Abstract
Crosstalk between co-stimulatory and -inhibitory ligands are a prominent node of immune cell regulation. Mounting evidence points towards a critical role for CD155, the poliovirus receptor, in suppressing T cell function, particularly in cancer. However, relative to other known co-stimulatory/co-inhibitory ligands (e.g. CD86, CD80, PD-L1), the physiological functions of CD155 and the mechanisms controlling its expression remain unclear. We discovered that CD155 expression is co-regulated with PD-L1 on tumor-associated macrophages (TAMs), is transcriptionally regulated by persistently active Aryl hydrocarbon Receptor (AhR), and can be targeted for suppression via AhR inhibition in vivo. Therapeutic inhibition of AhR reversed tumor immunosuppression in an immune competent murine tumor model, and markers of AhR activity were highly correlated with TAM markers in human glioblastomas. Thus, CD155 functions within a broader, AhR-controlled macrophage activation phenotype that can be targeted to reverse tumor immunosuppression.
Introduction
Elucidating the biology of immune checkpoints and co-stimulatory molecules inspired the development of effective therapeutics for cancer, autoimmunity, and organ transplantation. CD155, originally discovered as the poliovirus receptor (PVR) (1), is an immune checkpoint gaining interest as a target for cancer immunotherapy (2). CD155 is virtually universally expressed in solid neoplasia (3) and genetic ablation of the murine CD155 ortholog (gene name PVR) restricts tumor growth and metastases and bolsters the antitumor efficacy of anti-PD1 and anti-CTLA4 (4). This indicated a non-redundant role for CD155 as an immune checkpoint (4).
However, targeting CD155 itself for therapy is hindered by its widespread expression outside of the tumor site, e.g. on vascular endothelial cells (3) or spinal cord anterior horn motor neurons (5). Moreover, CD155 binding-activated receptors have both activating (e.g. DNAM-1) (6) and suppressive (e.g. TIGIT) (7) roles in shaping the tumor immune landscape. Thus, unraveling the biological context(s) and mechanisms of CD155 expression control may reveal more tractable routes to target this immune checkpoint. Given the functional importance of CD155 (4) and PD-L1 (8) in myeloid cells, the influence of PD-L1 on cancer immune resistance (9), as well as the pivotal role of antigen presenting cells in determining T cell function, we investigated the biological role of CD155 expression in TAMs/human macrophages.
Here, we define a relationship between CD155 and PD-L1 expression on TAMs, induced by signals that have historically been associated with ‘classical’ or ‘alternative’ activation in macrophages, and discover a mechanism of CD155 transcriptional regulation via persistently active AhR. Inhibiting AhR mitigated CD155 expression on TAMs and reversed tumor-intrinsic immune suppression in a murine immunocompetent tumor model, revealing a node at which the CD155 checkpoint may be clinically targeted.
Materials and Methods
Analysis of GBM Samples
De-identified surgically resected tumor tissue was collected from consented subjects under an IRB-approved protocol. GBM tumor tissue was collected within 1h of resection by the Duke Preston Robert Tisch Brain Tumor Center BioRepository. Specimens were dissociated in RPMI-1640 containing 100μg/mL Liberase-TM (Sigma-Aldrich) and 10μg/mL DNAse I (Roche) for 20min at 37°C with agitation. Single cell suspensions were filtered through 70μM and 40μM cell strainers (Olympus Plastics), washed in PBS (Gibco), and reconstituted in PBS containing 2% FBS (Sigma-Aldrich) with 1:20 Human Tru-Stain FcX™ block (Biolegend). Cell suspensions were stained with antibodies against CD45-BUV395 (BD Biosciences), CD14-BV421, CD33-BV510, HLA-DR-BV786, CD31-FITC, CD3/19-BUV737, CD11b-APC, CD16-BV711, CD15-APC-fire7, and either CD155-PE and PD-L1-BV605 or isotype control-PE and -BV605 antibody (all BioLegend); followed by washing and reconstitution in 7-AAD containing PBS+2%FBS. Cells were gated for appropriate size on SSC-A and FSC-A; single cells by proportionate FSC-H and FSC-A size; live cells by 7-AADNeg; non-endothelial cells by CD31-FITC-ANeg; CD45 by CD45-BUV396-A+, non NK, B, or T Cells by CD56-BUV737-A-, CD19-BUV737-A-, and CD3-BUV737-A-; and for macrophages by CD11b-APC-A+, CD16-BV711-A+,and CD14-BV421-A+.
Materials
LPS (Invivogen), IL-4, M-CSF, CH223191, SR1 (Stemcell), JW-67, PMA, GNF-351 and SB203580 (Sigma-Aldrich) were reconstituted per manufacturer’s instructions; concentrations are noted in figure legends. Leukopaks (Stemcell Technologies) from 4 different de-identified donors were processed using Leucosep™ tubes (Greiner Bio-One) and Ficoll-Paque™ Plus (GE healthcare) following the manufacturer’s instructions to isolate PBMCs. E0771 cells (a gift from Greg Palmer, Duke Univ.) were grown in high-glucose DMEM (Gibco) supplemented with 10% FBS. All cell lines were confirmed mycoplasma free. THP-1 and U937 cells (both ATCC) were cultured in suspension in RPMI 1640 supplemented with 10% FBS, antibiotics, and 0.1% β-mercaptoethanol (THP-1; Sigma).
In vitro experiments
Frozen PBMCs were thawed in 10mL AIM-V media (Invitrogen), and incubated in 2mL AIM-V media containing 10μg/mL DNAse I (Roche) (15min). Cells were spun down and plated at a density of 1×106 PBMCs per well in 6-well plates in DMEM (Gibco) containing 10% FBS, in the presence of 50μg/mL M-CSF (Stemcell Technologies) for 7 days. THP-1 and U937 cells were plated at a concentration of 1.2×106 cells per well (35mm well) and differentiated using PMA (100μg/mL for THP-1, 50μg/mL for U937) for 48h (THP-1) or 72h (U937). Media were changed and cells were allowed to rest (24h) before treatment. Unless otherwise noted, IL-4 or LPS treatment was for 24h.
Immunoblot
Immunoblots were performed as reported earlier (10) with antibodies against CD155, PD-L1, p-STAT6(Y641), STAT6, p-p38, p38, α-tubulin, p-NFκB(S536), NFκB, p-JNK, JNK, p-STAT1 (Y701), STAT1, p-MK2(T334), MK2, AhR, PARP, GAPDH (Cell Signaling Technology). Unless otherwise noted, immunoblots were performed using whole cell lysates. Where relevant, densitometric quantifications are shown as a change in protein level normalized to tubulin compared to control. In the case of CD155 and PD-L1 induction, fold change post IL-4 or LPS treatment is shown in comparison to unstimulated cells treated with the same inhibitor or siRNA. In the case of STAT6 and AhR, fold-change is shown in comparison to untreated cells.
siRNA, Fractionation, RT-qPCR
SiRNA against STAT6, AhR, or All-Stars negative control siRNA (Qiagen) were complexed with lipofectamine LTX following the manufacturer’s instructions, delivering 100pmol of siRNA per 35mm dish for 6h. Treatment of siRNA-treated MDMs occurred 36h after transfection. For cell fractionation, NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo cat# 78835) were used according to manufacturer’s protocol. For RT-qPCR, cells were pelleted and lysed in TRIZOL for total RNA extraction as described previously (11). RNA samples were treated with GeneJET RNA Cleanup and Concentration Micro Kit (Thermo K0842) according to the manufacturer’s protocol. Cocktails of 30ng sample RNA, 10μL of 2x, 1μL of probe (human PVR [Hs00197846_m1] and AHRR [Hs01005075_m1] and human and mouse 18s [Hs03003631_g1] and CYP1B1 [Hs00164383_m1, Mm00487229_m1], all Thermo Fisher) were pipetted into 96 well-plates in triplicate for each sample and analyzed as previously described (11). Where 18s was not detected in a sample, data were excluded.
