Abstract
Objective
To determine the incidence of epilepsy among Medicare beneficiaries with a new diagnosis of Alzheimer dementia (AD) or Parkinson disease (PD).
Methods
Retrospective cohort study of Medicare beneficiaries with an incident diagnosis of AD or PD in the year 2009. The 5-year incidence of epilepsy was examined by sociodemographic characteristics, comorbidities and neurodegenerative disease status. Cox regression models examined the association of neurodegenerative disease with incident epilepsy, adjusting for demographic characteristics and medical comorbidities.
Results
We identified 178,593 individuals with incident AD and 104,157 individuals with incident PD among 34,054,293 Medicare beneficiaries with complete data in 2009. Epilepsy was diagnosed in 4.45% (7,956) of AD patients and 4.81% (5,010) of PD patients between 2009 and 2014, approximately twice as frequently as in the control sample. Minority race/ethnicity was associated with increased risk of incident epilepsy. Among individuals with AD and PD, stroke was associated with increased epilepsy risk. Traumatic brain injury (TBI) was associated with increased epilepsy risk for individuals with PD. Depression was also associated with incident epilepsy (AD adjusted hazard ratio (AHR): 1.23 (1.17–1.29), PD AHR: 1.45 (1.37–1.54)). In PD only, a history of hip fracture (AHR, 1.35 (1.17–1.57)) and diabetes (AHR, 1.11 (1.05–1.18) were also associated with increased risk of epilepsy.
Conclusion
Incident epilepsy is more frequently diagnosed among neurodegenerative disease patients, particularly when preceded by a diagnosis of depression, TBI or stroke. Further studies into the differences in epilepsy risk between these two populations may help elucidate different mechanisms of epileptogenesis.
Keywords: Epilepsy, Dementia, Alzheimer, Parkinson, Incidence, Epidemiology
Key points
Epilepsy is often seen in neurodegenerative disease.
The five-year epilepsy incidence rates among Medicare beneficiaries with Alzheimer Dementia and Parkinson disease are almost double the rate in those without a neurodegenerative diagnosis.
Depression is independently associated with developing epilepsy in the neurodegenerative population.
Introduction
Despite longstanding awareness that the cell death and disruption of brain architecture seen in neurodegenerative diseases can also lead to epilepsy, limited data exists on epilepsy burden among Alzheimer dementia (AD) and Parkinson disease (PD) populations. As the older adult population continues to rapidly grow, we should expect the burden of neurodegenerative disease and associated epilepsy to increase [1, 2]. Therefore, large-scale characterizations of epilepsy in the neurodegenerative population are critical to planning future healthcare delivery models.
Epilepsy outcomes (like outcomes in PD and AD) are known to be improved through differential treatment choices [3–8], many of which are mediated by specialty care [9–12]. Coordinating care for this population of vulnerable, complex neurologic patients first requires a careful characterization of the population at risk, including the identification and quantification of any socioeconomic disparities or comorbid conditions that impact the risk of developing epilepsy or may impact treatment paradigms.
In order to begin to address these knowledge gaps, our primary study objective was to determine the 5-year incidence rate of epilepsy in adults over 65 years of age with a new diagnosis of AD or PD. Our secondary objective was to determine the extent to which sociodemographic characteristics or comorbid neurological disorders are associated with incident epilepsy in these populations.
Methods
Data
This study used data from the Medicare programme, which administers health insurance to approximately 96% of the US population over age 65 [13]. We used data from multiple Medicare research identifiable files containing individual-level data that can be linked between datasets and over time using a unique encrypted beneficiary identification code. The Medicare beneficiary summary file base segment contains enrolment, eligibility data, sociodemographic and residential characteristics (such as race, age, sex, postal code) and date of death. The Chronic Condition Warehouse (CCW) files contain both indicators and date of diagnosis variables for 67 common and disabling conditions, including AD and epilepsy. The condition variables are derived using algorithms that search all Medicare claims data for specific diagnosis or procedure codes. PD is not present in the CCW; therefore, the Carrier file was used to identify all individuals diagnosed with PD in Medicare files.
