Abstract
Introduction:
Despite increases in surgical capacity in Malawi, minimal data exist on post-operative complications. Identifying surgical management gaps and targeting quality improvement requires detailed, longitudinal complications and outcome data that assess surgical safety and efficacy.
Methods:
We conducted a six-month prospective, observational study of patients >12 years following laparotomy at a tertiary hospital in Lilongwe, Malawi. Outcomes included post-operative complications and mortality. The senior-most rounding physician determined complication diagnoses. Bivariate and Poisson regression analyses identified predictors of mortality.
Results:
Only patients undergoing emergent laparotomy (77.8%) died before discharge, so analysis excluded elective cases. Of 189 patients included, the median age was 33.5 (IQR 22–50.5), 22 (12.2%) had prior abdominal surgery, and 11 (12.1%) were HIV-positive. Gastrointestinal perforation was the most common diagnosis (35.5%). The most common procedures were primary gastrointestinal repair (24.9%), diverting ostomy (21.2%), and bowel resection with anastomosis (16.4%). Overall post-operative mortality was 14.8%. Intraabdominal complication occurred in 17 (9.0%) patients, of whom 8 (47.1%) died. Older age (RR 1.05, 95% CI 1.02–1.08, p<0.001) and intraabdominal complication (RR 2.88, 95% CI 1.28–6.46, p=0.01) increased the relative risk of mortality. Preoperative diagnosis, surgical intervention type, and symptom-to-surgery time did not increase the relative risk of mortality.
Conclusions:
The incidence of complications and mortality following laparotomy at a large referral hospital in Malawi is high. Older age and intraabdominal complications increase the risk of death. Strategies to improve operative mortality in Malawi should prioritize post-operative surveillance and management and continued outcomes reporting.
Keywords: Laparotomy, mortality, Malawi
Introduction
The global burden of disease has begun to shift from communicable to non-communicable diseases, such as traumatic injury, cancer, and cardiovascular disease. Surgical management for both chronic and acute surgical diseases is recognized as an essential and cost-effective intervention in improving global population health [1–4]. At present, the poorest third of the world’s population receives only 3–5% of global surgical procedures [2, 5]. Africa has the highest burden of surgically-treatable disease in terms of disability-associated life years (DALYs) [6]. In sub-Saharan Africa, there is limited surgical access due to delayed referrals from district-level facilities, unreliable infrastructure, and provider shortages. For those able to access surgical care, post-operative morbidity and mortality are significant concerns. The African Surgical Outcomes Study (ASOS) demonstrated that surgical patients have twice the global average mortality rate, despite being generally younger with fewer preoperative comorbidities [7–9]. Laparotomy, one of three essential Bellwether procedures [10], represents a higher proportion of operations and carries a two- to three-fold increased mortality rate in low-income countries compared to high-income countries [9]. Though the severity of the disease necessitating the laparotomy and the surgical acuity may be drivers of increased mortality outcomes, the identification, and management of post-operative complications may be considered a proxy for surgical quality.
Limited post-operative surveillance due to staffing shortages with high patient volumes and limited diagnostic adjuncts are known as potential drivers of the surgical mortality gap between resource-rich and resource-poor countries [7, 10]. Measuring the quality of surgical care is complex, and post-operative mortality is a recommended global indicator for estimating the quality of surgical care. [11–13] However, measures of morbidity, particularly post-operative complications, in the assessment of the quality of surgical care delivery is necessary as surgical systems continue to develop in resource-limited settings.
Malawi is a small country in sub-Saharan Africa, with a population of 18 million and an average life expectancy of 64 years [14]. As of 2018, it was ranked 172 out of 189 countries in terms of its human development index. Like many other African nations, Malawi’s health care system is structured according to a colonial three-tiered referral system of health centers (primary care), district hospitals (some surgical capacity), and regional hospitals (full general, obstetric, and orthopedic surgical capacity). In Malawi specifically, four tertiary-level facilities and many smaller district-level facilities are equipped to provide surgical care. A 2007 survey of the country estimated that the Malawi Ministry of Health provides 60% of the formal health care services in the country, the Christian Health Association of Malawi (CHAM), an additional 37%, and the rest by local government and private practitioners [15]. Non-physician clinical officers compose 77% and 95% of the country’s surgical and anesthetic workforce, respectively [15]. The estimated average general surgeon density is 0.36 per 100,000 people, compared with 4.7 per 100,00 people in the United States [15]. Despite recent increases in the number of physician surgeons trained and practicing in Malawi [16], access to surgical care in the country is still limited [15, 17–21], and detailed post-operative outcomes data is scarce. The study’s primary objective was to determine the frequency of complications following laparotomy and quantify their impact on mortality in a tertiary care center in Malawi.
