Abstract
The present study used a novel mouse model with proximal tubule-specific knockout of AT1a receptors in the kidney, PT-Agtr1a−/−, to test the hypothesis that intratubular angiotensin II (Ang II) and AT1a receptors in the proximal tubules are required for maintaining normal blood pressure and the development of Ang II-induced hypertension. Twenty-six groups (n=6–15 per group) of adult male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice were infused with Ang II (1.5 mg/kg/day, i.p.), or overexpressed an intracellular Ang II fusion protein in the proximal tubules for 2 weeks. Basal telemetry blood pressure were ~15 ± 3 mmHg lower in PT-Agtr1a−/− than wild-type mice and ~13 ± 3 mmHg higher than Agtr1a−/− mice (P<0.01). Basal glomerular filtration was ~23.9% higher (P<0.01), whereas fractional proximal tubule Na+ reabsorption was lower in PT-Agtr1a−/− mice (P<0.01). Deletion of AT1a receptors in the proximal tubules augmented the pressure-natriuresis response (P<0.01), and natriuretic responses to salt loading or Ang III infusion (P<0.01). Ang II induced hypertension in wild-type, PT-Agtr1a−/− and PT-Nhe3−/− mice, but the pressor response was ~16 ± 2 mmHg lower in PT-Agtr1a−/− and PT-Nhe3−/−mice (P<0.01). Deletion of AT1a receptors or NHE3 in the proximal tubules attenuated ~50% of Ang II-induced hypertension in wild-type mice (P<0.01) but blocked intracellular Ang II fusion protein-induced hypertension in PT-Agtr1a−/− mice (P<0.01). Taken together, the results of the present study provide new insights into the critical role of intratubular Ang II/AT1 (AT1a)/NHE3 pathways in the proximal tubules in normal blood pressure control and the development of Ang II-induced hypertension.
Keywords: Angiotensin II, AT1a receptor, hypertension, pressure-Natriuresis, proximal Tubule
Summary
The present study used a novel mouse model to demonstrate for the 1st time that genetic deletion of AT1a receptors selectively in the proximal tubules of the kidney lowers basal blood pressure and attenuates Ang II-induced hypertension by promoting the pressure-natriuresis response and natriuretic responses to acute and chronic salt loading or to Ang III that preferably binds and stimulates AT2 receptors in the proximal tubules. These results provide new insights into the important role of the Ang II/AT1a/NHE3 pathway in the proximal tubules of the kidney in basal blood pressure control and the development of Ang II-dependent hypertension.
Graphical Abstract

High blood pressure, or hypertension, is well recognized as a multifactorial disorder with diverse genetic, neural, cardiovascular, renal, and lifestyle mechanisms. However, the kidney stands out as one of the most important organs significantly contributing to the regulation of blood pressure in health and the development of hypertension in disease.1–3 The primary role of the kidney is not only to get rid of all wastes and reabsorption of all vital electrolytes, nutrients and proteins, but also functions as an important endocrine organ to produce endocrine, paracrine and intracrine factors. 4–6 Indeed, the kidney expresses a robust intratubular renin-angiotensin system (RAS) with all major components, including angiotensinogen (AGT), renin, angiotensin-converting enzyme (ACE), angiotensin II (Ang II), AT1 (AT1a) and AT2 receptor. 7,8 The expression of the intratubular RAS is especially predominant in the early nephron segments of the kidney, namely the proximal tubules.7,8 Although the proximal tubules also generate Ang I, Ang (1–7), Ang III, or Ang (3–8) and express receptors or binding sites for these Ang peptides, 6,9,10 Ang II via activation of AT1 (AT1a) receptors and the Na+/H+ exchanger 3 (NHE3) plays the most predominant role in regulating proximal tubule sodium (Na+) and fluid reabsorption that contributes to overall blood pressure homeostasis and body salt and fluid balance. 11–14
We and others have previously shown that Ang II exerts distinct biphasic actions on the expression or activity of the sodium (Na+) and hydrogen (H+) exchanger 3 (NHE3) in cultured proximal tubule cells in vitro, and proximal tubular sodium reabsorption in vivo.15–17 In vivo studies with free flow micropuncture or microperfusion of proximal tubules have shown that physiological concentrations of Ang II stimulates, whereas pathophysiological levels of Ang II inhibits proximal tubular Na+ transport in rats or mice.18,19 It is less clear, however, whether and how these in vitro or local actions of Ang II via activation of AT1 (AT1a) receptors in the proximal tubules would impact on basal blood pressure homeostasis and the development of Ang II-dependent hypertension. It has been suggested that the distal nephron tubular segments perhaps play more important long-term roles because any alterations or perturbations of proximal tubular Na+ reabsorption are expected to be fully compensated by the actions of downstream tubular segments. 20 However, two recent studies using genetically modified, proximal tubule-specific, AT1a receptor-deficient mouse models showed that intratubular Ang II and AT1a receptors directly regulate blood pressure with or without compensatory expression of NHE3, NaPi2 (the sodium and phosphate cotransporter 2), Na+/K+-ATPase, NCC (the sodium chloride cotransporter), NKCC2 (the sodium and potassium cotransporter 2), ENaC (the epithelial sodium channel) in proximal tubular or distal nephron segments. 11,12 Both studies used the Cre/LoxP approach to generate mutant mice with proximal tubule-specific AT1a-deficient mice, with Gurley et al. using the PEPCK-Cre/Agtr1aflox11 and Li et al. using the KAP2-iCre/Agtr1aflox approach, respectively. 12 However, PEPCK reportedly not only expresses in the proximal tubules, but also in other nephron segments of the kidney, as well as in epithelial cells in many extrarenal tissues, such as liver, white and brown fat tissues, jejunum, ileum, and sublingual gland. 21,22 KAP2, the kidney androgen-regulated protein, is expressed not only in the proximal tubules in the outer medulla, but also in the uterus. 23,24 Whether and to what extent the PEPCK or KAP2 promoter-driven deletion of AT1a receptors in the proximal tubules of the kidney and extrarenal tissues would alter the basal blood pressure phenotype and the development of Ang II-dependent hypertension as well as underlying mechanisms involved remain poorly understood.
Against this background, we have generated a novel mutant mouse model with proximal tubule-specific deletion of AT1 (AT1a) receptors in the kidney using the iL1-SGLT2-Cre/Agtr1aflox approach. The scientific premise is that the SGLT2 (the sodium and glucose cotransporter 2) promoter is very specific to the proximal tubules, because SGLT2 is expressed primarily, if not exclusively, in the S1 and S2 segments of the proximal tubules, and little Sglt2 is expressed beyond the end of S3 segment of the proximal tubules, i.e., in distal nephron segments in the medulla and extrarenal tissues. 25,26 In the present study, we used this new mutant mouse model to test the hypothesis that intratubular Ang II and AT1 (AT1a) receptors in the proximal tubules are required for maintaining basal blood pressure homeostasis and the full development of Ang II-dependent hypertension. Specifically, we systemically determined whether proximal tubule-specific deletion of AT1a receptors in the kidney will: a) lower basal blood pressure; b) alter glomerular filtration; c) augment the pressure-natriuresis response; d) increase the natriuretic responses to acute or chronic salt loading or to Ang III, and e) attenuate circulating/extracellular and intratubular/intracellular Ang II-induced hypertension. The results of the present study provide new evidence for and new insights into the critical role of intratubular Ang II/AT1 (AT1a) receptors/NHE3 pathway in the proximal tubules in the physiological regulation of blood pressure and the full development of Ang II-dependent hypertension in mice.
Methods
The authors will make all methods and materials including adenoviral constructs, in vivo transfection protocols, protocols for genotyping mutant mice with global or proximal tubule-specific deletion of AT1a receptors or NHE3, surviving and non-surviving surgical protocols, experimental protocols, and all supporting raw data available to other researchers. A detailed section of Methods and Materials is provided in the ONLINE SUPPLEMENT.
