Abstract
Being able to vizualize a pathogen at a site of interaction with a host is an aesthetically appealing idea and the resulting images can be both informative as well as enjoyable to view. Moreover, the approaches used to derive these images can be powerful in terms of offering data unobtainable by other methods. In this article, we review three primary modalities for live imaging Borrelia spirochetes: whole animal imaging, intravital microscopy and live cell imaging. Each method has strengths and weaknesses, which we review, as well as specific purposes for which they are optimally utilized. Live imaging borriliae is a relatively recent development and there was a need of a review to cover the area. Here, in addition to the methods themselves, we also review areas of spirochete biology that have been significantly impacted by live imaging and present a collection of images associated with the forward motion in the field driven by imaging studies.
Introduction
Imaging has become one of the primary methods for the analysis of pathogen-host interactions and imaging of living cells and organisms has recently made a large contribution to our knowledge of B. burgdorferi pathogenesis. The focus on imaging of live pathogens and or host cells/animals can provide powerful in situ results on dynamic interactions that are not obtainable using spirochetes grown in culture or analyzed in vitro or after tissue fixation. This article reviews currently available technologies for live imaging that have been used on B. burgdorferi and that have revealed significant new findings (Radolf and Samuels, 2021). We start with the view from 10,000 feet, which allows visualization of B. burgdorferi infection of the entire animal. This is followed by a lower altitude sighting of specific organ systems or anatomical sites by high resolution, real-time imaging of live spirochetes in a living animal. Finally, we focus on live imaging in cellular systems. Each approach can provide unique and important information. The major contributions that these methodologies have made to our current understanding of Lyme disease pathogenesis will be reviewed. This article cannot provide a comprehensive review of all the topics covered here, and non-imaging data from the papers cited below is not discussed; instead, the reader is referred to the original literature for complete details of the papers we examine.
The methodologies to be described here are highly varied, as are the processes studied and the resulting knowledge gained. Whole animal imaging makes use of bioluminescent probes and has been successfully used to study Borrelia pathogenesis including dissemination and colonization of the mouse using a variety of B. burgdorferi mutant strains. By coupling specific gene promoters to luminescent probes, the methodology has also proven useful to study the localized expression in the mouse of several spirochete genes following infection. Intravital microscopy has opened a new area of study in terms of B. burgdorferi-endothelial interactions, the innate immune response to B. burgdorferi infection, vascular transmigration, the localized effectiveness of antibiotic treatment in the mouse and B. burgdorferi motility and chemotaxis during infection. Intravital imaging allows the study of these processes at specific locations in a living mouse at high resolution and in real-time. Finally, live cell imaging provides the highest resolution analysis and has allowed for novel, in-depth of study of B. burgdorferi-tick and B. burgdorferi-mammalian cell interactions by the imaging of dissected whole mount infected tick midguts and salivary glands and B. burgdorferi-cell interactions in chambers mimicking vascular flow. Although widely diverse in technical methodology, the approaches described below are tethered to each other through their common use of light to image the desired processes.
Whole Animal Imaging
Bioluminescent in vivo imaging (BLI) is a widely utilized technique to investigate a variety of questions from tumor development, immune response and host-pathogen interactions to bacterial pathogenesis (Contag et al., 1995; Rehemtulla et al., 2000; Sadikot et al., 2002; Jenkins et al., 2003; Hutchens and Luker, 2007; Lienenklaus et al., 2009; Andreu et al., 2011; Xu et al., 2016). The first publication of bioluminescent bacteria was Contag et al. (Contag et al., 1995) in Salmonella typhimurium utilizing the luciferase gene from the firefly (Photinus pyralis) to spatiotemporally track infection in mice. This method provides a satellite view of infection as it progresses in mice and is not designed for an assessment of pathogenesis on a cellular level. Bioluminescence is the result of an enzymatic reaction by luciferase with the D-luciferin substrate, oxygen, and ATP (Figure 1). The robust signal is emitted from viable cells with relatively low background. Over the years, a variety of luciferase alleles from insects and marine organisms have been developed to capture light emission from deep tissues within mammalian hosts (Zhang et al., 1999; Waidmann et al., 2011; Xu et al., 2016). To capture images and quantify bioluminescence emission from mammalian tissue, a couple-charged device (CCD) camera is required. BLI was first implemented with B. burgdorferi using a constitutively expressed codon optimized Photinus pyralis luciferase gene on a multicopy shuttle vector (designated as Bbluc; (Blevins et al., 2007; Hyde et al., 2011). This work was followed by the development of single copy Bbluc, a borrelial codon optimized Renilla luciferase (RlucBb), and several fluorescent proteins with potential use in the murine model (Chan et al., 2015; Adams et al., 2017; Takacs et al., 2018).
Figure 1. The implementation of bioluminescent imaging (BLI) in Borrelia burgdorferi.
(A) Luciferase enzymology. Enzymology of luciferase that results in light formation. This reaction requires ATP and dissolved oxygen. (B) Construction of a luciferase containing plasmid for BLI following infection with B. burgdorferi. The Bbluc gene was cloned into the pBBE22 shuttle vector, which contains the 25 kb linear plasmid (lp25) pncA (bbe22) gene, to generate pBBE22luc. B. burgdorferi strains lacking the lp25 plasmid are non-infectious. The presence of pncA together with Bbluc promotes simultaneous positive selection for the maintenance of this plasmid during infection and the constitutive expression of Bbluc. (C) In vivo tracking of luciferase-expressing B. burgdorferi. A ventral view of Balb/C mice infected with 105 B. burgdorferi constitutively expressing Bbluc. The images are from the same 4 mice imaged at 1h out to 91 days (d). All mice imaged were given D-luciferin except the mouse on the left, which is infected with infectious B. burgdorferi with no Bbluc gene; the mouse on the right is infected with B. burgdorferi containing Bbluc but lacking the pncA (bbe22) gene required for persistence. The presence of the pncA and luc genes is indicated at the top left. For the images shown, the signal range averaged between 5.5 x 103 to 1.0 x 105 photons per second (p/s).