Methods
We performed a six-month prospective observational study of adult patients admitted to the general surgery service and underwent laparotomy at Kamuzu Central Hospital (KCH) in Lilongwe, Malawi, from October 2019 through March 2020. KCH is a 900-bed tertiary public hospital and referral center for the central region of Malawi. KCH has six operating theatres, two of which are used daily for general surgery. Over the study period, there were five consultant general surgeons, two surgical clinical officers, and at least three surgery registrars assigned to the general surgery service. KCH has a five-bed intensive care unit (ICU) with a 1:2 nurse to patient ratio. There is capability for endotracheal intubation and ventilation and all necessary ICU monitoring except invasive blood pressure. Also, there is an eight-bed high-dependency unit (HDU) that functions as a step-down unit with a 1: 4 nurse to patient ratio with telemetry and pulse oximetry, and availability of noninvasive ventilation.
In this study, we included all adult patients undergoing abdominal surgery at KCH and admitted postoperatively to the general surgery ward, HDU, or ICU. Patients discharged and readmitted within 30-days of their initial operation were included in the analysis (n = 7) with any reoperation coded as management of a complication such that patients were not duplicated in the analysis. We excluded children (aged ≤ 12 years) and patients missing outcomes data from the study cohort. The primary outcomes were in-hospital mortality and post-operative complications. Data collected include basic demographics, smoking status, HIV status, past surgical history, time of first symptoms (emergent cases only), preoperative hemoglobin, diagnosis, and operation details. We recorded information on pre- and post-operative diagnoses as well as operation details from the admission and operation notes. We determined preoperative hemoglobin from the most recent full blood count before surgery. We defined anemia as preoperative hemoglobin in the mild, moderate, or severe range based on sex-specific WHO definitions [22]. We recorded post-operative complications as defined by the European Perioperative Clinical Outcome (EPCO) definitions utilized in the African Surgical Outcomes Study [7].
We followed patients from post-operative day zero to either death or discharge. They were evaluated by a surgery registrar on rounds three or four times per week and a consultant surgeon at least once per week. The lead author was responsible for prospectively recording complications diagnosed by the most senior physician on rounds, usually a registrar or a consultant surgeon. The researchers made no attempts to change usual standard practices other than clarifying findings and management plans. Some post-operative complications necessarily require diagnostic adjuncts not readily available in our setting. Many complications were certainly under-recognized in this study cohort, namely non-abdominal complications such as urinary tract infections, pneumonia, and deep vein thromboses. We did not record complications retrospectively diagnosed after death. Analysis for this study of laparotomy patients was limited to abdominal complications, including surgical site infections, intraabdominal infections, anastomotic or repair leaks, fascial dehiscence, and enterocutaneous fistulas. Superficial versus deep surgical site infections could not be reliably distinguished on clinical examination and were classified together as surgical site infections.
We used univariate analysis was used to determine data distribution for continuous variables. The study population was analyzed using descriptive statistics in the overall population and stratified by outcome (death or discharge). We performed a bivariate analysis based on mortality. To compare the distribution of outcome across patient and operative variables, we utilized χ2 for categorical variables, Student’s t-test for normally distributed continuous variables, and Kruskal-Wallis for non-normally distributed continuous variables.
We performed multivariable Poisson regression modeling to determine patient and operative characteristics that affected the relative risk of mortality. A priori, we included in the model any variables potentially associated with in-hospital post-operative mortality, including age, sex, smoking status, HIV status, moderate to severe preoperative anemia, consultant surgeon involvement, time from symptom onset to surgical intervention, post-operative complications, American Society of Anesthesiologist (ASA) Physical Status Classification, and surgical wound class. We utilized a backward elimination approach to reduce error and improve precision (narrowing of confidence intervals) in the model. We removed HIV status, smoking status, and symptom to surgery time from the model based on this methodology.
We utilized StataCorp v16.0, College Station, Texas, for all statistical analyses. We reported confidence intervals at 95%, and we set alpha at 0.05 for this study. The Malawi National Health Science Research Committee and the University of North Carolina Institutional Review Board approved this study and the need for informed consent was waived.
