Abstract
Background
Measuring fluid status during intraoperative hemorrhage is challenging, but detection and quantification of fluid overload is far more difficult. Using a porcine model of hemorrhage and over-resuscitation, it is hypothesized that centrally obtained hemodynamic parameters will predict volume status more accurately than peripherally obtained vital signs.
Methods
Eight anesthetized female pigs were hemorrhaged at 30 mL/min to a blood loss of 400 mL. After each 100 mL of hemorrhage, vital signs (heart rate, systolic blood pressure [SBP], mean arterial pressure [MAP], diastolic blood pressure [DBP], pulse pressure, pulse pressure variation) and centrally obtained hemodynamic parameters (mean pulmonary artery pressure [MPAP], pulmonary capillary wedge pressure [PCWP], central venous pressure [CVP] and cardiac output [CO]) were obtained. Blood volume was restored, and the pigs were over-resuscitated with 2,500 mL of crystalloid, collecting parameters after each 500 mL bolus. Hemorrhage and resuscitation phases were analyzed separately to determine differences among parameters over the range of volume. Conformity of parameters during hemorrhage or over-resuscitation was assessed.
Results
Over the course of hemorrhage, changes from baseline euvolemia were observed in vital signs SBP, DBP, MAP after 100 mL of blood loss. Central hemodynamic parameters MPAP and PCWP were changed after 200 mL blood loss, and CVP, after 300 mL of blood loss. Over the course of resuscitative volume overload, changes were observed from baseline euvolemia in MPAP and CVP after 500 mL resuscitation, in PCWP after 1,000 mL resuscitation and CO after 2,500 mL resuscitation. In contrast to hemorrhage, vital sign parameters did not change during over-resuscitation. The strongest linear correlation was observed with PCWP in both hemorrhage (r2=0.99) and volume overload (r2=0.98).
Conclusions
Pulmonary capillary wedge pressure is the most accurate parameter to track both hemorrhage and over-resuscitation, demonstrating the unmet clinical need for a less-invasive PCWP equivalent.
Introduction:
Fluid management is the most common therapeutic intervention during anesthesia and can dramatically influence surgical outcomes.1,2 Maintaining accurate fluid replacement (“Goal Directed Fluid Therapy;” GDFT) is associated with decreased 30-day postoperative morbidity and mortality.3,4 Inadequate resuscitation can lead to systemic hypoperfusion and its sequelae. Conversely, excessive resuscitation, particularly in patients with diminished cardiopulmonary reserve, can result in pulmonary and peripheral edema, increased ventilator requirements and mortality.5
Clinical signs of hypovolemia such as decreased urine output, altered mentation and decreased skin turgor lack precision and represent delayed manifestations of intravascular volume loss.6,7 Vital signs including heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) are routinely monitored for all surgical procedures and used as indicators of volume status. Central venous catheters can be used to determine the central venous pressure (CVP), a surrogate for preload, whose usefulness is confounded by variability in intrathoracic pressure, peripheral vascular tone and cardiac function.8 A pulmonary arterial catheter (PAC) can provide more accurate measures of volume status using central measures of cardiac filling, including mean pulmonary arterial pressure (MPAP), cardiac output (CO), and pulmonary capillary wedge pressure (PCWP), though imperfect, often considered the gold-standard for intravascular volume status.9 However, PACs are difficult to use accurately and have been associated with potentially severe complications such as pneumothorax and pulmonary artery rupture.9
Less invasive methods such as transthoracic or transesophageal echocardiography can provide critical information about preload and cardiac function, though these techniques requires user expertise and can be cumbersome.10 Additional non-invasive surrogates such as noninvasive cardiac output monitoring (NICOM; Baxter, Deerfield, IL) use principles of thoracic bioimpedence or bioreactance to estimate CO, though are prone to motion artifact, require absence of dysrhythmia and are not validated in heart failure and cardiogenic shock.11,12 Vital signs and central hemodynamic parameters are measurements reflecting a single given time point, and are termed static measurements. Cardiovascular parameters reflective of real-time changes in preload indices over a given respiratory cycle are referred to a dynamic measurements, and include pulse pressure variation (PPV), stroke volume variation (SVV) and systolic pressure variation.13 These parameters are more accurate than vital signs in predicting fluid responsiveness, the ability to generate an increase in stroke volume proportional to the administered volume.14 However, they require high tidal volumes during mechanical ventilation, regular HR, and heavy sedation for accuracy.15 Thus, to improve intraoperative monitoring of volume shifts, it is imperative to understand how vital signs and central hemodynamic parameters change throughout the entire spectrum of volume changes during anesthetic management, from hypovolemia to euvolemia to hypervolemia.
In this investigation, a porcine model of controlled hemorrhage followed by resuscitation and subsequent over-resuscitation was used to analyze static and dynamic peripherally and centrally obtained hemodynamic measurements. The hypothesis was that centrally obtained hemodynamic parameters would be most accurate in assessing volume status over the course of moderate hemorrhage and over-resuscitation, as alternative and less invasive parameters commonly used clinically have limitations that may restrict the ability to sustain accurate fluid management during anesthesia.
Materials and Methods:
The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee (protocol M1800176-00), and National Institute of Health Guidelines for the care and use of laboratory animals were strictly followed. This experiment utilized a series of eight sequential 40-45 kg female Yorkshire pigs (Oak Hill Genetics, Ewing, IL) of approximately 12 weeks of age, used in the order received. The gender was chosen to facilitate easier urinary catheterization. Sample size was determined based on analogous experiments from our research group, considering the principle of reduction of animal specimens though sufficiently powering the study to mitigate the need for further animal use.16,17 No formal statistical power calculation was conducted. Experiments were performed in the Vanderbilt University Animal Operating Room (OR) facility, starting in the early morning. No randomization or blinding was employed.
