Abstract
Cadmium is toxic to the ovaries in animal studies, but its association with diminished ovarian reserve in women is not established. We investigated urinary cadmium, a biomarker of long-term exposure, in relation to diminished ovarian reserve, as indicated by elevated serum follicle-stimulating hormone concentrations (≥10 IU/L), in women aged 35–49 years (unweighted n = 1,681). Using data from the Third National Health and Nutrition Examination Survey (1988–1994), we conducted Poisson regression to estimate adjusted relative risks and 95% confidence intervals. Because the best approach to correcting for urinary dilution in spot samples with creatinine remains controversial, we employed 3 approaches: standardization, covariate adjustment, and covariate-adjusted standardization. Our data suggested a modest association with standardization (highest quartile vs. lowest: relative risk (RR) = 1.3, 95% confidence interval (CI): 0.8, 1.9; P for trend = 0.06) and covariate-adjusted standardization (highest quartile vs. lowest: RR = 1.3, 95% CI: 0.9, 1.9; P for trend = 0.05) and a stronger association with covariate adjustment (highest quartile vs. lowest: RR = 1.8, 95% CI: 1.2, 2.9; P for trend = 0.01). The stronger association with covariate adjustment may reflect bias from conditioning on urinary creatinine, a collider in the hypothesized causal pathway. We conclude that cadmium may contribute to ovarian aging in women and that careful consideration of the creatinine adjustment approach is needed to minimize bias.
Keywords: cadmium, creatinine adjustment, follicle-stimulating hormone, ovarian reserve
Abbreviations
- AMH
anti-Müllerian hormone
- BIA
bioelectrical impedance analysis
- CI
confidence interval
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- NHANES III
Third National Health and Nutrition Examination Survey
- RR
relative risk
Editor’s note: An invited commentary on this article appears on page 125.
The ovarian reserve comprises a pool of primordial follicles and is central to the longevity of ovarian function (1); depletion of this ovarian follicle pool with ovarian aging leads to infertility and menopause (1, 2). The loss of primordial follicles in reproductive-age women occurs from the continuous activation of primordial follicles and the subsequent atresia of all but a small fraction that become the ovulatory follicles of each menstrual cycle (1, 3, 4). Primordial follicle loss can also occur with exposure to ovarian toxicants. For example, substantial loss of primordial follicles has been observed with exposure to the chemotherapeutic agent cyclophosphamide (5, 6); this loss is associated with an increased incidence of premature ovarian failure and earlier menopause (7–9), placing women at risk for conditions such as cardiovascular disease and osteoporosis at a younger age (10, 11). Chronic exposure to low doses of environmental contaminants that are toxic to ovarian primordial follicles may also hasten the depletion of the ovarian reserve.
One such environmental contaminant that may adversely affect ovarian reserve is cadmium. Cadmium is a toxic metal that is released into the environment through human industrial activities; it strongly binds to organic matter in soil and is subsequently taken up by plants (12). Cadmium released to water accumulates in aquatic organisms (12). As a result, exposure to cadmium in the United States is common. The US general population is primarily exposed through the inhalation of cigarette smoke (from cadmium in tobacco leaves) and the ingestion of contaminated foods, such as leafy vegetables, grains, peanuts, sunflower seeds, soybeans, shellfish, and organ meat (12–14). Limited data from animal studies suggest that exposure to cadmium can result in the destruction of primordial and primary follicles (15–17). In women, cadmium has been detected in ovarian tissue and follicular fluid (18–20) and appears to accumulate within this organ (19).
Few human studies have investigated cadmium in relation to ovarian reserve. Two widely used biomarkers of ovarian reserve are anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) (21). AMH is secreted from small ovarian preantral and antral follicles, and AMH concentrations decline with the depletion of the primordial follicle pool (21). FSH is inhibited by inhibin B and estradiol secreted from growing ovarian follicles; the decrease in production of these ovarian hormones with follicular pool depletion results in elevated FSH secretion (22). Although several studies have investigated cadmium in relation to reproductive hormones, including AMH and FSH (23–28), most of these studies examined reproductive hormones to understand the role of cadmium in hormonal alterations and menstrual cycle function (23, 25, 28) or in the etiology of bone health (24) and breast cancer (27). To our knowledge, only 1 study was designed to evaluate cadmium in relation to ovarian reserve. That study, conducted among 283 premenopausal women in South Korea, found a decline in ovarian reserve (as measured by AMH) with increasing blood cadmium concentrations (26).
Since the measurement of cadmium in blood captures both recent and chronic exposure (29), we were interested in further investigating the association between cadmium and ovarian reserve using a long-term marker of cadmium exposure—cadmium measured in urine. Cadmium accumulates in the body, particularly the liver and kidneys, due to the lack of efficient excretion mechanisms (12); it has an estimated biological half-life in the kidneys of 10–30 years (30). The purpose of this study was to evaluate urinary cadmium in relation to serum FSH in a nationally representative sample of US women.
