Abstract
Bioengineered neural tissues help advance our understanding of neurodevelopment, regeneration, and neural disease; however, it remains unclear whether they can replicate higher-order functions including cognition. Building upon technical achievements in the fields of biomaterials, tissue engineering, and cell biology, investigators have generated an assortment of artificial brain structures and co-cultured circuits. Though they have displayed basic electrochemical signaling, their capacities to generate minimal patterns of information processing suggestive of high-order cognitive analogues have not yet been explored. Here, we review the current state of neural tissue engineering and consider the possibility of a study of cognition in vitro. We adopt a practical definition of minimal cognition, anticipate problems of measurement, and discuss solutions toward a study of cognition in a dish.
Keywords: neural tissue engineering, minimal cognition, in vitro, bioengineering, information processing
Reverse engineering the black box
Brains are collections of biological tissues with extraordinary capacities. Indeed, all of human experience, from feelings of love and affection to indifference, fatigue, and the most intense sensations of pain, are products of brain function. Decision-making, moral reasoning, abstraction, and all other features of cognition may be operationally complex, but they are all fundamentally born of information processing within biological networks. For at least several thousands of years [1], humans have understood the relative significance of the brain among other organs as a determinant of thought and behaviour; however, the mechanisms of the mind have long-remained partially obscured within a black box and without a blueprint.
Historically, it has been assumed that a comprehensive picture of brain function will eventually be realized by manipulating and measuring the brains of animals and their dissociated or post-mortem parts. Consequently, cognitive researchers, philosophers, and neuroscientists have revealed a wealth of information about brain functions and their neural substrates. Beginning with functional assessments of patients – usually survivors of brain injuries [2,3] or surgical interventions [4,5] – we first linked regional insults with particular losses or changes to thought and behaviour. Today, ever more sophisticated neuroimaging tools are being actively developed [6,7] to increase the spatial and temporal resolutions of structural and functional brain maps. However, there are limitations to the study of brain function which are intrinsic to the assumptions that underly conventional approaches in the cognitive sciences. In pursuit of a significant leap forward in the field, it may be necessary to embrace a new paradigm with alternative assumptions.
Neural tissue engineering, the process of creating artificial brain-like specimens in the laboratory, may represent a fundamentally disruptive tool in the hands of cognitive scientists. Building custom, iterative, and modular brains in search of loss- or gain-of-function has become a practical reality of biomedical engineering. Recent reports of bioengineered neural tissues displaying increasingly complex functional profiles [8,9] indicate their potential suitability as models to investigate higher-order phenomena such as cognition. And with the integration of technologies such as designer receptors exclusively activated by designer drugs (DREADDs) [10], optogenetic actuators (see Glossary) [11], and genome editing [12], customizable and miniaturized brain models represent flexible tools to explore new frontiers in the cognitive sciences. Even without a blueprint of the mind, bioengineers can now design and build limitless variations on the black box and asses their comparative functional potential. In this review, the current state of neural tissue engineering is discussed in relation to the possibilities and impediments to a study of higher-order brain functions “in a dish”.
A novel approach to fuel discovery
Major breakthroughs in neuroscience have been attributed to the development of specialized tools and techniques that have allowed investigators to ask questions which would otherwise go unasked. From the histology and microscopy of Golgi [13] and Cajal [14] that launched a century of neuroanatomical investigation, to Hodgkin and Huxley’s [15] seminal electrophysiological measurements that inspired a revolution in molecular neuroscience [16,17], the impact of new and relevant tools cannot be understated. Indeed, the contemporary method of optogenetically stimulating animal brains [18,19], which involves the precise activation of sub-millimeter neural targets, is fundamentally derived from and built upon the seminal works of brain stimulation pioneers such as Delgado [20,21], and Penfield [22,23], who sought to evoke neural activity by applying comparatively crude injections of current. This uninterrupted chain of innovation has fueled tremendous discovery; however, the vast majority of modern neuroscientific tools and techniques are shaped by a common set of assumptions.
One core assumption of modern neuroscience is that the systematic observation and manipulation of animal brains and cells will eventually reveal all that can be known about neural signaling, cognition, behaviour, and even consciousness. With few exceptions, the brain is treated as an object to be probed, stimulated, ablated, dissociated, imaged, genetically manipulated, or otherwise imposed on by instrumentation. The default neuroanatomy, microcircuitry, and neurophysiology of biological organisms – products of natural selection, ontogeny, and maturation – are taken as granted. In pursuit of their hypotheses, most investigators design experiments beginning with the assumption of normative brain structure-function relationships. This sensible and effective strategy continues to yield important information and is the current gold standard approach in the field.
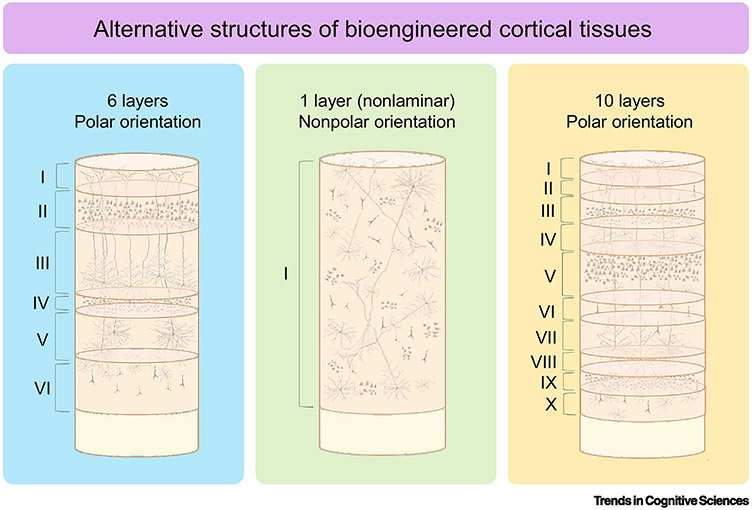
There is, however, a radically different approach to neuroscientific investigation that is becoming increasingly accessible. Unlike conventional methods, neural tissue engineering begins without a normative model of the brain. Rather, the investigator is tasked with defining the parameters of the neural substrate, building brain tissues de novo from the bottom-up, and then using them as tools to answer specific questions [24-26]. Instead of probing the pre-assembled brains of animals, investigators are now free to design and build artificial circuits and pathways that differ from their naturally selected counterparts and to push systems to their extremes in search of first principles that underlie brain function. Indeed, artificial neural tissues are not limited by inborn developmental morphology or structural-functional templates found in nature. And as it is conceivable that some or all higher-order cognitive functions may be substrate-independent [27,28], the rationale and means to test the independence hypothesis are now beginning to converge. That is, it may be possible for the same functions to be displayed by or emerge from completely different spatio-organizational cell configurations, which is consistent with concept of degeneracy [29]. With neural tissue engineering, a 1-, 10-, or 100-layered neocortex could be generated, and its functions compared to other artificial tissues with alternative cytoarchitectonic configurations (Fig. 1). The option to experimentally control factors such as cell density, the biochemical makeup of the extracellular matrices (ECM), cell type ratios (e.g., excitatory vs inhibitory neurons), the degree of myelination, and the orientation of fibers is a quintessential feature of neural tissue engineering.
