Abstract
Background:
Oncology settings increasingly use patient experience data to evaluate clinical performance. Given that older patients with hematologic malignancies are a high-need and high-risk population, this study examined factors associated with patient-reported health care experiences during the first year of their cancer diagnosis.
Methods:
Cross-sectional study using the 2000–2015 SEER-CAHPS® data to examine patient experiences of Medicare enrollees with a primary diagnosis of leukemia or lymphoma. The primary outcomes were three CAHPS assessments: overall care, personal doctor and health plan overall. We estimated case-mix adjusted and fully adjusted associations between factors (i.e., clinical and sociodemographic) and the CAHPS outcomes using bivariate statistical tests and multiple linear regression.
Results:
The final sample included 1,151 patients, with 431 diagnosed with leukemia and 720 diagnosed with lymphoma (median time from diagnosis to survey 6 months). Patients who completed the survey further apart from the diagnosis date reported significantly higher adjusted ratings of care overall (p=.008) than those closer to diagnosis. American Indian/Alaska Native, Asian, and Pacific Islander patients had lower adjusted ratings of care overall (p= .003) than Non-Hispanic White patients. Multimorbidity was significantly associated with higher adjusted personal doctor ratings (p=.003).
Conclusions:
Unfavorable patient experience ratings among vulnerable and under-represented older adults diagnosed with hematologic malignancies warrant targeted efforts to measure and improve care quality. Future measurement of experiences of cancer care soon after diagnosis, coupled with careful sampling of high-priority populations, will inform oncology leaders and clinicians on strategies to improve care for high-risk, high-cost populations.
Keywords: hematologic diseases, Consumer Assessment of Healthcare Providers and Systems (CAHPS), patient experience, health care quality
Precis:
Racial minority patients with hematologic malignancies reported worse experiences with health care and clinicians than white patients. Targeted efforts are needed to address shortfalls in the quality of cancer care and refine policy-relevant uses of patient experience outcomes.
Introduction
Leukemia and lymphoma, malignant neoplasms of the hematopoietic system, will account for approximately 176,000 cancer diagnoses in the US every year.1 Principally a disease of older adults, the median age of diagnosis is 64 years.2 Treatment regimens for hematologic malignancies in older adults include systemic, high-dose chemotherapy with or without radiotherapy and immunotherapy (e.g., Rituximab for chronic lymphocytic leukemia [CLL] or non-Hodgkin lymphoma).3,4 Problems arise during active cancer treatment from unmet information needs, delays in accessing care, and limited social assistance.5,6 The potential for patient harm during treatment of hematologic malignancies is notable; 5-year survival rates are generally poor, chemotherapy agents frequently bear irrevocable toxicities, and treatments are difficult to coordinate efficiently across multiple health care providers.7 Furthermore, pitfalls in quality of care are difficult to address due to limited data on the patient-reported health care experiences, especially among the hematologic malignancy population.
Patient-centeredness is a core pillar of health care quality, which emphasizes soliciting the patient’s preferences and values during care delivery.8 Patient-centeredness is a relatively understudied outcome measure in cancer care quality research, despite the plan by the Centers for Medicare & Medicaid Services (CMS) to withhold payments to hospitals with subpar patient experience scores.9 Furthermore, many hospitals and cancer centers tie favorable patient experience ratings to clinicians’ financial incentives. Clinicians, scientists, and policy-makers universally recognize the important of a patient-centered health care delivery environment to achieve high-value cancer care.10 To our knowledge, few systematic investigations have examined the patient experiences of adults diagnosed with hematologic malignancies.
Accordingly, the purpose of this study was to examine factors influencing patient experiences in care during the first year of diagnosis in older adults diagnosed with leukemia and lymphoma. We hypothesized that subjects from under-represented backgrounds, those in the acute phases of treatment, and those diagnosed with leukemia (versus lymphoma) would report less favorable experiences of care.
Methods
Study design and setting
This study was a cross-sectional analysis of existing data from the National Cancer Institute (NCI) Surveillance, Epidemiology and End-Results and Consumer Assessment of Healthcare Providers and Systems (SEER-CAHPS) linked resources. The SEER-CAHPS resource prepared data from participants in the National Cancer Institute’s SEER registry in addition to their Medicare enrollment data and any completed Medicare CAHPS patient experience surveys.11 Briefly, population-based registries in the SEER program collected and received health data from patients diagnosed with cancer at hospitals, ambulatory settings and other clinics within an entire state or metropolitan area.12
The study’s conceptual framework was derived from Andersen and Aday’s behavioral model of health care utilization.13 The framework posited that individual characteristics and exposures to health care interventions during care for cancer would influence the health care experiences reported by patients. The institutional review board of the authors’ university deemed the study exempt from human subjects’ approval.
The SEER registry collected detailed tumor and demographic variables from participants who received care at participating cancer centers in registry areas. As of 2019, the SEER registries covered geographic areas with approximately 107 million people (34.6% of the US population), comparable to the general US population in measures of socioeconomic status (i.e., poverty and education), race, and ethnicity.12
The CMS uses several survey instruments, with standardized protocols for collection and analysis, to examine individuals’ experiences with health care professionals, systems, and health plans.14 For this study, the Medicare CAHPS survey assessed domains of experience with care from health care professionals, systems, and insurance plans. Domains of the Medicare CAHPS survey were grouped into two categories: global ratings (i.e., one-item assessments of a single construct) or subscale measures (i.e., composite scores of multiple items).15
Participants
We included patients enrolled in Medicare Fee-for-Service (FFS) and Medicare Advantage (MA) plans with a primary (i.e., first cancer) diagnosis of leukemia or lymphoma. Additional eligibility criteria required that enrollees were at least age 65 at the time of diagnosis and the year of diagnosis was 2000–2015. We included cases if enrollees completed the survey in the first twelve months of the primary cancer diagnosis. The final sample contained 1,151 eligible cases. Figure 1 displayed the eligibility criterion flow diagram.