Mice, Vaccination and Analysis of Spleens
C57BL/6J female mice were purchased from Jackson Laboratory and were used in accordance with Duke IACUC-approved protocols. Mice were co-housed with littermates at a maximum of five animals per cage. For immunization, Complete Freund’s Adjuvant and Incomplete Freund’s Adjuvant (Invivogen) were diluted to 50% in PBS and complexed to chicken Ovalbumin protein (Sigma-Aldrich) for treatment of 8-week old female C57BL/6J mice with 50μg of OVA protein in 200μL of adjuvant/PBS solution or PBS. Mice were challenged with 50μg OVA in 200μL one week after vaccination or treated with vehicle control. Mice were treated with 40μg of SR1 diluted in 5% DMSO/95% olive oil or vehicle control 48h and 1h before vaccination, and every 48h after vaccination. Mice were euthanized 48h after challenge and spleens were harvested and stored in ice cold PBS, crushed through 70μM strainers in PBS and pelleted. The splenic cell pellet was reconstituted in red blood cell lysis buffer (Sigma), processed through 40μM strainers, washed with PBS + 2% FBS and spun down for flow staining. Single cell suspensions were stained with CD45.2-BUV395, NK1.1-BV421, CD11c-BV510, F4/80-BV605, CD11b-BV711, CD19-FITC, CD3-FITC, 7-AAD, and either CD155-PE and PD-L1-APC or isotype control-PE and isotype control-APC as shown in Figure 4A–D. Cells were gated on FSC-A and SSC-A for appropriate size, on FSC-A and FSC-H for single cells, live by negative for 7-AAD, CD45.2 positive by CD45.2-APC-Cy7-A+, non-T and non-B cells by CD19-FITC-ANeg and CD3-FITC-ANeg, non NK cells by NK1.1-BV421-ANeg, CD11b positive by CD11b-BV711-A+, and for macrophages by SSC-A and F4/80-BV605+. Splenic cells shown in Figure 4E were stained with Zombie Aqua-BV510 (1h, Biolegend), blocked with mouse FC Block (30min, Biolegend), and stained with CD45.2-BUV395, NK1.1-BV421, Ly6G-BV605, CD11b-BV711, CD19-FITC, CD3-FITC, F4/80-PE-Cy5, CD11c-APC-Cy7 and either CD155-PE and PD-L1-BV786 or isotype control-PE and isotype control-BV786. Cells were gated for appropriately sized single cells, for live by Zombie Aqua-BV510Neg, CD45.2 positive by CD45.2-BUV396-A+, non-T and non-B Cells by CD19-FITC-A- and CD3-FITC-A-, non NK cells by NK1.1-BV421-A-, non-neutrophils by Ly6G-BV605-A-, non-DCs by CD11c-APC-Cy7-, and for macrophages by CD11b-BV711-A+ and F4/80-PE-Cy5-A+.
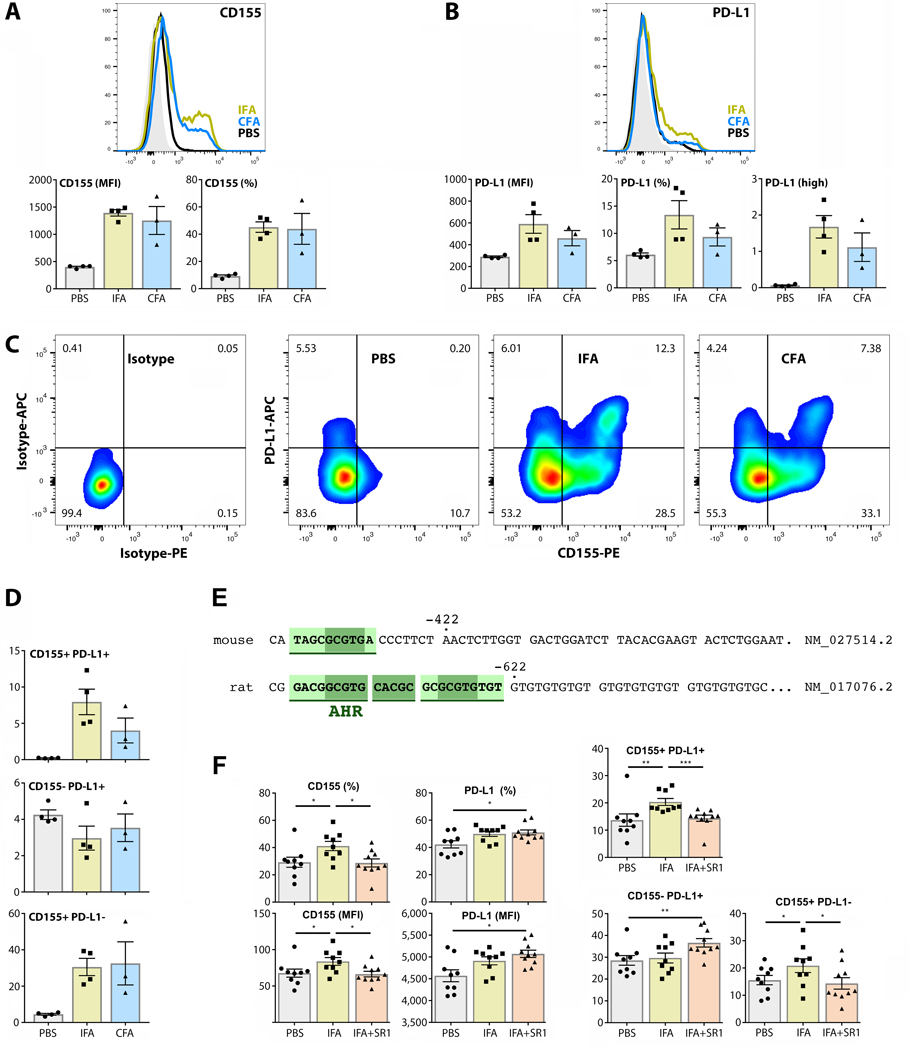
FIGURE 4.
CD155 and PD-L1 are induced in Th1 and Th2 contexts in murine splenic macrophages. (A-D) CD155 and PD-L1 expression, and co-expression of both, were measured by flow cytometry on murine splenic macrophages after mock (PBS), CFA (Th1) or IFA (Th2) immunization with OVA challenge. Panel (C) shows isotype controls and representative flow cytometry results from one animal in each of the experimental groups. Panel (D) shows that PD-L1 induction only occurred on macrophages with elevated CD155 expression. (N=4 for PBS and IFA; n=3 for CFA). (E) The mPVR region upstream of the transcriptional start site contains a DRE identified as an AhR responsive site by JASPAR analysis (compare to PVR in Figure 3A). (F) Mice were treated with vehicle control or SR1 48hrs and 1h before IFA immunization or PBS treatment, and 48hrs and 1h before PBS (mock) immunization or OVA challenge. CD155 and PD-L1 expression was measured as in (A-C). (N=9 for PBS and IFA; n=10 for IFA+SR1).