General anesthesia was induced using a standard, widely utilized induction combination of ketamine (2.2 mg/kg)/xylazine (2.2 mg/kg)/telazol (4.4 mg/kg) administered through an intravenous catheter placed in an ear vein, and maintained with 1% isoflurane (Primal, Boston, MA).18,19 Pigs were intubated and maintained on volume-control ventilation at a tidal volume of 8 mL/kg, with respiratory rate titrated to an end-tidal CO2 of 35-40 mmHg, and a positive end-expiratory pressure of 5 cm H20.20 Intravenous unfractionated heparin was administered as a 10,000 international unit bolus initially, with 5,000 additional units every two hours.
Surgical exposure of bilateral internal jugular veins allowed for placement of a PAC (Edwards Lifesciences, Irvine, CA) and an 8.5 French catheter for blood removal. An arterial line was placed in the internal carotid artery and used to record continuous measurements of HR, SBP, DBP and MAP. Pulse pressure (PP) was taken as the difference between SBP and DBP, and PPV was calculated as the difference between peak pulse pressure at inspiration and expiration during the respiratory cycle.21 Using Lab Chart 8 (ADInstruments, Colorado Springs, CO), one-hundred pulse cycles were selected and input into the blood pressure module. The offline analysis was selected with the arterial pressure signal having a minimum peak height of 5 mmHg, and a minimum height of 5% of the peak height was used. From this analysis, the following parameters were obtained from the signal: HR, SBP, DBP, MAP, PP, and PPV. The PAC was used to transduce MPAP and CVP. Cardiac output was obtained through the PAC using thermodilution. PCWP was obtained at end-expiration after inflation of the PAC balloon with 1.5 mL air and confirmation of restricted right to left blood flow through appropriate change in the pulmonary artery pressure waveform.
After induction and preparation, baseline hemodynamic parameters were obtained (baseline PCWP was 9±2 mmHg [mean±standard deviation]) after thirty minutes of equilibration to mitigate any potential sympathomimetic tachycardia or hypertensive effect of ketamine. Using a mechanical roller-pump, blood was removed at 30 mL per minute, a flow rate chosen to approximate human hemorrhage.16 A total of 400 mL of blood was drawn, representing 10-15% of total blood volume.22 All vital signs and central hemodynamic measurements were obtained after each 100 mL of blood volume was removed, up to 400 mL. The entire hemorrhaged blood volume was returned at a rate of 100 mL per minute until post-hemorrhagic euvolemia was restored. Next, PlasmaLyte (37°C; Baxter) was infused at a rate of 100 mL/min via the same mechanical roller-pump, stopping after each 500 mL bolus for hemodynamic measurements. PlasmaLyte was infused up through 2,500 mL of fluid. Upon completion of the experiment, the pigs were euthanized with sodium pentobarbital (125 mg/kg). No randomization or blinding was used as all the pigs were subjected to the same intervention.
Statistical Analysis
Vital signs and central hemodynamic parameters at each measured volume were reported as means ± standard deviations. The primary outcome measures were strength of linear correlation during both hemorrhage and over-resuscitation phases, as defined by the square of the linear regression correlation coefficient (r2). The r2, representing the variance between the group means accounted for by the linear correlation, was used as the primary measure of goodness of fit.23 The r2 values ranged from 0.00 (no correlation) to 1.00 (perfect linear correlation). All statistical tests compare hemodynamic values among different volume statuses, with pigs (n=8) as the unit of analysis. Baseline (0 mL) values for all parameters were all found to be normally distributed among the eight pigs via the Shapiro-Wilk test; as such, parametric statistical comparison tests were used for analysis and outliers were not considered.
Hemorrhage and resuscitation phases were analyzed separately using one-way Analysis of Variance (ANOVA) with each of the eight pigs representing a repeated measure, to determine whether there were differences among these parameters over the range of volume. Tukey’s post-hoc test of multiple comparisons was used to determine at which volume point a change represented a significant difference from baseline. Volume-based changes in hemodynamic parameters were characterized by simple linear regression analysis, to measure correlation of measured parameters to volume status (volume status was taken as the independent variable and the measured parameter as the dependent variable).24 Parameters that conformed best to a linear trend line over a given course of volume changes were deemed best suited for use as a surrogate for intravascular volume status in that linearity provides optimal predictability of the degree of change expected by a specific volume perturbation.25
It could not be assumed that all parameters would return to their initial euvolemic baseline following blood return after hemorrhage. Therefore, values of all vital signs and central hemodynamic parameters were also compared at their pre-hemorrhagic and resuscitated euvolemic (0 mL) states. Comparisons between all parameters at states of both pre-hemorrhagic and resuscitated euvolemia were performed, using the paired Student’s t test to characterize whether these parameters differed between the two euvolemic states.
A two-tailed P value < 0.05 represented the standard for statistical significance in all analyses. Statistical analysis was conducted using GraphPad Prism 13 (GraphPad Software, San Diego, CA).
Results:
Hemorrhage
There were no missing or excluded data and all animals (n=8 pigs) survived, and were included in the analysis. There was no observed change in HR throughout hemorrhage (P=0.665). Systolic blood pressure (P<0.001), DBP (P<0.001) and MAP (P<0.001) significantly decreased with increasing volume of hemorrhage; changes in SBP, DBP, and MAP were all significant after the first 100 mL of blood removal (representing approximately 3-4% of the total blood volume).22 Pulse pressure (P=0.145) and PPV (P=0.160) were not significantly different over the course of hemorrhage. The central hemodynamic parameters MPAP (P<0.0001), PCWP (P<0.0001) and CVP (P=0.004) significantly decreased over the course of hemorrhage. Significant changes in these three measurements were first realized at hemorrhage volumes of 200 mL, 200 mL, and 300 mL, respectively. In contrast, CO did not significantly decrease over the course of hemorrhage (P=0.092). Mean values of all parameters during hemorrhage are summarized in Table 1. Simple linear correlations of the means of values from all eight pigs at each of the five-volume states achieved during hemorrhage were determined (Table 2). Heart rate had little correlation (r2=0.22) with bled volume, while SBP, DBP, MAP, PP and PPV all demonstrated linear conformity with an r2>0.80. All central hemodynamic parameters demonstrated an r2≥0.98.
