Abstract
BACKGROUND:
Brain volumes in regions such as the hippocampus and amygdala have been associated with risk for development of PTSD. The objective of this study was to determine whether a set of regional brain volumes, measured by MRI at 2 weeks following mTBI (GCS 13–15), are predictive of PTSD at 3- and 6-months post-injury.
METHODS:
This study uses data from TRACK-TBI, a prospective longitudinal study of patients with mTBI. We included patients (N = 421) assessed after evaluation in the Emergency Department, and at 2 weeks (including MRI), 3-, and 6-months post-injury. Probable PTSD diagnosis (PCL-5 score ≥ 33) was the outcome. The Freesurfer 6.0 processing pipeline was used to perform volumetric analysis of 3D T1-weighted MRI at 3 Tesla. Brain regions selected a priori for volumetric analyses were insula, hippocampus, amygdala, superior frontal cortex, rostral and caudal anterior cingulate, and lateral and medial orbitofrontal cortex.
RESULTS:
77 (18.3%) and 70 (16.6%) patients had probable PTSD at 3- and 6-months. A composite volume derived as the first principal component (PC1) incorporating 73.8% of the variance in insula, superior frontal cortex, and rostral and caudal cingulate contributed to prediction of 3-month (but not 6-month) PTSD in multivariable models incorporating other established risk factors.
CONCLUSIONS:
Results, while in need of replication, provide support for a brain reserve hypothesis of PTSD and proof-of-principle for how prediction of at-risk individuals might be accomplished to enhance prognostic accuracy and to enrich clinical prevention trials for individuals at highest risk of PTSD following mTBI.
Keywords: posttraumatic stress disorder (PTSD), brain, Insula, cingulate, Amygdala, traumatic brain injury (TBI)
INTRODUCTION
Posttraumatic stress disorder (PTSD) is an important mental disorder caused by exposure to traumatic stress. Whereas the traumatic stress may be psychological in nature (e.g., threat of injury or death), it may also involve physical injury (e.g., fractures or gunshot wounds). Numerous, well-replicated risk factors for PTSD following trauma have been established (1). These include perceived likelihood of death at the time of trauma, presence of concomitant physical injury, female sex, low education, low IQ, and a history of childhood maltreatment. Genetic and epigenetic risk factors for PTSD are also being identified (2).
Brain morphometric measures have also been identified and replicated as risk factors for development or persistence of PTSD following exposure to traumatic stress. Brain structures of persons with and without PTSD, differ on average, (3) with the former having smaller hippocampal (4–6) and cortical (7) volumes, and reduced cortical thickness (8).
Traumatic brain injury (TBI) is an injury that is especially liable to result in PTSD, though the pathological bases for this co-occurrence are as yet incompletely understood (9–13). Given the findings of structural brain differences between patients with PTSD and healthy controls, there is particular interest in determining the prognostic utility of brain measurement for subsequent PTSD. Among patients with mild TBI (Glasgow Coma Scale [GCS] 13–15), risk factors for PTSD post-TBI include female sex, cause of injury (intentional harm vs. accidental), and history of pre-existing mental health disorder (14). However, these are imperfect predictors of PTSD symptoms; brain imaging represents a promising objective avenue for developing more accurate early biomarkers. Magnetic resonance imaging (MRI) is especially suited for this purpose because of its wide availability, safety, convenience, and excellent image quality.
Few studies have addressed the issue of brain morphometry in PTSD in relation to comorbid mTBI or with reference to outcomes. In a small (N=30) study of patients with PTSD, larger anterior cingulate volume at baseline was shown to be a predictor of recovery (15). In another small study, patients with mTBI with significant PTSD symptoms (n = 12) were found to have larger entorhinal cortex volumes than patient with mTBI without significant PTSD symptoms (n = 27) (16).
In the present study, we investigated MRI quantification of global and regional brain volumes as early biomarkers of PTSD, assessed with the PTSD Checklist for DSM-5 (PCL-5) (17). We limited analysis to the following structures based on pre-existing evidence for their involvement in PTSD: insula, hippocampus, amygdala, superior frontal gyrus, rostral and caudal ACC, medial and lateral orbitofrontal gyri (5–7, 18, 19). We explored whether early MRI volumetrics (obtained at 2 weeks after mTBI) are predictive of PTSD at 3- and 6-months post-injury using the 3 Tesla (3T) high-resolution brain MRI scans of patients with mTBI from the multicenter Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study.
