Abstract
This review summarizes recent literature addressing the association of short sleep duration, shift work, and obstructive sleep apnea, with hypertension risk, blood pressure (BP) levels, and 24-h ambulatory BP. Observational studies demonstrate that subjectively assessed short sleep increases hypertension risk, though conflicting results are observed in studies of objectively assessed short sleep. Intervention studies demonstrate that mild and severe sleep restriction are associated with higher BP. Rotating and night shift work are associated with hypertension as shift work may exacerbate the detrimental impact of short sleep on BP. Further, studies demonstrate that shift work may increase nighttime BP and reduce BP control in hypertension patients. Finally, moderate to severe obstructive sleep apnea is associated with hypertension, particularly resistant hypertension. Obstructive sleep apnea is also associated with abnormal 24-h ambulatory BP profiles, including higher daytime and nighttime BP, non-dipping BP, and a higher morning surge. Continuous positive airway pressure treatment may lower BP and improve BP dipping. In conclusion, efforts should be made to educate patients and healthcare providers about the importance of identifying and treating sleep disturbances for hypertension prevention and management. Empirically supported sleep health interventions represent a critical next step to advance this research area and establish causality.
Keywords: short sleep duration, shift work, obstructive sleep apnea, blood pressure, hypertension
Introduction
Sleep is a multi-dimensional health behavior. Sleep disturbances are ubiquitous among US adults.1-5 Sleep disturbances may result from several etiologies including unhealthy sleep behaviors (the most prevalent and widely studied being short sleep duration), presence of sleep disorders such as obstructive sleep apnea (OSA), a form of sleep disordered breathing characterized by complete or partial upper airflow cessation during sleep, and/or recurrent circadian disruption with misalignment of the timing and regularity of lifestyle behaviors and endogenous circadian rhythms within a 24-h period, often induced by shift work.6,7 More than a third of US adults sleep less than the recommended 7h/night.1,2 Among night shift workers, who make up ~25% of the US workforce8 , the prevalence of a sleep duration <7h is 62%.3,4 Shift workers also report higher prevalence of poor sleep quality and insomnia4 and are vulnerable to recurrent circadian misalignment.6 Finally, overall prevalence of mild to severe OSA among adults aged 30-70y has been estimated at 26%; prevalence rates vary by age, sex, and BMI, but are generally increasing particularly among obese adults.5,9
Short sleep, shift work, and OSA are associated with hypertension (HTN) risk.10-12 OSA is considered a secondary cause of HTN.9 Although a growing body of evidence has linked short sleep and shift work to HTN risk10,12 , neither are included as risk factors in HTN guidelines. Furthermore, it is unclear whether short sleep and shift work share common underlying mechanisms.11
This review summarizes recent literature (2015-2020) addressing the association of short sleep, shift work, and OSA, with prevalent and incident HTN, clinic systolic blood pressure (SBP) and diastolic BP (DBP), and abnormalities in ambulatory BP among adults. To our knowledge, this represents the first review of these sleep disturbances, taken together, in relation to HTN, clinic BP, and measures out-of-clinic BP level and diurnal pattern, which are more strongly related to future cardiovascular disease risk than clinic blood pressure. We also review the distinct and common mechanisms that may underlie the associations of these sleep disturbances with HTN, given that sleep problems tend to cluster. Lastly, we highlight the methodological limitations of existing studies and the remaining knowledge gaps, which may help guide future observational and experimental studies within this research area.
Short Sleep Duration and BP
A 2016 American Heart Association (AHA) scientific statement on sleep and cardiometabolic health concluded that there is strong epidemiological evidence that self-reported short sleep duration, defined using different cut-offs (≤5h, ≤6h, or ≤7h), is a risk factor for HTN.7 As discussed in a 2019 review on sleep duration and BP12 , most studies utilizing self-reported sleep duration have reported higher HTN risk among short sleepers, whereas those utilizing objectively-measured sleep duration have yielded conflicting results.13-16 Short sleep in combination with sleep disorders may be particularly harmful. Among 7,107 OSA patients, objectively-assessed sleep duration between 5-6 h and <5h increased the odds of HTN by 45% and 80%, respectively.15 Extremely short sleep duration (<5h) was more detrimental than OSA in terms of HTN risk suggesting that short sleep in OSA may be a target for HTN prevention.
The association of short sleep with the circadian pattern of BP, typically assessed using 24-h ambulatory BP monitoring (ABPM), is not well-characterized12 , despite the strong association of abnormalities in 24-h ambulatory BP patterns including non-dipping BP (<10% reduction in nighttime BP) with cardiovascular disease (CVD) morbidity and mortality.9 Two recently published studies17,18 have examined objectively-assessed short sleep duration, utilizing wrist actigraphy, and 24-h ambulatory BP. In a post-hoc analysis of data from the Lifestyle Modification in Blood Pressure Lowering Study (LIMBS) of adults aged 18-80y and the Penn Icelandic Sleep Apnea Study (PISA) of adults aged 40-65y, mean 24-h SBP was 12.7 mmHg higher and 4.7 mmHg higher respectively, among participants with sleep duration <7h vs. ≥7h (p<0.01).17 In LIMBS, the greatest difference was observed for daytime SBP (12.1 mmHg, p<0.001), but significant differences were also reported for nighttime SBP (9.3 mmHg, p=0.029). Shorter sleep duration was also associated with higher 24-h SBP, independent of nighttime and office BP. Similarly, in the longitudinal North Texas Heart Study (n=300 adults, 50% women, age: 21-70y), shorter actigraphy-derived sleep duration was associated with higher daytime and nighttime BP.18 A temporal trend between sleep duration and BP the next day was observed, as shorter sleep duration on one night was associated with higher SBP the following day.
Intervention studies examining the effect of sleep restriction on BP are limited. In a small study (n=20, mean age: 31.6y), 24-h shift-related short-term sleep deprivation (24-h shift with ~3h of sleep) increased SBP (+5.7 mmHg; p=0.011) and DBP (+6.3 mmHg, p=0.009).19 Similarly, in a study (n=30, mean age: 26.7y) of acute stress-induced arousal and its association with sleep, self-reported and objectively-assessed short sleep duration (<6h) were moderately correlated with increased morning BP.20
Two trials investigated the effects of sleep restriction on 24-h ambulatory BP.21,22 In a randomized controlled trial (n=45, mean age: 32y,), participants assigned to repeated sleep restriction (4h of sleep/night for 3 nights, followed by recovery sleep of 8h, repeated 4 times in succession) vs. a control condition (8h/night)21 , had non-dipping DBP and higher BP during blocks of sleep restriction. These data demonstrate that repeated exposure to shortened sleep reduces BP dipping, despite intermittent catch-up sleep. Whether frequent shortened sleep may increase HTN risk due to non-dipping BP is unknown.
