Abstract
Hypoxia during pregnancy is a major contributor to the pathogenesis of preeclampsia and intrauterine growth restriction. Our recent studies revealed that pregnancy-induced uterine vascular adaptation depended on the enhanced Ca2+ spark/spontaneous transient outward current (STOC) coupling and hypoxia during gestation diminished this adaption. In the present study, we test the hypothesis of a mechanistic link of microRNA-210 (miR-210) in hypoxia-impaired Ca2+ spark/STOC coupling in uterine arteries. Pregnant ewes acclimatized to high altitude (3,801 m) hypoxia for ~110 days significantly increased circulation levels of miR-210 in both the ewe and her fetus. Treatment of uterine arteries from high-altitude animals with the antagomir miR-210-LNA recovered hypoxia-repressed STOCs in pregnant ewes and restored the hormonal regulation of STOCs in non-pregnant animals. In uterine arteries from low-altitude control animals, miR-210 mimic suppressed STOCs in pregnant ewes and inhibited the hormonal regulation of STOCs in non-pregnant animals. Mechanistically, miR-210 directly targeted and downregulated type 2 ryanodine receptor and large-conductance Ca2+-activated K+ channel β1 subunit, resulting in significant decreases in Ca2+ sparks and STOCs in uterine arteries. In addition, miR-210 indirectly decreased STOCs by targeting ten-eleven translocation methylcytosine dioxygenase. Together, the present study revealed a mechanistic link of miR-210 in hypoxia-induced repression of Ca2+ spark/STOC coupling in uterine arteries during gestation, providing novel insights into the understanding of pregnancy complications associated with hypoxia and the potential therapeutic targets.
Keywords: pregnancy, preeclampsia, IUGR, Ca2+ sparks, STOCs, ryanodine receptor, BKCa channel β1 subunit, TET1
Summary
The present study demonstrates that elevated miR-210 is necessary and sufficient to inhibit Ca2+ spark/STOC coupling in uterine arterial adaptation to gestational hypoxia by downregulating RyR2, BKCa β1 subunit and TET1. The study provides new insights into the understanding of fundamental mechanisms underlying programming of vascular dysfunction caused by gestational hypoxia, impacting on maternal cardiovascular health and developmental plasticity.
Graphical Abstract
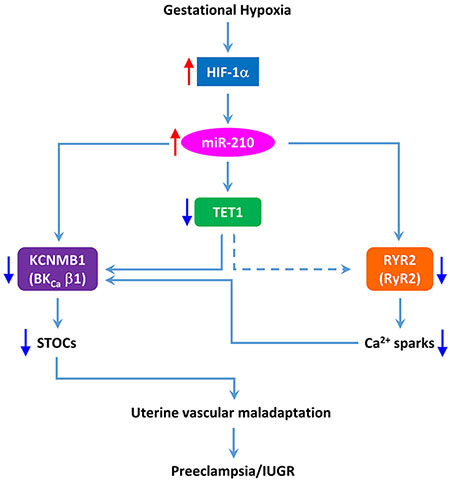
Introduction
Hypoxia is a common and severe stress to an organism’s homeostatic mechanisms, and hypoxia during gestation is associated with significantly increased incidence of maternal complications of preeclampsia, adversely impacting fetal development and increasing subsequent risk for cardiovascular and metabolic disease.1 Pregnant women at high altitude have a two- to four-fold increase in the incidence of preeclampsia, which is often proceeded by a decrease in uteroplacental blood flow.2–5 Extensive human and animal studies have revealed a causative role of increased uterine vascular resistance and placental hypoxia in preeclampsia and intrauterine growth restriction (IUGR) associated with hypoxia in pregnancy. In an animal model of pregnant sheep acclimatized to high altitude hypoxia, we have found that pregnant ewes are similar to humans in that they have an increase in uterine vascular resistance and an elevation in maternal systemic blood pressure.6, 7 Yet, much remains unknown of the mechanisms underlying maternal cardiovascular adaptation to long-term hypoxia during gestation. It should be noted that the impact of hypoxia on the uterine circulation is complex and contradictory observations have been reported in humans and animals. Reduced uterine artery resistance index was also observed in high-altitude human pregnancy and in a rat model of IUGR induced by chronic hypoxia,8, 9 which may represent a compensatory mechanism. Furthermore, nature selection has enabled native Andeans to have greater uterine blood flow during pregnancy than European women at high altitude and protect them from hypoxia-associated IUGR.10
Hypoxia via HIF1α robustly upregulates microRNA-210 (miR-210), which together with HIF1α are master regulators of the cellular hypoxic response.11–17 MiR-210 is specifically induced by HIF1α, but not HIF2α.18, 19 Mature miR-210 of 22 nt is highly homologous across species and is identical among the human, sheep and rodent. Of importance, an increase in miR-210 appears to be a common feature in preeclampsia and gestational hypoxia. Thus, elevated placental expression of miR-210 and circulating miR-210 levels have been demonstrated in both preeclampsia and pregnancy at high altitude in pregnant women.20–27 We have demonstrated significant increases in HIF1α and miR-210 in uterine arteries in pregnant sheep acclimatized to high altitude hypoxia.6, 28 It should be emphasized that in addition to miR-210, many other miRs in the uteroplacental unit are also altered in preeclampsia/IUGR and contribute to the pathogenesis of these pregnancy complications.29
Spontaneous transient outward currents (STOCs) at physiological membrane potentials of vascular smooth muscle cells fundamentally regulate vascular myogenic tone and blood flow in an organ, as well as arterial blood pressure.30–40 We demonstrated in sheep that pregnancy significantly increased STOCs in uterine arteries.41 Of importance, our recent study revealed that high altitude hypoxia suppressed this pregnancy-induced upregulation of STOCs in uterine arteries,42 identifying an exciting and novel regulatory target in programming of uterine vascular malfunction in response to gestational hypoxia. STOCs are mediated by the large-conductance Ca2+-activated K+ (BKCa) channel that is mainly activated by highly localized and concentrated Ca2+ release events through the opening of ryanodine receptors (RyRs) in the sarcoplasmic reticulum, termed Ca2+ sparks.30, 39 Ca2+ sparks and STOCs are tightly coupled in that the appearance of a Ca2+ spark almost always transiently activates a group of BKCa channels.43 All three subtypes of RyRs, RyR1–3, exist in vascular smooth muscle cells. Our recent studies demonstrated that pregnancy significantly increased the expression of all three RyR subtypes in uterine arteries and enhanced the co-localization of RyR2 with the β1 subunit of BKCa channel in the uterine artery.41, 44 In addition, we demonstrated that high altitude hypoxia suppressed pregnancy-induced upregulation of RyR2 in uterine arteries 42 and knockdown of RyR2 with siRNAs significantly decreased Ca2+ spark frequency and suppressed STOCs in uterine arteries.44
A question of great relevance is whether and to what extent the elevation of miR-210 mediates the dysregulation of Ca2+ spark/STOC coupling in programming of uterine vascular malfunction in response to gestational hypoxia. In the present study, we revealed a mechanistic link of miR-210 in hypoxia-induced repression of Ca2+ spark/STOC coupling in uterine arteries during gestation by targeting both type 2 ryanodine receptor and BKCa β1 subunit, resulting in significant decreases in Ca2+ sparks and STOCs in uterine arteries. These findings provide novel insights into the mechanistic understanding of pregnancy complications associated with hypoxia and the potential therapeutic targets.
Methods
The authors declare that all supporting data are available within the article.
Animal model and tissue preparation
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. After tissue collection, animals were euthanized via intravenous injection of 15 mL T-61 solution (Hoechst-Rousel, Somervile, NJ), according to American Veterinary Medical Association guidelines.
Time-dated pregnant and non-pregnant sheep (Ovis aries) were obtained from Nebeker Ranch (~300 m above sea level, ewe PaO2: ~102 mm Hg), Lancaster, CA. For high-altitude hypoxic treatment, non-pregnant and pregnant ewes when they were able to be confirmed pregnancy with ultrasound around 30 days of gestation were transported to and maintained at the White Mountain Research Station (altitude of 3,801 m, ewe PaO2: ~60 mm Hg), White Mountain, Bishop, CA. After the treatment, near-term (~142–145 days of gestation) pregnant and non-pregnant ewes were transported to Loma Linda University where they were studied. This high-altitude sheep “model” has been well-established and extensively studied over the past three decades. As we reported in early studies, although this model did not result in pre-term labor or overtly fetal growth restriction, per se, the high-altitude fetuses showed moderate arterial hypoxemia, significantly elevated mean arterial blood pressure and ~25% decreased combined cardiac output. In addition, high-altitude hypoxia had profound effects on maternal uterine vascular and placental adaptation to pregnancy, and on developmental plasticity of cerebrovascular, neural, cardiac, pulmonary, endocrine, metabolic and other systems.1 Uterine arteries were isolated from non-pregnant and pregnant ewes bearing either singleton or twin fetuses. For pregnant ewes with a singleton fetus, uterine arteries were isolated from the uterine horn bearing the fetus. Animals were anesthetized with intravenous injection of propofol (2 mg/kg) followed by intubation, and anesthesia was maintained on 1.5% to 3.0% isoflurane balanced in O2 throughout the surgery. An incision was made in the abdomen and the uterus was exposed. The resistance-sized uterine artery segments (~200 μm in diameter) were isolated and removed without stretching and placed into a physiological salt solution (PSS) containing (in mmol/L) 130.0 NaCl, 10.0 HEPES, 6.0 glucose, 4.0 KCl, 4.0 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.18 KH2PO4, and 0.025 EDTA (pH 7.4). For tissue culture, uterine arteries were placed in a culture dish containing 5 mL of phenol red-free DMEM supplemented with 1% charcoal-stripped fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin and incubated at 37 °C in humidified incubators for 48 hours, as described previously.45, 46 For the hormonal treatment, uterine arteries from non-pregnant sheep were incubated in the presence of vehicle DMSO or 17β-estradiol (E2β, Sigma, St. Louis, MO) (0.3 nmol/L) plus progesterone (P4, Sigma) (100.0 nmol/L), which mimicked the circulating levels of these two hormones in pregnancy, as reported previously.45, 46 Following the treatment, the subsequent experiments were conducted in the absence of vehicle or hormones.