Figure 1. Alternative structures of bioengineered cortical tissues.
Hypothetical structures of tissue engineered cerebral cortices that vary by number of layers (laminae) and cellular orientation (polar or non-polar). The default neocortex (left, blue) could be re-configured into a non-laminar, non-polar structure (center, orange), or a hyper-laminated, polar structure (right, green) to test relevant hypotheses.
It may be said that neural tissue engineering is less restrictive than conventional approaches, even if at the occasional cost of immediate translational relevance [30]. However, significant efforts to model neuropathology are currently underway, where traumatic brain injury [31,32] and neurodegeneration [33,34] have already been recapitulated as minimal models of disease in vitro. To create models of brain disease, investigators construct artificial tissues using diseased donor cells [35] or induce pathology by simulating etiologically relevant conditions within the culture media or environment (e.g., toxic compounds, radiation) [36]. Techniques such as bioprinting – which involves the precise extrusion of single cells into custom, patterned arrays – and scaffold-based 3D cell culture – a method that emphasizes the importance of a cell’s microenvironment – have been used to fabricate an assortment of brain tissues [37,38] and even biomimetic support structures such as the neurovascular unit [39]. With each technical advancement, it has become evident that artificial neural tissues are trending toward increased physiological relevancy, surpassing traditional, monolayer-based cell culture (e.g., increased complexity, co-culture compartmentalization, stratification) [40] and even rivaling organotypic slice culture without its main drawback of extensively damaged or fully severed tract systems [41,42].
As the line between in vivo and in vitro blurs, with neural organoids now displaying electrophysiological signatures reminiscent of animal electroencephalographic (EEG) profiles [43], there is a realistic implication that artificial brain tissues will soon have the capacity to display information processing patterns characteristic of prototypic thought and reflect other cognitive processes at a rudimentary level. Thus, there is a primary need to develop experimental paradigms with which higher-order cognitive phenomena including memory, reasoning, problem-solving, decision-making or their information processing analogues can be reliably measured in vitro. After all, without measurement, we may never appreciate the functional consequences of our creations.
Applying neural tissue engineering to the study of cognition
Major components of the nervous system have been recapitulated as in vitro tissue models including the cerebral cortex [44-46], forebrain [46], midbrain [47], hypothalamus [48], hippocampus [49], and cerebellum [50]. Interstitial fluid flow within the brain’s microenvironment has also been recreated in vitro [51]. Consistent with the purposes of modelling, only select features of specific in vivo brain regions are recapitulated. Current practical strategies in neural tissue engineering aim for “minimal” models of the brain that satisfy some but not all criteria. Only features that are considered necessary a priori for the purposes of a particular demonstration or experiment are included in the design and fabrication of artificial neural tissues. Therefore, it must be said that one shortcoming of current models is that they often fail to fully capture the sum total properties inherent to the brain regions they purport to model – whether or not those properties are critical to function. However, as the number of minimal brain models expands, it becomes relevant to consider which structures are most likely to display signs of cognitive processing. Among the structures that have been successfully modeled, the cerebral cortex is perhaps the most likely to exhibit minimal cognition given its important role in perception, problem-solving, decision-making, and consciousness. While the creation and assessment of individual artificial brain components is expected to yield important information, efforts are now turning to the equally important integration of complex circuits including the cortical-hippocampal interface [52] to model higher order brain functions such as memory encoding and retrieval. To date, there have been no reports of a bioengineered thalamocortical circuit – a milestone that would permit the measurement of information processing events relevant to consciousness including temporal binding [53]. However, as methods of 3D culture and co-culture continue to develop rapidly, systems-level constructs are eagerly anticipated.
It is important to consider the contemporary means by which tissues are generated in the laboratory, their associated impact on function, and the intrinsic limitations of each technique. Several distinct methods of tissue engineering have been developed with relevant applications in regenerative medicine, drug screening, disease modelling, and the study of neurological development [54]. The most common tools include neural spheroids [55], neural organoids [56], scaffold-based constructs [57], microfluidic devices [58], and bioprinted tissues [59]. As indicated by a recent report, bioprinting can be used to create “smart materials” with a capacity to simulate developmental, biophysically-driven processes such as cortical folding [60]. Neural organoids notoriously exhibit embryonic-like phenotypes [61] which is a recognized limitation when generalizing results to mature brains. However, in the hands of cognitive scientists, neural organoids may represent a means to identify cognitive developmental milestones associated with embryogenesis. Similarly, investigations of evolutionary brain development may now be possible with chimpanzee [62] and other non-human primate brain organoids.
Bioengineered neural tissues are currently assembled using primary cultures, immortalized cells lines, or by differentiation of induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) [54] and are typically embedded within protein composites that simulate the ECM [63] and other components of the tissue microenvironment. As reviewed in detail elsewhere [64], the size, scalability, longevity, replicability, and functional capacities of bioengineered neural tissues vary greatly, offering a range of possible advantages and disadvantages as model systems. It is, however, unknown how these parameters may impact the possibility of displaying minimal cognition.
To achieve a study of cognition in vitro, investigators will need to be willing to ask questions not normally associated with cell culture or tissue engineering: Can artificial neural tissues discriminate between closely related stimuli? Can they display percepts? If so, what is the associated difference threshold? Can they be operantly conditioned? If so, what would constitute “reinforcement” or “punishment” in vitro? Can we measure the equivalent of “attention span” in vitro? How might signs of intelligence or experience be inferred? Questions such as these may arouse knee-jerk calls to avoid anthropomorphization; however, cognition is uncontroversially a feature of some biological systems and its expression by laboratory-generated neural tissues is a nontrivial possibility. Indeed, determining whether or not cognition can be displayed in vitro – like any other scientific pursuit – begins with the willingness to formulate a falsifiable hypothesis, gather empirical data, and dispassionately adopt or abandon an evidence-based conclusion. Interdisciplinary teams comprised of cognitive scientists, engineers, biologists, psychologists, neuroscientists, computer scientists, and ethicists will be required to undertake a study of thought, behaviour, and experience “in a dish”.