Because the best approach to correcting for urinary dilution in spot urine samples with urinary creatinine remains controversial, we additionally compared the estimates of the cadmium-FSH association obtained using 3 approaches—standardization, covariate adjustment, and covariate-adjusted standardization. Urinary dilution correction methods are performed to account for differences in environmental contaminant concentrations attributable to interindividual variation in urine concentrations due to hydration status at the time of spot urine sample collection (31). However, a recent study suggested that standardization and covariate adjustment, 2 commonly used approaches, may result in biased results in causal scenarios where disease risk factors also affect creatinine concentrations (32). Standardization is the division of the environmental contaminant concentration by the concentration of creatinine; this method assumes constant excretion of urinary creatinine across individuals and may introduce bias from interindividual variations in urinary creatinine not due to hydration (32). Covariate adjustment is implemented by including creatinine as a covariate in the regression model; this method assumes that creatinine operates as a confounder of the exposure-outcome relationship. However, in some causal scenarios creatinine may instead act as a collider (32, 33), a variable affected by 2 other variables on a causal pathway whose adjustment may result in confounding (34). This could occur if urinary creatinine is affected by other relevant factors (e.g., age and race) related to the outcome of interest, in addition to hydration. To avoid these potential sources of bias, a new method was recently developed: covariate-adjusted standardization (32). This method standardizes the environmental contaminant concentration by the estimated proportion of creatinine solely attributable to hydration.
METHODS
Data source
We used data from the Third National Health and Nutrition Examination Survey (NHANES III), a national, cross-sectional study conducted in 1988–1994 by the National Center for Health Statistics (35). NHANES III was designed to measure the health and nutritional status of a nationwide sample of noninstitutionalized US civilians aged 2 months or more. Data collection in NHANES III included a standardized in-person interview, biological sample collection, a physical examination, and laboratory procedures and has been described in detail elsewhere (35, 36).
Study population
Our strategy for the selection of the study population was similar to that employed in a prior study of demographic, reproductive, and behavioral characteristics and elevated FSH using data from NHANES III (37). We limited our study population to women aged 35–49 years (unweighted n = 2,205) given our interest in ovarian aging before the median age of natural menopause in industrialized countries (ages 50–52 years) (38) and included both premenopausal and postmenopausal women. Postmenopausal women were included because the measurement of cadmium in urine is indicative of long-term exposure and likely characterizes exposure before the rise in FSH with ovarian aging. We excluded women with factors that precluded the assessment of FSH: women with a history of bilateral oophorectomy (n = 132) or whose status was missing (n = 1) or uncertain (women who had a hysterectomy but were missing oophorectomy data) (n = 7); those who were currently pregnant (n = 23), were breastfeeding (n = 15), or had taken oral contraceptives in the last 3 months (n = 82); and those whose menses had ceased because of chemotherapy or radiation (n = 9). We also excluded women with missing data on FSH, luteinizing hormone (LH), cadmium, or creatinine level (n = 185) and those with an estimated glomerular filtration rate less than 60 mL/minute/1.73 m2, which is indicative of chronic kidney disease (n = 9) (39). Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (39). In the equation, we used available NHANES III data on serum creatinine, age, and race, and we calibrated the serum creatinine values as previously described (40).
Since the collection of blood samples in NHANES III was not timed to the menstrual cycle, we excluded women with LH:FSH ratios greater than 2, in whom an increase in FSH may reflect the gonadotropin surge that precedes ovulation (n = 61) (37). We included women reporting use of hormone replacement therapy, because hormone replacement therapy does not suppress FSH levels into the normal physiological range (41). In addition, information on hormone replacement therapy was only ascertained in a subset of women (18%) who reported a history of hysterectomy or no menses in the previous 2 months (and were not currently pregnant or breastfeeding). After all of the exclusions, the unweighted sample size for this study was 1,681, of whom 65 women were postmenopausal.
Urinary cadmium and creatinine measurement
Spot urine samples were collected from study participants and measured for concentrations of cadmium by the Nutritional Biochemistry Branch of the Division of Environmental Health Laboratory Sciences at the Centers for Disease Control and Prevention using atomic absorption spectrometry with Zeeman background correction (36, 42). The limit of detection was 0.01 ng/mL (43); none of the laboratory-reported values were below the detection limit.
Creatinine concentration (mg/dL) was measured in urine at the University of Minnesota School of Medicine (Department of Pediatrics; Minneapolis, Minnesota) on the basis of a Jaffé rate reaction using a Beckman Synchron AS/ASTRA clinical analyzer (Beckman Coulter Inc., Brea, California) (36). Although the limit of detection was 1 mg/dL, values less than 10 mg/dL were considered “statistically suspect,” and the laboratory substituted the value of 7.9 mg/dL for them (unweighted n = 10) (43).
FSH and LH measurement
Serum FSH and LH were measured in NHANES III by the Department of Endocrinology at the University of Massachusetts Medical Center (Worcester, Massachusetts) using an immunoradiometric assay (FSH MAIAclone and LH MAIAclone; Ciba Corning Diagnostic Corporation, East Walpole, Massachusetts) (36). The limit of detection for FSH and LH was 0.15 IU/L (43). FSH and LH were detected in nearly all study participants; values less than the limit of detection were replaced by the value of the limit of detection divided by the square root of 2 (for FSH, unweighted n = 1; for LH, unweighted n = 2) (44).