MATERIALS AND METHODS
Participants
Patients were enrolled at 11 academic Level 1 trauma centers in the United States within 24 hours of injury, following evaluation in the Emergency Department (ED) for TBI as part of the prospective TRACK-TBI study (20); all received a computed tomography (CT) scan per order of the evaluating ED physician. Exclusion criteria included: significant polytrauma that would interfere with follow-up; penetrating TBI; prisoners or patients in custody; pregnancy; patients on psychiatric “hold”; being non-English or non-Spanish speaking; having a contraindication to MRI; major debilitating mental (e.g., schizophrenia or bipolar disorder) or neurological disorders (e.g., stroke, dementia) or any other disorder that would interfere with assessment and follow-up or provision of informed consent. Written consent was obtained from all subjects to participate in a protocol approved by the University of California, San Francisco Institutional Review Board (IRB) and by the IRBs at participating sites.
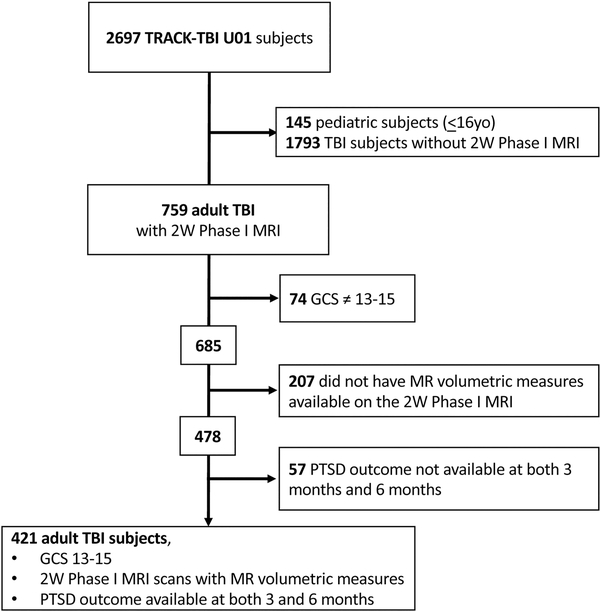
This paper analyzed a subset of the TRACK-TBI U01 cohort with the following criteria: adult (age ≥17), GCS ED arrival scores of 13–15, PCL-5 outcome measure collected at both 3 months and 6 months, with MR volumetric measures analyzed from a research-acquired 3D T1-weighted MRI at 2 weeks after injury. (Figure: CONSORT DIAGRAM). Subjects not included in the analysis due to missing PCL-5 outcomes (N=57) did not differ significantly from those who were included (N=421) on any sociodemographic, clinical, or injury-related parameter.
Figure:
CONSORT DIAGRAM
Measures
Within 24 hours of injury, demographic and clinical characteristics of the patients were collected, including age, sex, race, ethnicity, years of education, history of psychiatric conditions including substance abuse, prior history of TBI, and the cause of the recent injury, categorized as incidental falls, road traffic incidents, violence/assaults, and other causes. Glasgow Coma Scale scores (21) were acquired at the time of presentation to the ED. All patients in the analyses reported here had ED admission GCS score of 13–15.
PTSD Checklist for DSM-5 (PCL-5) was obtained to measure past-month posttraumatic stress disorder symptoms. The range of the scale is 0–80. Signal detection analyses against a clinical gold standard have shown that PCL-5 scores of 31 to 33 are optimally efficient for diagnosing PTSD (17). We used scores of ≥33 to indicate probable PTSD. PCL-5 subscales of reexperiencing, avoidance, negative cognitions and mood, and hyperarousal were also examined as continuous measures.
For the 3-month follow-up, the assessment was completed at mean = 3 months (IQR: 2.9–3.1, range 2.7–3.5) from the time of injury; for the 6-month follow-up the assessment was completed at mean = 6 months (IQR: 5.9–6.2, range 5.4–6.7) from the time of injury.
MR Image Acquisition
Patients underwent 3D T1-weighted imaging at approximately 2 weeks after injury using 3T MRI scanners. Harmonization across MRI platforms was achieved with the Alzheimer Disease Neuroimaging Initiative (ADNI) standard, including the ADNI phantom for monitoring any geometric distortions (22). Briefly, this acquisition consisted of a sagittal 3D Fast Spoiled Gradient Echo (FSPGR) T1-weighted sequence (General Electric) or a sagittal 3D Magnetization-Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted sequence (Philips and Siemens) using 8-channel (General Electric and Philips) or 12-channel (Siemens) head radiofrequency coils and at a spatial resolution of 1.0 × 1.0 × 1.2 mm. The imaging protocol also included 3D T2-weighted fluid attenuated inversion recovery (FLAIR), 3D T2*-weighted gradient echo and 3D T2-weighted sequences for radiological interpretation of abnormal MRI findings.