Study design and sample
We performed a retrospective cohort study of Medicare beneficiaries (ages 65 and older) with an incident diagnosis of PD or AD in 2009. To designate a diagnosis as incident, we required each individual to have at least 2 years of Medicare coverage with no neurodegenerative diagnosis (no PD, no AD, and no other forms of dementia) as well as no epilepsy diagnoses in any care setting. All potential subjects therefore had complete Medicare coverage as of 1 January 2007 and had continuous inpatient and outpatient care coverage before the neurodegenerative diagnosis in 2009. We excluded individuals also receiving benefits from the Medicaid programme, which provides additional coverage to Medicare beneficiaries who meet poverty criteria or have a qualifying mental or physical disability. Individuals concurrent Medicaid enrolment were excluded to remove potential confounding from pre-existing physical, intellectual disabilities such as cerebral palsy, quadriplegia, severe traumatic brain injuries or genetic neurodevelopmental disorders.
Epilepsy and AD diagnoses were determined by query of the 2009 CCW data. The CCW epilepsy indicator variable was based on the presence of at least one inpatient or two outpatient claims with a diagnosis code of 345.x, according to the International Classification of Diseases, Ninth Revision (ICD-9). Previous studies support the use of this algorithm [14, 15]. Alzheimer disease was indicated for beneficiaries with at least one inpatient, skilled nursing facility, home health agency, health options plan or carrier claim with diagnosis code 331.0 in a 3-year period. We identified individuals with PD based on the presence of at least two claims with a diagnosis of 332.0 in the Carrier file. The two resulting incident neurodegenerative cohorts formed the case study samples for all analyses. Control groups for each sample were created by removing observations with missing values for any adjustment variables and then creating a random 10% sample of Medicare beneficiaries without the given neurodegenerative condition. Patients in the control sample who later acquired the given neurodegenerative condition between 1 January 2009 and 31 December 2014 were excluded.
Sensitivity analysis
We also conducted a sensitivity analysis where we removed patients that had a diagnosis of the other neurodegenerative disease in subsequent years. More specifically, for our AD cohort, we excluded any cases with a PD diagnosis after their incident AD diagnosis and any controls with a PD diagnosis after 1 January 2009. This was also done for our PD cohort where we removed those with an AD diagnosis. We then re-ran each of our models.
Patient characteristics
We extracted data on beneficiary age, sex and race. Age in 2009 was extracted as a continuous variable. Race and ethnicity were not coded separately in Medicare data until recently, therefore available demographic categorizations were: ‘White’, ‘Black’, ‘Asian’, ‘Hispanic’, ‘Native North American’ and ‘other/unknown’. Finally, the CCW was queried to identify the following comorbid conditions: myocardial infarction, atrial fibrillation, cataracts, heart failure, chronic kidney disease, female breast cancer, colorectal cancer, endometrial cancer, lung cancer, prostate cancer, chronic obstructive pulmonary disease, depression, diabetes, glaucoma, hip or pelvic fracture, ischemic heart disease, osteoporosis, rheumatoid or osteoarthritis. The extraction algorithms for all health conditions considered in the study are presented in Appendix 1.
Outcomes
The primary outcome was incident epilepsy, measured through 31 December 2014. Incident epilepsy was defined as epilepsy occurring after the diagnosis date of the neurodegenerative condition or after 1 January 2009 for controls. End of follow-up was the minimum of death date or 31 December 2014.
Statistical analyses
Demographic characteristics were compared using chi-squared tests for categorical variables and t-tests for continuous variables. To examine patient characteristics associated with incident epilepsy, we built multivariable cause-specific Cox regression models with Efron ties to estimate the risk of epilepsy for each variable of interest within our two neurodegenerative populations. Time to epilepsy was defined as the time from neurologic diagnosis date to first epilepsy diagnosis date for PD and AD cases, and for controls as time from 1 January 2009 to first epilepsy diagnosis date. Patients were censored on mortality date or 31 December 2014. We then ran both cause-specific Cox regression and Fine and Gray subdistribution hazard models to measure the association of each neurodegenerative disease with epilepsy compared with controls while adjusting for patient risk factors [16]. Model variables were initially selected a priori based on clinical knowledge, review of existing literature and variable availability. The statistical analyses for our study were performed using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA). Figures 1 and 2 were generated using DistillerSR Forest Plot Generator from Evidence Partners.
