Abstract
CCR6+ CXCR3+ CCR4- CD4+ memory T cells, termed Th1*, are important for long-term immunity to Mycobacterium tuberculosis (MTB) and the pathogenesis of autoimmune diseases. Th1* cells express a unique set of lineage-specific transcription factors characteristic of both Th1 and Th17 cells, and display distinct gene expression profiles compared to other CD4+ T cell subsets. To examine molecules and signaling pathways important for the effector function of Th1* cells, we performed loss-of-function screening of genes selectively enriched in the Th1* subset. The genetic screen yielded candidates whose depletion significantly impaired T cell receptor (TCR)-induced interferon gamma (IFNγ) production. These included genes previously linked to IFNγ or MTB susceptibility and novel candidates, such as ISOC1, encoding a metabolic enzyme of unknown function in mammalian cells. ISOC1-depleted T cells, which produced less IFNγ and IL-17, displayed defects in oxidative phosphorylation and glycolysis, and impairment of pyrimidine metabolic pathway. Supplementation with extracellular pyrimidines rescued both bioenergetics and IFNγ production in ISOC1-deficient T cells, indicating that pyrimidine metabolism is a key driver of effector functions in CD4+ T cells and Th1* cells. Results provide new insights into the immune-stimulatory function of ISOC1 as well as the particular metabolic requirements of human memory T cells, providing a novel resource for understanding long-term T cell-driven responses.
Keywords: CD4 T cells, mycobacterium tuberculosis, interferon gamma, pyrimidine metabolism
Introduction
IFNγ production by CD4+ T cells plays an essential role in persistent control of infections, including latent Mycobacterium tuberculosis (MTB) (1–4). In humans, genetic mutations resulting in IFNγ deficiency predispose to mycobacterial diseases (5), and animal studies show that mice lacking IFNγ or IFNγ-regulating factors such as T-bet, IL-12, STAT1 are highly susceptible to mycobacteria infection (6–9). Non-conventional IFNγ-producing CD4+ T cells termed Th1* cells account for >80% of MTB-reactive memory T cells in the periphery (10–13). Th1* cells exhibit a CCR6+CXCR3+CCR4- phenotype and share lineage-specific characteristics with conventional Th1 (CCR6-CXCR3+CCR4-) and Th17 (CCR6+CXCR3-CCR4+) memory cells (10, 14–17), including expression of T-bet and RORC, the transcriptional regulator of IL-17 production. Th1* cells are significantly expanded in latent TB infection (LTBI) (10), and IFNγ-producing Th1* cells comprise more than 50% of MTB-specific cells during active MTB disease (17), supporting a protective role in MTB control. Th1* cells are also linked to pro-inflammatory signaling in rheumatoid arthritis, multiple sclerosis, and Crohn’s disease (14, 16, 18). Thus, this unique subset is important in the context of protective responses to infection and in controlling autoimmunity and inflammation.
Previous studies provided transcriptomic analysis of immune signature genes in human Th1* cells (10, 19). More than 400 genes demonstrate differential expression in Th1* compared to Th1, Th2, and Th17 populations (10). Functional network analysis of genes selectively up-regulated in Th1* shows enrichment of factors linked to T cell proliferation, effector functions, and MTB immunity such as CCR2 (20), RORC (21), and components of IL-23 signaling (22–26). We reasoned that these genes are involved in Th1* effector function. Here we report a loss-of-function screen of immune signature genes in endogenous human Th1* cells. New regulators of IFNγ production included ISOC1, a metabolic enzyme of unknown function in mammalian cells. We find that loss of ISOC1 reduced IFNγ and IL-17 production by perturbing cellular pyrimidine metabolism. Results demonstrate the utility of functional screening for validating immune signature genes, and highlight the importance of metabolic fitness for the adaptive immunity.
Materials and Methods
Gene Selection Strategy
For the RNAi screen, 209 genes were selected based upon enriched expression in Th1* cells compared to Th1, Th2, and Th17 (10). To prioritize functionally relevant genes in this subset, gene regulatory network (GRN) analysis using the C3NET approach (27) was performed using the published gene expression dataset for Th1* cells (10) and additional Th1* and activated CD4+ T cell datasets from the DICE database (28). For each gene, C3NET identifies paired genes showing the highest co-expression as ‘neighbors’ in the regulatory network, and genes with multiple neighbors are central network ‘hub’ genes. We utilized number of neighbors for each gene in the network analysis to rank the Th1* enriched genes. Combining this information with gene ontology, a set of 100 gene-specific shRNAs (including a non-targeting shRNA) was used for screening, with T-bet and LCK included as established positive regulators of IFNγ.
Isolation of human CD4 T cells and cell sorting
PBMC were isolated from healthy donor whole blood at the LJI Clinical Core and normal blood donor program (VD-057). PBMC were purified by density gradient centrifugation using Lymphoprep (Cosmo Bio, USA). Isolated cells were resuspended in HI-FBS FBS (Sigma-Aldrich) containing 10% DMSO and cryopreserved in liquid N2. CD4+ cells were positively selected and isolated using CD4 dynabeads and magnetic separation (Dynal, Life Technologies), according to the manufacturer’s protocol. After purification, ~ 98% of cells were CD4+ as assessed by flow cytometry. For isolation of Th1* and other memory cell subsets, PBMC were labeled with positive and negative selection antibodies (10). Briefly, viable lymphocytes were identified by forward/side scatter and Live/Dead Aqua (eBioscience) staining. Dump gating excluded CD8, CD14, CD19-positive populations and dead cells. CD4+CD3+CD25- memory cells were identified based on CD45RA and CCR7 expression (i.e. excluding CD45RA+CCR7+ naive cells). Cells were sorted in CCR6+CXCR3+CCR4- (Th1*), CCR6+CXCR3-CCR4+ (Th17), CCR6-CXCR3+CCR4- (Th1), and CCR6-CXCR3-, CCR4+ (Th2) subsets. Th1* cells were further expanded in culture for screening experiments. Cell sorting was performed on a BD FACSAria-3 or FACSAria-4 Fusion instruments. The antibodies used were: CD4 APCEf780 (RPA-T4) and CD45RA eF450 (HI100) (from eBioscience), CD3 Alexa Fluor 700 (UCHT1), CXCR3 APC (IC6), CCR6 biotin (11A9), CD19 V500 (HIB19), CD14 V500 (145E2), CD8 V500 (RPA-T8), CD25 FITC, CCR4 PECy7 (1G1) (all from BD), CCR7 PerCPCy5.5 (G043H7) (BioLegend).
T cell culture and stimulation
Purified T cells were maintained in IMDM containing 5% HI-FBS (Sigma-Aldrich), 2% human serum (CellGro), and 25 μg/ml gentamicin (Gibco), denoted as T cell medium. Freshly sorted cells were stimulated with anti-CD3/CD28 coated dynabeads (Invitrogen) at 1:1 cell:bead ratio, and expanded in T cell medium supplemented with 60 U/ml recombinant IL-2 (eBioscience). Cell numbers were determined by Trypan Blue staining using Countess cell counter (Thermo Scientific) or Moxi Z cell counter (Orflo Technologies).
Human shRNA library and lentivirus production
Genome-wide human lentiviral shRNAs (Sigma-Mission TRC) were obtained at the LJI Functional Genomics Core (29, 30). shRNA clones were collected from master glycerol stock plates using Beckman Biomek FXp liquid handler, and amplified in LB medium in deep-well 96-well plates. Plasmid DNA was isolated using Omega E.Z.N.A. Plasmid DNA Mini kit and quantified on a NanoDrop One (Thermo Scientific). In the screen, four distinct shRNAs for were combined into gene-specific pools during bacterial culture after normalization by optical density. The culture was then used for plasmid DNA purification using GenElute HP 96 Well Plasmid Miniprep kit (Sigma-Aldrich) and lentivirus production. For validation of selected genes, 3–5 individual shRNAs were tested for each gene. Lentivirus for pooled or individual shRNAs were produced in HEK293T cells plated in 6-well plates in antibiotic-free DMEM (ThermoFisher Scientific) supplemented with glutamine and FBS. Cells cultured in 10% FBS-containing DMEM were transfected with 375 ng shRNA plasmid and 375 ng of 3:1 (psPAX2:pMD2.G) lentiviral packaging mix using jetPRIME transfection reagent (Polyplus). Packaging plasmids were obtained from AddGene. After 24 hrs, the media was changed to the culture media containing 30% FBS. Viral supernatants were collected at post-transfection days 2 and 3, filtered through 0.45 μm low protein-binding filters, aliquoted and stored at −80 °C. Titers of pooled shRNA viruses used in the primary screen were measured using p24 ELISA kit (ZeptoMetrix). Non-targeting control corresponds to SHC002 from the TRC library.
Lentivirus transduction
Cells were stimulated for 1–2 days with anti-CD3/CD28 coated dynabeads (Life Technologies) at a 1:1 bead to cell ratio prior to transduction with shRNA lentiviruses. After removal of dynabeads, cells were infected with high-titer shRNA lentiviruses (MOI ~8–10) in 96-well plates (100 μl viral supernatants per ~9×105 cells per well). Cells were transduced in triplicate wells (assigned for each shRNA target). Polybrene (Sigma-Aldrich) was added to the mixture at a final concentration 8 μg/ml. Cells were centrifuged at 2000 rpm for ~ 2hrs at 30 °C; viral media was removed after 4 hr and replaced with T cell medium containing 60 U/ml IL-2. One day after transduction, puromycin (InvivoGen) was added at a final concentration 2 μg/ml. Cells were cultured for 3–4 days in IL-2 containing T cell medium in the presence of puromycin, followed by re-stimulation with anti-CD3/CD28 dynabeads (in the absence of added IL-2). Viable cells were analyzed for anti-CD3/28-induced IFNγ production and used in other downstream analyses. For several experiments, functional viral titers were determined by titrating viral preparations in the presence and absence of puromycin (Fig. 1A). Titration curves were fit according to the Poisson distribution. Briefly, fraction of infected cells P follows equation P = (1 – e-m), where m is multiplicity of infection (MOI). For a given dose of viral stock V, P is a variable that can be experimentally quantified by dividing the number of viable puromycin-resistant cells by the control cell number in the absence of puromycin. The corresponding MOI is a function of an unknown variable, viral titer T; MOI can be expressed as T*V/N, where N is the number of cells in the sample. Therefore, P can be expressed as (1-e-T*V/N). However, experimental titration curves show that quantification can be confounded by the presence of transduction-resistant cells that are not infected at increasing amounts of virus (Fig 1A). Thus, an additional parameter, a fraction of “transducible” cells (Amax), is introduced to the analysis. The resulting equation P = Amax *(1 – e -T*V/ Amax *N) was used for non-linear regression analysis of titration curves to determine the viral titer.