Statistical analyses
Given the cross-sectional study design and the inclusion of both premenopausal and postmenopausal women, we modeled FSH as a binary outcome to characterize the rise in FSH with ovarian aging. We defined an elevated FSH level as a concentration greater than or equal to 10 IU/L based on the use of this cutpoint in clinical practice to signify diminished ovarian reserve (45). To directly estimate the relative risk and 95% confidence interval for the association between urinary cadmium level and elevated FSH, we used Poisson regression, accounting for the complex survey sampling design. Since the dose-response relationship may be nonlinear, we categorized urinary cadmium into quartiles using the weighted distribution among the study population. We selected factors for adjustment a priori using a directed acyclic graph (Figure 1) and adjusted for age at sample collection (years; continuous), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican-American, or other), and smoking status (never smoker, former smoker, current smoker of <20 cigarettes/day, or current smoker of ≥20 cigarettes/day). We additionally adjusted for history of unilateral oophorectomy (yes, no) and history of hysterectomy (yes, no), since both factors are associated with increased FSH concentrations (46).
Figure 1.
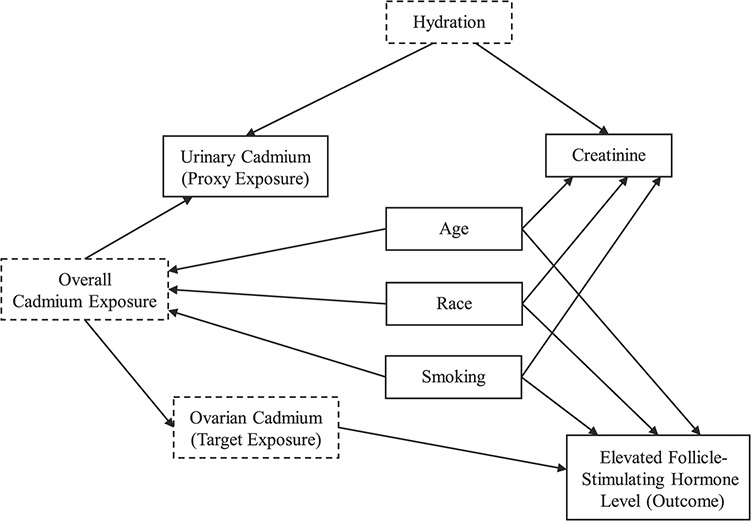
Directed acyclic graph of the hypothesized causal pathway between urinary cadmium concentrations and elevated follicle-stimulating hormone concentrations, Third National Health and Nutrition Examination Survey, 1988–1994. Variables with solid lines were observed; those with dashed lines were unobserved. For simplicity, unilateral oophorectomy and hysterectomy, which were hypothesized to directly affect follicle-stimulating hormone levels, are not included in the figure.
We corrected for urinary dilution using urinary creatinine concentration, by applying 3 methods. First, we used standardization, in which we divided the cadmium concentrations (ng/mL) by the observed creatinine concentrations (mg/dL) and multiplied by 100. Second, we adjusted for creatinine as a continuous covariate in the multivariable-adjusted regression model of cadmium (ng/mL) and elevated FSH. Third, we used covariate-adjusted standardization (32). For this method, we first fitted a model of the natural logarithm of creatinine as a function of covariates that chronically and directly affect creatinine outside of hydration (47, 48): age at sample collection (years; continuous), fat-free mass (kg; continuous), body mass index (weight (kg)/height (m)2; continuous), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican-American, or other), smoking status (never smoker, former smoker, current smoker of <20 cigarettes/day, or current smoker of ≥20 cigarettes/day), alcohol consumption (never drinker, former drinker, or current drinker), frequency of physical exercise (0, < 3, or ≥3 times per week), and reported history of diabetes when not pregnant (yes, no). The categories of alcohol consumption were determined using data on the ever consumption of at least 12 alcoholic drinks and the consumption of at least 12 drinks in the past 12 months. Weekly exercise frequency was calculated by summing the frequency of 8 activities over the prior month (jogging, bicycling, swimming, aerobics/aerobic dancing, other dancing, calisthenics, gardening, and weight-lifting) and dividing by 4. Fat-free mass was estimated using an established equation for females (−9.529 + (0.168 × weight) + (0.696 × (height2/resistance)) + 0.016 × resistance), where resistance is estimated by bioelectrical impedance analysis (BIA) using the RJL BIA instrumentation (RJL Systems, Inc., Clinton Township, Michigan) (49). Given that BIA was measured in NHANES III using the Valhalla 1990B BIA instrument (Valhalla Scientific, San Diego, California), we converted these BIA resistance values to the equivalent RJL 101 BIA instrument resistance values using the equation for females (RJL resistance = 9.6 + 0.96 Valhalla resistance) (50). From the fitted model, we estimated the linear prediction of the log creatinine values and exponentiated these predicted log values to obtain the fitted creatinine values. Lastly, we calculated the ratio of observed creatinine to fitted creatinine and divided the cadmium concentrations by this ratio.
