Abstract
The dysregulated sepsis-induced cytokine storm evoked during systemic infection consists of biphasic and interconnected pro- and anti-inflammatory responses. The contrasting inflammatory cytokine responses determine the severity of the septic event, lymphopenia, host survival, and the ensuing long-lasting immunoparalysis state. Natural Killer (NK) cells, due to their capacity to elaborate pro- (i.e., IFNγ) and anti-inflammatory (i.e., IL-10) responses, exist at the inflection of sepsis-induced inflammatory responses. Thus, NK cell activity could be beneficial or detrimental during sepsis. Here, we demonstrate that murine NK cells promote host survival during sepsis by limiting the scope and duration of the cytokine storm. Specifically, NK cell-derived IL-10, produced in response to IL-15, is relevant to clinical manifestations in septic patients and critical for survival during sepsis. This role of NK cells demonstrates that regulatory mechanisms of classical inflammatory cells are beneficial and critical for controlling systemic inflammation, a notion relevant for therapeutic interventions during dysregulated infection-induced inflammatory responses.
Introduction
Sepsis is a dysregulated systemic inflammatory response, comprised of both pro- and anti-inflammatory cytokines, that results in significant host morbidity and mortality (1; 2). In the last 30 years advancements in early intervention and resuscitation strategies have reduced early patient mortality, yet every 6 seconds 9 people will develop sepsis and 2 of those will die (3). While considerable effort has been made to develop therapeutic strategies, the result has dishearteningly been the failure of 100+ phase II and III clinical trials targeted at the pro-inflammatory component of the cytokine storm (4). However, targeting only the inflammatory components of sepsis belies the complexity of the septic event whose dualistic pro- and anti-inflammatory components exist synchronously (5; 6). Thus, it is pertinent to re-interrogate the relationship between pro- and anti-inflammatory cytokines produced during the septic event to potentially redefine our approach to developing therapeutic strategies for sepsis.
Immune cells are often ascribed uniform roles during sepsis related to their canonical cytokine production, wherein cells that conventionally produce either pro- or anti-inflammatory cytokines are considered detrimental or beneficial, respectively, to host survival. For example, natural killer (NK) cells are considered to be detrimental to host survival during the cytokine storm due to their well-described production of the pro-inflammatory cytokines (7–9). Contextually, NK cells are an innate lymphoid population that performs effector functions (i.e., cytokine production and cytotoxicity) in response to both direct receptor interaction and cytokine stimulation (10; 11). This classical view of NK cells as inflammatory effectors conforms nicely with their proposed detrimental role during a septic event by further exaggerating the cytokine storm. Indeed, during the septic event NK cells produce pro-inflammatory IFNγ in response to the other inflammatory signals produced during the cytokine storm (e.g., IL-15) (12–14). As a consequence, NK cells are generally considered to participate in a detrimental positive feedback loop that increases the severity of the septic cytokine storm.
This proposed positive feedback loop of NK cell activation must, in some way, be balanced by anti-inflammatory negative feedback, given that not all individuals who develop sepsis will succumb to the cytokine storm (15). Notably, NK cells can counterbalance inflammation through the production of IL-10 and thereby limit immune-mediated pathology during systemic infection (16–18). NK cells also produce IL-10 following stimulation with some of the same cytokines which evoke pro-inflammatory cytokine production (e.g., IL-15) (19; 20). Thus, because of the dualistic inflammatory states of sepsis and capacity of NK cells to participate in this dualism, re-evaluation of the relationship between NK cells, cytokine storm, and sepsis severity may provide insights for therapeutic advancement.
Herein, we demonstrate that NK cells promote host survival during sepsis by limiting the scope and duration of the cytokine storm. Our data show that while NK cells do initially respond to IL-15 by producing IFNγ during sepsis, they subsequently convert to being IL-10 producers. Further, NK cells produced IL-10 during sepsis in an IL-15 dependent manner and this NK cell-derived IL-10 promoted host survival during sepsis. Importantly, we observed enhanced NK cell IL-10 production in septic patients indicating this regulatory role may have clinical significance for framing the interrelationship of the pro- and anti-inflammatory phases of sepsis. The present study elucidates a previously undescribed regulatory mechanism of NK cells during sepsis, revealing further complexity in the relationship between NK cells and the cytokine storm, that points to potential therapeutic avenues for minimizing the scope and duration of overactive inflammatory responses.
Materials and Methods
Ethics statement
Experimental procedures using mice were approved by University of Iowa Animal Care and Use Committee under ACURF protocol #6121915 and #9101915. The experiments performed followed Office of Laboratory Animal Welfare guidelines and PHS Policy on Humane Care and Use of Laboratory Animals. Cervical dislocation was used as the euthanasia method of all experimental mice.
Mice
Inbred C57Bl/6 (B6; Thy1.2/1.2 and Thy1.1/1.1) mice were purchased from the National Cancer Institute (Frederick, MD) and maintained in the animal facilities at the University of Iowa at the appropriate biosafety level. Il10 BAC-in transgene (IL-10bit) mice were acquired from Dr. Casey Weaver (21). NCR1-CreERT2 Rosa26-tdTomato mice (22) were kindly provided by Dr. Joseph Sun. IL-10flox/flox mice were kindly provided by Dr. Axel Roers (23). NCR1-CreERT2 IL-10flox/flox Rosa26-tdTomato Thy1.1/1.1 mice were generated by first crossing IL-10flox/flox mice with Thy1.1/1.1 mice. IL-10flox/flox Thy1.1/1.1 were then crossed and subsequently backcrossed with NCR1-CreERT2 Rosa26-tdTomato to generate NCR1-CreERT2+/− IL-10flox/flox Rosa26-tdTomato Thy1.1/1.1 and IL-10flox/flox Rosa26-tdTomato Thy1.1/1.1 mice. Oral gavage of tamoxifen (Sigma; 200μg tamoxifen/g of body weight) in corn oil (Sigma) over the course of 3–5 days was sufficient to induce Cre excision of the loxP-flanked IL-10 exon 1 (IL-10flox) and the flanked stop codon (Rosa26-TdTomato).
Institutional Setting and IRB Approval
Patients were recruited at the University of Iowa Hospitals and Clinics, an 811-bed academic tertiary care center. Blood sample acquisition, patient data collection, and analysis were approved by the University of Iowa Institutional Review Board (ID #201804822). Informed consent was obtained from patients or their legally authorized representatives.
Sepsis Patient Selection and Data Collection
Subjects 18 years of age or older meeting Sepsis-3 criteria for sepsis or septic shock (2) secondary to intra-abdominal infection, soft tissue infection, bloodstream infection, or pneumonia were enrolled. Exclusion criteria were infection requiring antibiotics in the past month, hospitalization for infection in the past year, and chemotherapy or radiation within the past year were excluded. Demographics and baseline characteristics including age, gender, race, APACHE II score, SOFA score, and presence of septic shock were collected. EDTA-treated blood samples were collected within 24 hours of presentation.
Healthy Control Patient Selection and Data Collection
Healthy volunteers 25 to 80 years of age were recruited from University of Iowa faculty, staff, and graduate/professional students. Exclusion criteria were signs or symptoms of active infections, infection requiring antibiotics within the past month, infection requiring hospitalization in the past year, and chemotherapy or radiation in the past year. Demographic data including age, gender, and race were collected. EDTA-treated blood samples were collected at an initial visit to our research clinic.
Human Cell Isolation and cryopreservation
Human cell isolation was adjusted from the previously described methodology (24). Briefly, whole blood was centrifuged, and plasma removed. ACK red blood cell lysis buffer was then added to the cell pellet and rested for 5 min at room temperature. Cells were again centrifuged, and supernatant was removed. Lysis and centrifugation was repeated 1–2 additional times. Cells were then washed with PBS 3 times before being counted and resuspended in cell freeze media (90%FCS [Hyclone] 10%DMSO [Fischer Scientific]). Cells were then stored at −80°C until use. When used in vitro, PBL were rapidly thawed and placed into warmed complete media. Cells were then washed 3 times with warmed media and aggregates filtered prior to use.
Antibody administration
NK depletion:
NK cell depletion was performed as previously described (25). Briefly, mice were depleted of NK cells by administration of αNK1.1 depleting antibody (2 doses of 300μg i.p. q.d.). Control mice were given the same amount of rat IgG. Where applicable the second dose of antibody was given immediately post-surgery, following abdominal closure.
IL-15 blockade:
IL-15 blockade was performed as previously described (26; 27). Briefly, mice were treated with 7.5μg of αIL-15/R, a chain antibody (eBioscience) that recognizes both unbound, soluble IL-15 and IL-15 complexed with the IL-15 receptor alpha to inhibit IL-15 signaling, the day prior to surgery and then daily thereafter for 3 days.
Cell isolation
Peripheral blood was collected by submandibular cheek bleeds to obtain PBL. Single-cell suspensions from spleen were generated after mashing tissue through 70 μm cell strainer without enzymatic digestion.
Flow cytometry, peptides and cytokine detection
Flow cytometry data were acquired on a FACSCanto (BD Biosciences, San Diego, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). To determine expression of cell surface proteins, mAb were incubated at 4°C for 20–30 min and cells were fixed using Cytofix/Cytoperm Solution (BD Biosciences) and, in some instances, followed by incubation with mAb for an additional 20–30 min to detect intracellular proteins. The following mAb clones were used to stain murine samples: NK1.1 (PK136; eBioscience), CD3 (17A2; eBioscience), NKp46 (29A1.4; eBioscience), IFNγ (XMG1.2; eBioscience), Thy1.1 (HIS51; eBioscience), CD49b (DX5; Biolegend), CD122 (TM-b1; eBioscience), and CD19 (MB19–1; eBioscience). For cytokine staining following in vitro stimulation BFA (BD Biosciences) was added during the last four hours of stimulation. The following mAb clones were used staining of patient samples: CD45 (HI30; Tonbo), CD3 (OKT3; Tonbo), CD19 (HIB19; Tonbo), CD56 (MY31; Tonbo), and IL-10 (JES5–2A5; Tonbo).
Cecal ligation and puncture (CLP) model of sepsis induction
Mice were anesthetized with ketamine/xylazine (University of Iowa, Office of Animal Resources), the abdomen was shaved and disinfected with Betadine (Purdue Products), and a midline incision was made (28). The distal third of the cecum was ligated with Perma-Hand Silk (Ethicon), punctured once (for CLP20) or twice (for CLP50) using a 25-gauge needle, and a small amount of fecal matter extruded out of each puncture. The cecum was then returned to abdomen, the peritoneum was closed with 641G Perma-Hand Silk (Ethicon), and skin sealed using surgical Vetbond (3M). Following surgery, 1 mL PBS was administered s.c. to provide post-surgery fluid resuscitation. Bupivacaine (Hospira) was administered at the incision site, and flunixin meglumine (Phoenix) was administered for postoperative analgesia. Sham mice underwent identical surgery excluding cecal ligation and puncture. Clinical disease was scored according to the following system: Grooming 0-normal, 1-piloerection, 2-ruffled; Gait 0-normal, 1-ataxic, 2-none; Mobility 0-normal, 1-reduced, 2-immobile; Body Position 0-Full extension, 1-hunched, 2-moribund.