Is an in vitro study of minimal cognition possible?
Before considering the technical details involving the assembly or measurement of artificial brain tissues with which to study cognition in vitro, it is first necessary to ask whether or not such a thing is possible in principle. The major products of brain function can be divided into two broad categories: cognition and behaviour. Cognition or mental processes classically include functions such as thought, experience, and sensation while behaviour is restricted to functions that terminate as motor outputs. In the brain, perception, attention, memory, problem solving, decision-making, the encoding of language – each cognitive function is emergent of billions of interconnected neural and glial cells engaged in a continuous transfer of information. Because current brain models are minimally representative of their in vivo analogues and mental processes, as currently understood, are not directly applicable to in vitro systems, it is necessary to establish a definition of minimal cognition on which to base further study. It may also be necessary to consider the minimal equivalents of specific cognitive phenomena such as “minimal reasoning” or “minimal memory”.
Fortunately, the concept of minimal cognition [65] has received mounting attention in recent years [66-69]. While it may be important to distinguish between the anthropocentric view of cognition, minimal cognitive phenomena, and reflexive or adaptive ontogenetic processes, there is evidence to support the view of cognition as a ubiquitous phenomenon [70]. An up-to-date and thorough review of minimal cognition was recently published, where information processing capacities of biochemical circuits and networks are considered representational of the lower-bound fundaments of biological cognition [70]. In the author’s view, neurons – and by extension, brains – are otherwise commonplace biocomputational systems that have been optimized for speed and distance. Therefore, as we consider the possibility of studying minimal cognition in vitro, we will regard information processing as the central operational variable. As we will discuss, mental processes are paired to measurable information processing patterns, representing one path toward a study of cognitive features in vitro; however, simulating behaviour in vitro with the use of embodied cultures may also be necessary to verify the content of information represented within artificial neural tissues. If, as is conventionally assumed, the bases of cognition and behaviour can be reduced to information processing within brain tissues, and artificial neural tissues have already displayed patterned activations [54,71,72] as well as a compatibility as grafts or implants with living hosts [73], we submit that a minimal form of cognition in vitro may have already been achieved but has not yet been verified. In practice, there are some key challenges associated with the measurement and inference of cognition within the in vitro context that are worth discussing.
The problem of measurement and current approaches
It is unfortunately the case that cognitive abilities are not subject to direct observation or measurement. Rather, they are inferred either by measurement of information processing within brains or coupled metabolism (e.g., EEG, fMRI, PET) [74], by the observation and quantification of operationalized behaviours (e.g., facial expressions, gestures, ambulation, pressing a lever) [75], or by assessing the content of introspective self-report (i.e., interpretable language) [76] which, with few exceptions, is absent in non-human laboratory animals. Several reliable information processing signatures are known to correlate with mental processes including event-related potentials such as the P300 component which most consistently involves activation of temporal, parietal, and prefrontal cortices [77] and is associated with decision-making [78]. Similarly, cross-frequency coupling of theta (~7Hz) and gamma (~40Hz) oscillations is known to correlate with sensory and memory processes [79]. When studying human brains, there is little reason to suspect that these information processing signatures are randomly associated with differential mental processes across individuals. Rather, there is a normative assumption of structure-function relationships that is empirically verifiable. Since the invention of EEG, alpha (~10Hz) rhythms detected at occipital sensors have been and remain a hallmark of the resting baseline condition – particularly if amplified by the participant closing their eyes. While similar signatures may be detectable in artificial neural tissues, it is unclear whether assumptions of coupled mental processes would survive the transition to a study of minimal cognition in vitro. Nevertheless, it may be necessary to deliberately model these structures and characterize artificial neural tissues as a function of their information processing capacities and their ranked complexity or analogous properties in relation to human-based observations.
Most studies that have assessed information processing in artificial neural tissues have been limited to functionally immature neural organoids. However, measuring connectivity coupled to planar microelectrode arrays (MEAs), investigators have supplied evidence of complex network formation with both spontaneous and evoked potentials indicative of organized function including the innervation of animal spinal tissue and contraction of muscle [80]. Complex phenomena such as the startle fear response have also been inferred by increased freezing to conditioned auditory stimuli in mice transplanted with human brain organoids [81]. On the basis of studies such as these, investigators [82] have recently suggested that the Perturbational Complexity Index – a tool developed to assess the information content of the brains of coma patients relative to healthy controls using transcranial magnetic stimulation [83] – could be adapted to assess cognitive features related to dementia in neural organoids.
Without any prior knowledge concerning which information processing patterns correlate with specific mental processes, behaviour (or self-report) is a necessary condition to infer cognition. Consider the difficulties associated with measuring cognition in patients with complete paralysis, as is the case in locked-in syndrome (LIS) [84]. If not for some very limited eye movement [85] and assistive technologies that convert brain activity into interpretable outputs [86], inference of cognition in LIS patients – which is now thought to be present – would not be possible. And, importantly, it is precisely those conversions from brain activity to interpretable outputs that functionally replace behaviour as the relevant and quantifiable outputs in addition to eye movements.
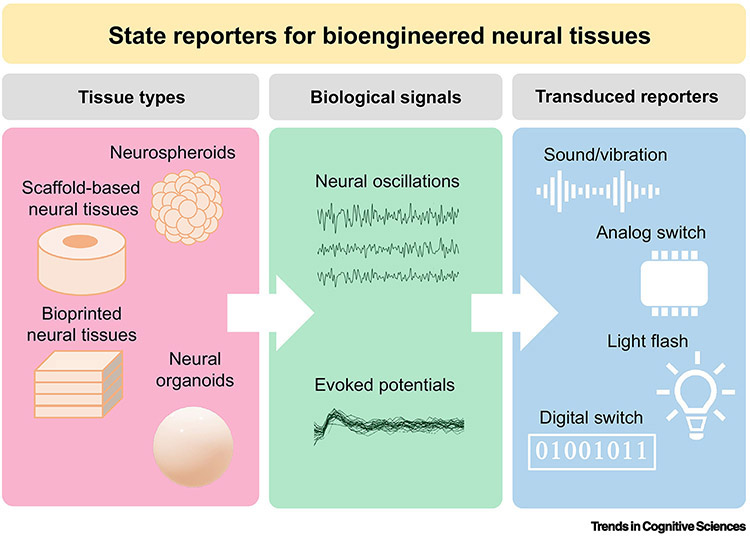
An assumption of normative brain structure and function is valuable in cognitive research. One implication of shared structure-function relationships across human brains is the possibility of reverse inference [87] which involves interpreting neuroimaging data to draw meaningful conclusions about the participant’s perception on the basis of brain activity alone [88,89]. Reverse inference is only made possible by an accumulation of empirical measurement involving human participants with conserved brain structure self-reporting their perceptions while undergoing neuroimaging. Consequently, investigators are now able to make accurate predictions about the experiences of participants without asking directly. To illustrate the importance of normative assumptions of brain structure-function relationships, consider the thought experiment of performing functional neuroimaging on a disembodied brain whose cognitive-behavioural outputs, uncoupled from their typical correlative anatomy, are mapped randomly across the cortices. Isolated from any sensory organ or a neuromuscular interface with which to report experience, mapping function would not be possible. Like the brains of living organisms, artificial neural tissues will require terminal outputs that are essentially non-neural with which to signal their operationalized internal states. Rather than measuring action potentials or network activity, the “state reporter” would summarize the global functional state of the system and collapse to a discrete output signal among a finite set of possible signals. One solution would involve a brain-computer interface (BCI) that would effectively transduce neural activity to digital representations or outputs as lights, sounds, and vibrations (Fig. 2).
