Abstract
To determine the efficacy of inorganic nitrite supplementation on endothelial function in humans and mechanisms of action, we performed: 1) a randomized, placebo-controlled, parallel-group clinical trial with sodium nitrite (80 mg/day, 12 weeks) in older adults (n=49, 68±1 yr); and 2) reverse translation experiments in young (6 mo) and old (27 mo) c57BL/6 mice. In the clinical trial, sodium nitrite increased plasma nitrite (p<0.05) and was well-tolerated. Brachial artery flow-mediated dilation (endothelial function) was increased 28% vs. baseline after nitrite supplementation (p<0.05), but unchanged with placebo. Nitrotyrosine, a marker of oxidative stress, was reduced by 45% vs. baseline in biopsied endothelial cells after nitrite, but not placebo, treatment. Plasma from nitrite-treated, but not placebo-treated, subjects decreased whole-cell (CellROX) and mitochondria-specific (MitoSOX) reactive oxygen species (ROS) in cultured human umbilical vein endothelial cells (p<0.05). Old mice (OC, n=9) had ~30% lower ex vivo carotid artery endothelium-dependent dilation (EDD) vs. young mice (YC, n=9) due to reduced nitric oxide (NO) bioavailability (p<0.05). Nitrite supplementation (drinking water, 50 mg/L, 8 weeks) restored EDD and NO bioavailability in old mice (ON, n=10) to YC. Mitochondrial ROS suppression of EDD was present in OC (increased EDD with a mitochondrial-targeted antioxidant, p<0.05), but not in YC or ON. A mitochondrial ROS-inducer (rotenone) further impaired EDD in OC (p<0.05); YC and ON were protected. Markers of mitochondrial health were greater in aorta of ON vs. OC (p<0.05). Inorganic nitrite supplementation improves endothelial function with aging by increasing NO, decreasing mitochondrial ROS/oxidative stress and increasing mitochondrial stress resistance.
Keywords: reactive oxygen species, nitric oxide, stress resistance
Graphical Abstract
Introduction
Aging is the main risk factor for cardiovascular diseases (CVD), which remain the leading cause of morbidity and mortality in the developed world.1, 2 The age-associated increase in CVD risk is largely due to the development of vascular dysfunction, a major manifestation of which is vascular endothelial dysfunction.3–5 A key mechanism contributing to endothelial dysfunction with aging is a decline in the bioavailability of nitric oxide (NO) secondary to increases in oxidative stress,4, 6 which, in turn, is a consequence of age-related impairments in mitochondrial health favoring excess superoxide-associated reactive oxygen species (ROS) production.7, 8
Inorganic nitrite is a cellular-protective molecule and the major storage form of NO in tissues.9 Biochemically, as a key intermediary of the nitrate-nitrite-NO pathway, nitrite undergoes a one-step reduction to increase NO bioavailability and signaling.9, 10 Preclinical findings from our laboratory show that oral treatment with inorganic nitrite completely reverses age-related endothelial dysfunction in old mice by increasing NO bioavailability with no effects in young mice.11 As a first step in translating these findings to humans, we subsequently conducted a dose-ranging, feasibility-focused pilot study and found that 80 and 160 mg/day of oral sodium nitrite supplementation for 12 weeks improved NO-mediated endothelial function (brachial artery flow-mediated dilation [FMD]) to a similar extent in a small group of healthy adults 50–79 years of age.12
NO decreases mitochondrial ROS via post-translational modification of mitochondrial proteins and/or modulation of electron transport chain activity.13, 14 NO also augments signaling pathways to activate mitochondrial biogenesis and antioxidant capacity, including peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1a) and nuclear factor-erythroid-2-related factor 2 (NRF2) signaling, and the AMP-activated protein kinase pathway.15–18 These regulators, in turn, promote increases in mitochondrial mass and expression of metabolic and antioxidant processes that collectively act to prevent excess mitochondrial ROS and maintain a pool of healthy mitochondria.15–18 With aging, these processes are dysregulated, which contributes to age-related vascular dysfunction.7, 8 However, the efficacy of inorganic nitrite and associated elevations in NO bioavailability for reversing mitochondrial ROS-related endothelial dysfunction has not been investigated.
Accordingly, the main goal of the current study was to determine the efficacy of sodium nitrite for improving brachial artery FMD (primary outcome) in healthy older adults by conducting a parallel-design, randomized, placebo-controlled clinical trial with 12-week treatment arms. In this clinical trial, we also sought to obtain novel mechanistic insight on the role of decreased oxidative stress in mediating the beneficial effects of inorganic nitrite by assessing oxidant modification in biopsied endothelial cells and the influence and identity of changes in plasma “circulating factors” on total and mitochondrial-specific ROS bioactivity using tandem “plasma exposure” experiments in human endothelial cell culture and targeted plasma metabolomics analyses. Our secondary goal was to interrogate the role of improvements in mitochondrial health and decreases in mitochondrial ROS as mediators of nitrite-associated improvements in endothelial function. To accomplish this aim, we conducted “reverse-translation” experiments in young and old mice evaluating the effects of age and sodium nitrite treatment on mitochondrial ROS-mediated suppression of endothelial function and accompanying signaling pathways.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Clinical Studies
All procedures involving human subjects were reviewed and approved by the Institutional Review Board at the University of Colorado Boulder. The benefits, nature, and risks of the study procedures were explained to all participants. Written informed consent was obtained from all subjects before enrollment in the study. This study was registered on ClinicalTrials.gov (NCT02393742). All measurements were performed at the University of Colorado Boulder Clinical Translational Research Center (CTRC).
Participants.