Animals
Wild-type (C57BL/6J or littermates) and global AT1a receptor-deficient mice, Agtr1a−/− or B6.129P2-Agtr1atm1Unc/J (Stock No: 002682), were purchased from Jackson Laboratories and bred in this laboratory as previously described.27,28 Proximal tubule-specific AT1a receptor-deficient mice, PT-Agtr1a−/−, were generated and bred in the this laboratory using the iL1-Sglt2-Cre/Agtr1a-floxed approach, as we described recently for PT-Nhe3−/− mice.13,14 The iL1-Sglt2-Cre mice were originally obtained from Rubera et al., 25 whereas Agtr1a-floxed mice, C57BL/6N-Agtr1atm1Uky/J (Stock No: 016211), were generated by Rateri et al. 29 and purchased from Jackson laboratories. C57BL/6N-Agtr1atm1Uky/J mice were crossed with iL1-Sglt2-Cre mice to generate PT-Agtr1a−/− mice for the studies in the present study (Figures S1–S3). The use of adult male (>12-week-old) wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice were approved by the Institutional Animal Care and Use Committees of the University of Mississippi Medical Center and Tulane University School of Medicine, respectively.
Experimental Protocols
Basal blood pressure, glomerular filtration rate (GFR), proximal tubule Na+ reabsorption, and urinary Na+ excretion phenotypes in conscious adult male wild-type, Agtr1a−/−, PT-Agtr1a−/− mice
Two groups (n=12–14) of adult male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice were used to determine their basal systolic, diastolic and mean arterial blood pressure using both implanted telemetry (Data Science) and noninvasive tail-cuff approach (Visitech, NC).13,14,27,28 Two groups (n=9–11) of wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice were used to determine their whole-kidney glomerular filtration rate (GFR) using the transdermal GFR monitoring approach with FITC-sinistrin, as described (Figure S4).13,14,30 Two additional groups (n=8–11) of wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice were used to determine the whole-kidney proximal tubule Na+ reabsorption, 24 h drinking, diet, urinary Na+, K+, Cl- excretion and urine osmolality using metabolic cage and the noninvasive lithium clearance technique as we described previously. 13,14,17,27
Glomerular and proximal tubule ultrastructure in adult male WT, global Agtr1a−/−, and PT-Agtr1a−/− mice
To determine whether deletion of AT1a receptors may alter the ultrastructure of histological phenotypes, one group each (n=8–10) of adult male wild-type, global Agtr1a−/− and PT-Agtr1a−/− mice were euthanized, the kidneys collected and processed for high resolution electron microscopic imaging of the glomerular and proximal tubular ultrastructure.13,14,31 Glomerular mesangial, endothelial, and epithelial cells and podocytes, as well as brush border membranes, microvillar, and mitochondria of proximal tubules were closely examined at 6,430X for glomeruli and 31,900X for the proximal tubules, respectively. 13,14,31
The pressure-natriuresis response in adult male wild-type and PT-Agtr1a−/− mice
To determine whether deletion of AT1a receptors selectively in the proximal tubules of the kidney augments the pressure-natriuresis response, two groups (n=7–9) of adult male wild-type and PT-Agtr1a−/− mice were anesthetized, and prepared for the standard pressure-natriuresis studies, as described. 13,14,32
The natriuretic responses to acute saline expansion and a high salt diet in adult male wild-type and PT-Agtr1a−/− mice
To determine whether proximal tubule-specific deletion of AT1a receptors in the kidney would augment the acute natriuretic response to acute saline expansion, three groups of wild-type and PT-Agtr1a−/− mice (n=8–12 per group) were treated with or without intraperitoneal injection of saline (0.9% NaCl), or water (H2O), at 10% body weight.13,14 In the chronic experimental protocol, two groups of wild-type and PT-Agtr1a−/− mice (n=6–10 per group) were treated with or without 2% NaCl for 2 weeks. 13,14 Their natriuretic responses were compared between wild-type and PT-Agtr1a−/− mice accordingly.
The blood pressure response to intratubular overexpression of an intracellular Ang II fusion protein selectively in the proximal tubules of adult male wild-type, global Agtr1a−/− and PT-Agtr1a−/− mice
To determine the roles of systemic, intratubular or intracellular AT1a receptors, two groups of male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice (n=8–11 per group) were anesthetized and the kidneys exposed to induce adenovirus-mediated overexpression of an intracellular cyan fluorescent Ang II fusion protein, Ad-Sglt2-ECFP/Ang II, selectively in the proximal tubules. 28,31,33 Systolic, diastolic and mean blood pressure responses were measured at baseline and weekly after the expression of Ad-Sglt2-ECFP/Ang II was induced selectively in the proximal tubules for 2 weeks.
The development of Ang II-induced hypertension in adult male wild-type, global Agtr1a−/−, PT-Agtr1a−/− and PT-Nhe3−/− mice
To determine whether deletion of AT1a receptors or NHE3 in the proximal tubules attenuate Ang II-induced hypertension, both acute and long-term pressor responses to Ang II was determined under anesthetized and conscious conditions, respectively. Two groups each of male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice (n=7–9 per group) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and cannulated with catheters in the left carotid artery and jugular vein for measurement of intraarterial blood pressure, and for the infusion of saline or increasing pressor doses of Ang II (10, 50, and 100 ng/min, i.v.), as we described.14,17,34 Three groups (n=12–14 per group) of adult male wild-type, global Agtr1a−/− and PT-Agtr1a−/−, or PT-Nhe3−/− mice were infused with or without a pressor dose of Ang II via an osmotic minipump (1.5 mg/kg/day, i.p.) for 2 weeks, or concurrently treated with the AT1 receptor blocker losartan (20 mg/kg/day, p.o.), as we described previously. 14,17,34
Role of reactive oxygen species or superoxide in Ang II-induced hypertension in adult male wild-type and PT-Agtr1a−/− mice
To determine whether genetic deletion of AT1a receptors selectively in the proximal tubules of the kidney attenuates Ang II-induced hypertension via interactions with ROS or superoxide, two groups (n=7–10 per group) of adult male wild-type and PT-Agtr1a−/− mice were treated without or with Tempol, a superoxide dismutase-mimetic (50 mg/kg/day, p.o.) for 2 weeks. Systolic, diastolic, and mean arterial pressure, and 24 h urinary Na+ and K+ excretion was determined before and weekly after Tempol treatment.35
The natriuretic responses to Ang III in adult male wild-type and PT-Agtr1a−/− mice
In the proximal tubules of the kidney, Ang III reportedly binds and stimulates AT2 receptors to induce a natriuretic response.9,36 To determine whether the natriuretic response to Ang III is augmented in PT-Agtr1a−/− mice, adult male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice (n =6 for each group) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and infused with saline or increasing doses of Ang III, at 10, 50, and 100 ng/min, i.v., respectively, for 60 min each. The blood pressure and natriuretic responses to Ang III were determined. Three additional groups of wild-type and PT-Agtr1a−/− mice (n=5–8) were infused without or with Ang III or concurrent treatment of Ang III and the AT2 receptor blocker PD123319 (20 mg/kg/day, p.o.) at an equivalent pressor dose of Ang II, i.e., 1.5 mg/kg body wt./day, i.p., via osmotic minipump for 2 weeks. The blood pressure and natriuretic responses to Ang III were determined at baseline and weekly after Ang III infusion. 14,17,33,34
Statistical analysis
All experimental data are presented as mean ± SEM. The differences in all responses between different groups of male and female wild-type, global Agtr1a−/− and PT-Agtr1a−/− mice including systolic, diastolic and mean arterial pressure, 24 h urinary Na+ excretion, the pressure-natriuretic response, the hypertensive responses to Ang II, and the natriuretic responses to salt loading or Ang III were first analyzed using one-way ANOVA, followed by Student’s unpaired t test if a significant response between groups of wild-type and PT-Agtr1a−/− mice was detected. The significance of statistical differences between responses were set P<0.05.
Results
Effects of proximal tubule-specific deletion of AT1a receptors in the kidney on basal general phenotypes in PT-Agtr1a−/− mice
Figure S2 shows specific genotyping results in PT-Agtr1a−/− mice with an expected mutant band at 262 bp, compared with expected bands at 262 and and 202 bp, respectively, in heterozygous mutant mice, and an expected wild-type band at 202 bp, respectively. Figure S3 confirms that compared with wild-type mice, AT1a receptor expression in the proximal tubules of the kidney was nearly undetectable in wild-type mice, and markedly decreased by >90% in PT-Agtr1a−/− mice. Table S1 summarizes and compares general phenotypes in age- and body weight-matched adult male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice. Compared with wild-type mice, proximal tubule-specific deletion of AT1a receptors had no effects on body wt., heart wt., kidney wt., the heart wt. to or kidney wt. to body wt. ratios, drinking and hematocrit. However, blood volume, urinary Na+, K+, and Cl- concentrations were signicicantly increased over those of wild-type mice, whereas urine osmolality was significantly decreased in PT-Agtr1a−/− mice. Global deletion of AT1a receptors also significantly increased urinary Na+, K+, and Cl- concentrations and decreased urine osmolality, which were greater than in PT-Agtr1a−/− mice (Table S1).