Table 1-.
Measured parameters by fluid status: hemorrhage phase
| Fluid Status: | 0 mL | −100 mL | −200 mL | −300 mL | −400 mL | P |
|---|---|---|---|---|---|---|
| Vital Sign Parameters | ||||||
| Heart Rate (beats per minute) | 95±11 | 95±12 | 95±14 | 95±15. | 96±15 | 0.665 |
| SBP (mmHg) | 100±12 | 95±12* | 88±12*** | 82±13** | 78±13* | <0.001 |
| DBP (mmHg) | 68±12 | 63±13* | 58±15* | 53±16** | 49±15** | <0.001 |
| MAP (mmHg) | 83±12 | 77±13* | 71±14** | 64±15** | 60±15** | <0.001 |
| Pulse Pressure (mmHg) | 32±6 | 32±6 | 30±8 | 29±7 | 29±6 | 0.145 |
| Pulse Pressure Variation | 16±11 | 17±12 | 18±8 | 18±5 | 21±11 | 0.160 |
| Central Hemodynamic Parameters | ||||||
| MPAP (mmHg) | 16±3 | 15±4 | 14±4* | 13±3**** | 12±4**** | <0.0001 |
| PCWP (mmHg) | 9±2 | 7±3 | 6.±3* | 5±3** | 4±3*** | <0.0001 |
| CVP (mmHg) | 7±4 | 6±4 | 5±4 | 4±4* | 4±4* | 0.004 |
| CO (L/min) | 3.9±0.7 | 3.8±0.7 | 3.7±0.7 | 3.5±0.7 | 3.4±0.8 | 0.092 |
Values are reported with their standard deviations
P values reflect one-way ANOVA analysis
Relative to 0 milliliters (mL):
P<0.05
P≤0.01
P≤0.001
P≤0.0001
Table 2-.
Linear correlation of all parameters with fluid status
| Hemodynamic Parameter |
r2 (hemorrhage) | r2 (resuscitation) |
|---|---|---|
| Heart Rate (beats per minute) | 0.22 | 0.15 |
| SBP (mmHg) | 0.99 | 0.40 |
| DBP (mmHg) | 0.99 | 0.72 |
| MAP (mmHg) | 0.99 | 0.79 |
| Pulse Pressure (mmHg) | 0.91 | 0.41 |
| Pulse Pressure Variation | 0.84 | 0.74 |
| MPAP (mmHg) | 0.99 | 0.89 |
| PCWP (mmHg) | 0.99 | 0.98 |
| CVP (mmHg) | 0.99 | 0.93 |
| CO (L/min) | 0.98 | 0.95 |
r2 (hemorrhage) refers to the square of the correlation coefficient (between 0 and 1) used to assess goodness-of-fit of the linear relationship between the hemodynamic parameter and the amount of blood hemorrhaged over the course of hemorrhage up to −400 mL. r2 (resuscitation) similarly assessed the linear correlation between the hemodynamic parameter and the amount of excess fluid infused over the course of overload resuscitation, from 0 mL to 2,500 mL.
Resuscitation and volume overload
As with the hemorrhagic phase, there were no missing or excluded data and all animals (n=8) survived, and were included in the analysis. There was no observed change in HR (P=0.183), SBP (P=0.750), DBP (P=0.700), MAP (P=0.669) and PP (P=0.421) throughout resuscitation and volume overload. Pulse pressure variation too was not significant over the course of resuscitation and volume overload (P=0.055). The central hemodynamic parameters MPAP, PCWP, CVP and CO significantly increased over the course of resuscitation and volume overload (P<0.0001 for all). Both MPAP and CVP were significantly greater than their euvolemic values after administration of 500 mL PlasmaLyte, while PCWP and CO were significantly greater at PlasmaLyte volumes of 1,000 mL and 2,500 mL, respectively. Mean values of all parameters during resuscitation and volume overload are summarized in Table 3. Simple linear correlations of the means of values from all eight pigs at each of the six volume states during this phase were determined (Table 2). All vital signs (HR, SBP, DBP, MAP, PP and PPV) demonstrated a linear correlation with resuscitative volume status of r2<0.80. The central hemodynamic parameters MPAP (r2=0.89), PCWP (r2=0.98), CVP (r2=0.93) and CO (r2=0.95) demonstrated strong linear correlations.
Table 3-.