One-hundred and fifty healthy men and postmenopausal women aged 60–80 years were recruited for the study. Eighty-five of the participants screened did not meet inclusion criteria. Fifty-six eligible participants were randomized to receive placebo (n=24) or sodium nitrite (n=32). Enrolled participants were excluded or withdrew from the study for the following reasons: subject burden (n=1), change in health status (n=1), and side effects to the intervention (n=5) (Figure S1). Additional participant details and inclusion/exclusion criteria are provided in the Data Supplement.
Study design, randomization, and intervention.
The study design was a 12-week randomized, double-blind, placebo-controlled, parallel design clinical trial. Randomization was performed by a member of the study team not involved in the assessment of outcomes, and a block randomization scheme stratified for age (60–69 and 70–79 years) and sex was used. Participants were randomized to either receive placebo or sodium nitrite (80 mg), taken orally as 40 mg twice per day in the morning and at night.12 This dose was selected based on its similar efficacy vs. 160 mg daily in our pilot study.12 Additional details on the intervention, measurements19 and participant characteristics20 are provided in the Data Supplement.
Plasma nitrite levels.
To determine the acute effects of sodium nitrite or placebo capsule ingestion on plasma nitrite levels, plasma was collected 4 hours after ingestion of the first capsule. The timing of this blood sample was based on pharmacokinetic data from the manufacturer for capturing peak plasma concentrations.21 Plasma nitrite levels were also measured at week 12 of the intervention 12–18 hours after sodium nitrite or placebo ingestion to assess the nonacute effects of supplementation on circulating nitrite levels. Plasma nitrite levels were assayed as described previously.22 Additional details are provided in the Data Supplement.
Vascular endothelial function.
Brachial artery FMD (using a five-minute forearm cuff occlusion) was assessed via high-resolution ultrasonography (Toshiba Xario XG), as described previously.23, 24 Measurements are expressed as percentage and absolute change from baseline diameter.24 Additional details are provided in the Data Supplement.
Endothelial cell biopsies, isolation and assessment of nitrotyrosine.
Endothelial cells were obtained from antecubital veins via endovascular biopsy, isolated and assessed for abundance of nitrotyrosine, a marker of oxidative stress, as described previously.25 All endothelial cell fluorescence data are reported as ratios to human umbilical vein endothelial cell protein expression and normalized to pre-intervention (baseline) values. Additional details are provided in the Data Supplement.
Plasma exposure experiments for whole-cell and mitochondrial ROS production.
Human umbilical vein endothelial cells (HUVECs) were plated in 96-well culture plates and incubated under standard conditions (37°C, 5% CO2, humidified). After 2–4 passages, cells were cultured for 24h in basal media supplemented with 10% plasma from a subset of subjects (N=9 for placebo-treated and N=11 for sodium nitrite treated subjects) collected before and after sodium nitrite and placebo (in triplicate).26, 27 Cells were incubated with the fluorescent probes CellROX Deep Red (ThermoFisher; to detect whole-cell ROS bioactivity), MitoSOX (ThermoFisher; to detect mitochondrial ROS [mtROS] bioactivity) and MitoTracker (ThermoFisher; to control for differences in mitochondrial volume) and analyzed by quantitative fluorescence microscopy.28 All fluorescence data are normalized to pre-intervention (baseline) values.28
Plasma Metabolomics.
A targeted metabolomics analysis focusing on 134 central carbon and nitrogen metabolites was performed on plasma from the subjects’ blood samples (N=8 for placebo-treated and N=10 for sodium nitrite treated subjects) that were used for the cell culture plasma exposure experiments, as described previously28–32 (see Data Supplement for details).
Preclinical Studies
All animal protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder. Male c57BL/6 mice, an established model of age-associated vascular endothelial dysfunction,33, 34 were purchased from the aging colony at the National Institute on Aging at ~6 or ~25 months of age and allowed to acclimate to our facilities for 2 weeks prior to beginning treatment. Young control mice (n=9) continued on normal drinking water, while old mice were randomly assigned to control (n=9, regular drinking water) or nitrite treatment (n=10), which received sodium nitrite (50 mg/L, a dose previously reported to be efficacious11, 35) in the drinking water for eight weeks.
Vascular endothelial function.
Endothelium-dependent dilation (EDD) in response to increasing doses of acetylcholine (ACh, Sigma Aldrich) and endothelium-independent dilation in response to increasing concentrations of the exogenous NO donor sodium nitroprusside (SNP, Sigma Aldrich) were measured in isolated carotid arteries as previously described.7, 11, 36 Further details, including all pharmacological agents used for pharmaco-dissection, are provided in the Data Supplement. The thoracic aorta was excised, dissected free of surrounding tissue and stored for later assessment of protein expression by standard Western blotting techniques as described elsewhere.11, 37 Details on procedures and antibodies are provided in the Data Supplement.
Data analysis.
Statistical analyses were performed with GraphPad Prism version 8. Data are expressed as mean ± standard error (SEM). Statistical significance was set a priori at α=0.05. Additional descriptions of the statistical approaches are provided in the Data Supplement.
Results
Clinical Trial
Participant characteristics.
There were no differences in participant characteristics (age, sex, body mass index and waist to hip ratio) and clinical or physiological blood markers (cholesterol, triglycerides, glucose, C-reactive protein, oxidized LDL and norepinephrine) between placebo and nitrite conditions at baseline or following the intervention (all p>0.05) (Table 1). Blood pressure did not differ between groups at baseline or in response to treatment (p>0.05, Table 1). Inclusion of baseline blood pressure as a covariate in our analysis did not influence the blood pressure responses to treatment (p>0.05).
Table 1.