Figure 2. State reporters for bioengineered neural tissues.
To assess cognitive and behavioural capacities in vitro, the functional activations of bioengineered neural tissues are transduced to non-neural signals that signal the state of the system. Tissue constructs (left panel) assembled using a variety of neural tissue engineering techniques will generate a range of biological signals (center panel) that, within the context of a specific paradigm, can yield important information about the functional state of the system. Signals can then be transduced (right panel) to trigger events that are analogous to response patterns observed in behaving organisms.
One consequence of extending a neural network’s influence on objects or effectors beyond the network itself is the creation of an embodied culture, functionally substituting behaviour. Embodied neural networks plated on MEAs have already been used to control robots [90], operate computer-generated animal bodies [91] and pilot simulated aircraft [92]. Simulations of embodied neural networks have also displayed features of learning and goal-directed behaviour such as moving toward or staying within user-defined areas [93]. Perhaps most relevant are the recent demonstrations of 3D human neuromuscular organoids that can contract and generate central circuits integral to reflexes and other motor responses [94,95]. Indeed, a three-part “assemboid” combining cortical, spinal, and muscular tissues was recently reported, thus recapitulating the major components of the corticospinal tract, which is the executive pathway associated with volitional movement in humans [96]. Similar strategies are simultaneously recapitulating extrapyramidal motor circuits [97]. Therefore, the technical challenges associated with imbuing neural cultures with the capacity to interact with the external world is already being addressed; though admittedly, efforts remain preliminary and progress is expected to be incremental. Once equipped with the means to signal the state of the neural tissue, it will be possible to perform assessments using behaviourist techniques including operant paradigms. Tasks involving lever pressing [98], orienting [99], or maze solving [100] could potentially be adapted for the in vitro context, facilitated by BCIs coupled to MEAs or similar electrophysiological devices. Pre-defined amplitude or frequency thresholds could serve as triggers to select specific outputs or ON-OFF states. To that end, identifying methods to positively or negatively reinforce artificial brain tissues would represent a significant achievement. Once established, it may be possible to apply a behaviourist approach as a first-order approximation to the study of higher function in vitro.
What about a study of consciousness in vitro?
What can be achieved using neural tissue engineering is a question that must be continually revisited with each incremental innovation. While currently impractical, to fully replicate the structure of the brain such that the artificially-generated tissue is at every level of analysis – from the proteins that make up the cells and the precise composition of the extracellular matrix linking neurons and glia – indistinguishable from its natural template would be, by definition, to create a tissue that can experience. To suppose otherwise would be to admit that consciousness does not emerge from brain matter. Indeed, some combination of neural substrates that together form the human brain is responsible for the generation of the kind of information processing that we call consciousness. The longstanding reliance upon the established brain structures of living organisms as the subjects of investigation has been fruitful [22, 101, 102] but may be limiting. Of course, there are many neural correlates of consciousness (NCCs) [103] including important circuits [104] and signature oscillatory patterns [105]. It may also be claimed that several brain regions represent necessary but not sufficient [106] generators of consciousness including the cerebral cortices and the arousal pathways [107,108]. However, a definitive locus or group of loci that are solely equipped to generate consciousness have not yet been identified despite our access to highly detailed neuroanatomical maps [109-111] and a wealth of functional imaging data [112,113].
If, as has been hypothesized, consciousness is an emergent process [114], a neural engineering approach will, by addition and subtraction of modules, reveal the point at which the system’s function becomes more than the sum of its parts. Indeed, the process of bioengineering structural NCCs may represent a path toward an understanding of causal mechanisms. However, verification of consciousness represents a significant hurdle in its own right [115] because consciousness may be a fundamentally private phenomenon – inaccessible to anything outside the system that generates it [116]. Distinguish building a tissue that has the capacity to experience from a secondary problem: determining whether we succeeded. Indeed, there is no satisfactory definition or test of consciousness that does not rely upon indirect effects or inference; determining how phenomenal experiences emerge from matter is a genuinely hard problem [117]. Once the measurement problem is addressed, which is longstanding [118], there are potentially limitless neural substrates which could be tested. Just as comparative anatomy highlighted the remarkable overlap between species which were thought to be phylogenetically unrelated, we submit that a comparative study of cognition across a very large set of iterative artificial neural tissues is of equal importance as we attempt to understand the biological origins of phenomena such as thought, intelligence, and even consciousness.
Concluding remarks
Neural tissue engineering is beginning to emerge as a useful tool to fuel discovery with the promise to help identify and delineate causal mechanisms. While most neural tissue engineering is currently performed in the interest of recapitulating native brain structure and function to model disease states, the prospect of studying cognition in vitro should be considered seriously and explored in parallel. Indeed, the prospect of developing an in vitro approach to cognitive science provides several new investigate paths (see Outstanding Questions). As examples of ever-more sophisticated information processing in artificial neural tissues begins to accumulate, it may also be relevant to consider the obligations we might have to these potentially sentient constructs. It will perhaps be necessary to develop an ethics that may or may not distinguish between brains and their laboratory-generated counterparts [119]. Just as the content of experience, especially suffering, is considered in non-human animals, the same curtesy will likely be extended to artificial neural tissues in due time assuming bioengineers will one day recapitulate cognition in a dish.
Outstanding questions.
What are the fundamental underlying principles of a cognitive system and to what degree are they determined by the microscopic cytoarchitecture of their primary neural substrates?