The effect of prolonged mild sleep restriction on BP has been examined in one randomized, crossover trial of 24 pre-menopausal women with overweight/obese or at-risk for obesity, who completed a 6-week intervention (delayed bedtime by 1.5h) and 6 weeks of habitual sleep with a 6-week washout between phases.22 There was a significant sleep × week interaction on clinic SBP (p=0.036), characterized by an increase in weekly SBP over time in the sleep restriction vs. habitual sleep phase. Additionally, 24-hour DBP, wake DBP, and mean arterial BP were higher after 6 weeks of sleep restriction compared with habitual sleep. Psychological variables (perceived stress, stressful events and distress, and lower resilience) did not mediate the association between sleep restriction and BP, suggesting mechanisms independent of psychological stressors may be at play.
Shift Work and BP
Shift work refers to a work schedule that falls outside the traditional 9:00AM–5:00PM workday and may encompass evening or night shifts, early morning shifts, and rotating shifts.
Shift work is associated with CVD morbidity and mortality23 , and recent studies continue to support the hypothesis that shift work increases HTN risk.24-27 HTN incidence is higher among shift workers who experience repeated and prolonged exposure to elevated BP during periods of shift work.10,23 A 2017 meta-analysis of 27 observational studies demonstrated that shift work is associated with 31% and 10% higher odds of HTN in cohort studies and cross-sectional studies, respectively.10 In cohort studies, rotating shift work was associated with 34% higher HTN risk (OR[95%CI]:1.34[1.08–1.67]). Cohort data on night shift work and HTN are limited, but the pooled estimate from cross-sectional studies was non-significant (OR[95%CI]:1.07[0.85–1.35]).10
Recent studies support the hypothesis that shift work increases HTN risk.24-27 In a cross-sectional study of 1,953 male workers (including 1,075 shift workers) in China, individuals with rotating and night shift work (11PM to 7AM for 4 days, followed by 3PM to 11PM for 4 days, followed by 7AM to 3PM for 4 days with 1-2 off days between the night, afternoon, and day shifts) had 51% higher odds of having SBP/DBP ≥140/90 mmHg compared to day workers.24 In another study of 4,519 Chinese men, where shift work was assessed as the frequency of working a shift from 12:00PM to 8:00AM, frequent vs. never shift-workers had 20% higher odds of having SBP/DBP ≥140/90 mmHg.25 Shift work also modified the association of combined short sleep and poor sleep quality on HTN, as odds were 43% and 97% higher among those who reported an occasional and frequent shift work schedule, respectively, suggesting that night shift-work may further exacerbate the detrimental effects of short sleep and poor sleep quality on BP.25
Likewise, in a cross-sectional Brazilian study of 2,588 female nurses, current or former night shift workers, who reported working from 7:00PM-7:00AM at least once a week, had 68% higher odds of self-reported HTN compared to day workers.26 Notably, odds of HTN were 24% lower in night workers who reported on-shift napping, suggesting that napping may mitigate the detrimental effects of sleep restriction and circadian misalignment on BP in shift workers, but this finding warrants replication in prospective studies and in other populations with objectively-assessed BP.
Prospective data addressing the effects of shift type and rotations on HTN risk are limited. In a 2019 US prospective cohort study of 2,151 workers at manufacturing facilities (2003-2013), associations between rotational (switching to a different shift based on start time) and night work (working a shift ≥3h between 11:00PM-6:00AM) and incident HTN were evaluated.27 Workers with mostly night work and frequent rotations (≥50% night and ≥10% rotation) had 4-fold higher HTN risk compared to non-night workers. Associations were strongest among those with 95–100% night work (i.e. “permanent night workers” who are consistently experiencing circadian disruption) and among those who engage in both night and rotational work. Whether these associations persist over time requires further study. Similarly, in a prospective cohort study of 2,079 shift workers and 5,341 day workers from Ontario, Canada (age: 35-69y), history of shift work was associated with 21% and 26% higher risk of incident HTN in men and women, respectively, over the 12y follow-up period.28
The impact of acute exposure to shift work on ambulatory BP was investigated in a 2020 systematic review and meta-analysis of 50 published papers between 1980-2018.29 On average, BP measured during any sleep period separate from shift work was lower by 17.5 mmHg for SBP and 5.4 mmHg DBP compared to BP measured during shift work, but the epidemiological evidence was deemed heterogeneous due to wide variation in shift worker type, shift schedules, and regularity of BP measurements between studies. Overall, most existing studies compare BP during one shift workday to BP on a rest/leisure day. Additional research on the acute and long-term impact of shift work on BP during sleep is warranted, particularly among those with elevated clinic or daytime BP. The impact of shift work on long-term BP control in shift workers with HTN also needs further investigation. A Korean study of >600,000 adults showed that BP control rates in those with HTN who are taking HTN medication are lower in night vs. day workers.30 Whether night shift work exacerbates uncontrolled BP in those with HTN or impacts adherence to medications is another area for further research.