Figure 1.
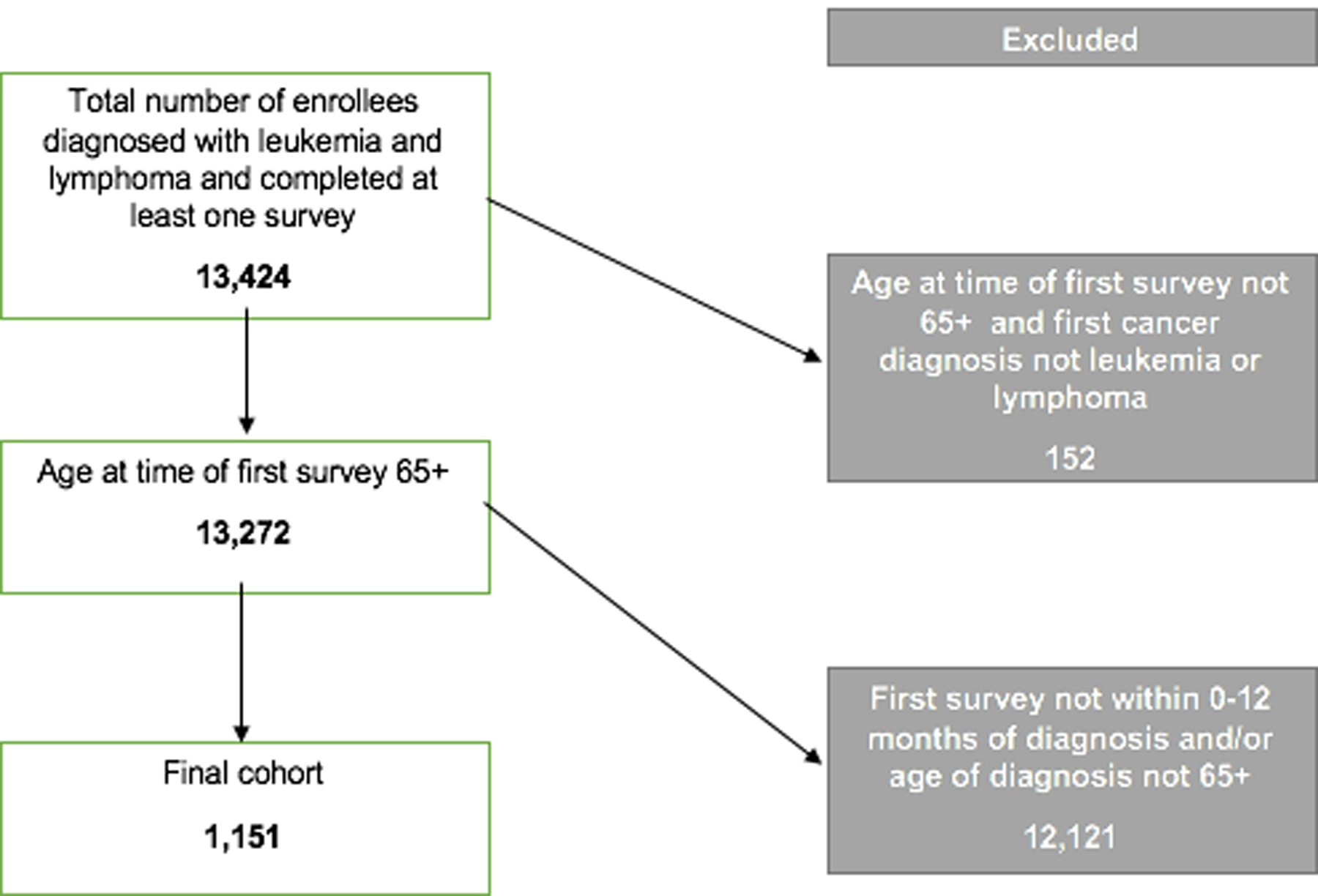
Eligibility criteria flow diagram.
We considered cases ineligible for complete case analysis if the survey contained missing items on the global ratings of care overall, personal doctor overall, or health plan overall. Therefore, we excluded 219 cases (19.0%) from the global rating of care model, 240 cases (20.8%) from the personal doctor overall model, and 110 cases (9.5%) from the health plan overall model.
Measures
The Medicare CAHPS survey was the primary patient instrument. CMS distributes CAHPS surveys in English, Spanish, and Chinese language versions. Historically, the Medicare CAHPS survey does not yield an exceptional response rate despite using a mixed modal approach (i.e., mail and telephone survey recruitment). The median annual Medicare CAHPS response rate from 2000–2015 for Medicare Advantage surveys was 63.25% (IQR 33.6) and 56.3% for Fee-for-Service surveys (IQR 25.2).15 Prior investigations reported statistically significant differences in global ratings at approximately 10% (i.e., less than a 1-unit difference) with robust, large sample sizes.16,17 We compared the adjusted CAHPS outcomes by covariate to understand substantive significance (i.e., clinically-meaningful difference) and not solely statistical significance.18
CAHPS Global Ratings.
The primary outcome measures were the CAHPS global ratings of care overall, personal doctor, and health plan. These variables measured enrollees’ perceptions of their health care, personal doctor, and health plan each with 11-point scales. The recall period for the global ratings was over the last six months. The items were scored on a “0” to “10” scale, which a score of “10” represented “best care possible” and a score of “0” meant “worst care possible”. Satisfactory psychometric performance and clinical face validity were previously demonstrated in the context of cancer care.19,20
CAHPS composite scales.