Tumor Implantation, Treatment, Dissociation, Flow Cytometry
E0771 cells were injected into the fat pad of 8-week old female C57BL/6J mice. When tumors were >10mm3, mice were treated with 40 μg SR1 in 200μL olive oil i.p. or vehicle control every 48h until euthanasia at 5 or 14 days after starting treatment. Tumors were harvested and stored in ice cold PBS until processing. Tumors were minced and dissociated in RPMI 1640 containing 100μg/mL Liberase-TM (Sigma-Aldrich) and 10μg/mL DNAse I (Roche) for 20min at 37°C with agitation. Dissociated cell suspensions were centrifuged (500G x 3min) and supernatant was retained for cytokine analysis. The pellet was reconstituted in FACS buffer and filtered through 70 μM and 40 μM strainers, washed, and reconstituted in PBS + 2% FBS for staining. Prior to staining, ~10% of volume was separated, centrifuged, and lysed in TRIZOL solution for RNA analysis. Single cell suspension was stained with Zombie Aqua-BV510 (Biolegend) for one hour, blocked with FC block (Biolegend) for 1 hour, and stained and gated as previously described for Figure 4E. For T Cell analysis, cell suspensions were stained with CD45.2-BUV395 (BD Bioscience), CD4-FITC, CD8-BV421, CTLA4-BV605, OX40-BV711, CD25-PE, TIGIT-PE-TexasRed, FoxP3-PE-Cy5, and CD3-APC (all Biolegend). Cells were gated for appropriate size, single cells, live cells, and CD45.2 as described above. Cells were selected for CD3 positive by CD3-APC-A, for CD8 positive by CD8-BV421-A positive and CD4-FITC-A negative or TRegs by CD4-FITC-A positive and CD8-BV421-A negative followed by FoxP3-PE-Cy5-A positive.
Cytokine analysis
LEGENDplex™ (BioLegend) assays were used to measure cytokines with the Mouse Macrophage/Microglia, or Mouse Th Cytokine panels on a BD Fortessa X-20 flow cytometer. LEGENDplex software was used to determine analyte concentrations per manufacturer’s instructions. Minimum threshold values were shown if cytokine concentrations were below sensitivity of detection (automatically determined by analysis software); in rare cases where analyte concentration exceeded maximum threshold values, the maximum value was shown. Tumor homogenate cytokine analysis was performed as previously described (12).
Statistical analyses, TCGA analysis
Assay-specific statistical tests are indicated in the corresponding figures. GraphPad Prism 8 was used to perform all statistical analyses and plot data. GBM patient data were obtained from the TCGA Research Network: https://www.cancer.gov/tcga and were analyzed on cbioportal.org (13, 14) for Spearman correlation and p values modified for multiple comparison (q values).
Results
‘Classically’ and ‘alternatively’ activated macrophages have elevated levels of CD155 and PD-L1 expression
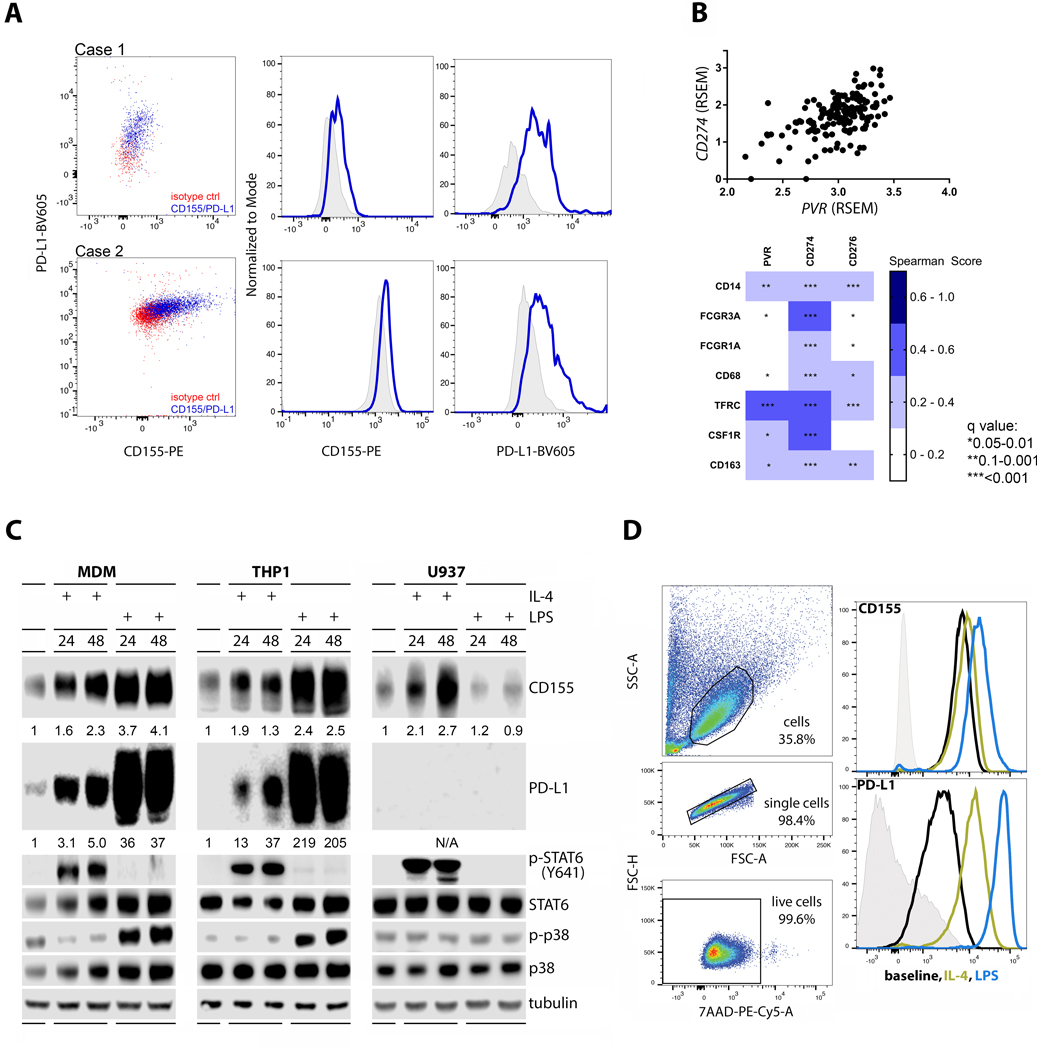
Reverberating with prior observations (15), analyses of WHO grade IV malignant glioma (glioblastoma, GBM) patient ex vivo tumor specimens revealed expression of CD155 and PD-L1 on TAMs (Fig. 1A). Indeed, PVR RNA expression was correlated with PD-L1 (CD274) and with multiple prominent myeloid cell markers in GBM (TCGA; Fig. 1B). Macrophages constitute a large percentage of tumor-associated myeloid cells in GBM (16), exhibiting phenotypes on a broad polarization spectrum (17). Conventionally, ‘classic’ macrophage activation, which historically has been simulated in vitro upon lipopolysaccharide (LPS) or interferon (IFN) γ stimulation, is contrarian to ‘alternative’ activation simulated upon stimulation with IL-4 (18). Based on the historical record with these stimuli, we investigated CD155 and PD-L1 expression in response to LPS and IL-4 stimulation of macrophage-lineage cells. We found that both LPS and IL-4 induced CD155 (and PD-L1) within 24h after treatment of primary human monocyte-derived macrophages (MDMs), and in phorbol ester (TPA)-differentiated acute monocytic leukemia (THP-1) cells, indicating THP-1 cells an appropriate model for our node of interest (Fig. 1C). IL-4 and LPS treatment elicited the expected, canonical signaling response with STAT6(Y641) and p38 MAPK phosphorylation in both cell types, respectively. TPA-differentiated U937 (histiocytic lymphoma) cells, commonly used for studies in cells of monocytic lineage, neither yielded PD-L1 expression at baseline or upon IL-4 or LPS stimulation, nor responded in a canonical manner to LPS [i.e. induction of p-p38 MAPK (19)]. However, U937 recapitulated IL-4 stimulated CD155 induction seen in MDMs and in THP-1 cells (Fig. 1C). Flow cytometry analyses of MDMs confirmed that increased CD155 expression, monitored by immunoblot in Figure 1C, occurred at the cell surface (Fig. 1D).