Methodology
Most bioluminescent borrelial strains employ a luciferase gene that is encoded on a shuttle vector and maintained through in vivo selective pressure (Hyde et al., 2011; Wager et al., 2015; Zhi et al., 2015; Skare et al., 2016; Adams et al., 2017). A multicopy plasmid-encoded form of luciferase is necessary for a high level of sensitivity in order to decipher subtle phenotypic differences. To facilitate this, constitutively expressed Bbluc, expressed from the strong flaB promoter (PflaB), was introduced into pBBE22 within a strain background that used positive selection for pncA (bbe22) to maintain the presence of the luc gene during infection for up to 91 days post-infection without an added antibiotic (Figure 1; (Hyde et al., 2011; Wager et al., 2015; Zhi et al., 2015; Skare et al., 2016; Medina-Perez et al., 2020; Saputra et al., 2020). It has since been demonstrated that antibiotic supplementation in drinking water can maintain luc-based bioluminescence throughout a 10 day period of murine infection (Adams et al., 2016).
Experimentation using luciferase-based imaging with B. burgdorferi has been restricted to needle inoculation of mice although one study evaluated the acquisition of bioluminescent B. burgdorferi in crushed tick samples (Adams et al., 2017). Initially, Balb/c mice were used given the low skin content of melanin to maximize the acquired bioluminescent signal (Hyde et al., 2011; Wager et al., 2015; Skare et al., 2016; Saputra et al., 2020). More recently, however, C57 and C3H mice have been utilized, albeit at a lower level of sensitivity (Zhi et al., 2015; Medina-Perez et al., 2020). The advantage of needle inoculation is the ability to infect with a known amount of B. burgdorferi. To image, the mice are anesthetized using isoflurane and injected intraperitoneally with D-luciferin, the substrate for BbLuc, and then placed in an imager with a CCD camera that can detect light emission (Hyde and Skare, 2018). Following injection of the D-luciferin substrate, the mice can be imaged for up to 45 minutes; however, the signal does proportionally decrease over time. It is important to include a background control mouse in each image for every group of mice and time point for accurate analysis of the quantified bioluminescent signal. There are multiple acceptable approaches to control for background. One approach is to infect all the mice in the group with bioluminescent B. burgdorferi and not inject one mouse with D-luciferin (Hyde et al., 2011; Wager et al., 2015; Zhi et al., 2015; Hyde and Skare, 2018; Medina-Perez et al., 2020). Alternatively, one mouse in each group can be infected with B. burgdorferi lacking luciferase (Figure 1) or encoding a promoterless luciferase and then treated with D-luciferin as with the other mice in the group.
A challenge in experimental B. burgdorferi infection is determining tissue specific localization during later stages of disease. For deeper tissue colonization, mice can be treated with D-luciferin, sacrificed, organs harvested, incubated in additional substrate, and imaged (Skare et al., 2016; Saputra et al., 2020). Imaging of individual tissues allows the direct quantification of bioluminescence as a representation of bacterial burden or gene expression without further manipulation as would be necessary using real-time PCR.
Advantages
The greatest advantage of whole animal imaging is the ability to track infection of B. burgdorferi in a living animal over an extended period of time and observe quantifiable changes in infectivity, dissemination, and gene expression by non-invasive means. Of particular note, the in vivo bioluminescent evaluation of borrelial gene regulation involves environmental conditions that cannot be recapitulated under in vitro conditions or in dialysis membrane chambers incubated in rat peritoneal cavities. The low level of background luminescence, which often plagues fluorescence techniques, contributes to increased sensitivity and detection. Another distinguishing feature from fluorescence is that light emission is an indicator of bacterial viability (Figure 1). The requirement for oxygen and ATP as reaction co-factors for light production is found only in viable cells, whereas a decline in fluorescence indicates degradation of the protein and, therefore, diminished excitability. This sensitivity has proven to be particularly important in the comparison of mutants in various genes relative to the parental and complemented strains (Hyde et al., 2011; Wager et al., 2015; Zhi et al., 2015; Skare et al., 2016; Medina-Perez et al., 2020). Inherent to this, the number of animals required to get an assessment of infection is significantly reduced, and the same animals are repeatedly monitored, providing a less variable and more cohesive kinetic evaluation of infection.
Disadvantages
B. burgdorferi bioluminescent imaging is limited by the strength of the emitted signal, which is dependent on the number of bacteria present, strength of the promoter driving Bbluc, and the copy number of Bbluc. BLI in B. burgdorferi has been utilized primarily with the robust, constitutively expressed flaB promoter driving Bbluc expression encoded on a multicopy shuttle vector (Figure 1). Weaker promoters linked to Bbluc reduce the sensitivity of detection or possibly are not detectable above background levels. The quantifiable range of bioluminescence is 600 to 60,000 counts for valid analyses of bioluminescence imaging (Hyde and Skare, 2018). One other potential issue is the use of a multicopy Bbluc to visualize light emitting B. burgdorferi. To date, bioluminescence driven by flaB or other promoters on a multicopy plasmid has correlated well with borrelial burden or transcript levels, respectively, but this may not be the case for every gene of interest (Hyde et al., 2011; Skare et al., 2016; Saputra et al., 2020).