Obstructive Sleep Apnea and BP
OSA which is caused by a collapse of the upper airway during sleep leading to transient asphyxia and experiences of hypoxemia, brain arousals, sleep problems, and daytime somnolence5,31 is a risk factor for HTN and is highly prevalent in HTN patients (30-50%), particularly those with resistant HTN (~80%).32,33 The prevention and treatment of OSA is considered a possible target for lowering CVD risk.31,34
OSA, particularly moderate to severe OSA, is associated with prevalent and incident HTN.31 A 2018 meta-analysis of 26 studies demonstrated that OSA was associated with ~3-fold higher resistant HTN risk (OR[95%CI]:2.84[1.70-3.98]).35 Further, mild (apnea-hypopnea index (AHI)>5), moderate (AHI>15), and severe OSA (AHI>30) was associated with 18%, 32%, and 56% higher risk of HTN, respectively, and a dose-response relationship was observed. Sub-group analyses by ethnicity and sex demonstrated that associations are stronger in Caucasian vs. Asian adults and males vs. females.35 Strong associations between OSA and resistant HTN were also recently demonstrated among Black adults in the Jackson Heart Study.36 In that study, 913 participants (mean age: 64.0±10.6y) completed in-home polysomnography and clinic BP assessments between 2012-2016.36 Participants with moderate or severe OSA, defined as a respiratory event index ≥15, had 2-fold greater odds of resistant HTN.36
OSA may also impact 24-h ambulatory BP patterns. A 2019 meta-analysis of 1,562 OSA patients showed that the prevalence of non-dipping BP was 59.1%.37 When comparing OSA patients to controls, OSA was associated with 47% greater odds of having non-dipping BP, while moderate to severe OSA was associated with 67% higher odds of having non-dipping BP. Longitudinal data demonstrated a dose-response relationship between OSA severity at baseline and odds of developing a non-dipping SBP profile.38 In a clinic-based sample of 100 HTN patients (mean age: 58±10y)39 , 10.5% of dippers vs. 43.5% of non-dippers had an AHI ≥15, indicative of moderate to severe OSA. AHI also predicted the magnitude of BP dipping (β =−0.288, p=0.03), suggesting that patients with non-dipping BP are at high risk of OSA. Similarly, a cross-sectional study of 153 patients (median age: 62y) showed that individuals with a reverse BP dipping pattern (SBP ratio>1.00) had ~4-fold higher odds for OSA.40 When BP dipping patterns were defined based on DBP ratios, individuals with both non-dipping (0.90<DBP ratio≤1.00) and reverse BP dipping patterns (DBP ratio>1.00) had 2.7-fold and 3.5-fold higher odds of OSA, respectively.40
Higher AHI, indicative of greater OSA severity, has also been linked to higher morning and evening mean BP, and higher morning surge (exaggerated increase in BP from nighttime to early morning).31,41-44 Limited data, mostly from male participants, also suggest that masked HTN (normal clinic BP with high ambulatory BP) is prevalent among individuals with moderate to severe OSA.45-48 Finally, OSA treatments including continuous positive airway pressure (CPAP) and mandibular advancement devices may lower CVD risk, as they have been associated with reduced BP.49,50 Short-term CPAP treatment may also help decrease diurnal BP variability and may convert “non-dippers” into a dipping 24-h BP profile especially among patients with resistant HTN.50,51
Sex and Racial/Ethnic Differences
Women may be more prone to the effects of short sleep duration on HTN risk, particularly during young adulthood. In a meta-analysis of cross-sectional studies52 , sleeping ≤5 h or ≤6 h was associated with 36% higher odds of HTN among women only. Similarly, in a meta-analysis by Wang et al. sleeping ≤ 5 h vs. 7 h was associated with 68% higher HTN risk among women, but null results were observed for men. However, evidence from US populations is mixed. Sex differences were not reported for the relation between short sleep and HTN in the Coronary Artery Risk Development in Young Adults (CARDIA) study53 , the Sleep and Heart Health Study54 , or the Hispanic Community Health Study/Study of Latinos sleep study.13 Data from the Western New York Study55 and the Behavioral Risk Factor Surveillance System and National Health Interview Survey (NHIS)56 demonstrate that short sleep is related to elevated HTN risk in women. In the Nurses’ Health Study, sleeping ≤5 h or 6 h vs. 7 h was associated with up to 25% higher odds for HTN57 , but sleeping ≤5 h was associated with a 20% higher risk of incident HTN only in women aged <50 y.57 Consistent with these results, in the Western New York Study, the association between short sleep and HTN was stronger among pre-menopausal women, in whom short sleep was associated with >3-fold higher odds for HTN vs. 49% higher risk observed in postmenopausal women.55
On the other hand, it appears that the association of moderate to severe OSA with HTN may be stronger in men compared to women. In the prospective Vitoria Sleep Cohort of >1000 Spanish adults aged 30 to 70 years (43.7% male), although there was no significant association between OSA and hypertension risk and the study revealed no sex differences58, in a subsequent analysis, moderate to severe OSA was associated with >2-fold higher odds of having moderate to severe hypertension, defined as SBP ≥160 and DBP ≥ 100 mm Hg, in men but not women.59 However, sex differences in the cardiovascular impact of OSA is an area in the nascent stages of characterization, and additional research is needed to decipher how OSA frequency and severity in men and women contribute to sex differences in HTN burden. Similarly, evidence is limited regarding sex differences in the effect of shift work on HTN risk. In the Canadian Community Health Survey, history of shift work was associated with higher HTN rates in both men and women.28 Whether women or men are more prone to the cardiovascular consequences of shift work warrants further investigation.
Regarding racial/ethnic differences in the influence of sleep disturbances on BP, as we report previously, short sleep duration is likely a contributor to racial disparities in HTN.12 According to the NHIS, Black adults are 41% more likely than whites to report being short sleepers60 , and objectively-assessed sleep data from the Chicago Area Sleep Study indicate that Black adults sleep ~48 minutes less than white adults.61 In the NHIS, associations of sleep duration with HTN risk varied by race, as Black adults who slept <6h vs. 6-8h were 34% more likely to report HTN than their white counterparts.62 Similarly, in the CARDIA study, sleep duration mediated the difference in DBP change over time between Black and white adults.53 Shift work may also be a stronger risk factor for HTN among Black adults. In the Nurses’ Health Study, rotating night shift work was associated with 81% higher risk for incident HTN in Black adults, but no increase in risk was observed in white adults.63 In another study, Black women working a night shift were more likely to have a non-dipper BP profile (defined as <10% drop in SBP during sleep) compared to women of other races.64
Differences by race/ethnicity have also been reported for the relation between OSA and HTN. In the 2007–2008 National Health and Nutrition Examination Survey, probable OSA (derived from self-reported data on OSA diagnosis, snorting, gasping or stopping breathing during sleep, and snoring) was associated with 69% and 40% higher odds of HTN among Hispanics/Latinos and white but not Black adults.65 In models stratified by both race/ethnicity and BMI, probable OSA was associated with >4-fold, 65%, and 2-fold greater odds of HTN in among Black adults who were overweight, white adults who were overweight, and Hispanic/Latino adults who were obese, respectively. Studies linking OSA with measures of diurnal BP variation, including nocturnal BP dipping, and differences by race/ethnicity are lacking.
Potential Mechanisms Underlying the Association of Sleep Disturbances with Hypertension
Short sleep, shift work, and OSA have adverse consequences on BP and circadian patterns and may alter HTN risk through some shared behavioral, psychological, and physiologic pathways (Figure 1), though underlying mechanisms are not fully elucidated.11 Sleep deprivation and OSA have been associated with increased sympathetic activity and reduced parasympathetic activation during sleep.66-68 In the Multi-Ethnic Study of Atherosclerosis, sleeping <6 h was linked to markers of lower levels of parasympathetic tone, higher levels of sympathetic tone, and lower high-frequency heart rate variability.69 Disturbances in autonomic balance are associated with HTN and non-dipping BP.66,70,71 OSA patients experience chronic intermittent hypoxia, sleep fragmentation, and arousals with subsequent sympathetic activation and increased inflammation and oxidative stress, which collectively lead to dysfunction of the vascular endothelium and are known HTN risk factors.67
Figure 1. Possible Mechanisms Underlying Sleep Disturbances with Hypertension.