Cytokine analysis
Multiplex cytokine analysis was performed via BioRad Bio-plex Pro Mouse Cytokine 46- and 23-plex as well as Procarta Plex Human 45-plex according to the manufacturer’s instructions for plasma cytokine analysis. Multiplex was analyzed on BioRad Bio-Plex (Luminex 200) analyzer in the University of Iowa Flow Cytometry core facility.
IFNγ, IL-15, and IL-10 ELISAs (ELISA MAX Deluxe Set, Biolegend) were performed according to the manufacturer’s instructions.
RNA sequencing and Gene Set Enrichment Analysis
The RNA-seq. gene expression data are from a previously published data set deposited at the NCBI GEO (accession number GSE114739 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi).
Statistical analysis
Unless stated otherwise data were analyzed using Prism8 software (GraphPad) using two-tailed Student t-test (for 2 individual groups, if unequal variance Mann-Whitney U test was used), one-way ANOVA with Bonferroni post-hoc test (for >2 individual groups, if unequal variance Kruskal-Wallis with Dunn’s post-hoc test was used), two-way ANOVA (for multiparametric analysis of 2 or more individual groups, pairing was used for samples that came from the same animal), Fisher’s exact test (for categorical data from 2 individual groups) with a confidence interval of >95% to determine significance (*p ≤ 0.05). Mantel-Cox test was used for comparison of Kaplan-Meier survival curves. Cytokine data were log transformed for correlation analysis to correct for heteroscedasticity observed in the distribution of raw data (29). Data are presented as standard error of the mean.
Results
NK cells promote host survival and limit the duration of the cytokine storm during sepsis.
NK cells act as pro-inflammatory mediators of the septic cytokine storm (7–9). However, given the duality of cytokine responses during sepsis and capacity of NK cells to participate in this dualism we sought to understand how NK cells broadly influence sepsis disease severity. To this end NK cells were depleted with αNK1.1 depleting antibody as previously described (7–9), achieving NK cell depletion that lasted up to 10 days after the initial Ab administration (Fig 1A–D). Further, we utilized a cecal-ligation-and-puncture (CLP) model for septic induction for its robust yet adjustable severity that effectively mimics clinical manifestations of sepsis (28; 30; 31). By varying the number of cecal punctures, severity of the septic event was modulated thereby enabling the interrogation of the role of NK cells during differential disease severity (Fig 1E). No difference in disease morbidity or mortality was observed when comparing NK-replete (IgG-treated) or -depleted (αNK1.1-treated) sham (control) and CLP20 (low disease severity) surgery mice (Fig 1F–I). However, while no difference was observed in the morbidity of NK-replete and -depleted mice following CLP50 (high disease severity) surgery (Fig 1J), NK-depleted animals appeared to be meaningfully more susceptible to CLP50-induced mortality (Fig 1K). Endpoint survival analysis (via chi-squared and logistic regression) and Pearson’s R correlation analysis further supported the trend toward enhanced mortality among NK-depleted hosts undergoing CLP50 surgery (Fig S1). Further we noted that the apparent enhanced mortality in the depleted group occurred after day 2, a time at which the cytokine storm has typically resolved in this model(15). We, therefore, evaluated the mortality from day 2 forward and found it to be significantly (p=0.0319) increased in the NK-depleted mice. These data suggest NK cells may promote long-term host survival after sepsis. Though it is notable that NK1.1 depletion impacts NKT cells it has previously been reported that CD1d-deficient animals (which only lack NKT cells) are less susceptible to sepsis mortality (32) suggesting that the increased susceptibility of depleted animals is associated with NK cells.
Figure 1: NK cell depletion leads to enhanced mortality late after sepsis induction.
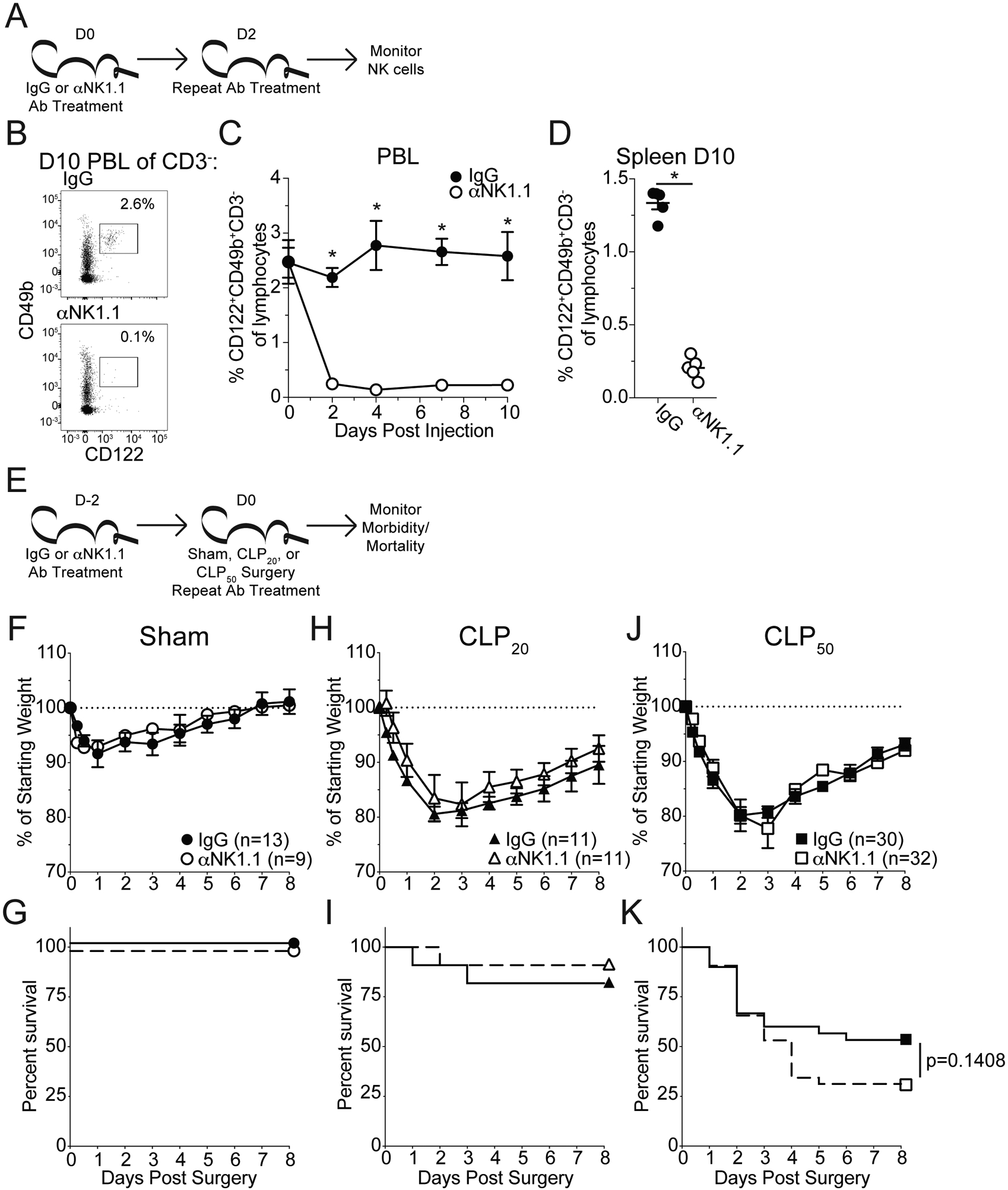
A) Experimental Design: The frequency of NK cells (CD3−CD122+CD49b+) in the PBL of mice every other day for 10 days. Mice were administered either control IgG or αNK1.1 depleting antibody on days 0 and 2 (after monitoring PBL). The frequency of NK cells in the spleen was assessed at D10. (B) Representative Gating: Gate of CD122+CD49b+ cells in the PBL 10 days after the initial depletion. Prior gating is on CD3− lymphocytes. Frequencies are of total lymphocytes. (C) Frequency of NK cells in the PBL throughout the following administration of control IgG or αNK1.1 antibody. (D) Frequency of NK cells in the spleen 10 days after the initial depletion. Data are representative from at least 3 independent experiments with 3–5 mice per group. E) Experimental Design: 2 days prior to surgery mice were administered either control IgG or αNK1.1 depleting antibody. On the day of surgery mice underwent either Sham (no ligation or puncture), CLP20 (ligation and 1 puncture), or CLP50 (ligation and 2 punctures) and received a second antibody injection. (F,G) Sham, (H,I) CLP20, and (J,K) CLP50 were monitored for (F,H,J) weight loss and (G,I,K) mortality daily for 8 days. Morbidity and mortality data are cumulative from 3 independent experiments with 9–32 mice per group. Mortality data is displayed as Kaplan-Meier curves. Error bars represent standard error of the mean.
To understand how NK depletion may be influencing the cytokine storm, plasma cytokines were assessed prior to and at 6, 12, 24, and 48hrs post-CLP50 surgery from NK-sufficient or -deficient animals. While a cytokine storm was evident in the NK-sufficient animals, the scope of the cytokine storm was significantly increased in NK-depleted counterparts (Fig 2A). Further, when the concentrations of IL-6, IL-1β, and IFNγ (cytokines associated with sepsis severity (32–37)) were assessed, NK-depleted CLP50 hosts had an extended duration, but not an increased magnitude, of the septic cytokine storm relative to NK-replete mice (Fig 2B–D). Notably, NK cell depletion did not influence the magnitude of the IFNγ response suggesting that while NK cells contribute IFNγ during sepsis other cells may compensate in their absence (Fig 2C). Thus, the data in Figures 1 and 2 collectively show that NK cells promote survival during sepsis by limiting the scope and duration of the cytokine storm.
Figure 2: NK cell depletion leads to a prolonged cytokine storm.