Participant and Clinical Blood Characteristics
| Placebo | Nitrite | |||
|---|---|---|---|---|
| Subject Characteristics | Baseline | End-Intervention | Baseline | End-Intervention |
| Age, yr | 68±1 | — | 67±1 | — |
| Males/females, n | 12/11, 23 | — | 13/13, 26 | — |
| SBP, mmHg | 130±3 | 130±2 | 126±3 | 129±3 |
| DBP, mmHg | 75±2 | 75±2 | 74±2 | 75±2 |
| Body mass, kg | 73.8±2.6 | 74.4±2.4 | 74.4±2.6 | 74.1±2.6 |
| Body mass index, kg/m2 | 26.0±0.7 | 26.0±0.7 | 25.5±0.7 | 25.5±0.7 |
| Waist circumference, cm | 86±2 | 86±2 | 84±2 | 87±3 |
| Waist:hip ratio, U | 0.86±0.02 | 0.85±0.02 | 0.84±0.02 | 0.84±0.02 |
| Total Cholesterol, mg/dl | 175±7 | 177±7 | 173±6 | 174±6 |
| HDL Cholesterol, mg/dl | 52±3 | 51±3 | 55±3 | 57±3 |
| LDL Cholesterol, mg/dl | 110±6 | 106±6 | 102±6 | 100±6 |
| Triglycerides, mg/dl | 87±8 | 98±8 | 82±7 | 85±8 |
| Glucose, mg/dl | 82±4 | 83±3 | 88±7 | 88±7 |
| C-reactive protein, mg/l | 1.00±0.31 | 1.15±0.36 | 0.94±0.14 | 1.16±0.25 |
| Oxidized LDL, U/l | 74±4 | 75±5 | 65±5 | 66±4 |
| Norepinephrine, pg/ml | 369±20 | 363±32 | 396±23 | 392±25 |
Data are mean±SEM; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; placebo n=22, nitrite n=25 for all blood values.
Safety and tolerability.
All treatment-emergent adverse events were expected, and the majority were mild (Table S1). Three participants taking sodium nitrite reported dizziness or lightheadedness, three reported fatigue, one reported anxiety and one reported nausea. One participant on sodium nitrite had a severe allergic reaction to nitrite requiring medical attention (serious expected adverse event); this participant was withdrawn from the study (see Data Supplement for details).
Sodium nitrite acutely increases plasma nitrite levels.
Ingestion of sodium nitrite, but not placebo, effectively increased plasma nitrite levels 4 hours after ingestion (p<0.05, Figure S2). As expected based on the established pharmacokinetics of nitrite,21 concentrations ~12 hours after the last dose were not different from baseline post-intervention (p>0.05, Figure S2).
Sodium nitrite improves vascular endothelial function.
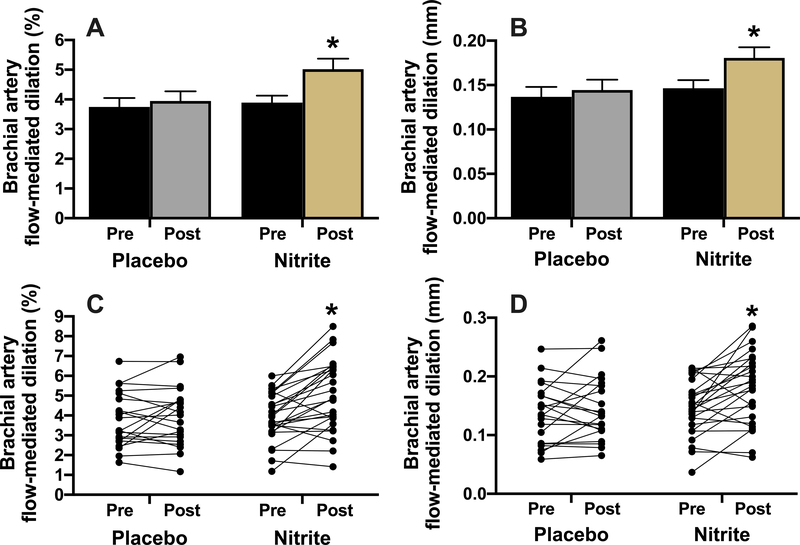
Brachial artery FMD was increased by 28% with chronic sodium nitrite supplementation but unchanged with placebo (p<0.05, Figure 1). Other brachial artery parameters were not different between conditions (p>0.05, Table S2).
Figure 1.
Brachial artery flow-mediated dilation (FMD) expressed as percent (A and C; treatment x time p<0.05) and absolute (B and D; treatment x time p<0.05) change before and after 12 weeks of placebo (N=21) or sodium nitrite supplementation (N=26). Values are presented as mean±SEM and as individual responses (lower panels). *p<0.05 vs. pre-intervention, within group.
Sodium nitrite decreases endothelial cell oxidative stress.
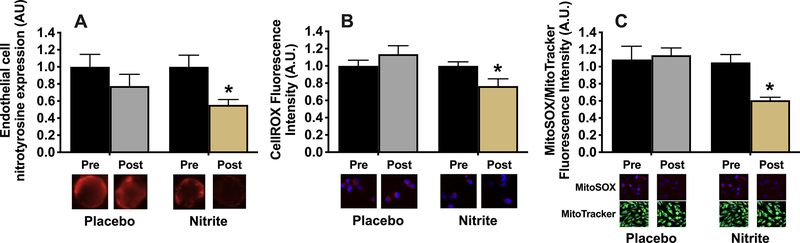
The abundance of nitrotyrosine, a marker of oxidative modification of proteins (i.e., oxidative stress), was ~45% lower (p<0.05) among individual biopsied endothelial cells obtained from our subjects after sodium nitrite supplementation compared with baseline, whereas there was no difference (p>0.05) in subjects before vs. after placebo treatment (Figure 2A). In sodium nitrite-supplemented subjects, ex vivo treatment of cultured HUVECs with plasma obtained 12 hours after the last dosing (i.e., when nitrite levels had returned to baseline levels) resulted in an ~25% decrease (p<0.05) in basal whole cell ROS bioactivity (CellROX fluorescence, Figure 2B) and an ~35% reduction in mitochondrial-specific ROS bioactivity (MitoSOX fluorescence, Figure 2C) without altering mitochondrial abundance, as assessed by MitoTracker fluorescence. In contrast, whole cell and mitochondrial-specific ROS bioactivity were unchanged (p>0.05) in HUVECS exposed to plasma obtained from subjects before vs. after placebo treatment (Figures 2B–C).