Results
Over the study period, we accrued 373 post-operative patients. Of these, 252 patients underwent laparotomy for varying indications, and 243 (96.4%) had sufficiently complete outcomes data to be included in the analysis (Figure 1). No patients undergoing elective abdominal surgery died, so analysis was restricted to emergent cases (n = 189, 77.8%). Of laparotomies, 156 (82.5%) were male with a median age of 33.5 years (IQR 22 – 50.5) and the median post-operative length of stay of 6 days (IQR 4 – 8) (Table 1). Of tested patients, 12.1 % were HIV-positive, and 12.2% had a history of prior abdominal surgery. At least one abdominal complication occurred in 25 (13.2%) cases. Surgical site infections occurred in 13 (6.9%) patients, and at least one intraabdominal complication was diagnosed in 17 (9.0%) patients. Intraabdominal complications included 8 intraabdominal infections, 11 anastomotic or repair leaks, and 6 instances of abdominal fascial dehiscence. Unplanned reoperation was undertaken for 13 (76.5%) patients with intraabdominal complications. The overall mortality for patients undergoing laparotomy was 14.8%. The median time to post-operative death was 4.5 days (IQR 1.5 – 9). No patients died intraoperatively, and one patient died in post-anesthesia recovery.
Fig 1.
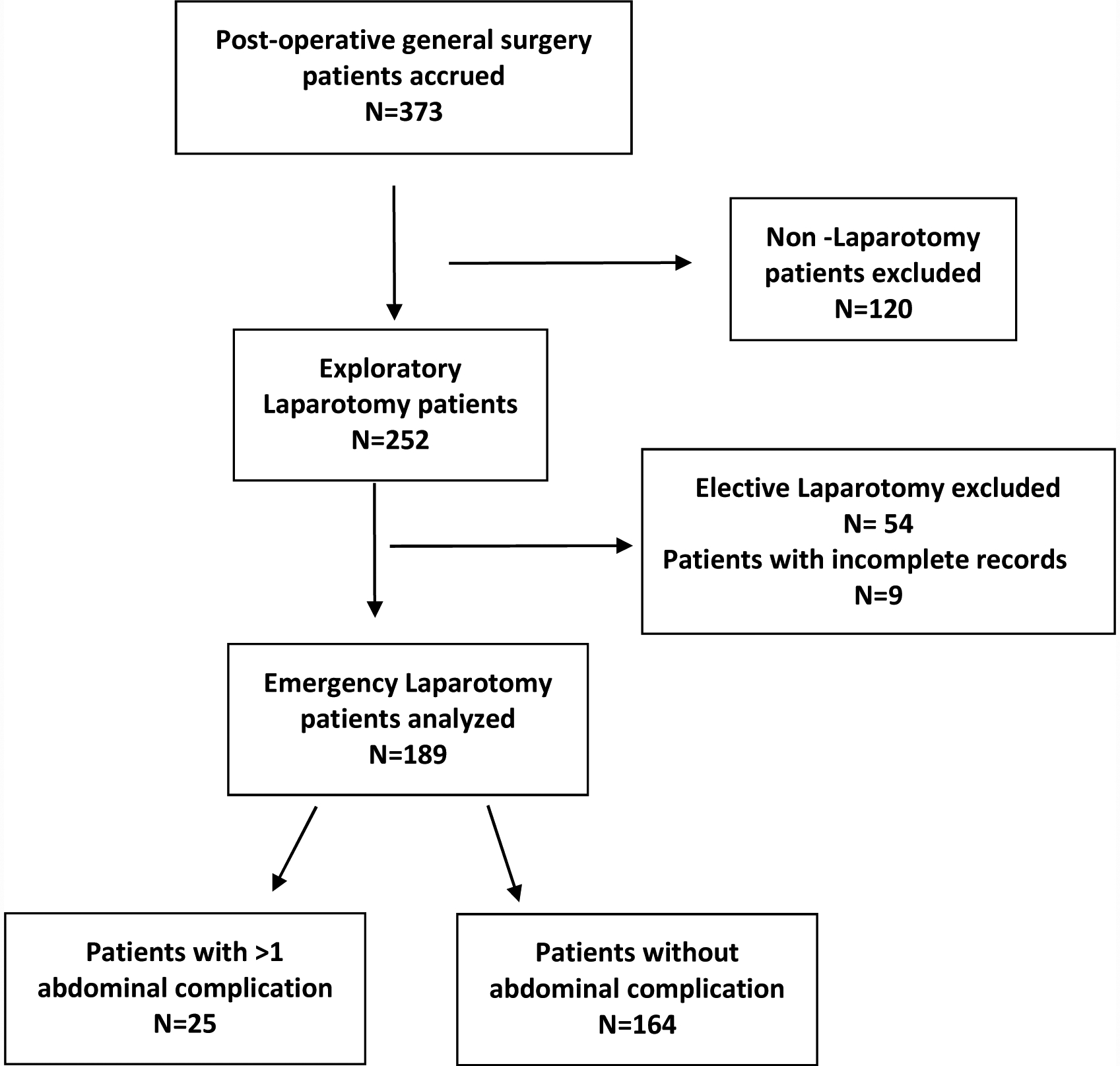
Patient inclusion and exclusion criteria and frequency of abdominal complications
Table 1.