One issue with whole animal imaging is the lack of resolution of individual bacterial cells; that is, the signal only indicates localization within the skin in a broad sense as organisms disseminate intracutaneously (Waidmann et al., 2011). In vivo imaging of the whole animal or individual tissues provides limited insight into the molecular mechanisms of pathogenesis. Other techniques outlined in this review involve individual B. burgdorferi cells interacting in vivo with the host vasculature and tissues. Another limitation of whole animal imaging is the inability to detect and quantify B. burgdorferi in deeper tissues; thus, signals in tissues such as the heart, bladder, tibiotarsal joint, and lymph nodes are not accurate. As indicated above, and outlined in more detail below, organs can be imaged ex vivo to determine borrelial localization, but this requires invasive techniques and more mice if several time points are needed (Skare et al., 2016; Saputra et al., 2020).
Phenotypic characterization of luciferase expressing B. burgdorferi strains
The ability to detect light-emitting B. burgdorferi has provided important insight into how Lyme disease spirochetes can establish and maintain an infection in a small cohort of mice both temporally and spatially (Hyde et al., 2011). This technology was used initially to assess the phenotype of a B. burgdorferi bbk32 mutant that exhibited an apparent conflicting phenotype in the literature (Li et al., 2006; Seshu et al., 2006). The differences observed between these three week end point studies was attributed to the infectious dose used; specifically, when a high and non-physiologically relevant load was used (e.g., 105 spirochetes), very little difference was observed between the infection of the B. burgdorferi bbk32 mutant relative to its parent (Li et al., 2006; Seshu et al., 2006). However, when a lower and more physiological infectious dose was used (e.g., 103 spirochetes) a significant difference in infectivity was observed (Seshu et al., 2006). When the B. burgdorferi bbk32 mutant was transformed with the Bbluc construct (Figure 2), the resulting data demonstrated an attenuated phenotype at both the lower and higher dose and provided additional quantitative infection kinetics of the B. burgdorferi bbk32 mutant (Hyde et al., 2011). Specifically, the overall signal was significantly less starting at 7 days and was 4.1- to 7.7-fold less at all time points (Hyde et al., 2011). For the 105 dose, the light signal for the B.burgdorferi bbk32 mutant was 3-fold less than that for the parent strain at day 7 (Hyde et al., 2011).
Figure 2. Tracking phenotypes of B. burgdorferi mutants using BLI.

Infectivity of the wild type parent strain (ML23 pBBE22luc), a B. burgdorferi bbk32 mutant (JS315 pBBE22luc), and a B. burgdorferi dbpBA deletion (JF105 pBBE22luc) were compared in Balb/c mice. Mice were infected intradermally at 103 B. burgdorferi and imaged. The mouse on the far left is infected equivalently as the others but was not given D-luciferin substrate (denoted with a “minus”). Mice given substrate are indicated with a “plus”. All images for each time point and strain shown were standardized to the same photon per second range. This figure is reproduced from (Hyde et al., 2011; Zhi et al., 2015) with permission from John Wiley and Sons, Inc.
Imaging values also correlated well with qPCR. The B. burgdorferi bbk32 mutant showed significantly reduced bacterial burdens by qPCR in the skin, lymph node, and joint, independent of dose (Hyde et al., 2011). Total genomic B. burgdorferi DNA from infected skin correlated well with bioluminescent imaging, indicating that the detection of light emitting B. burgdorferi is a valid approach for quantifying borrelial burdens during active infection (Hyde et al., 2011). This method also has the added advantage of deciphering subtle differences in infectivity not otherwise apparent without tracking genomic equivalents via qPCR at discrete time points. While a valuable and valid means to determine infectivity, qPCR at multiple time points has the disadvantage of being expensive, laborious, and, most importantly, requires a large number of animals to obtain relevant data.
The experiments discussed above used multicopy Bbluc to detect light emitting B. burgdorferi following infection. Another group integrated the Bbluc gene and a linked antibiotic resistance locus into the strain N40 bbe02 restriction/modification system gene (Chan et al., 2015). By virtue of its construction, this strain was more competent for transformation and gained bioluminescence. When imaged, however, the signal detected was not uniform between the mice tested, presumably due to the low inoculum doses used (10, 100, and 103), which were, in some instances, at or below the 50% infectious dose for B. burgdorferi (ID50).
Having established bioluminescent imaging as a method to characterize and quantify B. burgdorferi infectivity, numerous subsequent studies used this approach to track mutant phenotypes in live mice. In a manner similar to the B. burgdorferi bbk32 mutant, the inactivation of bb0744 (p83/100), as well as the non-coding RNA ittA, resulted in a significantly attenuated phenotype relative to the infectious parent strain (Wager et al., 2015; Medina-Perez et al., 2020). Other mutants that are severely impaired in their ability to cause experimental infection, including B. burgdorferi dbpBA and bba33, were vetted using bioluminescence, either confirming (for dbpBA mutants) or demonstrating (for bba33) their loss of virulence (Blevins et al., 2008; Shi et al., 2008; Weening et al., 2008; Hyde et al., 2011; Zhi et al., 2015). Typical images of “wildtype” (parent), subtle, or pronounced phenotypic differences are shown in Figure 2. Note that other borrelial genes have been targeted but did not yield an infectivity defect via imaging or other metrics of infection (unpublished). These analyses reinforced the strength of this approach, notably, that a phenotype, be it mild or profound, can be evaluated in a limited but significant pool of experimentally infected mice in a sensitive and timely manner.