FIGURE 1.
Macrophages induce CD155 and PD-L1 expression after stimulation with IL-4 or LPS. (A) Two patient ex vivo GBM samples were analyzed by flow cytometry to assess surface expression of CD155 and PD-L1. (B) TCGA samples were analyzed for correlation between expression of immune checkpoints (PVR, CD274, CD276) and macrophage markers CD14, FCGR3A, FCGR1A, CD68, TFRC, CSF1R, and CD163. The immune checkpoints analyzed showed statistically significant correlation to macrophage markers (bottom panel). CD274 and PVR showed strong correlation to each other (top panel). (C) MDMs were differentiated in MCSF for 7 days and treated with IL-4 or LPS for the final 24 or 48h. THP-1 and U937 monocytes were differentiated in PMA and treated with IL-4 or LPS for 24 or 48h post differentiation. (D) Flow analysis was performed on MDMs treated as in (A). IL-4 and LPS stimulation induced elevated CD155 and PD-L1 surface expression. Data shown in (C) and (D) are representative of three repeats.
Induction of CD155 expression on macrophages is STAT6 (after IL-4 treatment) and p38 MAPK (after LPS treatment) dependent
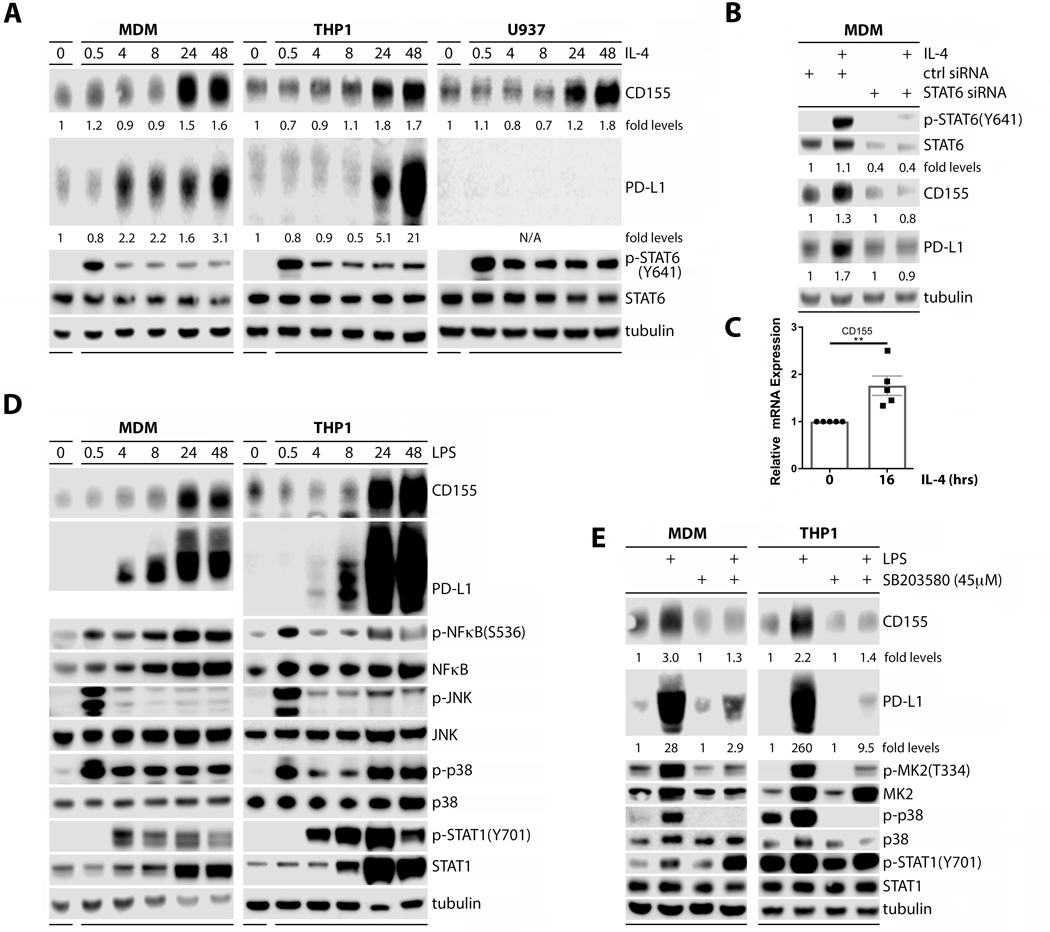
To decipher cell signaling cascades leading to CD155 and PD-L1 induction, we first performed detailed time course assays with IL-4 and LPS stimulation in our monocytic lineage panel (LPS stimulation was only tested in MDMs and THP-1s) (Fig. 2). IL-4 treatment produced canonical STAT6(Y641) phosphorylation within 30min, followed by CD155 and PD-L1 induction with a 4–24h delay (Fig. 2A). Transient, siRNA-mediated depletion of STAT6 in MDMs greatly diminished STAT6/p-STAT6(Y641) levels, and abrogated CD155 and PD-L1 induction after 24h of stimulation with IL-4 (Fig. 2B). Quantification of CD155 mRNA by RT-qPCR after IL-4 treatment (16h) showed induction at the transcript level (Fig. 2C). Thus, in aggregate, the time course of CD155/PD-L1 induction, dependency on STAT6, and the IL-4-induced increase of CD155 template, indicate a transcriptional response in line with the canonical signal transduction pathway induced by IL-4 receptor activation (20).
FIGURE 2.
Induction of CD155 on macrophages is a STAT6 (IL-4 treatment) and p38 MAPK (LPS treatment) dependent transcriptional response. (A) MDMs or differentiated THP-1/U937 cells were treated with IL-4 and the signaling response documented by immunoblot. (B) Transient STAT6 depletion with siRNA prior to IL-4 stimulation abrogated CD155/PD-L1 induction in MDMs. (C) RT-qPCR analyses of PVR transcript in THP-1 cells after IL-4 stimulation (16h) (n=5). (D) MDMs and differentiated THP-1 cells were treated with LPS and the signaling response documented by immunoblot. (E) MDMs or THP-1 cells were treated with SB203580 prior to LPS stimulation and the signaling response was recorded by immunoblot. Data shown in (A), (B), (D), and (E) are representative of three repeats.
CD155/PD-L1 induction upon LPS treatment mirrored the response to IL-4 (Fig. 2D). Canonical downstream toll-like receptor 4 (TLR4) signals—p-p38 MAPK, p-JNK and p-NFκB(S536)—were evident by 30min, followed by delayed induction of CD155 and PD-L1 at 4–24h (Fig. 2D). We also observed delayed (relative to p38/Jnk/NFκB phosphorylation) activation of STAT1, evident as p-STAT1(Y701) accumulation by ~4h after LPS stimulation (Fig. 2C). P38 MAPK inhibition with SB203580, evident as blockade of p38-downstream MK2(T334) phosphorylation, demonstrated that LPS-induced CD155/PD-L1 in MDMs and THP-1 cells depends on p38 MAPK activation (Fig. 2E). Thus, distinct signaling networks influence CD155 and PD-L1 induction: these may reflect divergent regulatory pathways, or may involve a shared, convergent signaling nexus downstream of STAT6 and p38.