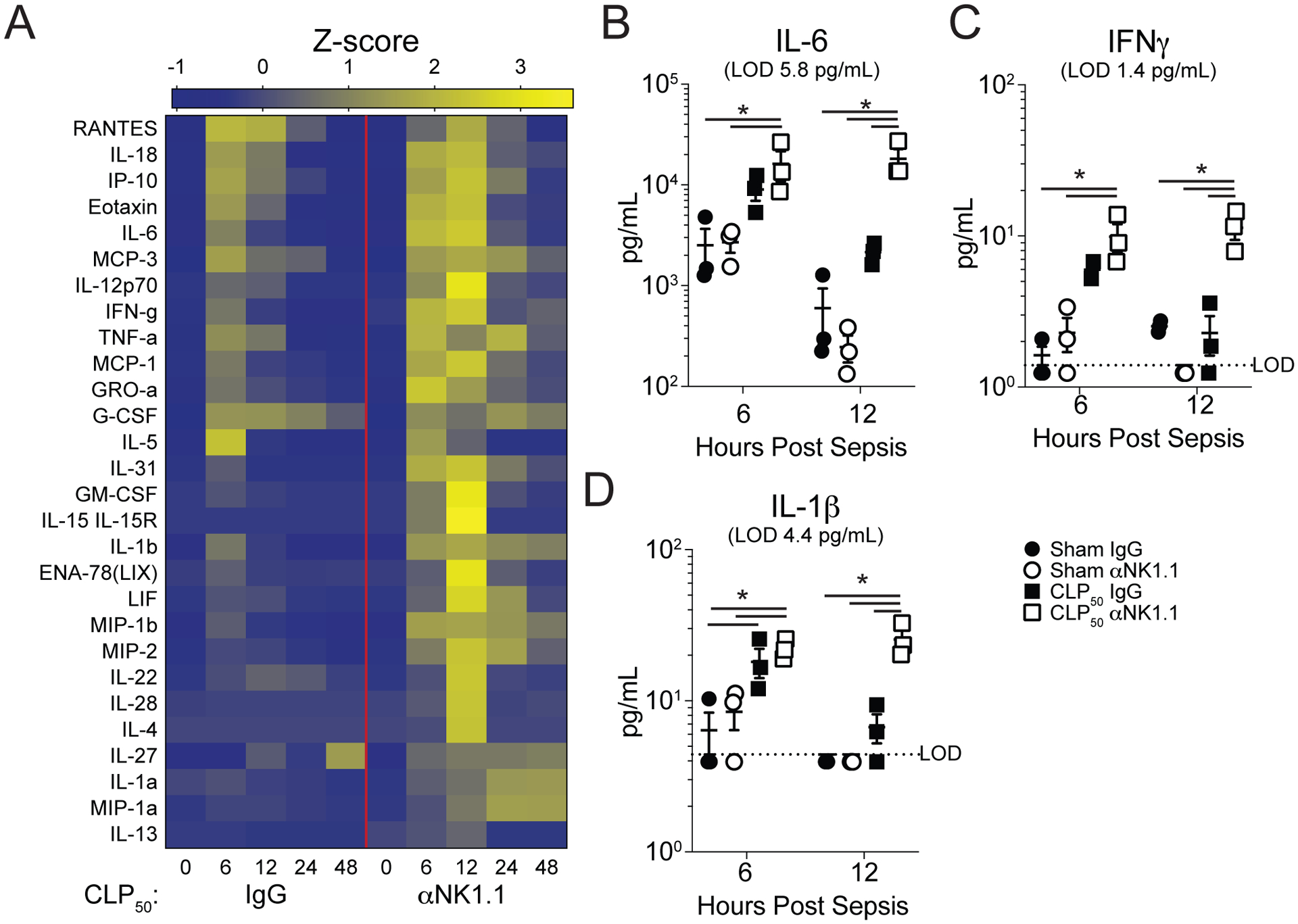
A) Heatmap of plasma cytokines in IgG- and αNK1.1-treated CLP50 hosts prior to surgery and at 6, 12, 24, and 48hrs post-surgery. Plasma (B) IL-6, (C) IFNγ, and (D) IL-1β concentration at 6 and 12 hrs after surgery in Sham and CLP50 mice that received either control IgG or αNK1.1 depleting antibody. Plasma cytokine data are from a single experiment with 3 mice per group. * = p<0.05. Error bars represent standard error of the mean.
IL-15 is consumed by NK cells during sepsis and elicits NK IL-10 production.
The presence of IL-15 in the cytokine storm of NK-depleted hosts (Fig 2A) prompted further interrogation given its critical role in NK cell survival/homeostasis (38; 39). Indeed, IL-15 was significantly elevated in the plasma of NK-depleted hosts relative to sham surgical controls and non-depleted septic counterparts (Fig 3A). NK cells avidly consume IL-15, which is a tightly regulated cytokine due to its potent inflammatory and lymphoproliferative properties (40–42). Thus, the increase in plasma IL-15 potentially reflects the absence of IL-15 consumption by NK cells. To address how IL-15 influences NK cell cytokine production whole splenocyte cultures were treated with rIL-15 and supernatant was analyzed for cytokine production after 24 or 48hrs. Similar to prior work, IL-15 administration led to increased IFNγ production (Fig 3B), however, an increase in IL-10 production was also noted after 48hrs of culturing (Fig 3C) (17; 43). NK cells can produce IL-10 in response to IL-15 as observed in systemic malarial and Listeria infections (19; 20). To define the capacity and kinetics of NK cell production of IFNγ and IL-10 in response to IL-15, splenocytes from IL-10bit C57Bl/6 mice, which express Thy1.1 during active IL-10 transcription(21), were stimulated with either low or high dose rIL-15 in vitro for 24 or 48hrs (Fig 4A). Low dose IL-15 served as the control, due to the reliance of NK cells on IL-15 for survival (38; 39). Interestingly, there was a temporal distinction in the production of IFNγ and IL-10 by NK cells such that increased IFNγ and little IL-10 was detected at 24hrs while little IFNγ and increased IL-10 was detected at 48hrs (Fig 4B–D).
Figure 3: NK cells consume IL-15 during sepsis and IL-15 induces production of IL-10.
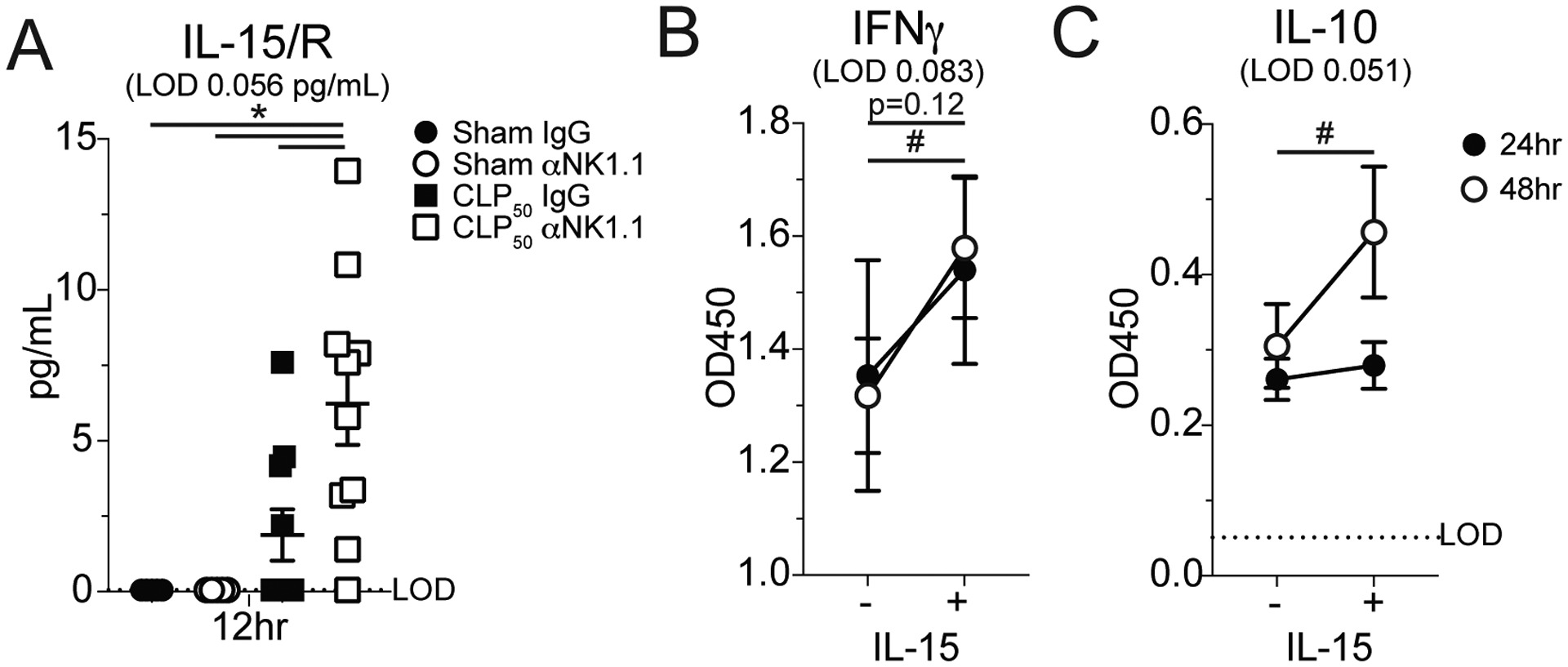
A) Plasma IL-15 concentration at 12 hrs after surgery in Sham and CLP50 mice that received either control IgG or αNK1.1 depleting antibody. OD450 reading for (B) IFNγ and (C) IL-10 in the supernatant of splenocytes treated with either 0 or 104 pg/mL IL-15 for either 24 or 48hrs. All data are representative from at least 2 independent experiments with at least 4–5 mice per group. (A) * = p<0.05, (B,C) *=p<0.05 for 24hrs #=p<0.05 for 48 hrs. Error bars represent standard error of the mean.
Figure 4: NK cells produce IL-10 in an IL-15-dependent manner.