Effects of proximal tubule-specific deletion of AT1a receptors in the kidney on glomerular and proximal tubular ultrastructures in PT-Agtr1a−/− mice
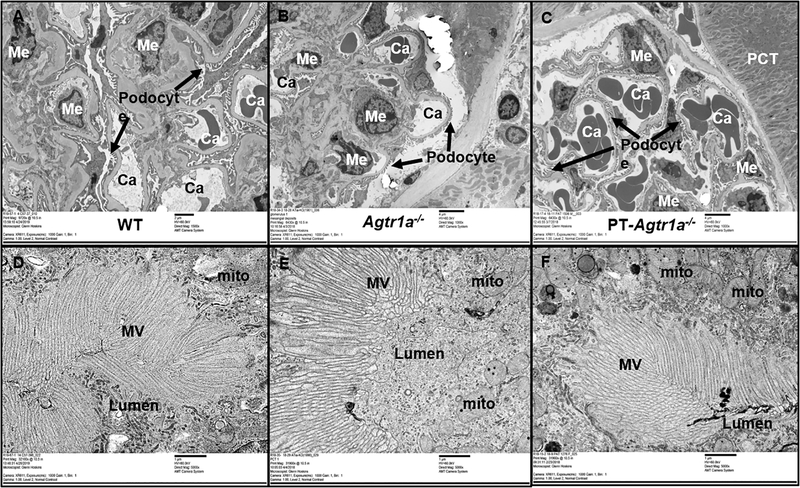
Figure 1 shows high resolution electron microscopic images of the representative glomerular and proximal tubular ultrastructures in wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice. As a general rule, glomerular and proximal tubular ultrastructures of wild-type mice were used for comparisons. Glomerular capillaries, epithelial cells, endothelial cells, mesangial cells, and podocytes were all clearly visualized and tightly organized in wild-type mice (Fig. 1A). In the proximal tubules of wild-type mice (Fig. 1D), the lumen, microvillars of the brush borders, and mitochondria were all normally organized or oriented toward the lumen, as expected. In global Agtr1a−/− mice, glomerular endothelial, epithelial and mesangial cells were largely similar to those of wild-type mice, but podocytes were thinner, whereas Bowman’s capsule was wider and the basement membrane thickened, compared with wild-type mice (Fig. 1B). The proximal tubular lumen opened, whereas the microvillars are slightly thicker, possibly due to low blood pressure or low glomerular perfusion pressure in global Agtr1a−/− mice (Fig. 1E). In PT-Agtr1a−/− mice, glomerular (Fig. 1C) and proximal tubular ultrastructures (Fig. 1F) were similar to those of wild-type mice.
Figure 1.
The effects of global or proximal tubule-specific deletion of AT1a receptors in the kidney on glomerular and proximal tubular ultrastructure in adult male wild-type (WT), global Agtr1a−/−, and PT-Agtr1a−/− mice. A & D: high resolution electron microscopic (EM) micrographs showing the ultrastructure of representative glomerulus (9,720 X) and proximal tubules (32,100 X) in adult male WT mice. B & E: EM micrographs showing the ultrastructure of representative glomerulus (6,430 X) and proximal tubules (32,100 X) in adult male Agtr1a−/− mice. C & F: EM micrographs showing the ultrastructure of representative glomerulus (6,430 X) and proximal tubules (32,100 X) in adult male PT-Agtr1a−/− mice. In global Agtr1a−/− mice, glomerular endothelial, epithelial and mesangial cells were largely similar to those of WT mice, but podocytes were thinner, whereas Bowman’s capsule was wider and the basement thickened, compared with WT mice. There were no significant differences in glomerular and proximal tubular ultrastructure between wild-type and PT-Agtr1a−/− mice. Abbreviations: Ca, capillaries; Me, mesangial cell; Mito, mitochondria; MV, microvilli of the proximal tubule; N=6 per group.
Proximal tubule-specific deletion of AT1a receptors in the kidney lowers basal blood pressure, and increases glomerular filtration rate and urinary natriuretic responses in PT-Agtr1a−/− mice
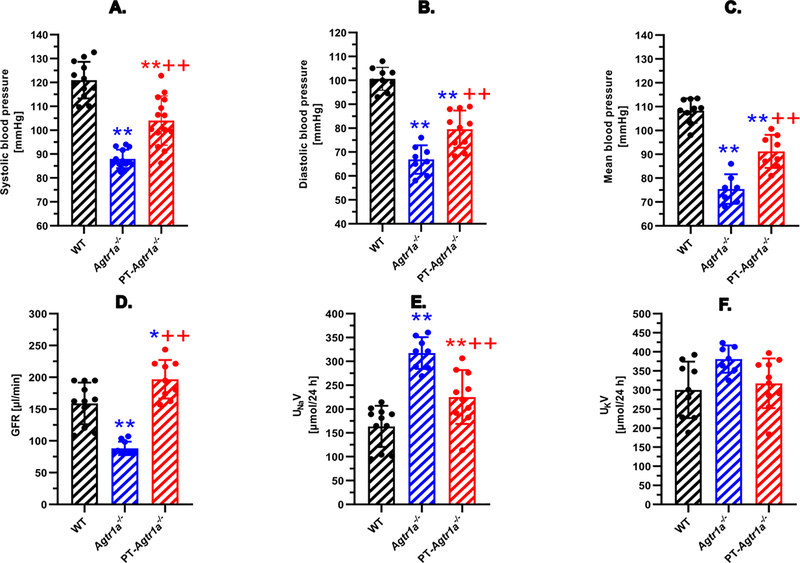
Figure 2 compares basal systolic (A), diastolic (B), and mean arterial blood pressure (C), glomerular filtration rate (GFR) (D), and 24 h urinary Na+ (E) and K+ excretion rates (F) in wild-type (WT), global Agtr1a−/−, and PT-Agtr1a−/− mice. Deletion of AT1a recetors in all tissures of global Agtr1a−/− mice markedly decreased systolic, diastolic, and mean blood pressure by ~ 30 ± 4 mmHg (n=13, P<0.01 vs. WT, n=12). Deletion of AT1a receptors selectively in the proximal tubules of PT-Agtr1a−/− mice, however, significantly lowered basal systolic, diastolic, and mean blood pressure by ~ 15 ± 3 mmHg (n=14, P<0.01 vs. WT or Agtr1a−/−). GFR was markedly decreased in global Agtr1a−/− mice, as expected with markedly decreased blood pressures and renal perfusion pressure (n=10, P<0.01 vs. WT, n=11) (D). Interestingly, GFR was significantly and consistently increased in PT-Agtr1a−/− mice by ~23% (n=9, P<0.01 vs. WT or Agtr1a−/− mice). Likewise, 24 h urine excretion (not shown) and urinary Na+ excretion were significantly increased in global Agtr1a−/− (n=8, P<0.01 vs. WT) and PT-Agtr1a−/− mice (n=10, P<0.01 vs. WT). 24 h urinary K+ excretion remained unaltered.
Figure 2.
Basal systolic, diastolic and mean arterial blood pressure, glomerular filtration rate (GFR), and urinary Na+ and K+ excretory phenotypes in adult male wild-type, global Agtr1a−/−, and PT-Agtr1a−/− mice. Global deletion of AT1a receptors decreases basal blood pressure by ~30 ± 3 mmHg in Agtr1a−/− mice, whereas proximal tubule-specific deletion of AT1a receptors lowers basal blood pressure by ~15 ± 3 mmHg in PT-Agtr1a−/−mice (A-C). Global deletion of AT1a receptors decreases in Agtr1a−/− mice, whereas proximal tubule-specific deletion of AT1a receptors increases GFR in PT-Agtr1a−/− mice, respectively (D). Both global and proximal tubule-specific deletion of AT1a receptors induced significant natriuresis (E) without altering K+ excretion (F). *P<0.05 or **P<0.01 vs. WT mice; ++P<0.01 vs. global Agtr1a−/− mice.