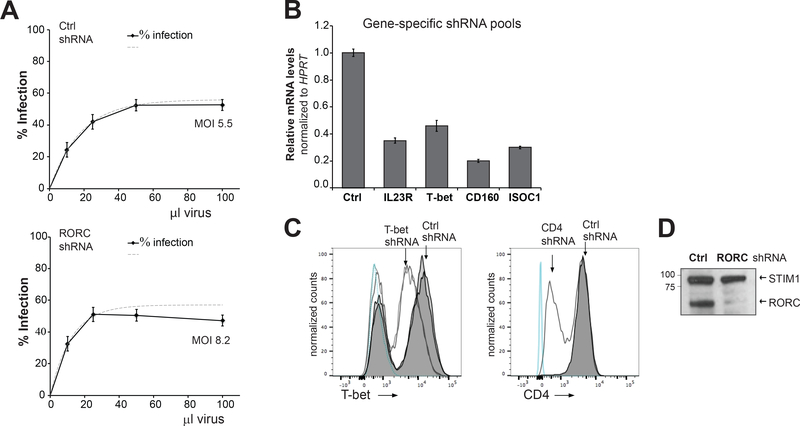
FIGURE 1. Lentiviral shRNA transduction and knockdown efficiency in human T cells.
(A) Titration of shRNA lentiviruses in CD4+ T cells. Dotted lines represent best-fit curves generated using non-linear regression. Cells (~105 cells/well) were infected in 96-well plates with 10–100 μl shRNA viruses and cultured with or without puromycin (2 μg/ml) for 3 days. Control (Ctrl) is a non-targeting SHC002 shRNA from the Sigma-Mission TRC library. Percentage viable cells was measured using ATP levels. Percentage infection for viral dose, the titer and MOI was quantified as described in Methods. Data are mean ± S.E.M., n=4 replicate samples. (B) Knockdown efficiency produced by pooled shRNAs targeting indicated genes in Th* cells. Relative mRNA levels were measured by RT-qPCR; data are mean ± S.E.M., n=3 replicate samples. (C,D) Validation of protein knockdown. Expression of T-bet, CD4, and RORC in control and shRNA-transduced CD4+ T cells was measured by flow cytometry (C) or western blot (D). Double lines indicate replicate samples, and blue lines indicate unstained cells (C). STIM1 was used as a loading control (D). Data are representative of two independent experiments using two to three replicates per experimental group. Paired t-test was used to analyze statistical significance.
IFNγ ELISA and ATP assays
IFNγ in cell supernatants was measured using human IFNγ ELISA kit (Invitrogen) according to manufacturer’s protocol. Cells plated at 5×105 cells/100 μl per well of a 96-well plate were stimulated with anti-CD3/28 dynabeads for 24 hrs, unless otherwise indicated. Background signal was assessed using unstimulated cells. After stimulation, cells were transferred to U-bottom 96-well plates and centrifuged for 5 min (800 g). Supernatants (50 μl from each well) were collected and stored at −80 °C prior to analysis. Cell viability was measured using ATP-based CellTiter-Glo viability assay (Promega). After supernatant collection, cells pellets were resuspended in 100 μl T cell media and 20 μl aliquots from each well were transferred to black-wall 96-well plates (Costar) for ATP measurements. IFNγ (OD450) and ATP (luminescence) were measured using a PE Envision-1800 high throughput microplate reader. ATP values were used to normalize IFNγ levels. To correct for confounding inhibitory effects on ATP, dose-dependent effects of azide on ATP production and IFNγ levels were measured (Fig 3B). The non-linear relationship was fit into a third order polynomial function (31). This model was used to calculate normalized IFNγ levels. Screening data are expressed as percentages of corresponding values obtained with control (non-targeting) shRNAs. IFNγ concentrations in the supernatants were determined based on the calibration curve (using human IFNγ standards) generated on each ELISA plate. A non-linear (quadratic) regression was applied to fit the data.
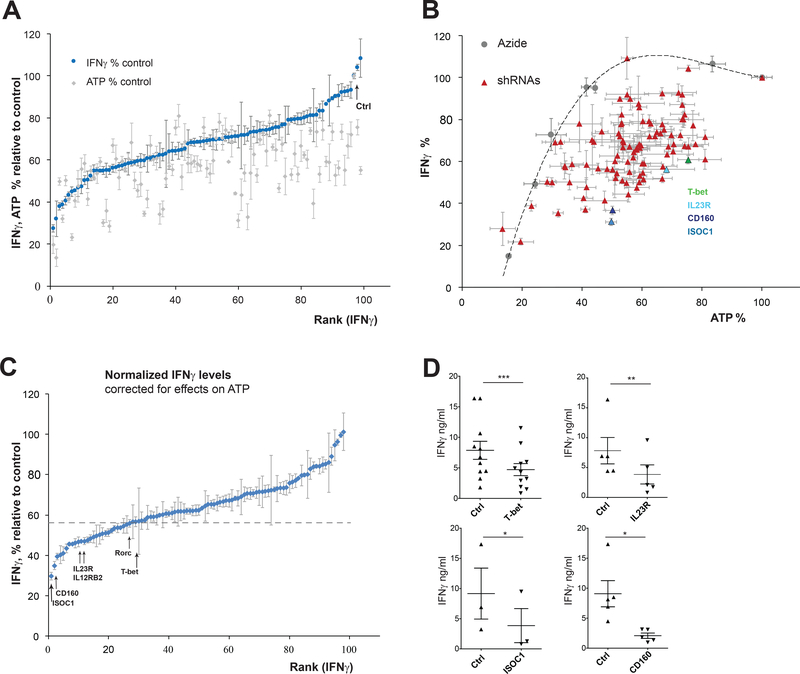
FIGURE 3. Loss-of-function RNAi screen for regulators of IFNγ production in Th1* cells.
(A) IFNγ and ATP levels in Th1* cells transduced with gene-specific shRNA pools. Data are expressed as percentages corresponding values in cells transduced with non-targeting SHC002 shRNA (Ctrl). Experimental set-up depicted in Fig. 2E. (B) IFNγ and ATP data from (A) compared with azide, an inhibitor of ATP production. Dose-dependent effects of azide titrated in the concentration range 0–20 mM on IFNγ and ATP levels were measured, and the relationship was fit into a third order polynomial function (dotted line) used for IFNγ data normalization. Results for T-bet, IL23R, CD160 and ISOC shRNAs are highlighted in different colors. (C) Normalized IFNγ data corrected for shRNA-dependent effects on ATP; dotted line indicates an arbitrary cut-off based on the positive control (T-bet shRNA). Data in (A-C) are shown as mean ± S.E.M of 3–6 replicate samples. (See Tables S1, S2 for IFNγ and ATP data sets). (D) Effects of selected shRNA pools on IFNγ levels. Data are representative of three independent experiments using three to eleven replicates per experimental group and shown as mean ± S.E.M. Paired t-test was used to analyze statistical significance.
IL-17 ELISA
IL-17 in cell supernatants was measured using human IL-17 ELISA kit (Sigma) according to the manufacturer’s protocol and as described above for IFNγ, except that 60–80 μl supernatant was analyzed. IL-17 concentrations were determined from a calibration curve using IL-17 standards and a non-linear (quadratic) regression analysis.
Flow-cytometry and intracellular IFNγ measurements
Intracellular IFNγ was measured in anti-CD3/28-stimulated cells. After 24 hrs stimulation, fixation & permeabilization buffer (Affymetrix-eBioscience) was added prior to staining for intracellular proteins. Brefeldin A (Sigma-Aldrich) was added at 10 μg/ml during last 4 hrs of stimulation. Prior to fixation, cells were stained with Live/Dead Aqua or Violet fixable viability dyes (Invitrogen). Fixed and permeabilized cells were stained with IFNγ-FITC (eBioscience) or IFNγ-APC antibody (BD Pharmingen) for 40 min at room temperature. Other antibodies used for surface and intracellular staining were: CD160-APC, CD4-APCef780 or CD4-FITC (all from eBioscience), and T-bet-PE (Miltenyi Biotec Inc). For T-bet staining, samples of anti-CD3/28-stimulated cells were prepared using fixation & permeabilization buffer set for transcription factors and nuclear proteins (Affymetrix-eBioscience). Samples were acquired in a BD FACSCanto-II instrument and analyzed using FlowJo10.2 software.
Cell viability and proliferation
Proliferation of anti-CD3/28-stimulated CD4 T cells was measured by carboxyfluorescein dilution. Cells were labeled using CellTrace CFSE cell proliferation kit (Thermo Scientific) following the manufacturer’s protocol. After 2 or 3 days stimulation with anti-CD3/28 dynabeads (at a 1:1 bead to cell ratio), carboxyfluorescein-loaded cells were analyzed by flow cytometry. CFSE staining at day 0 (in unstimulated cells) was performed in select experiments to verify equal CFSE loading in control and test samples. Cell survival during cell culture was assessed using Moxi Z cell counter (Orflo Technologies). Alternatively, cells were counted after Trypan Blue staining using Countess automated cell counter (Thermo Scientific). Apoptosis was measured using FITC-labeled Annexin V/Propidium iodide (PI) staining kit (BD Pharmingen) and flow cytometry. Initial forward and side scatter gating excluded most of dead (PI-positive) cells from further analysis. Early apoptosis was quantified as a percentage of Annexin V-positive and PI-negative cells. Samples were analyzed in BD FACSCanto-II instrument within 1 hour after staining.