Measurement of Ca2+ sparks
Ca2+ sparks were measured in endothelium-denuded uterine arteries loaded with the Ca2+ sensitive dye Fluo-4 AM and using a Zeiss LSM 710 NLO laser scanning confocal imaging workstation on an inverted microscope platform (Zeiss Axio Observer Z1).41 The endothelium was mechanically disrupted by gently pulling a silver wire across the intimal surface of the uterine arterial segments 5 times, with confirmation by visual analysis of the preparations on the confocal microscope after loading the tissue with Fluo-4. Arterial segments were incubated with 10 μmol/L Fluo-4 AM (ThermoFisher, Waltham, Massachusetts) dissolved in DMSO along with 0.1% pluronic F127 (ThermoFisher) for 2 hours at room temperature. Tissues were then washed for 30 minutes to allow dye esterification and then cut into linear strips. The arterial segments were pinned to Sylgard blocks and placed in an open bath imaging chamber mounted on the confocal imaging stage. Cells were illuminated at 488 nm with a krypton argon laser and the emitted light was collected using a photomultiplier tube. Line scans were imaged at 529 frames s−1 with the emission signal recorded at 493–622 nm. The acquisition period for Ca2+ spark recordings was 18.9 s. The resultant pixel size ranged from 0.021 to 0.1 μm per pixel. To ensure that sparks within the cell were imaged, the pinhole was adjusted to provide an imaging depth of 2.5 μm. This depth is roughly equivalent to the width of 50% of the cell based on morphological examination of live preparations. Line scans were analyzed using Sparklab 4.2.1 to characterize Ca2+ spark parameters such as frequency (sparks/μm/s), amplitude (F/F0), spatial size (the full width at half maximum, FWHM) and duration (the full duration at half maximum, FDHM). The fractional fluorescence intensity was calculated as F/F0 = F - baseline/F0 - baseline, where baseline is the intensity from a region of interest with no cells, F is the fluorescence intensity for the region of interest, and F0 is the fluorescence intensity during a period from the beginning of the recording when there was no Ca2+ activity.
Measurement of STOCs
Arterial smooth muscle cells were enzymatically dissociated from resistance-sized uterine arteries as described previously.41 Briefly, uterine arteries were minced and incubated (37 °C, 10 minutes) in low-Ca2+ HEPES-buffered physiological salt (PSS) solution containing (in mmol/L) 140.0 NaCl, 5.0 KCl, 0.1 CaCl2, 1.2 MgCl2, 10.0 HEPES, and 10.0 glucose (pH 7.4). Vessels were then exposed to a two-step digestion process that involved: 1) a 60-minute incubation in low-Ca2+ HEPES-buffered PSS (37 °C) containing 1.5 mg/ml papain (Worthington Biochemical; Lakewood, NJ), 1.5 mg/ml dithiothreitol (MilliporeSigma, St. Louis, MO), and 1.5 mg/ml bovine serum albumin (MilliporeSigma); and 2) a 60-minute incubation in low-Ca2+ HEPES-buffered PSS (37 °C) containing 1.5 mg/ml collagenase IV (Worthington), and 1.5 mg/ml bovine serum albumin (MilliporeSigma). Following the enzyme treatment, tissues were washed with low Ca2+ HEPES-buffered PSS. Single smooth muscle cells were released by gently inverting the tube(s) containing low Ca2+ HEPES-buffered PSS and digested tissues several times. The cells were kept at 4 °C and experiments were conducted within 6 hours of cell isolation. STOCs were recorded in the whole-cell configuration of the perforated patch-clamp technique using an EPC 10 patch-clamp amplifier with Patchmaster software (HEKA, Lambrecht/Pfalz, Germany) at room temperature as previously described.41 Briefly, cell suspension drops were placed in a recording chamber, and adherent cells were continuously superfused with HEPES-buffered PSS containing (in mmol/L) 140.0 NaCl, 5.0 KCl, 1.8 CaCl2, 1.2 MgCl2, 10.0 HEPES, and 10.0 glucose (pH 7.4). Only relaxed and spindle-shaped myocytes were used for recording. Micropipettes were pulled from borosilicate glass and had resistances of 2 to 5 megaohm (mΩ) when filled with the pipette solution containing (in mmol/L) 140.0 KCl, 1.0 MgCl2, 5.0 Na2ATP, 5.0 EGTA, 10.0 HEPES (pH 7.2) with 250 μg/ml amphotericin B. Membrane currents were recorded while the cells were held at steady membrane potentials between −50 and 10 mV in 10 mV-increments. STOCs were analyzed with Mini Analysis program (Synaptosoft, Leonia, NJ) with a threshold for detection setting at 10 pA. The currents were normalized to cell capacitance and expressed as picoampere per picofarad (pA/pF).
Measurement of plasma miR-210
Plasma miRs were purified using miRNeasy serum/plasma kit (Qiagen, Germantown, MD) according to the manufacturers’ instructions. MiR-210 was subsequently measured using miScript II RT kit (Qiagen) following the manufacturers’ instructions. Relative gene expression of miR-210 was calculated by 2−ΔΔCT method.47
Transfection of microRNA mimic and antagomir
Tissue transfection was conducted as described previously.6, 48 Briefly, the mixtures of miR-210 mimic (Qiagen), miR-210-LNA (Qiagen), or AllStars negative controls (Qiagen), with HiPerfect transfection reagent (Qiagen) and Opti-MEM 1 (ThermoFisher) were prepared and incubated for ~30 minutes at room temperature. The mixtures were subsequently added to DMEM containing 1% charcoal-stripped fetal bovine serum in a 6-well plate (final concentration of 100 nmol/L) maintained in an incubator at 37 °C for 48 hours.
Knockdown of genes encoding ten-eleven translocation methylcytosine dioxygenase 1 (TET1) and BKCa β1 subunit
To knockdown TET1 or BKCa β1 subunit encoding gene KCNMB1, resistance-sized uterine arteries of pregnant sheep were transfected with TET1 or KCNMB1 siRNAs (Dharmacon Inc, Lafayette, CO), as described previously.49 Target or scrambled siRNAs were mixed with HiPerfect Transfection Reagent (Qiagen) and Opti-MEM I (ThermoFisher) for ~30 minutes at room temperature and subsequently added into DMEM/F12 supplemented with 1% charcoal-stripped fetal bovine serum. Tissues were incubated in the medium containing target or scrambled siRNAs at a final concentration of 100 nmol/L in an incubator at 37 °C for 48 hours. Our previous studies showed that siRNAs of TET1 and KCNMB1 successfully knocked down TET1 and KCNMB1, respectively, in uterine arteries.49
Luciferase reporter assay
Luciferase reporter assay was performed as previously described.6 A 249-bp 3′UTR segment of ovine KCNMB1 mRNA harboring the mature miR-210 target was cloned into the pmirGLO vector (Promega) between XhoI (5′) and Xba I (3′). This final construct was designated pmiR-XBX. For luciferase assays, uterine arterial smooth muscle cells were transfected with pmiR-XBX or pmirGLO (for the vector control) together with miR-210 mimic or negative control (Qiagen) using X-tremeGENE HP DNA transfection reagent (Roche, Indianapolis, IN) following the manufacturer’s instructions. The cells were processed using the Dual-Luciferase Reporter Assay System (Promega) 48 hours after transfection.
RNA-induced silencing complex-immunoprecipitation (RISC-IP) assay
RISC-IP assay was performed as previously described.50 Uterine arterial smooth muscle cells were transfected 100 nmol/L miR-210 mimic or negative control (Qiagen) by using HiPerfect transfection reagent (Qiagen) according to the manufacturer’s instructions. Cells were harvested 24 hours after transfection and washed in ice-cold PBS followed by complete lysis buffer (Active Motif) at 4 °C for 10 minutes. RISC-IP of the lysate was conducted using the miRNA Target IP Kit (Active Motif) according to the manufacturer’s instructions. The RNA was extracted with phenol/chloroform/isoamyl alcohol (25:24:1, Fisher Scientific) once, chloroform once and precipitated and resuspended in RNase-free water. The precipitated RNA was subjected to RT-qPCR using primers specific for the sheep RyR2 3′-UTR. β-actin was used as an internal control. The relative abundance of RyR2 transcript pulled down by Ago1/2/3 antibody was calculated by the 2−ΔΔCT method and is presented as the fold induction relative to the control. Primers used were 5′-CACACACATGCACACACACACACTC (forward) and 5’-TGGCAGTTCACAACATCAGCAAG (reverse) for RyR2 and 5′-GCAGGTCATCACCATCGGCAAT (forward) and 5’-ACCGTGTTGGCGTAGAGGTCCT (reverse) for β-actin.
Western immunoblotting
Protein abundance of RyR2 in uterine arteries was measured as described previously.41 Briefly, tissues were homogenized in a lysis buffer followed by centrifugation at 4 °C for 10 minutes at 10,000g, and the supernatants were collected. Samples with equal proteins were loaded onto 4-12% PAGEr™ Gold Gels (Lonza, Allendale, NJ) and were separated by electrophoresis at 100 V for 2-2.5 hours. Proteins were then transferred onto nitrocellulose membranes. After blocking nonspecific binding sites by dry milk, membranes were incubated with primary antibodies (1:1000 dilution) against RyR2 (AB9080, EMD Millipore, Billerica, MA). The specificity of RyR2 antibody was confirmed previously.41 After washing, membranes were incubated with secondary horseradish peroxidase–conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. Results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software (Kodak, Rochester, NY).
Statistical analysis
Data were expressed as means ± SEM obtained from the number of experimental animals. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Differences were evaluated for statistical significance (P<0.05) by ANOVA or t test where appropriate.
Results
High altitude pregnancy elevated miR-210 in maternal and fetal circulations
High-altitude pregnancy elevated uteroplacental miR-210.6, 27 A human study also revealed that circulating miR-210 was increased in high-altitude pregnancy.51 We then examined whether high altitude hypoxia altered circulating miR-210 in pregnant sheep. We found that pregnant ewes acclimatized to high altitude hypoxia were similar to humans in that they had a significant increase in miR-210 in both maternal and fetal circulations (Figure 1).