Figure 1.
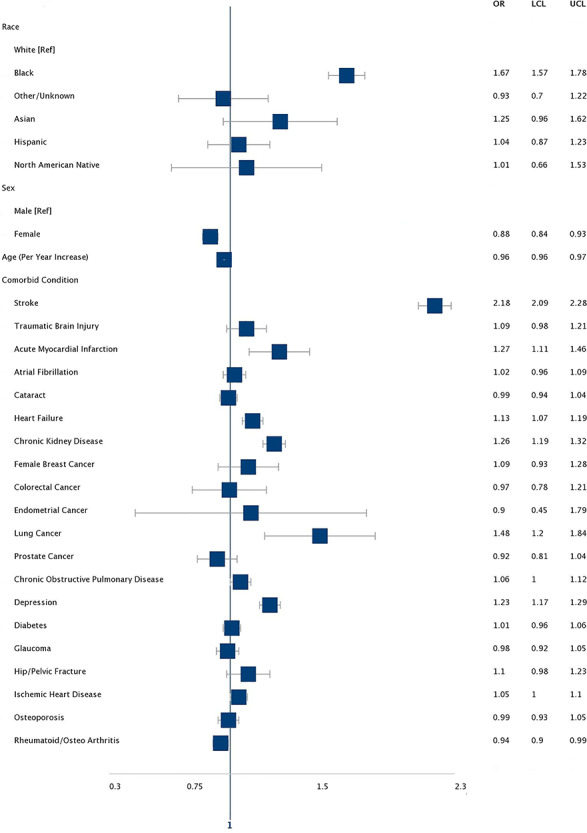
AHR for risk factors for incident epilepsy among medicare beneficiaries with AD. Asterisk denotes models included all the variables in the table.
Figure 2.
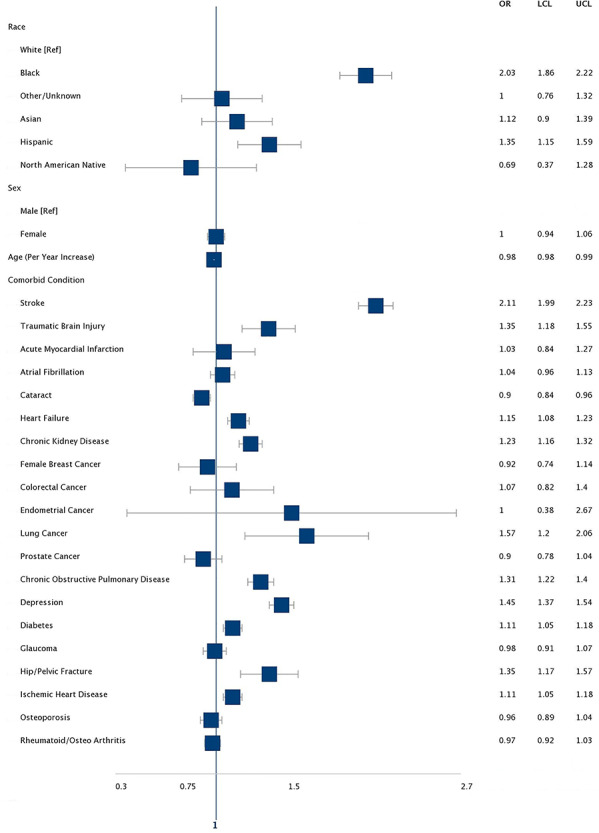
AHR for risk factors for incident epilepsy among medicare beneficiaries with PD. Asterisk denotes models included all the variables in the table.
Standard protocol approvals, registrations and patient consents
This study was approved by the University of Pennsylvania’s Institutional Review Board.
Data availability statement
Research level US Medicare data are available for purchase through an application process overseen by the Research Data Assistance Center [17].
Results
We identified a total of 34,054,293 people, ages 65 and above, who had been enrolled in Medicare for at least 2 years on 1 January 2009 and did not have concurrent Medicaid. Of these, 178,593 subjects had a new diagnosis of AD, and 104,157 subjects had a new diagnosis of PD in 2009. Over the 5 year follow-up period, 63,319 PD patients and 126,362 AD patients died, for a median follow-up time of 1,413 days for the PD cohort and 1,085 days for the AD cohort.