MR Image Processing and Analysis
Cortical reconstruction and volumetric segmentation of the 3D T1-weighted images were performed with the FreeSurfer Version 6.0 processing framework (http://surfer.nmr.mgh.harvard.edu/). This process includes motion correction, removal of nonbrain tissue (23), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (24, 25), intensity normalization (26), tessellation of the gray matter - white matter boundary, automated topology correction, and surface deformation (27, 28) to produce representations of cortical thickness. Using the entire 3-dimensional MR volume in segmentation and deformation procedures, the representation of cortical thickness is calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex (29). The produced maps use spatial intensity gradients, are not restricted to the voxel resolution of the original data and can detect submillimeter group differences. Using an automated labeling system based on the Desikan-Killiany Atlas the cortex was divided into 33 gyral regions in each hemisphere (https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation). In addition to the Freesurfer 6.0 automated Quality Control (QC) process, all segmented scans were visually inspected by an experienced operator and segmentations were manually corrected when necessary.
As noted above, we limited analysis to the following structures based on pre-existing evidence for their involvement in PTSD and computed estimated volumes according to standard FreeSurfer volume segmentation: insula, hippocampus, amygdala, superior frontal gyrus, rostral and caudal ACC, medial and lateral orbitofrontal gyri and total intracranial volume (ICV). None of the laterality indices for ROIs showed significant association with PTSD at 3 or 6-months post-injury in the bivariate analyses; therefore, for paired structures, the total volume as the sum of left and right hemispheres was used in all analyses.
The CT and MRI scans were interpreted by a board-certified neuroradiologist blinded to the patients’ clinical information using the NIH Common Data Elements (CDEs) for TBI pathoanatomic classification (30). Patients with any acute abnormal CT or MRI findings related to the recent injury were categorized as “CT positive” or “MRI positive,” respectively. Most CT findings were small contusions and small subarachnoid hemorrhages whereas MRI findings largely consisted of microbleeds due to hemorrhagic axonal injury and small contusions; neither CT or MRI positivity were significantly associated with PTSD outcomes, and so they were not included in any of the analyses.
Statistical Analysis
Bivariate and multivariable (adjusting for sociodemographic characteristics and other known PTSD risk factors including sex, race, ethnicity, education, prior TBI, history of psychiatric illness, cause of injury, and early PTSD symptoms at 2 weeks) associations between individual standardized brain volume metrics and probable PTSD outcomes at 3- and 6-months post-injury were analyzed for each of the 8 preselected regions using logistic regression. Benjamini-Hochberg’s method (31) was used to adjust for multiple testing. To avoid problems with multicollinearity when simultaneously including multiple regions in the models, principal components analysis (PCA) was conducted on four volumetric measures (Insula, Superior frontal, Rostral Anterior Cingulate, and Caudal Anterior Cingulate), chosen because they were associated with 3-month PTSD outcome at Benjamini-Hochberg FDR-adjusted p ≤ 0.20 in the multivariable analyses. Subsequent multivariable models evaluated the association between the first principal component (PC1) as a composite score – which explained the majority of the variance in those four regional volumes– and the PTSD outcomes, adjusting for known demographic and clinical risk factors.
The composite of these 4 regions (PC1) was also tested for association with sub-domains of PTSD symptoms, as measured by the PCL-5 subscales, at 3- and 6-months post-injury. The distribution of PCL-5 subscales were skewed with excessive zeros, so a zero-inflated negative binomial (ZINB) regression model was used to jointly assess the associations of brain volumes with presence or absence of PCL symptoms (OR component) and with severity of the symptoms (FC component). Statistical analyses were performed in R version 3.6.1 (http://www.r-project.org).
RESULTS
Demographic and clinical characteristics of the study participants are shown in Table 1. Approximately two-thirds of the sample was male, and mean age of the sample was 38.7 (SD 16.1) years. Consistent with our findings in a larger sample of TRACK-TBI patients (14), of whom the present sample is a subset who had MRIs (FIGURE: CONSORT DIAGRAM), characteristics most strongly associated with risk for PTSD at either the 3- or 6-month follow-up included pre-injury psychiatric history, prior TBI, and less education.
Table 1.
Demographic and clinical characteristics.