Are some cognitive capacities “substrate-independent” and others “substrate-dependent”?
Which neural tissue engineering approach is best-suited to fabricate artificial brain-like tissue with which to study higher-order functions?
Will a co-cultured, artificial circuit involving the pairing of multiple cell and tissue types be required to generate minimal cognition or will a single structure and cell type be sufficient?
Can building cognitive tissues bottom-up allow investigators to move past conventional neural correlates of consciousness and eventually reveal the neural causes of consciousness?
Will the laminar and polar architectures of the cerebral cortices be integral to generating minimal cognition or can they be replaced with alternative tissue architectures without loss of function?
Highlights.
tissue engineering requires designing and assembling customizable brain tissues using specialized techniques ranging from bioprinting to growing neural organoids
Artificial cerebral cortices, hippocampi, and specialized circuits have been bioengineered, displaying complex physiological signatures including patterned oscillations
In principle, artificial neural tissues can display minimal cognition in vitro as indicated by specialized information processing patterns
Equipping bioengineered neural tissues with appropriate interfaces as embodied cultures will allow investigators to ask and answer macro-scale questions not normally reserved for in vitro studies
The prospect of building brains to generate minimal cognition is becoming more realistic with current innovations of neural tissue engineering
Acknowledgements
We thank the NIH (P41EB027062, R01NS092847) for support for the underlying studies that contributed to this review.
Glossary
- 3D culture/co-culture
one (3D culture) or many (3D co-culture) cell types suspended in a stable, three-dimensional structure that approximates real-life physiological conditions
- Biomimetic
an artificial method or structure that mimics or simulates a natural biological processs
- Bioprinting
a process involving the patterning of cells into artificial tissues using biological “inks” (e.g., alginate, agarose, collagen, gelatin)
- Degeneracy
a property of biological units or pathways that can perform similar functions and are effectively interchangeable
- Embodied cultures
an in vitro system that has been functionally coupled to an effector that acts on the external world
- Microfluidic devices
thin, chip devices that house cells within defined spaces connected by narrow channels (~15μm) that allow for the highly controlled passage of fluids
- Minimal cognition
a basic level of cognition that satisfies the minimum necessary conditions to qualify as a mental process
- Neural organoids
miniature (~4mm wide) brains that express embryonic-like morphology and that are formed using (pluripotent) stem cells that can become many different kinds of tissues
- Neural spheroids
three-dimensional, spherical aggregates of neural cells caused by limiting adhesion (attachment) to flat surfaces where cells would normally form two-dimensional films
- Optogenetic actuators
light-activated ion channels such as channelrhodopsin that allow for precise control of neural tissues at the cellular level
- Scaffold-based constructs
neural cells embedded within customizable, preconstructed, three-dimensional environments, typically made of synthetic or biomaterial-derived polymers
- Substrate-independent
the independence of the mind or cognition from any one particular substrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman RP, & Goodrich JT (1999). The edwin smith surgical papyrus. Child's Nervous System, 15(6-7), 281–284. DOI: 10.1007/s003810050395 [DOI] [PubMed] [Google Scholar]
- 2.Broca P (1861). Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletin et Memoires de la Societe anatomique de Paris, 6, 330–357. [Google Scholar]
- 3.Luria AR (1948/1963). Restoration of Function After Brain Injury. New York: Macmillan [Google Scholar]
- 4.Penfield W, & Flanigin H (1950). Surgical therapy of temporal lobe seizures. AMA Archives of Neurology & Psychiatry, 64(4), 491–500. DOI: 10.1001/archneurpsyc.1950.02310280003001 [DOI] [PubMed] [Google Scholar]
- 5.Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry, 20(1), 11. DOI: 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu L, Zhang Y, Wang S, Yap PT, & Shen D (2020). Synthesized 7T MRI from 3T MRI via deep learning in spatial and wavelet domains. Medical Image Analysis, 62, 101663. DOI: 10.1016/j.media.2020.101663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbagh D, Ablin P, Varoquaux G, Gramfort A, & Engemann DA (2020). Predictive regression modeling with MEG/EEG: from source power to signals and cognitive states. NeuroImage, 116893. DOI: 10.1016/j.neuroimage.2020.116893 [DOI] [PubMed] [Google Scholar]
- 8.Fedorchak NJ, Iyer N, & Ashton RS (2020, June). Bioengineering tissue morphogenesis and function in human neural organoids. Seminars in Cell & Developmental Biology. In press. 10.1016/j.semcdb.2020.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zafeiriou MP et al. (2020). Developmental GABA polarity switch and neuronal plasticity in Bioengineered Neuronal Organoids. Nature communications, 11(1), 1–12. DOI: 10.1038/s41467-020-17521-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban DJ, & Roth BL (2015). DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annual Review of Pharmacology and Toxicology, 55, 399–417. DOI: 10.1146/annurev-pharmtox-010814-124803 [DOI] [PubMed] [Google Scholar]
- 11.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, & De Lecea L (2007). Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature, 450(7168), 420–424. DOI: 10.1038/nature06310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidenreich M, & Zhang F (2016). Applications of CRISPR–Cas systems in neuroscience. Nature Reviews Neuroscience, 17(1), 36. DOI: 10.1038/nrn.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merico G (1999). Microscopy in Camillo Golgi's times. Journal of the History of the Neurosciences, 8(2), 113–120. DOI: 10.1076/jhin.8.2.113.1837 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Lopez P, Garcia-Marin V, & Freire M (2010). The histological slides and drawings of Cajal. Frontiers in Neuroanatomy, 4, 9. DOI: 10.3389/neuro.05.009.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgkin AL, & Huxley AF (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500–544. DOI: 10.1113/jphysiol.1952.sp004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick DA, Shu Y, & Yu Y (2007). Hodgkin and Huxley model—still standing?. Nature, 445(7123), E1–E2. DOI: 10.1038/nature05523 [DOI] [PubMed] [Google Scholar]