Alzheimer dementia
Table 1 displays baseline characteristics of individuals who were newly diagnosed with AD as compared with controls in our older adult sample. Medicare beneficiaries with AD were most frequently white (88.4%), female (65.3%) and were older (median age 84) than controls. Individuals with AD were more often diagnosed with every chronic condition investigated except endometrial cancer (0.1%), female breast cancer (1.9%) and cataract (19.7%).
Table 1.
Baseline characteristics of Medicare beneficiaries with AD and PD, 2009
| Neurodegenerative cohort | AD | PD | ||||
|---|---|---|---|---|---|---|
| Characteristic | Cases n, column % | Controls n, column % | P value, Chi-square | Cases n, column % | Controls n, column % | P value, Chi-square |
| Race | ||||||
| White | 157,848 (88.4) | 2,163,056 (85.4) | <0.0001 | 92,807 (89.1) | 2,520,294 (85.4) | <0.0001 |
| Black | 15,487 (8.7) | 231,671 (9.1) | 6,069 (5.8) | 271,482 (9.2) | ||
| Other/Unknown | 1,058 (0.6) | 38,609 (1.5) | 1,088 (1) | 43,258 (1.5) | ||
| Asian | 1,021 (0.6) | 40,114 (1.6) | 1,680 (1.6) | 45,697 (1.5) | ||
| Hispanic | 2,746 (1.5) | 46,091 (1.8) | 2,193 (2.1) | 55,002 (1.9) | ||
| North American native | 433 (0.2) | 12,803 (0.5) | 320 (0.3) | 14,584 (0.5) | ||
| Sex | ||||||
| Male | 61,977 (34.7) | 1,126,854 (44.5) | <0.0001 | 51,354 (49.3) | 1,281,053 (43.4) | <0.0001 |
| Female | 116,616 (65.3) | 1,405,490 (55.5) | 52,803 (50.7) | 1,669,264 (56.6) | ||
| Median age (IQR) | 84 (78–88) | 73 (68–80) | <0.0001 | 80 (75–86) | 74 (68–81) | <0.0001 |
| Comorbid condition | ||||||
| Stroke | 50,081 (28) | 314,697 (12.4) | <0.0001 | 30,032 (28.8) | 394,031 (13.4) | <0.0001 |
| Traumatic brain injury | 5,775 (3.2) | 20,018 (0.8) | <0.0001 | 2,777 (2.7) | 27,968 (0.9) | <0.0001 |
| Acute myocardial infarction | 4,915 (2.8) | 23,572 (0.9) | <0.0001 | 2020 (1.9) | 28,112 (1) | <0.0001 |
| Atrial fibrillation | 31,754 (17.8) | 202,180 (8) | <0.0001 | 16,184 (15.5) | 243,585 (8.3) | <0.0001 |
| Cataract | 35,133 (19.7) | 539,290 (21.3) | <0.0001 | 25,193 (24.2) | 625,957 (21.2) | <0.0001 |
| Heart failure | 67,202 (37.6) | 409,814 (16.2) | <0.0001 | 34,873 (33.5) | 499,754 (16.9) | <0.0001 |
| Chronic kidney disease | 50,787 (28.4) | 343,212 (13.6) | <0.0001 | 27,216 (26.1) | 413,549 (14) | <0.0001 |
| Female breast cancer | 3,399 (1.9) | 50,807 (2) | 0.0026 | 1778 (1.7) | 58,871 (2) | <0.0001 |
| Colorectal cancer | 2,253 (1.3) | 23,705 (0.9) | <0.0001 | 1,157 (1.1) | 27,695 (0.9) | <0.0001 |
| Endometrial cancer | 222 (0.1) | 4,113 (0.2) | <0.0001 | 85 (0.1) | 4,678 (0.2) | <0.0001 |
| Lung cancer | 2,142 (1.2) | 26,139 (1) | <0.0001 | 1,058 (1) | 29,835 (1) | 0.8859 |
| Prostate cancer | 6,038 (3.4) | 75,003 (3) | <0.0001 | 4,913 (4.7) | 86,315 (2.9) | <0.0001 |
| Chronic obstructive pulmonary disease | 37,223 (20.8) | 275,920 (10.9) | <0.0001 | 19,627 (18.8) | 328,114 (11.1) | <0.0001 |
| Depression | 66,371 (37.2) | 316,116 (12.5) | <0.0001 | 34,917 (33.5) | 388,131 (13.2) | <0.0001 |
| Diabetes | 59,703 (33.4) | 690,590 (27.3) | <0.0001 | 37,686 (36.2) | 812,920 (27.6) | <0.0001 |
| Glaucoma | 20,130 (11.3) | 266,017 (10.5) | <0.0001 | 12,228 (11.7) | 311,620 (10.6) | <0.0001 |
| Hip/Pelvic fracture | 9,698 (5.4) | 17,463 (0.7) | <0.0001 | 3,673 (3.5) | 23,398 (0.8) | <0.0001 |
| Ischemic heart disease | 94,178 (52.7) | 803,369 (31.7) | <0.0001 | 53,682 (51.5) | 958,641 (32.5) | <0.0001 |