Use of imaging to track the expression of B. burgdorferi genes by transcriptional reporters
B. burgdorferi undergoes complex gene regulation in the mammalian host and BLI enables spatiotemporal tracking of changes in gene expression. Promoters for ospC and dbpBA were linked to luciferase, PospC-luc and PdbpBA-luc, respectively, to monitor the borrelial response to the distinct stages of pathogenesis (Figures 3 and 4) (Skare et al., 2016; Saputra et al., 2020). Bioluminescent reporter strains were normalized for changes in borrelial burden quantified from the constitutively expressed luciferase strain PflaB-luc. These studies clearly demarcated the unique expression patterns of ospC and dbpBA despite both belonging to the RpoS regulon (Hubner et al., 2001; Caimano et al., 2004; Yang et al., 2005; Ouyang et al., 2012; Skare et al., 2016; Saputra et al., 2020). dbpBA was robustly expressed throughout infection, at times exceeding emission from PflaB-luc (Figure 3) (Saputra et al., 2020). Given the long held paradigm that ospC is needed early in infection (Grimm et al., 2004; Tilly et al., 2006; Tilly et al., 2009), the observation that ospC expression was detectable 21 days post-infection, was surprising (Skare et al., 2016). Imaging of individual tissues demonstrated distinct tissue specific expression that changed over the course of infection. These results demonstrated that ospC and dbpBA are induced in the skin and heart early in infection after which expression declines at these sites (Figure 4). Consistent with the aforementioned atypical late expression of ospC, ospC levels were higher later in the infectious process in the bladder and joint, suggesting that OspC may be important in secondary colonization at these sites or persistence within these tissues. In the inguinal lymph node and tibiotarsal joint, dbpBA reached the highest level of expression after 21 days. Interestingly, ospC was not detectable in the lymph node. These studies highlight the utility of in vivo imaging to quantify the differential expression patterns of targeted B. burgdorferi genes in a tissue tropic fashion.
Figure 3. Tracking B. burgdorferi gene expression in infected mice using distinct reporter constructs. Imaging of infected mice with various reporters.
Balb/c mice were infected with 105 infectious B. burgdorferi containing PflaB-luc, Pospc-luc, or PdbpBA-luc reporters and imaged at the time points indicated. The control mouse (“-“) was treated as described in Figure 2. All reporters were normalized for interstrain comparison to the spectral emission scale shown below the images. Images reproduced from (Skare et al., 2016; Saputra et al., 2020).
Figure 4. Ex vivo gene expression in tissues in B. burgdorferi infected mice. (A) Imaging of reporter constructs in infectious B. burgdorferi within heart and joint tissues.
Heart and tibiotarsal joint from mice infected with infectious B. burgdorferi containing PflaB-luc, Pospc-luc, or PdbpBA-luc were imaged at the time points indicated. Control mice were treated as described in Figure 2. Images for the PflaB-luc, Pospc-luc, and PdbpBA-luc expression are set on individual scales to display the full spectrum of bioluminescence. Images reproduced from (Skare et al., 2016; Saputra et al., 2020).
In addition to tracking the expression of known borrelial genes, luciferase based reporters have been used to characterize promoters that are induced following experimental infection of mice. Several of the promoters tested in this way were identified using the BbIVET “promoter trap” approach designed to identify promoters that function during infection (Ellis et al., 2013). This system uses randomly cloned fragments of B. burgdorferi DNA to drive expression of pncA (bbe22), an essential gene for borrelial infectivity. Several of the promoters isolated, including those that drive primary, internal, or antisense transcripts were re-tested using the Bbluc allele (Adams et al., 2017). These studies confirmed that in vivo reporters can be utilized in B. burgdorferi to study genes spatially and temporally expressed in vivo and, further, track quantifiable differences in live, experimentally infected mice.
Intravital Microscopy
Methodology
Intravital microscopy (IVM) allows the direct visualization of a pathogen at work in a living host, a goal sought for many years and that provides a gold standard for pathogen-host interactions. IVM has been used for decades to investigate a variety of biological processes in living animals (Secklehner et al., 2017). However, its use to investigate bacterial pathogen-host interactions is a relatively recent development with the first report appearing in 2005 (Laschke et al., 2005). An important feature of this methodology is being able to discriminate between the pathogen and the infected host. This is typically accomplished by fluorescent labelling of the pathogen, either by staining or, preferably, by expression of a fluorescent marker, such as GFP, coupled with staining of host cells with fluorescent antibodies or other fluorescent markers. Finally, accessibility of the tissue desired for imaging is an important feature of this methodology, which usually requires surgical preparation of the area of interest prior to imaging.