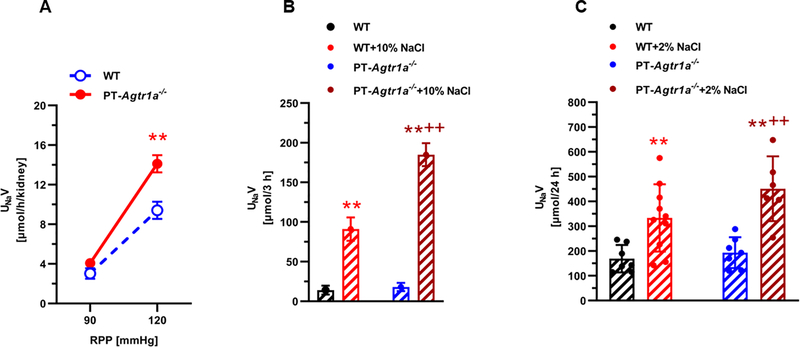
Proximal tubule-specific deletion of AT1a receptors in the kidney augments the pressure-natriuresis response and natriuretic responses to acute saline expansion and chronic high salt diet
Proximal tubule-specific deletion of AT1a receptors in the kidney significantly augmented the pressure-natriuresis response to an increase of renal perfusion pressure of ~30 mmHg in PT-Agtr1a−/− mice (P<0.01, n=8) (Fig. 3). Indeed, urinary Na+ excretion (UNaV) increased from 3.03 ± 0.14 at ~ 90 mmHg to 9.41 ± 0.17 μmol/per h/per kidney at ~120 mmHg in wild-type mice (P<0.01). By comparison, urinary Na+ excretion increased from 4.07 ± 0.33 at ~ 90 mmHg to 14.10 ± 0.88 μmol/per h/per kidney at ~120 mmHg in PT-Agtr1a−/− mice (P<0.01) (Fig. 3A). In response to acute saline expansion (10% of body wt., i.p.), urinary Na+ excretion (UNaV) increased ~6.5-fold in wild-type mice, whereas it increased >10-fold in PT-Agtr1a−/− mice (P<0.01 vs. WT) (Fig. 3B). When fed with 2% high salt diet for 2 weeks, UNaV increased ~1.9-fold in wild-type mice, whereas it increased 2.5-fold in PT-Agtr1a−/− mice (P<0.01 vs. WT), respectively (Fig. 3C).
Figure 3.
Proximal tubule-specific deletion of AT1a receptors in the kidney augments the pressure-natriuresis response (A) and the natriuretic response to acute saline expansion (B) or chronic high salt diet (C) in adult male PT-Agtr1a−/− mice. In response to an increase of ~30 mmHg in renal perfusion pressure (RPP), the pressure-natriureiss response increased ~3-fold in wild-type mice, whereas the response increased ~4-fold in PT-Agtr1a−/− mice (A, **P<0.01). In response to 10% saline expansion, the natriuretic response increased >6-fold in wild-type, but 10-fold in PT-Agtr1a−/− mice (B, **P<0.01 vs. control; ++P<0.0 vs. wild-type). Finally in response to 2% high salt diet for 2 weeks, 24 h urinary Na+ excretion increased 1.9-fold in Agtr1a−/− and 2.5-fold in PT-Agtr1a−/− mice, respectively (C, **P<0.01 vs. control; ++P<0.0 vs. wild-type).
Proximal tubule-specific deletion of AT1a receptors in the kidney attenuates intracellular Ang II- or systemic/extracellular Ang-induced hypertension in PT-Agtr1a−/− mice
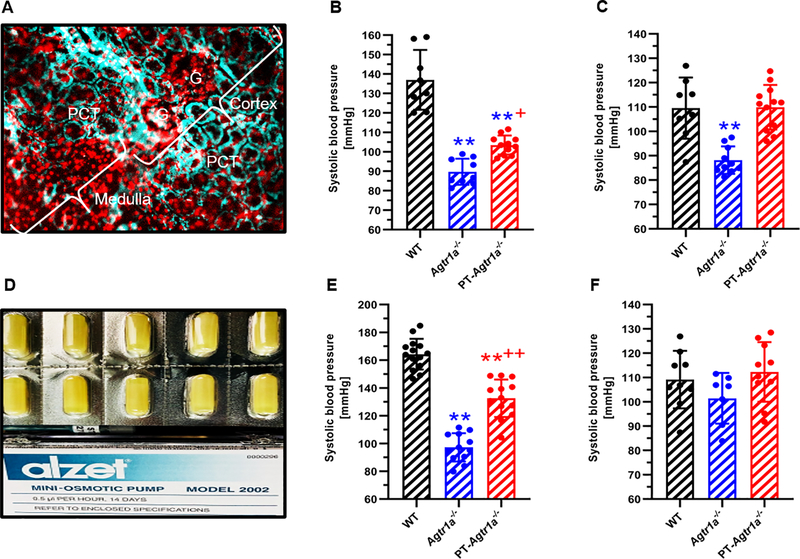
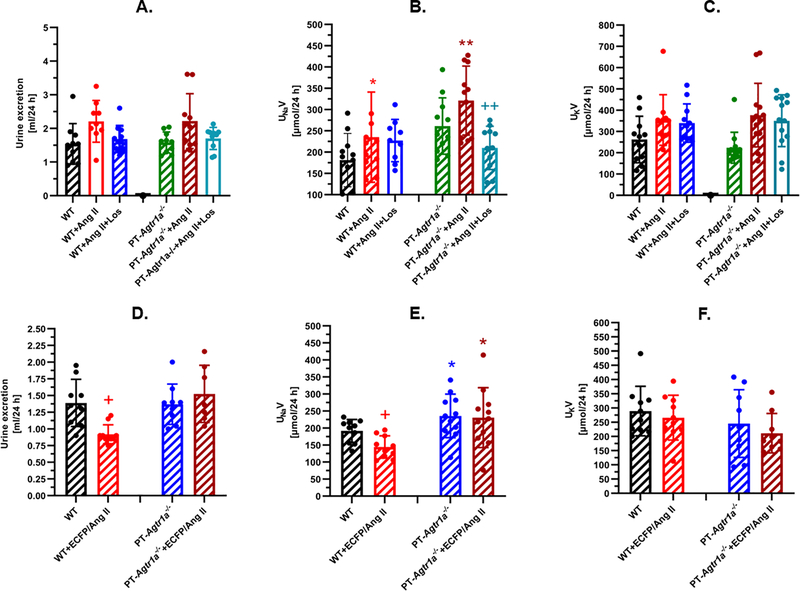
Figure 4A–4C show the effect of proximal tubule AT1a receptor knockout on the blood pressure response to adenovirus-mediated overexpression of an intracellular Ang II fusion protein, Ad-sglt2-ECFP/Ang II, selectively in the proximal tubules of the kidney. In wild-type mice, the expression of Ad-sglt2-ECFP/Ang II in the proximal tubulels increased blood pressure from 118 ± 3 mmgHg to 137 ± 5 mmHg (n=8, P<0.01 vs. control) (Fig. 4B). The expression of Ad-sglt2-ECFP/Ang II in the proximal tubules had no effect on blood pressure in global Agtr1a−/− mice (90 ± 3 mmHg, n=9, n.s.) (Fig. 4B). By comparison, the blood pressure-elevating effect of Ad-sglt2-ECFP/Ang II expression in the proximal tubules was completely attenuated in PT-Agtr1a−/− mice (105 ± 5 mmHg, n=11, P<0.01 vs. WT) (Fig. 4B). Concurrent treatment with losartan completely blocked the blood pressure-elevating effects of Ad-sglt2-ECFP/Ang II expression selectively in the proximal tubules of the kidney (Fig. 4C).
Figure 4.