Figure 1. High-altitude pregnancy increased circulating miR-210.
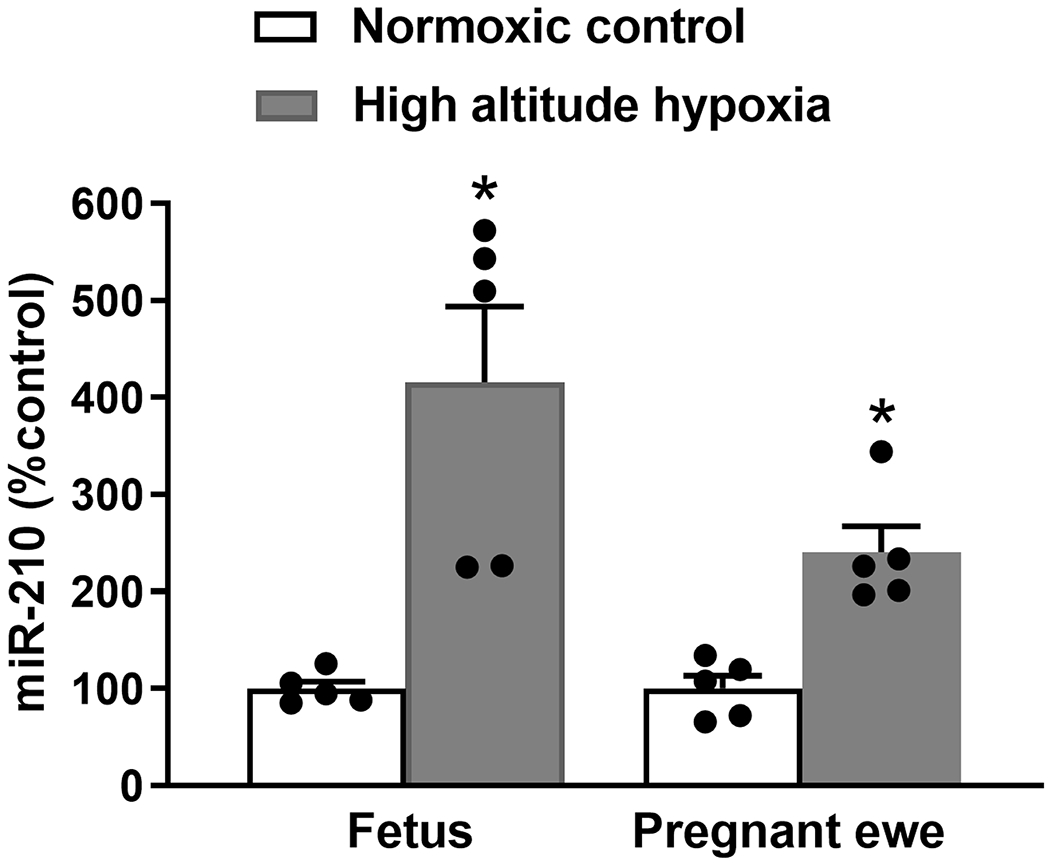
Levels of miR-210 in the maternal and fetal circulations of normoxic control and high-altitude hypoxia were determined. Data are means ± SEM of 5 animals of each group; independent-samples t-test; *P < 0.05, high-altitude hypoxia versus normoxic control.
MiR-210-LNA reversed hypoxia-induced suppression of STOCs in uterine arteries
Our recent study demonstrated that high altitude hypoxia suppressed pregnancy-induced upregulation of STOCs in uterine arteries.42 In addition, we showed a significant increase in miR-210 in uterine arteries in pregnant sheep acclimatized to high altitude hypoxia, which was blocked by transfecting the miR-210 inhibitor miR-210-LNA.48 To reveal a causal effect of elevated miR-210 on repressed STOCs in uterine arteries of high-altitude pregnant animals, we examined whether inhibition of endogenous miR-210 by miR-210-LNA could reverse the effect of hypoxia on STOCs in uterine arteries. To this end, uterine arteries of high-altitude pregnant animals were transfected with negative control or miR-210-LNA under 10.5% O2. Compared to negative control, miR-210-LNA markedly increased STOC frequency and amplitude in uterine arteries of high-altitude pregnant animals (Figures 2A and 2B). In addition, miR-210-LNA shifted STOC occurrence to a more negative membrane potential (from −20 mV to −40 mV), similar to that observed in normoxic control animals.
Figure 2. MiR-210-LNA reversed the effect of hypoxia on STOCs in uterine arteries of high-altitude animals.
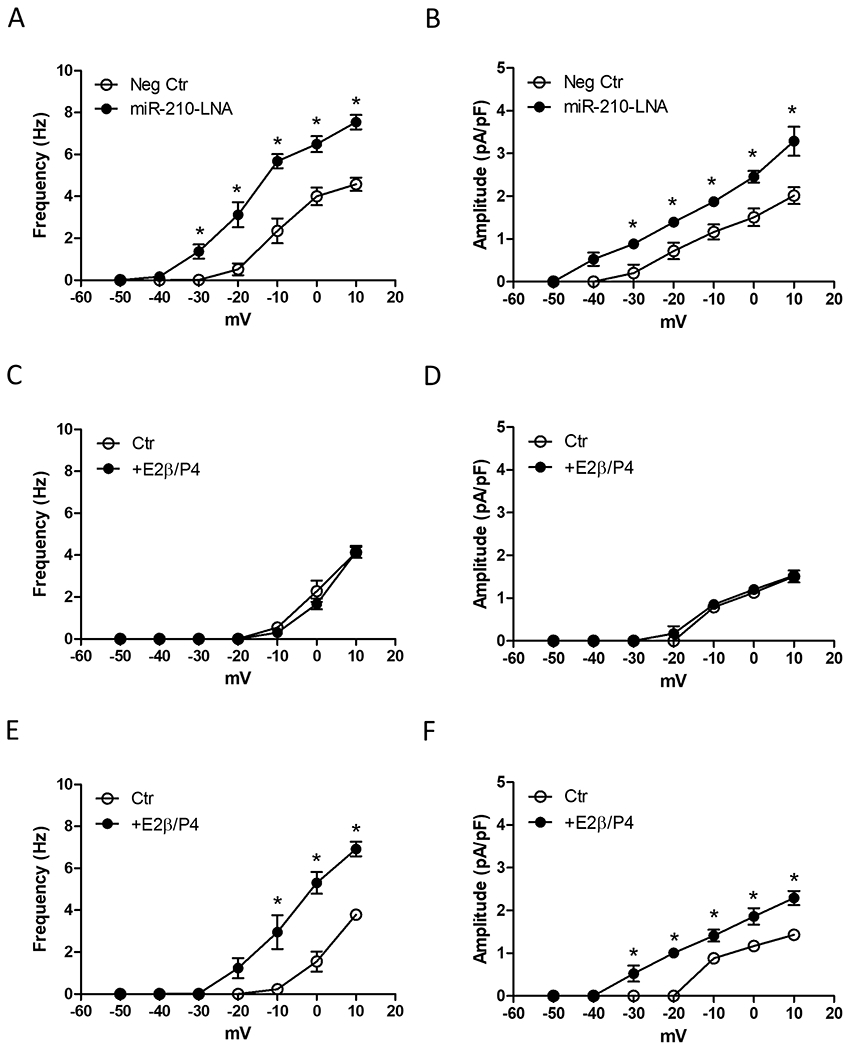
A and B, Uterine arteries from high-altitude pregnant sheep were transfected with 100 nmol/L miR-210-LNA or negative control (Neg Ctr) under 10.5% O2 for 48 hours and STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group. *P < 0.05, miR-210-LNA versus Neg Ctr. C - F, Uterine arteries from high-altitude nonpregnant sheep were transfected with Neg Ctr (C, D) or 100 nmol/L miR-210-LNA (E, F) under 10.5% O2 for 48 hours, in the presence of vehicle control (Ctr) or 17β-estradiol (E2β 0.3 nmol/L)/progesterone (P4; 100.0 nmol/L). STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, E2β/P4 versus Ctr.
MiR-210-LNA restored hormonal regulation of STOCs in uterine arteries
Steroid hormones play a pivotal role in the adaptation of uterine circulation during pregnancy. Previously, we demonstrated that the ex vivo treatment of uterine arteries from nonpregnant animals with estrogen and progesterone significantly increased both STOC frequency and amplitude.41 This hormonal regulation of STOCs was rescinded in uterine arteries of non-pregnant animals acclimatized to high altitude hypoxia. Thus, in contrast to that observed in normoxic control animals, the ex vivo hormonal treatment of uterine arteries from high altitude non-pregnant sheep failed to affect STOC frequency and amplitude (Figures 2C and 2D). Of importance, blockade of miR-210 with miR-210-LNA restored the hormonal regulation of STOCs in uterine arteries of non-pregnant animals acclimatized to high altitude hypoxia (Figures 2E and 2F).
MiR-210 mimic imitated the effect of gestational hypoxia and suppressed STOCs in uterine arteries
We next determine whether and to what extent miR-210 mimic has a direct effect in suppressing STOCs in uterine arteries. Uterine arteries from normoxic control pregnant sheep were treated with miR-210 mimic and STOCs were measured after the treatment. As shown in Figures 3A and 3B, miR-210 treatment shifted STOC occurrence to a more positive membrane potential (from −40 mV to −20 mV) and significantly suppressed both STOC frequency and amplitude. Indeed, the effect of miR-210 imitated that of high-altitude hypoxia, and STOCs recorded in uterine arteries of control animals in the presence of miR-210 were not significantly different from those observed in uterine arteries of pregnant ewes acclimatized to high altitude hypoxia (Figures 2A and 2B). In addition, miR-210 inhibited the ex vivo hormonal regulation of STOCs in uterine arteries. Uterine arteries from control non-pregnant animals were treated ex vivo with estrogen and progesterone under 21.0% O2 for 48 hours in the absence or presence of miR-210 mimic. In the absence of miR-210 mimic, the hormonal treatment significantly increased both STOC frequency and amplitude (Figures 3C and 3D). In contrast, in the presence of miR-210 mimic, uterine arteries responded like the tissues from no-pregnant ewes acclimatized to high altitude hypoxia and lost the effect of ex vivo hormonal regulation of STOCs (Figures 3E and 3F).