Early intravital imaging was performed using standard epifluorescence microscopy. More recently, however, more sophisticated microscopes providing superior resolution and imaging are available. Current microscopes of choice are either the spinning disk laser confocal microscope (SDLCM) (Oreopoulos et al., 2014) or a confocal multi-photon microscope (MPM) (Larson, 2011). The spinning disk allows imaging through multiple pinholes, thereby blocking out of focus light from both the excitation and emission light paths. The SDLCM allows high resolution imaging and rapid image acquisition. In contrast, MPM uses near infrared light where the sum of two or more photons reaching the sample provides the energy required to excite the fluorophore. The long wavelength light reduces light scattering and allows deep imaging into the sample and reduces photodamage. MPM also allows direct imaging of certain highly ordered molecular structures (eg collagen) without a fluorophore, through second harmonic generation. In this process, two near infrared photons merge their energies into a single emitted photon of visible light. Confocal microscopes of both SDLCM and MPM types also can be used for 3D reconstructions, allowing a more realistic analysis of processes in living organisms. Microscopes for intravital imaging need to be equipped with an appropriate stage, objectives and equipment for stabilizing an anesthetized living mouse for microscopic observation (Moriarty et al., 2008; Masedunskas et al., 2012; Belperron et al., 2018).
Advantages
The major advantage of IVM is that it allows for the direct visualization of a live pathogen in a living organism at high resolution and in real-time. Digital video images can be acquired and quantification is possible in terms of fluorescence intensity, velocity measurements and enumeration of spirochetes. Mechanistic studies are possible by coupling the imaging with genetics, injection of peptides, antibodies and small or macromolecules. Multichannel fluorescence experiments and 3D reconstructions are routinely performed. Combining intravital imaging with the above modalities offers an unprecedented glimpse of pathogenic mechanisms in a variety of locations.
Disadvantages
IVM is labor intensive and usually requires a high level of surgical skill with mice or other small animals and appropriate state-of-the-art microscopes specifically setup for intravital imaging. It relies on sites that can be effectively imaged (eg, skin, lung, liver, heart, spleen, brain, cremaster) (Secklehner et al., 2017) and the surgical expertise required to prepare each tissue for imaging. Intravital imaging typically requires a large number of pathogens to be present in order to locate and image the infecting organism. In the case of B. burgdorferi, IVM may require infection modalities that differ from tick inoculation and may therefore be subject to perturbations from a natural infection. Clarity and resolution can suffer sometimes from small movements due to respiration by the mouse. Finally, the methodology is definitely not high throughput.
B. burgdorferi-endothelial interactions
Hematogenous dissemination is an important, but poorly understood, facet of Lyme disease pathogenesis (Wormser, 2006). By disseminating to a variety of organ systems, B. burgdorferi can cause a wide variety of clinical manifestations and symptoms. But how do spirochetes manage to grab hold of the vascular endothelium in the presence of the extremely strong shear forces found in the bloodstream and then cross the endothelium to infect the parenchyma? This process is not effectively or realistically studied outside of the mouse, and it was the starting point for intravital imaging of B. burgdorferi by Moriarty and coworkers (Moriarty et al., 2008). The experimental system used continues to provide valuable information on B. burgdorferi-endothelial interactions and on the vascular transmigration process.
In the first Borrelia intravital imaging experiments, a high concentration of B. burgdorferi expressing GFP from a shuttle vector was injected directly into the bloodstream via the jugular or femoral veins of anesthetized mice (resulting in about 107 spirochetes/ml in the blood). Spirochetes were also visible in the ear at two weeks post-infection; however, this paper focused on B. burgdorferi-vascular interactions immediately after inoculation, when significant numbers of organisms could be observed. The vasculature was labelled with fluorescent CD31 (PECAM-1) antibody; a representative micrograph is shown in Figure 5. This work showed that, similar to leukocytes (Filippi, 2019), spirochetes engage in multi-stage interactions with the endothelium, including transient tethering interactions, short-lived dragging interactions and stationary adhesion to the vasculature (Figure 6). The vast majority of short-term interactions occurred on the surface of endothelial cells, while 75% of stationary adhesions were localized to endothelial junctions, suggesting that transmigration might be a paracellular process. Escaping spirochetes were present only at about 0.1% of the observed spirochete-endothelial interactions and were, therefore, difficult to reliably observe with any confidence. 3D reconstructions also were performed to accurately analyze placement of spirochetes within or outside the observed post-capillary venules. An average displacement velocity of 3.4 μm/min (range 1.1-8.1) was measured for B. burgdorferi escaping from post-capillary venules.
Figure 5.

Intravital microscopy to study B. burgdorferi-microvascular interactions. Spinning disk confocal microscopy of GFP-expressing, infectious B. burgdorferi in a post-capillary venule of a living mouse. The vasculature was visualized with an AlexaFluor555-conjugated antibody to CD31 (PECAM-1). Image from (Norman et al., 2008).
Figure 6.
Schematic summarizing multi-stage interactions of B. burgdorferi with the mouse microvasculature based upon IVM (Moriarty et al., 2008; Norman et al., 2008). Tethering and dragging interactions comprise the essential first step of interactions with the microvasculature and are mechanistically distinct from each other regarding BBK32 interactions with Fn and GAGs, respectively (Moriarty et al., 2012). The tethering and dragging interactions are also mechanistically divergent from subsequent stationary adhesions. Stationary adhesion requires additional host and or spirochete factors and result in a redistribution of most spirochetes from cellular locations to endothelial junctions. Vascular transmigration requires P66 (Kumar et al., 2015). Figure modified from (Norman et al., 2008).