Measured parameters by fluid status: resuscitation and overload phase
| Fluid Status: | 0 mL | 500 mL | 1,000 mL | 1,500 mL | 2,000 mL | 2,500 mL | P |
|---|---|---|---|---|---|---|---|
| Vital Sign Parameters | |||||||
| Heart Rate (beats per minute) | 99±16 | 96±14 | 95±13 | 95±13 | 99±9 | 101±9 | 0.183 |
| SBP (mmHg) | 89±9 | 89±13 | 89±15 | 89±15 | 91±13 | 91±12 | 0.750 |
| DBP (mmHg) | 58±11 | 57±14 | 57±15 | 57±14 | 59±12 | 59±12 | 0.700 |
| MAP (mmHg) | 72±10 | 71±14 | 72±16 | 72±15 | 73±13 | 74±12 | 0.669 |
| Pulse Pressure (mmHg) | 33±6 | 32±7 | 32±7 | 32±7 | 32±7 | 32±7 | 0.421 |
| Pulse Pressure Variation | 20±12 | 16±11 | 11±3 | 10±4 | 8±2 | 11±7* | 0.055 |
| Central Hemodynamic Parameters | |||||||
| MPAP (mmHg) | 17±4 | 20±5* | 22±5*** | 23±5**** | 23±4**** | 24±4*** | <0.0001 |
| PCWP (mmHg) | 8±3 | 10±3 | 12±4* | 15±3**** | 16±3*** | 17±3**** | <0.0001 |
| CVP (mmHg) | 6±4 | 9±4* | 11±4*** | 13±4**** | 13±4**** | 14±4**** | <0.0001 |
| CO (L/min) | 4.3±1.3 | 4.4±1.1 | 4.6±0.88 | 5.1±1.3 | 5.1±1.5 | 5.7±1.0** | <0.0001 |
Values are reported with their standard deviations
P values reflect one-way ANOVA analysis
Relative to 0 milliliters (mL):
P<0.05
P≤0.01
P≤0.001
P≤0.0001
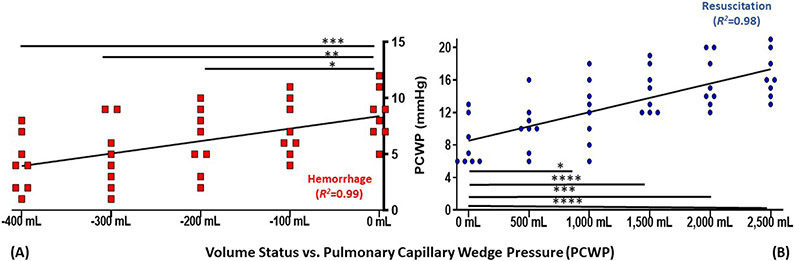
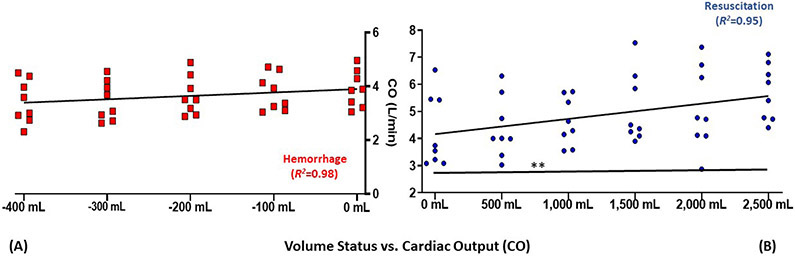
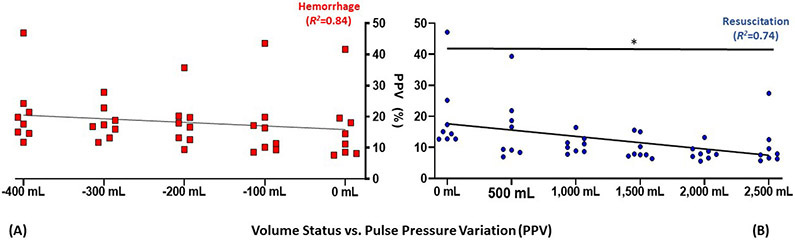
Pulse pressure variation, as the dynamic measurement assessed in this study, PCWP, as the gold-standard, and CO, a representative indicator of central filling assumed to be proportional to volume were examined graphically. The hemorrhage-resuscitation-overload sequences for these variables are depicted in Figure 1, Figure 2, and Figure 3, respectively.
Figure 1-. Graphical depiction of pulmonary capillary wedge pressure (PCWP) during (A) whole blood hemorrhage and (B) crystalloid resuscitation.
Trend line reflects linear regression of means; points reflect each replicate measurement (n=8)
Relative to 0 mL: *P<0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001
mL, millilters
Figure 2-. Graphical depiction of cardiac output (CO) during (A) whole blood hemorrhage and (B) crystalloid resuscitation.
Trend line reflects linear regression of means; points reflect each replicate measurement (n=8)
Relative to 0 mL: **P≤0.01
mL, millilters
Figure 3-. Graphical depiction of pulse pressure variation (PPV) during (A) whole blood hemorrhage and (B) crystalloid resuscitation.
Trend line reflects linear regression of means; points reflect each replicate measurement (n=8)
Relative to 0 mL: *P<0.05
mL, millilters
The parameters SBP, DBP and MAP had still not returned to baseline upon blood volume reinfusion. All other parameters were not significantly different between both euvolemic states (Table 4).
Table 4-.
Comparison of pre-hemorrhagic and resuscitated (post-hemorrhagic) euvolemia
| Parameter | Pre-hemorrhagic Euvolemia | Resuscitated Euvolemia | P |
|---|---|---|---|
| Vital Sign Parameters | |||
| Heart Rate (beats per minute) | 95±11 | 99±16 | 0.300 |
| SBP (mmHg) | 100±12 | 90±9 | 0.008 |
| DBP (mmHg) | 68±12 | 58±11 | 0.005 |
| MAP (mmHg) | 83±12 | 72±10 | 0.006 |
| Pulse Pressure (mmHg) | 32±6 | 33±6 | 0.849 |
| Pulse Pressure Variation | 16±11 | 20±12 | 0.217 |
| Central Hemodynamic Parameters | |||
| MPAP (mmHg) | 16±3 | 17±4 | 0.624 |
| PCWP (mmHg) | 9±2 | 8±3 | 0.584 |
| CVP (mmHg) | 7±4 | 6±4 | 0.487 |
| CO (L/min) | 3.9±0.7 | 4.3±1.3 | 0.283 |
Values are reported with their standard deviations
Discussion:
This investigation provides a comprehensive analysis of vital signs and centrally derived hemodynamic parameters in relation to progressive perturbation of intravascular volume in a porcine model. The model included both hemorrhage for volume loss and resuscitation/volume overload with crystalloid solution to simulate volume overload in a controlled resuscitation such as elective or urgent major surgery. While the assessed indices have previously been characterized in controlled hemorrhage models, there is a dearth of data in analogous models of volume overload. The principal finding is that blood pressure values and centrally obtained hemodynamic indices accurately and consistently change with progressive hemorrhage, while only centrally obtained parameters MPAP, PCWP, CVP and CO change with volume overload resuscitation.