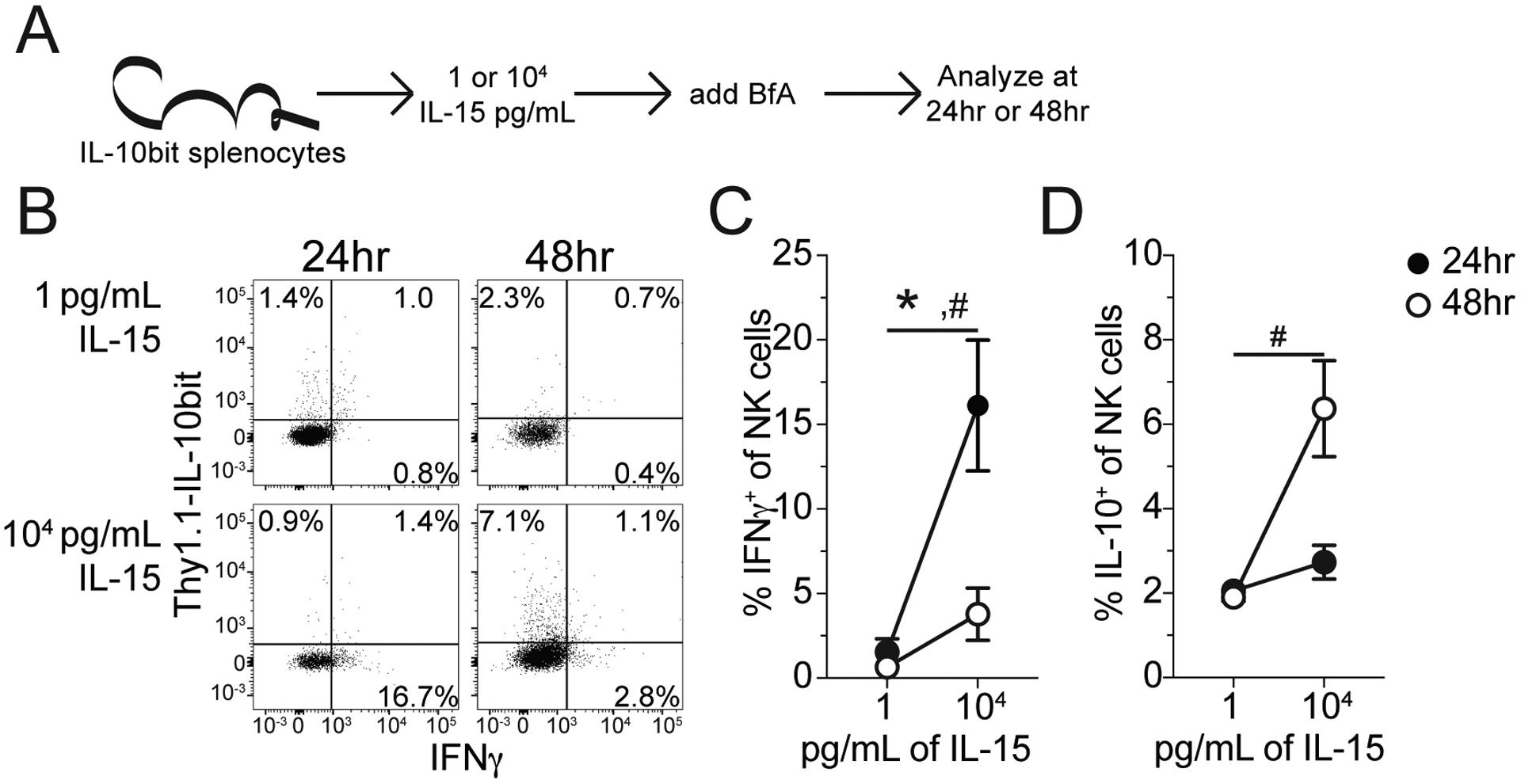
(A) Experimental Design: Splenocytes were harvested from IL-10bit mice, cells express Thy1.1 when IL-10 is expressed. IL-10bit splenocytes were then stimulated with either 1 or 104 pg/mL of IL-15 for either 24 or 48hrs. BfA was added during the last 4hrs of stimulation (either 20 or 44hrs, respectively) and the frequency of IFNγ and IL-10 expressing NK cells (NK1.1+CD3−) was determined by ICS. (B) Representative Staining: NK cells expressing Thy1.1 (IL-10) and IFNγ at 24 or 48hrs post stimulation with either 1 or 104 pg/mL IL-15. Frequency of (C) IFNγ- and (D) IL-10-expressing NK at 24 or 48hrs post stimulation with either 1 or 104 pg/mL of IL-15. Data are representative from 2 independent experiments with at least 4–5 mice per group. * = p<0.05 for 24hrs #=p<0.05 for 48 hrs. Error bars represent standard error of the mean.
Our in vitro studies with IL-15-stimulated NK cells revealed interesting data regarding IFNγ and IL-10 production, but we realize the in vitro stimulations with a single cytokine likely do not capture the complexity and global influence of the entire sepsis-induced cytokine storm on NK cells. Thus, IL-10 expression by NK cells was interrogated in septic hosts. Importantly, IL-10 gene expression was observed in sorted NK cells 24hrs after surgery that resolved by 48hrs (Fig S2B), demonstrating NK IL-10 production occurs in septic hosts. The difference in kinetics of the IL-10 response between the in vitro IL-15 stimulation and in vivo response to sepsis likely reflects the complexity of the stimulations. Further, IL-10 production was observed in NK cells from IL-10bit mice following both CLP20 and CLP50 (Fig S2C). These data indicate that NK cells produce IL-10 in the context of the sepsis-induced cytokine storm and IL-10 production is relevant even when mortality is not anticipated.
To determine whether NK cell production of IL-10 during sepsis was IL-15-dependent, IL-10bit mice were treated daily with either control IgG or αIL-15/R blocking antibody, which targets both unbound, soluble IL-15 and IL-15 complexed with the IL-15 receptor alpha, beginning a day prior to CLP20 surgery. The frequency of IL-10-producing NK cells (as measured by Thy1.1 expression) in the PBL was assessed longitudinally while those in the spleen were assessed 48hrs post-surgery and plasma IL-10 was assessed 24hrs post-surgery (Fig 5A). Consistent with our in vitro studies, IL-15/R blockade significantly reduced the frequency of IL-10-producing NK cells in the PBL (Fig 5B) and led to a trending decrease in the spleen (Fig 5C). The reduced frequency of IL-10-producing NK cells culminated in a significant reduction in the concentration of plasma IL-10 24hrs post-surgery (Fig 5D). Importantly, the dosage of the anti-IL-15 antibody, while sufficient to block IL-10 production by NK cells, did not reduce the frequency or number of NK cells (Fig 5E), nor did it make them more sensitive to sepsis-induced cell loss (Fig 5F). Thus, our data in Figures 3–5 collectively demonstrate NK cells produce IL-10 during sepsis in an IL-15-dependent manner.
Figure 5: NK cells produce IL-10 during sepsis in an IL-15-dependent manner.
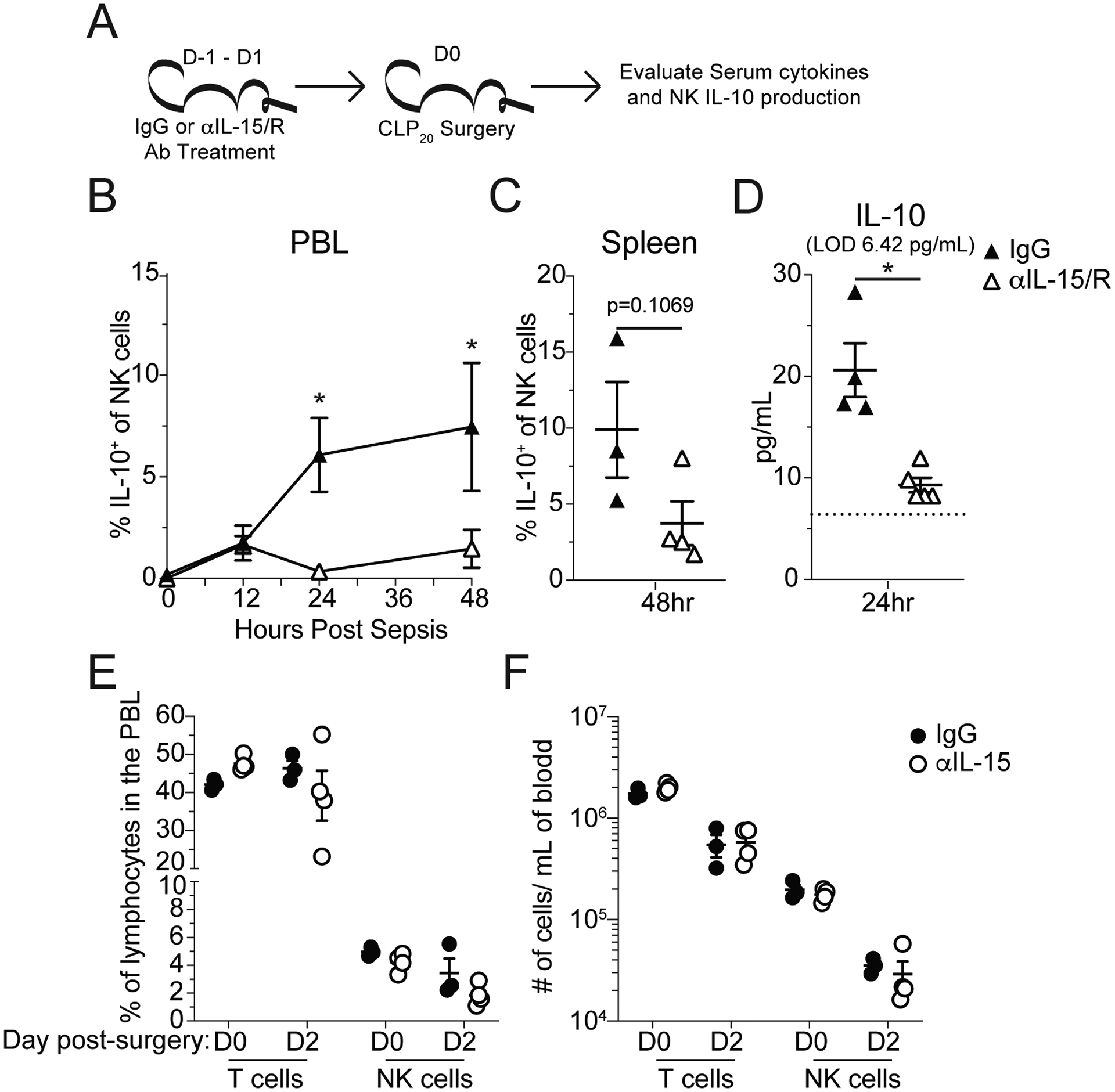
A) Experimental Design: Mice were given either control IgG or αIL-15/R blocking antibody, which inhibits both unbound, soluble IL-15 and IL-15 complexed with the IL-15 receptor alpha, 1 day prior to surgery and repeated on the day of sham or CLP20 surgery (Day 0) and Day 1 post-surgery. IL-10 production by NK cells and plasma IL-10 concentrations were monitored. (B) Frequency of IL-10-producing NK cells in the PBL of mice treated with either control IgG or αIL-15/R blocking antibody was assessed prior to and 12, 24, and 48hrs after surgery. (C) Frequency of IL-10-producing NK cells in the spleens of mice treated with either control IgG or αIL-15/R blocking antibody was assessed 48hrs after surgery. (D) Plasma IL-10 concentration of either control IgG or αIL-15/R blocking antibody treated mice 24hrs after surgery. Frequency (E) and number (F) of T cells and NK cells on the day of and 2 days after CLP20 surgery (1 and 3 days after initiation of IL-15 blockade, respectively). All data are representative from at least 2 independent experiments with at least 4–5 mice per group. * = p<0.05. Error bars represent standard error of the mean.