A second IVM study of B. burgdorferi-vascular interactions by the same group (Norman et al., 2008) focused on mechanistic issues involved in the pathogen-host endothelial interactions and coupled a variety of experimental approaches with IVM. These included the use of gain of function genetic constructs as well as antibodies, peptides and macromolecules to block B. burgdorferi-endothelial interactions. Tethering and dragging interactions were shown to be mechanistically distinct from stationary adhesions. Initial interactions were found to be mediated by host fibronectin (Fn) and glycoaminoglycans (GAGs), macromolecules known to interact with a variety of bacterial pathogens, and by the B. burgdorferi Fn and GAG binding protein BBK32. A subsequent IVM study further dissected the role of BBK32 by using BBK32 specifically reduced in either Fn or GAG binding (Moriarty et al., 2012). These studies showed that Fn binding by BBK32 is responsible for tethering interactions which provide the first point of contact for spirochetes careening through the vasculature under shear force. This molecular braking permits subsequent BBK32-GAG interactions that mediate spirochete dragging (Figure 7). This study also showed that RevA, RevB and BB0347 did not provide adhesion activity under shear stress in gain of function strains and that another unknown adhesin (Adhesin X) imparts strong adhesive properties to B. burgdorferi in the absence of BBK32. Further characterization of BBK32-endothelial interactions is discussed in the live cell imaging section.
Figure 7.
BBK-mediated tethering and dragging steps of vascular adhesion through distinct macromolecular interactions involving distinct Fn and GAG binding regions. Figure from (Moriarty et al., 2012), with permission from John Wiley and Sons, Inc.
The B. burgdorferi gain of function vascular adhesion system described above has also been used to assess the endothelial adhesion of Tp0751 (Pallilysin) from Treponema pallidum by expression of this protein in B. burgdorferi followed by IVM (Kao et al., 2017). Pallilysin was found to strongly interact with post-capillary venules in living mice and was also characterized by live cell imaging as noted in that section below.
The innate immune response to B. burgdorferi
IVM has also been used to study the hepatic intravascular immune response to B. burgdorferi (Lee et al., 2010). Imaging of the liver after intravenous injection of B. burgdorferi revealed that Kupffer cells ingested the spirochetes and induced iNKT cell clustering via CXCR3. Subsequently, direct contact between Kupffer cells and iNKT cells through the antigen-presenting molecule CD1D resulted in iNKT cell activation that reduced B. burgdorferi dissemination specifically into the knee joint and surrounding tissue.
A follow-up study by the same group (Lee et al., 2014) used multichannel spinning-disk intravital microscopy of joints and found iNKT cells adjacent to and surrounding the peripheral joint vasculature (Figure 8). The iNKT cells were positioned to ambush spirochetes at the vessel wall and interrupted transmigration of the spirochetes into the joints. B. burgdorferi that escaped iNKT cell immune surveillance and transmigrated into the joint tissue were subjected to attack by extravascular iNKT cells (Figure 9), which killed the spirochetes using a granzyme-dependent pathway. The combined results suggest that iNKT cells comprise a potent immune surveillance system and cytotoxic barrier for B. burgdorferi transmigration into joint tissue. This is further supported by the large increase in B. burgdorferi found specifically in joint tissue in Cd1d−/− mice, which are deficient in iNKT cells. This finding was important for the development of an intravital assay to monitor the transmigration ability of B. burgdorferi (Kumar et al., 2015) as described in a subsequent section.
Figure 8.

3D reconstruction of iNKT cells in the mouse joint from IVM images. The iNKT cells are green (CXCR6-GFP, where most GFP-expressing extravascular cells were iNKT cells) and the vasculature red (stained with AlexaFluor555-conjugated antibody to PECAM-1. Image reprinted from (Lee et al., 2014).
Figure 9.
Spirochete attack by an extravascular iNKT cell in CXCR6-GFP mouse joint where most GFP-expressing extravascular cells were iNKT cells. The image on the left shows a motile tomato-labelled extravascular spirochete (red arrow) and a stationary iNKT cell (yellow arrow). The right image shows the path of spirochete migration (red dots) and the spirochete captured by the iNKT cell. The captured spirochete was rendered immobile for the remainder of the experiment (1 hour). Image reprinted from (Lee et al., 2014).
Antibiotic treatment of B. burgdorferi-infected mice
Two-photon confocal IVM has been used to visualize GFP-expressing B. burgdorferi in the dermis and calcaneal tendon of needle-inoculated and tick-infected Myd88−/− mice (Bockenstedt et al., 2012). The use of Myd88−/− mice deficient in the Toll-like receptor adaptor molecule MyD88 results in up to a 100-fold increase in spirochete burden (Barthold et al., 1992; Bolz et al., 2004; Liu et al., 2004; Wang et al., 2004) and, therefore, facilitates microscopic observation. Using real-time IVM, the authors reported that most B. burgdorferi were found moving along collagen fibers in the ear as can be seen in Figure 10. Treatment with ceftriaxone resulted in a dramatic disappearance of spirochetes in the dermis and tendons 24 hours after starting antibiotic treatment (Figure 11). Interestingly, at this time, two morphological transformations of elongated spirochetes into spherical forms were captured by video imaging. A very important finding from this study was that, although antibiotic treatment with ceftriaxone or doxycycline effectively killed B. burgdorferi, antigenic spirochete remnants were observed between 2 and 10 weeks after completion of treatment. Spirochete immunogenic and inflammatory antigens also were found in the joints of antibiotic-treated mice, suggesting that they might play a role in triggering the development of Lyme arthritis and/or other autoimmune sequelae.
Figure 10.