Real-time quantitative PCR
Total RNA was isolated from ~1–2×106 cells using Quick-RNA MiniPrep kit (Zymo Research). RNA concentrations were obtained on a NanoDrop spectrophotometer; cDNA was reverse-transcribed from 5 μg RNA with qScript cDNA SuperMix (Quanta Biosciences). Primers and probes for real time qPCR analysis were predesigned Taqman Gene Expression Assays (Applied Biosystems) including: TBX21 (Hs00894392_m1), CD160 (Hs00199894_m1), HPRT1 (Hs02800695_m1), ISOC1 (Hs0011372_m1), IL23R (Hs00332759_m1). Measurements were done in BioRad CFX96 qPCR instrument. All values were normalized to mRNA levels of the housekeeping gene HPRT1 (hypoxantine phosphoribosyl transferase).
Deep labeling, metabolite extraction, and LC-MS/MS
Untargeted analysis of endogenous metabolites in CD4+ T cells was performed using “deep 13C labeling” of amino acids and glucose (32). CD4+ cells transduced with non-targeting and ISOC1 shRNAs were cultured in RPMI-based 13C-labeled (Cambridge Isotopes) media supplemented with 25 μg/ml gentamicin and 5% FBS and IL-2 (60 U/ml). Transduction and selection of shRNA-expressing cells was performed as described above in 13C-labeled medium and 1 μg/ml puromycin 24 hours after transduction. Cells were cultured for 5 days in the labeling media, and media fresh added every day. In parallel, control and ISOC1 shRNA cells were cultured in unlabeled media under the same conditions. On day 4, cells were transferred to puromycin-free labeled or unlabeled media and stimulated with anti-CD3/CD28. On day 5, cells (~1.5X106 for triplicate samples per condition) were centrifuged and supernatant aliquots were collected and frozen at −80 °C. Cell pellets were washed with cold PBS and stored at −80 °C. Cell pellets were lysed in cold 4:1 methanol:water, cycled three times between 40 °C and −80 °C for 30 second intervals. Extracts were centrifuged at 14,000 RPM for 10 minutes at 4 °C to sediment insoluble cell debris. Supernatants were removed and dried via vacuum concentration at 40 °C until dry, before resuspension in cold 4:1 methanol:water. Extracts were incubated at −20 °C for 30 minutes to sediment any remaining insoluble material, which was removed through additional centrifugation at 14,000 RPM for 10 minutes at 4 °C. LC-MS/MS was performed as described (32). Briefly, chromatography was performed using a Thermo Vanquish UHPLC system (Thermo Scientific) with a ZIC-pHILIC polymeric column (150 mm x 2.1 mm x 5 μm) (Sequant) at 25 °C. All cell samples used an injection volume of 5 μL, constituting 80,000 cell equivalents. Mobile phases were: A) 20 mM ammonium bicarbonate in water, pH 9.60, and B) acetonitrile. Gradient mobile phase followed a 19 minutes course, starting at 90 % B for 2 minutes, followed by a linear gradient to 55 % B at 16 minutes, sustained for 3 additional minutes. The column was then re-equilibrated for 11 minutes at 90 % B. Positive and negative ions were generated from a heated electrospray ionization source coupled to a Thermo Q-Exactive Orbitrap mass spectrometer. MS1 spectra were acquired at 35,000 resolution with 100 ms ion trap time; MS2 spectra were acquired at 17,500 resolution and 50 ms ion trap time from a 1.0 m/z range around parent MS1 and with a normalized collision energy of 35 V. Mass range was 67–1000 m/z. Sheath and auxiliary gas flow rates were 40 and 20 units, respectively, with sweep gas flow rate at 2 units. Spray voltage was 3.5 kV for positive mode and 2.5 kV for negative mode. Capillary inlet and auxiliary gas heater temperatures were 275 °C and 350 °C, respectively. LC-MS/MS feature extraction, putative HMDB identification, and mass isotopomer enrichment analysis were performed as previously described (32).
Metabolic flux
Energy metabolism fluxes was assessed using Seahorse XFe96 analyzer (Agilent). Optimized assay medium contained DMEM base (Sigma D5030–1L), 1.85 g/L NaCl, 5 mM HEPES, pH 7.6 (at room temperature) and 3 mg/L Phenol Red supplemented with 10 mM glucose, 10 mM pyruvate, 4 mM glutamine. Cells were acutely attached to Seahorse-manufactured 96-well cell culture plates pre-coated with Cell-Tak (Corning, # 354240). Cell-Tak was used essentially as described in the manufacturer’s protocol, except that coating density of 12 μg/cm2 (1.3 μg/well in 10 μl) was applied. For seeding, cells were pelleted (5 min at 400 g), washed with the assay medium, plated at a density of 3 ×105 cells per well and spun down (5 min at 500 g) for attachment 30 min before the assay. Prior to seeding, cell concentrations were determined from triplicate live cell counts using the Moxi Z cell counter. Equal cell numbers were verified in each well by microscopy. For stimulation, anti-CD3/CD28-coated dynabeads were washed with PBS + 0.1% BSA twice followed by wash with the assay buffer and added to the cells in the ratio 2:1 immediately prior to plating. Oxygen Consumption Rate (OCR) was measured as the indicator of mitochondrial activity. Extracellular Acidification Rate (ECAR) reflects the rate of lactate production from glucose, as well as carbon dioxide production from glycolytically produced pyruvate. Thus, ECAR serves as a semi-quantitative measure of glycolytic flux. Contribution of non-glycolytic acidification in ECAR was derived from responses of cells incubated in the absence of added glucose and subtracted from the responses in complete, glucose-containing medium to determine glycolyic fluxes. The basal state of stimulated or unstimulated cells was followed for 30 minutes by which time TCR-mediated bioenergetics responses manifested. Monitoring was followed by addition of mitochondrial inhibitors (Sigma-Aldrich): 2 μg/ml oligomycin, 2 pulses of 150 μM uncoupler DNP and 2 μM respiratory inhibitor myxothiazol to assess resting respiration (an indicator of mitochondrial membrane intactness), maximal respiratory capacity and non-mitochondrial/baseline oxygen consumption, respectively. ECAR measurements corrected for non-glycolytic component were used to determine basal and maximally stimulated glycolytic activity; the latter was induced by oligomycin but increased over time and reached maximum in the last reading in the presence of myxothiazol. A conversion factor of ECAR units (mpH/min) to acid production rates (pmol H+/min) was determined in separate experiment by injecting known amount of acid to the medium, and was found to be 6.1.
Western blotting
Cells were centrifuged at a low speed and pellets resuspended in 50 μl ice-cold lysis buffer containing 0.5% Nonidet P-40, 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 1X Complete protease inhibitor mixture (Roche). After 20 min on ice, whole cell lysates were centrifuged at 15,000xg for 15 min. Protein concentrations were determined by Pierce BCA protein assay (Thermo Scientific). Whole cell extracts were loaded on NuPage 4%−12% Bis-Tris gels (Life Technologies) at 20–40 μg per lane. Gels were run at 200 V for 42 min and proteins transferred to nitrocellulose membrane (BioRad) at 30 V for 75 min. Membranes were blocked in 2.5% milk overnight at 4 °C and probed with primary antibodies for RORC, ISOC1 (Aviva Systems Biology), and STIM1 (Cell Signaling) and secondary horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies (Sigma Aldrich). Proteins were detected using ECL (GE Healthcare) or SuperSignal West Femto reagents (Thermo Scientific).
Statistical analysis.
Student’s t-test and two-way analysis of variance (ANOVA) with post-hoc Bonferroni tests were performed using GraphPad Prism software. Symbols: ***, p<0.001; **, p<0.01; *, p<0.05.
Results
Loss-of-function screening of immune signature genes in endogenous Th1* uncovers regulators of IFNγ production
To characterize the Th1* immune signature, we designed an RNA interference (RNAi) screen examining IFNγ production. Target genes were selected based upon enrichment in Th1* cells and gene network analysis prioritizing regulatory or signaling proteins (Table S1). Lentiviral transduction in CD4+ T cells yielded ~50% shRNA-expressing, puromycin-resistant cells (Fig. 1A). Higher viral doses did not significantly increase transduction efficiency, and cell viability decreased at MOI >10. Pooled shRNA preparations used in the screen typically elicited 60–80% target mRNA and protein depletion after puromycin selection (Fig. 1B–D).