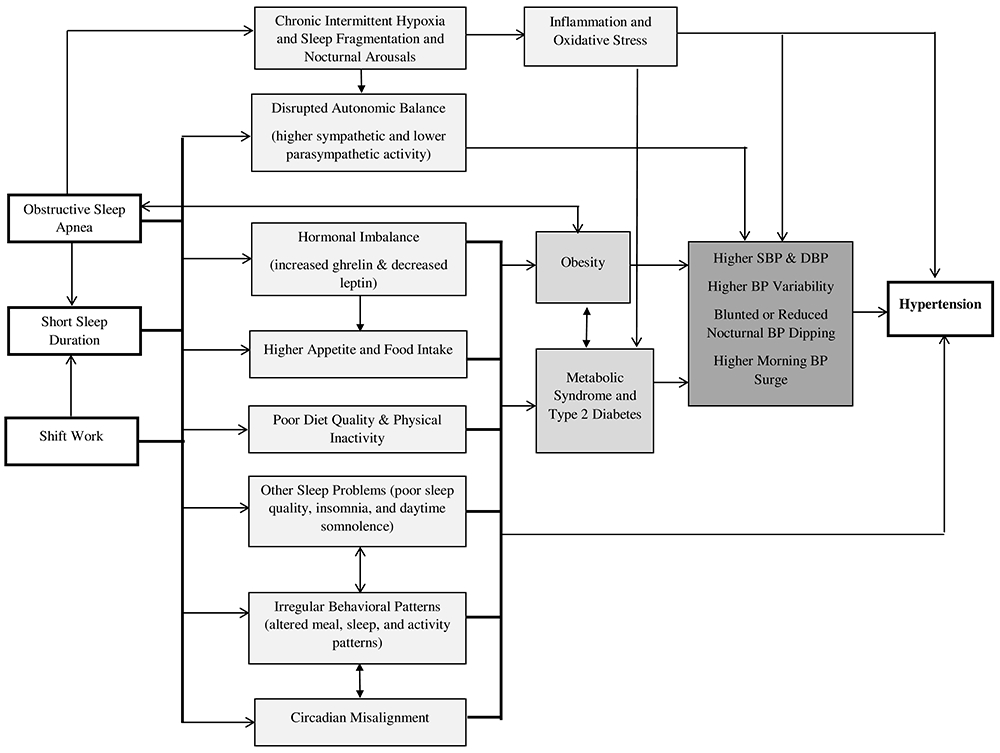
Short sleep, shift work, and obstructive sleep apnea may increase hypertension risk through several physiological mechanisms including disturbed autonomic balance, hormonal imbalances, inflammation and oxidative stress, greater predisposition to obesity, metabolic syndrome, and type 2 diabetes, and unhealthy lifestyle behaviors. These behavioral and physiological factors may lead to higher blood pressure and abnormalities in 24-h ambulatory patterns, predisposing to hypertension.
Short sleep, shift work, and OSA are all independently associated with weight gain and obesity72-74 and are associated with higher incidence of metabolic syndrome and type 2 diabetes.75-77 Patients with metabolic syndrome and type 2 diabetes have up to 2-fold greater risk of developing HTN78 , and both have been shown to act as partial mediators of the association of short sleep and shift work with HTN.57,79,80
The associations of sleep disturbances with HTN as well as with obesity and type 2 diabetes (which predispose to HTN) may be explained, at least in part, by the higher prevalence of unhealthy behaviors in short sleepers, shift workers, and OSA patients. Sleep restriction and shift work are also related to decreases in leptin, increases in ghrelin, increased appetite and hunger (with particular cravings for sweets, startch, and salty snacks), higher energy intake, and poor overall diet quality.81-85 OSA patients also report lower diet quality.86 Shorter sleep has been linked to lower physical activity87 , and physical activity is reduced with increasing daytime sleepiness that is characteristic of OSA.74 In shift workers, physical inactivity has been shown to mediate associations between shift work and HTN.80 Physical inactivity, higher dietary intake, and poor diet quality are independent predictors of HTN9 , and lead to obesity and its metabolic sequela, which likely lie in the causal pathway linking sleep disturbances to HTN.
Other sleep problems may also underlie the association of short sleep, shift work, and OSA with HTN. Poor sleep quality, excessive daytime sleepiness, and insomnia symptoms are observed in short sleepers, shift workers, and OSA patients.80,88-90 The downstream effects of poor sleep quality, insomnia, and excessive daytime sleepiness on BP7 may contribute to the higher HTN risk associated with short sleep, shift work, and OSA. Short sleep and its adverse health consequences are also likely one of the mechanisms underlying the association of shift work and separately, OSA, with HTN risk.11,12 Shift workers, particularly those who are older in age and morning chronotypes (reflecting morning preference for daily activities), are more prone to short sleep.91 Similarly, shorter sleep, likely due to increased nocturnal awakenings, has been reported in OSA patients, with the shortest sleep observed in those with severe OSA.92
Habitual short sleep and shift work can increase HTN risk by disrupting circadian rhythmicity and leading to circadian misalignment.29,66,93 The suprachiasmatic nucleus, which controls the endogenous circadian rhythm, may become metabolically flattened and arrhythmic due to restricted sleep and chronically inverted behavioral cycles that are mismatched with 24-h light/dark cycles.66,94-96 Furthermore, short sleep and shift work are associated with activity at unconventional circadian times leading to a desynchrony between the master clock in the brain and the peripheral clocks in the organs, thus creating a state of metabolic dysfunction that predisposes to HTN.66,97,98 Short sleepers and shift workers may also have irregular eating behaviors including longer food intake hours, increased nighttime eating and meal timing variability.81,97 Collectively, these behaviors disrupt circadian rhythmicity, including 24-h BP diurnal patterns66,99 , leading to non-dipping BP, higher daytime, nighttime, and 24-h BP, and increased HTN risk.66,98,100,101
Furthermore, changes to circadian clock genes may represent another mechanism through which sleep disturbances lead to elevated BP. The central clock in the suprachiasmatic nucleus of the hypothalamus, which regulates the endogenous circadian rhythm, interacts with peripheral clocks located throughout the body including within the brain, nervous system, kidney, heart, and vasculature to regulate BP throughout the 24-h period.102 This central clock is entrained by external cues including light, temperature, humidity, and feeding times.103 Through interaction with peripheral clocks, the circadian clock controls various intracellular processes such as transcription, translation, and protein post-translational modifications including phosphorylation, acetylation, and ubiquitylation, as well as degradation.103 In fact, a group of transcription factors within the circadian clocks regulate gene expression through a series of translational feedback loops.104 Studies using mostly animal models have demonstrated that several peripheral circadian clock proteins and genes including Circadian Locomotor Output Cycles Kaput (CLOCK), aryl hydrocarbon receptor nuclear translocator–like protein 1 (also known as Bmal1), Period (per1, per2, and per3), and Cryptochrome (cry1, and cry2) are important regulators of the circadian pattern of BP.102,104 For example, whole-body knock out of the Bmal1 gene in mice leads to a loss of the circadian rhythm of BP compared to wild-type controls.104
Clock genes also play an important role in regulating sleep homeostasis. Several studies have demonstrated that sleep deprivation in adult rats can lead to changes in gene expression in the brain.105-108 This includes expression of the circadian clock genes per1 and per2.105,109,110 Studies in humans are also suggestive that sleep deprivation can affect gene expression and circadian disruption. In a study of 12 males, one night of sleep deprivation led to suppression of Bmal1.111 Similarly, in vitro studies have demonstrated that hypoxia can increase hypoxia inducible factor-1α (HIF-1α) levels which induces hypoxic response genes112 and can lead to circadian misalignment.113 HIF-1α is chronically elevated in OSA patients. A small study among 20 patients (10 with severe OSA vs 10 without OSA) showed that OSA patients had higher levels of HIF-1α. Higher levels of HIF-1α were correlated with higher peripheral clock proteins including Per1, cry1, Bmal, and CLOCK114 suggesting that OSA patients may be at higher risk of circadian dysregulation. While preliminary, these studies may provide possible insights as to how short sleep duration and separately hypoxia, may act at the molecular level by disrupting normal circadian rhythm, ultimately leading to higher BP.103,107,108