Proximal tubule-specific deletion of AT1a receptors in the kidney significantly attenuates hypertension induced by adenovirus-mediated overexpression of an intracellular Ang II fusion protein, Ad-sglt2-ECFP/Ang II, selectively in the proximal tubules of the kidney (A-C), or by systemic infusion of Ang II (D-F). Please note that systemic infusion of a pressor dose of Ang II for 2 weeks (1.5 mg/kg/day, i.p.) increased blood pressure >45 mmHg in wild-type mice, and this response to Ang II was halved in PT-Agtr1a−/− mice (B). Concurrent treatment of Ang II-infused wild-type or PT-Agtr1a−/− mice with the AT1 receptor blocker losartan (20 mg/kg/day, p.o.) normalized blood pressure to the basal level of wild-type mice (C). In response to proximal tubule-specific expression of Ad-sglt2-ECFP/Ang II, blood pressure increased 17 ± 3 mmHg in wild-type mice, and the response was blocked completely in PT-Agtr1a−/− mice (E), or by losartan (F). Ang II or Ad-sglt2-ECFP/Ang II had no effect on blood pressure in global Agtr1a−/− mice (B & E). **P<0.01 vs. wild-type; +P<0.05 or ++P<0.01 vs. Agtr1a−/− mice.
In response to a pressor dose (1.5 mg/kg/day, i.p.) of Ang II infusion for 2 weeks, systolic blood pressure increased ~43 ± 3 mmHg in wild-type mice (n=14, P<0.01, Fig. 4E) and the pressor response was blocked completely by concurrent treatment with the AT1 receptor blocker losartan (20 mg/kg/day, p.o.) (109 ± 4 mmHg, n=9, P<0.01 vs. Ang II) (Fig. 4F). In global Agtr1a−/− mice, the pressor response to Ang II was completely blocked, as expected (97 ± 3 mmHg, n=12, P<0.01 vs. WT)(Fig. 4E). In PT-Agtr1a−/− mice, the pressor response to Ang II was significantly attenuated by about 50% of wild-type mice to 133 ± 4 mmHg (n=13, P<0.01 vs. WT) (Fig. 4E), which was further normalized to control with concurrent losartan treatment (113 ± 4 mmHg, n=10, P<0.01 vs. Ang II) (Fig. 4F).
Proximal tubule-specific deletion of AT1a receptors in the kidney increases the natriuresis response to Ang II-induced hypertension in PT-Agtr1a−/− mice
Infusion of Ang II for 2 weeks induced small, but not significant, diuretic effects in both wild-type and PT-Agtr1a−/− mice (Fig. 5A), but increased 24 h urinary Na+ excretion in both wild-type and PT-Agtr1a−/− mice (P<0.05, Fig. 5B). 24 h urinary K+ excretion tended to be higher, but not significantly (Fig. 5C). Interestingly, losartan attenuated the natriuretic response to Ang II in PT-Agtr1a−/− mice, possibly due to the hypotensive effect of losartan (Fig. 5B). The expression of Ad-sglt2-ECFP/Ang II in the proximal tubules of the kidney decreased 24 h urine excretion (Fig. 5D) and urinary Na+ excretion (Fig. 5E) in wild-type (P<0.05 vs. control), but not in PT-Agtr1a−/− mice (Fig. 5D). However, both urinary water and Na+ excretion remained significantly higher in PT-Agtr1a−/− mice (P<0.05 vs. WT). Urinary K+ excretion was unaltered in response to Ad-sglt2-ECFP/Ang II expression in the proximal tubules of wild-type and PT-Agtr1a−/− mice (Fig. 5F).
Figure 5.
Effects of proximal tubule-specific deletion of AT1a receptors in the kidney on 24 h urine excretion, urinary Na+ and K+ excretion in adult male PT-Agtr1a−/− mice. Note that systemic Ang II infusion moderately increased 24 h urine and urinary Na+ excretion in both wild-type and PT-Agtr1a−/− mice (A & B) without altering urinary K+ excretion (C). By contrast, adenovirus-mediated expression of Ad-sglt2-ECFP/Ang II selectively in the proximal tubules induced antidiuretic and antinatriuretic responses in wild-type mice, but not in PT-Agtr1a−/− mice (D & E). *P<0.05 or **P<0.01 vs. wild-type mice. +P<0.05 or ++P<0.01 vs. wild-type control (D & E) or vs. Ang II in PT-Agtr1a−/− mice (B).
Role of proximal tubule NHE3 in Ang II-induced hypertension in PT-Agtr1a−/− mice
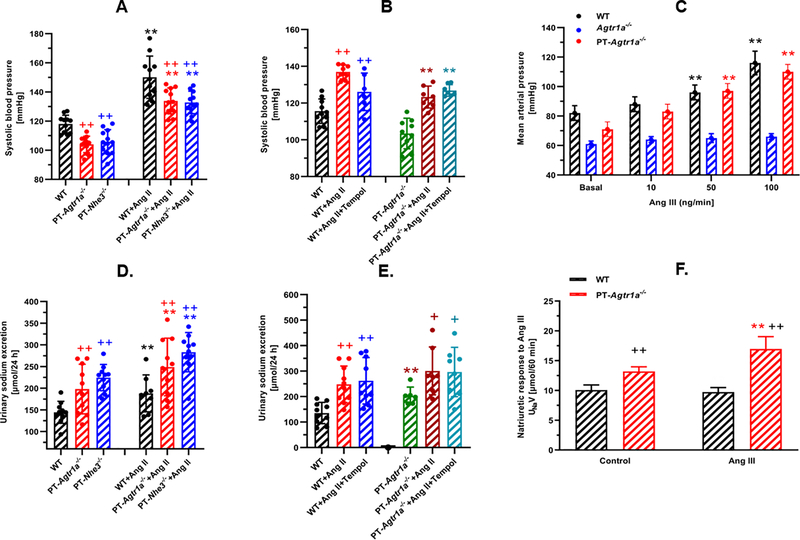
Since NHE3 in the proximal tubules is the downstream target of intratubular Ang II via activation of AT1 (AT1a) receptors, we compared basal blood pressure levels and the pressor response to Ang II-induced hypertension between PT-Agtr1a−/− and PT-Nhe3−/− mice. Figure 6A shows that there were no significant differences in basal blood pressure levels as well as their responses to 2-week infusion of a pressor dose of Ang II in PT-Agtr1a−/− and PT-Nhe3−/− mice. 24 h urinary Na+ excretion was also similar between PT-Agtr1a−/− and PT-Nhe3−/− mice (Fig. 6D).
Figure 6.
Roles of the Na+/H+ exchanger 3 (NHE3), superoxide, and Ang III in the blood pressure and natriuretic responses to Ang II-induced hypertension in PT-Nhe3−/− and PT-Agtr1a−/− mice. Note that proximal tubule-specific deletion of either NHE3 or AT1a receptors in the kidney lowered basal blood pressure and attenuated Ang II-induced hypertension to a similar extent (A), and induced natriuretic responses to a similar degree under basal conditions and during Ang II-induced hypertension (D). Concurrent Tempol treatment had no effect on Ang II-induced hypertension (B) and natriuretic responses in wild-type and PT-Agtr1a−/− mice (E). At physiological concentration (10 ng/min, i.v.), Ang III had no effect on blood pressure (C), but induced a significant natriuretic response in PT-Agtr1a−/− mice (F). **P<0.01 vs. control wild-type (A), PT-Nhe3−/− or PT-Agtr1a−/− mice. +P<0.05 or ++P<0.01 vs. their respective controls or during Ang II infusion.
Role of superoxide in Ang II-induced hypertension in PT-Agtr1a−/− mice
In wild-type mice, concurrent treatment of Ang II-infused mice with a low and slow pressor dose (0.5 mg/kg/day, i.p.) and tempol (50 mg/kg/day, p.o.) for 2 weeks led to attenuation of blood pressure by ~15 ± 3 mmHg (Ang II: 141 ± 5 mmHg, n=10 vs. Ang II+Tempol: 126 ± 4 mmHg, n=6, P<0.05) (Fig. 6B). Tempol treatment, however, had no effect on Ang II-induced hypertension in PT-Agtr1a−/− mice (Ang II: 127 ± 3 mmHg, n=7 vs. Ang II+Tempol: 129 ± 4 mmHg, n=7, n.s.) (Fig. 6B). Tempol treatment also had no effects on 24 h urinary Na+ excretion in Ang II-infused WT and PT-Agtr1a−/− mice (Fig. 6D).