| PTSD at 3-months post-injury |
PTSD at 6-months post-injury |
||||||
|---|---|---|---|---|---|---|---|
| N | No (n=344) | Yes (n=77) | p-value | No (n=351) | Yes (n=70) | p-value | |
| Age (Mean (SD)) | 421 | 38.9 (16.5) | 37.8 (14.2) | .70 | 39.2 (16.5) | 36.6 (13.5) | .32 |
| Years of Education (Mean (SD)) | 417 | 14.3 (2.8) | 13.2 (2.2) | <.001 | 14.4 (2.7) | 12.8 (2.5) | <.001 |
| Patient Type | 421 | .61 | .89 | ||||
| ED Discharge | 175 | 140 (40.7%) | 35 (45.5%) | 145 (41.3%) | 30 (42.9%) | |
|
| Hospital admit no ICU | 170 | 139 (40.4%) | 31 (40.3%) | 141 (40.2%) | 29 (41.4%) | ||
| Hospital admit with ICU | 76 | 65 (18.9%) | 11 (14.3%) | 65 (18.5%) | 11 (15.7%) | ||
| Sex | 421 | .033 | .039 | ||||
| Female | 141 | 107 (31.1%) | 34 (44.2%) | 110 (31.3%) | 31 (44.3%) | ||
| Male | 280 | 237 (68.9%) | 43 (55.8%) | 241 (68.7%) | 39 (55.7%) | ||
| Race | 418 | .054 | <.001 | ||||
| White | 309 | 255 (74.3%) | 54 (72%) | 264 (75.6%) | 45 (65.2%) | ||
| Black | 76 | 57 (16.6%) | 19 (25.3%) | 53 (15.2%) | 23 (33.3%) | ||
| Other | 33 | 31 (9.0%) | 2 (2.7%) | 32 (9.2%) | 1 (1.5%) | ||
| Hispanic | 419 | .73 | .86 | ||||
| No | 349 | 287 (83.7%) | 62 (81.6%) | 292 (83.4%) | 57 (82.6%) | ||
| Yes | 70 | 56 (16.3%) | 14 (18.4%) | 58 (16.6%) | 12 (17.4%) | ||
| Injury Cause | 421 | .024 | .020 | ||||
| Road traffic incident | 267 | 216 (62.8%) | 51 (66.2%) | 219 (62.4%) | 48 (68.6%) | ||
| Incidental fall | 91 | 82 (23.8%) | 9 (11.7%) | 83 (23.7%) | 8 (11.4%) | ||
| Violence/assault | 23 | 15 (4.4%) | 8 (10.4%) | 15 (4.3%) | 8 (11.4%) | ||
| Other | 40 | 31 (9.0%) | 9 (11.7%) | 34 (9.7%) | 6 (8.6%) | ||
| Psychiatric History | 421 | <.001 | .002 | ||||
| No | 347 | 295 (85.8%) | 52 (67.5%) | 299 (85.2%) | 48 (68.6%) | ||
| Yes | 74 | 49 (14.2%) | 25 (32.5%) | 52 (14.8%) | 22 (31.4%) | ||
| Prior TBI | 420 | .003 | .019 | ||||
| No | 276 | 237 (69.1%) | 39 (50.7%) | 239 (68.3%) | 37 (52.9%) | ||
| Yes | 144 | 106 (30.9%) | 38 (49.4%) | 111 (31.7%) | 33 (47.1%) | ||
| CT | 417 | .26 | .028 | ||||
| CT− | 299 | 240 (70.4%) | 59 (77.6%) | 241 (69.5%) | 58 (82.9%) | ||
| CT+ | 118 | 101 (29.6%) | 17 (22.4%) | 106 (30.6%) | 12 (17.1%) | ||
| MRI | 421 | .80 | .19 | ||||
| MRI− | 234 | 190 (55.2%) | 44 (57.1%) | 190 (54.1%) | 44 (62.9%) | ||
| MRI+ | 187 | 154 (44.8%) | 33 (42.9%) | 161 (45.9%) | 26 (37.1%) | ||
Bivariate and multivariable associations between volumes in each of the 8 regions of interest and probable PTSD status at 3-months post-injury are shown in Table 2. Smaller volumes of three regions (superior frontal, rostral and caudal ACC) were individually predictive of PTSD at 3 months in the fully adjusted analyses (Table 2B, right panel). Insula also showed a trend (raw p-value=0.084, BH-adjusted p-value=0.168) (Table 2, right panel) Using principal components analysis (PCA), we determined that a single principal component (PC1) that incorporated 73.8% of the variance in those 4 regional volumes predicted 3-month PTSD, even in multivariable models incorporating other established risk markers including early (2-week) PTSD symptom severity (Table 3a). When considering individual symptom sub-domains at 3-months as measured by the PCL-5, severity and presence or absence (at a trend level) of hyperarousal symptoms was the only symptom sub-domain significantly predicted by PC1 (Table 3b).
Table 2.
Bivariate model 1* (left) and bivariate model 2** (right) association between each volumetric measure (standardized) and probable PTSD at 3-months post-injury.
| Bivariate Model 1 |
Bivariate Model 2 |
||||||
|---|---|---|---|---|---|---|---|
| AOR | 95% CI | p-value | AOR | 95% CI | p-value | BH-adjusted p-value | |
| Insula | .66 | .47–.94 | .020 | .66 | .41–1.06 | .084 | 0.168 |
| Hippocampus | 1.16 | .85–1.59 | .36 | 1.09 | .71–1.67 | .70 | 0.70 |
| Amygdala | 1.12 | .82–1.54 | .47 | 0.82 | .53–1.25 | .35 | 0.40 |
| Superior Frontal | .70 | .51–.96 | .026 | .53 | .34–.84 | .007 | 0.036 |
| Rostral Anterior Cingulate | .70 | .50–.96 | .028 | .58 | .37–.92 | .019 | 0.051 |
| Caudal Anterior Cingulate | .78 | .57–1.05 | .10 | .57 | .37–.87 | .009 | 0.036 |
| Medial Orbitofrontal | .87 | .64–1.19 | .38 | .82 | .54–1.24 | .35 | 0.40 |
| Lateral Orbitofrontal | .86 | .64–1.16 | .32 | .78 | .52–1.18 | .24 | 0.39 |
AOR = Adjusted odds ratio; BH = Benjamini-Hochberg method to adjust for multiplicity controlling for the family discovery rate
Models include the specified ROI and are adjusted for intracranial volume
Models include the specified ROI and are adjusted for intrancranial volume, sex, race, ethnicity, years of education, history of psychiatric illness, prior traumatic brain injury, injury cause, and 2-week PTSD symptom score
Table 3a.