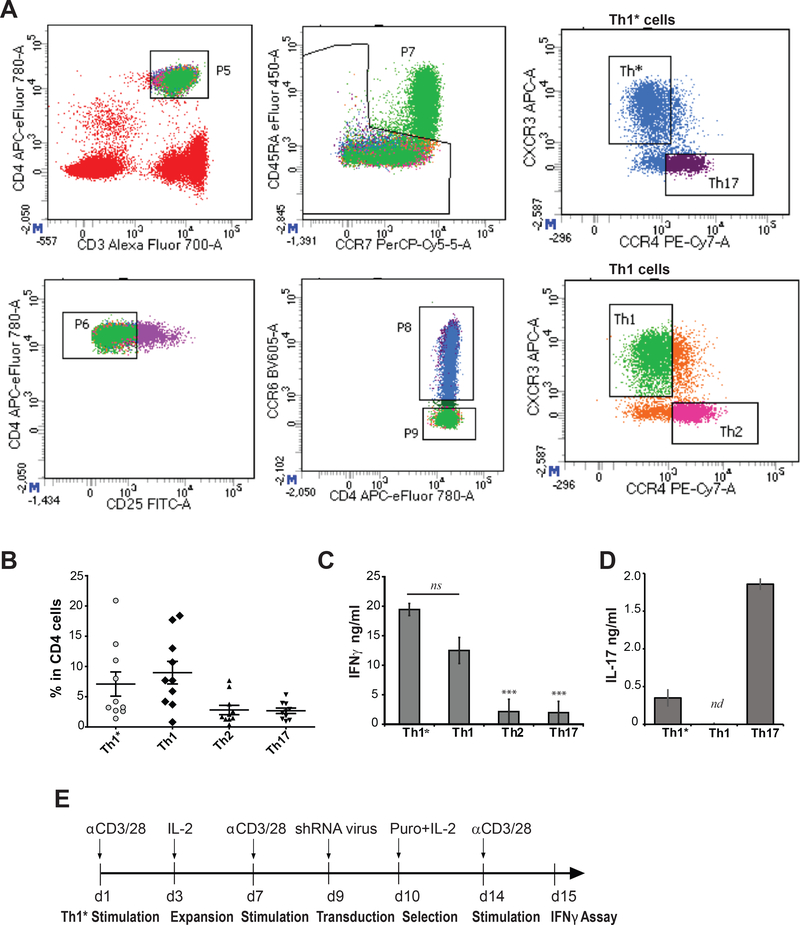
Th1*, Th1, Th2 and Th17 subsets were co-sorted from human PBMC (Fig. 2A). Endogenous Th1* cells typically constitute 2–21% of total CD4+ T cells in the periphery ((10) and Fig. 2B). Anti-CD3/28-stimulated Th1* co-produced IFNγ (Fig. 2C) and IL-17 (Fig. 2D), which was preserved after repeated polyclonal stimulations ex vivo. The screen was conducted using ex vivo expanded Th1* cells, by measuring secreted IFNγ in puromycin-selected cells after anti-CD3/28 stimulation (Fig. 2E). To normalize IFNγ levels, cell viability was measured post-stimulation by quantifying ATP. These measurements typically demonstrate linear dependence upon metabolically active cell numbers over a large dynamic range. However, ATP readouts varied significantly in the shRNA-expressing Th1* populations (Fig. 3A), likely due to effects upon cellular bioenergetics, puromycin sensitivity or other factors affecting cellular ATP levels. To reduce false-positives, IFNγ measurements were corrected for decreased ATP. Dose-dependent effects of the mitochondrial inhibitor azide on IFNγ production were examined, confirming a reciprocal relationship between acute inhibition of ATP synthesis and IFNγ levels. Interestingly, dependence of IFNγ on ATP was non-linear, in that two-fold decrease in ATP did not significantly affect IFNγ that was sharply decreased at >50% depletion of ATP (Fig. 3B). This implies that IFNγ production in CD4 T cells can be sustained under conditions of impaired mitochondrial function and energy production. In line with this, a previous study demonstrated that pre-activated CD4 T cells retained proliferative capacity in the presence of mitochondrial inhibitors for 2 days post-activation (33). Azide titration results justify utility of the ATP assay for measuring viable cells to normalize IFNγ in microplate format. The relationship between the two parameters was fit into a cubic polynomial function to generate a “calibration curve” for IFNγ values (Fig. 3B, 3C). In contrast to the relative independence of IFNγ production on acute ATP depletion using azide, inhibition of protein synthesis by puromycin profoundly affected IFNγ levels (Fig. S1). Accordingly, shRNA pools targeting ribosomal proteins RPL39 and RPL17 were among the top inhibitory candidates (Table 1). IFNγ and ATP data (Fig. 3A) and ATP-normalized IFNγ data (Fig. 3C) are presented in Table S1 and Table S2, respectively.
FIGURE 2. Phenotypic characteristics of Th1* cells and experimental strategy.
(A) Representative gating panels for sorting Th1*, Th1, Th2, and Th17 populations. Viable lymphocytes were selected by forward and side scatter gating and live/dead marker (not shown). CD4/CD3 population (panel 5) was sorted to select memory cells (CD45RA negative, panel 7), and CD4+CCR6+ (panel 8) and CD4+CCR6- (panel 9) compartments containing Th1*/Th17 and Th1/Th2 subsets, respectively. Other sorting details are described in Methods. (B) Th1*, Th1, Th17, and Th2 cell frequencies in total CD4+ population; n (number of donors) = 11. (C, D) IFNγ and IL-17 concentrations in supernatants from Th1* and the other subsets, stimulated with anti-CD3/28 dynabeads for 24 hrs. ns denotes non-significant, nd-not detected. (E) An experimental time-line established for shRNA screening in Th1* cells. Data are representative of three independent experiments using three replicates per experimental group and shown as mean ± S.E.M. Paired t-test was used to analyze statistical significance.
Table 1.
A ranked list of candidate genes and their functional annotations.
| Gene | % IFNγ | SEM | Protein annotation |
|---|---|---|---|
| ISOC1 | 29.8 | 1.8 | Isochorismatase domain-containing protein 1; a putative hydrolase of unknown function in mammalian cells |
| CD 160 | 34.9 | 2.0 | An Ig-like protein, binds MHC class I molecules; regulated IFNγ in NK cells; co-inhibitory and co-stimulatory functions |
| HSPE1 | 39.4 | 1.7 | Heat shock protein (HSP10) member 1. Multifunctional: mitochondrial protein folding; anti-inflammatory activity |
| IER5 | 40.3 | 4.4 | Immediate early response 5 protein encoded a p53 target gene; regulates cell proliferation and contributes to tumorigenesis |
| RPL39 | 40.9 | 3.4 | Ribosomal protein |
| RPL17 | 43.5 | 2.2 | Ribosomal protein |
| FILIP1L | 45.5 | 0.6 | Filamin A-interacting protein 1-like; cytoskeleton remodeling; inhibition of cancer cell migration; promotes apoptosis |
| GAB1 | 45.6 | 0.5 | GRB1-associated scaffolding/adapting protein, promotes type I interferon production; enhances MAPK/NF-kB activation |
| PREP | 46.1 | 2.3 | Prolyl-endopeptidase, multiple protein interactions (α-synuclein, GAPDH); dysregulated in autoimmune diseases |
| ZAK | 46.6 | 2.4 | Leucine zipper and sterile-α-motif-containing kinase, also classified as a MAP3K; TGF-β signaling; pro-inflammatory effects |
| NTN4 | 46.9 | 0.2 | Netrin 4, a laminin-based protein; regulates neurovascular interactions and angiogenesis; a putative anti-inflammatory factor |
| IL23R | 47.0 | 1.8 | IL23 receptor IL23-specific subunit, associates with IL12RB1 subunit; implicated in Th17 development; linked to MTB |
| IL12RB2 | 47.6 | 1.5 | IL12 receptor IL12-specific subunit, associates with IL12RB1 subunit; implicated in Th1 development; linked to MTB |
| CYP2E1 | 48.4 | 1.9 | A member of cytochrome 450 family of xenobiotic metabolizing enzymes; regulates IL-22, IL-17 |
| CASP4 | 49.2 | 3.0 | Apoptosis-related inflammatory caspase-4, involved in inflammasome activation, mediates innate immune responses |
| TMEM136 | 49.7 | 5.4 | Transmembrane protein 136 |
| LCK | 50.3 | 4.8 | Lymphocyte-specific Scr-family protein tyrosine kinase; multiple functions in Th1 and Th17 cell signaling |
| LTB | 50.8 | 2.9 | Lymphotoxin β (TNF-C); activates inflammatory responses; required for lymphoid organogenesis |
| LRRTM2 | 51.0 | 1.5 | Leucine-rich repeat transmembrane 2 protein; neuronal synapse development; unknown function in immune cells |
| TNFSF13B | 51.3 | 2.0 | TNF receptor superfamily member 13B cytokine, B-cell activating factor (BAFF); involved in T cell activation and survival |
| IL18RAP | 52.2 | 1.1 | IL18 receptor accessory protein; involved in IL18/IL12 signaling and IFNγ production; linked to chronic inflammatory disorders |
| SASH1 | 53.4 | 1.3 | SAM/SH1 domain containing 1, a scaffold protein; TLR4 signaling/innate immune response; a putative tumor suppressor |
| TMEM186 | 53.6 | 2.4 | Transmembrane protein 186 |
| IL1RL1 | 53.7 | 0.6 | IL1 receptor-like (ST2); receptor for IL-33; multifunctional in immune cells; a critical role in asthma and atopic dermatitis |
| RORC | 54.6 | 3.6 | RAR-related orphan receptor γ, transcription factor; leukocyte-specific RORγT regulates Th17/IL17 pathway; linked to MTB |
| PDE4D | 55.3 | 4.2 | Phosphodiesterase 4D, the cAMP hydrolyzing enzyme in immune cells; regulates TCR signaling, anti-inflammatory drug target |
| T-bet | 56.0 | 1.9 | T-box transcription factor TBX21; controls IFNγ expression in Th1; regulates effector function in other immune cells |
Numbers are IFNγ levels (means and S.E.M.) normalized per ATP as described in Fig. 3 and Methods, and expressed as percentages of control. (A 56% cutoff was selected based on the effect of shRNA targeting T-bet, the positive regulator of IFNγ production.) Highlighted in bold are the top two hits as well as genes previously implicated in IFNγ regulation and/or MTB. A complete list of screened genes and corresponding data sets are presented in Table S1.
Candidate emerging from the shRNA screen fell into diverse functional groups (Table 1). Among the positive regulators of IFNγ were proteins involved in MTB susceptibility (IL23R, IL12RB2) or enhanced T cell immunoreactivity (TNFSF13B/BAFF). Knockdown of the Th17 lineage-specific factor RORC decreased Th1* viability and IFNγ production, in agreement with a previous report (21). Candidates included proteins previously implicated in innate and adaptive immunity (Table 1), including the HSPE1-encoded chaperonin (HSP10), a potential therapeutic target in autoimmune diseases (34).