NK cell-derived IL-10 supports host survival during sepsis.
IL-10 has an established role as an anti-inflammatory cytokine and thus counterbalances the pro-inflammatory aspects of the cytokine storm. Thus, the production of IL-10 during sepsis may be a mechanism by which NK cells contribute to the regulation of inflammatory responses. Indeed, NK IL-10 production limits immunopathology during other systemic infections (19; 44). To determine the role of IL-10 during sepsis, wildtype and Il-10−/− mice underwent CLP50 surgery (Fig S3A). Similar to prior reports, Il-10−/− mice exhibited enhanced mortality (Fig S3B) suggesting IL-10 plays a critical role in promoting host survival during sepsis(45).
To address whether NK cell-derived IL-10 contributes to host survival during sepsis NCR1-CreERT2+/− Rosa26-tdTomato IL-10flox/flox mice were generated. These mice along with Rosa26-tdTomato IL-10flox/flox littermates were treated with tamoxifen daily for 5 days to knock out IL-10 expression in NK cells expressing the CreERT2 (Fig 6A). Depletion was assessed via induced tdTomato expression, wherein ~90% of NK cells had activated CreERT2 (Fig 6B) and NK cells composed >95% of the cells that had activated CreERT2 (Fig 6C) demonstrating the specificity of the IL-10 deficiency. Two days after the final tamoxifen treatment mice underwent CLP20 surgery and morbidity/mortality was monitored (Fig 6A). Mice whose NK cells lacked IL-10 (NCR1-CreERT2+/− Rosa26-tdTomato IL-10flox/flox) exhibited elevated weight loss, increased mortality, and prolonged signs of illness (Fig 6D–F). Importantly, this loss of IL-10 production by NK cells reduced host survival from 70% to 30% (Fig 6E), likely reflecting the capacity of NK cells to contribute solely as pro-inflammatory effectors (e.g., IFNγ). This loss of NK cell-derived IL-10 was also sufficient to reduce the amount of IL-10 present in the plasma by ~50% demonstrating their major contribution to the anti-inflammatory arm of the cytokine storm (Fig 6G). Furthermore, the specificity of the CreERT2 activation for NK cells precludes a contribution of NKT cells to this difference in survival, emphasizing the role of NK-derived IL-10. Thus, these data show NK cell-derived IL-10 is a critical facet of host survival during sepsis by limiting the scope and duration of the cytokine storm.
Figure 6: NK cell-derived IL-10 supports host survival during sepsis.
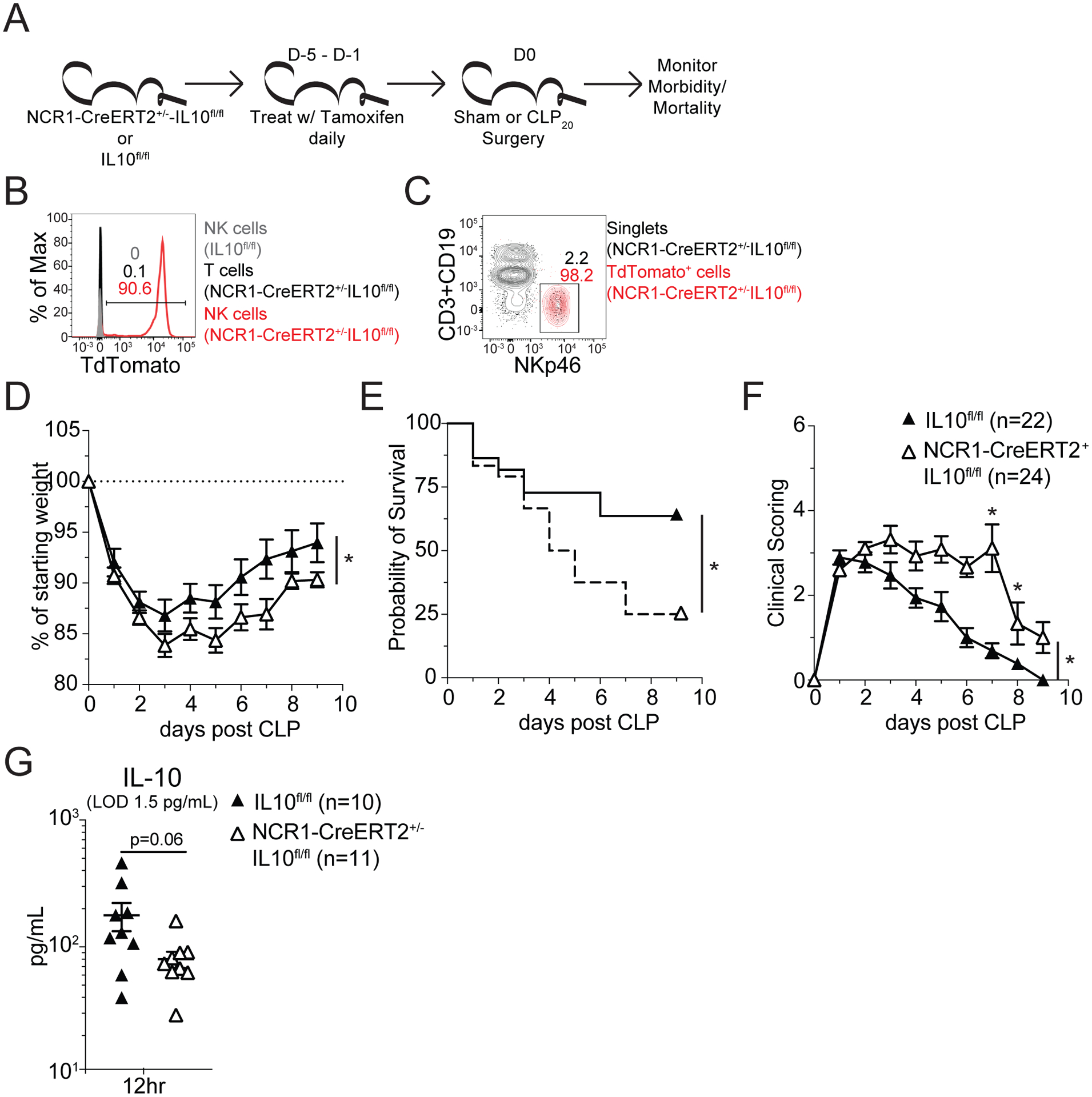
A) Experimental Design: NCR1-CreERT2+/− IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 and IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice were administered tamoxifen in corn oil daily, for 3–5 days, by oral gavage. Two days after the last tamoxifen dose mice underwent CLP20 surgery. Mice were then monitored for morbidity, by both weight loss and clinical scoring, and mortality. Representative flow profiles demonstrating successful Cre-mediated excision of LoxP sites on the day of surgery by assessing the frequency of (B) TdTomato-expressing cells among NK cells from IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice as wells among T cells and NK cells from NCR1-CreERT2+/− IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice and by assessing or (C) NK cells among both singlets and TdTomato-expressing cells in NCR1-CreERT2+/− IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice. (D) Weight loss, (E) mortality, and (F) clinical scoring of following CLP20 surgery of IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice (NK-IL10 sufficient) and NCR1-CreERT2+/− IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 (NK-IL10 deficient) mice. (G) Plasma IL-10 concentration of IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 mice (NK-IL10 sufficient) and NCR1-CreERT2+/− IL-10flox/flox Rosa26-TdTomato Thy1.1/1.1 (NK-IL10 deficient) mice 12hrs after CLP20 surgery. Morbidity and mortality data are combined from 2 independent experiment with 22–24 mice per group. Mortality data is displayed as Kaplan-Meier curves. Cytokine data are from a single experiment with 10–11 mice per group. * = p<0.05. Error bars represent standard error of the mean.
Increased IL-10 production by NK cells in septic patients correlates with severity of the cytokine storm.
To determine whether NK cell-derived IL-10 also plays a role in septic patients, PBL were collected from both septic patients and healthy controls. Septic patient samples were collected within 24hrs of admission to the ICU. Control and septic patients did not exhibit substantial demographic differences though septic patients exhibited severe disease (Table I). NK cells from patients were identified as CD45+CD3−CD19−CD14− cells (Fig 7A). NK cell subset analysis revealed a predominance of CD56dim NK cells in both the septic patients and healthy controls (Fig 7A,D). We did not observe lymphopenia in septic patients, which is associated with the immunoparalysis state following sepsis (46–50), indicating that analysis of these samples is more likely to reflect the on-going cytokine storm. However, there was increased production of IL-10 by NK cells observed in septic patients (Fig 7E, F). Thus, similar to observations in mice, NK cells in septic patients produce IL-10. We further observed that while IL-10, IFNγ, and IL-6 were all elevated in patient plasma (Fig 8A–C), the concentration of IL-10 correlated with the concentration of IL-6 (Fig 8D) but was inversely correlated with the concentration of IFNγ (Fig 8E). Correlations were performed with log transformed values to correct for heteroscedasticity in the distribution of raw data (29). The correlation of IL-10 with IL-6 (Fig 8D) is consistent with notion of IL-10 acting to regulate the pro-inflammatory cytokine storm. While the inverse correlation of IFNγ with IL-10 is consistent with our observation that NK cells produce either IL-10 or IFNγ at a given time (Fig 4). Thus, IL-10 production by NK cells, in response to IL-15, is a critical factor in host survival during sepsis by limiting the duration of the cytokine storm and is a conserved feature of between animal models and septic patients.