Multivariable logistic regression model assessing the association between risk factors, including early (2-week) PTSD symptom severity as assessed by the PCL-5, and PTSD at 3-months post- injury (n=405).
| OR | 95% CI | Chi-sq | p-value | |
|---|---|---|---|---|
| PC1 | .65 | 0.49–0.87 | 8.75 | .003 |
| ICV (standardized) | 2.03 | 1.19–3.48 | 6.67 | .01 |
| Male (ref: Female) | .72 | 0.31–1.68 | .58 | .45 |
| Black (ref: White/Other) | 1.05 | 0.42–2.63 | .01 | .92 |
| Hispanic (ref: White/Other) | 1.31 | 0.50–3.38 | .30 | .58 |
| Years of Education | .96 | 0.84–1.10 | .31 | .58 |
| Any psychiatric history (ref: None) | 1.89 | 0.84–4.28 | 2.35 | .13 |
| Any prior TBI (ref: None) | 1.63 | 0.81–3.28 | 1.90 | .17 |
| Violent injury cause (ref: Accidental) | 1.40 | 0.39–5.10 | .26 | .61 |
| PCL-5 Total Score at week 2 | 1.09 | 1.07–1.12 | 65.54 | < .001 |
PC1: first principal component that explained 73.8% of the variance in the regional volumes of the insula, superior frontal cortex, and rostral and caudal anterior cingulate.
Table 3b.
Multivariable zero-inflated negative binomial regression model assessing the association between risk factors and PCL-5 Hyperarousal subscale at 3-months post-injury (n=405)
| FC | 95% CI | p-value | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| PC1 | .94 | 0.88–0.99 | .031 | 1.27 | 0.99–1.64 | .06 |
| ICV (standardized) | 1.13 | 1.00–1.27 | .049 | .79 | 0.49–1.26 | .31 |
| Male (ref: Female) | .99 | 0.82–1.19 | .89 | .96 | 0.44–2.11 | .92 |
| Black (ref:White/Other) | 1.21 | 0.99–1.48 | .063 | 1.48 | 0.63–3.44 | .37 |
| Hispanic (ref: non-Hispanic) | 1.09 | 0.89–1.33 | 0.43 | 1.66 | 0.66–4.18 | .28 |
| Education (y) | .96 | 0.93–0.99 | 0.013 | 1.11 | 0.98–1.25 | .09 |
| Any psychiatric history (ref: None) | 1.16 | 0.96–1.40 | 0.128 | 2.06 | 0.77–5.52 | .15 |
| Any prior TBI (ref: None) | 1.17 | 0.997–1.37 | 0.055 | 1.35 | 0.68–2.67 | .39 |
| Violent injury cause* (ref: Accidental) | 1.05 | 0.79–1.39 | 0.76 | -- | -- | -- |
| PCL-5 Hyperarousal at week 2 | 1.07 | 1.05–1.08 | <0.001 | 1.35 | 1.20–1.50 | <0.001 |
PC1: first principal component that explained 73.8% of the variance in the regional volumes of the insula, superior frontal cortex, and rostral and caudal anterior cingulate
FC: Estimated fold change with 95% confidence interval of the predictor variable associated with severity of hyperarousal symptoms when the symptoms are present.
OR: Estimated odds ratio with 95% confidence interval of the predictor variable associated with presence (non-zero scores) vs. absence (zero scores) of hyperarousal symptoms.
Injury cause was not modeled in the zero-inflation part due to zero-count in one of the cells which would lead to an infinite confidence interval.
The 4-region composite (PC1) was not predictive of PTSD at 6 months in the multivariable model (Supplemental Table S1). However, when considering individual symptom sub-domains at 6 months as measured by the PCL-5, only severity (but not presence or absence) of hyperarousal symptoms was significantly predicted by a model that included PC1 (Table 4).
Table 4.