Figure 2.
Nitrotyrosine expression in endothelial cells obtained via endovascular biopsy from study participants before (pre) and after (post) 12 weeks of supplementation with sodium nitrite or placebo (A, N=14–15/group; group x time p=0.28). Total reactive oxygen species (ROS) bioactivity (CellROX signal) (B; treatment x time p<0.05) and mitochondrial ROS bioactivity normalized for mitochondrial volume (MitoSOX relative to MitoTracker signal) (C; treatment x time p<0.05) in cultured human umbilical vein endothelial cells treated with plasma (plasma exposure experiments) from a subset of participants pre and post 12 weeks of supplementation with sodium nitrite or placebo, N=9–11/group. Data are expressed relative to pre-intervention values within group and presented as mean±SEM. *p<0.05 vs. pre-intervention, within group.
Sodium nitrite alters circulating factors related to oxidative stress and antioxidant defense.
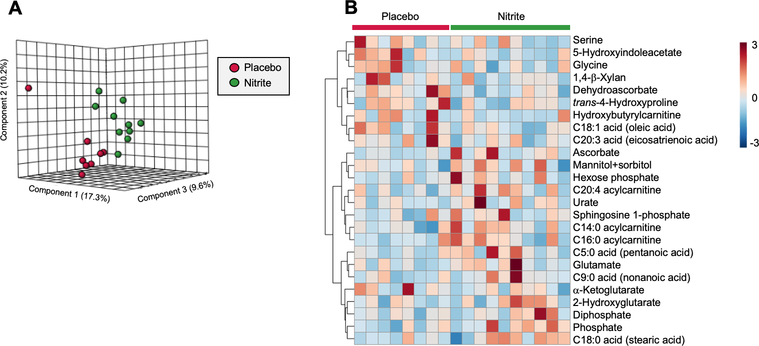
To identify changes in circulating molecular factors that may have contributed to the decrease in whole-cell and mitochondrial ROS bioactivity observed in HUVECs after exposure to plasma from our sodium nitrite supplemented subjects, we performed a targeted metabolomics analysis on plasma obtained before and after sodium nitrite (and placebo) treatment ina subset of subjects (N=8–10/group). We observed a distinction between the change in plasma metabolite profile in sodium nitrite vs. placebo treated subjects, as indicated by the separation of points in our partial least squares discriminant analysis (Figure 3A) and the supporting heat map of the results of our hierarchical clustering analysis of the top 25 metabolites that were differentially altered by the two treatment conditions (Figure 3B). Upon further analysis, we found that deydroascorbate, the oxidized form of the superoxide-scavenging metabolite ascorbate, was reduced in response to sodium nitrite supplementation (p<0.05 vs. placebo) (Figure 3 and Table S2), whereas ascorbate concentrations were increased (p=0.08 vs. placebo) (Figure 3 and Table S2). To determine the relations between these changes in plasma metabolites and corresponding changes in HUVEC ROS bioactivity, we performed linear regression analyses on the pooled samples from both the sodium nitrite supplemented and placebo treated subjects. The changes in plasma dehydroascorbate were positively related to the corresponding changes in HUVEC whole-cell (R2=0.43, p=0.004) and mitochondrial (R2=0.22, p=0.055) ROS bioactivity. There were no significant associations with changes in ascorbate concentrations before vs. after treatment.
Figure 3.
Targeted metabolomics analysis of plasma samples taken before and after chronic supplementation with sodium nitrite or placebo from a subset of subjects (N=8–10/group). 3-Dimensional partial least squares-discriminant analysis depicting the differential response to treatment (expressed as a fold change from baseline within each group) in the sodium nitrite and placebo-treated groups (A). Hierarchical clustering analysis of top 25 metabolites differentially altered by treatment between sodium nitrite and placebo-treated groups (each box represents a fold change from baseline for a given subject) (B).
Preclinical Studies
Nitrite treatment reverses age-related endothelial dysfunction by increasing NO bioavailability.
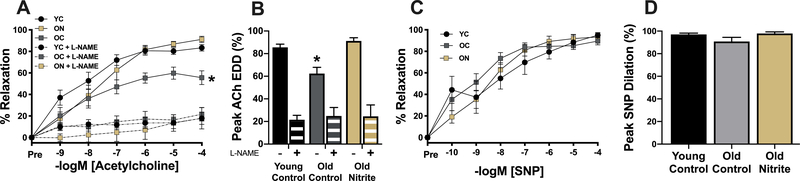
Carotid artery preconstriction to phenylephrine was not different between groups (p>0.05). EDD in carotid arteries was lower (p<0.05) in old control compared with young control mice (Figure 4A and B) and this impairment was mediated by reduced NO bioavailability, as indicated by a lack of group differences in the absence of NO after administration of the NO synthase inhibitor L-NAME (p>0.05, Figure 4A and B). Nitrite supplementation in old mice fully restored EDD and NO bioavailability (Figure 4A and B). There were no differences (p>0.05) among groups in the response to the NO donor sodium nitroprusside (endothelium-independent dilation, Figure 4C and D), indicating that the improvements in endothelial function were not related to changes in vascular smooth muscle sensitivity to NO.
Figure 4.