| Osteoporosis | 40,200 (22.5) | 315,295 (12.5) | <0.0001 | 19,790 (19) | 380,435 (12.9) | <0.0001 |
| Rheumatoid arthritis/osteoarthritis | 64,516 (36.1) | 536,944 (21.2) | <0.0001 | 35,714 (34.3) | 644,015 (21.8) | <0.0001 |
IQR = Interquartile range.
In our sample of older persons with AD, 4.45% (n = 7,956) developed epilepsy over the 5-year follow-up period as compared with only 2.21% (n = 55,976) of controls. This produced an adjusted hazard ratio (AHR) of 2.46 (95% (confidence interval) CI: 2.39–2.52). In addition, while accounting for the competing risk of mortality, we found that AD resulted in an increase in the cumulative incidence of epilepsy (AHR 1.85; 95% CI: 1.80–1.90). Characteristics significantly associated with incident epilepsy included a history of stroke (AHR 2.18) or depression (AHR 1.23), as well as Black race category (AHR 1.67). Every year increase in age was associated with a lower adjusted risk of epilepsy diagnosis (AHR 0.96 95% CI: 0.96–0.97) (see Appendix 2, Appendix 3, and Figure 1). After removing 12,787 (7.2%) AD patients and 45,894 (1.8) controls with a PD diagnosis in subsequent years, our results were similar (Appendix 4 and 5).
Parkinson disease
Table 1 displays baseline characteristics of individuals who were newly diagnosed with PD as compared with controls in our older adult sample. PD patients were more likely to be white (89.1%), male (49.3%) and older (median age 80) than controls. They were more likely to have every chronic condition investigated except for endometrial cancer (0.1%) and female breast cancer (1.7%).
In this national sample of newly diagnosed PD, 4.81% (n = 5,010) developed epilepsy over the 5-year follow-up period as compared with only 2.40% (n = 70,772) of controls. This results in an AHR of 2.01 (95% CI: 1.95–2.07), whereas, while accounting for the competing risk of mortality, we found that PD resulted in an increase in the cumulative incidence of epilepsy with a subdistribution hazard ratio of 1.64 (95% CI: 1.59–1.69. Stroke (AHR 2.11), TBI (AHR 1.35), Chronic Obstructive Pulmonary Disease (COPD) (AHR 1.31), depression (AHR 1.45), hip/pelvic fracture (AHR 1.35) as well as Black (AHR 2.03) or Hispanic race/ethnicity (AHR 1.35) were significantly associated with an increased risk of incident epilepsy. Increasing age appeared to lower epilepsy risk (AHR per year of age 0.98; 95% CI: 0.98–0.99). (Appendix 2, Appendix 3, and Figure 2). After removing 22,134 (21.3%) PD patients and 177,537 (6.0) controls with an AD diagnosis in subsequent years our results were similar (Appendix 4 and 5).
Discussion
In this study, we describe 5-year epilepsy incidence rates among Medicare beneficiaries with AD and PD that are almost double the rate in those without a neurodegenerative diagnosis. This description in a national sample of an increased rate of incident epilepsy is similar to that observed in other PD populations [18]. Prior cohort studies in AD cohorts more frequently examined a diagnosis of seizure rather than epilepsy, and this may account for the lower hazard ratio we found (around 1.9 as compared with prior reports up to 8) [19, 20]. Our findings in a large national sample; however, highlight the need for better characterization of the burden of epilepsy in an ageing population where neurodegenerative disease is expected to become increasingly prevalent.