The secondary outcome measures were three composite scores of the CAHPS survey (i.e. subscales): patient communication with physicians (i.e., “doctor communication”), getting care quickly, and getting needed care. From our data, the subscale internal consistency reliability Cronbach’s alpha coefficients for doctor communication, getting care quickly, and getting needed care were .88, .59, and .57, respectively. Previous analyses from the CMS found more favorable scale reliabilities.20
The doctor communication scale (four items) asked enrollees how their physician explained things clearly, listened carefully, showed respect, and spent enough time with them. The getting care quickly scale (three items) asked enrollees how often they received care as soon as needed when sick or injured, and received non-urgent appointments as soon as needed. The getting needed care scale (four items) asked enrollees how often it was easy for them to receive appointments with specialists, and obtain the care, tests, or treatments they needed through their health plan.
The scales were scored with a four-point scale (Never, Sometimes, Usually, Always), with one indicating “Never”, and four indicating “Always”. We used a linear mean scoring approach to transform the scale measures to a continuous scale from “0” to “100”, which “0” represented the worst possible rating and “100” the best possible rating.
Baseline variables.
Measures obtained from the SEER Patient Entitlement and Diagnostic Summary File (PEDSF) included: age, tumor histology,21 race/ethnicity, sex, month and year of diagnosis, and urban/rural status. Measures obtained from the CAHPS dataset included case-mix adjustment variables: age, education level, general health status, mental health status [Likert scale, excellent to poor], received help responding, proxy answered survey questions, Medicaid dual eligibility (yes/no), low income subsidy, Chinese language. Additional variables were: months from the first survey to diagnosis; plan survey type (FFS or MA); percentage of census tract in poverty; and number of comorbid health conditions excluding cancer (MA only). Using the tumor histology codes, we created a risk-adjustment measure for disease severity if the leukemia diagnosis was an acute leukemia, and if the lymphoma diagnosis was T-cell lymphoma, natural killer cell lymphoma, and peripheral T-cell lymphoma. We identified cases of patients diagnosed with both leukemia and myelodysplastic syndrome (MDS) and in the higher-risk group.
Statistical analyses
We compared the percentages of patients in various demographic and clinical subgroups with χ² tests. Bivariate analyses with the CAHPS case-mix adjustment compared the means of the CAHPS global ratings by the months of cancer diagnosis at time of survey, Medicare enrollment type and other covariates with one-way ANOVA. We used the alpha 0.05 level of significance for all statistical tests. We managed the datasets with SAS 9.4 (Cary, NC) and performed all analyses in Stata version 15 (College Station, TX).
We accounted for missing values of covariates and secondary outcomes deemed missing completely at random with multiple imputations procedures.22 Two hundred imputations were performed. The analytic software omitted the following cases and variables from the imputed dataset due to collinearity: Hispanic ethnicity, those who lived alone, and answered the survey through a proxy. We examined the model fit with the average relative increase in variance for imputed regressions and the r2 estimate for complete case models. As a sensitivity analysis, we examined the linear models with complete case data to assess the reliability of the imputations.
Results
Patient Characteristics
Table 1 displays patient demographic, clinical and social characteristics by primary tumor type. Of the 431 patients diagnosed with leukemia, 211 (18.3% total sample) were diagnosed with CLL and 109 (9.5%) with acute myeloid leukemia (AML). Of the 721 patients diagnosed with lymphoma, 681 were diagnosed with non-Hodgkin lymphoma (59.2%). The sample comprised primarily of Non-Hispanic Whites (86.8% of leukemia cases, 86.2% of lymphoma cases) with no comorbidity (74.5% of leukemia cases, 73.6% of lymphoma cases).
Table 1.
Characteristics of CAHPS respondents diagnosed with leukemia and lymphoma.
| Diagnosis | |||
|---|---|---|---|
| Leukemia (n=431) | Lymphoma (n= 720) | ||
| Characteristic | No. (%) | No. (%) | p-valuea |
| Age at diagnosis | .32 | ||
| 65–69 | 68 (15.8) | 138 (19.2) | |
| 70–74 | 112 (26.0) | 160 (22.2) | |
| 75–79 | 97 (22.5) | 171 (23.8) | |
| 80–84 | 80 (18.6) | 144 (20.0) | |
| 85+ | 74 (17.1) | 107 (14.8) | |
| Sex | <.001 | ||
| Male | 249 (57.7) | 331 (45.9) | |
| Female | 182 (42.3) | 389 (54.1) | |
| Race/Ethnicity | <.01 | ||
| Non-Hispanic White | 374 (86.8) | 621 (86.2) | |
| Non-Hispanic Black | 28 (6.5) | 27 (3.8) | |
| Hispanic and Non-Hispanic American Indian/Alaska Native, Asian, Pacific Islander | 29 (6.7) | 72 (10.0)) | |
| Education | .03 | ||
| 8th grade to high school | 80 (18.6) | 178 (24.7) | |
| High school graduate or GED | 115 (26.7) | 216 (30.0) | |
| Some college or higher | 208 (48.3) | 283 (39.3) | |
| Unknown | 28 (6.4) | 43 (6.0) | |
| SEER region at diagnosis | .30 | ||
| Northeast | 85 (19.7) | 126 (17.5) | |
| Midwest | 35 (8.1) | 54 (7.5) | |
| South | 73 (17.0) | 154 (21.4) | |
| West | 238 (55.2) | 386 (53.6) | |
| Comorbidity count** | .49 | ||
| 0 | 321 (74.5) | 530 (73.6) | |
| 1 | 69 (16.0) | 132 (18.3) | |
| 2+ | 41 (9.5) | 58 (8.1) | |
| Time from diagnosis to survey, months | .01 | ||
| 0–3 | 152 (32.3) | 196 (27.2) | |
| 4–7 | 122 (28.3) | 246 (34.2) | |
| 8–12 | 157 (36.4) | 278 (38.6) | |
| General health status | .63 | ||
| Excellent, very good, good | 287 (66.6) | 501 (69.6) | |
| Fair/poor | 131 (30.4) | 194 (26.9) | |
| Unknown | 13 (3.0) | 25 (3.5) | |
| Mental health status | .22 | ||
| Excellent, very good, good | 348 (80.7) | 594 (82.5) | |
| Fair/poor | 45 (10.4) | 57 (7.9) | |
| Unknown | 38 (8.9) | 69 (9.6) | |
| Urban/rural residency at diagnosis | .65 | ||
| Big Metro, Metro, Urban | 408 (94.7) | 666 (92.5) | |
| Less Urban, Rural | 23 (5.3) | 54 (7.5) | |
| Did a proxy help complete survey | .98 | ||
| No | 309 (71.7) | 514 (71.4) | |
| Yes | 55 (12.8) | 91 (12.6) | |
| Unknown | 67 (15.5) | 115 (16.0) | |
| Medicaid dual eligibility | .84 | ||
| No | 393 (91.2) | 654 (90.8) | |
| Yes | 38 (8.8) | 66 (9.2) | |
| Medicare advantage indicator | .73 | ||
| FFS type or PDP | 184 (42.7) | 300 (41.7) | |
| MA type | 247 (57.3) | 420 (58.3) | |
| Disease severity indicator | <.001 | ||
| No | 267 (62.1) | 673 (93.5) | |
| Yes | 164 (37.9) | 47 (6.5) | |
| Percentage of census tract in poverty | .17 | ||
| 0–<5% poverty | 115 (26.7) | 190 (26.3) | |
| 5–<10% poverty | 131 (30.4) | 183 (25.4) | |
| 10–<20% poverty | 123 (28.5) | 243 (33.8) | |
| 20–100% poverty | 62 (14.4) | 100 (13.9) | |
Abbreviations: CAHPS, Consumer Assessment of Healthcare Providers and Systems Survey; GED, general education development; SEER, surveillance epidemiology end-results registry.