Intraoperative fluid therapy is an element of the perioperative process in which there remains variability among anesthesiology teams. As Enhanced Recovery After Surgery (ERAS) protocols facilitate more cost-effective perioperative care, decreased complications, and shorter lengths of stay, there has been greater recognition of the importance of perioperative fluid management.26 Along with avoidance of opioids and maintenance of normothermia, perioperative GDFT is among the few key evidence-based tenets of successful ERAS protocols primarily influenced by the anesthesiology team.26
The importance of GDFT is perhaps most marked in cases requiring intentional fluid restriction such as major hepatic resection and thoracic surgery. Fluid restrictive approaches prevent acute lung injury and pneumonia after pulmonary resection, pneumonectomy and esophagectomy.27,28 However, intraoperative under-resuscitation poses the risk of systemic hypoperfusion. Incidence of acute kidney injury, the most common manifestation of perioperative fluid restriction, is estimated to be as high as 10% after thoracic surgery.29 Excess fluid during these procedures promotes pulmonary endothelial disruption, fills dependent and residual portions of lung, and can overwhelm the ability of intrathoracic lymphatics to effectively drain.27,30 Thus, both under- and over-resuscitation can have detrimental consequences, underscoring the significant clinical need for accurate monitoring of volume status to support GDFT during anesthetic management.
During hemorrhage, minimal change was observed with HR, consistent with class one hemorrhagic shock.31 Mitigation of early tachycardia can be explained by volume redistribution, hormonally activated compensatory vasoconstriction and parasympathetic reflexes.32 Excellent linear correlations for SBP, DBP and MAP were observed during hemorrhage, with clinically appreciable absolute changes of ~ 20 mmHg detected after 400 mL of hemorrhage. Blood pressure was preserved in early hemorrhage, though this is likely hemorrhage rate-dependent.33,34 Furthermore, SBP, DBP and MAP did not return to pre-hemorrhagic euvolemia values after re-infusion of removed blood, in contrast to other assessed parameters. These findings suggest limitations in using vital signs for detecting and quantifying hemorrhage intraoperatively or in the intensive care unit (ICU) setting.35 Heart rate and blood pressure are even less useful in detecting volume overload, though few studies have examined these changes in controlled experiments.1,36
The two most common dynamic parameters for fluid status assessment supported by most GDFT protocols and ERAS pathways are SVV and PPV. Both have improved sensitivity and specificity in predicting of fluid responsiveness relative to static measures such as CVP.25,37 PPV predicts fluid responsiveness in ventilated patients better than SVV, particularly in patients with lung-protective low tidal volume ventilator strategies, and was thus chosen for assessment in this study.14,38 As illustrated in Figure 3, PPV correlated well with progressive induction of class one hemorrhage, commensurate with absolute blood pressure parameters (SBP, MAP and DBP). Its performance during over-resuscitation was superior to HR, SBP, PP and CVP; however, it was inferior to MPAP, PCWP and CO when examined using linear regression. These results were consistent with the findings of Graham and colleagues in an analogous model of fluid status prediction during hemorrhage and resuscitation in smaller pigs.39 Graham and colleagues further concluded that PPV should not be used as a singular determinant for titration in GDFT, as it is influenced by multiple patient factors including autonomic tone, co-administered medications, and need for constant ventilator settings.39 Additional commonplace factors compromising its use include intra-abdominal hypertension, spontaneous ventilation, poor lung compliance and dysrhythmias.21
Cardiac output demonstrated a strong linear trend with both blood removal as well as fluid overload (Figure 2). As summarized by Mehta and Arora, multiple monitors have been developed for less invasive estimation of CO including the PiCCO system (PiCCO; Gentinge, Germany), the Non-invasive Cardiac Monitor (NICO; Novametrix Medical Systems, Wallingford, CT) system and the Endotracheal Cardiac Output Monitor (ECOM; Con-Med, Irving, CA).40 These devices still require cumbersome or restrictive conditions, including arterial cannulation, regulated ventilation and high tidal volumes. In this experiment, CO correlated linearly with volume status over the volume range examined; deviation is expected at the extremes of hemorrhage and volume overload due to the Starling relationship, however, this did not manifest in the utilized volume range.16
For cardiologists, PCWP is critical in assessing the hemodynamic effect of mitral valve pathology, pulmonary hypertension and left ventricular dysfunction. PCWP is also used to diagnose patients with acute congestive heart failure and guide diuretic therapy, and is a critical determinant of suitability for left ventricular assist device placement.41,42 Despite limited intraoperative adaptation by anesthesiologists, PCWP demonstrated the strongest linear correlation in detecting changes in both hemorrhage as well as volume overload, as illustrated in Figure 1. Its use for intraoperative assessment of cardiac filling and fluid responsiveness is only hindered by both the complexity of PAC usage, and the additional risk conferred by advancement into a distal pulmonary artery. PAC-guided resuscitation may even confer a benefit in trauma patients presenting with advanced hemorrhagic shock, suggesting the effort to place a PAC or use adjuncts such as echocardiography may be warranted in extreme circumstances, rather than relying on more easily obtainable measures.43 These data may perhaps not be surprising, as they confirm the relevance of PCWP as a gold-standard measure of fluid status.