Univariate and bivariate analysis of demographics, operative information, and postoperative abdominal complications over mortality.
| Overall n = 189 | Died n = 28 (14.8%) | Discharged n = 161 (85.2%) | p-value | |
|---|---|---|---|---|
| Age: median (IQR) | 33.5 (22 – 50.5) | 40 (29 – 60) | 32.5 (22 – 48) | 0.04 |
| Male sex: n (%) | 156 (82.5%) | 22 (78.6%) | 134 (83.2%) | 0.5 |
| Current smoker: n (%) | 27 (17.9%) | 5 (29.4%) | 22 (16.4%) | 0.2 |
| HIV-positive: n (%) | 11 (12.1%) | 2 (13.3%) | 9 (11.8%) | 0.9 |
| Prior abdominal surgery: n (%) | 22 (12.2%) | 5 (19.2%) | 17 (11.0%) | 0.2 |
| Symptom-to-surgery time, days: median (IQR) | 3 (2 – 6) | 5 (2 – 7) | 3 (2 – 6) | 0.2 |
| Preoperative hemoglobin, g/dL: mean (SD) | 13.5 (3.4) | 12.7 (3.0) | 13.6 (3.4) | 0.2 |
| Any anemia: n (%) | 52 (34.0%) | 11 (45.8%) | 41 (31.8%) | 0.2 |
| Moderate/Severe anemia: n (%) | 28 (18.3%) | 7 (29.2%) | 21 (16.3%) | 0.1 |
| Consultant involved: n (%) | 118 (62.4%) | 14 (50.0%) | 104 (64.6%) | 0.1 |
| Unplanned re-operation: n (%) | 13 (6.9%) | 5 (17.9%) | 8 (5.0%) | 0.01 |
| Surgical site infection: n (%) | 13 (6.9%) | 1 (3.6%) | 12 (7.5%) | 0.5 |
| Intraabdominal complication: n (%) | 17 (9.0%) | 8 (28.5%) | 9 (5.6%) | <0.001 |
| Intraabdominal infection | 8 (4.2%) | 3 (10.7%) | 5 (3.1%) | |
| Anastomotic or repair leak | 11 (14.1%)* | 7 (25.0%) | 4 (2.5%) | |
| Fascial dehiscence | 6 (3.2%) | 1 (3.6%) | 5 (3.1%) | |
| Enterocutaneous fistula | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| ASA Physical Status Classification: n (%) | 0.1 | |||
| 1 | 68 (44.4%) | 8 (34.8%) | 60 (46.2%) | |
| 2 | 54 (35.3%) | 8 (34.8%) | 46 (35.4%) | |
| 3 | 29 (19.0%) | 6 (26.1%) | 23 (17.7%) | |
| 4 | 1 (0.7%) | 1 (4.4%) | 0 (0.0%) | |
| 5 | 1 (0.7%) | 0 (0.0%) | 1 (0.8%) | |
| Wound Class: n (%) | 0.4 | |||
| Clean | 31 (16.5%) | 3 (10.7%) | 28 (17.5%) | |
| Clean-Contaminated | 34 (18.1%) | 4 (14.3%) | 30 (18.8%) | |
| Contaminated | 35 (18.6%) | 8 (28.6%) | 27 (16.9%) | |
| Dirty | 88 (46.8%) | 13 (46.4%) | 75 (46.9%) | |
| Postoperative disposition: n (%) | 0.008 | |||
| Ward | 153 (81.0%) | 17 (60.7%) | 136 (84.5%) | |
| HDU | 26 (13.8%) | 7 (25.0%) | 19 (11.8%) | |
| ICU | 10 (5.3%) | 4 (14.3%) | 6 (3.7%) | |
| Postoperative length of stay, days: median (IQR) | 6 (4 – 8) | 4 (1 – 9) | 6 (4 – 7) | 0.1 |
out of 78 patients undergoing bowel anastomosis or primary repair
The most common indications for laparotomy were gastrointestinal perforation (n=67, 35.5%) and intestinal volvulus (n=58, 30.7%). The most common procedures were primary gastrointestinal repair (n=47, 24.9%), creation of a diverting ostomy (n=40, 21.2%), and bowel resection with anastomosis (n=31, 16.4%), (Table 2). A consultant physician surgeon was involved in 62.4% of cases. Ten patients (5.3%) went to the ICU and 26 (13.8%) to the high-dependency unit (HDU) immediately postoperatively.
Table 2.