Table I:
Patient demographics.
| Overall | |||
|---|---|---|---|
| Patients | Septic (n=27) | Control (n=16) | p-value |
| Age (mean +/−SD) | 59.3 +/−16.3 | 51.6 +/−13.2 | ns |
| Male (%) | 40.7% | 37.5% | ns |
| Caucasian (%) | 100% | 81.3% | 0.0454 |
| APACHE II Score (mean +/−SD) | 11.1 +/−5.9 | ||
| SOFA Score (mean +/−SD) | 4.6 +/−4.3 | ||
| % in Septic Shock | 55.6% | ||
| Time Post-Admission (hrs) | 6.6 +/−5.3 | ||
| Cellular Data (Figure 7) | |||
| Patients | Septic (n=27) | Control (n=16) | p-value |
| Age (mean +/−SD) | 59.3 +/−16.3 | 51.6 +/−13.2 | ns |
| Male (%) | 40.7% | 37.5% | ns |
| Caucasian (%) | 100% | 81.3% | 0.0454 |
| APACHE II Score (mean +/−SD) | 11.1 +/−5.9 | ||
| SOFA Score (mean +/−SD) | 4.6 +/−4.3 | ||
| % in Septic Shock | 55.6% | ||
| Time Post-Admission (hrs) | 6.6 +/−5.3 | ||
| Cytokine Data (Figure 8) | |||
| Patients | Septic (n=14) | Control (n=12) | p-value |
| Age (mean +/−SD) | 62.6 +/−14.4 | 52.8 +/−10.8 | ns |
| Male (%) | 28.6% | 33.3% | ns |
| Caucasian (%) | 100% | 91.7% | ns |
| APACHE II Score (mean +/−SD) | 12.4 +/−4.6 | ||
| SOFA Score (mean +/−SD) | 4.4 +/−3.3 | ||
| % in Septic Shock | 78.6% | ||
| Time Post-Admission (hrs) | 11.64 +/−8.4 |
Figure 7: Septic patient NK cells have elevated IL-10 expression.
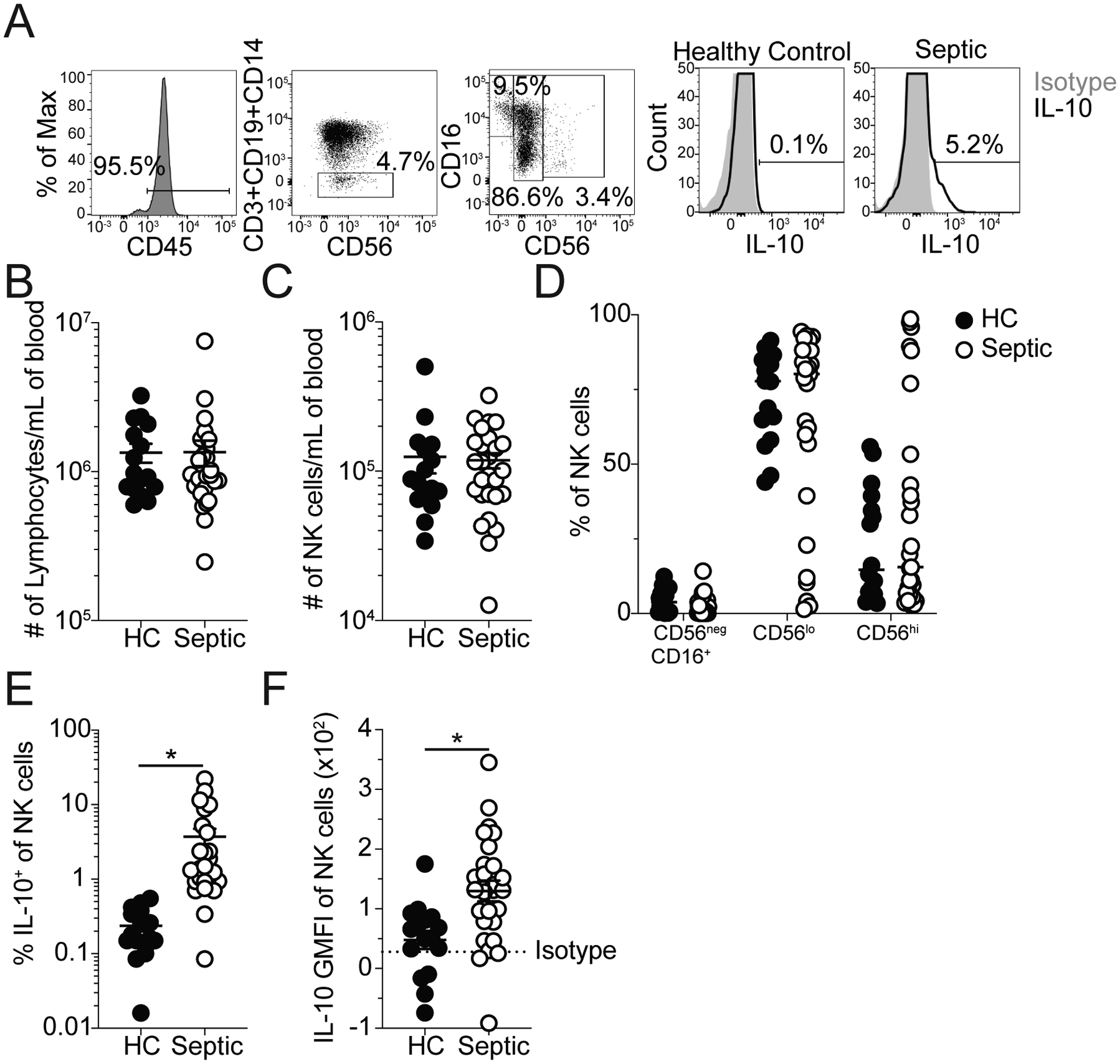
A) Representative Staining: NK cells (CD45+CD3−CD19−CD14− cells), CD56negCD16+, CD56lo, and CD56hi NK cells, and NK cells expressing IL-10 from healthy controls and septic patients (within 24hrs of hospital admission). Grey histograms indicate staining with isotype control antibody. The number of (B) total lymphocytes and (C) NK cells per mL of blood in healthy controls and septic patients. (D) Frequency of CD56negCD16+, CD56lo, and CD56hi NK cells in healthy controls and septic patients. The (E) frequency and (F) GMFI of IL-10 among all NK cells for both healthy controls and septic patients. Data are representative of 3 independent experiments with 16–27 patients per group. * = p<0.05. Error bars represent standard error of the mean. Dashed line indicates Isotype GMFI.
Figure 8: Positive correlation of plasma IL-10 with IL-6 in septic patients.
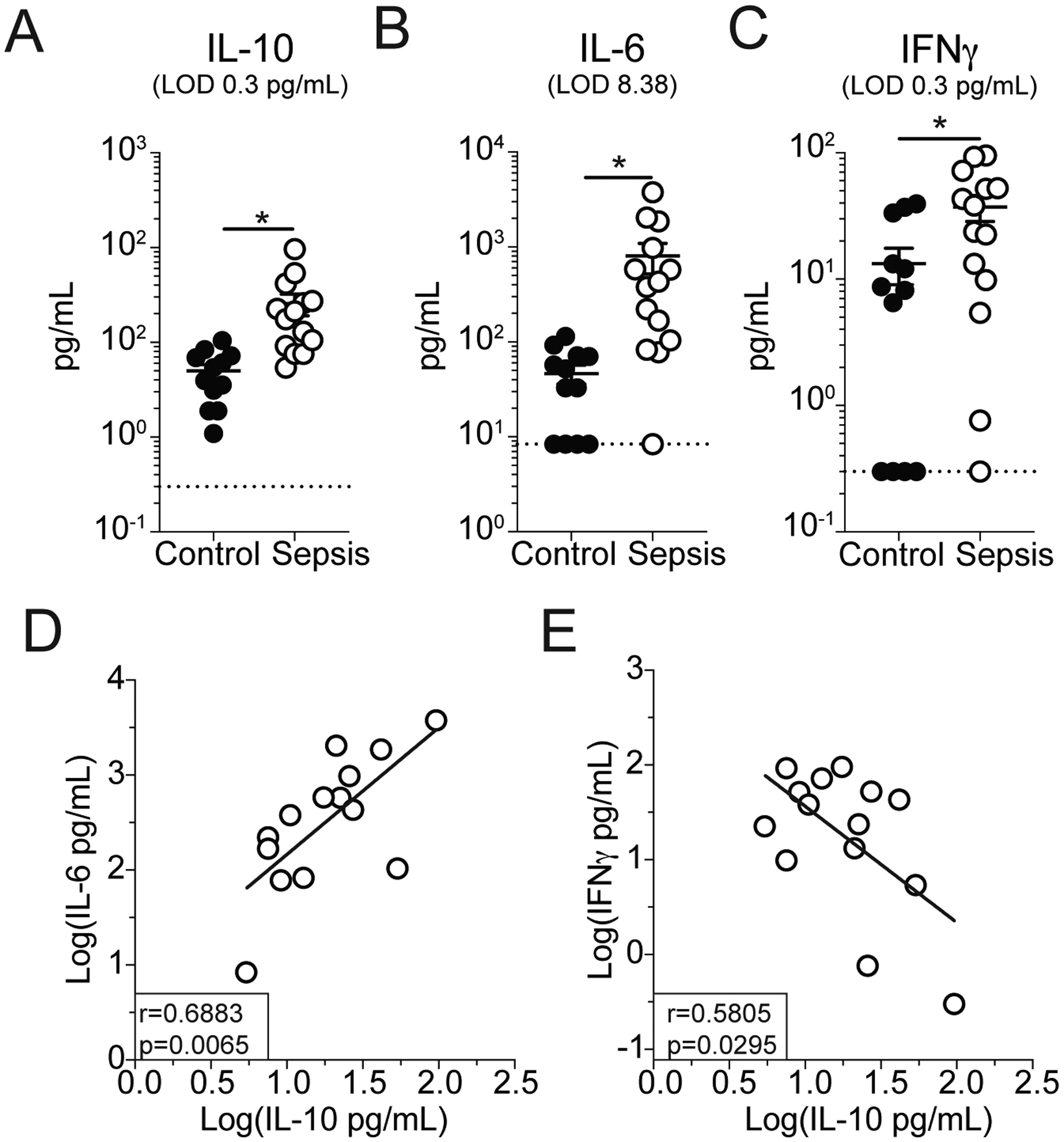
Plasma (A) IL-10, (B) IL-6, and (C) IFNγ concentration in healthy controls and septic patients within 24hrs of hospital admission. Correlation of plasma IL-10 with plasma (D) IL-6 or (E) IFNγ in septic patients. Data are log transformed to correct for heteroscedasticity in data distribution. Data are from a single experiment with 12–14 patients per group. * = p<0.05. Error bars represent standard error of the mean.
Discussion
Sepsis remains a significant and often fatal disease for which numerous clinical trials targeting the cytokine storm have failed (4). This is likely due to the complex inter-relationships of the various cytokines elicited and their corresponding effect on cells. Broadly, this cytokine release can be delineated into pro- and anti-inflammatory phases of the cytokine storm. Here, we demonstrated a linkage of this biphasic inflammatory response wherein pro-inflammatory IL-15 elicited anti-inflammatory IL-10 production by NK cells. This indicates that therapies which solely target the inflammatory aspects of the cytokine storm may subsequently impact the beneficial anti-inflammatory aspects. Thus, by disrupting the pro-inflammatory signals that are detrimental to the host, the anti-inflammatory signals that benefit the host may also become dysregulated. NK cells exemplify this complexity through their production of both pro-inflammatory (e.g., IFNγ) and anti-inflammatory (e.g., IL-10) cytokines during sepsis, in response to similar stimuli (17; 19; 20). This may contribute the difficulties experienced when attempting to utilize inflammatory cytokine blockade to reduce disease severity, wherein anti-inflammatory, protective immune responses may also be dampened.