Carotid artery endothelium-dependent dilation (EDD) to increasing doses of acetylcholine (A) and peak EDD (B) in the absence and presence of the nitric oxide (NO) synthase inhibitor L-NAME (NG-nitro-L-arginine methyl ester) in young (6 mo) control (YC) and old (27 mo) control (OC) mice and old mice supplemented with sodium nitrite for 8 weeks (ON). Carotid artery endothelium-independent dilation to increasing doses of the NO donor sodium nitroprusside (SNP) (C) and peak dilation to SNP (D). N=9–10/group. All data are mean±SEM. *p<0.05 vs. young control.
Nitrite treatment abolishes age-related mtROS-mediated suppression of endothelial function and improves resistance to acute mtROS stress in old mice.
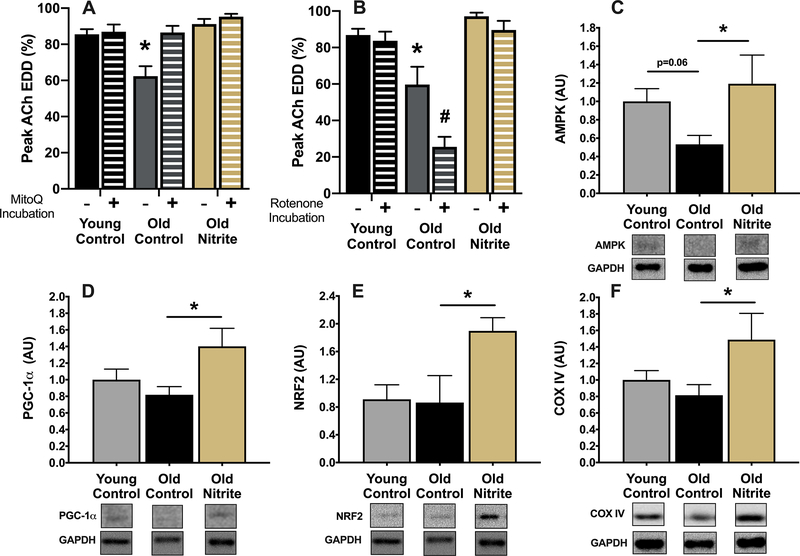
Incubation of carotid arteries with the mitochondria-specific antioxidant MitoQ completely restored EDD in old control animals but had no effect (p>0.05) in young control animals, indicating that aging is accompanied by tonic mtROS-mediated suppression of endothelial function (Figure 5A). In contrast, ex vivo MitoQ incubation had no effect (p>0.05) on EDD in old nitrite-supplemented animals, demonstrating complete amelioration of the mtROS-mediated suppression of function observed in old control mice (Figure 5A). Acute ex vivo incubation of arteries with 0.5 μM of the mitochondrial stressor rotenone to stimulate mtROS production7, 38–40 caused further impairment (p<0.05) in EDD in arteries of old control mice but had no effect (p>0.05) in young control mice or old nitrite-supplemented animals (Figure 5B). Together, these results suggest that increased mtROS contribute to the age-related decline in vascular endothelial function and that nitrite supplementation rescues endothelial function in old mice, in part by reducing vascular mitochondria-derived oxidative stress. Further, the data suggest that aging is associated with diminished resistance to an acute arterial mtROS challenge and that nitrite supplementation restores resistance to this stressor in old mice.
Figure 5.
Peak carotid artery endothelium-dependent dilation in the absence and presence of the mitochondrial-targeted antioxidant MitoQ (A) and the absence and presence of the mitochondrial stressor rotenone (B) in young (6 mo) control (YC) and old (27 mo) control (OC) mice and old mice supplemented with sodium nitrite for 8 weeks (ON). Aortic protein abundance of AMP-activated protein kinase (AMPK) (C), peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α) (D) and nuclear factor (erythroid-derived 2)-like 2 (NRF2) (E) and mitochondrial cytochrome C oxidase subunit IV (COXIV) (F) normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), with representative images from the same membrane below each panel. N=4–10/group. All data are mean±SEM. *p<0.05 vs. young control or for comparison indicated. #p<0.05 vs. absence of rotenone.
Nitrite treatment increases upstream regulators of mitochondrial function and protein markers of mitochondrial health.
Protein abundance of AMPK, a key energy-sensing enzyme and upstream regulator of mitochondrial function, was ~50% lower (p=0.06) in aorta of old compared to young mice (Figure 5C). Nitrite supplementation in old mice restored total aortic AMPK to levels similar to that of young controls (Figure 5C). Aortic protein expression of PGC-1a, NRF2, key regulators of mitochondrial biogenesis and health, and mitochondrial cytochrome C oxidase subunit IV (COXIV), a marker of mitochondrial mass, were not different (p>0.05) between young and old mice but were increased (p<0.05) in old nitrite-supplemented mice (Figure 5D, E and F). Collectively, these data suggest that nitrite supplementation may enhance mitochondrial health in the vasculature by boosting upstream signaling pathways, as well as markers of mitochondrial biogenesis.
Discussion
In the current study, we took the next translational step towards establishing sodium nitrite as a therapeutic strategy for healthy vascular aging by conducting a randomized, placebo-controlled, double-blind parallel group design clinical trial to determine the effects of sodium nitrite on vascular endothelial function in older adults. We show that sodium nitrite elevated plasma nitrite levels and was safe and well-tolerated over the 12-week treatment duration. Moreover, we demonstrate that sodium nitrite supplementation in older adults improves vascular endothelial function, which is associated with reductions in endothelial cell oxidative stress and alterations in circulating factors that decrease endothelial ROS bioactivity, primarily of a mitochondrial origin. To more comprehensively assess the role of nitrite-induced changes in vascular mitochondria in these observations, we also conducted parallel experiments in mice. Our results provide the first direct, cause and effect insight regarding the mitochondrial mechanisms by which nitrite reverses age-related endothelial dysfunction, which include attenuating mitochondrial ROS-related suppression of endothelial function and enhancing resistance to acute, mitochondria-associated stress. These latter changes were accompanied by evidence of favorable alterations in upstream signaling pathways that regulate mitochondrial biogenesis and health.