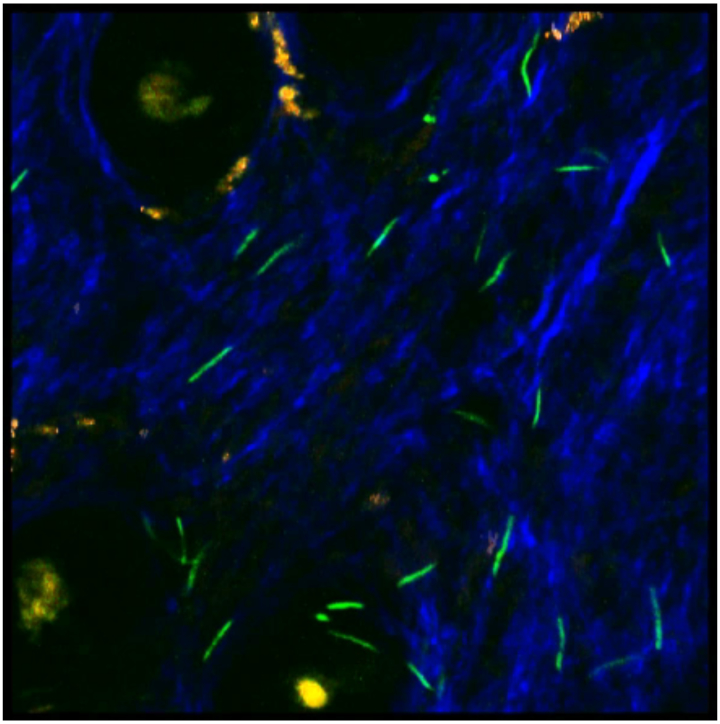
IVM of B. burgdorferi expressing GFP in the ear of an immunodeficient mouse 8 weeks post-infection, visualized by confocal two photon microscopy. Second harmonic generation of collagen results in a blue signal, melanin autofluoresces in all channels and appears white/orange, and the hair follicles auto fluoresce yellow (see Belperron et al., 2018). Image from Alexia Belperron and Linda Bockenstedt.
Figure 11.
A) B. burgdorferi expressing GFP in skin of a MyD88−/− mouse 22 days post-infection. The blue signal is from second harmonics of skin collagen. B) A dramatic decrease in spirochete numbers is shown after one day of ceftriaxone treatment. The arrow denotes a spirochete that subsequently became a spherical form (not shown). Images from (Bockenstedt et al., 2012), reprinted with permission from the American Society for Clinical Investigation.
Motility and chemotaxis of B. burgdorferi
The ability of Lyme disease spirochetes to disseminate in a vertebrate host is dependent upon their impressive flagella-driven motility (Sultan et al., 2013; Motaleb et al., 2015; Sultan et al., 2015). This motility has been studied by two-photon confocal IVM in the dermis of mouse ears following tick inoculation (Harman et al., 2012). Spirochete motility was observed to be similar to that previously reported in liquid and in gelatin. However, previously unobserved stationary but motile states were found. These were referred to as wriggling and lunging (Figure 12). The former remained at a fixed location but displayed movement as an undulating, traveling wave. The latter spirochetes were fixed at only one end while they underwent bending and relaxation in apparent attempts to free themselves from their attachment point. These stationary spirochetes are presumed to be tethered to the extracellular matrix by interactions strong enough to withstand the forces of spirochete motility. The IVM observations were compared with B. burgdorferi motility in gelatin and media to help establish in vitro conditions for studying spirochete motility that closely matched motility in the mouse dermis.
Figure 12.
A time course of GFP-expressing spirochete motility patterns in mouse dermis and gelatin. A) Tick-inoculated mouse dermis. B) 3% gelatin. Lunging (L), translocating (T), wriggling (W) and non-motile (N) spirochetes. The blue signal is from second harmonics of skin collagen. The scale bar is 10μm. This figure is reprinted from (Harman et al., 2012).
Two photon IVM microscopy also has been used to visualize Ixodes scapularis feeding on mice in real time and to study the response of nearby B. burgdorferi to the feeding ticks (Bockenstedt et al., 2014). Either rag−/− mice, lacking an acquired immune response, or MyD88−/− mice were infected either by needle inoculation or by infestation with infected nymphs. For IVM, unfed nymphs were placed on the ears of mice infected for at least two weeks with GFP-expressing B. burgdorferi. The characteristic motility behavior of the observed spirochetes was the same in wild-type, rag−/− and MyD88−/− mice. Restructuring of collagen fibers proximal to the bite site was observed and, although some spirochetes seemed unaffected by the presence of a feeding tick, others were observed to migrate in the direction of the hypostome, suggesting a chemotactic response. This work also supports the currently held belief that spirochetes are acquired from the skin rather than from the blood during tick feeding.
IVM has also been used to study the role of the chemotaxis regulator CheY3 (Novak et al., 2016). B. burgdorferi (wild-type or spirochetes with a disrupted cheY3 gene) were injected intradermally into the dorsal surface of the mouse ear. In comparison to wild-type B. burgdorferi, ΔcheY3 spirochetes displayed little translational motility in the mouse skin at six hours post-infection, with a progressive loss in motility over 48 hours. The spirochetes that were motile at early times displayed translational motility velocities similar to wild-type spirochetes but were unable to reverse direction and continued to run in the same direction (Figure 13). The inability of spirochetes tethered to the ECM to reverse direction and free themselves may be responsible for their loss of motility. ΔcheY3 spirochetes apparently were trapped near the inoculation site and, by 96 hours post-infection, all mutant spirochetes were cleared, while wild-type B. burgdorferi increased in numbers. The cheY spirochetes did not disseminate to other tissues. The IVM results clearly demonstrated an essential role for CheY3 in motility, viability and dissemination in the mouse.
Figure 13.