CD160 is a positive regulator of IFNγ production in Th1* cells
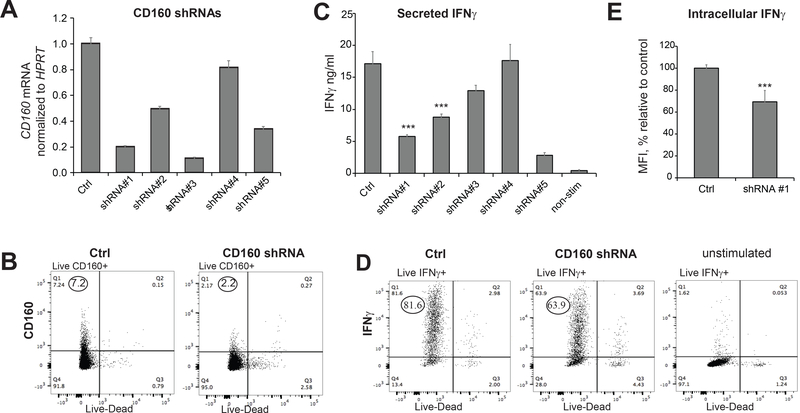
CD160 was a high-ranking positive regulator emerging from the screen (Table 1). CD160 binds MHC class I molecules and Herpes Virus Entry Mediator (HVEM), and is critical for IFNγ production in NK cells (35). The function of CD160 in T cells is less well understood, and is linked to stimulatory and inhibitory signals (36–40). Validation experiments confirmed that CD160 shRNA significantly decreased IFNγ production in Th1* cells isolated from different donors (Fig. 3D). We identified multiple individual CD160 shRNAs that reduced target gene and protein expression without affecting cell viability (Fig. 4A, 4B, Table S3). Reminiscent of CD4+ and CD8+ T cells (36), expression of CD160 in Th1* was detectable in a sub-population of cells (Fig. 4B). Measurements of secreted or intracellular IFNγ by ELISA and immunostaining confirmed that CD160 KD in Th1* cells decreased IFNγ production (Fig. 4C, 4D, 4E). Together, data support a stimulatory role for CD160 in memory CD4 T cells.
FIGURE 4. CD160 knockdown decreases IFNγ production in Th1* cells.
(A,B) Effects of individual CD160 shRNAs on CD160 mRNA and protein expression. Cells were transduced with shRNA SHC002 (Ctrl) and indicated shRNAs. shRNA sequences are listed in Table S3. (C) Effects of individual CD160 shRNAs on IFNγ production, as measured by ELISA. Constructs indicated in red produced significant cell death in puromycin-selected cells. (D, E) Intracellular IFNγ staining confirms decreased IFNγ production in CD160 KD Th1* cells. The values in (E) represent median fluorescent intensity (MFI) expressed as a percentage relative to cells transduced with control shRNA. Representative FACS data (D) shows intracellular IFNγ measurements. MFI was calculated from the upper left quadrant. Unstimulated cells are shown as negative control. Data are representative of at least two independent experiments using three replicates per experimental group and shown as mean ± S.E.M. Paired t-test was used to analyze statistical significance.
ISOC1 deficiency compromises CD4+ T cell effector functions by dysregulating pyrimidine metabolism
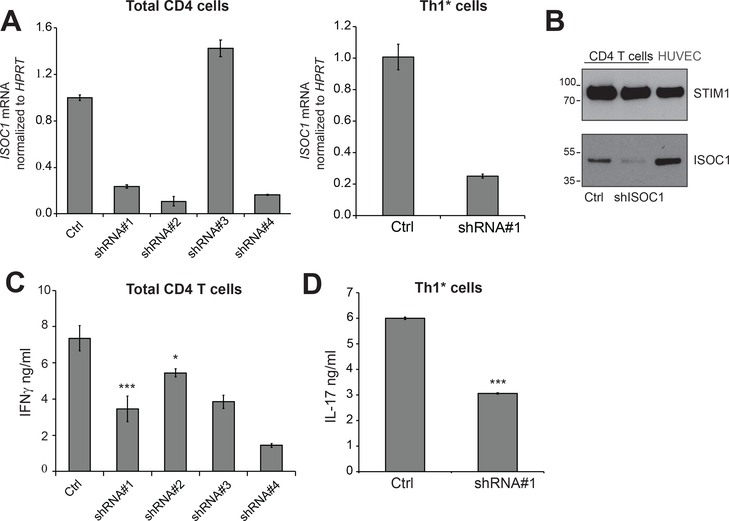
ISOC1 is an isochorismatase domain-containing protein of unknown function. In prokaryotes, isochorismatase (EC 3.3.2.1) catalyzes the conversion of isochorismate to 2,3-dihydroxybenzoate and pyruvate, however the metabolic function of its mammalian orthologs remains obscure. Transcriptome profiles demonstrated a 2-fold increase in ISOC1 expression in Th1* cells (10). The effect of ISOC1 depletion in reducing anti-CD3/28-stimulated IFNγ and IL-17 production was confirmed in bulk CD4 and Th1* cells transduced with individual ISOC-specific shRNAs (Fig. 5A–D).
FIGURE 5. ISOC1 knockdown compromises effector function of total CD4 and Th1* cells.
(A, B) Effects of individual shRNAs on ISOC1 mRNA was measured in total CD4+ T and Th1* cells. Corresponding shRNA sequences are listed in Table S3. Constructs in red produced significant cell death in puromycin-selected cells. (B) ISOC1 depletion by shRNA (#1) in total CD4+ T cells; endothelial cells (HUVEC) were used as reference sample; STIM1 was used as a loading control. (C) Total CD4+ T cells recapitulate the effect of ISOC1 depletion on IFNγ production. Data are mean ± S.E.M of 3 replicate samples. IFNγ was measured in cellular supernatants as described in Fig. 2. (D) Th1*-driven IL-17 production and effect of ISOC1 knockdown. Data are representative of at least two independent experiments using three to four replicates per experimental group and shown as mean ± S.E.M. Paired t-test was used to analyze statistical significance.
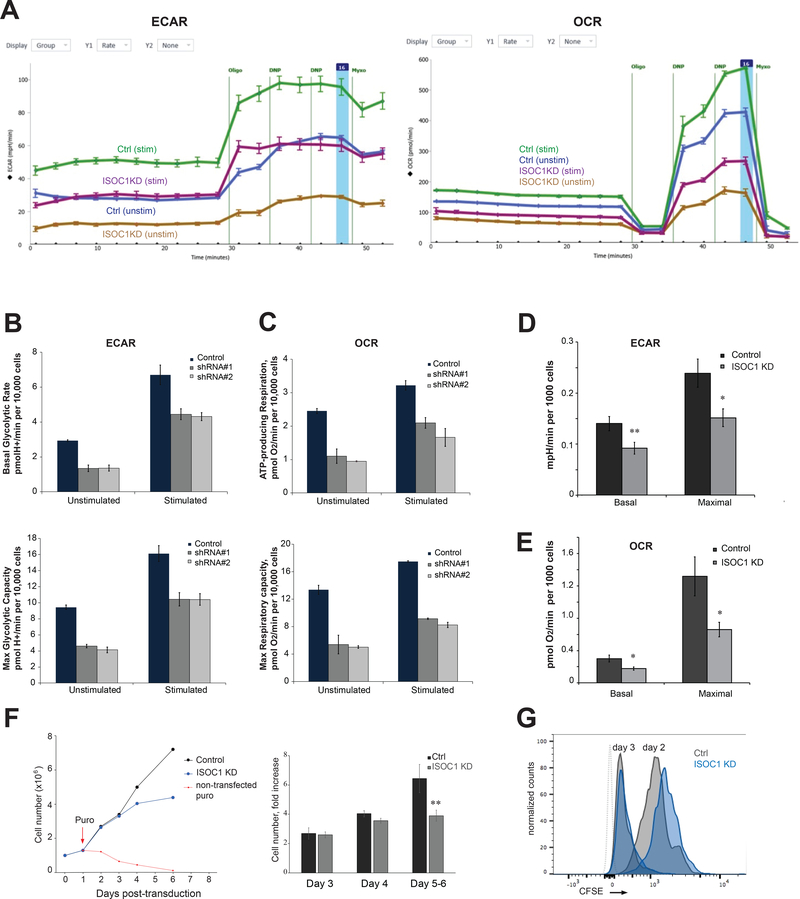
To explore ISOC1function, we performed metabolic analysis of ISOC1-deficient CD4+ T cells using validated ISOC1 shRNAs that produce efficient knockdown and minimal toxicity (Fig. 5A, 5B). To examine glycolysis and oxidative phosphorylation, respectively, extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured (Fig. 6A–E). T cell activation is accompanied by rapid up-regulation of glycolysis and dynamic changes in ECAR. Accordingly, anti-CD3/28-stimulated CD4 T cells demonstrated markedly increased ECAR versus non-stimulated cells (Fig. 6A). Consistent with the higher energy demands of activated T cells, mitochondrial respiration (OCR) was also increased upon anti-CD3/28 stimulation, as evidenced by a higher basal OCR within one hour after stimulation (Fig. 6C, 6E). Addition of oligomycin, an inhibitor of mitochondrial ATP synthase, suppressed mitochondrial respiration and enhanced ECAR, a reflection of increased glycolysis as a compensatory mechanism for ATP production. ISOC1 KD cells showed significant reduction in both ECAR and OCR (Fig. 6A–E) compared to controls. The basal and maximal ECAR, the latter measured in the presence of the respiratory inhibitor myxothiazol, were significantly decreased upon depletion of ISOC1. Likewise, basal (i.e. ATP-producing) and maximal (i.e. FCCP-stimulated) mitochondrial respiration were partially inhibited. Overall, ISOC1 depletion produced ~40% inhibition of mitochondrial respiration and glycolytic activity (Fig. 6D, 6E). Since both basal and maximal OCR and ECAR were equally suppressed in ISOC1 KD cells, our results suggest that ISOC1 deficiency broadly perturbs T cell metabolism. Impaired bioenergetics is consistent with reduced proliferative capacity of ISOC1 KD cells (Fig. 6F, 6G). Effects on cell numbers manifested after 6 days in culture (Fig. 6F), which likely reflects relatively low energy requirements of resting T cells. Delayed proliferation due to ISOC1 depletionalso manifested after TCR re-stimulation, which imposes high energy demands (Fig. 6G).
FIGURE 6. ISOC1 depletion in CD4 T cells compromises cellular bioenergetics and proliferation.