Figure 3. MiR-210 mimic inhibited STOCs in uterine arteries of normoxic control animals.
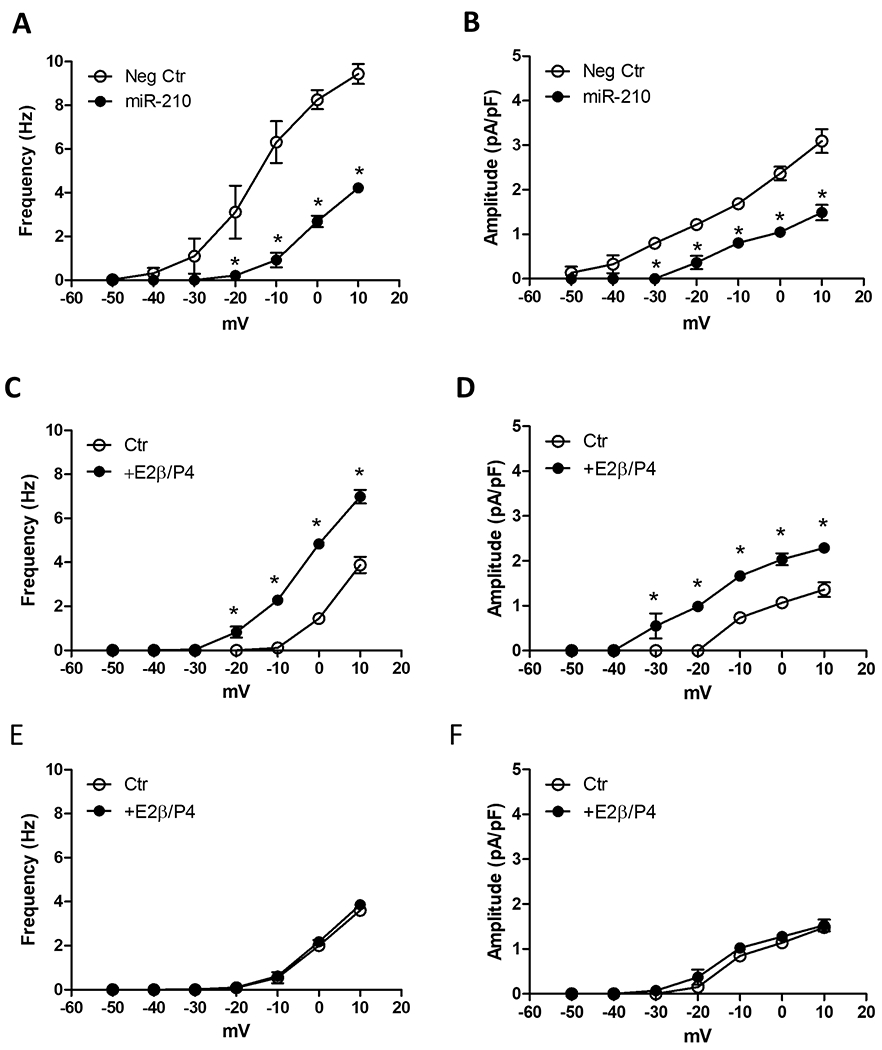
A and B, Uterine arteries from normoxic control pregnant sheep were transfected with 100 nmol/L miR-210 mimic (miR-210) or negative control (Neg Ctr) under 21.0% O2 for 48 hours and STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group. *P < 0.05, miR-210 versus Neg Ctr. C - F, Uterine arteries from normoxic control nonpregnant sheep were transfected with Neg Ctr (C, D) or 100 nmol/L miR-210 (E, F) under 21.0% O2 for 48 hours, in the presence of vehicle control (Ctr) or 17β-estradiol (E2β 0.3 nmol/L)/progesterone (P4; 100.0 nmol/L). STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 6 animals in each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, E2β/P4 versus Ctr.
MiR-210 directly targeted and downregulated RyR2 and Ca2+ sparks in uterine arteries
STOCs are manifestations of RyR-mediated Ca2+ sparks. Although all three subtypes of RyRs exist in vascular smooth muscle cells, their function in the regulation of Ca2+ sparks may be different. Our recent study identified an important role of RyR2 and demonstrated that knockdown of RyR2 with siRNAs significantly decreased Ca2+ sparks and suppressed STOCs in uterine arteries.44 In addition, we showed that high altitude hypoxia suppressed pregnancy-induced upregulation of RyR2 in uterine arteries.42 We thus investigated the effect of miR-210 in the regulation of RyR2 and Ca2+ sparks in uterine arteries. We identified a potential miR-210 complementary binding site in RyR2 3′-UTR using the sequence alignment program MultAlin (http://multalin.toulouse.inra.fr/multalin/multalin.html) (Figure 4A). We then determined whether RyR2 is genuinely targeted and regulated by miR-210. RISC-IP assay revealed that RyR2 mRNA was enriched by ~2-fold in miR-210-transfected uterine arteries of pregnant animals compared to negative control-treated tissues (Figure 4A), indicating that RyR2 is a direct target of miR-210. Moreover, miR-210 treatment significantly downregulated RyR2 protein abundance in uterine arteries compared to negative control (Figure 4B).
Figure 4. MiR-210 mimic downregulated RyR2 and Ca2+ sparks in uterine arteries.
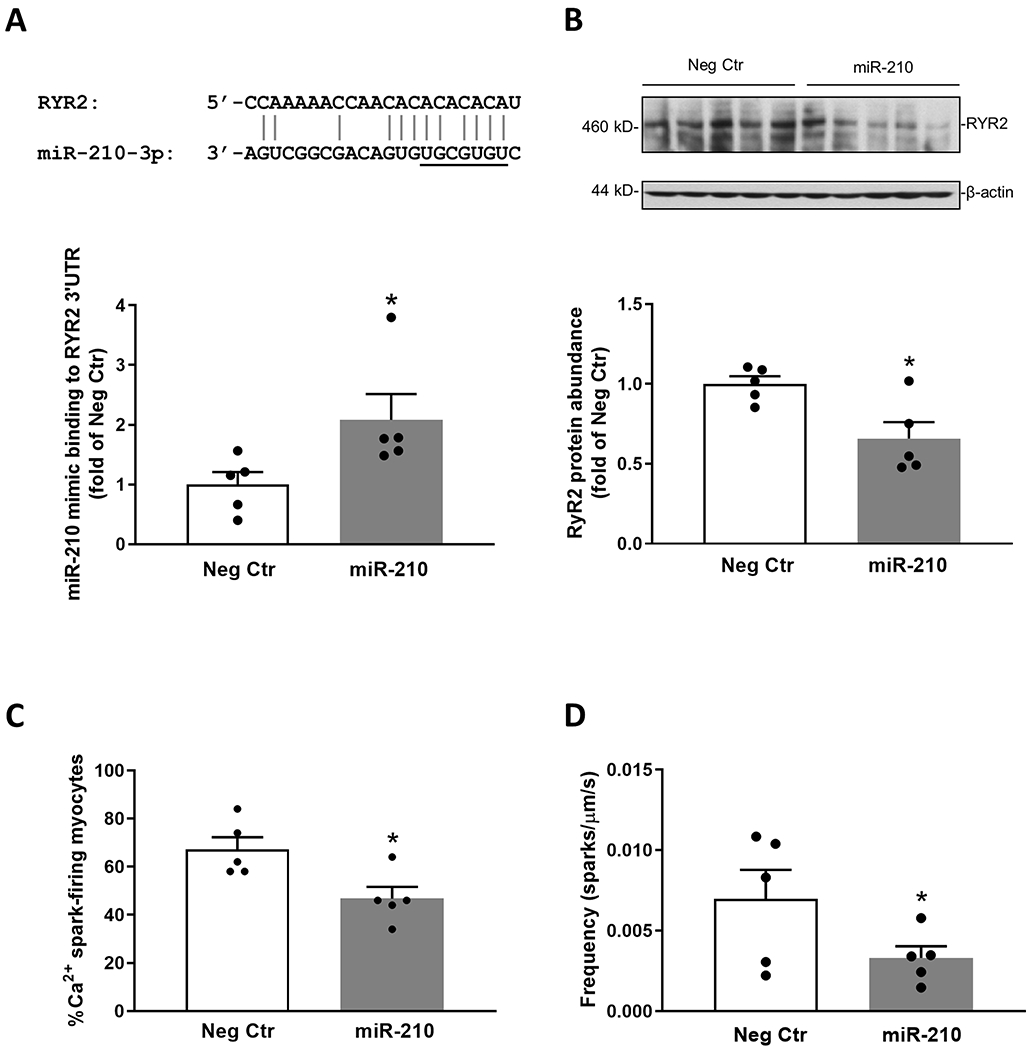
A. RyR2 is a bona fide target of miR-210 in uterine arteries. Schematic representation of alignment of the predicted miR-210 binding site to ovine RyR2 3′UTR is shown. Uterine arterial smooth muscle cells were treated with 100 nmol/L miR-210 mimic (miR-210) or negative control (Neg Ctr) for 24 hours and RISC-IP analysis of abundance of RyR2 3′UTR pulled down by Ago1/2/3 antibody was determined after the treatment. B - D. Uterine arteries from normoxic control pregnant sheep were treated with 100 nmol/L miR-210 or Neg Ctr under 21.0% O2 for 48 hours. RyR2 protein abundance (B), the percentage of myocytes with Ca2+ sparks (C) and Ca2+ spark frequency (sparks/μm/s) (D) were determined after the treatment. Data are means ± SEM of 5 animals in each group; independent-samples t-test; *P < 0.05, miR-210 versus Neg Ctr.