Proximal tubule-specific deletion of AT1a receptors in the kidney augments the natriuretic response to Ang III in anesthetized PT-Agtr1a−/− mice
Acute systemic infusion of Ang III induced concentration-dependent increases in mean arterial pressure in anesthetized wild-type and PT-Agtr1a−/− mice (Fig. 6C). In response to the lowest dose at 10 ng/min, Ang III has no significant effect on mean arterial pressure in wild-type and PT-Agtr1a−/− mice. When Ang III was infused at higher pharmacological concentrations, 50 ng/min and 100 ng/min, respectively, mean arterial pressure increased proportionally in wild-type and PT-Agtr1a−/− mice. However, the pressor response to Ang III was still significantly smaller than that induced by equivalent concentrations of Ang II (P<0.01). For example, blood pressure increased to above 160 mmHg in response to Ang II at 100 ng/min (not shown). Infusion of Ang III for 2 weeks at 1.5 mg/kg/day, i.p., slightly increased systolic blood pressure by ~8 ± 2 mmHg in WT mice (n=11, P<0.05), but not in PT-Agtr1a−/− mice (n=11, n.s.) (Figure S5). Concurrent treatment with the AT2 receptor blocker PD123319 at 20 mg/kg/day, p.o., had no further effect on Ang III-induced blood pressure responses in either wild-type or PT-Agtr1a−/− mice (Figure S5). Figure 6F show the urinary Na+ excretory responses (UNaV) to Ang III infusion in anesthetized wild-type and PT-Agtr1a−/− mice. Ang III infusion at 50 ng/min, i.v. had no effect on the natriuretic response in wild-type, but significantly increase the natriuretic response in PT-Agtr1a−/− mice (P<0.01 vs. WT). PD123319 slightly, but not significantly, alter the natriuretic response to Ang III in anesthetized wild-type or PT-Agtr1a−/− mice (not shown).
Discussion
Using a novel mouse model with genetic deletion of AT1a receptors selectively in the proximal tubules of the kidney, the present study mechanistically tested several hypotheses on: a) whether intratubular Ang II and AT1a receptors in the proximal tubules of the kidney are required for maintaining basal blood pressure homeostasis; b) whether intratubular Ang II and AT1a receptors in the proximal tubules are required for the development of Ang II-induced hypertension; c) whether the natriuretic responses to the increased pressure, acute saline expansion, or chronic high salt diet are augmented after the AT1a receptor is deleted from the proximal tubules; d) whether the Na+/H+ exchanger 3 (NHE3) in the proximal tubules and superoxide are required for Ang II-induced hypertension in PT-Agtr1a−/− mice; and e) whether the natriuretic response to Ang III is augmented after the AT1a receptor is deleted from the proximal tubules. The results of the present study clearly demonstrate that deletion of AT1a receptors selectively in the proximal tubules resulted in significant decreases of basal systolic, diastolic and mean arterial pressure by ~15 ± 3 mmHg in PT-Agtr1a−/− mice, compared with a decrease of ~30 ± 3 mmHg in adult male, age- and body wt.-matched global Agtr1a−/− mice. 27,28,37,38 Deletion of AT1a receptors selectively in the proximal tubules also significantly attenuated the hypertension in PT-Agtr1a−/− mice, induced either by a pressor dose of systemic Ang II infusion, or by adenovirus-mediated overexpression of an intracellular Ang II fusion protein, Ad-sglt2-ECFP/Ang II, selectively in the proximal tubules. Our results further demonstrate that these effects are associated with the augmentation of the natriuretic responses to increased blood pressure, acute saline expansion, chronic salt loading, and to Ang III that preferable binds and activates AT2 receptors in the proximal tubules.2,9,36 These phenotypic responses in PT-Agtr1a−/− mice are very similar to the mutant mice with proximal tubule-specific deletion of NHE3 in the kidney.13,14 Taken together, the present study strongly supports a critical role of intratubular Ang II and AT1a receptors in the proximal tubules in maintaining physiological blood pressure as well as in the development of Ang II-dependent hypertension.
Intratubular Ang II and AT1 (AT1a) receptors in the proximal nephron have previously been suggested to play a key role in the physiological regulation of sodium and fluid reabsorption that may directly or indirectly contribute to the blood pressure regulation and the development of hypertension. 5,7,16,17,19 However, this understanding is mostly indirect, because a direct cause and effect relationship is difficult to be determined using in vivo micropuncture or in vitro microperfusion of a single proximal tubule or using the whole-kidney integrative approach. Recently, a number of studies have used novel, state of the art approaches to determine the direct roles of AT1a receptors in the proximal tubules of the kidney.11,12,39 Crowley and his colleagues performed cross-kidney transplantation between wild-type and global Agtr1a−/− mice, and demonstrated for the 1st time virtually equivalent contributions of intrarenal versus extrarenal AT1a receptors to basal blood pressure homeostasis. 39 Gurley et al. 11 and Li et al. 12 used the PEKCK-Cre/Agtr1a-LoxP and KAP2-Cre/Agtr1a-LoxP approaches, respectively, to generate mutant mouse models with proximal tubule-specific deletion of AT1a receptors in the kidney. Both studies showed that their mouse models lowered basal blood pressure ~10 ± 3 mmHg than wild-type littermates. For the unknown reasons, one study found that there were no differences in the maximal pressor responses to bolus injection of pharmacological doses of Ang II, i.e., 10 μg/kg, i.v.,11 whereas the other reported no differences in blood pressure responses to 10-day Ang II infusion at 800 ng/kg/min, i.p., between wild-type and PT-KO mice. 12 The present study differs from these two studies both in the approach to generate the mutant mouse model, and in key phenotypic responses including basal blood pressure levels and the pressor and natriuretic responses to Ang II-induced hypertension. Instead of using PEKCK-Cre or KAP2-Cre approach, the present study used the iL1-SGLT2-Cre approach to generate our PT-Agtr1a−/− mice as PEKCK and KAP2 are reportedly not only expressed in the proximal tubules, but also in distal nephron segments and extrarenal epithelial tissues. 21–24 By contrast, SGLT2 is almost exclusively expressed in S1 and S2 segments of the proximal tubules.25,26 Indeed, Rubera et al. have demonstrated that the SGLT2 promoter drove Cre expression exclusively in the proximal tubules, not in the loop of Henle, distal tubules, or collecting ducts in the medulla. 25
With this novel iL1-SGLT2-Cre approach, the present study consistently demonstrated that basal blood pressure, as measured by the telemetry technique, was ~15 ± 3 mmHg lower in our PT-Agtr1a−/− than wild-type mice under control conditions. This response represents about 50% of the decreases in basal blood pressure previously reported in global Agtr1a−/− mice, 27,28,37,38 and is consistent with the cross-kidney transplantation study of Crowley et al.39 In response to systemic Ang II infusion, deletion of AT1a receptors selectively in the proximal tubules of PT-Agtr1a−/− mice also attenuated about 50% of increases in blood pressure responses to Ang II as seen in wild-type mice. Our results strongly suggest that intratubular AT1a receptors in the proximal tubules and the extra-proximal tubule and/or extrarenal AT1a receptors contribute about equally to the basal blood pressure control and the development of Ang II-induced hypertension. 39 This interpretation is further supported by the experiments, in which an intracellular Ang II fusion protein, Ad-sglt2-ECFP/Ang II, was expressed selectively in the proximal tubules of wild-type and PT-Agtr1a−/− mice in the present study. The latter proximal tubule-specific approach has the advantage over systemic Ang II infusion to exclude the actions of circulating Ang II acting on extrarenal AT1a receptors.28,31,34 The expression of intracellular Ang II in the proximal tubules consistently increased blood pressure by >15 to 18 mmHg in wild-type mice, 28,31,34 and this effect was completely blocked in PT-Agtr1a−/− mice. Thus, the results of the present study provide strong evidence for an important role of intratubular Ang II and AT1a receptors in the proximal tubules in basal blood pressure control and the development of Ang II-dependent hypertension.