(A) Seahorse XFe96 analyzer data output: ECAR (left panel) and OCR (right panel); vertical lines indicate sequential additions of oligomycin, DNP, and myxothiazol. Other experimental details are specified in Methods. (B). Basal (upper panel) and maximal (lower panel) ECAR in control and ISOC1 knockdown cells generated using two ISOC1 shRNA clones. (C) Basal (upper panel) and maximal (lower panel) OCR in control and ISOC1 knockdown cells generated using ISOC1 shISOC#1 and shISOC#2 (ISOC-KD1 and ISOC-KD2). The values in (B) and (C) represent mean ± S.E.M. (n=3 replicate wells) from the experiment depicted in panel (A). (D, E) Summary of Seahorse XFe96 analyzer measurements: 4 independent experiments similar to one shown in (A) were performed and averaged. Values for basal and maximal ECAR and OCR in stimulated control and ISOC1 KD cells are shown. Other details of data analysis are described in Methods. (F, G). Effects of ISOC1 shRNA on cell proliferation. (F) Viable cell numbers were measured during 5–6 days post-transduction/puromycin selection period; day 0 denotes the day of transduction, puromycin was added at day 1 as indicated. Left panel shows representative kinetics of control (black line) and ISOC1 KD (blue line) cell proliferation; red dotted line shows a time-dependent loss of non-transduced cells cultured in the presence of puromycin. Right panel shows a fold change in the number of control and ISOC1 KD cells at day 3, 4, and 5 or 6 post-transduction. (G) Control and ISOC1 KD cells were labeled with CFSE and continuously stimulated with anti-CD3/28 for 3 days. Proliferation was measured by carboxyfluorescein dilution at day 2 and 3 post-stimulation as indicated. Grey dotted line indicates unstained cells. Data are representative of three independent experiments using four to five replicates per experimental group and shown as mean ± S.E.M. Paired t-test was used to analyze statistical significance. *, ** correspond to p ≤0.05 and ≤0.01, respectively.
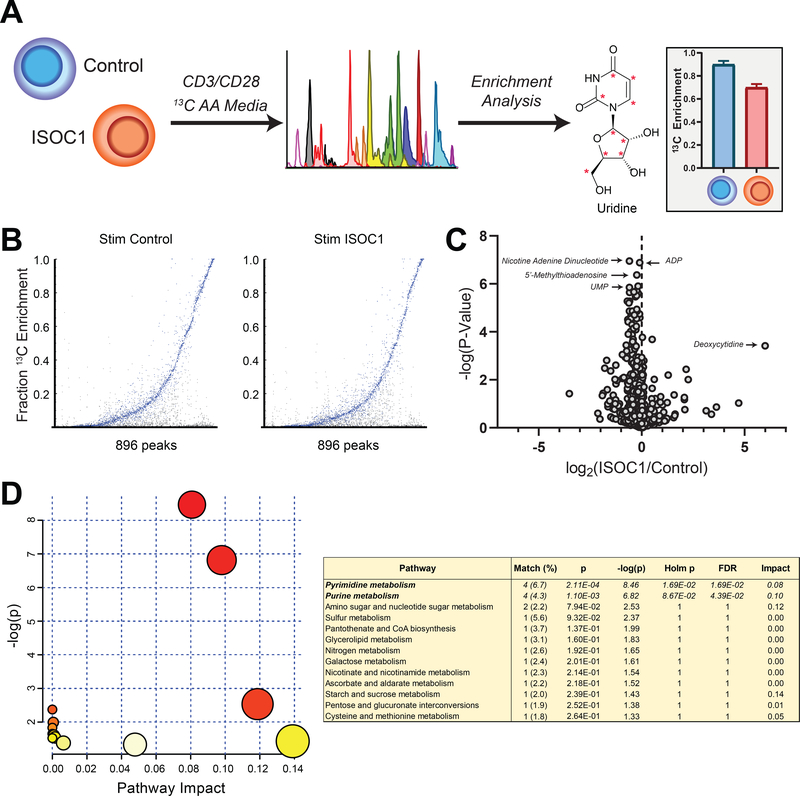
Next, we investigated how ISOC1 deficiency globally alters CD4 T cell metabolism by applying high-resolution mass spectrometry and untargeted metabolomics using deep 13C isotope labeling (32) (Fig. 7A). A total of 896 LC-MS isotopically-labelled features corresponding to known cellular metabolites were measured in control and ISOC1-depleted T cells (Fig. 7). To identify metabolic perturbations in ISOC1-depleted T cells, the differentially produced 13C-labelled metabolites were subjected to pathway enrichment analysis using MetaboAnalyst (41, 42), identifying the most prominently affected pathways as pyrimidine and purine biosynthesis (Fig. 7D). As both the building blocks for nucleic acid synthesis and bio-active signaling molecules, purine and pyrimidine nucleotides play a critical role in T cell survival, differentiation, and expansion (43). Thus, we reasoned that lower nucleotide levels in ISOC1-deficient T cells impairs IFNγ production by interfering with multiple biosynthetic pathways and accounting for the decline in bioenergetic parameters (Fig. 6).
FIGURE 7. Metabolomics of control and ISOC1 knockdown cells.
A) Schematic of Deep Labeling culturing (left), LC-MS/MS (middle) and enrichment analysis (right) in CD4 T cells depleted of ISOC1. (B) Label incorporation in 896 putatively identified metabolites by carbon-13 (13C) isotopomer fraction in control (left) or ISOC1 KD (right) anti-CD3/28-stimulated cells (n = 3 per condition). (C) Volcano plot of metabolite 13C fraction relative changes in ISOC1-depleted cells. (D) Pathway enrichment analysis by MetaboAnalyst of significantly enriched (13C > 5%) features changing significantly in ISOC1-depleted cells (p < 0.05 with Bonferroni multiple hypothesis correction). Data are representative of two independent experiments using four to five replicates per experimental group. Paired t-test was used to analyze statistical significance.
Nucleoside supplementation rescues T cell effector functions in ISOC1-deficient T cells
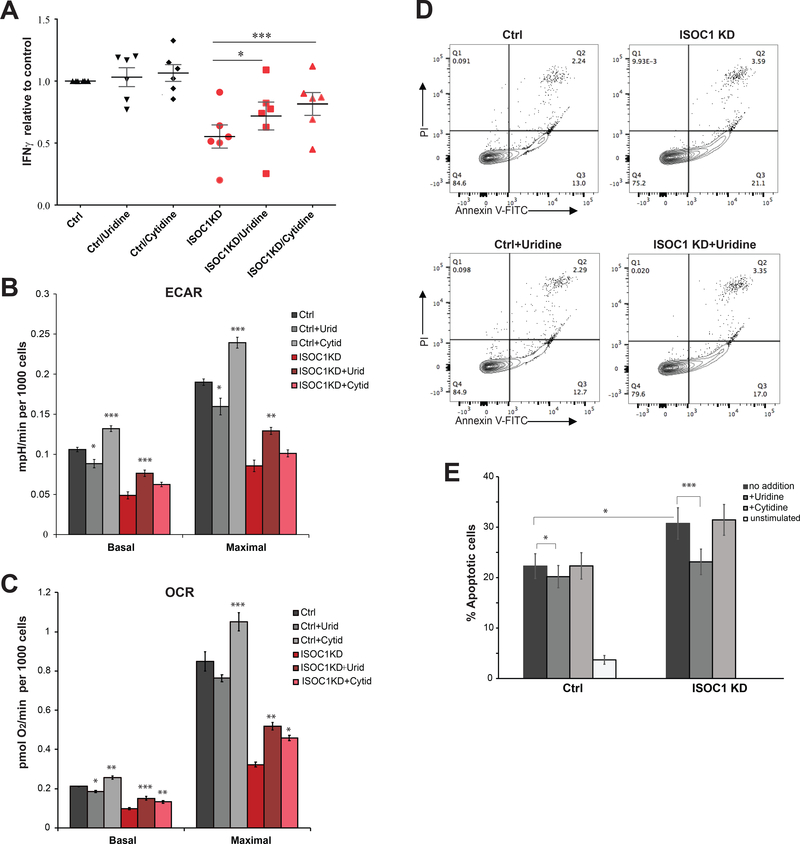
To further investigate the relationship between pyrimidine deficiency and diminished effector functions of ISOC1-deficient T cells, rescue experiments were performed by nucleoside supplementation. Nucleosides and pyrimidine bases such as uridine and cytidine are transported across the plasma membrane by facilitated diffusion via equilibrative nucleoside transporters (ENT) expressed in T cells. Previous studies demonstrate that uridine or cytidine supplementation (at 100–200 μM) interferes with chemical inhibitors of pyrimidine biosynthesis in lymphocytes (44). Exogenous cytidine or uridine was added to the growth media, resulting in potentiation of IFNγ production in ISOC1 KD cells (Fig. 8A) and negligible effects on control cells. Independent replicate experiments demonstrated significantly augmented IFNγ response in cytidine-supplemented ISOC1-deficient cells, with the less significant effect of uridine (p<0.05). ISOC1 KD moderately increased stimulation-dependent early apoptosis, which was reversed by uridine but not cytidine (Fig. 8D, 8E). Supporting the hypothesis that nucleotide deficiency compromises the metabolic demands of ISOC1-deficient T cells, supplementation of either uridine or cytidine stimulated ECAR and OCR (Fig. 8B, 8C). These data suggest that replenishment of nucleotide pools in ISOC1-deficient T cells enables increased energy demands and restores effector functions. Thus, our data highlight a novel regulatory role for ISOC1 and pyrimidine metabolism in TCR-mediated IFNγ production.
FIGURE 8. Supplementation with exogenous nucleosides partially rescues IFNγ production and energy metabolism in ISOC1 KD CD4 T cells.