While invasive hemodynamic parameters are the most accurate measures of volume status from hypovolemia to hypervolemia, there has been progress in the development of non-invasive surrogates for volume status as alluded to previously, some of which have gained widespread use in ICU and OR settings. Nonetheless, these data underscore the need for a non-invasive modality commensurate with PCWP to mitigate the need for a PAC while aiding in fluid titration. In contrast to direct measurements as often used with invasive catheters (e.g. PCWP, MPAP), peripherally obtained non-invasive intravascular fluid status is best obtained via interpretation of physiologic waveforms. Derived from photoplethysmogram waveform analysis, compensatory reserve index represents a validated measure of blood volume, useful for highly sensitive detection of small-volume hemorrhage and earlier detection of impending hemodynamic collapse.44 Approaches to arterial waveform interpretation include assessment of PPV, and various forms pulse wave analysis, recently and comprehensively summarized in Anesthesiology.40,45 Finally, though not yet applied clinically, venous waveform analysis has shown promise in detecting fluid status in both pigs and humans via a validated algorithm that considers harmonic amplitudes in fast Fourier transform spectra of peripherally and transcutaneously acquired venous waveforms to produce a “PCWP-equivalent.”16
There are multiple limitations to this study. Ostensibly, the introduction of human error inherent in data collection and interpretation influences the reliability of hemodynamic parameter measurements both within each pig, and among all pigs. The study used healthy female pigs and extrapolating to pathologic states and between genders would require additional studies with appropriate models.46 Moreover, females may respond better to post-hemorrhagic resuscitation than males, potentially lessening external validity of these findings.47 Furthermore, eight pigs were considered, a number thoughtfully chosen to minimize animal use but may be restrictive. This study aimed to critically assess parameters over the spectrum of volume status in a controlled series of clinically germane hemodynamic shifts. However, the sequences of rapid hemorrhage and initial blood resuscitation, and choice of crystalloid for over-resuscitation do not fully mirror an analogous clinical process such as elective surgery with intermittent blood loss, acute surgery with high volume blood loss, or trauma resuscitation. Next, while isoflurane anesthetic is regarded to have minimal effect on vital signs, cardiac and autonomic function, data suggest a blunted sympathetic response to hemorrhage and volume overload that would otherwise manifest in a non-anesthetized human may have occurred.48 Differential responses to hemorrhage and resuscitation among the pigs, as quantified by the standard deviations, were unavoidable and may be due to differences in lung compliance and cardiac function, among other factors.49 Additionally, extrapolation of these results to hemorrhage or resuscitation at faster, slower, or variable rates is limited.50 Finally, myocardial dysfunction may occur due to severe trauma and hemorrhage, a case in which superimposed cardiogenic shock physiology may hinder optimal performance of PCWP and other parameters. Conclusions on monitoring of severe shock, and gauging resuscitation in cases of potential myocardial compromise cannot be made.
This study suggests efficacy and utility of centrally obtained parameters in quantifying intraoperative fluid status throughout hemorrhage and volume overload resuscitation. Despite the recognized limitations, these results support PCWP as a useful measurement of volume status in hemorrhage, while novelly showing its relevancy in controlled volume overload. Given the significant limitations of PAC utilization, establishment of a peripherally obtained PCWP-equivalent for widespread use may be ideal and represents a critical unmet clinical need.
Acknowledgements:
The authors would like to acknowledge the animal care staff at Vanderbilt University, Nashville TN, USA, for their assistance in animal housing, day-to-day care, anesthesia and compliance with all Institutional Animal Care and Use Committee regulations.
Funding Statement: Support for this research was provided by Project # 1R01HL148244-01, from the National Institutes of Health, Potomac, MD, USA
Footnotes
Prior Presentations: This research has not yet been presented. Since the previous submission, it has been accepted as an oral presentation at the SCCM Virtual Care Congress (January 31, 2021-February 12, 2021; exact date, and time TBD)
Conflicts of Interest: Dr. Wise, Dr. Polcz, Dr. Beilman, Dr. Sobey, Dr. Leisy and Dr. Kiberenge declare no competing interests, or potential conflicts of interest. Kyle Hocking, PhD, is founder, CEO and president of VoluMetrix and is an inventor of intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. Colleen Brophy, MD, is founder and CMO of VoluMetrix and an inventor of intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix. Bret Alvis, MD, owns stock in VoluMetrix and is an inventor of intellectual property in the field of venous waveform analysis assigned to Vanderbilt and licensed to VoluMetrix and is married to the COO of VoluMetrix. None of the technology or intellectual property developed by VoluMetrix was considered or utilized at any point in this investigation.