Accumulating evidence supports the efficacy of targeting the nitrate-nitrite-NO pathway with inorganic nitrates or nitrites in states of NO-deficiency such as aging.10–12, 41–45 Indeed, preclinical findings from our laboratory show that oral nitrite supplementation rescues age-associated declines in endothelial function by restoring NO bioavailability.11 Based on these findings, we conducted a pilot study in small groups of older adults with sodium nitrite and found the optimal dose for further translation (80 mg/day, as studied here) and initial evidence that chronic administration is safe, tolerable and improves NO-mediated endothelial function.12 In the present investigation, we extend our previous findings in a larger cohort of adults free from clinical disease and demonstrate that 12 weeks of sodium nitrite improves brachial artery FMD, a well-established measure of NO-mediated endothelial function that is an independent predictor of risk of future CVD. Brachial artery FMD was increased by 1.1% units (a 28% improvement) with sodium nitrite without evidence of sex-specific effects. The magnitude of increase is greater than the clinically significant margin of a 1% difference, which, in cross-sectional comparisons, is associated with a 13% decrease in risk of future CV events.46 These data suggest that strategies that enhance nitrite levels may hold promise for reducing CVD risk with aging.
We have previously shown in endothelial cells obtained by endovascular biopsy that abundance of nitrotyrosine, a cellular marker of oxidative stress, is increased in older adults and inversely related with endothelial function (brachial artery FMD).47 Here, we show that chronic supplementation with sodium nitrite, but not placebo, reduces nitrotyrosine in biopsied endothelial cells, consistent with the notion of decreased endothelial oxidative stress. Indeed, a decrease in superoxide-related oxidative stress is likely necessary to observe a sodium nitrite supplementation-associated increase in NO bioavailability and enhanced endothelial function because of the prominent NO-scavenging effects of excess superoxide. To gain insight into how nitrite supplementation may be modulating endothelial cell oxidative stress, we utilized an innovative translational model involving treatment of cultured endothelial cells with plasma taken from subjects before and after sodium nitrite or placebo, allowing us to assess the influence of “circulating factors” in plasma on endothelial cell ROS.26–29 Importantly, cells were treated with plasma taken from subjects 12 hours after sodium nitrite consumption, when plasma nitrite levels had returned to baseline following in vivo uptake by tissues. This approach allowed us to evaluate the effects chronic nitrite supplementation-induced changes in the circulating milieu vs. direct effects of nitrite as a mechanism of nitrite-related reductions in endothelial oxidative stress. We found that plasma from nitrite-treated subjects reduced both whole-cell and mitochondrial ROS bioactivity. These results suggest that nitrite-mediated alterations in circulating factors are a novel mechanism of decreased total and mitochondrial oxidative stress in endothelial cells, which may be responsible, at least in part, for the beneficial effects of sodium nitrite supplementation on endothelial function
The broad translational scope of the present investigation precluded an exhaustive “omics” evaluation of all possible circulating factors that may have contributed to the observed effects of sodium nitrite supplementation on endothelial (HUVEC) ROS bioactivity. However, to establish proof of concept that our treatment had the ability to influence at least selective factors, we performed an exploratory targeted metabolomics analysis of 130 metabolites in subject plasma. We found evidence of an increase in the superoxide-scavenging metabolite ascorbate, suggestive of increased plasma antioxidant capacity with sodium nitrite compared with placebo treatment, whereas dehydroascorbate, which is generated following ascorbate oxidation by ROS, was reduced. These findings are consistent with a decrease in the systemic pro-oxidative environment of plasma after sodium nitrite treatment. In line with this notion, the magnitude of decrease in dehydroascorbate with treatment was associated with the decrease in both whole-cell and mitochondrial-specific ROS bioactivity. Although additional omics analyses will be required to obtain a more comprehensive understanding of the changes to the circulating milieu responsible for the antioxidative and vascular protective effects of sodium nitrite, using a focused (targeted) plasma metabolomics platform, these findings provide an example of the type of molecular events that may be involved.
To extend our ex vivo observations regarding the effects of sodium nitrite supplementation on endothelial mitochondrial ROS to in vivo endothelial function, we conducted mechanistic, reverse translation studies in mice. We found that nitrite supplementation improved vascular endothelial function in old mice by increasing NO bioavailability.11 We also observed that the age-associated endothelial dysfunction was mediated by excess mtROS, evidenced by a selective improvement in endothelial function in arteries of old mice upon incubation with mitochondria-specific antioxidant MitoQ.7 Endothelial dysfunction in old mice was accompanied by a decline in vascular mitochondrial health, indicated by reduced ability to resist an acute mtROS challenge (rotenone) in line with previous findings in our laboratory.7, 38, 48 Importantly, we extend these observations here by showing that nitrite supplementation in old mice reduces mtROS-mediated suppression of EDD and improves the ability of arteries to resist an acute mtROS challenge (i.e., enhances mitochondrial stress resistance).