CD155 induction in response to both IL-4 and LPS is dependent on AhR
Since STAT6(Y641) and p38 were phosphorylated within 0.5h of IL-4 or LPS stimulation, respectively, but CD155 expression levels only increased >4h thereafter, PVR induction likely is a transcriptional response to activation of multiple signaling relays. Therefore, we submitted the PVR region upstream of the known PVR transcriptional start site (21) for putative transcription factor binding sites to JASPAR analysis. Figure 3A depicts the promoter sequence upstream of the transcriptional start site in the PVR gene [GenBank reference standard (RefSeqGene) for PVR: NG_008781; (21)]. JASPAR analysis revealed the presence of a Dioxin-Responsive Element [DRE; defined by the substitution-intolerant core 5’-GCGTG-3’ (22)] ~310 nt upstream of the PVR transcriptional start site, indicating a possible Aryl hydrocarbon Receptor (AhR) responsive site (Fig. 3A). We used a previously established ‘position weight matrix’ examining the putative influence of the DRE sequence context on AhR binding. This matrix is based on analyses of the DRE sequence context within the promoter regions of a set of confirmed, bona fide AhR targets (22). The PVR upstream region DRE’s position weight matrix is >0.85, above the threshold established for DREs in the promoter regions of bona fide AhR target genes (22). Additionally, both STAT6 (23) and p38 (24) signaling have been linked to the regulation of AhR; and AhR signaling has recently been connected to PD-L1 expression (25). AhR is prominently implicated in TAM polarization in GBM (26), in mediating microglial CNS inflammation (27) and in LPS-induced macrophage inflammation (28). Therefore, an involvement of AhR in immunomodulatory regulation of PVR in macrophages was plausible.
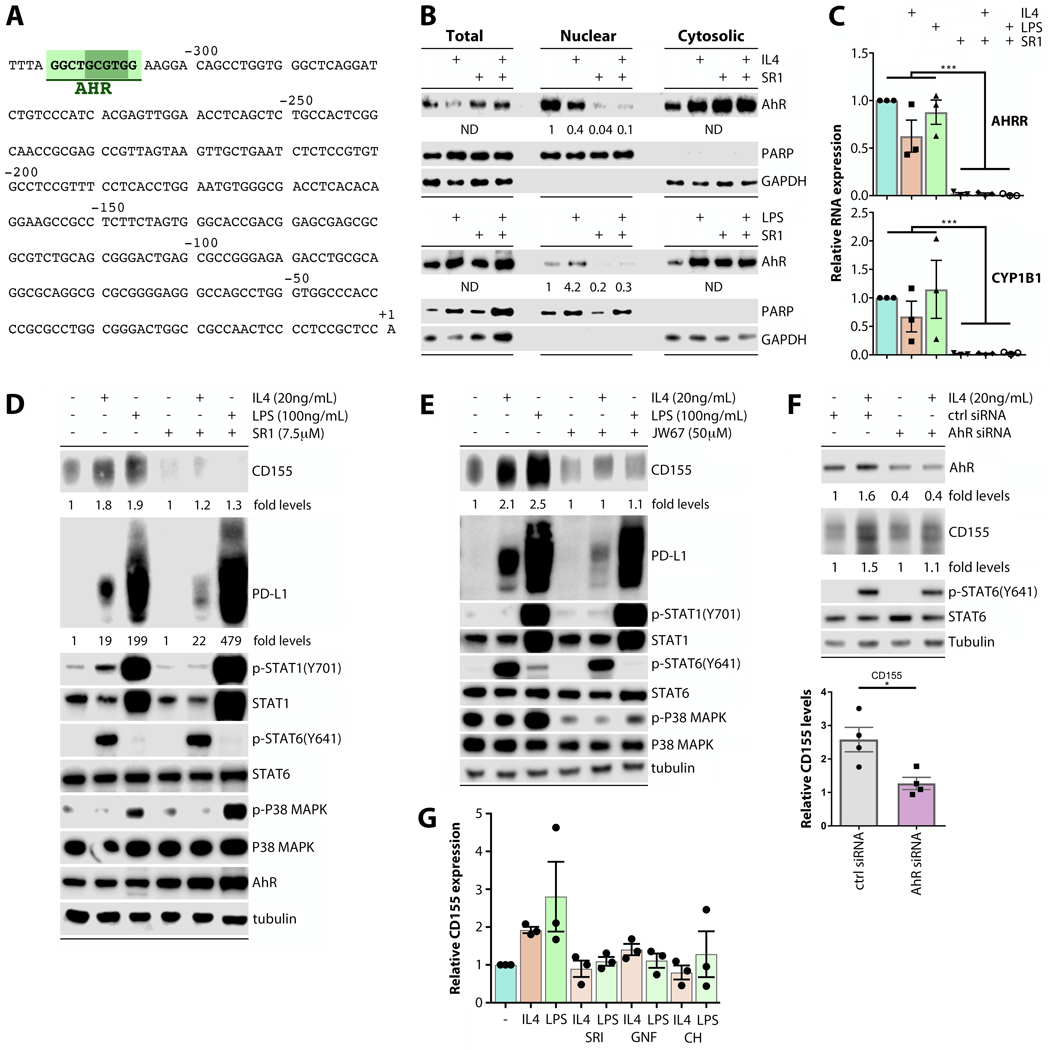
FIGURE 3.
CD155 induction in response to both IL-4 and LPS is dependent on AhR. (A) The PVR region upstream of the transcriptional start site contains a DRE identified as an AhR responsive site by JASPAR analysis. (B) THP-1 cells were pre-treated with AhR inhibitor SR1 24h prior to stimulation with IL-4 or LPS, fractionated, and analyzed for AhR levels by immunoblot. (C) THP-1 cells were treated as in (B) and RT-qPCR was performed to examine (AhR transcriptional target) AhRR and CYP1B1 transcript levels (n=5). (D) THP-1s were treated as in (B, C). CD155, PD-L1 levels and the STAT1, STAT6, p38 signaling response were analyzed by immunoblot. (E) THP-1 cells were pre-treated with β-catenin inhibitor JW-67 analyzed as in (D). (F) Transient AhR depletion with siRNA prior to IL-4 stimulation abrogated CD155 induction in THP-1 cells (n=4). (G) MDMs were pre-treated with AhR inhibitors SR1, GNF-351 (GNF), and CH223191 (CH), and CD155 expression was measured and quantified using immunoblot (n=3). Data in (B), (D), and (E) are representative of three repeats
AhR, upon activation by xenobiotic (e.g. polycyclic aromatic hydrocarbons; PAH) or intrinsic (e.g. tryptophan metabolite) ligands, is freed from chaperone interactions for nuclear translocation and association with the AhR nuclear translocator (ARNT). Primary products of AhR transcriptional activation are cytochrome P450-dependent monooxygenases (CYPs), catalyzers of PAH metabolism, and the negative feedback AhR Repressor (AhRR). Once inside nuclei, AhR is prone to degradation; however, AhR binding to β-catenin yields mutual stabilization with sustained promoter activity (29). The AhR:β-catenin form is associated with an altered transcriptional target profile, centered on CYP1B1 (29).
To unravel a possible involvement of AhR in CD155 induction upon macrophage stimulation, we used various small molecule inhibitors of AhR activation (Fig. 3B–D) and of AhR:β-catenin interaction (Fig. 3E). SR1 is a synthetic heterocyclic compound shown to bind AhR and inhibit its transcription factor activity (30). We confirmed this: immunoblot analyses in fractionated cell lysates revealed that SR1 led to cytosolic retention/blocked nuclear accumulation of AhR (Fig. 3B). Accordingly, (AhR transcriptional target) AhRR and CYP1B1 template expression was virtually abolished in SR1-treated THP-1 cells, either untreated or stimulated with IL-4 or LPS (Fig. 3C). SR1 prevented CD155 induction upon LPS and IL-4 stimulation in THP-1 cells, without disrupting canonical LPS and IL-4 induced signaling (Fig. 3D). PD-L1 induction was only modestly affected by AhR inhibition with SR1 after IL-4 treatment (Fig. 3D; we did not identify a DRE in the CD274 promoter), possibly indicating redundant and/or independent mechanisms inducing PD-L1 after macrophage activation.