We next determined whether miR-210 altered Ca2+ sparks in uterine arteries. In uterine arteries of pregnant animals transfected with negative control, Ca2+ sparks were observed in 67.2 ± 5.1% of myocytes. MiR-210 significantly reduced Ca2+ spark-firing myocytes to 46.8 ± 4.8 (Figure 4C), representing a ~30% decrease. Moreover, miR-210 also lowered Ca2+ spark frequency by ~50% (Figure 4D). Other Ca2+ spark firing characteristics such as amplitude (F/F0), width (FWHM) and duration (FDHM) in uterine arteries were not significantly altered by miR-210 (data not shown). Of interest, the effects of miR-210 on Ca2+ sparks are similar to those observed in the previously study, showing ~30% decrease in Ca2+ spark-firing myocytes and ~50% reduction in Ca2+ spark frequency in uterine arteries of pregnant ewes acclimatized to high altitude hypoxia.42
MiR-210 downregulated BKCa β1 subunit in uterine arteries
BKCa β1 subunit residing in the close proximity to RyRs within spatial boundaries of the Ca2+ microdomain functions as a Ca2+ sensor and is critical for the Ca2+ spark/STOC coupling.37, 52–54 Pregnancy increased the co-localization of RyR2 with the β1 subunit in the uterine artery.44 Potential miR-210 binding sequences were identified in the 3′-UTR of ovine β1 subunit encoding gene KCNMB1. As shown in Figure 5A, a co-transfection of pmirGLO-XBX containing a 249-bp 3′-UTR segment of ovine KCNMB1 harboring the miR-210 target sites and miR-210 mimic produced a significant decrease in the luciferase activity, demonstrating a direct interaction of miR-210 with the 3′-UTR of KCNMB1. This is consistent with the previous finding showing that miR-210 mimic downregulated the β1 subunit in uterine arteries.6 Of importance, knockdown of the β1 subunit with siRNAs significantly reduced both STOC frequency and amplitude (Figures 5B and 5C), recapitulating the effect of miR-210 on STOCs in uterine arteries (Figures 3A and 3B).
Figure 5. MiR-210 mimic downregulated BKCa β1 subunit encoding gene KCNMB1 and STOCs in uterine arteries.
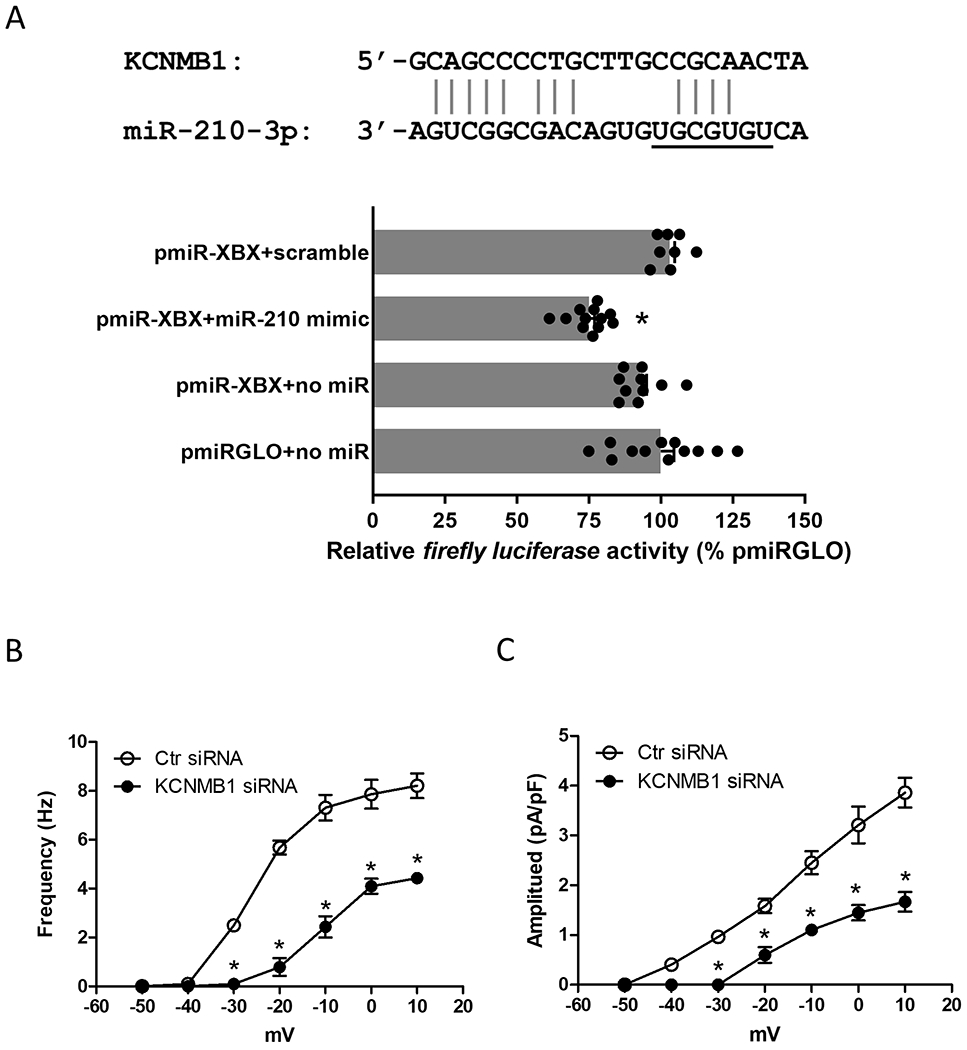
A, Schematic representation of alignment of the predicted miR-210 binding site to ovine KCNMB1 3′-UTR is shown. Uterine arterial smooth muscle cells were co-transfected with the constructs containing ovine KCNMB1 3′-UTR (pmiR-XBX) in the presence of 100 nmol/L miR-210 mimic (miR-210) or negative control (scramble) for 48 hours. Luciferase assays of various pmirGLO reporters were measured after the treatment. Data are means ± SEM from 8-12 assays; one-way ANOVA with the post hoc Bonferroni test; *P < 0.05, pmirGLO-XBX + miR-210 versus pmirGLO-XBX + scramble. B and C, Uterine arteries of normoxic control pregnant sheep were treated with scramble control (Ctr siRNA) or KCNMB1 siRNAs for 48 hours and STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, KCNMB1 siRNA versus Ctr siRNA.
Involvement of TET1 in miR-210-mediated regulation of STOCs in uterine arteries
TETs regulate active DNA demethylation. TET1 is a target of miR-210 and it was downregulated by miR-210 in uterine arteries.6 We thus determined the role of TET1 in the regulation of STOCs in uterine arteries. Uterine arteries from pregnant ewes were transfected with TET1 siRNAs and scramble controls. As shown in Figures 6A and 6B, TET1 siRNAs treatment significantly decreased both STOC frequency and amplitude in uterine arteries. To examine whether TET was involved in miR-210-LNA-mediated enhancement of STOCs in uterine arteries of pregnant animals acclimatized to high altitude hypoxia (Figures 2A and 2B), we examine the effect of miR-210-LNA on STOCs in the uterine arteries in the presence of a TET inhibitor, fumarate. As shown in Figures 6C and 6D, fumarate annulled the effect of miR-210-LNA to restore STOCs in uterine arteries of pregnant animals acclimatized to high altitude hypoxia.
Figure 6. Effect of TET1 on STOCs in uterine arteries.
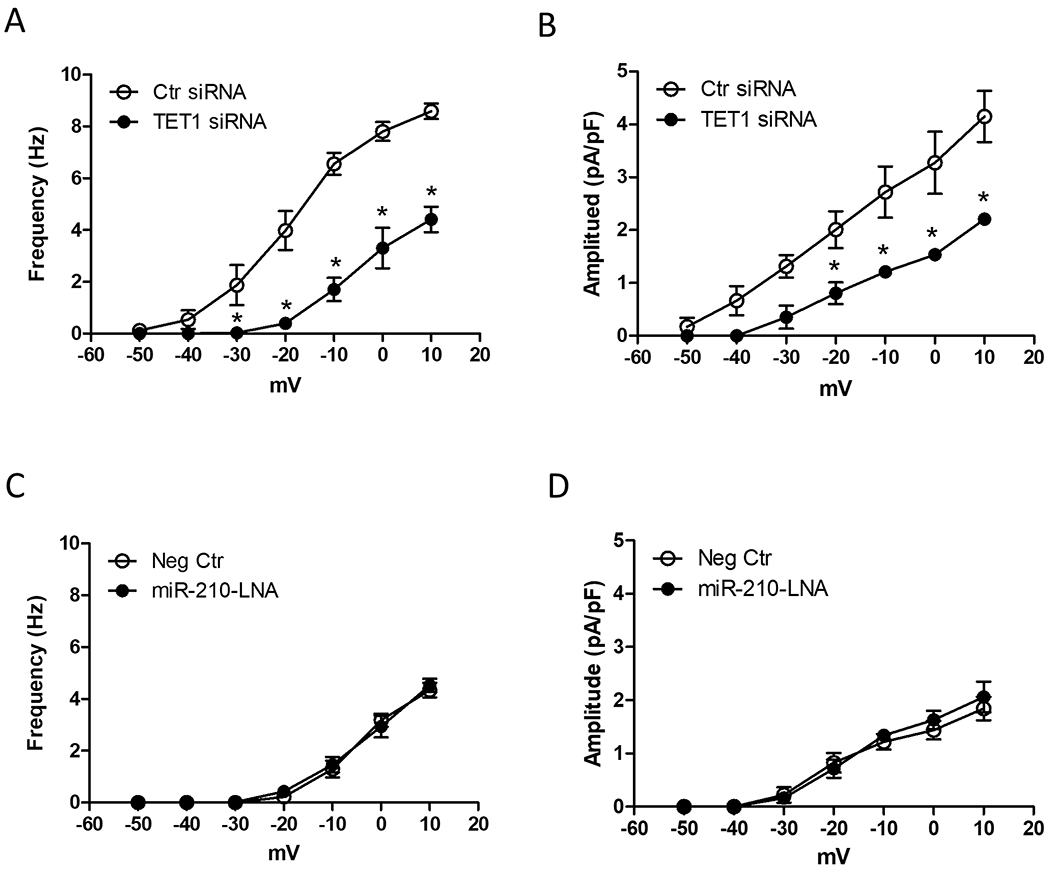
A and B, Uterine arteries of normoxic control pregnant sheep were treated with scramble control (Ctr siRNA) or TET1 siRNAs for 48 hours and STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, TET1 siRNA versus Ctr siRNA. C and D, Uterine arteries from high-altitude pregnant sheep were treated with 100 nmol/L miR-210-LNA or negative control (Neg Ctr) in the presence of 3 mmol/L monoethyl fumarate (MEF) under 10.5% O2 for 48 hours. STOC frequency and amplitude were measured after the treatment. Data are means ± SEM of 5 animals in each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test.