AMPK, PGC-1a and NRF2 are elements of critical energy-sensing pathways that regulate mitochondrial biogenesis, homeostasis and quality control processes.49 With aging, these pathways become dysregulated globally and may contribute to declines in mitochondrial health and function,50, 51 which, in turn, drive mtROS-related vascular dysfunction and increased arterial susceptibility to stress.7, 38 Indeed, we have shown that interventions that modulate these pathways enhance endothelial function by suppressing mtROS and increasing resistance to mitochondrial stressors.7, 38 Nitrite and NO can upregulate AMPK, an important mediator of mitochondrial function and biogenesis via signaling cascades involving PGC-1a and NRF2.51, 52 Additionally, NO can directly activate PGC-1a, thereby promoting mitochondrial biogenesis and increases in mitochondrial mass.53 Although we did not have sufficient aortic lysate to confidently assess the phosphorylated AMPK to total AMPK ratio (a commonly used marker of AMPK activation state), we observed marked increases in total AMPK, PGC-1a, NRF2 and COX IV - a marker of mitochondrial mass - in arteries of old mice after oral supplementation with sodium nitrite. These observations are consistent with the notion of nitrite-mediated enhancement of mitochondrial health as an upstream mechanism of reductions in mitochondrial ROS production and oxidative stress, and consequent improvements in endothelial function. Enhanced mitochondrial antioxidant capacity induced by activation of these signaling pathways also likely contributed to the preservation of endothelial function in response to the mitochondrial ROS-related stressor rotenone, reflecting increased endothelial stress resistance. Lastly, it is possible that increased NO bioavailability with nitrite may have directly reduced mitochondrial ROS production by posttranslational modification of mitochondrial proteins and/or effects on electron transport chain activity.13, 14
Taken together, our results indicate that the beneficial effects of nitrite supplementation on vascular mitochondrial health, including reductions in mtROS-mediated suppression of endothelial function, increases in resistance to acute mtROS stress, upregulation of pathways that promote mitochondrial biogenesis and homeostasis and increases in mitochondrial mass, may at least partially explain its efficacy for improving endothelial function in aging.
Perspectives
Here, we took the next step in the clinical translation of inorganic nitrite therapy for healthy vascular aging by showing that chronic supplementation is safe and well tolerated in older adults and improves vascular endothelial function, likely by decreasing excess mitochondria-derived ROS. We also provide novel preclinical evidence in support of the role of an amelioration of mitochondrial ROS-related suppression of endothelial function and susceptibility to a mitochondrial stressor, which are associated with evidence of improvements in markers of mitochondrial biogenesis and health. Collectively, our results provide support for the idea that inorganic nitrite, and potentially other therapeutic strategies acting on the nitrate-nitrite-NO pathway (e.g., inorganic nitrates), may be an effective treatment for improving vascular function and possibly decreasing the risk of CVD and other clinical disorders of aging, including hypertension, cognitive dysfunction and chronic kidney disease.
Supplementary Material
Novelty and Significance.
What Is New?
This trial establishes the efficacy of oral supplementation with sodium nitrite as a strategy for improving vascular endothelial function in older adults.
We provide the first evidence in humans that improvements in endothelial function with sodium nitrite is related to reductions in endothelial oxidative stress and changes in circulating factors that decrease total and mitochondrial-specific endothelial cell reactive oxygen species bioactivity.
We provide the first in vivo evidence that inorganic nitrite restores age-related endothelial dysfunction by decreasing mitochondrial reactive oxygen species and improving resistance to a mitochondrial reactive oxygen species-related stressor, likely via alterations in upstream mitochondrial signaling pathways associated with biogenesis and health.
What Is Relevant?
Older adults are at an increased risk for cardiovascular diseases in part because of vascular endothelial dysfunction. As such, it is important to establish evidence-based therapeutic options, and their associated mechanisms, to improve endothelial function in this group.
This study demonstrates that sodium nitrite improves endothelial function in older adults with impaired baseline vascular function and establishes reductions in endothelial cell oxidative stress and improvements in mitochondrial health/fitness as the major mechanisms of action.
Summary
Sodium nitrite supplementation improved vascular endothelial function in older adults, which was associated with decreased endothelial cell oxidative stress and total and mitochondrial reactive oxygen species bioactivity. Sodium nitrite improved endothelial function in old mice by ameliorating the tonic suppressive effects of excessive mitochondrial-specific reactive oxygen species and enhancing resistance to a mitochondrial stressor linked to improvements in mitochondrial fitness.
Acknowledgements
The authors thank the staff of the University of Colorado Boulder CTRC for their technical assistance.
Funding
This work was supported by NIH awards R01-AG013038, K01-DK115524, F31-AG047784, F32-AG053009, T32-AG000279 and NIH/NCATS Colorado CTSA Grant Number UL1-TR002535.