Bivariate analysis of diagnosis categories and operative intervention over mortality.
| Overall n = 189 | Died n = 28 (14.8%) | Discharged n = 161 (85.2%) | p-value | |
|---|---|---|---|---|
| Diagnostic Category: n (%) | ||||
| Ventral hernia | 6 (3. 2%) | 1 (3.6%) | 5 (3.1%) | 0.9 |
| Gastrointestinal perforation | 67 (35.5%) | 11 (39.3%) | 56 (34.8%) | 0.6 |
| Gastric | 24 (12.7%) | 4 (14.3%) | 20 (12.4%) | |
| Ileal | 13 (6.9%) | 1 (3.6%) | 12 (7.5%) | |
| Duodenal | 4 (2.1%) | 1 (3.6%) | 3 (1.9%) | |
| Jejunal | 3 (1.6%) | 1 (3.6%) | 2 (1.2%) | |
| Appendiceal | 8 (4.2%) | 1 (3.6%) | 7 (4.3%) | |
| Colonic | 1 (0.5%) | 1 (3.6%) | 0 (0.0%) | |
| Unspecified | 13 (7.4%) | 2 (7.2%) | 11 (6.8%) | |
| Volvulus | 58 (30.7%) | 7 (25.0%) | 51 (31.7%) | 0.5 |
| Midgut | 17 (9.0%) | 1 (3.6%) | 16 (9.9%) | |
| Sigmoid | 31 (16.4%) | 5 (17.9%) | 26 (16.1%) | |
| Compound | 10 (5.3%) | 1 (3.6%) | 9 (5.6%) | |
| Abdominal mass | 10 (5.3%) | 3 (10.7%) | 7 (4.4%) | 0.2 |
| Inflammation/Abscess | 18 (9.5%) | 2 (7.1%) | 16 (9.9%) | 0.6 |
| Solid organ injury | 12 (6.4%) | 1 (3.6%) | 11 (6.8%) | 0.5 |
| Other | 18 (9.5%) | 3 (10.7%) | 15 (9.3%) | 0.8 |
| Operation Category: n (%) | ||||
| Hernia repair | 5 (2. 7%) | 1 (3.6%) | 4 (2.5%) | 0.7 |
| Primary gastrointestinal repair | 47 (24.9%) | 6 (21.4%) | 41 (25.5%) | 0.6 |
| Bowel resection and anastomosis | 31 (16.4%) | 8 (28.6%) | 23 (14.3%) | 0.06 |
| Diverting ostomy | 40 (21.2%) | 5 (17.9%) | 35 (21.7%) | 0.6 |
| Appendectomy | 17 (9.0%) | 1 (3.6%) | 16 (9.9%) | 0.3 |
| Other clean abdomen | 27 (14.3%) | 3 (10.7%) | 24 (14.9%) | 0.6 |
| Other infected/dirty abdomen | 12 (4.6%) | 2 (7.1%) | 10 (6.2%) | 0.9 |
Bivariate analysis based on mortality demonstrated that the development of post-operative intra-abdominal complications (p < 0.001) and immediate post-operative requirement of HDU or ICU care (p = 0.008) were associated with increased mortality. Factors conferring an increased risk of mortality on multivariate Poisson regression modelling were older age (RR 1.05, 95% CI 1.02 – 1.08, p < 0.001) and intraabdominal complication (RR 2.88, 95% CI 1.28 – 6.46, p = 0.01) (Table 3).
Table 3.
Multivariate Poisson regression modelling demonstrates the relative risk of mortality for included patient and operative factors.
| Relative Risk | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Sex | 1.73 | 0.52 – 5.72 | 0.4 |
| Age | 1.05 | 1.02 – 1.08 | < 0.001 |
| Moderate/severe anemia | 1.45 | 0.32 – 6.52 | 0.6 |
| Consultant involved | 0.62 | 0.28 – 1.38 | 0.2 |
| Intra-abdominal complications | 2.88 | 1.28 – 6.46 | 0.01 |
| Wound class | |||
| Clean | Ref | --- | --- |
| Clean-contaminated | 1.99 | 0.24 – 16.19 | 0.5 |
| Contaminated | 5.46 | 0.70 – 42.86 | 0.1 |
| Dirty | 2.10 | 0.26 – 16.70 | 0.5 |
| ASA class | |||
| I | Ref | --- | --- |
| II | 1.24 | 0.46 – 3.33 | 0.7 |
| III | 2.45 | 0.69 – 8.66 | 0.2 |
| Postoperative Disposition | |||
| Ward | Ref | --- | --- |
| HDU (Step-down) | 1.74 | 0.47 – 6.40 | 0.4 |
| ICU | 5.40 | 0.69 – 44.68 | 0.1 |
Discussion
Laparotomy is an essential general surgical procedure worldwide and is associated with significantly elevated morbidity and mortality in low-resource compared to high-resource settings. In this prospective observational study of consecutive patients undergoing emergent laparotomy, at least one intraabdominal complication developed in 9.0% of these patients. The overall mortality was 14.8%. Multivariate Poisson regression indicated that intraabdominal complications were associated with a nearly 3-fold increase in relative risk of mortality.
