, et al. Prognostic factors in diabetic foot ulcer. Angiology. 2017:3319717694452. [DOI] [PubMed] [Google Scholar]
- 4.Brownrigg JRW, Hinchliffe RJ, Apelqvist J, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab. Res. Rev 2016;32:128–135. [DOI] [PubMed] [Google Scholar]
- 5.Chang JW, Heo W, Choi MSS, et al. The appropriate management algorithm for diabetic foot. Medicine (Baltimore). 2018;97(27):e11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlin MJ, Saltzman CL, Anderson RB. Mann’s Surgery of the Foot and Ankle. 9th ed. Mosby; 2013. [Google Scholar]
- 7.Gardner SE, Frantz RA. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol. Res. Nurs 2008;10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner SE, Hillis SL, Heilmann K, et al. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol 2003;41:5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 2004;39 Suppl 2:S115–122. [DOI] [PubMed] [Google Scholar]
- 12.Jneid J, Lavigne JP, La Scola B, Cassir N. The diabetic foot microbiota: A review. Hum. Microbiome J 2017;5–6:1–6. [Google Scholar]
- 13.Lee FE-H, Falsey AR, Halliley JL, et al. Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults. J. Infect. Dis 2010;202:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee FE-H, Halliley JL, Walsh EE, et al. Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect. J. Immunol. Baltim. Md 1950 2011;186:5514–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindbloom BJ, James ER, McGarvey WC. Osteomyelitis of the foot and ankle. Foot Ankle Clin. 2014;19:569–588. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA. Osteomyelitis of the foot in diabetic patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 1997;25:1318–1326. [DOI] [PubMed] [Google Scholar]
- 17.Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis 2012;54(12):1679–1684. [DOI] [PubMed] [Google Scholar]
- 18.Maljkovic Berry I, Melendrez MC, Bishop-Lilly KA, et al. Next generation sequencing and bioinformatics methodologies for infectious disease research and public health: approaches, applications, and considerations for development of laboratory capacity. J. Infect. Dis 2020;28:221(Suppl_3):S292–307. [DOI] [PubMed] [Google Scholar]
- 19.Marmor S, Bauer T, Desplaces N, et al. Multiplex antibody detection for noninvasive genus-level diagnosis of prosthetic joint infection. J Clin Microbiol. 2016;54(4):1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masters EA, Trombetta RP, Bentley KL de M, et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy.” Bone Res. 2019;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molines-Barroso RJ, Lázaro-Martínez JL, Aragón-Sánchez J, et al. Analysis of transfer lesions in patients who underwent surgery for diabetic foot ulcers located on the plantar aspect of the metatarsal heads. Diabet. Med. J. Br. Diabet. Assoc 2013;30:973–976. [DOI] [PubMed] [Google Scholar]
- 22.Nishitani K, Beck CA, Rosenberg AF, et al. A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clin. Orthop 2015;473:2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J. Orthop. Res 2015;33:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh I, Muthukrishnan G, Ninomiya MJ, et al. Tracking anti-Staphylococcus aureus antibodies produced in vivo and ex vivo during foot salvage therapy for diabetic foot infections reveals prognostic insights and evidence of diversified humoral immunity. Infect. Immun 2018;86(12):e00629–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osmon DR, Berbari EF, Berendt AR, et al. Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis 2013;56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 26.Palermo A, Nesterov-Mueller A. Serological number for characterization of circulating antibodies. Int. J. Mol. Sci 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percival SL, Malone M, Mayer D, et al. Role of anaerobes in polymicrobial communities and biofilms complicating diabetic foot ulcers. Int. Wound J 2018;15:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am. J. Surg 1998;176:5S–10S. [DOI] [PubMed] [Google Scholar]
- 29.Sade R, Jatothu D, Taruni, et al. Management of diabetic foot ulcers in a teaching hospital. Int. Surg. J 2017;4(9):3088–3091. [Google Scholar]
- 30.Saeed K, McLaren AC, Schwarz EM, et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthop. Res 2019;37:1007–1017. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz EM, Parvizi J, Gehrke T, et al. 2018 International Consensus Meeting on Musculoskeletal Infection: research priorities from the general assembly questions. J. Orthop. Res 2019;37:997–1006. [DOI] [PubMed] [Google Scholar]
- 32.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol 1999;37:3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sultan AR, Swierstra JW, Toom NAL, et al. Production of Staphylococcal Complement Inhibitor (SCIN) and other immune modulators during the early stages of Staphylococcus aureus biofilm formation in a mammalian cell culture medium. Infect. Immun 2018;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabur S, Eren MA, Çelik Y, et al. The major predictors of amputation and length of stay in diabetic patients with acute foot ulceration. Wien. Klin. Wochenschr 2015;127:45–50. [DOI] [PubMed] [Google Scholar]
- 35.Tarabichi M, Alvand A, Shohat N, et al. Diagnosis of Streptococcus canis periprosthetic joint infection: the utility of next-generation sequencing. Arthroplasty Today. 2018;4:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarabichi M, Shohat N, Goswami K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J. Bone Joint Surg. Am 2018;100:147–154. [DOI] [PubMed] [Google Scholar]
- 37.Tarabichi M, Shohat N, Goswami K, et al. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Jt. J 2018;100-B:127–133. [DOI] [PubMed] [Google Scholar]
- 38.Udovichenko OV, Maximova NV, Amosova MV, et al. Prevalence and prognostic value of depression and anxiety in patients with diabetic foot ulcers and possibilities of their treatment. Curr. Diabetes Rev 2017;13:97–106. [DOI] [PubMed] [Google Scholar]
- 39.Utsunomiya M, Tomita MT, Kinoshita MK, et al. Prognostic factor of diabetic and ischemic foot ulcer, multi-center, multi department observational study. Eur. Heart J 2017;38(Suppl_1):P1408. [Google Scholar]
- 40.Yang J-J, Chang T-W, Jiang Y, et al. Commensal Staphylococcus aureus provokes immunity to protect against skin infection of methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci 2018;19(5):1290. [DOI] [PMC free article] [PubMed] [Google Scholar]