Finally, sleep disturbances may increase HTN risk via their influence on psychological factors, including depression and anxiety. Indeed, a bi-directional association likely exists between sleep and depression and anxiety. Short versus normal sleep duration has been linked to 31% higher risk for depression.115 Co-morbid depression has been reported in OSA patients116 , and OSA is considered an independent risk factor for depression.117 Shift work disorder, which refers to sleep impairment (persistent and severe sleep disturbance during the sleep period and/or excessive sleepiness during the wake period) resulting from shift work, is also strongly associated with depressive symptoms and anxiety, particularly among female shift workers.118,119 Depression and anxiety have in turn been linked to reduced nighttime BP dipping, higher BP, and greater risk of prevalent and incident HTN.120
Knowledge Gaps and Future Research Directions
While recent studies add to the evidence base demonstrating that short sleep, shift work, and OSA are independently associated with HTN risk, higher BP and 24-h ambulatory BP, these data have a number of limitations. Although several sleep studies, nested within population-based cohorts, have objectively-assessed sleep measures, much of the existing literature on short sleep duration in relation to BP relies on self-reported sleep duration, which is only moderately correlated with objective sleep and has been shown to be systematically biased.121 Further, it is important to note that different questions/questionnaires were used for measurement of self-reported sleep duration and distinct metrics may be used to estimate subjective and objective sleep duration (e.g., reported bedtime vs. sleep onset time); this limits comparability of findings among studies. Thus, future studies with objectively assessed sleep (i.e. wrist actigraphy) that utilize consistent methodologies for estimating sleep duration are necessary to facilitate comparability of study findings and to move this field of research forward.
Most studies examining the association between short sleep and BP are cross-sectional. Studies published after the release of the 2017 AHA/ACC updated definition for HTN9 , continue to use the 140/80 mmHg threshold, which may lead to underestimation of the associations between sleep disturbances and prevalent HTN. Further, studies with longitudinal and/or continuous BP measurement are lacking. For example, changes in BP after exposure to multiple sequential shifts are not adequately captured. Importantly, few studies have examined racial/ethnic and/or sex differences or explored how structural level factors including racism, neighborhood environment (noise and poverty), and socio-economic status affect the association between sleep disturbances and HTN.
In studies of shift work, shift work type/duration/timing, occupation, and recovery time between shifts are often not considered. OSA studies often have limited sample sizes that may not account for OSA severity and the long-term effects of CPAP treatment on BP control. In addition, how sleep quality, sleep variability, and insomnia affect the associations of sleep duration, shift work, and OSA with BP remains unknown. Finally, it is notable that existing studies on sleep disturbances and BP significantly vary in adjustment for potential confounders, which limits comparability of findings across the studies. Studies may also have residual confounding, as data on other psychosocial factors, lifestyle behaviors (e.g., caffeine intake, alcohol use), chronotype, occupational exposures, underlying health status and co-morbidities, were not routinely assessed or accounted for in statistical analyses.
Therefore, critical knowledge gaps remain (Table 1). Additional prospective cohort studies, and/or ancillary studies, with multiple time points using objective sleep assessments are needed to elucidate the longitudinal associations of sleep disturbances with HTN risk and BP control, underlying mechanisms, and, interventions. Longitudinal assessment of lifestyle behaviors and out-of-office BP using ABPM or home blood pressure monitoring is needed. Finally, studies of how multi-dimensional sleep health contributes to HTN risk are needed.
Table 1.
Unanswered Research Questions on Sleep Disturbances and Blood Pressure*
| Sleep Disturbance Type | Questions |
|---|---|
| Short Sleep |
|
| Shift Work |
|
| Obstructive Sleep Apnea |
|
| General |
|
BP: blood pressure; CPAP: continuous positive airway pressure; CVD: cardiovascular disease; HTN: hypertension; OSA: obstructive sleep apnea
Conclusion
Sleep disorders and unhealthy sleep behaviors often occur concurrently. Empirically supported sleep health interventions represent a critical next step to advance this research area and establish causality. If supported, increased efforts should be made to identify and treat sleep disturbances for the prevention and management of HTN. Addressing the effects of sleep disturbances on BP may have significant public health impact for lowering the HTN burden, especially among populations at disproportionate risk.
Acknowledgments
Sources of Funding: NM is funded by a NIH/NHLBI K99/R00 Pathway to Independence Award (R00-HL148511). MA is funded by 18AMFDP34380732 from the AHA and from the NIH/NHLBI (K23 HL141682-01A1 and R01HL146636-01A1). CA is funded by the NIH/NHLBI (K23 HL125748) and AHRQ (R01HS024274). NW is funded by NIH/NHLBI (K23 HL125939 and K23 HL125939S1). NB is funded by the NIH/NHLBI (K23 HL163853-03 and R01 HL153382-01) and the Louis Katz Foundation and Victoria and Esther Aboodi Cardiology Researcher Fund.
References
- 1.Centers for Disease Control and Prevention. Short sleep duration among US adults. https://www.cdc.gov/sleep/data_statistics.html. Updated May 2, 2017. Accessed January 7, 2021.