The African Surgical Outcomes Study (ASOS), a 7-day prospective observational study of post-operative outcomes across 25 African counties, demonstrated 5.5% mortality among gastrointestinal and hepatobiliary surgery patients [7]. In a similar study of emergent abdominal operations across 58 countries, Bhangu et al. demonstrated overall 30-day mortality of 5.4%, which increased to 8.6% among countries with a low Human Development Index [9]. Country-level data in sub-Saharan Africa is still scarce [23], but available mortality estimates for general or abdominal surgery range from 4% to 11% [24–27]. As an indicator of surgical safety and quality, our institution’s high laparotomy mortality (14.8%) signals an urgent need for data-driven interventions.
The reason for the high mortality in our setting is multifactorial. Though we did not show that time from symptom onset to operative intervention impacts mortality, difficulties in accessing surgical care in Malawi are well known. At our institution, we have previously shown increased mortality in patients with peritonitis due to difficulty accessing timely surgical care and in-hospital delays to surgical intervention and in patients [28, 29]. There is also a high prevalence of malnutrition and anemia, which may impact post-operative recovery and the risk of complications. The importance of nutritional status in surgical outcomes is well established [30]. Unfortunately, in our setting, obtaining routine nutritional status markers such as albumin and pre-albumin in the preoperative period is impractical and expensive. Some degree of preoperative anemia was present in 34% of patients, more than half of which were moderately or severely anemic. However, our study did not demonstrate a significant impact of anemia on mortality. While increasing wound class has been reliably associated with increased rates of surgical site infections regardless of operation type [31], its relationship to mortality has not been well-described. Our study also failed to demonstrate an association between wound class and mortality.
The majority of cases in the overall cohort presented as surgical emergencies, and emergency surgery is a well-described, independent predictor of increased mortality in surgery patients globally [26, 32, 33]. The increased fraction of emergent presentations in resource-limited settings is indicative of the lack of timely access to definitive surgical care. Prin et al. studied this phenomenon and concluded that the emergency-to-elective surgery ratio could a proxy for egional access to surgery [34]. The high fraction of emergent surgeries in our study may yet be an underrepresentation of the exact volume of emergencies, as some patients may have died before surgical intervention.
Overall, neither the surgical diagnosis nor operative procedure was associated with mortality. These observations, in conjunction with the nearly 3-fold increased relative risk of mortality in patients who developed post-operative intra-abdominal complications, support recent literature in both high- and low-resource settings that indicate post-operative surveillance and management may be critical in determining patient outcomes [7, 10, 33, 35]. Numerous challenges to timely, safe, and effective post-operative care exist in limited-resource settings. These include surgical workforce shortages, hospital overcrowding, limited laboratory and imaging capacity, and inability to move patients to a monitored environment for higher levels of care, such as the intensive care unit. All of these factors severely limit the ability to recognize and manage post-operative deterioration.
Failure-to-rescue, defined as the rate of death following complications, has been widely used to assess the quality of post-operative care [10] and has been associated with institutional rather than patient factors [36]. Biccard et al. found that the prevalence of post-operative complications was lower across 25 African countries than the global prevalence, despite having twice the post-operative mortality [7]. All study patients who died had at least one diagnosed complication [7, 8, 10]. In contrast, most patients in our study died without the diagnosis of any complication, indicating not only failure to rescue but also failure to recognize deterioration and life-threatening complications in our patients. Unfortunately, due to limitations in diagnostic adjuncts at the time of this study, it was not possible to obtain more than speculative causes of death for most patients. Further, we acknowledge that at a tertiary referral center such as ours, high mortality may also indicate more advanced surgical disease in our cohort. Neither time from reported symptom onset to surgery nor the presence of preoperative anemia differed between patients who died versus those who survived to discharge. However, assessment of preoperative vital signs, mental status, and injury severity may be valuable in further evaluating predictors of post-operative outcomes.
In this study evaluating mortality among laparotomy patients suggests that interventions to optimize post-operative care may be the most valuable investments in improving surgical outcomes in our setting. Kamuzu Central Hospital, in partnership with the UNC Project has been training surgeons since 2009, and the number of surgeons operating in Malawi has increased over the past decade.