Pearson Chi-square test p-value.
Patients completed surveys most frequently between 8 to 12 months after their diagnosis (35.4% patients with leukemia, 38.6% with lymphoma, median time from diagnosis to survey 6 months). Twelve patients (1%) completed the survey in Spanish; no respondents completed the survey in Chinese.
Global Ratings of Care, Personal Doctor, and Health Plan and CAHPS scales
The global ratings of care and personal doctor varied significantly depending on when patients completed the survey during the first year of diagnosis, shown in Table 2.
Table 2.
Case-mix adjusted mean CAHPS global ratings and scales by months of cancer diagnosis at survey completion.
| Description | Measure | 0–3 months from diagnosis | 4–7 months from diagnosis | 8–12 months from diagnosis | p-value |
|---|---|---|---|---|---|
| Mean (95% CI) | |||||
| Global rating, single item | Care Overall | 8.5 (8.2, 8.8) | 8.6 (8.3, 8.9) | 8.8 (8.5, 9.2) | .001 |
| Personal Doctor Overall | 8.7 (8.4, 9.0) | 8.6 (8.3, 8.9) | 8.9 (8.7, 9.2) | .02 | |
| Health Plan Overall | 8.7 (8.3, 9.0) | 8.7 (8.4, 9.0) | 8.8 (8.5, 9.1) | .003 | |
| Composite scale, multi-item | Doctor Communication | 88.3 (84.7, 92.0) | 87.1 (83.6, 90.6) | 87.8 (84.4, 91.1) | .009 |
| Getting Care Quickly | 75.1 (70.2, 80.0) | 70.0 (65.3, 74.8) | 71.6 (67.1, 76.2) | .14 | |
| Getting Needed Care | 86.8 (82.5, 91.0) | 86.6 (82.6, 90.7) | 88.8 (84.9, 92.7) | .39 |
Abbreviations: CAHPS, Consumer Assessment of Healthcare Providers and Systems Survey; CI, confidence interval.
Adjusted with CAHPS case-mix procedures.
We observed significant case-mix adjusted differences in the mean global rating of care overall among racial and ethnic groups (p=.0003). The case-mix adjusted mean rating of care overall among Non-Hispanic American Indian/Alaska Native, Asian, and Pacific Islander patients was 8.2 (7.7–8.8), 8.4 (7.8–9.0) among Non-Hispanic Black patients, and 8.8 (8.5–9.1) among Non-Hispanic White patients.
We observed significant case-mix adjusted differences in the global rating of care overall (p=.02) by Medicare enrollment type (Table 3), but no significant differences in the other CAHPS measures. We observed no significant differences in the primary and secondary outcome measures by primary tumor type.
Table 3.
Case-mix adjusted mean CAHPS global ratings and scales by Medicare enrollment and survey type.
| Measure | FFS | FFS-PDP | MA | MA-PDP | MA-PPO | p-value |
|---|---|---|---|---|---|---|
| Mean (95% CI) | ||||||
| Care Overall | 8.4 (8.0, 8.9) | 9.0 (8.2, 9.7) | 8.5 (8.0, 9.0) | 8.4 (7.9, 8.8) | 8.4 (7.7, 9.1) | .02 |
| Personal Doctor Overall | 8.7 (8.1, 9.3) | 8.7 (8.1, 9.3) | 9.0 (8.6, 9.5) | 8.7 (8.3, 9.1) | 8.2 (7.6, 8.9) | .11 |
| Health Plan Overall | 8.8 (8.4, 9.2) | 8.7 (8.0, 9.4) | 8.7 (8.2, 9.2) | 8.9 (8.4, 9.4) | 8.4 (7.6, 9.1) | .30 |
| Doctor Communication | 88.5 (84.3, 92.7) | 86.6 (79.5, 93.8) | 89.1 (84.0, 94.1) | 87.2 (82.5, 91.9) | 87.6 (80.0, 95.3) | .79 |
| Getting Care Quickly | 72.8 (66.7, 78.8) | 78.2 (67.8, 88.7) | 76.3 (68.9, 83.7) | 74.3 (67.5, 81.2) | 78.8 (67.4, 90.3) | .45 |
| Getting Needed Care | 85.8 (79.9, 91.8) | 85.1 (76.1, 94.2) | 80.4 (73.4, 87.4) | 89.7 (83.0, 96.3) | 89.4 (78.5, 99.9) | .21 |
Abbreviations: CAHPS, consumer assessment of healthcare providers and systems; CI, confidence interval; FFS, fee-for-service; PDP, prescription drug plan; MA, Medicare advantage; PPO, preferred provider organization.