Given that creatinine may operate as a collider on the path between the exposure and outcome (Figure 1) and that adjustment may induce bias, we conducted an exploratory analysis with no adjustment for urinary creatinine concentration. We also explored the functional form of the dose-response relationship between cadmium and FSH using restricted cubic splines, with knots at the 5th, 35th, 65th, and 95th percentiles of the weighted urinary cadmium distribution and the first percentile designated as the reference urinary cadmium concentration; this analysis was conducted with all 3 approaches to correct for urinary dilution.
We conducted 2 sensitivity analyses. First, since the inhalation of cigarette smoke is a strong contributor to the body burden of cadmium and is toxic to ovarian primordial follicles (12, 51), we repeated the analyses among study participants who had not smoked cigarettes in the prior 10 years (unweighted n = 1,105). Second, to understand the impact of including postmenopausal women on our results, we repeated the analyses restricting the study population to premenopausal women (97% of the original study population; unweighted n = 1,615).
All analyses were conducted using Stata 14.2 (StataCorp LLC, College Station, Texas) using survey procedure commands to account for the complex survey sampling design and weighting of data. We considered α = 0.05 the level of statistical significance in all analyses.
RESULTS
In our study population, the geometric mean concentration of cadmium was 0.30 ng/mL (95% confidence interval (CI): 0.28, 0.32), and 26% of women had an elevated FSH level. Women with an elevated FSH level were more likely to be aged 45–49 years, to be non-Hispanic Black, to have 12 or fewer years of education, and to have a lower family income. These participants were also more likely to be former consumers of alcohol, to be current smokers of 20 or more cigarettes per day, to be menopausal, and to report a history of unilateral oophorectomy and/or hysterectomy and less likely to exercise 3 or more times per week in comparison with women whose FSH level was not elevated (Table 1).
Table 1.
Characteristics (%a) of Participants According to Elevated (≥10 IU/L) Follicle-Stimulating Hormone Level, Third National Health and Nutrition Examination Survey, 1988–1994
| FSH Level | ||
|---|---|---|
| Participant Characteristic | ≥10 IU/L | <10 IU/L |
| Age at sample collection, years | ||
| 35–39 | 12 | 45 |
| 40–44 | 33 | 39 |
| 45–49 | 55 | 17 |
| Race/ethnicity | ||
| Non-Hispanic White | 74 | 75 |
| Non-Hispanic Black | 13 | 10 |
| Mexican-American | 4 | 5 |
| Other | 9 | 10 |
| Education, years | ||
| <12 | 21 | 14 |
| 12 | 38 | 34 |
| >12 | 41 | 52 |
| Family income, dollarsb | ||
| <20,000 | 26 | 21 |
| ≥20,000 | 74 | 79 |
| Alcohol consumptionc | ||
| Never drinker | 16 | 15 |
| Former drinker | 44 | 34 |
| Current drinker | 41 | 51 |
| Smoking statusd | ||
| Never smoker | 43 | 57 |
| Former smoker | 22 | 22 |
| Current smoker, cigarettes/day | ||
| <20 | 13 | 10 |
| ≥20 | 22 | 11 |
| Physical exercise, times/week | ||
| 0 | 31 | 26 |
| <3 | 43 | 40 |
| ≥3 | 26 | 34 |
| Body mass indexe | ||
| <25.0 | 26 | 24 |
| 25.0–29.9 | 44 | 49 |
| ≥30.0 | 30 | 26 |
| Age at menarche, years | ||
| ≤11 | 24 | 21 |
| 12 | 22 | 29 |
| 13 | 26 | 23 |
| ≥14 | 28 | 27 |
| No. of live births | ||
| 0 | 16 | 18 |
| 1 | 13 | 13 |
| 2 | 35 | 37 |
| ≥3 | 37 | 32 |
| Unilateral oophorectomy | ||
| Yes | 11 | 4 |
| No | 89 | 96 |
| Hysterectomy | ||
| Yes | 21 | 9 |
| No | 79 | 91 |
| Postmenopausal status | ||
| Yes | 12 | 0.3 |
| No | 88 | 99.7 |
Abbreviation: FSH, follicle-stimulating hormone.
a Weighted percentage accounting for the complex survey sampling design, not including missing data. Some data were missing (unweighted n’s) for education (n = 5), family income (n = 23), smoking (n = 7), body mass index (n = 1), age at menarche (n = 14), unilateral oophorectomy (n = 5), hysterectomy (n = 2), and postmenopausal status (n = 1).
b Total combined family income in the last 12 months.
c Participants were considered never drinkers if they had never consumed at least 12 alcoholic drinks; former drinkers were defined as those who had ever consumed at least 12 drinks but reported not consuming at least 12 drinks in the past 12 months; and participants were defined as current drinkers if they had consumed at least 12 drinks in the past 12 months.
d Participants were considered never smokers if they had never smoked at least 100 cigarettes in their lifetime; former smokers were defined as those who had ever smoked at least 100 cigarettes but reported having quit smoking.
e Weight (kg)/height (m)2.