As LPS or IL-4 stimulation did not produce signs of induced AhR activity (eg. increased AhR nuclear accumulation, increased AHRR or CYP1B1 mRNA levels; Fig. 3B–C), we investigated persistent AhR activity resulting from β-catenin binding. JW67, a synthetic compound identified in a screen for WNT signaling inhibitors, accelerates β-catenin degradation (31). Similar to SR1, JW67 prevented CD155 induction in LPS/IL-4 treated THP-1 cells without disrupting canonical LPS/IL-4 signaling (Fig. 3E). In a pattern mirroring the response to AhR inhibition with SR1, PD-L1 induction was only modestly responsive in THP-1 cells stimulated with IL-4, but not LPS (Fig. 3E). Furthermore, siRNA-mediated depletion of AhR prior to IL-4 treatment of THP-1 cells prevented CD155 induction (Fig. 3F). We confirmed AhR-dependent CD155 induction in IL-4/LPS stimulated MDMs by quantitating immunoblots after treatment with SR1 and two additional AhR inhibitors (GNF-351 and CH223191) (Fig. 3G). Our investigations, showing that CD155 induction is abrogated by the inhibition of AhR transcriptional activity and of the AhR:β-catenin interface, indicate that persistently active AhR controls CD155 induction upon macrophage activation.
CD155 and PD-L1 are induced in Th1 and Th2 contexts in mouse splenic macrophages
Immune checkpoints generally exert their function within the context of immunological synapses, where their expression may also be influenced by T cell-secreted cytokines [e.g. IFNγ, IL-4; (32)]. Thus, we investigated if CD155 and PD-L1 induction on macrophages occurs in the context of adaptive immune responses in vivo. To this end, we vaccinated mice with synthetic Ovalbumin (OVA) peptide mixed in Complete- (CFA) or Incomplete Freud’s Adjuvant (IFA), respectively. By virtue of containing agonists for macrophage inducible Ca++-dependent lectin receptor (MINCLE), and for TLR2, 4, and 9, CFA elicits Th1-type responses, while IFA induces Th2-type responses. Mice were re-challenged with OVA one week after immunization, and euthanized 48h later. Their spleens were removed and splenic macrophages were harvested for flow cytometry (Fig. 4). Baseline CD155 expression increased broadly after CFA or IFA vaccination and OVA re-challenge (Fig. 4A). PD-L1 expression was also induced by both immunizations, but on a smaller proportion of macrophages than CD155 (Fig. 4B). Intriguingly, PD-L1 induction only occurred in combination with CD155 (Fig. 4C, D).
Mouse PVR [mPVR, a.k.a. Tage4 (33)] encodes for murine CD155, which is only 42% identical to its human ortholog (34) [RefSeqGene for mPVR: NM_027514.2; (35)]. We performed JASPAR analysis of the mPVR region upstream of the known transcriptional start site, similar to our approach to human PVR (see above). As human PVR, the mPVR upstream region carries a substitution-intolerant core DRE (22), in a similar position (~430 nt upstream of the transcriptional start site), suggesting AhR involvement in mPVR control. Lastly, we found a cluster of DREs, in a similar position (~620 nt upstream of the transcriptional start site) of the rat PVR gene [RefSeqGene for rat PVR: NM_017076.2] (Fig. 4E). Positional conservation of DREs within comparable distance to the transcriptional start site across homo, rattus and mus has been established as a predictor of AhR binding activity in putative target genes (22).
Conserved gene regulatory activity of the upstream regions of human and murine PVR is evident in transgenic mice expressing (the human poliovirus receptor) CD155 under control of either element. These mice displayed similar CD155 distribution patterns and pathogenic profiles upon poliovirus infection (36). To determine if in vivo induction of CD155 is AhR-dependent, as suggested by our in vitro data and JASPAR analysis of the mPVR upstream region (Fig. 4E), we treated mice with SR1 48hrs and 1h before IFA-OVA immunization, and 48hrs and 1h before OVA challenge. Reverberating with our in vitro findings, AhR inhibition prevented CD155 induction. In SR1-treated mice challenged with IFA, the percentage of CD155+ macrophages remained at baseline (Fig. 4F). Also, SR1 treatment had no effect on PD-L1 induction in vivo (Fig. 4F). Thus, AhR signaling controls CD155 induction during immunologic responses in vivo.
AhR activity mediates tumor immunosuppression, is associated with transcriptomic markers of TAMs and survival of patients with GBM
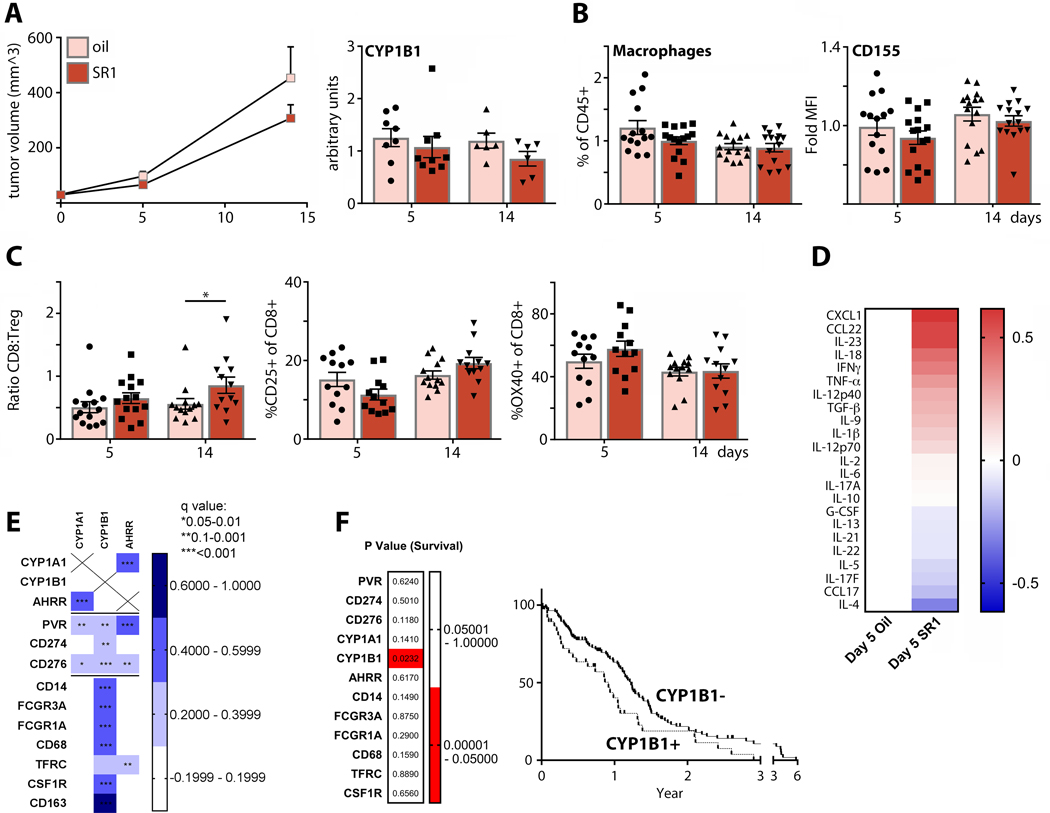
Both CD155 (4) and AhR (37) are implicated in cancer immune suppression and progression. Therefore, we investigated the implications of AhR signaling in a syngeneic, immunocompetent murine breast cancer model (E0771). AhR inhibition with SR1 modestly impaired tumor growth (Fig. 5A), repressed (AhR transcriptional target) CYP1B1 mRNA in tumors (Fig. 5A), decreased overall TAM levels (Fig. 5B), and diminished CD155 expression on TAMs (Fig. 5B). This was associated with a significantly elevated CD8:TReg ratio, an indication of more aggressive and less suppressive T cell phenotypes (Fig. 5C). CD25 (late) and OX40 (early) markers of CD8 T cell activation were elevated on intratumor CD8 T cells after SR1 treatment at 14 and 5 days, respectively (Fig. 5C). SR1 shifted intratumor cytokine profiles towards proinflammatory and Th1 phenotypes (Fig. 5D). Together these findings confirm a role for AhR in controlling TAM CD155 expression, TAM density, and tumor-intrinsic inflammation. To test if CD155/PD-L1 expression is linked to AhR in human tumors, we compared markers of AhR expression and of myeloid cells using the TCGA GBM cohort (Fig. 5E). We observed strong correlations between markers of both induced (CYP1A1, AHRR) and persistent (CYP1B1) AhR activity, with CD274, CD276, PVR expression (Fig. 5E). Both CYP1B1 (Fig. 5E) and CD274 (Fig. 1B) were highly correlated with macrophage markers. PVR showed strong correlation to markers of AhR activity (Fig. 5E). To determine which of these factors, if any, dictate patient outcome, we divided samples into low (z-score<0) and high (z-score>0) expression for each gene shown (Fig. 1F) and compared cohorts for survival. We only found a significant survival correlation for CYP1B1, indicating that the sustained AhR activity associated with AhR:β-catenin correlates with an unfavorable prognosis in GBM patients (Fig. 5F). In aggregate, we uncovered a role for AhR in controlling CD155 expression on TAMs; and defined a role for AhR in shaping the immunosuppressive tumor microenvironment, e.g. in GBM.