This study has significant limitations. While this is, to our knowledge, the most extensive prospective cohort study of post-operative complications in Malawi, our findings’ generalizability is limited by our sample size. In Malawi, severe limitations in diagnostic capacity and history taking may restrict the ability to assess comorbidities necessary in accurately determining ASA Physical Status Classification. Furthermore, there were significant impediments to discovering the actual frequency of post-operative complications, the most important of which was the lack of complete and regular patient assessments due to high patient volume and limited diagnostic adjuncts. Lastly, we conducted this study at a referral hospital equipped with multiple operating rooms and at least one on-call physician surgeon at any given time. As such, these results are unlikely generalizable to the district level, where operative and surveillance capacity are limited. As the capacity for general surgery increases at district facilities, their inclusion in surgical outcomes research will be valuable in informing national surgical plans [37].
Conclusion
Though surgical capacity has increased in Malawi, the high post-operative mortality at one of the largest providers of public surgical care in the country indicates an urgent need for interventions targeting surgical safety and quality. Abdominal complications are a robust, independent predictor of mortality following laparotomy, indicating the importance of post-operative surveillance for timely recognition of patient deterioration and early intervention. Interventions that facilitate such surveillance may be high-value, particularly in health systems with limited resources.
Highlight.
Outcomes following laparotomy may be considered a proxy for surgical quality
At least one intraabdominal complication was diagnosed in 9.3% of patients
The overall mortality for patients undergoing laparotomy was 14.8%
Post-operative intraabdominal complication increased relative risk of mortality five-fold
Failure to rescue is a contributor to post-operative deaths in this setting.
Acknowledgment
The authors gratefully acknowledge the consultant and trainee surgeons in the department of surgery at Kamuzu Central Hospital for their support in data collection.
Funding
John Sincavage is supported jointly by the US Department of State and Fogarty International Center, Grant #D43TW009340-08S3 Fulbright-Fogarty. Laura Purcell was supported by the National Institutes for Health, Fogarty International Center, Grant # D43TW009340.
Funding: This study was supported in part by the Fogarty-Fulbright and NIH Fogarty International Center Postdoctoral Research Fellowship to John Sincavage and Dr. Purcell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Mathers CD and Loncar D, Projections of Global Mortality and Burden of Disease from 2002 to 2030 (Projections of Global Mortality). PLoS Medicine, 2006. 3(11): p. e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meara JG, et al. , Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet, 2015. 386(9993): p. 569. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, et al. , Surgery and Obstetric Care are Highly Cost-Effective Interventions in a Sub-Saharan African District Hospital: A Three-Month Single-Institution Study of Surgical Costs and Outcomes. World Journal Of Surgery, 2016. 40(1): p. 14. [DOI] [PubMed] [Google Scholar]
- 4.Grimes CE, et al. , Cost-effectiveness of Surgery in Low- and Middle-income Countries: A Systematic Review. World Journal Of Surgery, 2014. 38(1): p. 252. [DOI] [PubMed] [Google Scholar]
- 5.Weiser TG, et al. , An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet, 2008. 372(9633): p. 139. [DOI] [PubMed] [Google Scholar]
- 6.Debas Haile T., G. R, McCord Colin, and Thind Amardeep, Surgery, in Disease Control Priorities in Developing Countries, 2nd edition, Jamison J.G.B. Dean T, Measham Anthony R, Alleyne George, Claeson Mariam, Evans David B, Jha Prabhat, Mills Anne, and Musgrove Philip, Editor. 2006, The World Bank/Oxford University Press: New York. [PubMed] [Google Scholar]
- 7.Biccard BM, et al. , Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. Lancet, 2018. 391(10130): p. 1589. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad T, et al. , Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Bja: British Journal Of Anaesthesia, 2016. 117(5): p. 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhangu A, et al. , Mortality of emergency abdominal surgery in high-, middle- and low-income countries. British Journal of Surgery, 2016. 103(8): p. 971. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad T, et al. , Use of failure-to-rescue to identify international variation in postoperative care in low-, middle- and high-income countries: a 7-day cohort study of elective surgery. British Journal of Anaesthesia, 2017. 119(2): p. 258. [DOI] [PubMed] [Google Scholar]
- 11.Watters D, et al. , Perioperative Mortality Rate (POMR): A Global Indicator of Access to Safe Surgery and Anaesthesia. World Journal Of Surgery, 2015. 39(4): p. 856. [DOI] [PubMed] [Google Scholar]
- 12.Weiser TG, et al. , Standardised metrics for global surgical surveillance. Lancet, 2009. 374(9695): p. 1113. [DOI] [PubMed] [Google Scholar]