Multivariable zero-inflated negative binomial regression model assessing the association between risk factors and PCL-5 Hyperarousal subscale at 6-months post-injury (n=405).
| FC | 95% CI | p-value | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| PC1 | .89 | .83–.95 | .001 | 1.11 | .83–1.49 | .47 |
| ICV (standardized) | 1.16 | 1.02–1.32 | .029 | .88 | .50–1.53 | .65 |
| Male (ref: Female) | 1.07 | .87–1.32 | .52 | .61 | .23–1.64 | .33 |
| Black (ref: White/Other) | 1.13 | .91–1.41 | .26 | 1.57 | .57–4.30 | .38 |
| Hispanic (ref: non-Hispanic) | 1.07 | .86–1.34 | .55 | 2.48 | .72–8.49 | .15 |
| Years of Education | .97 | .94–.997 | .033 | 1.07 | .94–1.22 | .31 |
| Any psychiatric history (ref: None) | 1.13 | .92–1.39 | .24 | 2.16 | .64–7.27 | .21 |
| Any prior TBI (ref: None) | 1.13 | .96–1.34 | .15 | .97 | .47–1.99 | .93 |
| Violent injury cause (ref: Accidental) | 1.40 | 1.05–1.88 | .024 | -- | -- | -- |
| PCL-5 Hyperarousal at week 2 | 1.08 | 1.07–1.10 | <.001 | 1.26 | 1.09–1.46 | .002 |
PC1: first principal component that explained 73.8% of the variance in the regional volumes of the insula, superior frontal cortex, and rostral and caudal anterior cingulate.
FC: Estimated fold change with 95% confidence interval of the predictor variable associated with severity of hyperarousal symptoms when the symptoms are present.
OR: Estimated odds ratio with 95% confidence interval of the predictor variable associated with presence (non-zero scores) vs. absence (zero scores) of hyperarousal symptoms.
Injury cause was not modeled in the zero-inflation part due to zero-count in one of the cells which would lead to an infinite confidence interval.
DISCUSSION
In this analysis of a pre-specified cohort from the TRACK-TBI study, regional brain volumes on structural MRI at 2-weeks post-injury were analyzed in relation to PTSD outcomes at 3- and 6-months post-injury. Smaller insula, superior frontal cortex, and rostral and caudal anterior cingulate volumes at 2 weeks following mTBI each contributed to prediction of PTSD at 3 months (but not 6 months). TBI is known to be an important risk factor for PTSD development following injury (12, 14); the current study provides important data on the relationship between volumetric brain measures and PTSD in the context of acute mTBI.
Reduced gray matter volume and decreased cortical thickness in patients with PTSD have been consistently reported in the anterior cingulate (6, 7) prefrontal cortex (8), and hippocampus (5, 6). Reduced volumes of the hippocampus, ACC and prefrontal cortex are vulnerability factors for PTSD, as is increased connectivity of the salience network and the default mode network, as recently reviewed (32). We therefore limited our investigation of volumes to regions within these structures. We created a single principal component (PC1) that explained much (74%) of the variance in the regional volumes of the insula, superior frontal cortex, and rostral and caudal anterior cingulate to avoid multicollinearity issues. The observation that larger brain volumes indicate less likelihood of symptoms at 3 months lends support to the concept of brain reserve (33) as an important factor in resilience to PTSD (34–37).
There were no significant interactions of brain volume metrics with prior TBI for PTSD outcomes at either 3- or 6-months post injury. We found that PC1 predicted PTSD at 3 months and, importantly, that it had predictive value even in models where 2-week PTSD symptoms (measured with the PCL-5) were included. This finding suggests that biological variables such as regional brain volumes can contribute to PTSD risk prediction beyond the value of early symptom measurement alone. We wish to emphasize however, that this study provides proof-of-principle only; the actual magnitude of the increase in risk prediction provided by regional brain volume measurement – while statistically significant – was small in effect compared with the predictive value of early (e.g., 2-week post-injury) symptom measurement and would be, at most, of marginal prognostic benefit.
For reasons that are not presently understood, 2-week PTSD symptoms continued to predict 6-month PTSD outcomes, but brain volumes (including PC1) did not. It may be the case that as time from the TBI advances, multiple biological and psychosocial factors intervene to lessen the importance of these factors. Interestingly, however, whereas overall PTSD symptoms were not predicted by regional brain volumes at 6-months post-injury, they continued to significantly predict severity of the PTSD subdomains, hyperarousal symptoms, as they had at 3-months post-injury. It may be the case that differences in regional brain volumes found in this study reflect a set of structures and their interrelationships that subserve elements of arousal that are dysfunctional in PTSD. This is a hypothesis that deserves testing with more direct focus on neuropsychological functions that parallel the trajectory of hyperarousal symptoms during the longitudinal course of PTSD after mTBI.