Adjusted with CAHPS case-mix procedures.
Factors associated with patient experiences
In preliminary unadjusted regression models, we found a significant and positive association between CAHPS scales and global ratings of care, personal doctor (p< .001 for each). This further demonstrated the convergent construct validity of the doctor communication, getting care quickly, and getting needed care scales with the global ratings. Therefore, we built new models to examine the CAHPS scales as outcome variables instead of covariates.
In fully adjusted models, completing the survey 8 to 12 months after diagnosis compared to 0 to 3 months was associated with a higher global rating of care (β .39, p= .008), see Table 4. Compared to Non-Hispanic Whites, American Indian/Alaska Native, Asian, and Pacific Islander patients had lower adjusted ratings of care overall (β −.73, p=.003), see Figure 2.
Table 4.
Fully adjusted association between characteristics and CAHPS domains.
| Global Ratingsa | Subscalesa | |||||
|---|---|---|---|---|---|---|
| Variables | Care Rating | Doctor Rating | Plan Rating | Doctor Comm. | Get Care Quick | Get Needed Care |
| Coefficient (95% CI) | ||||||
| Female (v Male) | −.15 (−.37, .08) | −.01 (−.22, .20) | .11 (−.12, .35) | 1.00 (−1.32, 3.31) | 2.85 (−.42, 6.13) | .23 (−2.44, 2.89) |
| Age (vs 65–70) | ||||||
| 70–74 | −.04 (−.41, .33) | −.15 (−.49, .20) | −.07 (−.45, .31) | −2.84 (−6.99, 1.30) | −.34 (−6.72, 6.03) | −5.70 (−10.6, −.80)* |
| 75–79 | −.08 (−.28, .45) | −.09 (−.25, .42) | .31 (−.07, .69) | −1.90 (−5.94, 2.14) | 1.40 (−4.66, 7.46) | .68 (−3.95, 5.31) |
| 80–85 | .23 (−.14, .60) | .18 (−.16, .53) | .44 (.05, .83)* | −.38 (−4.34, 3.58) | .71 (−5.14, 6.55) | .86 (−3.85, 5.57) |
| 85+ | −.02 (−.43, .38) | .07 (−.31, .45) | .44 (.02, .85)* | −1.38 (−5.74, 2.97) | 3.95 (−2.57, 10.5) | 1.57 (−3.62, 6.75) |
| Race/Ethnicity (v Non-Hispanic White) | ||||||
| Non-Hispanic Black | −.21 (−.77, .35) | −.17 (−.71, .37) | −.05 (−.61, .52) | −4.02 (−10.2, 2.19) | −2.18 (−10.9, 6.54) | 2.28 (−5.21, 9.77) |
| Non-Hispanic American Indian/Alaska Native, Asian, Pacific Islander | −.73 (−1.21, −.25)** | −.26 (−.72, .20) | −.28 (−.77, .22) | −2.86 (−8.22, 2.50) | −3.26 (−11.3, 4.80) | −4.52 (−10.8, 1.78) |
| Months of cancer diagnosis | ||||||
| 4–7mo (v 0–3) | .17 (−.11, .45) | −.03 (−.29, .24) | .27 (−.03.57) | −1.58 (−4.64, 1.49) | −2.45 (−6.85, 1.96) | −.19 (−3.72, 3.34) |
| 8–12mo (v 0–3) | .39 (.10, .69)** | .20 (−.08, .48) | .16 (−.14, .46) | −.76 (−4.04, 2.52) | .82 (−4.27, 5.90) | .12 (−3.63, 3.88) |
| Education (v 8th grade to high school) | ||||||
| High school graduate or GED | .07 (−.26, .39) | .19 (−.11, .48) | .09 (−.24, .43) | 2.32 (−1.18, 5.82) | −2.16 (−7.19, 2.87) | −.01 (−4.24, 4.22) |
| Some college or higher | −.10 ( −.42, .23) | .03 (−.27, .33) | −.28 (−.62, .05) | .56 (−2.84, 3.96) | −1.33 (−6.38, 3.71) | .85 (−3.38, 5.08) |
| General health status | ||||||
| Excellent, very good, good (v fair, poor) | .15 (−.12, .43) | .13 (−.12, .39) | .42 (.12, .71)** | 3.93 (1.00, 6.86)** | .25 (−3.95, 4.46) | 2.11 (−1.31, 5.54) |
| Comorbidity count | −.02 (−.18, .14) | .26 (.10, .42)** | .13 (−.04, .31) | 1.43 (−.37, 3.22) | 1.21 (−1.27, 3.68) | 1.42 (−.65, 3.49) |
| Medicaid dual eligible | ||||||
| Yes (v no) | −.10 (−.53, .32) | .43 (.04, .83)* | −.10 (−.36, .15) | 1.64 (−2.86, 6.14) | −4.88 (−11.5, 1.74) | 2.54 (−2.72, 7.81) |
| Urban-Rural status | ||||||
| Less Urban, Rural (v Big Metro, Metro, Urban) | .15 (−.35, .66) | .20 (−.26, .66) | −.05 (−.57, .47) | .76 (−4.52, 6.05) | −.47 (−8.32, 7.39) | −2.41 (−8.90, 4.08) |
Additionally adjusted with CAHPS case mix procedures, MA enrollment, if a proxy answered survey questions, SEER region of residence, percentage at poverty level, mental health status, tumor type, and tumor severity.
p<.05
p<.01
p<.001.
Figure 2.