Understanding the distribution of the burden of epilepsy in neurodegenerative populations is acutely important not only because of the expected growth of this segment of the population, but also because epilepsy treatment is of great clinical importance to these patients. Patients with dementia and other degenerative conditions may experience both non-epileptic staring or confusional episodes as well as seizures, and these events can lead to prescription of ineffective anti-epileptic drugs and often to emergency department visits and/or hospitalizations. Furthermore, there is a clear association between outpatient epilepsy care quality and health outcomes, suggesting that prompt identification and treatment of epilepsy in these patients can improve their health outcomes [3, 4].
Our study included all Medicare beneficiaries regardless of functional status and focused on neurodegenerative disease population. In this study, we did find an elevated risk of epilepsy associated with black race, which has been found in a community-dwelling study of older patients [21]. There was also no evidence of sex differences in epilepsy risk in the PD population. The different study design and sample populations likely explain the difference in findings and highlight the need to further explore the aetiology of the epilepsy in neurodegenerative populations with focus on race and sex subgroups. We found that age was not associated with increased risk of epilepsy incidence when neurodegenerative disease was also present. This inverse association with age may be because patients who are diagnosed at younger ages are more likely to have more rapidly progressive versions of their respective neurodegenerative disease and therefore more likely to develop epilepsy at younger ages. Alternatively, the risk of mortality with age may lead to censoring before the development of epilepsy. Ultimately, further studies with information on individual disease severity information will be helpful to clarify the reason for this inverse association.
We found that traditional risk factors such as stroke and TBI are associated with incident epilepsy in the PD population. Interestingly, our results highlight co-morbid depression as a risk factor for a future diagnosis of epilepsy in both PD and AD patients. Depression is known to be associated with PD, but occurs at any stage of the disease and can pre-date motor symptoms [22]. Similarly, depression has long been associated with AD and is associated with increased risk of development of AD. Our results showing increased risk of developing epilepsy in AD and PD patient with depression support the idea that depression is a result of neurodegeneration and that depression may be a marker of increased disease burden and global brain network disruption.
Limitations
This study is unique in that it uses a national sample of US adults over age 65 to describe 5-year epilepsy incidence in two neurodegenerative populations—AD and PD, provides evidence that epilepsy incidence is significantly increased, and presents an opportunity to improve outcomes in our ageing population. This study, however, has several possible limitations. First, our study assessed the Medicare population during 2009–14, and may not reflect the current experience. In addition, administrative claims data contain codes produced for billing and documentation purposes, and so, do not allow for a detailed examination of socioeconomic background, functional status or disease severity, which would all be helpful factors in understanding the variance in epilepsy burden in this population. Furthermore, administrative databases do not allow for the granular diagnostic information available in clinical notes, so while validated ICD-9-CM based algorithms were used to identify new epilepsy, we suspect that these may still be underrepresented. Furthermore, in order to have a more homogenous cohort, we removed cases and controls who later developed a second neurogenerative disease (AD or PD) although our sensitivity analysis suggests this did not significantly impact our results. Finally, there is a known lag-time between epilepsy onset and diagnosis [23, 24]. Our results, therefore, can only describe epilepsy diagnosis, which is most likely not true disease onset.
Conclusions
We assessed a large cohort of Medicare beneficiaries and present national data on epilepsy incidence in older adults with new-onset AD or PD. As the population continues to age, we should expect a greater burden of epilepsy among older adults. In particular, persons with neurogenerative disease will be disproportionately affected. These findings can inform the further study of the complex treatment regimens needed for multiple neurologic comorbidities in an aged population. Such epidemiologic knowledge will provide the foundation for the thoughtful design of future healthcare delivery models that will incorporate real-time data with multi-disciplinary, practice-changing interventions to improve outcomes in older adults suffering from neurodegeneration and epilepsy.