References:
- 1.Malbrain M, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, De Laet I, Minini A, Wong A, Ince C, Muckart D, Mythen M, Caironi P, Van Regenmortel N: Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann Intensive Care 2020; 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sander M, Schneck E, Habicher M: Management of perioperative volume therapy - monitoring and pitfalls. Korean J Anesthesiol 2020; 73: 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aya HD, Cecconi M, Hamilton M, Rhodes A: Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth 2013; 110: 510–7 [DOI] [PubMed] [Google Scholar]
- 4.Som A, Maitra S, Bhattacharjee S, Baidya DK: Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth 2017; 31: 66–81 [DOI] [PubMed] [Google Scholar]
- 5.Assaad S, Kratzert WB, Shelley B, Friedman MB, Perrino A Jr.: Assessment of Pulmonary Edema: Principles and Practice. J Cardiothorac Vasc Anesth 2018; 32: 901–914 [DOI] [PubMed] [Google Scholar]
- 6.Bonasso PC, Sexton KW, Hayat MA, Wu J, Jensen HK, Jensen MO, Burford JM, Dassinger MS: Venous Physiology Predicts Dehydration in the Pediatric Population. J Surg Res 2019; 238: 232–239 [DOI] [PubMed] [Google Scholar]
- 7.Jozwiak M, Monnet X, Teboul JL: Prediction of fluid responsiveness in ventilated patients. Ann Transl Med 2018; 6: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondergaard S, Parkin G, Aneman A: Central venous pressure: we need to bring clinical use into physiological context. Acta Anaesthesiol Scand 2015; 59: 552–60 [DOI] [PubMed] [Google Scholar]
- 9.Joubert I J MFM: The assessment of intravascular volume. South African Journal of Anaesthesia and Analgesia 2007; 15: 33–36 [Google Scholar]
- 10.Whitener S, Konoske R, Mark JB: Pulmonary artery catheter. Best Pract Res Clin Anaesthesiol 2014; 28: 323–35 [DOI] [PubMed] [Google Scholar]
- 11.Saugel B, Thiele RH, Hapfelmeier A, Cannesson M: Technological Assessment and Objective Evaluation of Minimally Invasive and Noninvasive Cardiac Output Monitoring Systems. Anesthesiology 2020; 133: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rali AS, Buechler T, Van Gotten B, Waters A, Shah Z, Haglund N, Sauer A: Non-Invasive Cardiac Output Monitoring in Cardiogenic Shock: The NICOM Study. J Card Fail 2020; 26: 160–165 [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Kaufman DA, Marik PE, Shapiro NI, Levett DZH, Whittle J, MacLeod DB, Chappell D, Lacey J, Woodcock T, Mitchell K, Malbrain M, Woodcock TM, Martin D, Imray CHE, Manning MW, Howe H, Grocott MPW, Mythen MG, Gan TJ, Miller TE: Perioperative Quality Initiative (POQI) consensus statement on fundamental concepts in perioperative fluid management: fluid responsiveness and venous capacitance. Perioper Med (Lond) 2020; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michard F, Lopes MR, Auler JO Jr.: Pulse pressure variation: beyond the fluid management of patients with shock. Crit Care 2007; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantari K, Chang JN, Ronco C, Rosner MH: Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int 2013; 83: 1017–28 [DOI] [PubMed] [Google Scholar]
- 16.Alvis BD, McCallister R, Polcz M, Lima JLO, Sobey JH, Brophy DR, Miles M, Brophy C, Hocking K: Non-Invasive Venous waveform Analysis (NIVA) for monitoring blood loss in human blood donors and validation in a porcine hemorrhage model. J Clin Anesth 2020; 61: 109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polcz M, Hocking KM, Chang D, Leisy P, Sobey JH, Huston J, Eagle S, Brophy C, Alvis BD: A brief report on the effects of vasoactive agents on peripheral venous waveforms in a porcine model. JRSM Cardiovasc Dis 2020; 9: 2048004020940857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise ES, Hocking KM, Luo W, Feldman DL, Song J, Komalavilas P, Cheung-Flynn J, Brophy CM: Traditional graft preparation decreases physiologic responses, diminishes viscoelasticity, and reduces cellular viability of the conduit: A porcine saphenous vein model. Vasc Med 2016; 21: 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radovancevic B, Eichstaedt HC, Tamez D, Patel V, Eya K, Nolden LK, Byler D, Cohen D, Frazier OH: Prolonged controlled hemorrhagic shock in a swine model: is there a role for mechanical circulatory assistance? ASAIO J 2003; 49: 721–6 [DOI] [PubMed] [Google Scholar]
- 20.Wise ES, Hocking KM, Kavic SM: Prediction of excess weight loss after laparoscopic Roux-en-Y gastric bypass: data from an artificial neural network. Surg Endosc 2016; 30: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teboul JL, Monnet X, Chemla D, Michard F: Arterial Pulse Pressure Variation with Mechanical Ventilation. Am J Respir Crit Care Med 2019; 199: 22–31 [DOI] [PubMed] [Google Scholar]
- 22.McGlone J, Pond WG: Pig production : biological principles and applications. Australia ; Clifton Park, NY, Thomson/Delmar Learning, 2003 [Google Scholar]
- 23.Baguley T: Serious stats : a guide to advanced statistics for the behavioral sciences. Houndmills, Basingstoke, Hampshire England: ; New York, Palgrave Macmillan, 2012 [Google Scholar]
- 24.Hocking KM, Sileshi B, Baudenbacher FJ, Boyer RB, Kohorst KL, Brophy CM, Eagle SS: Peripheral Venous Waveform Analysis for Detecting Hemorrhage and Iatrogenic Volume Overload in a Porcine Model. Shock 2016; 46: 447–52 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Jeon Y, Bahk JH, Gil NS, Hong DM, Kim JH, Kim HJ: Pulse pressure variation as a predictor of fluid responsiveness during one-lung ventilation for lung surgery using thoracotomy: randomised controlled study. Eur J Anaesthesiol 2011; 28: 39–44 [DOI] [PubMed] [Google Scholar]