Our data suggested a positive association between quartiles of urinary cadmium concentration and elevated FSH using any of the 3 approaches to correct for urinary dilution (Table 2). However, the magnitude of the association varied, depending on the method used for correction. We observed modest associations when we used standardization (highest quartile vs. lowest: relative risk (RR) = 1.3, 95% CI: 0.8, 1.9; P for trend = 0.06) and covariate-adjusted standardization (highest quartile vs. lowest: RR = 1.3, 95% CI: 0.9, 1.9; P for trend = 0.05). The association was stronger in magnitude when creatinine was included in the regression model as a covariate (highest quartile vs. lowest: RR = 1.8, 95% CI: 1.2, 2.9; P for trend = 0.01).
Table 2.
Associations Between Quartiles of Urinary Cadmium Concentration and Elevated (≥10 IU/L) Follicle-Stimulating Hormone Level Among Women Aged 35–49 Years (Unweighted n = 1,681), Considering 3 Approaches to Creatinine Adjustment, Third National Health and Nutrition Examination Survey, 1988–1994
| FSH Level, % a | Age-Adjusted Model b | Multivariable-Adjusted Model c | ||||
|---|---|---|---|---|---|---|
|
Type of Adjustment and Quartile of Exposure |
≥10 IU/L | <10 IU/L | RR | 95% CI | RR | 95% CI |
| Standardized cadmium concentration, μg/gd | ||||||
| Q1 (<0.24) | 18 | 28 | 1.0 | Referent | 1.0 | Referent |
| Q2 (0.24–0.41) | 17 | 28 | 0.9 | 0.6, 1.4 | 0.9 | 0.6, 1.4 |
| Q3 (0.41–0.72)e | 28 | 24 | 1.3 | 0.9, 2.0 | 1.2 | 0.8, 1.7 |
| Q4 (>0.72) | 37 | 21 | 1.6 | 1.1, 2.3 | 1.3 | 0.8, 1.9 |
| P for trend | <0.001 | 0.06 | ||||
| Covariate adjustment, ng/mLf | ||||||
| Q1 (<0.16) | 17 | 26 | 1.0 | Referent | 1.0 | Referent |
| Q2 (0.16–0.38) | 24 | 28 | 1.4 | 0.9, 2.3 | 1.4 | 0.9, 2.2 |
| Q3 (0.39–0.77) | 27 | 24 | 1.8 | 1.2, 2.7 | 1.6 | 1.0, 2.3 |
| Q4 (>0.77) | 31 | 23 | 2.3 | 1.5, 3.6 | 1.8 | 1.2, 2.9 |
| P for trend | <0.001 | 0.01 | ||||
| Covariate-adjusted standardization, ng/mLg | ||||||
| Q1 (<0.181) | 19 | 27 | 1.0 | Referent | 1.0 | Referent |
| Q2 (0.181–0.333)h | 17 | 28 | 0.9 | 0.6, 1.5 | 0.9 | 0.6, 1.4 |
| Q3 (0.333–0.608) | 29 | 24 | 1.4 | 1.0, 2.1 | 1.3 | 0.9, 1.9 |
| Q4 (>0.608) | 35 | 21 | 1.6 | 1.2, 2.3 | 1.3 | 0.9, 1.9 |
| P for trend | <0.001 | 0.05 | ||||
Abbreviations: CI, confidence interval; FSH, follicle-stimulating hormone; Q, quartile; RR, relative risk.
a Weighted percentage incorporating the complex survey sampling design.
b Results were adjusted for exact age at sample collection (years; continuous).
c Results were adjusted for exact age at sample collection, race/ethnicity, smoking status, history of unilateral oophorectomy, and history of hysterectomy.
d Cadmium concentrations were divided by urinary creatinine concentrations and multiplied by 100.
e The lower value for quartile 3 was 0.4103 before rounding.
f Urinary creatinine was included in the regression model as a covariate.
g Cadmium concentrations were divided by the ratio of observed creatinine concentrations and predicted creatinine concentrations. Predicted creatinine concentrations were determined by fitting a model for ln-transformed creatinine as a function of exact age at sample collection, fat-free mass, body mass index, race/ethnicity, smoking, alcohol consumption, frequency of exercise in the past month, and history of nongestational diabetes.
h The upper value for quartile 2 was 0.3326 before rounding.
In our exploratory analyses without creatinine adjustment (see Web Table 1, available at https://academic.oup.com/aje), we observed estimates of association similar to those obtained using standardization and covariate-adjusted standardization. When we repeated the analyses using restricted cubic splines, the dose-response relationship was similar to that characterized using quartiles (Web Figure 1).
In our sensitivity analyses restricting the study population to participants who had not smoked in the prior 10 years, the impact of restriction on the estimates of association appeared to differ by the approach used for urinary creatinine adjustment (Web Table 2). The associations were attenuated when using standardization and covariate-adjusted standardization. In contrast, the magnitude of association from the multivariable-adjusted analysis appeared somewhat stronger when using covariate adjustment. When we restricted the study population to premenopausal women, our estimates of the association between urinary cadmium and elevated FSH were similar to those obtained in the entire study population, across all the methods used for creatinine adjustment (Web Table 3).