- 2.Liu Y Prevalence of healthy sleep duration among adults—United states, 2014. MMWR. Morbid Mortal Wkly Rep. 2016;65(6):137–141. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Short sleep duration among workers—United states, 2010. MMWR morb mortal wkly rep. 2012; 61:281–28. [PubMed] [Google Scholar]
- 4.Yong LC, Li J, Calvert GM. Sleep-related problems in the US working population: Prevalence and association with shiftwork status. Occup Environ Med. 2017;74(2):93–104. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Lagsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG. Shift work and vascular events: Systematic review and meta-analysis. BMJ. 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Onge M, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the american heart association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman HR, Agarwal S, Caldwell JA, Fulgoni VL III. Demographics, sleep, and daily patterns of caffeine intake of shift workers in a nationally representative sample of the US adult population. Sleep. 2020;43(3):zsz240. [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017:24430. [DOI] [PubMed] [Google Scholar]
- 10.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: A systematic review and meta-analysis. J Hypertens. 2017;35(10):1929–1937. [DOI] [PubMed] [Google Scholar]
- 11.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. [DOI] [PubMed] [Google Scholar]
- 12.Makarem N, Shechter A, Carnethon MR, Mullington JM, Hall MH, Abdalla M. Sleep duration and blood pressure: Recent advances and future directions. Curr Hypertens Rep. 2019;21(5):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, Loredo JS, Reid KJ, Zee PC, Mossavar-Rahmani Y, Patel SR. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto T, Murase K, Tabara Y, Gozal D, Smith D, Minami T, Tachikawa R, Tanizawa K, Oga T, Nagashima S, et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: The Nagahama study. Sleep. 2018; 41(7). [DOI] [PubMed] [Google Scholar]
- 15.Ren R, Covassin N, Yang L, Li Y, Zhang Y, Zhou J, Tan L, Li T, Li X, Wang Y, et al. Objective but not subjective short sleep duration is associated with hypertension in obstructive sleep apnea. Hypertension. 2018;72(3):610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drager LF, Santos RB, Silva WA, Parise BK, Giatti S, Aielo AN, Souza SP, Furlan SF, Lorenzi-Filho G, Lotufo PA, Bensenor IM, et al. OSA, short sleep duration, and their interactions with sleepiness and cardiometabolic risk factors in adults: The ELSA-brasil study. Chest. 2019;155(6):1190–1198. [DOI] [PubMed] [Google Scholar]
- 17.Shulman R, Cohen DL, Grandner MA, Gislason T, Pack AI, Kuna ST, Townsend RR, Cohen JB. Sleep duration and 24-hour ambulatory blood pressure in adults not on antihypertensive medications. J Clin Hypertens. 2018;20(12):1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle CY, Ruiz JM, Taylor DJ, Smyth JW, Flores M, Dietch JR, Ahn C, Allison M, Smith TW, Uchino BN. Associations between objective sleep and ambulatory blood pressure in a community sample. Psychosom Med. 2019;81(6):545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuetting DL, Feisst A, Sprinkart AM, Homsi R, Luetkens J, Thomas D, Schild HH, Dabir D. Effects of a 24-hr-shift-related short-term sleep deprivation on cardiac function: A cardiac magnetic resonance-based study. J Sleep Res. 2018:e12665. [DOI] [PubMed] [Google Scholar]
- 20.Chen IY, Jarrin DC, Ivers H, Morin CM. Investigating psychological and physiological responses to the trier social stress test in young adults with insomnia. Sleep Med. 2017;40:11–22. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. J Hypertens. 2017;35(6):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St-Onge M, Campbell A, Aggarwal B, Taylor JL, Spruill TM, RoyChoudhury A. Mild sleep restriction increases 24-hour ambulatory blood pressure in premenopausal women with no indication of mediation by psychological effects. Am Heart J. 2020;223:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose–response relationship. Scand J Work Environ Health. 2018;44(3):229–238 [DOI] [PubMed] [Google Scholar]
- 24.Yeom JH, Sim CS, Lee J, Yun SH, Park SJ, Yoo CI, Sung JH. Effect of shift work on hypertension: Cross sectional study. Ann Occup Environ Med. 2017;29(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu K, Chen J, Wang L, Wang C, Ding R, Wu S, Hu D. Association of sleep duration, sleep quality and shift-work schedule in relation to hypertension prevalence in chinese adult males: A cross-sectional survey. Int J Environ Res Public Health. 2017;14(2):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotenberg L, Silva-Costa A, Vasconcellos-Silva PR, Griep RH. Work schedule and self-reported hypertension–the potential beneficial role of on-shift naps for night workers. Chronobiol Int. 2016;33(6):697–705. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson JM, Costello S, Neophytou AM, Balmes JR, Bradshaw PT, Cullen MR, Eisen EA. Night and rotational work exposure within the last 12 months and risk of incident hypertension. Scand J Work Environ Health. 2019;45(3):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahim A, McIsaac MA, Aronson KJ, Smith PM, Tranmer JE. The associations of shift work, sleep quality and incident of hypertension in ontario adults: A population-based study. Can J Cardiol. 2020. 10.1016/j.cjca.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 29.Patterson PD, Mountz KA, Budd CT, Bubb JL, Hsin AU, Weaver MD, Turner RL, Platt TE, Guyette FX, Martin-Gill C, Buysse DJ. Impact of shift work on blood pressure among emergency medical services clinicians and related shift workers: A systematic review and meta-analysis. Sleep Health. 2020;6(3):387–398. [DOI] [PubMed] [Google Scholar]
- 30.Park J, Shin S, Kang Y, Rhie J. Effect of night shift work on the control of hypertension and diabetes in workers taking medication. Ann Occup Environ Med. 2019;31(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres G, Sánchez-de-la-Torre M, Barbé F. Relationship between OSA and hypertension. Chest. 2015;148(3):824–832. [DOI] [PubMed] [Google Scholar]
- 32.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ. 2000;320(7233):479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin EJ, Muntner P, Bittencourt MS. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 35.Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, Xing W, Wang W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DA, Thomas SJ, Abdalla M, Guo N, Yano Y, Rueschman M, Tanner RM, Mittleman MA, Calhoun DA, Wilson JG, Muntner P. Association between sleep apnea and blood pressure control among blacks: Jackson heart sleep study. Circulation. 2019;139(10):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Blood pressure non-dipping and obstructive sleep apnea syndrome: A meta-analysis. Journal of clinical medicine. 2019;8(9):1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the wisconsin sleep cohort study. Sleep. 2008;31(6):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crinion SJ, Ryan S, Kleinerova J, Kent BD, Gallagher J, Ledwidge M, McDonald K, McNicholas WT.. Nondipping nocturnal blood pressure predicts sleep apnea in patients with hypertension. J Clin Sleep Med. 2019;15(7):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genta-Pereira DC, Furlan SF, Omote DQ, Giorgi DM, Bortolotto LA, Lorenzi-Filho G, Drager LF. Nondipping blood pressure patterns predict obstructive sleep apnea in patients undergoing ambulatory blood pressure monitoring. Hypertension. 2018;72(4):979–985. [DOI] [PubMed] [Google Scholar]