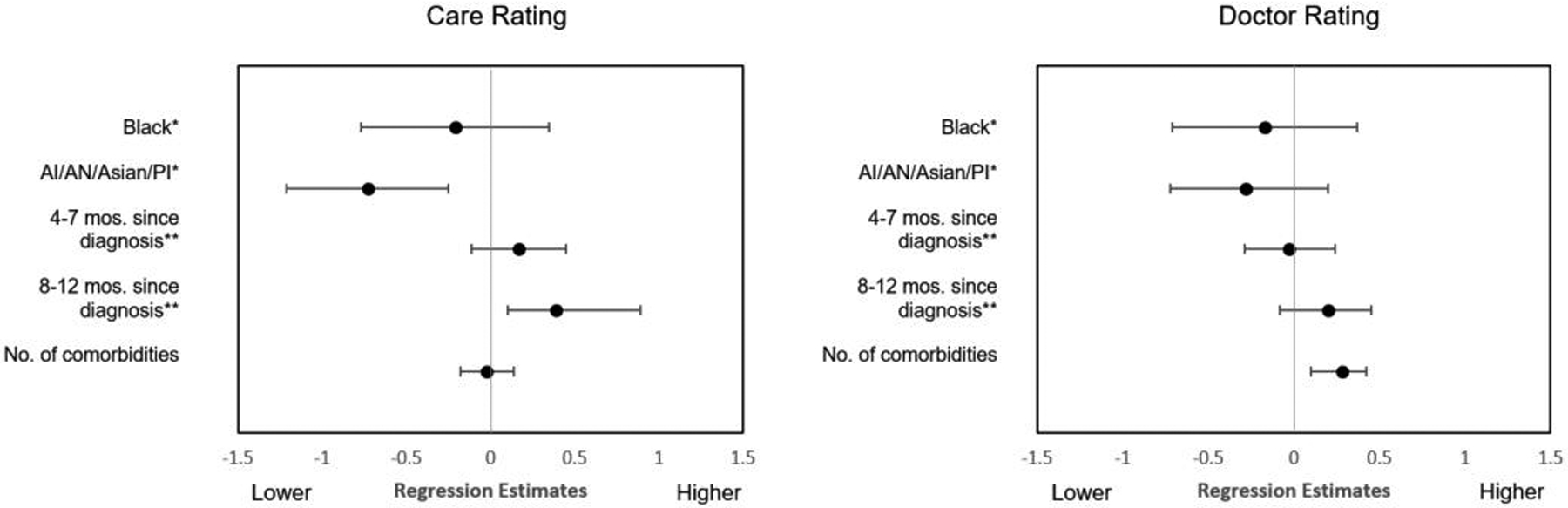
Fully adjusted regression estimates with 95% confidence intervals for the “Care” and “Doctor” CAHPS global rating models.
Each additional comorbidity (β .26, p=.003), as well as being dually eligible for Medicaid (β .43, p=.03), was significantly associated with a higher adjusted personal doctor rating. Favorable general health status was associated with significantly higher ratings of the health plan (p<.01) and doctor communication (p<.01).
In sensitivity analyses with complete cases only, we found no major differences in the direction or magnitude of regression coefficients.
Discussion
We found variations in perceptions of health care and interactions with providers among older adults diagnosed with hematologic malignancies in this secondary analysis of SEER-CAHPS data. After adjusting for known contributors to favorable health care experiences,23,24 ratings of care were more favorable in patients diagnosed with leukemia and lymphoma for greater than 8 months. In the context of hematologic cancer treatment, the first three months represent the acute treatment phase with multiple exposures to health care settings and personnel for myelosuppressive systemic therapy and potentially external beam radiation.25 Compared to solid tumors, hematologic cancer treatment for older adults usually requires chemotherapy with greater potential for myelotoxicity if the malignancy is of an acute or chronic leukemia morphology,26 or if lymphoma tumor burden is high.27 Along these lines, it is possible patients died earlier on and could not complete the survey given the high mortality of specific tumor sub-types in the first months of therapy (e.g., acute leukemia, T-cell lymphoma, and Burkitt lymphoma).28 This implication highlights the gravitas of delivery of care in this population and the need to capture patients’ voices early on to better understand experiences of care, preferences, and subsequent outcomes (e.g., what went wrong – patient factors, health system factors, physician factors, etc.).
Compared to previous research in patients diagnosed with solid tumors,29 our study observed lower unadjusted mean ratings on the getting care quickly scale, approximately a 10-point difference. It is important to note that diagnosis of hematologic malignancies are frequently delayed in older adults due to problems with oncology referrals, limited knowledge of hematologic pathologies among primary care providers, and masking of symptoms due to comorbidities.30,31 Findings from our study underscore the needed integration of geriatricians into routine care, given the critical role of geriatric assessments in clinical management of older patients with hematologic malignancies.32 Reduced intensity treatments, compared to the standard of care, may be preferred for older patients with a higher physiologic age and multiple comorbidities.33
Although we observed significant, albeit modest, differences in the overall experience of care in a relatively homogenous sample, this is a needed area of methodologic improvement in future patient experiences in cancer care research. Our findings were consistent with other literature showing that predictors of CAHPS item response and non-extreme value response (i.e., not “0” or “10”) included age younger than 70 years, non-Hispanic White race and ethnicity, and high education levels.34,35 We suspect that increased cases of Hispanics and non-White patients in this study would still identify a significant, negative relationship between race and ethnicity and global ratings, consistent with other SEER-CAHPS findings.36,37
The relationship between favorable patient experiences and hospital performance in surgical quality was studied previously, which showed a significant relationship between favorable patient experiences (i.e., HCAHPS measures) and mortality, failure to rescue, and minor surgical complications.38 Patient experience outcomes are currently used for public reporting and evaluating clinical performance (i.e., value-based purchasing).14,39 Our findings suggest that implementation of instruments targeted for cancer care (i.e., the CAHPS Cancer Care survey)40 should be administered in the acute treatment phases to capture health care delivery during clinically-important periods. Oncology practice leaders may find more clinically useful approaches of capturing objective measures of patient-clinician communication and receiving timely care while mitigating survey response bias, such as clinician audit and feedback and focus groups with patients.