Footnotes
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Disclosures
Sodium nitrite and placebo capsules were provided by Tony Giordano at TheraVasc. NSB is an inventor on dozens of issued U.S and international patents on nitrite and nitric oxide compositions and receives royalties from these patents from the University of Texas. NSB is also a Founder and shareholder in HumanN, Inc. Pneuma Nitric Oxide, LLC and Nitric Oxide Innovations, LLD. All other authors have no declarations of interest to disclose.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr., Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP, American Heart Association Advocacy Coordinating C, Council on Cardiovascular Disease in the Y, Council on the Kidney in Cardiovascular D, Council on E, Prevention, Council on Cardiovascular N, Council on A, Thrombosis, Vascular B, Council on Clinical C and Stroke C. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–90. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG and Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. [DOI] [PubMed] [Google Scholar]
- 4.Seals DR, Jablonski KL and Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120:357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widlansky ME, Gokce N, Keaney JF Jr. and Vita JA. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42:1149–60. [DOI] [PubMed] [Google Scholar]
- 6.Donato AJ, Machin DR and Lesniewski LA. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ Res. 2018;123:825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP and Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP and Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. Journal of cell science. 2010;123:2533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler AR and Feelisch M. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation. 2008;117:2151–9. [DOI] [PubMed] [Google Scholar]
- 10.Sindler AL, Devan AE, Fleenor BS and Seals DR. Inorganic nitrite supplementation for healthy arterial aging. Journal of applied physiology. 2014;116:463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ and Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging cell. 2011;10:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T and Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. Journal of applied physiology. 2016;120:416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikalov SI, Mayorov VI and Panov AV. Physiological Levels of Nitric Oxide Diminish Mitochondrial Superoxide. Potential Role of Mitochondrial Dinitrosyl Iron Complexes and Nitrosothiols. Frontiers in physiology. 2017;8:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erusalimsky JD and Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–31. [DOI] [PubMed] [Google Scholar]
- 15.Geary K, Knaub LA, Schauer IE, Keller AC, Watson PA, Miller MW, Garat CV, Nadeau KJ, Cree-Green M, Pugazhenthi S, Regensteiner JG, Klemm DJ and Reusch JE. Targeting mitochondria to restore failed adaptation to exercise in diabetes. Biochem Soc Trans. 2014;42:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ and Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. Journal of cardiovascular pharmacology. 2015;65:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MW, Knaub LA, Olivera-Fragoso LF, Keller AC, Balasubramaniam V, Watson PA and Reusch JE. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am J Physiol Heart Circ Physiol. 2013;304:H1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S and Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. [DOI] [PubMed] [Google Scholar]
- 19.RAaMR Lohman TG. Anthropometric Standardization Reference Manual. Human Kinetics. 1988. [Google Scholar]
- 20.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC and Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Medicine and science in sports and exercise. 1997;29:S1–205. [PubMed] [Google Scholar]
- 21.Greenway FL, Predmore BL, Flanagan DR, Giordano T, Qiu Y, Brandon A, Lefer DJ, Patel RP and Kevil CG. Single-dose pharmacokinetics of different oral sodium nitrite formulations in diabetes patients. Diabetes technology & therapeutics. 2012;14:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryan NS and Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskurza I, Monahan KD, Robinson JA and Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris RA, Nishiyama SK, Wray DW and Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donato AJ, Black AD, Jablonski KL, Gano LB and Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging cell. 2008;7:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunt VE, Wiedenfeld-Needham K, Comrada LN and Minson CT. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol. 2018;596:4831–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Miguelez P, Gregg J, Seigler N, Bass L, Thomas J, Pollock JS, Sullivan JC, Dillard TA and Harris RA. Acute Tetrahydrobiopterin Improves Endothelial Function in Patients With COPD. Chest. 2018;154:597–606. [DOI] [PubMed] [Google Scholar]
- 28.Ballak DB, Brunt VE, Sapinsley ZJ, Ziemba BP, Richey JJ, Zigler MC, Johnson LC, Gioscia-Ryan RA, Culp-Hill R, Eisenmesser EZ, D’Alessandro A, Dinarello CA and Seals DR. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging cell. 2020;19:e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton ZS, Brunt VE, Hutton DA, VanDongen NS, D’Alessandro A, Reisz JA, Ziemba BP and Seals DR. Doxorubicin-Induced Oxidative Stress and Endothelial Dysfunction in Conduit Arteries Is Prevented by Mitochondrial-Specific Antioxidant Treatment. JACC CardioOncol. 2020;2:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehrke S, Rice S, Stefanoni D, Wilkerson RB, Nemkov T, Reisz JA, Hansen KC, Lucas A, Cabrales P, Drew K and D’Alessandro A. Red Blood Cell Metabolic Responses to Torpor and Arousal in the Hibernator Arctic Ground Squirrel. J Proteome Res. 2019;18:1827–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemkov T, Hansen KC and D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clasquin MF, Melamud E and Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinformatics. 2012;Chapter 14:Unit14 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprott RL and Ramirez I. Current Inbred and Hybrid Rat and Mouse Models for Gereontological Research. ILAR J. 1997;38:104–109. [DOI] [PubMed] [Google Scholar]
- 34.Brown KA, Didion SP, Andresen JJ and Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–6. [DOI] [PubMed] [Google Scholar]
- 35.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB and Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Experimental gerontology. 2012;47:588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL and Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleenor BS, Seals DR, Zigler ML and Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging cell. 2012;11:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL and Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging. 2016;8:2897–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weir CJ, Gibson IF and Martin W. Effects of metabolic inhibitors on endothelium-dependent and endothelium-independent vasodilatation of rat and rabbit aorta. British journal of pharmacology. 1991;102:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA and Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–25. [DOI] [PubMed] [Google Scholar]
- 41.Jackson JK, Patterson AJ, MacDonald-Wicks LK, Oldmeadow C and McEvoy MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr Rev. 2018;76:348–371. [DOI] [PubMed] [Google Scholar]
- 42.Kapil V, Khambata RS, Robertson A, Caulfield MJ and Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundberg JO, Weitzberg E and Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature reviews Drug discovery. 2008;7:156–67. [DOI] [PubMed] [Google Scholar]
- 44.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG and Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. The American journal of clinical nutrition. 2016;103:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughan KS, Levine A, Helbling N, Anthony S, DeLany JP, Stefanovic-Racic M, Goodpaster BH and Gladwin MT. Effects of Oral Sodium Nitrite on Blood Pressure, Insulin Sensitivity, and Intima-Media Arterial Thickening in Adults With Hypertension and Metabolic Syndrome. Hypertension. 2020:HYPERTENSIONAHA12014930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaba Y, Chen JA and Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–40. [DOI] [PubMed] [Google Scholar]
- 47.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE and Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. [DOI] [PubMed] [Google Scholar]
- 48.LaRocca TJ, Hearon CM, Jr., Henson GD and Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Experimental gerontology. 2014;58:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura-Clapier R, Garnier A and Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovascular research. 2008;79:208–17. [DOI] [PubMed] [Google Scholar]
- 50.Martens CR and Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594:7177–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH and Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell metabolism. 2007;5:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundberg JO, Carlstrom M and Weitzberg E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell metabolism. 2018;28:9–22. [DOI] [PubMed] [Google Scholar]
- 53.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH and Criswell DS. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588:3551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.