- 26.Ljungqvist O, Scott M, Fearon KC: Enhanced Recovery After Surgery: A Review. JAMA Surg 2017; 152: 292–298 [DOI] [PubMed] [Google Scholar]
- 27.Chau EH, Slinger P: Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014; 18: 36–44 [DOI] [PubMed] [Google Scholar]
- 28.Marret E, Miled F, Bazelly B, El Metaoua S, de Montblanc J, Quesnel C, Fulgencio JP, Bonnet F: Risk and protective factors for major complications after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2010; 10: 936–9 [DOI] [PubMed] [Google Scholar]
- 29.Cardinale D, Cosentino N, Moltrasio M, Sandri MT, Petrella F, Colombo A, Bacchiani G, Tessitore A, Bonomi A, Veglia F, Salvatici M, Cipolla CM, Marenzi G, Spaggiari L: Acute kidney injury after lung cancer surgery: Incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer 2018; 123: 155–159 [DOI] [PubMed] [Google Scholar]
- 30.Licker M, Fauconnet P, Villiger Y, Tschopp JM: Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009; 22: 61–7 [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez G, Reines HD, Wulf-Gutierrez ME: Clinical review: hemorrhagic shock. Crit Care 2004; 8: 373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klabunde RE: Cardiovascular physiology concepts, 2nd edition. Philadelphia, PA, Lippincott Williams & Wilkins/Wolters Kluwer, 2012 [Google Scholar]
- 33.Sondeen JL, Dubick MA, Holcomb JB, Wade CE: Uncontrolled hemorrhage differs from volume- or pressure-matched controlled hemorrhage in swine. Shock 2007; 28: 426–33 [DOI] [PubMed] [Google Scholar]
- 34.Frankel DA, Acosta JA, Anjaria DJ, Porcides RD, Wolf PL, Coimbra R, Hoyt DB: Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J Surg Res 2007; 143: 276–80 [DOI] [PubMed] [Google Scholar]
- 35.Schultz WM, I: Vital signs after haemorrhage- Caution is appropriate. Trends in Anaesthesia and Critical Care 2015; 5: 89–92 [Google Scholar]
- 36.Claure-Del Granado R, Mehta RL: Fluid overload in the ICU: evaluation and management. BMC Nephrol 2016; 17: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherpanath TG, Geerts BF, Lagrand WK, Schultz MJ, Groeneveld AB: Basic concepts of fluid responsiveness. Neth Heart J 2013; 21: 530–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarado Sanchez JI; Caicedo Ruiz JDDF, J.J.l Ospina-Tascon GA; Cruz Martinez LE: Use of Pulse Pressure Variation as Predictor of Fluid Responsiveness in Patients Ventilated With Low Tidal Volume: A Systematic Review and Meta-Analysis. Clin Med Insights Circ Respir Pulm Med. 2020; 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham MR, McCrea K, Girling LG: Pulse pressure variability during hemorrhage and reinfusion in piglets: effects of age and tidal volume. Can J Anaesth 2014; 61: 533–42 [DOI] [PubMed] [Google Scholar]
- 40.Mehta Y, Arora D: Newer methods of cardiac output monitoring. World J Cardiol 2014; 6: 1022–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thayer K, Zweck E, Hernandez-Montfort J, Garan AR, Mahr C, Burkhoff D, Kapur N: Pulmonary Artery Catheter Usage and Mortality in Cardiogenic Shock. J Heart Lung Transplant 2020; 39: S54–S55 [Google Scholar]
- 42.Villela MA, Taleb I, Selzman C, Stehlik J, Dranow E, Wever-Pinzon O, Nativi-Nicolau J, McKellar S, Kemeyou L, Gilbert E, Koliopoulou A, Drakos S: Efficacy of Left Ventricular Assist Device Therapy in Cold and Dry Chronic Heart Failure Patients. J Heart Lung Transplant 2020; 39: S434–S435 [Google Scholar]
- 43.Friese RS, Shafi S, Gentilello LM: Pulmonary artery catheter use is associated with reduced mortality in severely injured patients: a National Trauma Data Bank analysis of 53,312 patients. Crit Care Med 2006; 34: 1597–601 [DOI] [PubMed] [Google Scholar]
- 44.Stewart CL, Mulligan J, Grudic GZ, Talley ME, Jurkovich GJ, Moulton SL: The Compensatory Reserve Index Following Injury: Results of a Prospective Clinical Trial. Shock 2016; 46: 61–7 [DOI] [PubMed] [Google Scholar]
- 45.Kouz K, Scheeren TWL, de Backer D, Saugel B: Pulse Wave Analysis to Estimate Cardiac Output. Anesthesiology 2020 [DOI] [PubMed] [Google Scholar]
- 46.Vutskits L, Clark JD, Kharasch ED: Reporting Laboratory and Animal Research in ANESTHESIOLOGY: The Importance of Sex as a Biologic Variable. Anesthesiology 2019; 131: 949–952 [DOI] [PubMed] [Google Scholar]
- 47.McKinley BA, Kozar RA, Cocanour CS, Valdivia A, Sailors RM, Ware DN, Moore FA: Standardized trauma resuscitation: female hearts respond better. Arch Surg 2002; 137: 578–83; discussion 583-4 [DOI] [PubMed] [Google Scholar]
- 48.Aneman A, Ponten J, Fandriks L, Eisenhofer G, Friberg P, Biber B: Splanchnic and renal sympathetic activity in relation to hemodynamics during isoflurane administration in pigs. Anesth Analg 1995; 80: 135–42 [DOI] [PubMed] [Google Scholar]
- 49.Diaz F, Erranz B, Donoso A, Salomon T, Cruces P: Influence of tidal volume on pulse pressure variation and stroke volume variation during experimental intra-abdominal hypertension. BMC Anesthesiol 2015; 15: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanala UR, Johanning JM, Pipinos II, High RR, Larsen G, Velander WH, Carlson MA: Fluid administration rate for uncontrolled intraabdominal hemorrhage in swine. PLoS One 2018; 13: e0207708. [DOI] [PMC free article] [PubMed] [Google Scholar]