FIGURE 5.
AhR activity mediates tumor immunosuppression, and is associated with transcriptomic markers of TAMs and survival in GBM patients. (A; left panel) Mice bearing orthotopic E0771 tumors were treated every 48h with SR1 or vehicle control, and tumor volume was monitored (n=30 for day 5, n=15 for day 14). (Right panel) RT-qPCR was performed on tumor total RNA to assess CYP1B1 expression (n=8 for day 5 oil, n=9 for day 5 SR1, n=6 for day 14oil and SR1). (B) TAM (n=14 for day 5 oil, n=15 for day 5 SR1 and day 14 oil and SR1) and (C) intratumor T cell abundance (n=14 for day 5 oil and SR1, n=13 for day 14 oil, n=12 for day 14 SR1) were measured by flow cytometry. Tumor infiltrating T cell phenotyping was performed to assess changes in the CD8:TReg ratio, levels of CD25 expression, and OX40 expression. (D) Cytokine expression in post-dissociation tumor supernatant was measured by multiplex analysis and the ratio of vehicle (n=10) vs. SR1-treated (n=11) tumors plotted. (E) TCGA GBM database was analyzed for correlation of immune checkpoints (PVR, CD274, CD276), AhR activity markers (CYP1A1, CYP1B1, and AHRR), and macrophage markers (CD14, FCGR3A, FCGR1A, CD68, TFRC, CSF1R, and CD163). (F) TCGA GBM samples were separated by high (z-scores>0) and low (z-scores<0) expression of genes listed in (E) and compared for survival. Only sorting by high and low CYP1B1 expression yielded significant difference in survival.
Discussion
Targeting of CD155 [with recombinant poliovirus; (38)] and its binding partner TIGIT (39) is being intensely pursued for cancer immunotherapy. Despite evidence for inhibitory and activating influences on immune effector cell subsets, depending on engagement with its various binding partners, recent insight indicates an immune suppressive role for CD155 in cancer (4). Indeed, CD155 is virtually universally expressed on neoplastic cells of solid cancers (2, 3, 40). Our work identifies persistently active AhR signaling as a mechanism controlling CD155 expression on macrophages in various activation contexts, including within the microenvironment of neoplastic lesions. Thus, AhR signaling represents a broader biological program exploited by tumors to institute immune subversion and tumor progression. CD155, AhR, and PD-L1 expression were associated with TAM markers in human gliomas; and AhR inhibition in vivo reduced TAM density in murine tumors. Together, these findings confirm that AhR signaling modulates suppressive TAM phenotypes (26), including via the CD155 immune checkpoint.
The observation that macrophage activation-induced PD-L1 only occurs in combination with induced CD155 indicates a generic role for both immune checkpoints in inflammation. Yet, nuances in the regulation of CD155 vs PD-L1 (e.g. AhR-independent PD-L1 induction by LPS), in addition to their engagement of distinct T cell/NK cell receptors, indicate varied contributions in a broader network of gene expression programs that fine-tune immune responses. Expression of multiple co-inhibitory receptors by myeloid cells underscores a weakness of targeting single molecules for cancer immunotherapy.
AhR is best known for its role in recognizing xenobiotic chemicals and initiating transcriptional responses to metabolize them (41). However, AhR also assumes immune suppressive and stimulatory roles in innate and adaptive immunity (42, 43). Thus, fittingly, CD155 with competing immune suppressive vs. stimulatory activities, is a transcriptional target of AhR. AhR control of the immune system is multifaceted and stretches well beyond CD155. For example, AhR signaling contributes to IDO expression, dendritic cell immunogenicity (44), TReg induction (45), and Th17 polarization (42). Thus, the observed effects of AhR inhibition on the tumor microenvironment are due to events beyond restricted CD155 expression. We propose that CD155 belongs to a broader AhR-directed gene expression program that, in concert with other effector molecules, sculpts immunological responses.
AhR inhibition only had modest tumor-suppressive effects, despite an increased CD8:TReg ratio, lower TAM density, downregulation of CD155 expression on TAMs, and increased intratumor inflammatory cytokine signatures. Therefore, while AhR inhibition may be therapeutically attractive, our findings suggest it is insufficient to mediate tumor regression on its own. Moreover, antitumor effects of AhR inhibition have previously been documented elsewhere (26). AhR inhibition did not restrict PD-L1 expression on macrophages in vivo, possibly indicating that combination with PD1/PD-L1 blockade may be necessary to enhance antitumor effects of AhR inhibition. Indeed, combining (mouse ortholog) CD155 depletion with immune checkpoint blockade mediated antitumor efficacy in an immunocompetent mouse tumor model (46).
In conclusion, persistently active AhR signaling controls expression of the immune checkpoint CD155 in macrophages. Thus, AhR inhibition may provide a therapeutically tractable approach to target CD155 in cancer. Our work further supports mounting evidence for an AhR-driven immune axis that determines immune homeostasis, immune cell differentiation/activation, and function; and places CD155 as an effector molecule within this signaling network.
Key Points.
CD155 is induced on macrophages in response to stimulation by IL-4 and LPS.
CD155 induction is dependent on persistently active AhR.
CD155 can be targeted in vivo via AhR inhibition.
Acknowledgements.
We thank the Duke Preston Robert Tisch Brain Tumor Center BioRepository for providing explant GBM tumor tissue, Greg Palmer for providing E0771 cells, and Elena Dobrikova, Mikhail Dobrikov, Jonathan Kastan, and Wafa Hassen for insightful comments and technical support.
Grant Support. This project was supported by Public Health Service Grant R01 NS108773 (M.G.); Kirschstein National Research Service Award F32CA224593 (M.C.B.) and the National Cancer Center Breast Cancer Fellowship (M.C.B).
Footnotes
Disclosures. M.G. is an inventor of intellectual property licensed to-, holds equity in-, and is an advisor and compensated consultant of Istari Oncology, Inc. M.C.B. is an inventor of intellectual property licensed to Istari Oncology, Inc.