Given this complexity it is pertinent to reflect on approaches used to target the cytokine storm. Specifically, methods of uncoupling the pro- and anti-inflammatory aspects of sepsis may effectuate enhanced survival when utilized in conjunction with pro-inflammatory cytokine blockade. With regard to NK cells specifically, Clark et al. demonstrated that activation of STAT3 activation, in response to cis-presentation of IL-15 and IL-10ra signaling, is required for IL-10 production (20). Therefore, STAT3 activation may initially present as a potential therapeutic target for inducing IL-10 production, however, it is also implicated in the production of inflammatory cytokines, in particular IL-6 which is associated with sepsis severity (51; 52). Importantly, administration of tocilizumab (i.e., IL-6 blockade) has some benefit in CLP models though has only recently begun to be explored clinically for sepsis (53; 54). These observations suggest STAT3 activation in combination with IL-6 blockade has the potential to decouple the biphasic components of sepsis and thereby promote survival. We also observed relatively few IFNγ and IL-10 co-producing NK cells, consistent with the observation by Clark et al. during Listeria infection (17). So, while NK cells have the capacity to contribute either IFNγ or IL-10 there is clearly regulation that establishes which of these a cell will contribute at a given time. Thus, further interrogation into the molecular switches between these differential responses may promote therapeutic tuning of cytokine responses in order to maintain the balance between pathogen clearance and immunopathology. Yet it is worth reflecting on the redundant nature of many inflammatory cytokines and cellular mediators within the context of sepsis and how singular focus on individual cells or cytokines is still likely to be too narrow for effectuating broad improvement in management of the cytokine storm.
Broadly, our characterization of counterbalancing mechanisms demonstrates that while cells are often described by their most canonical effector function, they must also retain the ability to offset their own action. Other examples of this include the production of IL-10 by memory CD8 T cells or IFNγ by Tregs (55–58). Thus, when dysregulation of cytokine responses occurs, as in sepsis, it may also be of benefit to look beyond the conventional cellular antagonist to a response and instead interrogate some of the mechanisms by which cells are losing their capacity to counterbalance their conventional effector functions. A relevant example can be found in SARS-CoV-2 infection where patients can experience acute respiratory distress syndrome (ARDS), along with an associated cytokine storm (consisting of both pro- and anti-inflammatory cytokines) and subsequent organ failure which parallels other pneumonic septic insults (59–62). Further, NK cells, among other lymphocytes, experience a severe numerical and functional loss after resolution of the infection (62–66) mirroring the immunoparalysis phase of sepsis (67–74). Thus, by reflecting on the congruence of immune dysregulation during major systemic infections therapeutic strategies may both improve and complicate therapeutic interventions. Such an approach could lead to more efficacious management of sepsis, SARS-CoV-2, and other dysregulated inflammatory responses.
Finally, it is relevant to reflect on how the duality of NK cells during the septic event may go on to influence the subsequent development of immunoparalysis, which is characterized by the numerical and functional impairment of numerous lymphocyte populations (15; 46; 47; 69–75). It is intriguing to postulate that those NK cells that survive the sepsis-induced lymphopenia may subsequently be primed to produce IL-10, promote anti-inflammatory activity, and variably reduce host capacity to respond to cancer and infection. Indeed, sepsis does alter the transcriptional profile of NK cells (47). With regard to patients, this may lead to a shift toward CD56− CD16+ NK cells that have been described in the context of chronic viral infections with reduced functional capability (76–78), similar to the described impairments of NK cells after septic insult. While we did not observe such a shift at this early timepoint, the production of IL-10 may serve as a prelude to this phenotypic and functional change. Thus, IL-10 production by NK cells may be consequential to/ indicative of both the cytokine storm and the subsequent immunoparalysis state.
Supplementary Material
Key Points:
NK cells produce IL-10 during sepsis in an IL-15-dependent manner.
NK cell-derived IL-10 promotes host survival during sepsis.
NK cell-derived IL-10 is relevant to septic patients.
Acknowledgements
Members of the Badovinac lab for helpful discussion.
Supported by NIH Grants R01AI114543 (V.P.B. and J.T.H.), R21AI147064 (V.P.B.), R35GM134880 (V.P.B.), R21AI151183 (V.P.B. and J.T.H), R01GM115462 (T.S.G.), R01AI125446 (N.S.B.), R01AI127481 (N.S.B.), R01AI42767 (J.T.H.), R01AI85515 (J.T.H.), R01AI100527 (J.T.H.), T32AI007511 (I.J.J.), T32AI007485 (I.J.J.) and a Veterans Health Administration Merit Review Award I01BX001324 (T.S.G.)
Footnotes
The authors declare no competing interest.
References
- 1.CDC. 2020. Sepsis: Data & Reports. In CDC, https://www.cdc.gov/sepsis/datareports/index.html. [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour C, and et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, and Naghavi M. 2020. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall JC 2014. Why have clinical trials in sepsis failed? Trends in Molecular Medicine 20:195–203. [DOI] [PubMed] [Google Scholar]
- 5.Delano MJ, and Ward PA. 2016. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunological Reviews 274:330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danahy DB, Strother RK, Badovinac VP, and Griffith TS. 2016. Clinical and Experimental Sepsis Impairs CD8 T-Cell-Mediated Immunity. Critical Reviews in Immunology 36:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H, Geng T, Chen P, Wang M, Hu J, Kang L, Song W, and Tang H. 2016. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Scientific Reports 6:27711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etogo AO, Nunez J, Lin CY, Toliver-Kinsky TE, and Sherwood ER. 2008. NK but Not CD1-Restricted NKT Cells Facilitate Systemic Inflammation during Polymicrobial Intra-Abdominal Sepsis. The Journal of Immunology 180:6334–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin G, P. NK, Liming L, B. JK, and S. ER 2018. The biology of natural killer cells during sepsis. Immunology 153:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinet L, and Smyth MJ. 2015. Balancing natural killer cell activation through paired receptors. Nature Reviews Immunology 15:243–254. [DOI] [PubMed] [Google Scholar]
- 11.Freeman BE, Raué H-P, Hill AB, and Slifka MK. 2015. Cytokine-Mediated Activation of NK Cells during Viral Infection. Journal of Virology 89:7922–7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero CR, Herzig DS, Etogo A, Nunez J, Mahmoudizad R, Fang G, Murphey ED, Toliver‐Kinsky T, and Sherwood ER. 2010. The role of interferon‐γ in the pathogenesis of acute intra‐abdominal sepsis. Journal of Leukocyte Biology 88:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Luan L, Patil NK, Wang J, Bohannon JK, Rabacal W, Fensterheim BA, Hernandez A, and Sherwood ER. 2017. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. The Journal of Immunology 198:1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiche L, Forel J-M, Thomas G, Farnarier C, Vely F, Bléry M, Papazian L, and Vivier E. 2011. The role of natural killer cells in sepsis. J Biomed Biotechnol 2011:986491–986491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huggins MA, Sjaastad FV, Pierson M, Kucaba TA, Swanson W, Staley C, Weingarden AR, Jensen IJ, Danahy DB, Badovinac VP, Jameson SC, Vezys V, Masopust D, Khoruts A, Griffith TS, and Hamilton SE. 2019. Microbial Exposure Enhances Immunity to Pathogens Recognized by TLR2 but Increases Susceptibility to Cytokine Storm through TLR4 Sensitization. Cell Reports 28:1729–1743.e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, and Moretta L. 2007. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. European Journal of Immunology 37:445–455. [DOI] [PubMed] [Google Scholar]
- 17.Clark SE, Filak HC, Guthrie BS, Schmidt RL, Jamieson A, Merkel P, Knight V, Cole CM, Raulet DH, and Lenz LL. 2016. Bacterial Manipulation of NK Cell Regulatory Activity Increases Susceptibility to Listeria monocytogenes Infection. PLOS Pathogens 12:e1005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S-H, Kim K-S, Fodil-Cornu N, Vidal SM, and Biron CA. 2009. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. Journal of Experimental Medicine 206:2235–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrack KS, Huggins MA, Taras E, Dougherty P, Henzler CM, Yang R, Alter S, Jeng EK, Wong HC, Felices M, Cichocki F, Miller JS, Hart GT, Johnson AJ, Jameson SC, and Hamilton SE. 2018. Interleukin-15 Complex Treatment Protects Mice from Cerebral Malaria by Inducing Interleukin-10-Producing Natural Killer Cells. Immunity 48:760–772.e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark SE, Burrack KS, Jameson SC, Hamilton SE, and Lenz LL. 2019. NK Cell IL-10 Production Requires IL-15 and IL-10 Driven STAT3 Activation. Frontiers in Immunology 10:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, and Weaver CT. 2007. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nature Immunology 8:931–941. [DOI] [PubMed] [Google Scholar]
- 22.Nabekura T, and Lanier LL. 2016. Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. Journal of Experimental Medicine 213:2745–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, and Müller W 2004. T Cell–specific Inactivation of the Interleukin 10 Gene in Mice Results in Enhanced T Cell Responses but Normal Innate Responses to Lipopolysaccharide or Skin Irritation. Journal of Experimental Medicine 200:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer FT, Denson JL, and Burchiel SW. 2017. Isolation, Cryopreservation, and Immunophenotyping of Human Peripheral Blood Mononuclear Cells. Current Protocols in Toxicology 74:18.20.11–18.20.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, and Clambey ET. 2015. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti–Asialo-GM1 Antibody. The Journal of Immunology 195:4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee GA, Lin T-N, Chen C-Y, Mau S-Y, Huang W-Z, Kao Y-C, Ma R.-y., and Liao N-S. 2018. Interleukin 15 blockade protects the brain from cerebral ischemia-reperfusion injury. Brain, Behavior, and Immunity 73:562–570. [DOI] [PubMed] [Google Scholar]
- 27.Mackay Laura K., Wynne-Jones E, Freestone D, Pellicci Daniel G., Mielke Lisa A., Newman Dane M., Braun A, Masson F, Kallies A, Belz Gabrielle T., and Carbone Francis R.. 2015. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43:1101–1111. [DOI] [PubMed] [Google Scholar]
- 28.Sjaastad FV, Jensen IJ, Berton RR, Badovinac VP, and Griffith TS. 2020. Inducing Experimental Polymicrobial Sepsis by Cecal Ligation and Puncture. Current Protocols in Immunology 131:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartlett MS 1947. The Use of Transformations. Biometrics 3:39–52. [PubMed] [Google Scholar]