- 41.Cho JS, Ihm S, Kim CJ, Park MW, Her SH, Park GM, Kim TS. Obstructive sleep apnea using Watch-PAT 200 is independently associated with an increase in morning blood pressure surge in never-treated hypertensive patients. J Clin Hypertens. 2015;17(9):675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavie-Nevo K, Pillar G. Evening–morning differences in blood pressure in sleep apnea syndrome: Effect of gender. Am J Hypertens. 2006;19(10):1064–1069. [DOI] [PubMed] [Google Scholar]
- 43.Ting H, Lo H, Chang S, Chung AH, Kuan PC, Yuan SC, Huang CN, Lee SD. Post-to pre-overnight sleep systolic blood pressures are associated with sleep respiratory disturbance, pro-inflammatory state and metabolic situation in patients with sleep-disordered breathing. Sleep Med. 2009;10(7):720–725. [DOI] [PubMed] [Google Scholar]
- 44.Mokros L, Kuczynski W, Franczak L, Bialasiewicz P. Morning diastolic blood pressure may be independently associated with severity of obstructive sleep apnea in non-hypertensive patients: A cross-sectional study. J Clin Sleep Med. 2017;13(7):905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrone O, Bonsignore MR. Blood-pressure variability in patients with obstructive sleep apnea: Current perspectives. Nat Science Sleep. 2018;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baguet JP, Boutin I, Barone-Rochette G, Levy P, Tamisier R, Pierre H, Boggetto-Graham L, Pépin JL. Hypertension diagnosis in obstructive sleep apnea: Self or 24-hour ambulatory blood pressure monitoring? Int J Cardiol. 2013;167(5):2346–2347. [DOI] [PubMed] [Google Scholar]
- 47.Baguet JP, Levy P, Barone-Rochette G, Tamisier R, Pierre H, Peeters M, Mallion JM, Pépin JL. Masked hypertension in obstructive sleep apnea syndrome. J Hypertens. 2008;26(5):885–892. [DOI] [PubMed] [Google Scholar]
- 48.Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DM, Lorenzi-Filho G, Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23(3):249–254. [DOI] [PubMed] [Google Scholar]
- 49.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: A systematic review and meta-analysis. JAMA. 2015;314(21):2280–2293. [DOI] [PubMed] [Google Scholar]
- 50.Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, Narkiewicz K, Minuz P. Effect of CPAP on blood pressure in patients with OSA/hypopnea: A systematic review and meta-analysis. Chest. 2014;145(4):762–771. [DOI] [PubMed] [Google Scholar]
- 51.Iftikhar IH, Valentine CW, Bittencourt LR, Cohen DL, Fedson AC, Gíslason T, Penzel T, Phillips CL, Yu-sheng L, Pack AI, Magalang UJ. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: A meta-analysis. J Hypertens. 2014;32(12):2341–50; discussion 2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: A systematic review and meta-analysis. Sleep Med. 2013;14(4):324–332. [DOI] [PubMed] [Google Scholar]
- 53.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch Intern Med. 2009;169(11):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: The sleep heart health study. Sleep. 2006;29(8):1009–1014. [DOI] [PubMed] [Google Scholar]
- 55.Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, Freudenheim JL, Kandala NB, Miller MA, Trevisan M. A population-based study of reduced sleep duration and hypertension: The strongest association may be in premenopausal women. J Hypertens. 2010;28(5):896–902. [DOI] [PubMed] [Google Scholar]
- 56.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep duration and hypertension: Analysis of> 700,000 adults by age and sex. J Clin Sleep Med. 2018;14(06):1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: Results from the nurses’ health study. Am J Hypertens. 2013;26(7):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cano-Pumarega I, Durán-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martínez-Null C, de Miguel J, Egea C, Cancelo L, Álvarez A, Fernández-Bolaños M. Obstructive sleep apnea and systemic hypertension: Longitudinal study in the general population: The vitoria sleep cohort. Am J Resp Crit Care Med. 2011;184(11):1299–1304. [DOI] [PubMed] [Google Scholar]
- 59.Cano-Pumarega I, Barbé F, Esteban A, Egea C, Durán-Cantolla J, Montserrat JM, Muria B, de la Torre MS, Fernández AA. Sleep apnea and hypertension: Are there sex differences? the vitoria sleep cohort. Chest. 2017;152(4):742–750. [DOI] [PubMed] [Google Scholar]
- 60.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carnethon MR, De Chavez PJ, Zee PC, Kim KY, Liu K, Goldberger JJ, Ng J, Knutson KL. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago area sleep study. Sleep Med. 2016;18:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandey A, Williams N, Donat M, Ceide M, Brimah P, Ogedegbe G, McFarlane SI, Jean-Louis G. Linking sleep to hypertension: Greater risk for blacks. Int J Hypertens. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African–Americans. J Hypertens. 2012;30(1):61–66. [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32(3):417–423. [DOI] [PubMed] [Google Scholar]
- 65.Sands-Lincoln M, Grandner M, Whinnery J, Keenan BT, Jackson N, Gurubhagavatula I. The association between obstructive sleep apnea and hypertension by race/ethnicity in a nationally representative sample. J Clin Hypertens. 2013;15(8):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27(10):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep. 2009;32(4):447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68(1):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castro-Diehl C, Roux AVD, Redline S, Seeman T, McKinley P, Sloan R, Shea S. Sleep duration and quality in relation to autonomic nervous system measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39(11):1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: Ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–1814. [DOI] [PubMed] [Google Scholar]
- 72.Cappuccio FP, Taggart FM, Kandala N, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun M, Feng W, Wang F, Li P, Li Z, Li M, Tse G, Vlaanderen J, Vermeulen R, Tse LA. Meta-analysis on shift work and risks of specific obesity types. Obesity Rev. 2018;19(1):28–40. [DOI] [PubMed] [Google Scholar]
- 74.Carter III R, Watenpaugh DE. Obesity and obstructive sleep apnea: Or is it OSA and obesity? Pathophysiology. 2008;15(2):71–77. [DOI] [PubMed] [Google Scholar]
- 75.Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S, Li M, Miao X, Chan EY, Tang JL, Wong MC. Meta-analysis on night shift work and risk of metabolic syndrome. Obesity Rev. 2014;15(9):709–720. [DOI] [PubMed] [Google Scholar]
- 76.Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–78. [DOI] [PubMed] [Google Scholar]