- 13.Felizaire M-R, et al. , Perioperative Mortality Rates as a Health Metric for Acute Abdominal Surgery in Low- and Middle-Income Countries: A Systematic Review and Future Recommendations. World Journal Of Surgery, 2019. 43(8): p. 1880. [DOI] [PubMed] [Google Scholar]
- 14.Bank TW, Life Expectancy at Birth - Malawi. 2018, The World Bank Group. [Google Scholar]
- 15.Henry JA, et al. , Surgical and anaesthetic capacity of hospitals in Malawi: key insights. Health Policy And Planning, 2015. 30(8): p. 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell LN, et al. , District General Hospital Surgical Capacity and Mortality Trends in Patients with Acute Abdomen in Malawi. World Journal Of Surgery, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varela C, et al. , Untreated surgical conditions in Malawi: A randomised cross-sectional nationwide household survey. Malawi Medical Journal, 2017. 29(3): p. 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varela C, et al. , TRANSPORTATION BARRIERS TO ACCESS HEALTH CARE FOR SURGICAL CONDITIONS IN MALAWI a cross sectional nationwide household survey. BMC Public Health, 2019. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prin M, et al. , High Elective Surgery Cancellation Rate in Malawi Primarily Due to Infrastructural Limitations. World Journal Of Surgery, 2018. 42(6): p. 1597. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski J, et al. , ‘I think we are going to leave these cases’. Obstacles to surgery in rural Malawi: a qualitative study of provider perspectives. Tropical Medicine & International Health, 2018. 23(10): p. 1141. [DOI] [PubMed] [Google Scholar]
- 21.Tindall A, et al. , Surgical facilities available at district hospitals in Malawi. Malawi Medical Journal : The Journal Of Medical Association Of Malawi, 2007. 19(1): p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011, World Health Organization. [Google Scholar]
- 23.Hoyler M, et al. , Shortage of Doctors, Shortage of Data: A Review of the Global Surgery, Obstetrics, and Anesthesia Workforce Literature. World Journal Of Surgery, 2014. 38(2): p. 269. [DOI] [PubMed] [Google Scholar]
- 24.Rickard J, Ntakiyiruta G, and Chu K, Associations with Perioperative Mortality Rate at a Major Referral Hospital in Rwanda. World Journal Of Surgery, 2016. 40(4): p. 784. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GA, et al. , Surgical volume and postoperative mortality rate at a referral hospital in Western Uganda: Measuring the Lancet Commission on Global Surgery indicators in low-resource settings. Surgery, 2017. 161(6): p. 1710. [DOI] [PubMed] [Google Scholar]
- 26.Biccard BM and Madiba TE, The South African surgical outcomes study: a 7-day prospective observational cohort study. SAMJ South African Medical Journal, 2015. 105: p. 465.+. [DOI] [PubMed] [Google Scholar]
- 27.Osinaike B, et al. , Nigerian surgical outcomes – Report of a 7-day prospective cohort study and external validation of the African surgical outcomes study surgical risk calculator. International Journal of Surgery, 2019. 68: p. 148. [DOI] [PubMed] [Google Scholar]
- 28.Gallaher JR, et al. , Outcomes of Peritonitis in Sub-Saharan Africa: An Issue of Access to Surgical Care. Journal of the American College of Surgeons, 2015. 221(4): p. S87. [Google Scholar]
- 29.Maine RG, et al. , Effect of in-hospital delays on surgical mortality for emergency general surgery conditions at a tertiary hospital in Malawi. BJS Open, 2019. 3(3): p. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mambou Tebou CG, et al. , Impact of perioperative nutritional status on the outcome of abdominal surgery in a sub-Saharan Africa setting. Bmc Research Notes, 2017. 10(1): p. 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortega G, et al. , An Evaluation of Surgical Site Infections by Wound Classification System Using the ACS-NSQIP. Journal of Surgical Research, 2012. 174(1): p. 33. [DOI] [PubMed] [Google Scholar]
- 32.Jawad M, et al. , Swedish surgical outcomes study (SweSOS): An observational study on 30-day and 1-year mortality after surgery. European Journal Of Anaesthesiology, 2016. 33(5): p. 317. [DOI] [PubMed] [Google Scholar]
- 33.Khuri SF, et al. , Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Annals Of Surgery, 2005. 242(3): p. 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prin M, et al. , Emergency-to-Elective Surgery Ratio: A Global Indicator of Access to Surgical Care. World Journal Of Surgery, 2018. 42(7): p. 1971. [DOI] [PubMed] [Google Scholar]
- 35.Lillie E, et al. , Avoidable perioperative mortality at the University Teaching Hospital, Lusaka, Zambia: a retrospective cohort study. Canadian Journal Of Anesthesia/Journal Canadien D&Apos;Anesthésie, 2015. 62(12): p. 1259. [DOI] [PubMed] [Google Scholar]
- 36.Silber JH, et al. , Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Medical Care, 1992. 30(7): p. 615. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski J, Bijlmakers L, and Brugha R, Global Surgery - Informing National Strategies for Scaling Up Surgery in Sub-Saharan Africa. International journal of health policy and management, 2018. 7(6): p. 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]