- 30.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, and Efron PA. 2010. Cecal Ligation and Puncture. Current Protocols in Immunology 91:19.13.11–19.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dejager L, Pinheiro I, Dejonckheere E, and Libert C. 2011. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends in Microbiology 19:198–208. [DOI] [PubMed] [Google Scholar]
- 32.Kim EY, Ner-Gaon H, Varon J, Cullen AM, Guo J, Choi J, Barragan-Bradford D, Higuera A, Pinilla-Vera M, Short SAP, Arciniegas-Rubio A, Tamura T, Leaf DE, Baron RM, Shay T, and Brenner MB. 2020. Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-γ in NK cells. The Journal of Clinical Investigation 130:3238–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Zhang H, Yin Y.-l., Guo W.-z., Ma Y.-q., Wang Y.-b., Shu C, and Dong L.-q.. 2016. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine 88:126–135. [DOI] [PubMed] [Google Scholar]
- 34.Qiao Z, Wang W, Yin L, Luo P, Greven J, Horst K, and Hildebrand F. 2018. Using IL-6 concentrations in the first 24 h following trauma to predict immunological complications and mortality in trauma patients: a meta-analysis. European Journal of Trauma and Emergency Surgery 44:679–687. [DOI] [PubMed] [Google Scholar]
- 35.Qiu X, Zhang L, Tong Y, Qu Y, Wang H, and Mu D. 2018. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: A meta-analysis. Medicine 97:e13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binnie A, Tsang JLY, and dos Santos CC. 2014. Biomarkers in acute respiratory distress syndrome. Current Opinion in Critical Care 20:47–55. [DOI] [PubMed] [Google Scholar]
- 37.Que Y-A, Delodder F, Guessous I, Graf R, Bain M, Calandra T, Liaudet L, and Eggimann P. 2012. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Critical Care 16:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nandagopal N, Ali AK, Komal AK, and Lee S-H. 2014. The Critical Role of IL-15–PI3K–mTOR Pathway in Natural Killer Cell Effector Functions. Frontiers in Immunology 5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Luan L, Patil NK, and Sherwood ER. 2017. Immunobiology of the IL-15/IL-15Rα complex as an antitumor and antiviral agent. Cytokine & Growth Factor Reviews 38:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anthony SM, Rivas SC, Colpitts SL, Howard ME, Stonier SW, and Schluns KS. 2016. Inflammatory Signals Regulate IL-15 in Response to Lymphodepletion. The Journal of Immunology 196:4544–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillerey C, Huntington ND, and Smyth MJ. 2016. Targeting natural killer cells in cancer immunotherapy. Nature Immunology 17:1025–1036. [DOI] [PubMed] [Google Scholar]
- 42.Ali AK, Nandagopal N, and Lee S-H. 2015. IL-15–PI3K–AKT–mTOR: A Critical Pathway in the Life Journey of Natural Killer Cells. Frontiers in Immunology 6:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JY, Lee SH, Yoon S-R, Park Y-J, Jung H, Kim T-D, and Choi I. 2011. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol Cells 32:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, Johnson LL, Smiley ST, and Mohrs M. 2009. Systemic but Not Local Infections Elicit Immunosuppressive IL-10 Production by Natural Killer Cells. Cell Host & Microbe 6:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latifi SQ, O’Riordan MA, and Levine AD. 2002. Interleukin-10 Controls the Onset of Irreversible Septic Shock. Infection and Immunity 70:4441–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen IJ, Sjaastad FV, Griffith TS, and Badovinac VP. 2018. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. The Journal of Immunology 200:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen IJ, Winborn CS, Fosdick MG, Shao P, Tremblay MM, Shan Q, Tripathy SK, Snyder CM, Xue H-H, Griffith TS, Houtman JC, and Badovinac VP. 2018. Polymicrobial sepsis influences NK-cell-mediated immunity by diminishing NK-cell-intrinsic receptor-mediated effector responses to viral ligands or infections. PLOS Pathogens 14:e1007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, and Karl IE. 2001. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+ T Lymphocytes in Humans. The Journal of Immunology 166:6952–6963. [DOI] [PubMed] [Google Scholar]
- 49.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, and Hotchkiss RS. 2010. IL-15 Prevents Apoptosis, Reverses Innate and Adaptive Immune Dysfunction, and Improves Survival in Sepsis. The Journal of Immunology 184:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shindo Y, McDonough JS, Chang KC, Ramachandra M, Sasikumar PG, and Hotchkiss RS. 2017. Anti-PD-L1 peptide improves survival in sepsis. Journal of Surgical Research 208:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, and Jenkins BJ. 2011. IL-6 Trans-Signaling Modulates TLR4-Dependent Inflammatory Responses via STAT3. The Journal of Immunology 186:1199–1208. [DOI] [PubMed] [Google Scholar]
- 52.Hodge DR, Hurt EM, and Farrar WL. 2005. The role of IL-6 and STAT3 in inflammation and cancer. European Journal of Cancer 41:2502–2512. [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim YF, Moussa RA, Bayoumi AMA, and Ahmed A-SF. 2020. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacology 28:215–230. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka T, Narazaki M, and Kishimoto T. 2016. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 8:959–970. [DOI] [PubMed] [Google Scholar]
- 55.Sun J, Madan R, Karp CL, and Braciale TJ. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature Medicine 15:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koenecke C, Lee C-W, Thamm K, Föhse L, Schafferus M, Mittrücker H-W, Floess S, Huehn J, Ganser A, Förster R, and Prinz I. 2012. IFN-γ Production by Allogeneic Foxp3+ Regulatory T Cells Is Essential for Preventing Experimental Graft-versus-Host Disease. The Journal of Immunology 189:2890–2896. [DOI] [PubMed] [Google Scholar]
- 57.Trandem K, Zhao J, Fleming E, and Perlman S. 2011. Highly Activated Cytotoxic CD8 T Cells Express Protective IL-10 at the Peak of Coronavirus-Induced Encephalitis. The Journal of Immunology 186:3642–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominguez-Villar M, Baecher-Allan CM, and Hafler DA. 2011. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nature Medicine 17:673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami M-E, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, and Koutsoukou A. 2020. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host & Microbe 27:992–1000.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson JG, Simpson LJ, Ferreira A-M, Rustagi A, Roque JA, Asuni A, Ranganath T, Grant PM, Subramanian AK, Rosenberg-Hasson Y, Maecker H, Holmes S, Levitt JE, Blish C, and Rogers AJ. 2020. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 5:e140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remy KE, Mazer M, Striker DA, Ellebedy AH, Walton AH, Unsinger J, Blood TM, Mudd PA, Yi DJ, Mannion DA, Osborne DF, Martin RS, Anand NJ, Bosanquet JP, Blood J, Drewry AM, Caldwell CC, Turnbull IR, Brakenridge SC, Moldwawer LL, and Hotchkiss RS. 2020. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 5:e140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sariol A, and Perlman S. 2020. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 53:248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, Qian J, Wu L, Chen Y, Chen Y, and Lin X. 2020. COVID-19 pneumonia: CD8+ T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clinical Immunology 218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, Song S, Ma Z, Mo P, and Zhang Y. 2020. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. The Journal of Infectious Diseases 221:1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajaram S, Canaday LM, Ochayon DE, Rangel KM, Ali A, Gyurova IE, Krishnamurthy D, Fletcher JS, Reighard SD, Cox A, Weirauch MT, Kottyan LC, Deshmukh H, Zacharias WJ, Borchers MT, and Waggoner SN. 2020. The Promise and Peril of Natural Killer Cell Therapies in Pulmonary Infection. Immunity 52:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, and Wei H. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences 117:10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Condotta SA, Khan SH, Rai D, Griffith TS, and Badovinac VP. 2015. Polymicrobial Sepsis Increases Susceptibility to Chronic Viral Infection and Exacerbates CD8+ T Cell Exhaustion. The Journal of Immunology 195:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Condotta SA, Rai D, James BR, Griffith TS, and Badovinac VP. 2013. Sustained and Incomplete Recovery of Naive CD8+ T Cell Precursors after Sepsis Contributes to Impaired CD8+ T Cell Responses to Infection. The Journal of Immunology 190:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue H-H, Harty JT, Griffith TS, and Badovinac VP. 2017. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLOS Pathogens 13:e1006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danahy DB, Kurup SP, Winborn CS, Jensen IJ, Harty JT, Griffith TS, and Badovinac VP. 2019. Sepsis-Induced State of Immunoparalysis Is Defined by Diminished CD8 T Cell–Mediated Antitumor Immunity. The Journal of Immunology 203:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin MD, Badovinac VP, and Griffith TS. 2020. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Frontiers in Immunology 11:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sjaastad FV, Kucaba TA, Dileepan T, Swanson W, Dail C, Cabrera-Perez J, Murphy KA, Badovinac VP, and Griffith TS. 2020. Polymicrobial Sepsis Impairs Antigen-Specific Memory CD4 T Cell-Mediated Immunity. Frontiers in Immunology 11:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, Legge KL, and Badovinac VP. 2016. Polymicrobial Sepsis Diminishes Dendritic Cell Numbers and Function Directly Contributing to Impaired Primary CD8 T Cell Responses In Vivo. The Journal of Immunology 197:4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, Kucaba TA, Badovinac VP, and Griffith TS. 2015. Alterations in Antigen-Specific Naive CD4 T Cell Precursors after Sepsis Impairs Their Responsiveness to Pathogen Challenge. The Journal of Immunology 194:1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen IJ, Jensen SN, Sjaastad FV, Gibson-Corley KN, Dileepan T, Griffith TS, Mangalam AK, and Badovinac VP. 2020. Sepsis impedes EAE disease development and diminishes autoantigen-specific naïve CD4 T cells. eLife 9:e55800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith SL, Kennedy PR, Stacey KB, Worboys JD, Yarwood A, Seo S, Solloa EH, Mistretta B, Chatterjee SS, Gunaratne P, Allette K, Wang Y-C, Smith ML, Sebra R, Mace EM, Horowitz A, Thomson W, Martin P, Eyre S, and Davis DM. 2020. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Advances 4:1388–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pollmann J, Rölle A, Hofmann M, and Cerwenka A. 2017. Hepatitis C Virus and Human Cytomegalovirus—Natural Killer Cell Subsets in Persistent Viral Infections. Frontiers in Immunology 8:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Björkström NK, Ljunggren H-G, and Sandberg JK. 2010. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends in Immunology 31:401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