DISCUSSION
Regardless of the approach used to correct for urinary dilution using urinary creatinine, our data suggested a positive association between urinary cadmium concentration and elevated FSH levels. This association is biologically plausible. Limited animal studies investigating the direct effects of cadmium on ovarian tissue have observed a loss of primordial and primary ovarian follicles and an increased proportion of atretic follicles after the administration of cadmium (15–17).
Our findings are consistent with the only prior study designed to evaluate exposure to cadmium in relation to the ovarian reserve (26). In a South Korean population of 283 premenopausal women aged 30–45 years, Lee et al. (26) reported a decline in ovarian reserve with increasing concentrations of blood cadmium. That study used AMH as the biomarker of ovarian reserve. AMH is secreted by small granulosa cells in the ovary and is largely considered gonadotropin-independent (52), which allows it to be measured with oral contraceptive use, with pregnancy, and without regard to menstrual cycle timing (53). However, measurement of AMH is limited by the lack of an international standardized assay (21).
We observed that the magnitude of the estimated association between urinary cadmium and elevated FSH depended on the approach used to correct for urinary dilution using urinary creatinine. We observed a modest association when we corrected for urinary dilution using standardization. Standardization assumes that the excretion of urinary creatinine is similar across individuals. However, creatinine, an end product of creatine phosphate metabolism in the skeletal muscle (48), varies with skeletal muscle mass and related factors, such as age, race, body mass index, and fat-free mass (47, 48). Using standardization, bias may be introduced by measurement error from the person-to-person variations in urinary creatinine excretion, outside of hydration.
In contrast, when creatinine was included in the model as a covariate, we observed estimates of the association that were stronger in magnitude than those obtained using the other 2 approaches. The inclusion of urinary creatinine as a covariate in the regression model has been recommended in order to evaluate the independent effect of an environmental contaminant and to avoid potential bias that may occur with standardization due to urinary creatinine’s being associated with the outcome (47). However, O’Brien et al. (32) proposed causal scenarios in which creatinine does not operate as a confounder but instead operates as a collider on the path between environmental contaminant exposure and disease. Applying that conceptual framework to our study (Figure 1), controlling for creatinine as a covariate in the regression model may induce bias by opening several backdoor paths between cadmium and FSH, through hydration, age, race, and smoking. In our exploratory analysis with no adjustment for creatinine, we observed estimates of the association similar to those obtained using standardization and covariate-adjusted standardization. The adjustment for variables on the backdoor paths opened by adjusting for a collider may not have been adequate to minimize bias if other open backdoor paths remain from unmeasured variables that are common ancestors of creatinine and FSH. In addition, covariate adjustment (or no adjustment for creatinine) does not control for interindividual variations in urinary creatinine excretion (32). Hence, the possibility exists that the greater magnitude of association observed with covariate adjustment is due to bias from adjusting for a collider and residual measurement error.
Covariate-adjusted standardization was developed to avoid sources of bias that may arise with standardization and covariate adjustment in specific causal scenarios where disease risk factors are associated with creatinine concentrations (32). The method does not condition on urinary creatinine (a potential collider) and accounts for the between-subject variation in creatinine concentrations, outside of hydration. Despite the methodological advancement of this approach, in our study we observed similar estimates of association using standardization and covariate-adjusted standardization. Our ability to detect a difference between methods may have been limited by the examination of a relatively homogeneous study population with regard to sex and age (i.e., women aged 35–49 years) and the extent to which the known predictors of urinary creatinine measured in NHANES III could isolate the proportion of creatinine solely attributable to hydration (32).
Our study had several limitations. First, we did not have data on other measures of urinary dilution. The comparison of our results with those obtained using other urinary metrics that may minimize measurement error under a similar causal scenario, such as urinary flow rates and cadmium excretion rates (33), would have further informed our investigation of the association between urinary cadmium and FSH. Second, cadmium is a known nephrotoxicant, and its impact on renal function may affect the concentrations of cadmium and creatinine in urine (31). To mitigate this concern, we excluded women with estimated glomerular filtration rates indicative of chronic kidney disease. Third, we excluded NHANES III participants with factors that preclude the measurement of FSH (such as hormonal contraceptive use, pregnancy, and lactation), which may affect the generalizability of our study findings. Fourth, given the cross-sectional study design, we were only able to evaluate cadmium exposure in relation to the ovarian reserve at a single time point, not its trajectory over time. Fifth, although cadmium measured in urine characterizes long-term exposure, it is possible that exposure to cadmium prior to that captured using the urinary biomarker may contribute to ovarian aging. Lastly, FSH measurement in NHANES III was untimed to the menstrual cycle. Measurement error is expected, as FSH level varies throughout the menstrual cycle (21). To minimize this source of measurement error, we excluded women with an LH:FSH ratio greater than 2, in whom the rise in FSH may be indicative of the preovulatory gonadotropin surge (37). Despite the potential limitations associated with its measurement, FSH is a widely recognized biomarker of ovarian reserve with established international reference standards (54, 55). The rise in FSH indicative of ovarian decline can be detected in women aged 35–49 years (56, 57) before overt changes in menstrual characteristics (45).