- 17.Schwiening CJ (2012). A brief historical perspective: Hodgkin and Huxley. The Journal of Physiology, 590(11), 2571–2575. DOI: 10.1113/jphysiol.2012.230458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deisseroth K (2011). Optogenetics. Nature Methods, 8(1), 26–29. DOI: 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yizhar O, Fenno LE, Davidson TJ, Mogri M, & Deisseroth K (2011). Optogenetics in neural systems. Neuron, 71(1), 9–34. DOI: 10.1016/j.neuron.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Delgado JM, Roberts WW, & Miller NE (1954). Learning motivated by electrical stimulation of the brain. American Journal of Physiology-Legacy Content, 179(3), 587–593. DOI: 10.1152/ajplegacy.1954.179.3.587 [DOI] [PubMed] [Google Scholar]
- 21.Delgado JM (1964). Free behavior and brain stimulation. In International Review of Neurobiology, 6, 349–449. Academic Press. DOI: 10.1016/s0074-7742(08)60773-4 [DOI] [PubMed] [Google Scholar]
- 22.Penfield W, & Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60(4), 389–443. DOI: 10.1093/brain/60.4.389 [DOI] [Google Scholar]
- 23.Penfield W (1958). Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proceedings of the National Academy of Sciences of the United States of America, 44(2), 51. DOI: 10.1073/pnas.44.2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nisbet DR, Crompton KE, Horne MK, Finkelstein DI, & Forsythe JS (2008). Neural tissue engineering of the CNS using hydrogels. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 87(1), 251–263. DOI: 10.1002/jbm.b.31000 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt CE, & Leach JB (2003). Neural tissue engineering: strategies for repair and regeneration. Annual Review of Biomedical Engineering, 5(1), 293–347. DOI: 10.1146/annurev.bioeng.5.011303.120731 [DOI] [PubMed] [Google Scholar]
- 26.Willerth SM, & Sakiyama-Elbert SE (2007). Approaches to neural tissue engineering using scaffolds for drug delivery. Advanced Drug Delivery Reviews, 59(4-5), 325–338. DOI: 10.1016/j.addr.2007.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bostrom N (2003). Are we living in a computer simulation?. The Philosophical Quarterly, 53(211), 243–255. DOI: 10.1111/1467-9213.00309 [DOI] [Google Scholar]
- 28.Moon K, & Pae H (2019). Making Sense of Consciousness as Integrated Information: Evolution and Issues of Integrated Information Theory. Journal of Cognitive Science, 20(1), 1–52. DOI: 10.17791/jcs.2019.20.1.1 [DOI] [Google Scholar]
- 29.Edelman GM, & Gally JA (2001). Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences, 98(24), 13763–13768. DOI: 10.1073/pnas.231499798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin I (2014). Engineered tissues as customized organ germs. Tissue Engineering Part A, 20(7-8), 1132–1133. DOI: 10.1073/pnas.1411975111 [DOI] [PubMed] [Google Scholar]
- 31.Tian WM, et al. (2005). Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Engineering, 11(3-4), 513–525. DOI: 10.1089/ten.2005.11.513 [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Yan Y, Wang X, Xiong Z, Lin F, Wu R, & Zhang R (2007). Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. Journal of Bioactive and Compatible Polymers, 22(1), 19–29. DOI: 10.1177/0883911506074025 [DOI] [Google Scholar]
- 33.Raja WK, et al. (2016). Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PloS One, 11(9). DOI: 10.1371/journal.pone.0161969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouleau N, et al. (2020). A Long-Living Bioengineered Neural Tissue Platform to Study Neurodegeneration. Macromolecular Bioscience. 20(3), 1–8. DOI: 10.1002/mabi.202000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penney J, Ralvenius WT, & Tsai LH (2020). Modeling Alzheimer’s disease with iPSC-derived brain cells. Molecular psychiatry, 25(1), 148–167. DOI: 10.1038/S41380-019-0468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowling P, & Clynes M (2011). Conditioned media from cell lines: A complementary model to clinical specimens for the discovery of disease-specific biomarkers. Proteomics, 11(4), 794–804. DOI: 10.1002/pmic.201000530 [DOI] [PubMed] [Google Scholar]
- 37.Chwalek K, Tang-Schomer MD, Omenetto FG, & Kaplan DL (2015). In vitro bioengineered model of cortical brain tissue. Nature Protocols, 10(9), 1362. DOI: 10.1038/nprot.2015.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, et al. (2018). Advances in 3D bioprinting for neural tissue engineering. Advanced Biosystems, 2(4), 1700213. DOI: 10.1002/adbi.201700213 [DOI] [Google Scholar]
- 39.Potjewyd G, Moxon S, Wang T, Domingos M, & Hooper NM (2018). Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends in Biotechnology, 36(4), 457–472. DOI: 10.1016/j.tibtech.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 40.Lovett ML, Nieland TJ, Dingle YTL, & Kaplan DL (2020). Innovations in 3D Tissue Models of Human Brain Physiology and Diseases. Advanced Functional Materials, 1909146. DOI: 10.1002/adfm.201909146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Simoni A, & Lily MY (2006). Preparation of organotypic hippocampal slice cultures: interface method. Nature Protocols, 1(3), 1439. DOI: 10.1038/nprot.2006.228 [DOI] [PubMed] [Google Scholar]
- 42.Gähwiler BH, Capogna M, Debanne D, McKinney RA, & Thompson SM (1997). Organotypic slice cultures: a technique has come of age. Trends in Neurosciences, 20(10), 471–477. DOI: 10.1016/s0166-2236(97)01122-3 [DOI] [PubMed] [Google Scholar]
- 43.Trujillo CA, et al. (2019). Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell, 25(4), 558–569. DOI: 10.1016/j.stem.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lancaster MA, & Knoblich JA (2014). Generation of cerebral organoids from human pluripotent stem cells. Nature Protocols, 9(10), 2329. DOI: 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozano R, et al. (2015). 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials, 67, 264–273. DOI: 10.1016/j.biomaterials.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 46.Kadoshima T, et al. (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell–derived neocortex. Proceedings of the National Academy of Sciences, 110(50), 20284–20289. DOI: 10.1073/pnas.1315710110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo J, et al. (2016). Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell, 19(2), 248–257. DOI: 10.1016/j.stem.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian X, et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell, 165(5), 1238–1254. DOI: 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi H, et al. (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nature Communications, 6(1), 1–11. DOI: 10.1038/ncomms9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, & Sasai Y (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Reports, 10(4), 537–550. DOI: 10.1016/j.celrep.2014.12.051 [DOI] [PubMed] [Google Scholar]
- 51.Park J, et al. (2015). Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer's disease. Lab on a Chip, 15(1), 141–150. DOI: 10.1039/C4LC00962B [DOI] [PubMed] [Google Scholar]