(A) Effects of ISOC1 shRNA on IFNγ production in the presence of uridine or cytidine (100 μM). Nucleosides were added to culture media 2 days prior to 48h stimulation with anti-CD3/28. On the day of stimulation, cells were counted and plated at equal concentration (50 000 cells/100 μl) in fresh T cell media with or without uridine or cytidine. IFNγ in supernatants was normalized to control cells transduced with control shRNA and cultured without nucleoside supplementation. Data in (A) were generated in 6 independent experiments with cells derived from different donors; each symbol represents data from an individual experiment. Statistical analysis was done using repeated measures two-way ANOVA. The Bonferroni post hoc test was used to analyze differences between groups with following results: ctrl vs ISOC1 KD, p<0.01; for ISOC1 KD group, uridine vs no additions, p<0.05, cytidine vs no additions, p<0.001. (B, C) Uridine or cytidine supplementation increases ECAR (B) and OCR (C) in ISOC1 KD cells. Seahorse XFe96 analyzer measurements were done as described in Fig. 6 and Methods. Cells were cultured in the presence of added uridine or cytidine (100 μM) for 2 days prior to ECAR/OCR analysis. Uridine or cytidine (100 μM) were also added to the Seahorse assay media during cell stimulation and data acquisition. Values in (B, C) are mean ± S.E.M. (D, E). Uridine supplementation decreases anti-CD3/28 stimulation-dependent apoptosis. (D) An example of apoptosis measurements measured by Annexin V/PI staining. (E) Percentages of early apoptotic cells (PI-negative, Annexin V-positive) quantified from 9 independent experiments (mean ± S.E.M.). Data are representative of three to nine independent experiments using three replicates per experimental group. Paired t-test was used to analyze statistical significance between groups: ctrl vs ISOC1 KD, p<0.05; ctrl vs ctrl+uridine, p<0.05; ISOC1 KD vs ISOC1 KD+uridine, p<0.001. Nucleosides, when present, were added to the culture media (at 100 μM) two days prior to stimulation of the cells with anti-CD3/28.
Discussion
The function of most candidates emerging from unbiased molecular profiling or genome-based network predictions is unknown. To understand how gene expression signatures can influence specific disease outcomes, it is imperative to understand the function of signature genes in a given cell type. Here we report a functional analysis of immune signature genes enriched in Th1* cells, also known as Th1/Th17 cells due to co-expression of IFNγ and IL-17 (10). Genes from the Th1* signature group were prioritized for RNAi screening based upon a predictive gene network analysis pinpointing regulatory and signaling proteins. This “targeted” genomics approach can explain the relatively high number of hits with functional significance in the screen. In addition to T-bet and RORC, our results supported substantial contribution of genes previously implicated in MTB and/or regulation of effector functions (Table 1).
Among positive regulators of IFNγ production, CD160 was unexpected as it is linked to HVEM-dependent inhibitory effect on TCR-mediated CD4+ T cell activation (36). However, the role of CD160 in T cell effector function is complex, since other studies support a co-stimulatory function in T cells (37, 38). Likewise, in other immune cells CD160 acts as both a positive and negative regulator of antigen-induced signals, depending on the specific receptor/ligand involved. In NK cells, CD160 is critical for IFNγ production (35), whereas it acts as a negative regulator of NKT cells in early innate immune response (45). Previously described unconventional mechanism of HVEM activation upon CD160 engagement (40) further highlights versatile function of this protein. In the context of MTB immunity, CD160 expression is downregulated in CD8 T cells isolated from actively infected TB patients versus LTBI subjects, although differences in cytokine profiles were not detected between these groups (46). Interestingly, detectable CD160 expression is limited to a minor subset (8–10%) of Th1* cells, whereas its downregulation produced an inhibitory effect on IFNγ production in the entire population (data not shown). This result warrants further investigation. Other potentially interesting candidates emerging from the screen include cancer-relevant apoptotic factors IER5 (a p53 target gene) and mitochondrial chaperonin HSPE1/HSP10 (47, 48). IER5 promotes apoptosis in cancer cells (49) and was one of the markedly down-regulated pro-apoptotic factors in transcriptional profiles of LTBI subjects versus uninfected control (19). Together with concomitant upregulation of anti-apoptotic proteins, these data indicate an enhanced propensity of Th1* cells to survival (10, 19). HSP10 is a multifunctional protein that is involved in pro-caspase 3 activation (47) and has been also implicated in promoting tumorigenesis and alleviating pro-inflammatory immune responses (34). As a component of the mitochondrial protein quality control system HSP10 may play an auxiliary role in IFNγ production via maintaining mitochondrial functional integrity.
With respect to novel potential regulators required for optimal IFNγ response, we were intrigued by the prominent effect of ISOC1, a recently identified isochorismase domain-containing protein presumed to elicit metabolic activity. ISOC1 expression is upregulated in several cancers (50–52), and its depletion markedly reduces proliferation of tumorigenic cells (50, 52). Additionally, ISOC1 was identified among top 10 differentially expressed genes in a mouse strain highly susceptible to Staphylococcus aureus and other pathogens (53), suggesting a regulatory role in the immune response. However, literature data related to the functional significance of ISOC1 are rare, and its molecular function is unknown. Comprehensive metabolomic profiling of ISOC1 knockdown CD4 T cells linked ISOC1 deficiency to severely compromised cellular pyrimidine and purine metabolism. Furthermore, bioenergetic flux analysis demonstrated a rapid stimulation of glycolysis by anti-CD3/28 addition that was abrogated in ISOC1 KD cells. Of note, the signaling pathways controlling acute upregulation of glycolysis, known as “glycolytic switch”, in activated hematopoietic cells are incompletely defined, as is its mechanistic link to cytokine production (33, 54–57). It has been suggested that glycolysis drives TCR-mediated IFNγ synthesis via a posttranscriptional mechanism rather than fueling ATP production (33, 54), providing an explanation as to why glycolysis, which is inefficient compared to oxidative phosphorylation and yields only two molecules of ATP per glucose, is indispensable for activated T cell proliferation and IFNγ production (33). We observed that mitochondria in stimulated CD4 T cells also rapidly responded to increased energy demands, as indicated by enhanced oxygen consumption in different metabolic states. Since both mitochondrial and glycolytic function were equally suppressed in ISOC1 knockdown cells, our results suggest that ISOC1 deficiency broadly perturbs cellular metabolism. This drives decreased IFNγ and IL-17 expression, slower proliferation and, accordingly, reduced cellular energy demands. Supporting ISOC1-depent metabolic defects, supplementation of ISOC1-defficient cells with exogenous nucleosides restored IFNγ production and potentiated mitochondrial and glycolytic energy transduction. Notably, our data indicate that ISOC1 regulates IFNγ production in CD4+ T cells and the Th1* subset.
Our results are supported by reports of compromised lymphocyte function after treatment with immune-suppressive inhibitors of pyrimidine biosynthesis, which was rescued by supplementation of cytidine or uridine (43, 44, 58). Further highlighting the role of pyrimidine metabolism in T cell abundance, loss-of-function mutations in the gene encoding the de novo pyrimidine synthesis enzyme CTP synthase 1 suppressed lymphocyte proliferation and induced T cell apoptosis, which was restored by the addition of cytidine (59). Our functional analysis of CD4+ T cells indicates that compromised nucleotide metabolism has downstream repercussions on CD4 T effector function, glycolysis, and mitochondrial respiration. As noted above, inhibitors of pyrimidine biosynthesis have potential therapeutic applications in ameliorating inflammation by restricting lymphocyte proliferation. Conversely, a fully functional memory T cell immune response is critically important to combat MTB and other infections. In these scenarios, targeting the pyrimidine supply could be explored as a beneficial approach. In conclusion, this study provides a necessary functional dimension to Th1* immune signature genes and rationalizes further studies of their roles in MTB-specific and other immune cells.
Supplementary Material
Key Points.
Functional analysis of Th1* immune signature genes uncovers novel regulators of IFNγ
Loss of ISOC1 impairs CD4+ T cell pyrimidine metabolism and cytokine production
Acknowledgments:
We thank David Freeman and Mehak Kaur for general technical assistance, and Dr. Benjamin Schmiedel for helpful advice.
1. This work was supported by National Institutes of Health (NIH) grants R01CA199376 and U01DE028227 awarded to S. Sharma and an Infrastructure Operating Fund (IOF) grant to S. Sharma under the Human Immune Profiling Center (HIPC) grant U19AI118626 to A. Sette; NIH S10OD020025 and R01ES027595 to M. Jain; I. Mathews is supported by NIH T32GM007752, F31CA236405 and a SPARK award from the LJI Board of Directors.