This study has several limitations worthy of comment. Due to the cross-sectional design of the study, the relationship between variables should be interpreted as associations and not causal relationships. Low internal consistency reliability for the getting care quickly and getting needed care scales in our data were an unavoidable limitation, however CMS found better estimates of scale reliability in plan-level analyses. The annual Medicare-CAHPS response rates have declined below 50% since 2011,15 the CAHPS program considered this satisfactory for a nationally-distributed survey.41 Barriers to CAHPS mixed-mode survey response perhaps included increased time demands of the average citizen and growth in unsolicited telephone calls with caller identification, resulting in call blocking.42 Finally, the Medicare CAHPS survey is intended to measure experiences of general health care in Medicare beneficiaries; survey items were not developed to reflect cancer care. Recognizing these limitations, the SEER-CAHPS linked resources allowed for a feasible investigation into health care experiences during the first year of diagnosis in older adults diagnosed with leukemia and lymphoma.
To the authors’ knowledge, this is the first registry-based investigation into factors associated with favorable patient experiences among older adults diagnosed with hematologic malignancies. Our findings provide important methodological and clinical practice considerations. Experiences with care and physicians varied significantly during the first year of a hematologic cancer diagnosis. There is room for improvement in measuring patient experiences with care during cancer treatment trajectories, notably in terms of sample selection and temporality of survey administration. Using patient experiences to improve the highest-risk and highest-cost treatment phases will benefit quality, equity and clinical outcomes in the long term by transforming care towards a more robust, patient-centered paradigm.
Acknowledgments:
The content was solely the responsibility of the authors and does not represent the official views of the American Cancer Society or the United States Department of Health and Human Services. We are grateful for Dr. Michelle Mollica and team at the SEER-CAHPS program at the National Cancer Institute.
Funding: Supported in part by the American Cancer Society Doctoral Degree Scholarship in Cancer Nursing (33507-DSCN-19-048-01-SCN), Hillman Scholars Program in Nursing Innovation, and the Jonas Nurse Scholars Program. Dr. Friese is supported by The University of Michigan Rogel Cancer Center (P30-CA-046592).
Footnotes
Conflicts of Interest: No disclosures.
Availability of data: Not applicable.
Code availability: Not applicable.
References
- 1.Leukemia and Lymphoma Society. Facts and Statistics, General Blood Cancers. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview/facts-and-statistics. Published 2019. Accessed February 1, 2020.
- 2.Rathnasabapathy R, Lancet JE. Management of Acute Myelogenous Leukemia in the Elderly. Cancer Control. 2003;10(6):469–477. doi: 10.1177/107327480301000605 [DOI] [PubMed] [Google Scholar]
- 3.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003 [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. External Beam Radiation Therapy. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation/external-beam-radiation-therapy.html. Published February 2017. Accessed March 25, 2019.
- 5.Wagner EH, Aiello Bowles EJ, Greene SM, et al. The quality of cancer patient experience: perspectives of patients, family members, providers and experts. BMJ Quality & Safety. 2010;19(6):484–489. doi: 10.1136/qshc.2010.042374 [DOI] [PubMed] [Google Scholar]
- 6.Crawford R, Sully K, Conroy R, et al. Patient-Centered Insights on Treatment Decision Making and Living with Acute Myeloid Leukemia and Other Hematologic Cancers. Patient. 2020;13(1):83–102. doi: 10.1007/s40271-019-00384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131(5):515–524. doi: 10.1182/blood-2017-09-746420 [DOI] [PubMed] [Google Scholar]
- 8.Mollica MA. Patient experiences of cancer care: scoping review, future directions, and introduction of a new data resource: Surveillance Epidemiology and End Results-Consumer Assessment of Healthcare Providers and Systems (SEER-CAHPS). Patient Experience Journal. 2017;4(1):103–121. [Google Scholar]
- 9.Agarwal AK, Hahn L, Merchant RM, Rosin R. Handcrafting the Patient Experience. NEJM Catalyst. 2019;5(5). doi: 10.1056/CAT.19.0618 [DOI] [Google Scholar]
- 10.Blayney DW, Simon MK, Podtschaske B, et al. Critical Lessons From High-Value Oncology Practices. JAMA Oncol. 2018;4(2):164. doi: 10.1001/jamaoncol.2017.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla N, Urato M, Ambs A, et al. Unveiling SEER-CAHPS®: A New Data Resource for Quality of Care Research. Journal of General Internal Medicine. 2015;30(5):641–650. doi: 10.1007/s11606-014-3162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. About the SEER Program. SEER. https://seer.cancer.gov/about/overview.html. Published 2020. Accessed January 20, 2020.
- 13.Andersen RM. Revisiting the Behavioral Model and Access to Medical Care: Does it Matter? Journal of Health and Social Behavior. 1995;36(1):1. doi: 10.2307/2137284 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. Consumer Assessment of Healthcare Providers & Systems (CAHPS). https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/CAHPS. Published 2019. Accessed January 30, 2020.
- 15.SEER-CAHPS®. National Cancer Institute. https://healthcaredelivery.cancer.gov/seer-cahps/overview/description.html. Published September 2018. Accessed December 11, 2018.