Discussion
The present study provides novel evidence of a mechanistic link of miR-210 in the hypoxia-induced repression of Ca2+ spark/STOC coupling in uterine arteries during gestation by targeting both type 2 ryanodine receptor and BKCa β1 subunit, resulting in significant decreases in Ca2+ sparks and STOCs that are of fundamental importance in programming of uterine vascular malfunction in response to gestational hypoxia. The major findings are: 1) hypoxia during gestation elevated miR-210 in maternal and fetal circulations, 2) inhibition of endogenous miR-210 by miR-210-LNA reversed hypoxia-induced suppression of STOCs in uterine arteries in pregnant ewes acclimatized to gestational hypoxia, 3) miR-210-LNA restored the ex vivo hormonal regulation of STOCs in uterine arteries of non-pregnant animals exposed to high altitude hypoxia, 4) miR-210 mimic imitated the effect of gestational hypoxia and suppressed STOCs in uterine arteries of pregnant ewes, and inhibited the ex vivo hormonal regulation of STOCs in uterine arteries of non-pregnant ewes, 5) miR-210 directly targeted and downregulated RyR2 and Ca2+ sparks in uterine arteries, 6) miR-210 downregulated BKCa β1 subunit resulting in a decrease in STOCs of uterine arteries, 7) TET1 was involved in the miR-210-mediated inhibition of STOCs in uterine arteries in response to gestational hypoxia.
Extensive human and animal studies have revealed a causative role of increased uterine vascular resistance and placental hypoxia in preeclampsia and IUGR associated with hypoxia in pregnancy.1 In an animal model of pregnant sheep acclimatized to high altitude hypoxia, we have found that pregnant ewes are similar to humans in that they have an increase in uterine vascular resistance and elevation in maternal systemic blood pressure.6, 7 Although much remains unknown of the mechanisms underlying maternal cardiovascular adaptation to long-term hypoxia during gestation, hypoxia via HIF1α robustly upregulates miR210, which together with HIF1α are master regulators of the cellular hypoxic response. Our previous studies demonstrated that HIF1α and miR-210 were significantly increased in uterine arteries in pregnant sheep acclimatized to high altitude hypoxia.6, 28 In the present study, we showed that pregnant ewes of high-altitude hypoxia had a significant increase in miR-210 in both maternal and fetal circulations. Previous studies in humans revealed that increased miR-210 is a common feature in preeclampsia and gestational hypoxia, showing elevated placental expression of miR-210 and circulating miR2-10 levels in both preeclampsia and pregnancy at high altitude in pregnant women.20–27 Similarly, hypoxia-induced miR-210 was found to be a major contributor to the development of pulmonary hypertension.55, 56 In addition to being biomarkers, recent evidence of endocrine and disease-modifying extracellular microRNAs in the circulation expanded our understanding of the systemic physiology of miRNA activity in the vasculature and beyond. It has been shown that miR-223 in the circulation is absorbed by vascular smooth muscle cells to regulate proliferation, migration and apoptosis by targeting insulin-like growth factor 1 receptor encoding gene IGF1R.57 In addition, it has been demonstrated that chronic hypoxia upregulates circulating miR-210 originated from the bone marrow, which has long-range tissue uptake and endocrine activity, acting on pulmonary arteries to promote pulmonary hypertension.58 Of importance, mature miR-210 of 22 nt is highly homologous across species and is identical among the human, sheep and rodent, suggesting a translational potential of miR-210 in the preclinical study.
The finding that inhibition of endogenous miR-210 by miR-210-LNA reversed hypoxia-induced suppression of STOCs in uterine arteries revealed a causal effect of elevated miR-210 on repressed STOCs in uterine arteries of high-altitude pregnant animals. STOCs at physiological membrane potentials of vascular smooth muscle cells are a key determinant of vascular myogenic tone and blood flow in an organ, as well as arterial blood pressure. STOCs produce K+ efflux mediated by BKCa channels, and cause membrane hyperpolarization resulting in vascular smooth muscle cell relaxation. We demonstrated recently in sheep that pregnancy significantly increased STOCs in uterine arteries,41 and hypoxia during gestation suppressed this pregnancy-induced upregulation of STOCs in uterine arteries.42 In addition, the previous study showed that miR-210 antagomir miR-210-LNA blocked the hypoxia-induced increase of miR-210 in uterine arteries of pregnant sheep.48 In the present study, we found that miR-210-LNA shifted STOC occurrence to a more negative membrane potential (from −20 mV to −40 mV) and markedly increased STOC frequency and amplitude in uterine arteries of high-altitude pregnant animals. Indeed, the blockade of miR-210 restored STOCs in uterine arteries of high-altitude pregnant ewes to those seen in normoxic control animals. In addition, miR-210-LNA recovered the ex vivo hormonal regulation of STOCs in uterine arteries of animals exposed to high altitude hypoxia. Steroid hormones play a pivotal role in the adaptation of uterine circulation during pregnancy. Previously, we showed that the ex vivo treatment of uterine arteries from nonpregnant animals with estrogen and progesterone significantly increased both STOC frequency and amplitude,41 and this hormonal regulation of STOCs was rescinded in uterine arteries of non-pregnant animals exposed to high altitude hypoxia.42 The present finding that inhibition of miR-210 with miR-210-LNA restored the ex vivo hormonal regulation of STOCs in uterine arteries of non-pregnant animals acclimatized to high altitude hypoxia revealed a mechanism of elevated miR-210 in hypoxia-induced repression of hormonal regulation of uterine arterial adaptation. Further evidence of a mechanistic link of miR-210 in the hypoxia-mediated effect on STOCs in uterine arteries comes from the present finding that miR-210 mimic had a direct effect in suppressing STOCs in uterine arteries. Indeed, the effect of miR-210 recapitulated that of high-altitude hypoxia, and STOCs recorded in uterine arteries of control pregnant animals in the presence of miR-210 mimic were not significantly different from those observed in uterine arteries of pregnant ewes acclimatized to high altitude hypoxia. In addition, miR-210 imitated the effect of hypoxia and inhibited the ex vivo hormonal regulation of STOCs in uterine arteries. In the presence of miR-210 mimic, uterine arteries responded in the same manner as those from non-pregnant ewes acclimatized to high altitude hypoxia, losing the effect of hormonal regulation of STOCs. Taken together, these findings provide evidence that elevated miR-210 is necessary and sufficient in mediating hypoxia-induced repression of STOCs in uterine arterial adaptation during pregnancy.
STOCs are activated by Ca2+ sparks. The functional coupling between Ca2+ sparks and STOCs is highly efficient that virtually a one-to-one relationship exists between Ca2+ sparks and STOCs. In animal models, abnormal Ca2+ spark/STOC coupling has been associated with cardiovascular dysfunction associated with hypertension, subarachnoid hemorrhage, shock, diabetes, obesity, and Duchenne muscular dystrophy.31, 32, 40, 59–63 Ca2+ sparks are mediated by RyRs. All 3 subtypes of RyRs, RyR1, RyR2, and RyR3 are expressed in vascular smooth muscle cells. Although both RyR1 and RyR2 constitute independent Ca2+ sparks, RyR2 is primarily located in the subplasmalemmal region of the endoplasmic reticulum53, 64 and is the predominant mediator of Ca2+ sparks in vascular smooth muscle.44, 65, 66 We showed that RyR2 knockdown decreased both Ca2+ sparks and STOCs in uterine arteries.44 In addition, we demonstrated that RyR2 played a key role in the regulation of Ca2+ sparks in uterine arterial adaptation to pregnancy, and that high-altitude hypoxia suppressed pregnancy-induced upregulation of RyR2 and Ca2+ sparks in uterine arteries.41, 42, 44 Of interest, the present study revealed that RyR2 is a direct target of miR-210. We showed that miR-210 mimic treatment significantly reduced RyR2 protein abundance in uterine arteries, which is consistent with the finding that gestational hypoxia suppressed pregnancy-induced RyR2 expression in uterine arteries.42 Of importance, miR-210 mimic recapitulated the effect of gestational hypoxia and reduced Ca2+ spark-firing myocytes and Ca2+ spark frequency to the levels observed in uterine arteries of pregnant ewes acclimatized to high altitude hypoxia.42 These findings suggest that increased miR-210 is sufficient in the downregulation of RyR2 and the reduction in Ca2+ sparks in uterine arterial response to hypoxia during pregnancy.
RyR2 is colocalized with the BKCa channel in vascular smooth muscle cells.62 Our recent study revealed that pregnancy significantly increased the co-localization of RyR2 with BKCa β1 subunit in the uterine artery.44 This spatial organization of close proximity facilitates the β1 subunit to sense Ca2+ sparks and promote Ca2+ spark/STOC coupling, which is proportional to the size of RyR/BKCa cluster.32, 62, 67 The BKCa β1 subunit plays a vital role in determining the coupling efficiency of Ca2+ sparks and STOCs, which is dynamically regulated under various physiological and pathophysiological conditions. In addition to RyR2, we identified that the BKCa β1 subunit is also a target of miR-210 by demonstrating a direct interaction of miR-210 with the 3′-UTR of KCNMB1. Our previous studies showed that miR-210 and hypoxia during gestation decreased BKCa channel β1 protein abundance in uterine arteries, which was restored by miR-210-LNA.6, 48 The present finding that knockdown of the β1 subunit recapitulated the effect of miR-210 and suppressed both STOC frequency and amplitude in uterine arteries revealed a causal role of miR-210-meidated downregulation of BKCa β1 subunit in hypoxia-induced repression of STOCs in uterine arteries.