- 52.Kato-Negishi M, Morimoto Y, Onoe H, & Takeuchi S (2013). Millimeter-Sized Neural Building Blocks for 3D Heterogeneous Neural Network Assembly. Advanced Healthcare Materials, 2(12), 1564–1570. DOI: 10.1002/adhm.201300052 [DOI] [PubMed] [Google Scholar]
- 53.Engel AK, & Singer W (2001). Temporal binding and the neural correlates of sensory awareness. Trends in Cognitive Sciences, 5(1), 16–25. DOI: 10.1016/s1364-6613(00)01568-0 [DOI] [PubMed] [Google Scholar]
- 54.Zhuang P, Sun AX, An J, Chua CK, & Chew SY (2018). 3D neural tissue models: From spheroids to bioprinting. Biomaterials, 154, 113–133. DOI: 10.1016/j.biomaterials.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 55.Dingle YTL, et al. (2015). Three-dimensional neural spheroid culture: an in vitro model for cortical studies. Tissue Engineering Part C: Methods, 21(12), 1274–1283. DOI: 10.1089/ten.TEC.2015.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Lullo E, & Kriegstein AR (2017). The use of brain organoids to investigate neural development and disease. Nature Reviews Neuroscience, 18(10), 573. DOI: 10.1038/nrn.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang-Schomer MD, et al. (2014). Bioengineered functional brain-like cortical tissue. Proceedings of the National Academy of Sciences, 111(38), 13811–13816. DOI: 10.1073/pnas.1324214111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bang S, Na S, Jang JM, Kim J, & Jeon NL (2016). Engineering-aligned 3D neural circuit in microfluidic device. Advanced healthcare materials, 5(1), 159–166. DOI: 10.1002/adhm.201500397 [DOI] [PubMed] [Google Scholar]
- 59.Knowlton S, Anand S, Shah T, & Tasoglu S (2018). Bioprinting for neural tissue engineering. Trends in Neurosciences, 41(1), 31–46. DOI: 10.1016/j.tins.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 60.Esworthy TJ, et al. (2019). Advanced 4D-bioprinting technologies for brain tissue modeling and study. International Journal of Smart and Nano Materials, 10(3), 177–204. DOI: 10.1080/19475411.2019.1631899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian X, Song H, & Ming GL (2019). Brain organoids: advances, applications and challenges. Development, 146(8). DOI: 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollen AA et al. (2019). Establishing cerebral organoids as models of human-specific brain evolution. Cell, 176(4), 743–756. DOI: 10.1016/j.cell.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sood D, et al. (2016). Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomaterials Science & Engineering, 2(1), 131–140. DOI: 10.1021/acsbiomaterials.5b00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birey F et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature, 545(7652), 54–59. DOI: 10.1038/nature22330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keijzer FA (2003). Making decisions does not suffice for minimal cognition. Adaptive Behavior, 11, 266–269. DOI: 10.1177/1059712303114006 [DOI] [Google Scholar]
- 66.Bich L, & Moreno A (2016). The role of regulation in the origin and synthetic modelling of minimal cognition. Biosystems, 148, 12–21. DOI: 10.1016/j.biosystems.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 67.Hanczyc MM, & Ikegami T (2010). Chemical basis for minimal cognition. Artificial life, 16(3), 233–243. DOI: 10.1162/artl_a_00002 [DOI] [PubMed] [Google Scholar]
- 68.Keijzer FA (2017). Evolutionary convergence and biologically embodied cognition. Interface Focus, 7(3), 20160123. DOI: 10.1098/rsfs.2016.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Duijn M, Keijzer F, & Franken D (2006). Principles of minimal cognition: Casting cognition as sensorimotor coordination. Adaptive Behavior, 14(2), 157–170. DOI: 10.1177/105971230601400207 [DOI] [Google Scholar]
- 70.Lyon P (2019). Of what is “minimal cognition” the half-baked version?. Adaptive Behavior, 1059712319871360. DOI: 10.1177/1059712319871360 [DOI] [Google Scholar]
- 71.Gu Q, et al. (2016). Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Advanced Healthcare Materials, 5(12), 1429–1438. DOI: 10.1002/adhm.201600095 [DOI] [PubMed] [Google Scholar]
- 72.Mahoney MJ, & Anseth KS (2006). Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials, 27(10), 2265–2274. DOI: 10.1016/j.biomaterials.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 73.Mansour AA, et al. (2018). An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology, 36(5), 432. DOI: 10.1038/nbt.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poldrack RA (2006). Can cognitive processes be inferred from neuroimaging data?. Trends in Cognitive Sciences, 10(2), 59–63. DOI: 10.1016/j.tics.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 75.Shettleworth SJ (2001). Animal cognition and animal behaviour. Animal Behaviour, 61(2), 277–286. DOI: 10.1006/anbe.2000.1606 [DOI] [Google Scholar]
- 76.Mitchell JP (2009). Inferences about mental states. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1309–1316. DOI: 10.1098/rstb.2008.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soltani M, & Knight RT (2000). Neural origins of the P300. Critical Reviews™ in Neurobiology, 14(3-4). DOI: 10.1615/CRITREVNEUROBIOL.V14.I3-4.20 [DOI] [PubMed] [Google Scholar]
- 78.Twomey DM, Murphy PR, Kelly SP, & O'Connell RG (2015). The classic P300 encodes a build-to-threshold decision variable. European journal of neuroscience, 42(1), 1636–1643. DOI: 10.1111/ejn.12936 [DOI] [PubMed] [Google Scholar]
- 79.Lisman JE, & Jensen O (2013). The theta-gamma neural code. Neuron,77(6), 1002–1016. DOI: 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giandomenico SL et al. (2019). Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nature neuroscience, 22(4), 669–679. DOI: 10.1038/s41593-019-0350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong X et al. 2020. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Molecular psychiatry, 1–13. DOI: 10.1038/S41380-020-00910-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ooi L et al. (2020). If Human Brain Organoids Are the Answer to Understanding Dementia, What Are the Questions?. The Neuroscientist, 1073858420912404. DOI: 10.1177/1073858420912404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casali AG et al. (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Science translational medicine, 5(198), 198ra105–198ra105. DOI: 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- 84.Schnakers C, et al. (2008). Cognitive function in the locked-in syndrome. Journal of Neurology, 255(3), 323–330. DOI: 10.1007/s00415-008-0544-0 [DOI] [PubMed] [Google Scholar]
- 85.Smith E, & Delargy M (2005). Locked-in syndrome. Bmj, 330(7488), 406–409. DOI: 10.1136/bmj.330.7488.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Combaz A, et al. (2013). A comparison of two spelling brain-computer interfaces based on visual P3 and SSVEP in locked-in syndrome. PloS One, 8(9). DOI: 10.1371/journal.pone.0073691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutzler F (2014). Reverse inference is not a fallacy per se: Cognitive processes can be inferred from functional imaging data. Neuroimage, 84, 1061–1069. DOI: 10.1016/j.neuroimage.2012.12.075 [DOI] [PubMed] [Google Scholar]