Supplementary Material
Contributor Information
Leah J Blank, Department of Neurology, Division of Health Outcomes and Knowledge Translational Research, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Emily K Acton, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Neurology Translational Center of Excellence for Neuroepidemiology and Neurological Outcomes Research, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Dylan Thibault, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Neurology Translational Center of Excellence for Neuroepidemiology and Neurological Outcomes Research, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Allison W Willis, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Neurology Translational Center of Excellence for Neuroepidemiology and Neurological Outcomes Research, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Leonard Davis Institute of Health Economics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The first author was supported by the Mirowski Family Fund as well as the Pharmacoepidemiology Training Grant (T32-NS-061779) and the Department of Neurology at the University of Pennsylvania.
References
- 1. Sillanpää M, Lastunen S, Helenius H, Schmidt D. Regional differences and secular trends in the incidence of epilepsy in Finland: a nationwide 23-year registry study. Epilepsia 2011; 52: 1857–67. [DOI] [PubMed] [Google Scholar]
- 2. Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States Population Estimates and Projections. Curerent Population Reports; 2014. www.census.gov/population (4 March 2019. date last accessed).
- 3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001; 345: 311–8. [DOI] [PubMed] [Google Scholar]
- 4. Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology 2016; 86: 1938–44. [DOI] [PubMed] [Google Scholar]
- 5. Granbichler CA, Oberaigner W, Kuchukhidze G et al. Decrease in mortality of adult epilepsy patients since 1980: lessons learned from a hospital-based cohort. Eur J Neurol 2017; 24: 667–72. [DOI] [PubMed] [Google Scholar]
- 6. Vossler DG, Weingarten M, Gidal BE. American Epilepsy Society treatments committee the AEST. Summary of antiepileptic drugs available in the United States of America: WORKING TOWARD A WORLD WITHOUT EPILEPSY. Epilepsy Curr 2018; 18: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrell MJ. RNS system in epilepsy study group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77: 1295–304. [DOI] [PubMed] [Google Scholar]
- 8. Salanova V, Witt T, Worth R et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015; 84: 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011; 77: 851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odenheimer G, Borson S, Sanders AE et al. Quality improvement in neurology: dementia management quality measures. Neurology 2013; 81: 1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reuben DB, Roth CP, Frank JC et al. Assessing care of vulnerable Elders-Alzheimer’s disease: a pilot study of a practice redesign intervention to improve the quality of dementia care. J Am Geriatr Soc 2010; 58: 324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology 2006; 67: 1592–9. [DOI] [PubMed] [Google Scholar]
- 13. Erdem E, Korda H, Haffer SC, Sennett C. Medicare claims data as public use files: a new tool for public health surveillance. J Public Health Manag Pract 2014; 20: 445–52. [DOI] [PubMed] [Google Scholar]
- 14. Tu K, Wang M, Jaakkimainen RL et al. Assessing the validity of using administrative data to identify patients with epilepsy. Epilepsia 2014; 55: 335–43. [DOI] [PubMed] [Google Scholar]
- 15. Jette N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia 2010; 51: 62–9. [DOI] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 17. ResDAC . Research Data Assistance Center. https://www.resdac.org/ (13 February 13 2019, date last accessed).
- 18. Gruntz K, Bloechliger M, Becker C, et al. Parkinson disease and the risk of epileptic seizures 2018; 83: 363–74. [DOI] [PubMed] [Google Scholar]
- 19. Irizarry MC, Jin S, He F et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 2012; 69: 368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarmeas N, Honig LS, Choi H et al. Seizures in Alzheimer disease. Arch Neurol 2009; 66: 992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain SA, Haut SR, Lipton RB, Derby C, Markowitz SY, Shinnar S. Incidence of epilepsy in a racially diverse, community-dwelling, elderly cohort: results from the Einstein aging study. Epilepsy Res 2006; 71: 195–205. [DOI] [PubMed] [Google Scholar]
- 22. Marsh L. Depression and Parkinson’s disease: current knowledge. Curr Neurol Neurosci Rep 2013; 13: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martínez-Juárez IE, Funes B, Moreno-Castellanos JC et al. A comparison of waiting times for assessment and epilepsy surgery between a Canadian and a Mexican referral center. Epilepsia Open 2017; 2: 453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia 2001; 42: 464–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research level US Medicare data are available for purchase through an application process overseen by the Research Data Assistance Center [17].