In addition to directly targeting and downregulating RyR2 and the β1 subunit, the present study also revealed the involvement of TET1 in miR-210-mediated regulation of STOCs in uterine arteries. TETs regulate active DNA demethylation. Previously, we demonstrated that gestational hypoxia-induced miR-210 directly targeted and downregulated TET1, leading to KCNMB1 hypermethylation and the repression of β1 subunit in uterine arteries.6, 48 In addition to the β1 subunit, hypermethylation of RyR2 promoter was found to be associated with reduced RyR2 expression.68 Thus, miR-210 targeting TET1 provides an additional mechanism in the downregulation of RyR2 and the β1 subunit and the inhibition of Ca2+ spark/STOC coupling in uterine arteries. Given that a miR can target many transcripts, it is not surprising that miR-210 interacts with TET1, RyR2 and KCNMB1. Similarly, miR-29b was also found to directly target both TET1 and KCNMB1.69, 70 The finding that miR-210 downregulates TET is highly intriguing because TET via its effect of DNA demethylation may regulate the expression of many other genes, including RyR2 and KCNMB1 in uterine arteries. Of interest, the downregulation of uteroplacental TETs also occurred in preeclampsia.71–73 Thus, it is likely that the impairment of STOCs in uterine arteries of pregnant animals acclimatized to high-altitude hypoxia is conferred in part by miR-210-induced TET1 downregulation and the resultant repression of RyR2 and KCNMB1. This notion is supported by the observations that 1) the suppressed STOCs by miR-210 and hypoxia were simulated by TET1 knockdown with siRNAs, 2) the restoration of STOCs by miR-210-LNA in uterine arteries of gestational hypoxia was offset by the TET inhibitor fumarate.
Perspective
Human and animal studies have revealed a causative role of increased uterine vascular resistance and placental hypoxia in preeclampsia and fetal intrauterine growth restriction associated with gestational hypoxia. Pregnant women at high altitude have a two- to four-fold increase in the incidence of preeclampsia, which is often proceeded by a decrease in uteroplacental blood flow. Similar to humans, we have shown that pregnant sheep at high altitude have an increase in uterine vascular resistance and elevation in maternal systemic blood pressure. Our recent study revealed a regulatory target of repressed STOCs in programming of uterine vascular malfunction in response to gestational hypoxia. The present study provides evidence of a mechanistic link of miR-210 in hypoxia-mediated impairment of Ca2+ spark/STOC coupling in uterine arteries during gestation in an animal model of pregnant ewes acclimatized to high-altitude hypoxia. We demonstrated that elevated miR-210 is necessary and sufficient in inhibiting STOCs in uterine arterial adaptation to gestational hypoxia. MiR-210 directly targeted and downregulated RyR2 and BKCa β1 subunit, repressing Ca2+ sparks and STOCs in uterine arteries. In addition, miR-210 indirectly decreased STOCs by targeting TET1. Of importance, overexpression of HIF-1α-responsive miR-210 and downregulation of TET1 and RyR2 have been constantly detected in the circulation and/or uteroplacental tissues in preeclampsia and IUGR.27, 74–77 Thus, the present study provides new insights into the understanding of fundamental mechanisms underlying programming of vascular dysfunction caused by gestational hypoxia, impacting on maternal cardiovascular health and developmental plasticity. Given that miR-210 is a master regulator of cellular hypoxic response, and STOCs are fundamentally important in regulating vascular tone and pressure in virtually all vascular beds, the present study has a broad impact in the comprehensive understanding of molecular mechanisms in the regulation of vascular function and hemodynamics. Although the focus of the present study is to investigate miR-210-mediated regulation of STOCs in uterine arterial smooth muscle, the endothelium also plays an important role in the adaptation of uterine circulation during pregnancy78–80 and endothelial dysfunction contributes to the pathogenesis of preeclampsia/IUGR.81 The role of miR-210 in regulating endothelial function remains an intriguing area of further investigation. Indeed, a recent study demonstrated that circulating miR-210 contributes to the development of hypoxia-induced pulmonary hypertension by targeting endothelial mitochondrial function.58 Although the present study highlights the potential of developing miR-210-targeting therapies for pregnancy complication associated with hypoxia, it should be noted that miR-210 also participates in proliferation, angiogenesis and other cellular processes.82 To be beneficial, the miR-210-targeting therapy should be efficiently delivered to the target tissue/cells and possess high potency and limited cytotoxicity/off-target effects.
Supplementary Material
Novelty and Significance.
What Is New?
Hypoxia during gestation elevates miR-210 in maternal and fetal circulations.
Inhibition of miR-210 reverses hypoxia-induced suppression of STOCs in uterine arteries.
MiR-210-LNA inhibits the effect of hypoxia and restores hormonal regulation of STOCs.
MiR-210 mimic recapitulates the effect of gestational hypoxia and suppresses STOCs.
MiR-210 inhibits the hormonal regulation of STOCs in uterine arteries.
MiR-210 directly targets and downregulates RyR2 and Ca2+ sparks in uterine arteries.
MiR-210 downregulates BKCa β1 subunit resulting in a decrease in STOCs.
TET1 is involved in the miR-210-mediated inhibition of STOCs in uterine arteries.
What Is Relevant?
The present study reveals a mechanistic link of miR-210 in hypoxia-induced repression of Ca2+ spark/STOC coupling in uterine arteries during gestation and provides novel insights into the understanding of pregnancy complications associated with hypoxia and potential therapeutic targets.
Acknowledgments
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Sources of Funding
This work was supported by National Institutes of Health Grants HD083132 (L. Z.), HL128209 (L. Z.), HL137649 (L. Z.), HL149608 (L. Z.).
Footnotes
Disclosures
None.
References
- 1.Ducsay CA, Goyal R, Pearce WJ, Wilson S, Hu XQ, Zhang L. Gestational hypoxia and developmental plasticity. Physiol Rev. 2018;98:1241–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: Regional and life-cycle perspectives. Am J Phys Anthropol. 1998;Suppl 27:25–64 [DOI] [PubMed] [Google Scholar]
- 3.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol (1985). 1995;79:15–22 [DOI] [PubMed] [Google Scholar]
- 4.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in colorado. Am J Obstet Gynecol. 1999;180:1161–1168 [DOI] [PubMed] [Google Scholar]
- 5.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol (1985). 1995;79:7–14 [DOI] [PubMed] [Google Scholar]
- 6.Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. Microrna-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance ca(2+)-activated k(+) channel expression and function in ovine uterine arteries. Hypertension. 2017;70:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krampl ER, Espinoza-Dorado J, Lees CC, Moscoso G, Bland JM, Campbell S. Maternal uterine artery doppler studies at high altitude and sea level. Ultrasound Obstet Gynecol. 2001;18:578–582 [DOI] [PubMed] [Google Scholar]
- 9.Aljunaidy MM, Morton JS, Cooke CL, Davidge ST. Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1068–R1075 [DOI] [PubMed] [Google Scholar]
- 10.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SY, Loscalzo J. Microrna-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: A master regulator of microrna biogenesis and activity. Free Radic Biol Med. 2013;64:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan YC, Banerjee J, Choi SY, Sen CK. Mir-210: The master hypoxamir. Microcirculation. 2012;19:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Lella Ezcurra AL, Bertolin AP, Melani M, Wappner P. Robustness of the hypoxic response: Another job for mirnas? Dev Dyn. 2012;241:1842–1848 [DOI] [PubMed] [Google Scholar]
- 15.Loscalzo J The cellular response to hypoxia: Tuning the system with micrornas. J Clin Invest. 2010;120:3815–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Le QT, Giaccia AJ. Mir-210--micromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez SR, Ma Q, Dasgupta C, Meng X, Zhang L. Microrna-210 suppresses glucocorticoid receptor expression in response to hypoxia in fetal rat cardiomyocytes. Oncotarget. 2017;8:80249–80264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. Hsa-mir-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348 [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, Takizawa T, Hirashima C, Takahashi K, Migita M, Ishikawa G, Yoneyama K, Asakura H, Izumi A, Matsubara S, Takeshita T, Takizawa T. Hydroxysteroid (17-beta) dehydrogenase 1 is dysregulated by mir-210 and mir-518c that are aberrantly expressed in preeclamptic placentas: A novel marker for predicting preeclampsia. Hypertension. 2012;59:265–273 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, Xin H, Sun S. Elevated levels of hypoxia-inducible microrna-210 in pre-eclampsia: New insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. Mir-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Zhou Y, Zhang Z. Mir-210: An important player in the pathogenesis of preeclampsia? J Cell Mol Med. 2012;16:943–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DB, Wang W. Human placental micrornas and preeclampsia. Biol Reprod. 2013;88:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Zhou H, Lin H, Qi J, Zhu C, Gao Z, Wang H. Circulating micrornas are elevated in plasma from severe preeclamptic pregnancies. Reproduction. 2012;143:389–397 [DOI] [PubMed] [Google Scholar]
- 26.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated micrornas in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31:781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colleoni F, Padmanabhan N, Yung HW, Watson ED, Cetin I, Tissot van Patot MC, Burton GJ, Murray AJ. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for mirna-210 and protein synthesis inhibition. PLoS One. 2013;8:e55194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D, Hu XQ, Huang X, Zhou J, Wilson SM, Yang S, Zhang L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS One. 2013;8:e73731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu XQ, Zhang L. Micrornas in uteroplacental vascular dysfunction. Cells. 2019;8: 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637 [DOI] [PubMed] [Google Scholar]
- 31.Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H. Hypersensitivity of bkca to ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res. 2007;101:493–502 [DOI] [PubMed] [Google Scholar]