There is a robust literature documenting smaller hippocampal volumes in PTSD (5, 6), but we did not find hippocampal volume to be predictive of PTSD in this study. It is unclear why our results differ from many others in this regard, but it is possible that PTSD occurring in the context of mTBI has a different brain structural basis than PTSD acquired via other types of trauma. In addition, given the more acute time frame of the current study (within 6 months of trauma), it may be that hippocampal volumes reflect PTSD at much further time points from injury. Our findings, in particular, of smaller posterior cingulate volume being associated with PTSD at 3-month follow-up does suggest that the default mode network may be involved, particularly in the genesis of PTSD, a hypothesis that has been supported by multiple resting-state functional connectivity studies in PTSD (38–41).
Our study has several noteworthy strengths and limitations. There are 2 major advantages and disadvantages to using MRI volumetrics for investigating brain structure in relation to PTSD symptoms following injury. First, an advantage is that it should be relatively insensitive to the physical effects of milder head trauma at the early subacute stage of injury. A potential disadvantage, though, is that we cannot be certain that the brain volumes obtained here at 2 weeks post-TBI are reflective of the pre-injury state or are influenced by variously evolving inury-related changes. A second advantage is that MRI volumetrics can be performed reproducibly with high precision across different MRI scanners of different types when a standardized acquisition protocol is used, enabling the aggregation of large multicenter datasets. The major disadvantage is that volumetrics cannot probe brain microstructure, function, or connectivity, which can be much more sensitive to inter-individual variation in brain organization relevant to complex mental disorders such as PTSD. It must also be noted that even though the principal component containing information about the 4 regions was statistically significant in the predictions shown, not all of its individual components withstood correction for multiple testing. This speaks to the need for replication of these results in larger samples. Another important limitation of the study is the fact that the PCL-5 assessment of PTSD symptoms was administered agnostic to the index trauma, i.e., participants responded based on their worst lifetime trauma, which may not have been the injury that resulted in their index TBI. Whereas we adjusted our models for pre-existing psychiatric history – which was the only information collected that might indicate pre-injury PTSD – we cannot know for certain whether the symptoms reported on the PCL-5 reflect de novo PTSD from the injury, or persistence of worsening of pre-injury PTSD.
In summary, we found that MRI volumetrics of several brain regions (insula, anterior and posterior cingulate, superior frontal cortex) early after mild traumatic brain injury was associated with PTSD prediction at 3- but not 6-months post-injury. Whereas the incremental effect size of inclusion of these volumetric measures was small and unlikely to be of clinical significance in their current form, results provide proof-of-principle for how prediction of at-risk individuals might be accomplished to enhance prognostic accuracy and to enrich clinical prevention trials for individuals at highest risk of PTSD following mTBI.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: This research was supported by the National Institutes of Health (grant U01NS086090) and US Department of Defense (grant W81XWH-14-2-0176). Abbott Laboratories provided funding for add-in TRACK-TBI clinical studies. One Mind provided funding for TRACK-TBI patients’ stipends and support to clinical sites.
Author Disclosures: Dr. Yuh had a patent for USPTO No. 62/269,778 pending. Dr. Manley received grants from the NINDS during the conduct of the study; research funding from the US Department of Energy, grants from the DoD, research funding from Abbott Laboratories, grants from the National Football League Scientific Advisory Board, and research funding from One Mind outside the submitted work; in addition, Dr. Manley had a patent for Interpretation and Quantification of Emergency Features on Head Computed Tomography issued. He served for 2 seasons as an unaffiliated neurologic consultant for home games of the Oakland Raiders; he was compensated $1500 per game for 6 games during the 2017 season but received no compensation for this work during the 2018 season. Dr. Stein received personal fees from Acadia, Amgen, Aptinyx, Bionomics, GW Pharma, and Janssen; as well as personal fees and stock options from Oxeia Biopharmaceuticals outside the submitted work. Dr. Diaz-Arrastia received personal fees and research funding from Neural Analytics Inc and travel reimbursement from Brain Box Solutions Inc outside the submitted work. Dr. Goldman received personal fees from Amgen, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Aspen Health Strategy Group, and Celgene outside the submitted work. Dr. Kreitzer received personal fees from Portola outside the submitted work. Dr. Mukherjee received grants from GE Healthcare and nonfinancial support from GE-NFL Head Health Initiative outside the submitted work; in addition, Dr. Mukherjee had a patent for USPTO No. 62/269,778 pending. Dr. Rosand received personal fees from Boehringer Ingelheim and New Beta Innovations outside the submitted work. Dr. Zafonte received royalties from Oakstone for an educational CD (Physical Medicine and Rehabilitation: a Comprehensive Review) and Demos publishing for serving as coeditor of Brain Injury Medicine. Dr. Zafonte serves or served on the scientific advisory boards of Myomo, Oxeia Biopharma, Biodirection, and Elminda. He also evaluates patients in the MGH Brain and Body-TRUST Program, which is funded by the National Football League Players Association. Dr. Zafonte served on the Mackey White Committee. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Atwoli L, Stein DJ, Koenen KC, McLaughlin KA (2015): Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 28:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daskalakis NP, Rijal CM, King C, Huckins LM, Ressler KJ (2018): Recent Genetics and Epigenetics Approaches to PTSD. Curr Psychiatry Rep. 20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, Abe O (2019): MRI findings in posttraumatic stress disorder. J Magn Reson Imaging. [DOI] [PubMed] [Google Scholar]