The present study further investigated the mechanisms by which intratubular Ang II and AT1a receptors in the proximal tubules contribute to blood pressure homeostasis and the development of Ang II-dependent hypertension. Previously, PEKCK-Cre-mediated deletion of AT1a receptors in the proximal tubules had no effects on basal levels of NHE3 and NaPi2 proteins in the proximal tubules, and NKCC2 proteins in the loop of Henle in the kidney of PTKO mice.11 GFR was found to decrease by ~29%, while urine osmolality remained unchanged, despite of a 63% decrease in proximal tubule fluid absorption in these mice.11 In a separate study, deletion of AT1a receptors in the proximal tubules by KAP2-Cre had no effects on 24 h urine excretion, urinary Na+ and K+ excretion, as well as urine osmolality, suggesting that proximal tubule-specific deletion has no effects on renal tubular electrolyte transport.12 The results of the present study are different from these studies in several ways. First, basal blood pressure was significantly lower, diuretic and natriuretic responses were greater, and urine osmolality was lower in our PT-Agtr1a−/− mice. Second, whole-kidney GFR as measured using the transdermal FITC-sinistrin approach was about 23% higher in PT-Agtr1a−/− mice. With this novel approach, we and others demonstrated that basal whole-kidney GFR in an adult wild-type mouse is about one-tenth of an adult rat’s GFR.14,30 The mechanisms underlying glomerular hyperfiltration in PT-Agtr1a−/− mice remains to be investigated, but this response may be likely similar to the increases in GFR during the administration of ACE inhibitors or ARBs.17,40,41 Indeed, deletion of AT1a receptors selectively in the proximal tubules is expected to block intratubular Ang II-stimulated proximal tubule reabsorption of NaCl and solutes, and significantly increase their delivery from the proximal tubules to the macula densa and juxtaglomerular cells, which may inhibit renin release and impair the tubuloglomerular feedback response. Third, we found that deletion of AT1a receptors selectively from the proximal tubules removes the well-recognized Na+-retaining actions of circulating and intratubular Ang II on NHE3 and other Na+ transporters or cotransporters in the proximal tubules of the kidney. This in turn augments the pressure-natriuresis responses and natriuretic responses to acute saline loading and chronic high salt diet in PT-Agtr1a−/− mice. This interpretation is consistent with our recent studies showing that deletion of NHE3 selectively in the proximal tubules of PT-Nhe3−/− mice lowers basal blood pressure also by increasing the pressure-natriuresis response and augmenting natriuretic responses to acute saline loading and chronic high salt diet in PT-Nhe3−/− mice.13,14 Fourth, our results show that deletion of AT1a receptors selectively from the proximal tubules attenuated Ang II-induced hypertension by ~50%, compared with when Ang II was infused systemically, whereas it completely blocked Ang II-dependent hypertension induced by adenovirus-mediated expression of an Ang II fusion protein selectively in the proximal tubules. Given that NHE3 is the key downstream target of intratubular and/or intracellular Ang II in the proximal tubules with very similar blood pressure, the pressor response to Ang II, and urinary Na+ excretory responses in PT-Nhe3−/− mice, proximal tubule NHE3 likely plays an important role in the observed blood pressure and renal response phenotypes in PT-Agtr1a−/− mice under basal conditions and during the development of Ang II-induced hypertension.13,14
Our previous in vitro autoradiographic Ang II receptor binding assays suggest that appropriate 10% to 15% of Ang II receptor binding sites in the proximal tubules of the kidney belong to AT2 receptors, suggesting that AT2 receptors may play a counterregulatory role against AT1 (AT1a) receptor-mediated, Ang II-stimulated proximal tubule Na+ reabsorption.2,42–44 Indeed, Li and Widdop have previously demonstrated this counterregulatory role of AT2 receptors in cardiovascular and renal hemodynamic responses to Ang II in the presence of AT1 receptor blockade.45 Kemp et al. have recently reported that Ang III, the major metabolite and analogue of Ang II, preferably binds to and stimulates AT2 receptors in the proximal tubules of the kidney.2,9,36 These authors further showed that interactions between Ang III and AT2 receptors induce significant natriuretic responses in part by internalizing NHE3 in the proximal tubules.2,9,36 Whether genetic deletion of AT1a receptors selectively from the proximal tubules of the kidney may augment the natriuretic responses to Ang III in PT-Agtr1a−/− mice has not been determined previously. In the present study, our data showed that Ang III significantly increased urinary Na+ excretion in anesthetized PT-Agtr1a−/− mice, compared with wild-type mice. This finding supports the studies of Kemp et al. 2,9,36 and suggests that in the absence of AT1 (AT1a) receptors in the proximal tubules, Ang III may physiologically stimulate AT2 receptors in the proximal tubules to induce a natriuretic response. Interestingly, however, long-term Ang III infusion for 2 weeks with or without concurrent blockade of AT2 receptors by PD123319 appears not to have significant effects on blood pressure and natriuretic responses in wild-type and PT-Agtr1a−/− mice, likely due to the compensatory responses to other vasoactive and Na+ transporters in the kidney. It may be necessary to further study the roles and mechanisms of Ang III and AT2 receptors in the regulation of proximal tubule Na+ reabsorption and therefore blood pressure homeostasis using novel mouse models with proximal tubule-specific deletion of AT2 receptors in the kidney.
Perspectives
In summary, our study was the first to use a novel genetically modified mouse model with proximal tubule-specific deletion of AT1a receptors in the kidney, PT-Agtr1a−/−, to directly test the hypothesis or the direct cause and effect relationship that AT1a receptors are required for normal blood pressure control and the development of Ang II-induced hypertension (Figure S6). This animal model is unique in that PT-Agtr1a−/− mice were generated for the 1st time using the iL1-SGLT2-Cre/Agtr1a-LoxP recombination approach. The promoter of SGLT2, or the Na+ and glucose co-transporter 2, to drive Cre expression in the proximal tubules of the kidney has previously been confirmed to be very specific to the S1 and S2 segments of the proximal tubules.13,14,25,26 Our PT-Agtr1a−/− mouse model is not androgen-inducible, nor is the KAP2-Cre or PEPCK-Cre-driven proximal tubule deletion of AT1a receptors.11,12 We demonstrated that deletion of AT1a receptors selectively in the proximal tubules of PT-Agtr1a−/− mice lowers basal blood pressure to the ~50% level of global knockout in Agtr1a−/− mice, attenuates ~50% of systemic Ang II-induced hypertension, and completely blocks intratubular/intracellular Ang II-induced hypertension. Proximal tubule-specific knockout of AT1a receptors is associated with the augmentation of the pressure-natriuresis response and natriuretic responses to acute salt loading, chronic high salt diet and to Ang III, but unlikely involves superoxide. These phenotypic responses in PT-Agtr1a−/− mice were similar to those of PT-Nhe3−/− mice with deletion of NHE3 selectively in the proximal tubules.13,14 Together, the similarity of blood pressure and renal transport or electrolyte excretory phenotypes in PT-Agtr1a−/− and PT-Nhe3−/− mice suggests that deletion of AT1a receptors will lead to inhibition of Ang II-stimulated NHE3 expression and activity and therefore Na+ and fluid reabsorption in the proximal tubules, which in turn contributes to lower basal blood pressure and attenuation of Ang II-induced hypertension in PT-Agtr1a−/− mice. Thus, the present study provides new evidence for, and new insights into the important role of the intratubular Ang II/AT1a receptor/NHE3 pathway in normal blood pressure control, the development and therapeutic target of Ang II-dependent hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
The Ang II/AT1a/NHE3 pathway has been suggested to play a critical role in the regulation of Na+ and fluid reabsorption in the proximal tubules of the kidney and the blood pressure control, but a direct cause and effect relationship between this pathway and basal blood pressure homeostasis and the development of Ang II-dependent hypertension has not been determined previously.
The present study used a novel mutant mouse model with genetic deletion of AT1a selectively in the proximal tubules of the kidney to demonstrate for the 1st time that the Ang II/AT1a/NHE3 pathway in the proximal tubules is required for maintenance of basal blood pressure homeostasis and the development of Ang II-dependent hypertension.
What Is Relevant?
The impaired pressure-natriuresis response and long-tern Na+ retention are not only the hallmarks of Ang II-dependent hypertension, but also the key mechanisms underlying many other forms of hypertension, such as human essential hypertension.
Since the Ang II/AT1a/NHE3 pathway in the proximal tubules contributes ~50% of basal blood pressure as maintained by the circulating and tissue renin-angiotensin system, as well as ~50% of Ang II-induced hypertension, the present study suggests that the Ang II/AT1a/NHE3 pathway in the proximal tubules of the kidney may be therapeutically targeted to treat Ang II-dependent hypertension.