A major strength of this study was the use of urinary cadmium level to characterize long-term exposure. The decades-long biological half-life of cadmium in the kidney (30) supports the assumption that the exposure preceded the outcome in our study, despite the cross-sectional study design. Our study was also strengthened by careful consideration of both the approach to correction for urinary dilution using urinary creatinine and the hypothesized exposure-outcome causal scenario, given the measurement of cadmium in a spot urine sample. The 2 common approaches used in practice—standardization and covariate adjustment—may induce bias in some hypothesized causal scenarios, and we observed that the magnitude of the association appeared to differ depending on the select creatinine adjustment approach used. Our ability to apply the 3 creatinine adjustment approaches considered in this study, particularly covariate-adjusted standardization, was supported by the rich data available in NHANES III.
In conclusion, data from our study suggest that cadmium may contribute to ovarian aging in women. In addition, the approach used to correct for urinary dilution may affect the estimation of the association and induce bias. Further research is warranted to understand the role of cadmium in the trajectory of the ovarian reserve, with careful consideration of the approach used to correct for urine diluteness when measuring cadmium in spot urine samples.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology and Biostatistics, College of Human Medicine, Michigan State University, East Lansing, Michigan (Kristen Upson); Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Kristen Upson, Katie M. O’Brien, Donna D. Baird); Clinical Research Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Janet E. Hall); and Stem Cells Toxicology Group, National Toxicology Program Laboratory, Division of the National Toxicology Program, National Institute of Environmental Health Sciences, Research Triangle, Park, North Carolina (Erik J. Tokar).
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (NIH) (grant R00NR017191 to K.U.) and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
We thank Drs. Allen Wilcox and Stephani Kim for reviewing an earlier version of this article.
This work was presented at the 31st Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Baltimore, Maryland, June 18–19, 2018, and the 51st Annual Meeting of the Society for Epidemiologic Research, Baltimore, Maryland, June 19–22, 2018.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Hoyer PB, ed. Ovarian Toxicology. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- 2. Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. [DOI] [PubMed] [Google Scholar]
- 3. Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris). 2010;71(3):132–143. [DOI] [PubMed] [Google Scholar]
- 4. Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5(1):e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen XY, Xia HX, Guan HY, et al. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci. 2016;17(6):Article 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meirow D, Lewis H, Nugent D, et al. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14(7):1903–1907. [DOI] [PubMed] [Google Scholar]
- 7. Koyama H, Wada T, Nishizawa Y, et al. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–1409. [DOI] [PubMed] [Google Scholar]
- 8. Warne GL, Fairley KF, Hobbs JB, et al. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–1162. [DOI] [PubMed] [Google Scholar]
- 9. McDermott EM, Powell RJ. Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis. 1996;55(4):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atsma F, Bartelink ML, Grobbee DE, et al. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279. [DOI] [PubMed] [Google Scholar]
- 11. van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14(6):525–530. [DOI] [PubMed] [Google Scholar]
- 12. Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services . Toxicological Profile for Cadmium. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2012. [Google Scholar]
- 13. Egan SK, Bolger PM, Carrington CD. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Environ Epidemiol. 2007;17(6):573–582. [DOI] [PubMed] [Google Scholar]
- 14. Egan SK, Tao SS, Pennington JA, et al. US Food and Drug Administration’s Total Diet Study: intake of nutritional and toxic elements, 1991–96. Food Addit Contam. 2002;19(2):103–125. [DOI] [PubMed] [Google Scholar]
- 15. Massányi P, Uhrín V, Valent M. Correlation relationship between cadmium accumulation and histological structures of ovary and uterus in rabbits. J Environ Sci Health A. 1997;32(5):1621–1635. [Google Scholar]
- 16. Nad P, Massanyi P, Skalicka M, et al. The effect of cadmium in combination with zinc and selenium on ovarian structure in Japanese quails. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(13):2017–2022. [DOI] [PubMed] [Google Scholar]
- 17. Weng S, Wang W, Li Y, et al. Continuous cadmium exposure from weaning to maturity induces downregulation of ovarian follicle development-related SCF/c-kit gene expression and the corresponding changes of DNA methylation/microRNA pattern. Toxicol Lett. 2014;225(3):367–377. [DOI] [PubMed] [Google Scholar]
- 18. Al-Saleh I, Coskun S, Mashhour A, et al. Exposure to heavy metals (lead, cadmium and mercury) and its effect on the outcome of in-vitro fertilization treatment. Int J Hyg Environ Health. 2008;211(5-6):560–579. [DOI] [PubMed] [Google Scholar]
- 19. Varga B, Zsolnai B, Paksy K, et al. Age dependent accumulation of cadmium in the human ovary. Reprod Toxicol. 1993;7(3):225–228. [DOI] [PubMed] [Google Scholar]