- 4.Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. (1996): Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological psychiatry. 40:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, et al. (2018): Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety. 35:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2018): Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 83:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S (2009): Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 66:1373–1382. [DOI] [PubMed] [Google Scholar]
- 8.Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, et al. (2017): Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 27:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson KF, Kehle SM, Meis LA, Greer N, Macdonald R, Rutks I, et al. (2011): Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. The Journal of head trauma rehabilitation. 26:103–115. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GB, Leite-Morris KA, Wang L, Rumbika KK, Heinrichs SC, Zeng X, et al. (2018): Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J Neurotrauma. 35:210–225. [DOI] [PubMed] [Google Scholar]
- 11.Stein MB, Kessler RC, Heeringa SG, Jain S, Campbell-Sills L, Colpe LJ, et al. (2015): Prospective Longitudinal Evaluation of the Effect of Deployment-Acquired Traumatic Brain Injury on Posttraumatic Stress and Related Disorders: Results From the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Am J Psychiatry. 172:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein MB, McAllister TW (2009): Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. The American journal of psychiatry. 166:768–776. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht JS, Abariga SA, Rao V, Wickwire EM (2020): Incidence of New Neuropsychiatric Disorder Diagnoses Following Traumatic Brain Injury. J Head Trauma Rehabil. [DOI] [PubMed] [Google Scholar]
- 14.Stein MB, Jain S, Giacino JT, Levin H, Dikmen S, Nelson LD, et al. (2019): Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry. 76:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickie EW, Brunet A, Akerib V, Armony JL (2013): Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol Med. 43:645–653. [DOI] [PubMed] [Google Scholar]
- 16.Lopez KC, Leary JB, Pham DL, Chou YY, Dsurney J, Chan L (2017): Brain Volume, Connectivity, and Neuropsychological Performance in Mild Traumatic Brain Injury: The Impact of Post-Traumatic Stress Disorder Symptoms. J Neurotrauma. 34:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. (2016): Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 28:1379–1391. [DOI] [PubMed] [Google Scholar]
- 18.O’Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J (2015): A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 232:1–33. [DOI] [PubMed] [Google Scholar]
- 19.Pietrzak RH, Averill LA, Abdallah CG, Neumeister A, Krystal JH, Levy I, et al. (2015): Amygdala-hippocampal volume and the phenotypic heterogeneity of posttraumatic stress disorder: a cross-sectional study. JAMA Psychiatry. 72:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, et al. (2013): Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 30:1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teasdale G, Jennett B (1976): Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 34:45–55. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. (2008): The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. (2004): A hybrid approach to the skull stripping problem in MRI. Neuroimage. 22:1060–1075. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- 26.Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. [DOI] [PubMed] [Google Scholar]
- 27.Fischl B, Liu A, Dale AM (2001): Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 20:70–80. [DOI] [PubMed] [Google Scholar]
- 28.Segonne F, Pacheco J, Fischl B (2007): Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 26:518–529. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, et al. (2013): Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 73:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 57:289–300. [Google Scholar]
- 32.Bolsinger J, Seifritz E, Kleim B, Manoliu A (2018): Neuroimaging Correlates of Resilience to Traumatic Events-A Comprehensive Review. Front Psychiatry. 9:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, et al. (2018): Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kremen WS, Koenen KC, Boake C, Purcell S, Eisen SA, Franz CE, et al. (2007): Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Arch Gen Psychiatry. 64:361–368. [DOI] [PubMed] [Google Scholar]
- 35.Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. (2009): Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 166:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen HJ, Andersen SB, Karstoft KI, Madsen T (2016): The influence of pre-deployment cognitive ability on post-traumatic stress disorder symptoms and trajectories: The Danish USPER follow-up study of Afghanistan veterans. J Affect Disord. 196:148–153. [DOI] [PubMed] [Google Scholar]
- 37.Polimanti R, Ratanatharathorn A, Maihofer AX, Choi KW, Stein MB, Morey RA, et al. (2019): Association of Economic Status and Educational Attainment With Posttraumatic Stress Disorder: A Mendelian Randomization Study. JAMA Netw Open. 2:e193447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, et al. (2018): Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. Neuroimage. 176:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M (2017): Default Mode Network Subsystems are Differentially Disrupted in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. (2016): Altered Default Mode Network (Dmn) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (Ptsd) in Combat Veterans of Afghanistan and Iraq. Depress Anxiety. 33:289–299. [DOI] [PubMed] [Google Scholar]
- 41.Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016): Aberrant Resting-State Brain Activity in Posttraumatic Stress Disorder: A Meta-Analysis and Systematic Review. Depress Anxiety. 33:592–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.