- 16.Morales LS, Elliott MN, Weech-Maldonado R, Hays RD. Differences in CAHPS® Adult Survey Reports and Ratings by Race and Ethnicity: An Analysis of the National CAHPS® Benchmarking Data 1.0. Health Services Research. 2001;36(3):595–617. [PMC free article] [PubMed] [Google Scholar]
- 17.Paddison CAM, Elliott MN, Haviland AM, et al. Experiences of Care Among Medicare Beneficiaries With ESRD: Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS) Survey Results. American Journal of Kidney Diseases. 2013;61(3):440–449. doi: 10.1053/j.ajkd.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 18.Quigley DD, Elliott MN, Setodji CM, Hays RD. Quantifying Magnitude of Group-Level Differences in Patient Experiences with Health Care. Health Serv Res. 2018;53:3027–3051. doi: 10.1111/1475-6773.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayanian JZ, Zaslavsky AM, Arora NK, et al. Patients’ Experiences With Care for Lung Cancer and Colorectal Cancer: Findings From the Cancer Care Outcomes Research and Surveillance Consortium. Journal of Clinical Oncology. 2010;28(27):4154–4161. doi: 10.1200/JCO.2009.27.3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Survey Instruments and Specifications. Medicare Advantage and Prescription Drug Plan CAHPS Survey. https://ma-pdpcahps.org/en/survey-instruments-and-specifications/. Published 2020. Accessed April 22, 2020.
- 21.National Cancer Institute. Site Recode ICD-O-3. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/siterecode/index.html. Published 2019. Accessed February 25, 2019.
- 22.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338(jun29 1):b2393–b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Case-Mix Adjustment Guidance for SEER-CAHPS Analyses. https://healthcaredelivery.cancer.gov/seer-cahps/researchers/adjustment_guidance.html. Published 2020. Accessed January 20, 2020.
- 24.Elliott MN, Haviland AM, Orr N, Hambarsoomian K, Cleary PD. How Do the Experiences of Medicare Beneficiary Subgroups Differ between Managed Care and Original Medicare?: Experiences of Medicare Beneficiary Subgroups. Health Services Research. 2011;46(4):1039–1058. doi: 10.1111/j.1475-6773.2011.01245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Systemic Therapy. https://www.moffitt.org/treatments/systemic-therapy/. Published 2018. Accessed November 30, 2018.
- 26.Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leukemia Research Reports. 2016;6:1–7. doi: 10.1016/j.lrr.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobbi PG, Broglia C, Di Giulio G, et al. The clinical value of tumor burden at diagnosis in Hodgkin lymphoma. Cancer. 2004;101(8):1824–1834. doi: 10.1002/cncr.20568 [DOI] [PubMed] [Google Scholar]
- 28.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes: 2016 US Lymphoid Malignancy Statistics by World Health Organization Subtypes. CA: A Cancer Journal for Clinicians. 2016;66(6):443–459. doi: 10.3322/caac.21357 [DOI] [PubMed] [Google Scholar]
- 29.Mollica MA, Weaver KE, McNeel TS, Kent EE. Examining urban and rural differences in perceived timeliness of care among cancer patients: A SEER-CAHPS study: Patient Experiences: Urban vs Rural. Cancer. 2018;124(15):3257–3265. doi: 10.1002/cncr.31541 [DOI] [PubMed] [Google Scholar]
- 30.Abel GA, Friese CR, Magazu LS, et al. Delays in referral and diagnosis for chronic hematologic malignancies: A literature review. Leukemia & Lymphoma. 2008;49(7):1352–1359. doi: 10.1080/10428190802124281 [DOI] [PubMed] [Google Scholar]
- 31.Friese CR, Abel GA, Magazu LS, Neville BA, Richardson LC, Earle CC. Diagnostic delay and complications for older adults with multiple myeloma. Leukemia & Lymphoma. 2009;50(3):392–400. doi: 10.1080/10428190902741471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. JCO. 2018;36(22):2326–2347. doi: 10.1200/JCO.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldoss I, Forman SJ, Pullarkat V. Acute Lymphoblastic Leukemia in the Older Adult. JOP. 2019;15(2):67–75. doi: 10.1200/JOP.18.00271 [DOI] [PubMed] [Google Scholar]
- 34.Elliott MN, Edwards C, Angeles J, Hambarsoomians K, Hays RD. Patterns of Unit and Item Nonresponse in the CAHPS® Hospital Survey: Unit and Item Nonresponse. Health Services Research. 2005;40(6p2):2096–2119. doi: 10.1111/j.1475-6773.2005.00476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott MN, Haviland AM, Kanouse DE, Hambarsoomian K, Hays RD. Adjusting for Subgroup Differences in Extreme Response Tendency in Ratings of Health Care: Impact on Disparity Estimates. Health Services Research. 2009;44(2p1):542–561. doi: 10.1111/j.1475-6773.2008.00922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halpern MT, Urato MP, Kent EE. The health care experience of patients with cancer during the last year of life: Analysis of the SEER-CAHPS data set: Health Care Ratings in Last Year of Life. Cancer. 2017;123(2):336–344. doi: 10.1002/cncr.30319 [DOI] [PubMed] [Google Scholar]
- 37.Farias AJ, Ochoa CY, Toledo G, Bang S-I, Hamilton AS, Du XL. Racial/ethnic differences in patient experiences with health care in association with earlier stage at breast cancer diagnosis: findings from the SEER-CAHPS data. Cancer Causes Control. 2020;31(1):13–23. doi: 10.1007/s10552-019-01254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacks GD, Lawson EH, Dawes AJ, et al. Relationship Between Hospital Performance on a Patient Satisfaction Survey and Surgical Quality. JAMA Surg. 2015;150(9):858. doi: 10.1001/jamasurg.2015.1108 [DOI] [PubMed] [Google Scholar]
- 39.Anhang Price R, Elliott MN, Zaslavsky AM, et al. Examining the Role of Patient Experience Surveys in Measuring Health Care Quality. Med Care Res Rev. 2014;71(5):522–554. doi: 10.1177/1077558714541480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evensen CT, Yost KJ, Keller S, et al. Development and Testing of the CAHPS Cancer Care Survey. JOP. August 2019:JOP.19.00039. doi: 10.1200/JOP.19.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CAHPS Surveys and Guidance. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/cahps/surveys-guidance/index.html. Published October 2018. Accessed November 30, 2018.
- 42.Pew Research Center. Collecting survey data. Pew Research Center Methods. https://www.pewresearch.org/methods/u-s-survey-research/collecting-survey-data/. Published 2019. Accessed March 27, 2019.