- 20. Younglai EV, Foster WG, Hughes EG, et al. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol. 2002;43(1):121–126. [DOI] [PubMed] [Google Scholar]
- 21. Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129–140. [DOI] [PubMed] [Google Scholar]
- 22. Racowsky C, Gelety TJ. The biology of the ovary. In: Bittar EE, Bittar N, eds. Reproductive Endocrinology and Biology. (Principles of Medical Biology, vol. 12). Stamford, CT: JAI Press, Inc.; 1998:77–102. [Google Scholar]
- 23. Christensen PS, Bonde JP, Bungum L, et al. Environmental cadmium and lead exposure and anti-Mullerian hormone in pregnant women. Reprod Toxicol. 2016;61:114–119. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42–60 years, NHANES III. Environ Res. 2010;110(1):105–111. [DOI] [PubMed] [Google Scholar]
- 25. Jackson LW, Howards PP, Wactawski-Wende J, et al. The association between cadmium, lead and mercury blood levels and reproductive hormones among healthy, premenopausal women. Hum Reprod. 2011;26(10):2887–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YM, Chung HW, Jeong K, et al. Association between cadmium and anti-Mullerian hormone in premenopausal women at particular ages. Ann Occup Environ Med. 2018;30:Article 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagata C, Konishi K, Goto Y, et al. Associations of urinary cadmium with circulating sex hormone levels in pre- and postmenopausal Japanese women. Environ Res. 2016;150:82–87. [DOI] [PubMed] [Google Scholar]
- 28. Pollack AZ, Schisterman EF, Goldman LR, et al. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119(8):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Järup L, Rogenfelt A, Elinder CG, et al. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health. 1983;9(4):327–331. [DOI] [PubMed] [Google Scholar]
- 30. Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. [DOI] [PubMed] [Google Scholar]
- 31. Weaver VM, Kotchmar DJ, Fadrowski JJ, et al. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. 2016;26(1):1–8. [DOI] [PubMed] [Google Scholar]
- 32. O’Brien KM, Upson K, Cook NR, et al. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124(2):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bulka CM, Mabila SL, Lash JP, et al. Arsenic and obesity: a comparison of urine dilution adjustment methods. Environ Health Perspect. 2017;125(8):087020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 35. National Center for Health Statistics . Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Hyattsville, MD: National Center for Health Statistics; 1994. [PubMed] [Google Scholar]
- 36. National Center for Health Statistics . Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 37. Cooper GS, Baird DD, Darden FR. Measures of menopausal status in relation to demographic, reproductive, and behavioral characteristics in a population-based study of women aged 35–49 years. Am J Epidemiol. 2001;153(12):1159–1165. [DOI] [PubMed] [Google Scholar]
- 38. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926. [DOI] [PubMed] [Google Scholar]
- 41. Gill S, Sharpless JL, Rado K, et al. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab. 2002;87(5):2290–2296. [DOI] [PubMed] [Google Scholar]
- 42. Pruszkowska E, Carnrick GR, Slavin W. Direct determination of cadmium in urine with use of a stabilized temperature platform furnace and Zeeman background correction. Clin Chem. 1983;29(3):477–480. [PubMed] [Google Scholar]
- 43. National Center for Health Statistics, Centers for Disease Control and Prevention . Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. NHANES III Laboratory Data File Documentation: Ages One Year and Older. (Catalog no. 76300). Hyattsville, MD: National Center for Health Statistics; 1996. https://wwwn.cdc.gov/nchs/data/nhanes3/1a/lab-acc.pdf. Revised September 2006. Accessed November 27, 2017. [Google Scholar]
- 44. Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76(5):874–878. [DOI] [PubMed] [Google Scholar]
- 46. Cooper GS, Thorp JM Jr. FSH levels in relation to hysterectomy and to unilateral oophorectomy. Obstet Gynecol. 1999;94(6):969–972. [DOI] [PubMed] [Google Scholar]
- 47. Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–627. [DOI] [PubMed] [Google Scholar]
- 49. Sun SS, Chumlea WC, Heymsfield SB, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77(2):331–340. [DOI] [PubMed] [Google Scholar]
- 50. Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26(12):1596–1609. [DOI] [PubMed] [Google Scholar]
- 51. Tuttle AM, Stampfli M, Foster WG. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum Reprod. 2009;24(6):1452–1459. [DOI] [PubMed] [Google Scholar]
- 52. Broer SL, Broekmans FJ, Laven JS, et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701. [DOI] [PubMed] [Google Scholar]
- 53. Kissell KA, Danaher MR, Schisterman EF, et al. Biological variability in serum anti-Mullerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Practice Committee of the American Society for Reproductive Medicine . Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9–e17. [DOI] [PubMed] [Google Scholar]
- 55. Rose MP. Follicle stimulating hormone international standards and reference preparations for the calibration of immunoassays and bioassays. Clin Chim Acta. 1998;273(2):103–117. [DOI] [PubMed] [Google Scholar]
- 56. Lee SJ, Lenton EA, Sexton L, et al. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod. 1988;3(7):851–855. [DOI] [PubMed] [Google Scholar]
- 57. Sowers MR, Zheng H, McConnell D, et al. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93(10):3958–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