Bibliography
- 1.Mendelsohn CL, Wimmer E, and Racaniello VR. 1989. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56: 855–865. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell JS, Madore J, Li XY, and Smyth MJ. 2019. Tumor intrinsic and extrinsic immune functions of CD155. Semin Cancer Biol. [DOI] [PubMed] [Google Scholar]
- 3.Chandramohan V, Bryant JD, Piao H, Keir ST, Lipp ES, Lefaivre M, Perkinson K, Bigner DD, Gromeier M, and McLendon RE. 2017. Validation of an Immunohistochemistry Assay for Detection of CD155, the Poliovirus Receptor, in Malignant Gliomas. Arch Pathol Lab Med 141: 1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li XY, Das I, Lepletier A, Addala V, Bald T, Stannard K, Barkauskas D, Liu J, Aguilera AR, Takeda K, Braun M, Nakamura K, Jacquelin S, Lane SW, Teng MW, Dougall WC, and Smyth MJ. 2018. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Gromeier M, Solecki D, Patel DD, and Wimmer E. 2000. Expression of the human poliovirus receptor/CD155 gene during development of the central nervous system: implications for the pathogenesis of poliomyelitis. Virology 273: 248–257. [DOI] [PubMed] [Google Scholar]
- 6.Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, and Smyth MJ. 2010. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol 184: 902–911. [DOI] [PubMed] [Google Scholar]
- 7.Lozano E, Dominguez-Villar M, Kuchroo V, and Hafler DA. 2012. The TIGIT/CD226 axis regulates human T cell function. J Immunol 188: 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, Vats P, Chinnaiyan AM, Lao CD, Lawrence TS, Wicha M, Hamanishi J, Mandai M, Kryczek I, and Zou W. 2018. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 128: 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE 3rd, Wistuba I, Chaft J, Rizvi NA, Spigel DR, Spira A, Hirsch FR, Cohen V, Smith D, Boyd Z, Miley N, Flynn S, Leveque V, Shames DS, Ballinger M, Mocci S, Shankar G, Funke R, Hampton G, Sandler A, Amler L, Mellman I, Chen DS, and Hegde PS. 2018. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A 115: E10119-E10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MC, and Gromeier M. 2017. MNK Controls mTORC1:Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep 18: 1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastan JP, Dobrikova EY, Bryant JD, and Gromeier M. 2020. CReP mediates selective translation initiation at the endoplasmic reticulum. Sci Adv 0036: eaba0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, Bigner DD, Gromeier M, and Nair SK. 2017. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, and Schultz N. 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, and Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Huang J, Xiong Y, Li S, and Liu Z. 2019. Large-scale analysis reveals the specific clinical and immune features of CD155 in glioma. Aging (Albany NY) 11: 5463–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, Rasmussen R, Dwivedi B, Seby S, Wolf SA, Gutmann DH, and Hambardzumyan D. 2017. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res 77: 2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, Thomas G, Zhou S, Wang Q, Elakkad A, Liebelt BD, Yaghi NK, Ezhilarasan R, Huang N, Weinberg JS, Prabhu SS, Rao G, Sawaya R, Langford LA, Bruner JM, Fuller GN, Bar-Or A, Li W, Colen RR, Curran MA, Bhat KP, Antel JP, Cooper LJ, Sulman EP, and Heimberger AB. 2016. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 19.Shoham S, Huang C, Chen JM, Golenbock DT, and Levitz SM. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol 166: 4620–4626. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, and Akira S. 1996. Essential role of Stat6 in IL-4 signalling. Nature 380: 627–630. [DOI] [PubMed] [Google Scholar]
- 21.Solecki D, Schwarz S, Wimmer E, Lipp M, and Bernhardt G. 1997. The promoters for human and monkey poliovirus receptors. Requirements for basic and cell type-specific activity. The Journal of biological chemistry 272: 5579–5586. [DOI] [PubMed] [Google Scholar]
- 22.Sun YV, Boverhof DR, Burgoon LD, Fielden MR, and Zacharewski TR. 2004. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res 32: 4512–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, Yasunaga S, Ikizawa K, Yanagihara Y, Kubo M, Kuriyama-Fujii Y, Sugita Y, Inokuchi A, and Izuhara K. 2005. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int Immunol 17: 797–805. [DOI] [PubMed] [Google Scholar]
- 24.Puga A, Ma C, and Marlowe JL. 2009. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 77: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, Fang WF, Zhou YC, Liang F, Zhang C, Huang YC, Liu Z, Fu YX, and Zhou GB. 2019. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun 10: 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, Gutierrez-Vazquez C, Kenison J, Tjon EC, Barroso A, Vandeventer T, de Lima KA, Rothweiler S, Mayo L, Ghannam S, Zandee S, Healy L, Sherr D, Farez MF, Prat A, Antel J, Reardon DA, Zhang H, Robson SC, Getz G, Weiner HL, and Quintana FJ. 2019. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 22: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, and Quintana FJ. 2018. Microglial control of astrocytes in response to microbial metabolites. Nature 557: 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, and Kishimoto T. 2009. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med 206: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler N, Awwad K, Fisslthaler B, Reis M, Devraj K, Corada M, Minardi SP, Dejana E, Plate KH, Fleming I, and Liebner S. 2016. beta-Catenin Is Required for Endothelial Cyp1b1 Regulation Influencing Metabolic Barrier Function. J Neurosci 36: 8921–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, and Cooke MP. 2010. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329: 1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, Dinh H, and Krauss S. 2011. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res 71: 197–205. [DOI] [PubMed] [Google Scholar]
- 32.Dustin ML 2014. The immunological synapse. Cancer Immunol Res 2: 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chadeneau C, LeCabellec M, LeMoullac B, Meflah K, and Denis MG. 1996. Over-expression of a novel member of the immunoglobulin superfamily in Min mouse intestinal adenomas. Int J Cancer 68: 817–821. [DOI] [PubMed] [Google Scholar]
- 34.Ravens I, Seth S, Forster R, and Bernhardt G. 2003. Characterization and identification of Tage4 as the murine orthologue of human poliovirus receptor/CD155. Biochem Biophys Res Commun 312: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 35.Chadeneau C, LeMoullac B, LeCabellec M, Mattei M, Meflah K, and Denis MG. 1996. Isolation and chromosomal location of mE4, a novel murine gene of the immunoglobulin superfamily. Mamm Genome 7: 636–637. [DOI] [PubMed] [Google Scholar]
- 36.Khan S, Toyoda H, Linehan M, Iwasaki A, Nomoto A, Bernhardt G, Cello J, and Wimmer E. 2014. Poliomyelitis in transgenic mice expressing CD155 under the control of the Tage4 promoter after oral and parenteral poliovirus inoculation. J Gen Virol 95: 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray IA, Patterson AD, and Perdew GH. 2014. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer 14: 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Vlahovic SJH,G, Harrison WT, McLendon RE, Ashley D, and Bigner DD. 2018. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med 379: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manieri NA, Chiang EY, and Grogan JL. 2017. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol 38: 20–28. [DOI] [PubMed] [Google Scholar]
- 40.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, and Wimmer E. 2000. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A 97: 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, and Fujii-Kuriyama Y. 1994. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem 269: 27337–27343. [PubMed] [Google Scholar]
- 42.Esser C, Rannug A, and Stockinger B. 2009. The aryl hydrocarbon receptor in immunity. Trends Immunol 30: 447–454. [DOI] [PubMed] [Google Scholar]
- 43.Shinde R, and McGaha TL. 2018. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol 39: 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, and Kishimoto T. 2010. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A 107: 19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, and Kerkvliet NI. 2005. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol 175: 4184–4188. [DOI] [PubMed] [Google Scholar]
- 46.Li XY, Das I, Lepletier A, Addala V, Bald T, Stannard K, Barkauskas D, Liu J, Aguilera AR, Takeda K, Braun M, Nakamura K, Jacquelin S, Lane SW, Teng MW, Dougall WC, and Smyth MJ. 2018. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J Clin Invest 128: 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]