- 88.Chang LJ, Yarkoni T, Khaw MW, & Sanfey AG (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex, 23(3), 739–749. DOI: 10.1093/cercor/bhs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieberman MD, & Eisenberger NI (2015). The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences, 112(49), 15250–15255. DOI: 10.1073/pnas.1515083112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kudoh SN, et al. (2011). Vitroid–the robot system with an interface between a living neuronal network and outer world. International Journal of Mechatronics and Manufacturing Systems, 4(2), 135–149. DOI: 10.1504/IJMMS.2011.039264 [DOI] [Google Scholar]
- 91.DeMarse TB, Wagenaar DA, Blau AW, & Potter SM (2001). The neurally controlled animat: biological brains acting with simulated bodies. Autonomous Robots, 11(3), 305–310. DOI: 10.1023/A:1012407611130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeMarse TB, & Dockendorf KP (2005, July). Adaptive flight control with living neuronal networks on microelectrode arrays. In Proceedings. 2005 IEEE International Joint Conference on Neural Networks, 2005. 3, 1548–1551. IEEE. DOI: 10.1109/IJCNN.2005.1556108 [DOI] [Google Scholar]
- 93.Chao ZC, Bakkum DJ, & Potter SM (2008). Shaping embodied neural networks for adaptive goal-directed behavior. PLoS Computational Biology, 4(3). DOI: 10.1371/journal.pcbi.1000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bakooshli MA et al. (2019). A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife, 8, e44530. DOI: 10.7554/eLife.44530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martins JMF et al. (2020). Self-organizing 3D human trunk neuromuscular organoids. Cell stem cell, 26(2), 172–186. DOI: 10.1016/j.stem.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 96.Andersen J et al. (2020). Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 183(7), 1913–1929.e26. DOI: 10.1016/j.cell.2020.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miura Y et al. (2020). Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nature Biotechnology, 38(12), 1421–1430. DOI: 10.1038/s41587-020-00763-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaham Y, Klein LC, Alvares K, & Grunberg NE (1993). Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacology Biochemistry and Behavior, 46(2), 315–322. DOI: 10.1016/0091-3057(93)90359-2 [DOI] [PubMed] [Google Scholar]
- 99.Koch J (1967). Conditioned orienting reactions in two-month-old infants. British Journal of Psychology, 58(1-2), 105–110. DOI: 10.1111/j.2044-8295.1967.tb01063.x [DOI] [PubMed] [Google Scholar]
- 100.Lee MG, et al. (2010). Operant conditioning of rat navigation using electrical stimulation for directional cues and rewards. Behavioural Processes, 84(3), 715–720. DOI: 10.1016/j.beproc.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 101.Hira R, et al. (2009). Transcranial optogenetic stimulation for functional mapping of the motor cortex. Journal of Neuroscience Methods, 179(2), 258–263. DOI: 10.1016/j.jneumeth.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 102.Lalancette-Hébert M, Gowing G, Simard A, Weng YC, & Kriz J (2007). Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. Journal of Neuroscience, 27(10), 2596–2605. DOI: 10.1523/JNEUROSCI.5360-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koch C, Massimini M, Boly M, & Tononi G (2016). Neural correlates of consciousness: progress and problems. Nature Reviews Neuroscience, 17(5), 307. DOI: 10.1038/nrn.2016.22 [DOI] [PubMed] [Google Scholar]
- 104.Llinás R, Ribary U, Contreras D, & Pedroarena C (1998). The neuronal basis for consciousness. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 353(1377), 1841–1849. DOI: 10.1098/rstb.1998.0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meador KJ, Ray PG, Echauz JR, Loring DW, & Vachtsevanos GJ (2002). Gamma coherence and conscious perception. Neurology, 59(6), 847–854. DOI: 10.1212/wnl.59.6.847 [DOI] [PubMed] [Google Scholar]
- 106.Tononi G, & Edelman GM (1998). Consciousness and complexity. Science, 282(5395), 1846–1851. DOI: 10.1126/science.282.5395.1846 [DOI] [PubMed] [Google Scholar]
- 107.Edlow BL, et al. (2012). Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. Journal of Neuropathology & Experimental Neurology, 71(6), 531–546. DOI: 10.1097/NEN.0b013e3182588293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Franks NP (2008). General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature Reviews Neuroscience, 9(5), 370–386. DOI: 10.1038/nrn2372 [DOI] [PubMed] [Google Scholar]
- 109.Hagmann P, et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biology, 6(7). DOI: 10.1371/journal.pbio.0060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orban GA, Van Essen D, & Vanduffel W (2004). Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences, 8(7), 315–324. DOI: 10.1016/j.tics.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 111.Van Essen DC, Drury HA, Joshi S, & Miller MI (1998). Functional and structural mapping of human cerebral cortex: solutions are in the surfaces. Proceedings of the National Academy of Sciences, 95(3), 788–795. DOI: 10.1073/pnas.95.3.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engel SA, et al. (1994). fMRI of human visual cortex. Nature. 525. DOI: 10.1038/369525a0 [DOI] [PubMed] [Google Scholar]
- 113.Logothetis NK, Pauls J, Augath M, Trinath T, & Oeltermann A (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157. DOI: 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- 114.Thompson E, & Varela FJ (2001). Radical embodiment: neural dynamics and consciousness. Trends in Cognitive Sciences, 5(10), 418–425. DOI: 10.1016/s1364-6613(00)01750-2 [DOI] [PubMed] [Google Scholar]
- 115.Seth AK, Dienes Z, Cleeremans A, Overgaard M, & Pessoa L (2008). Measuring consciousness: relating behavioural and neurophysiological approaches. Trends in Cognitive Sciences, 12(8), 314–321. DOI: 10.1016/j.tics.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crick F, & Koch C (2003). A framework for consciousness. Nature Neuroscience, 6(2), 119–126. DOI: 10.1038/nn0203-119 [DOI] [PubMed] [Google Scholar]
- 117.Chalmers DJ (1995). Facing up to the problem of consciousness. Journal of Consciousness Studies, 2(3), 200–219. DOI: 10.1093/acprof:oso/9780195311105.003.0001 [DOI] [Google Scholar]
- 118.Miller G (2005). What is the biological basis of consciousness?. Science, 309(5731), 79–79. DOI: 10.1126/science.309.5731.79 [DOI] [PubMed] [Google Scholar]
- 119.Cheshire JR, W. P (2020). Cerebral Organoids and the Threshold of Consciousness. Ethics & Medicine, 36(1), 27–3. [Google Scholar]