2. Abbreviations:
- MTB
mycobacterium tuberculosis
- LTBI
latent tuberculosis infection
- OCR
oxygen consumption rate
- ECAR
extracellular acidification rate
- KD
knockdown
References
- 1.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, and Behar SM. 2014. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyadova IV, and Panteleev AV. 2015. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediators Inflamm 2015: 854507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin PL, and Flynn JL. 2010. Understanding latent tuberculosis: a moving target. Journal of immunology 185: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai S, Mayer-Barber KD, and Barber DL. 2014. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr Opin Immunol 29: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, and van Dissel JT. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nature genetics 32: 97–105. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AM, Magram J, Ferrante J, and Orme IM. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. The Journal of experimental medicine 186: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, and Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine 178: 2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu HQ, Fisher-Hoch SP, and McCormick JB. 2011. Molecular immunity to mycobacteria: knowledge from the mutation and phenotype spectrum analysis of Mendelian susceptibility to mycobacterial diseases. Int J Infect Dis 15: e305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Both U, Kaforou M, Levin M, and Newton SM. 2015. Understanding immune protection against tuberculosis using RNA expression profiling. Vaccine 33: 5289–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arlehamn CL, Seumois G, Gerasimova A, Huang C, Fu Z, Yue X, Sette A, Vijayanand P, and Peters B. 2014. Transcriptional profile of tuberculosis antigen-specific T cells reveals novel multifunctional features. Journal of immunology 193: 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, McKinney DM, Kwok WW, Grey H, Sallusto F, Peters B, and Sette A. 2013. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS pathogens 9: e1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, Dorta G, Mazza-Stalder J, Bart PA, Roger T, Calandra T, Nicod L, and Harari A. 2013. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. European journal of immunology 43: 939–948. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, and Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology 8: 639–646. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, and Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F 2016. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu Rev Immunol 34: 317–334. [DOI] [PubMed] [Google Scholar]
- 16.Duhen T, and Campbell DJ. 2014. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. Journal of immunology 193: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikitina IY, Panteleev AV, Kosmiadi GA, Serdyuk YV, Nenasheva TA, Nikolaev AA, Gorelova LA, Radaeva TV, Kiseleva YY, Bozhenko VK, and Lyadova IV. 2018. Th1, Th17, and Th1Th17 Lymphocytes during Tuberculosis: Th1 Lymphocytes Predominate and Appear as Low-Differentiated CXCR3(+)CCR6(+) Cells in the Blood and Highly Differentiated CXCR3(+/−)CCR6(−) Cells in the Lungs. Journal of immunology 200: 2090–2103. [DOI] [PubMed] [Google Scholar]
- 18.van Langelaar J, van der Vuurst de Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, Dankers W, Verjans G, de Vries HE, Lubberts E, Hintzen RQ, and van Luijn MM. 2018. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain 141: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 19.Burel JG, Lindestam Arlehamn CS, Khan N, Seumois G, Greenbaum JA, Taplitz R, Gilman RH, Saito M, Vijayanand P, Sette A, and Peters B. 2018. Transcriptomic Analysis of CD4(+) T Cells Reveals Novel Immune Signatures of Latent Tuberculosis. Journal of immunology 200: 3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, and Ernst JD. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America 98: 7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, Wong N, Soudais C, Henderson LA, Marzouqa H, Shamma J, Gonzalez M, Martinez-Barricarte R, Okada C, Avery DT, Latorre D, Deswarte C, Jabot-Hanin F, Torrado E, Fountain J, Belkadi A, Itan Y, Boisson B, Migaud M, Arlehamn CSL, Sette A, Breton S, McCluskey J, Rossjohn J, de Villartay JP, Moshous D, Hambleton S, Latour S, Arkwright PD, Picard C, Lantz O, Engelhard D, Kobayashi M, Abel L, Cooper AM, Notarangelo LD, Boisson-Dupuis S, Puel A, Sallusto F, Bustamante J, Tangye SG, and Casanova JL. 2015. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader SA, and Cooper AM. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Selma W, and Boukadida J. 2012. IL23R(Arg381Gln) functional polymorphism is associated with active pulmonary tuberculosis severity. Clin Vaccine Immunol 19: 1188–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova JL, and Abel L. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 20: 581–620. [DOI] [PubMed] [Google Scholar]
- 25.Miller HE, and Robinson RT. 2012. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect Immun 80: 3828–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Barricarte R, Markle JG, Ma CS, Deenick EK, Ramirez-Alejo N, Mele F, Latorre D, Mahdaviani SA, Aytekin C, Mansouri D, Bryant VL, Jabot-Hanin F, Deswarte C, Nieto-Patlan A, Surace L, Kerner G, Itan Y, Jovic S, Avery DT, Wong N, Rao G, Patin E, Okada S, Bigio B, Boisson B, Rapaport F, Seeleuthner Y, Schmidt M, Ikinciogullari A, Dogu F, Tanir G, Tabarsi P, Bloursaz MR, Joseph JK, Heer A, Kong XF, Migaud M, Lazarov T, Geissmann F, Fleckenstein B, Arlehamn CL, Sette A, Puel A, Emile JF, van de Vosse E, Quintana-Murci L, Di Santo JP, Abel L, Boisson-Dupuis S, Bustamante J, Tangye SG, Sallusto F, and Casanova JL. 2018. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Science immunology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altay G, and Emmert-Streib F. 2010. Inferring the conservative causal core of gene regulatory networks. BMC Syst Biol 4: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmiedel BJ, Singh D, Madrigal A, Valdovino-Gonzalez AG, White BM, Zapardiel-Gonzalo J, Ha B, Altay G, Greenbaum JA, McVicker G, Seumois G, Rao A, Kronenberg M, Peters B, and Vijayanand P. 2018. Impact of Genetic Polymorphisms on Human Immune Cell Gene Expression. Cell 175: 1701–1715 e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, and Root DE. 2006. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124: 1283–1298. [DOI] [PubMed] [Google Scholar]
- 30.Root DE, Hacohen N, Hahn WC, Lander ES, and Sabatini DM. 2006. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat Methods 3: 715–719. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy WJ Jr and Gentle JE 1980. Statistical Computing Marcel Dekker, New-York. [Google Scholar]
- 32.Grankvist N, Watrous JD, Lagerborg KA, Lyutvinskiy Y, Jain M, and Nilsson R. 2018. Profiling the Metabolism of Human Cells by Deep (13)C Labeling. Cell Chem Biol 25: 1419–1427 e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, and Pearce EL. 2013. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia H, Halilou AI, Hu L, Cai W, Liu J, and Huang B. 2011. Heat shock protein 10 (Hsp10) in immune-related diseases: one coin, two sides. Int J Biochem Mol Biol 2: 47–57. [PMC free article] [PubMed] [Google Scholar]
- 35.Tu TC, Brown NK, Kim TJ, Wroblewska J, Yang X, Guo X, Lee SH, Kumar V, Lee KM, and Fu YX. 2015. CD160 is essential for NK-mediated IFN-gamma production. The Journal of experimental medicine 212: 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, and Freeman GJ. 2008. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nature immunology 9: 176–185. [DOI] [PubMed] [Google Scholar]
- 37.El-Far M, Pellerin C, Pilote L, Fortin JF, Lessard IA, Peretz Y, Wardrop E, Salois P, Bethell RC, Cordingley MG, and Kukolj G. 2014. CD160 isoforms and regulation of CD4 and CD8 T-cell responses. J Transl Med 12: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan CL, Peluso MJ, Drijvers JM, Mera CM, Grande SM, Brown KE, Godec J, Freeman GJ, and Sharpe AH. 2018. CD160 Stimulates CD8(+) T Cell Responses and Is Required for Optimal Protective Immunity to Listeria monocytogenes. Immunohorizons 2: 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolova M, Marie-Cardine A, Boumsell L, and Bensussan A. 2002. BY55/CD160 acts as a co-receptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int Immunol 14: 445–451. [DOI] [PubMed] [Google Scholar]
- 40.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, and Ware CF. 2009. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proceedings of the National Academy of Sciences of the United States of America 106: 6244–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia J, and Wishart DS. 2016. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics 55: 14 10 11–14 10 91. [DOI] [PubMed] [Google Scholar]
- 42.Xia J, Sinelnikov IV, Han B, and Wishart DS. 2015. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res 43: W251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quemeneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, and Genestier L. 2003. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. Journal of immunology 170: 4986–4995. [DOI] [PubMed] [Google Scholar]
- 44.Woo J, Lemster B, Tamura K, Starzl TE, and Thomson AW. 1993. The antilymphocytic activity of brequinar sodium and its potentiation by cytidine. Effects on lymphocyte proliferation and cytokine production. Transplantation 56: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim TJ, Park G, Kim J, Lim SA, Kim J, Im K, Shin MH, Fu YX, Del Rio ML, Rodriguez-Barbosa JI, Yee C, Suh KS, Kim SJ, Ha SJ, and Lee KM. 2019. CD160 serves as a negative regulator of NKT cells in acute hepatic injury. Nature communications 10: 3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, and Harari A. 2013. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. European journal of immunology 43: 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samali A, Cai J, Zhivotovsky B, Jones DP, and Orrenius S. 1999. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. The EMBO journal 18: 2040–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bie AS, Fernandez-Guerra P, Birkler RI, Nisemblat S, Pelnena D, Lu X, Deignan JL, Lee H, Dorrani N, Corydon TJ, Palmfeldt J, Bivina L, Azem A, Herman K, and Bross P. 2016. Effects of a Mutation in the HSPE1 Gene Encoding the Mitochondrial Co-chaperonin HSP10 and Its Potential Association with a Neurological and Developmental Disorder. Front Mol Biosci 3: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Tian M, Zhao H, He Y, Li F, Li X, Yu X, Ding K, Zhou P, and Wu Y. 2017. IER5 as a promising predictive marker promotes irradiation-induced apoptosis in cervical cancer tissues from patients undergoing chemoradiotherapy. Oncotarget 8: 36438–36448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng L, Zhao Y, Tang M, Luo Z, and Wang X. 2019. Knockdown of ISOC1 suppresses cell proliferation in pancreatic cancer in vitro. Oncol Lett 17: 4263–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaga R, Ikeda K, Boele J, Horie-Inoue K, Takayama K, Urano T, Kaida K, Carninci P, Kawai J, Hayashizaki Y, Ouchi Y, de Hoon M, and Inoue S. 2014. Systemic identification of estrogen-regulated genes in breast cancer cells through cap analysis of gene expression mapping. Biochem Biophys Res Commun 447: 531–536. [DOI] [PubMed] [Google Scholar]
- 52.Gao B, Zhao L, Wang F, Bai H, Li J, Li M, Hu X, Cao J, and GuiYing W. 2019. Knockdown of ISOC1 inhibits the proliferation and migration and induces the apoptosis of colon cancer cells through the AKT/GSK-3beta pathway. Carcinogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahn SH, Deshmukh H, Johnson N, Cowell LG, Rude TH, Scott WK, Nelson CL, Zaas AK, Marchuk DA, Keum S, Lamlertthon S, Sharma-Kuinkel BK, Sempowski GD, and Fowler VG Jr. 2010. Two genes on A/J chromosome 18 are associated with susceptibility to Staphylococcus aureus infection by combined microarray and QTL analyses. PLoS pathogens 6: e1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, Bae H, Xie J, Young HA, Wendell SG, and Delgoffe GM. 2018. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell reports 22: 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones N, Cronin JG, Dolton G, Panetti S, Schauenburg AJ, Galloway SAE, Sewell AK, Cole DK, Thornton CA, and Francis NJ. 2017. Metabolic Adaptation of Human CD4(+) and CD8(+) T-Cells to T-Cell Receptor-Mediated Stimulation. Frontiers in immunology 8: 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, and Hess C. 2013. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nature immunology 14: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 57.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, Zamboni N, Sallusto F, and Lanzavecchia A. 2016. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 167: 829–842 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimitrova P, Skapenko A, Herrmann ML, Schleyerbach R, Kalden JR, and Schulze-Koops H. 2002. Restriction of de novo pyrimidine biosynthesis inhibits Th1 cell activation and promotes Th2 cell differentiation. Journal of immunology 169: 3392–3399. [DOI] [PubMed] [Google Scholar]
- 59.Martin E, Palmic N, Sanquer S, Lenoir C, Hauck F, Mongellaz C, Fabrega S, Nitschke P, Esposti MD, Schwartzentruber J, Taylor N, Majewski J, Jabado N, Wynn RF, Picard C, Fischer A, Arkwright PD, and Latour S. 2014. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 510: 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.