- 77.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007;3(10):667–667. [DOI] [PubMed] [Google Scholar]
- 79.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: Analyses of the first national health and nutrition examination survey. Hypertension. 2006;47(5):833–839. [DOI] [PubMed] [Google Scholar]
- 80.Ohlander J, Keskin M, Stork J, Radon K. Shift work and hypertension: Prevalence and analysis of disease pathways in a german car manufacturing company. Am J Ind Med. 2015;58(5):549–560. [DOI] [PubMed] [Google Scholar]
- 81.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. [DOI] [PubMed] [Google Scholar]
- 83.Crispim CA, Waterhouse J, Dâmaso AR, Zimberg IZ, Padilha HG, Oyama LM, Tufik S, De Mello MT. Hormonal appetite control is altered by shift work: A preliminary study. Metab Clin Exp. 2011;60(12):1726–1735. [DOI] [PubMed] [Google Scholar]
- 84.James SM, Honn KA, Gaddameedhi S, Van Dongen HP. Shift work: Disrupted circadian rhythms and sleep—implications for health and well-being. Current Sleep Med Rep. 2017;3(2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hulsegge G, Boer JM, van der Beek, Allard J, Verschuren WM, Sluijs I, Vermeulen R, Proper KI. Shift workers have a similar diet quality but higher energy intake than day workers. Scand J Work Environ Health. 2016:459–468. [DOI] [PubMed] [Google Scholar]
- 86.Reid M, Maras JE, Shea S, Wood AC, Castro-Diehl C, Johnson DA, Huang T, Jacobs DR Jr, Crawford A, St-Onge MP, Redline S. Association between diet quality and sleep apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2019;42(1):zsy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McClain JJ, Lewin DS, Laposky AD, Kahle L, Berrigan D. Associations between physical activity, sedentary time, sleep duration and daytime sleepiness in US adults. Prev Med. 2014;66:68–73. [DOI] [PubMed] [Google Scholar]
- 88.Flo E, Pallesen S, Akerstedt T, Magerøy N, Moen BE, Grønli J, Nordhus IH, Bjorvatn B. Shift-related sleep problems vary according to work schedule. Occup Environ Med. 2013;70(4):238–245. [DOI] [PubMed] [Google Scholar]
- 89.Björnsdóttir E, Janson C, Gíslason T, Sigurdsson JF, Pack AI, Gehrman P, Benediktsdóttir B. Insomnia in untreated sleep apnea patients compared to controls. J Sleep Res. 2012;21(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hulsegge G, Loef B, van Kerkhof LW, Roenneberg T, van der Beek, Allard J, Proper KI. Shift work, sleep disturbances and social jetlag in healthcare workers. J Sleep Res. 2019;28(4):e12802. [DOI] [PubMed] [Google Scholar]
- 92.Chin K, Oga T, Takahashi K, Takegami M, Nakayama-Ashida Y, Wakamura T, Sumi K, Nakamura T, Horita S, Oka Y, Minami I. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in japan. Sleep. 2010;33(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–151. [DOI] [PubMed] [Google Scholar]
- 95.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. Hypothesis: Shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. 2003;52(11):2652–2656. [DOI] [PubMed] [Google Scholar]
- 97.St-Onge M, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal timing and frequency: Implications for cardiovascular disease preventionA scientific statement from the american heart association. Circulation. 2017;135(9):e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer FA. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goncharuk VD, Van Heerikhuize J, Dai J, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol. 2001;431(3):320–330. [DOI] [PubMed] [Google Scholar]
- 100.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawano Y Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34(3):281. [DOI] [PubMed] [Google Scholar]
- 102.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 2018;119:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Firsov D, Bonny O. Circadian rhythms and the kidney. Nat Rev Nephrol. 2018;14(10):626–635. [DOI] [PubMed] [Google Scholar]
- 104.Rhoads MK, Balagee V, Thomas SJ. Circadian regulation of blood pressure: Of mice and men. Curr Hypertens Rep. 2020;22(6):40-020-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A, Franken P, Lein ES, Kilduff TS. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci. 2008;28(28):7193–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41(1):35–43. [DOI] [PubMed] [Google Scholar]
- 107.Terao A, Wisor J, Peyron C, Apte-Deshpande A, Wurts SW, Edgar DM, Kilduff TS. Gene expression in the rat brain during sleep deprivation and recovery sleep: An affymetrix GeneChip® study. Neuroscience. 2006;137(2):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: A key function of sleep. Physiol Genomics. 2007;31(3):441–457. [DOI] [PubMed] [Google Scholar]
- 109.Wisor JP, O'Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. A role for cryptochromes in sleep regulation. BMC Neuroscience. 2002;3(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franken P, Thomason R, Heller HC, O'Hara BF. A non-circadian role for clock-genes in sleep homeostasis: A strain comparison. BMC Neuroscience. 2007;8(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ackermann K, Plomp R, Lao O, Middleton B, Revell VL, Skene DJ, Kayser M. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol Int. 2013;30(7):901–909. [DOI] [PubMed] [Google Scholar]
- 112.Jaspers T, Morrell M, Simonds A, Adcock I, Durham A. The role of hypoxia and the circadian rhythm in sleep apnoea. Eur Respir J. 2015;46(59): OA298. [Google Scholar]
- 113.Manella G, Aviram R, Bolshette N, Muvkadi S, Golik M, Smith DF, Asher G. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci U S A. 2020;117(1):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gabryelska A, Sochal M, Turkiewicz S, Białasiewicz P. Relationship between HIF-1 and circadian clock proteins in obstructive sleep apnea Patients—Preliminary study. J Clin Med. 2020;9(5):1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–670. [DOI] [PubMed] [Google Scholar]
- 116.BaHammam AS, Kendzerska T, Gupta R, Ramasubramanian C, Neubauer DN, Narasimhan M, Pandi-Perumal SR, Moscovitch A. Comorbid depression in obstructive sleep apnea: An under-recognized association. Sleep Breath. 2016;20(2):447–456. [DOI] [PubMed] [Google Scholar]
- 117.Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: Evidence and potential mechanisms. American J Geriatr Psychiatry. 2016;24(6):496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Booker LA, Sletten TL, Alvaro PK, Barnes M, Collins A, Chai-Coetzer CL, Naqvi A, McMahon M, Lockley SW, Rajaratnam SM, Howard ME. Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses. J Sleep Res. 2020;29(3):e12872. [DOI] [PubMed] [Google Scholar]
- 119.Torquati L, Mielke GI, Brown WJ, Burton NW, Kolbe-Alexander TL. Shift work and poor mental health: A meta-analysis of longitudinal studies. Am J Public Health. 2019;109(11):e13–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cohen BE, Edmondson D, Kronish IM. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28(11):1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19(6):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]