- 32.Koide M, Nystoriak MA, Krishnamoorthy G, O’Connor KP, Bonev AD, Nelson MT, Wellman GC. Reduced ca2+ spark activity after subarachnoid hemorrhage disables bk channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Zhang Y, Liu Y, Gu B, Cao R, Chen Y, Zhao T. Exercise prevents upregulation of ryrs-bkca coupling in cerebral arterial smooth muscle cells from spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2016;36:1607–1617 [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, Korthuis RJ, Davis MJ, Braun AP, Hill MA. Mechanisms underlying regional differences in the ca2+ sensitivity of bk(ca) current in arteriolar smooth muscle. J Physiol. 2013;591:1277–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan E, Kushner JS, Zakharov S, Nui XW, Chudasama N, Kelly C, Waase M, Doshi D, Liu G, Iwata S, Shiomi T, Katchman A, D’Armiento J, Homma S, Marx SO. Reduced vascular smooth muscle bk channel current underlies heart failure-induced vasoconstriction in mice. FASEB J. 2013;27:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of cav3.2 channels enhances the arterial myogenic response by modulating the ryr-bkca axis. Arterioscler Thromb Vasc Biol. 2015;35:1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted bk channel beta1 subunit gene feature abnormal ca(2+) spark/stoc coupling and elevated blood pressure. Circ Res. 2000;87:E53–60 [DOI] [PubMed] [Google Scholar]
- 38.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by ryr3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057 [DOI] [PubMed] [Google Scholar]
- 39.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to kca channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal ca2+ spark/stoc coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu XQ, Song R, Romero M, Dasgupta C, Huang X, Holguin MA, Williams V, Xiao D, Wilson SM, Zhang L. Pregnancy increases ca(2+) sparks/spontaneous transient outward currents and reduces uterine arterial myogenic tone. Hypertension. 2019;73:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu XQ, Song R, Romero M, Dasgupta C, Min J, Hatcher D, Xiao D, Blood A, Wilson SM, Zhang L. Gestational hypoxia inhibits pregnancy-induced upregulation of ca(2+) sparks and spontaneous transient outward currents in uterine arteries via heightened endoplasmic reticulum/oxidative stress. Hypertension. 2020;76:930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–256 [DOI] [PubMed] [Google Scholar]
- 44.Song R, Hu XQ, Romero M, Holguin MA, Kagabo W, Xiao D, Wilson SM, Zhang L. Ryanodine receptor subtypes regulate ca2+ sparks/stocs and myogenic tone of uterine arteries in pregnancy. Cardiovasc Res. 2020:cvaa089. doi: 10.1093/cvr/cvaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance ca(2+)-activated k(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 48.Hu XQ, Dasgupta C, Xiao J, Yang S, Zhang L. Long-term high altitude hypoxia during gestation suppresses large conductance ca(2+) -activated k(+) channel function in uterine arteries: A causal role for microrna-210. J Physiol. 2018;596:5891–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu XQ, Dasgupta C, Chen M, Xiao D, Huang X, Han L, Yang S, Xu Z, Zhang L. Pregnancy reprograms large-conductance ca(2+)-activated k(+) channel in uterine arteries: Roles of ten-eleven translocation methylcytosine dioxygenase 1-mediated active demethylation. Hypertension. 2017;69:1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B, Dasgupta C, Huang L, Meng X, Zhang L. Mirna-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol. 2019;17:976–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hale A, Lee C, Annis S, Min PK, Pande R, Creager MA, Julian CG, Moore LG, Mitsialis SA, Hwang SJ, Kourembanas S, Chan SY. An argonaute 2 switch regulates circulating mir-210 to coordinate hypoxic adaptation across cells. Biochim Biophys Acta. 2014;1843:2528–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M. Beta(1)-subunit of bk channels regulates arterial wall[ca(2+)] and diameter in mouse cerebral arteries. J Appl Physiol (1985). 2001;91:1350–1354 [DOI] [PubMed] [Google Scholar]
- 53.Lifshitz LM, Carmichael JD, Lai FA, Sorrentino V, Bellve K, Fogarty KE, ZhuGe R. Spatial organization of ryrs and bk channels underlying the activation of stocs by ca(2+) sparks in airway myocytes. J Gen Physiol. 2011;138:195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pritchard HAT, Gonzales AL, Pires PW, Drumm BT, Ko EA, Sanders KM, Hennig GW, Earley S. Microtubule structures underlying the sarcoplasmic reticulum support peripheral coupling sites to regulate smooth muscle contractility. Sci Signal. 2017;10:eaan2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gou D, Ramchandran R, Peng X, Yao L, Kang K, Sarkar J, Wang Z, Zhou G, Raj JU. Mir-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, et al. Genetic and hypoxic alterations of the microrna-210-iscu1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7:695–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan Z, Qin S, Li W, Wu W, Yang J, Chu M, Li X, Huo Y, Schaer GL, Wang S, Zhang C. An endocrine genetic signal between blood cells and vascular smooth muscle cells: Role of microrna-223 in smooth muscle function and atherogenesis. J Am Coll Cardiol. 2015;65:2526–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J, Florentin J, Tai YY, Torrino S, Ohayon L, Brzoska T, Tang Y, Yang J, Negi V, Woodcock CC, Risbano MG, Nouraie SM, Sundd P, Bertero T, Dutta P, Chan SY. Long range endocrine delivery of circulating mir-210 to endothelium promotes pulmonary hypertension. Circ Res. 2020;127:677–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971 [DOI] [PubMed] [Google Scholar]
- 60.McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, Scholfield CN, McGeown JG, Curtis TM. Diabetes downregulates large-conductance ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieves-Cintron M, Syed AU, Buonarati OR, Rigor RR, Nystoriak MA, Ghosh D, Sasse KC, Ward SM, Santana LF, Hell JW, Navedo MF. Impaired bkca channel function in native vascular smooth muscle from humans with type 2 diabetes. Sci Rep. 2017;7:14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pritchard HAT, Pires PW, Yamasaki E, Thakore P, Earley S. Nanoscale remodeling of ryanodine receptor cluster size underlies cerebral microvascular dysfunction in duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2018;115:E9745–E9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenstein AS, Kadir S, Csato V, Sugden SA, Baylie RA, Eisner DA, Nelson MT. Disruption of pressure-induced ca(2+) spark vasoregulation of resistance arteries, rather than endothelial dysfunction, underlies obesity-related hypertension. Hypertension. 2020;75:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L338–348 [DOI] [PubMed] [Google Scholar]
- 65.Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for ca(2+)-induced ca(2+) release in vascular myocytes. J Biol Chem. 2000;275:9596–9603 [DOI] [PubMed] [Google Scholar]
- 66.Kassmann M, Szijarto IA, Garcia-Prieto CF, Fan G, Schleifenbaum J, Anistan YM, Tabeling C, Shi Y, le Noble F, Witzenrath M, Huang Y, Marko L, Nelson MT, Gollasch M. Role of ryanodine type 2 receptors in elementary ca(2+) signaling in arteries and vascular adaptive responses. J Am Heart Assoc. 2019;8:e010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galice S, Xie Y, Yang Y, Sato D, Bers DM. Size matters: Ryanodine receptor cluster size affects arrhythmogenic sarcoplasmic reticulum calcium release. J Am Heart Assoc. 2018;7: e008724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt K, Molfenter B, Laureano NK, Tawk B, Bieg M, Hostench XP, Weichenhan D, Ullrich ND, Shang V, Richter D, et al. Somatic mutations and promotor methylation of the ryanodine receptor 2 is a common event in the pathogenesis of head and neck cancer. Int J Cancer. 2019;145:3299–3310 [DOI] [PubMed] [Google Scholar]
- 69.Tu J, Ng SH, Luk AC, Liao J, Jiang X, Feng B, Lun Mak KK, Rennert OM, Chan WY, Lee TL. Microrna-29b/tet1 regulatory axis epigenetically modulates mesendoderm differentiation in mouse embryonic stem cells. Nucleic Acids Res. 2015;43:7805–7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Babicheva A, Ayon RJ, Zhao T, Ek Vitorin JF, Pohl NM, Yamamura A, Yamamura H, Quinton BA, Ba M, Wu L, et al. Microrna-mediated downregulation of k(+) channels in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2020;318:L10–L26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Tang Y, Liu X, Zhou Q, Xiao X, Lan F, Li X, Hu R, Xiong Y, Peng T. 14-3-3 tau (ywhaq) gene promoter hypermethylation in human placenta of preeclampsia. Placenta. 2014;35:981–988 [DOI] [PubMed] [Google Scholar]
- 72.Li X, Wu C, Shen Y, Wang K, Tang L, Zhou M, Yang M, Pan T, Liu X, Xu W. Ten-eleven translocation 2 demethylates the mmp9 promoter, and its down-regulation in preeclampsia impairs trophoblast migration and invasion. J Biol Chem. 2018;293:10059–10070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma M, Zhou QJ, Xiong Y, Li B, Li XT. Preeclampsia is associated with hypermethylation of igf-1 promoter mediated by dnmt1. Am J Transl Res. 2018;10:16–39 [PMC free article] [PubMed] [Google Scholar]
- 74.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P, Hassan SS, Kim CJ. Distinct subsets of micrornas are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196:261 e261–266 [DOI] [PubMed] [Google Scholar]
- 75.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, Kim E, Lee J, Kim SY, Draghici S, Mittal P, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Mir-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011;179:590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitehead CL, Teh WT, Walker SP, Leung C, Larmour L, Tong S. Circulating micrornas in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One. 2013;8:e78487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biro O, Fothi A, Alasztics B, Nagy B, Orban TI, Rigo J, Jr. Circulating exosomal and argonaute-bound micrornas in preeclampsia. Gene. 2019;692:138–144 [DOI] [PubMed] [Google Scholar]
- 78.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. Vii. Estrogen and progesterone effects on enos. Am J Physiol Heart Circ Physiol. 2001;280:H1699–1705 [DOI] [PubMed] [Google Scholar]
- 80.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R245–258 [DOI] [PubMed] [Google Scholar]
- 81.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27–R44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bavelloni A, Ramazzotti G, Poli A, Piazzi M, Focaccia E, Blalock W, Faenza I. Mirna-210: A current overview. Anticancer Res. 2017;37:6511–6521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