Acknowledgements
We sincerely thank Dr. Julia L. Cook of Ochsner Clinic Foundation for providing the plasmid construct of ECFP/Ang II, and Drs. Isabelle Rubera and Michel Tauc of Université Côte d’Azur, France, for providing breeding pairs of iL1-sglt2-Cre mice for us to generate mutant mice with proximal tubule-specific deletion of AT1 (AT1a) receptors or NHE3 (the Na+/H+ exchanger 3) in the present study. We also thank Mr. Glenn Hoskins of the University of Mississippi Medical Center for performing electron microscopic imaging, and Elisa Miguel-Qin, Jessica Hoang, Rui Xu, and Rumana Hassan for excellent technical assistance over many years. The portions of the present study were presented previously as conference abstracts in J Hypertens. 36: e84, 2018; J Hypertens. 36: e92, 2018; JASN 2018 KIDNEY WEEK: TH-PO118; FASEB J. 33(1_supplement): 867.9, 2019; Kidney International Reports, 2019; 4(7): Supplement, S51; Hypertension. 2019; 74: AP2016; FASEB J, 33(S1), 2020; and Hypertension. 2020; 75: PO41, respectively.
Sources of Funding
This work was supported in part by grants from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; 2RO1DK067299–06A2 and 2R01DK067299–10A1; 1R01DK102429–01 and 2R01DK102429–03A1; and 1R01DK123144–01) to Dr. Jia L. Zhuo.
Footnotes
Disclosures of Conflict of Interest
None.
References
- 1.Guyton AC. Blood pressure control - special role of the kidneys and body fluids. Science 1991; 252(5014):1813–1816. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 2000; 35(1 Pt 2):155–163. [DOI] [PubMed] [Google Scholar]
- 3.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat. Med 2011; 17(11):1402–9. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo JL, Li XC. Proximal nephron. Compr Physiol. 2013; [3(3)]:1079–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobori H,Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59(3):251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol. 2012; 2(4):2733–52. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XC, Zhu D, Zheng X, Zhang J, Zhuo JL. Intratubular and intracellular renin-angiotensin system in the kidney: a unifying perspective in blood pressure control. Clin Sci (Lond) 2018;132(13):1383–1401. doi: 10.1042/CS20180121. Print 2018 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 2014; 4(3):1201–28. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemp BA, Bell JF, Rottkamp DM, et al. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60(2):387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am J Physiol Renal Physiol. 2006; 290(5):F1024–F1033. doi: 10.1152/ajprenal.00221.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurley SB, Riquier-Brison ADM, Schnermann J, et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13(4):469–475. doi: 10.1016/j.cmet.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Weatherford ET, Davis DR, et al. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1067–R1077. doi: 10.1152/ajpregu.00124.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XC, Soleimani M, Zhu D, et al. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) promotes the pressure-natriuresis response and lowers blood pressure in mice. Hypertension. 2018;72(6):1328–1336. doi: 10.1161/HYPERTENSIONAHA.118.10884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XC, Zhu D, Chen X, et al. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) in the kidney attenuates Ang II (angiotensin II)-induced hypertension in mice. Hypertension. 2019;74(3):526–535. doi: 10.1161/HYPERTENSIONAHA.119.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly AM, Harris PJ, Williams DA. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am J Physiol. 1995; 269(3 Pt 2):F374–F380. [DOI] [PubMed] [Google Scholar]
- 16.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol. 2010; 298(1):F177–F186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XC, Zhuo JL. Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int 2011; 80:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PJ, Young JA Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch 1977; 367: 295–297. https://doi.org/10.1007/BF00581370 [DOI] [PubMed] [Google Scholar]
- 19.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance II: impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol. 2012; 303(11):F1507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough AA, Nguyen MT. Maintaining balance under pressure: integrated regulation of renal transporters during hypertension. Hypertension. 2015;66(3):450–455. doi: 10.1161/HYPERTENSIONAHA.115.04593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short MK, Clouthier DE, Schaefer IM, Hammer RE, Magnuson MA, Beale EG. Tissue-specific, developmental, hormonal, and dietary regulation of rat phosphoenolpyruvate carboxykinase-human growth hormone fusion genes in transgenic mice. Mol Cell Biol 1992;12(3):1007–1020. doi: 10.1128/mcb.12.3.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beale EG, Clouthier DE, Hammer RE. Cell-specific expression of cytosolic phosphoenolpyruvate carboxykinase in transgenic mice. FASEB J 1992;6(15):3330–3337. doi: 10.1096/fasebj.6.15.1281456 [DOI] [PubMed] [Google Scholar]
- 23.Meseguer A, Catterall JF. Cell-specific expression of kidney androgen-regulated protein messenger RNA is under multihormonal control. Mol Endocrinol. 1990;4(8):1240–1248. doi: 10.1210/mend-4-8-1240 [DOI] [PubMed] [Google Scholar]
- 24.Malstrom SE, Tornavaca O, Meseguer A, Purchio AF, West DB. The characterization and hormonal regulation of kidney androgen-regulated protein (Kap)-luciferase transgenic mice. Toxicol Sci 2004;79(2):266–277. doi: 10.1093/toxsci/kfh125 [DOI] [PubMed] [Google Scholar]
- 25.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol 2004; 15(8):2050–2056. [DOI] [PubMed] [Google Scholar]
- 26.Wright EM. Renal Na+-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280(1):F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10 [DOI] [PubMed] [Google Scholar]
- 27.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of angiotensin II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2007; 293:F586–F593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT1a receptors induces blood pressure responses to intracellular angiotensin II in AT1a receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2013; 304:R588–R598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rateri DL, Moorleghen JJ, Balakrishnan A, et al. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res 2011;108(5):574–581. doi: 10.1161/CIRCRESAHA.110.222844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 2012; 303(5):F783–F788. [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Zhou X, Zhuo JL. Evidence for a physiological mitochondrial angiotensin II system in the kidney proximal tubules: novel roles of mitochondrial Ang II/AT1a/O2- and Ang II/AT2/NO signaling. Hypertension. 2020;76(1):121–132. doi: 10.1161/HYPERTENSIONAHA.119.13942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson DL, Raff H, Roman RJ. Influence of angiotensin II on pressure natriuresis and renal hemodynamics in volume-expanded rats. Am J Physiol 1991; 260(6 Pt 2):R1200–R1209. [DOI] [PubMed] [Google Scholar]
- 33.Li XC, Shull GE, Miguel-Qin E, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics. 47(10), 479–487. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol. 2011; 300:F1076–F1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiyama A, Fukui T, Fujisawa Y, et al. Systemic and Regional Hemodynamic Responses to Tempol in Angiotensin II-Infused Hypertensive Rats. Hypertension. 2001;37(1):77–83. doi: 10.1161/01.hyp.37.1.7736. [DOI] [PubMed] [Google Scholar]
- 36.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53(2):338–343. doi: 10.1161/HYPERTENSIONAHA.108.124198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: a role for AT1B receptors in blood pressure regulation. Am J Physiol. 1997;272(4 Pt 2):F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515 [DOI] [PubMed] [Google Scholar]
- 38.Cervenka L, Horácek V, Vanecková I, et al. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40(5):735–741. doi: 10.1161/01.hyp.0000036452.28493.74 [DOI] [PubMed] [Google Scholar]
- 39.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 2005;115(4):1092–1099. doi: 10.1172/JCI23378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuo JL, Thomas D, Harris PJ, Skinner SL. The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J Physiol. 1992;453:1–13. doi: 10.1113/jphysiol.1992.sp019214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris PJ, Zhuo JL, Skinner SL. Effects of angiotensins II and III on glomerulotubular balance in rats. Clin Exp Pharmacol Physiol. 1987;14(6):489–502. doi: 10.1111/j.1440-1681.1987.tb01505.x [DOI] [PubMed] [Google Scholar]
- 42.Zhuo JL, Song K, Harris PJ, Mendelsohn FA. In vitro autoradiography reveals predominantly AT1 angiotensin II receptors in rat kidney. Ren Physiol Biochem. 1992;15(5):231–239. doi: 10.1159/000173458 [DOI] [PubMed] [Google Scholar]
- 43.Zhuo JL, Dean R, MacGregor D, Alcorn D, Mendelsohn FA. Presence of angiotensin II AT2 receptor binding sites in the adventitia of human kidney vasculature. Clin Exp Pharmacol Physiol. 1996;23 Suppl 3:S147–S154. doi: 10.1111/j.1440-1681.1996.tb03077.x [DOI] [PubMed] [Google Scholar]
- 44.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30(5):1238–1246. doi: 10.1161/01.hyp.30.5.1238 [DOI] [PubMed] [Google Scholar]
- 45.Li XC, Widdop RE. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142(5):821–830. doi: 10.1038/sj.bjp.0705838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.