Contrasting motility behavior of dcheY3 and WT B. burgdorferi in mouse ear. Paths were tracked at six hours post-injection. Colored lines (except green) show the migration paths of the spirochete. Images from (Novak et al., 2016), with permission from John Wiley and Sons, Inc.
Vascular transmigration by B. burgdorferi
Little is currently known about the vascular transmigration step of B. burgdorferi dissemination. Data suggesting a paracellular path whereby spirochetes cross the endothelium at endothelial cell junctions have been reported (Comstock and Thomas, 1989, 1991). In contrast, data suggesting that transmigration occurs via a transcellular pathway have also been reported (Szczepanski et al., 1990). Moreover, little is known regarding the bacterial and host proteins and processes involved. The transmigration process was difficult to study by IVM using using the approach described above for vascular adhesion because looking for transmigrating spirochetes (0.1% of observed spirochetes) is akin to looking for a needle in a haystack. Moreover, transmigrated spirochetes rapidly leave the site of vascular escape in the skin, leaving no record. In addition, host defense systems can interfere with transmigration at several levels and B. burgdorferi genes involved in survival can be mistaken for genes playing a direct role in transmigration.
To circumvent these problems, an IVM transmigration assay was developed using information discovered in the study of the host innate immune response to B. burgdorferi described above (Lee et al., 2010; Lee et al., 2014). The assay uses the peripheral knee vasculature as the site of observation in Cd1d−/− mice, which lack iNKT cells. Use of Cd1d−/− mice is a crucial feature in this assay system as iNKT cells are the first line of defense against B. burgdorferi in mouse knee joint tissue (Lee et al., 2010; Lee et al., 2014). In wild-type mice, spirochetes are rapidly eliminated and unavailable for observation and enumeration. Moreover, the knee joint provides a stable environment where spirochetes remain in the local milieu and can be accurately counted after transmigration. Using this system, spirochetes were injected in the tail vein and visualized in knee joint tissue at 24 hours post-infection (Kumar et al., 2015); very little transmigration occurs before that time. Transmigrated spirochetes are clearly discernible from the vasculature (Figure 14) and display a reciprocating, translational motion outside the vessels. This IVM system was coupled with mutations in the genes encoding the Fn and GAG binding protein BBK32 and the integrin adhesin and porin, P66. Wild-type levels of transmigration were observed when bbk32 was disrupted; however, disruption of p66 resulted in a loss of transmigration. In particular, mutation of residues involved in integrin binding but not affecting the porin function abrogated transmigration, indicating a role for P66 and integrin binding in vascular transmigration of B. burgdorferi. In contrast, disruption of P66 did not influence vascular adhesion, suggesting that initial establishment of a borrelial-endothelial interaction is dependent upon BBK32 and perhaps other B. burgdorferi adhesins.
Figure 14.
Intravital microscopy to study B. burgdorferi vascular transmigration. Spinning disk confocal microscopy of GFP-expressing, infectious B. burgdorferi that escaped from the vasculature in the knee joint-proximal tissue of a living mouse. The vasculature was visualized with an AlexaFluor555-conjugated antibody to CD31 (PECAM-1). Image from (Kumar et al., 2015).
An important component of these IVM experiments was the monitoring of spirochete clearance from the vasculature. The removal of surface adhesins can affect the rate of spirochete clearance. This can result in an apparent decrease in transmigration that stems from a decrease in the number of spirochetes in the vasculature rather than an actual transmigration defect (Kumar et al., 2015).
More recently (Lin et al., 2020), another Borrelia adhesin has been shown to be required for vascular transmigration using IVM. The extracellular matrix binding activity of OspC is specific for fibronectin and dermatan sulphate, and ECM binding by OspC is required for extravasation. OspC does not appear to confer strong vascular adhesion, as BBK32 does, but likely interacts with the vascular endothelium, as P66 does, to promote transmigration subsequent to early tethering and dragging interactions that deaccelerate the spirochetes under the shear stress of bloodflow. The mechanism(s) by which P66 and OspC promote vascular transmigration is unknown at this time, however, it is tempting to speculate that they may induce endothelial signaling to promote cellular changes required for spirochetal transmigration.
Finally, a very recent IVM study (Tan et al., 2020) has revealed that the 24 hour lag observed for vascular transmigration of B. burgdorferi results from a requirement for endothelial activation to facilitate vascular escape. Extravasation is stimulated by a factor of three by host neutrophils, which do not recognize or clear the infecting spirochetes. The role of neutrophils appears to be the production of TNF-α, MCP-1 (CCL2) and IL-10, all of which can activate the endothelium for transmigration of B. burgdorferi. Figure 15 shows adherent and crawling neutrophils that do not recognize B. burgdorferi in the murine cremaster microvasculature.
Figure 15.

Spinning disk laser confocal micrograph showing endothelial activation in the murine cremaster microvasculature 24 hours after iv injection of GFP-expressing B. burgdorferi. Adherent and crawling neutrophils, physically distanced from spirochetes, were stained were with phycoerythin-conjugated anti-Ly6G (red) and venules were stained with Alexa Fluor 647-conjugated anti-PECAM1 (blue). See (Tan et al., 2020). Image from Björn Petri, Xi Tan and George Chaconas.
Live Cell Imaging Methodologies
Ex vivo live cell imaging of B. burgdorferi-tick interactions
Direct observation of B. burgdorferi behaviour and biology in ticks is challenging because their cuticle is strongly autofluorescent (Hammer et al., 2001). Multiple approaches have been used to overcome this problem over the past 50 years, including whole organ dissection and cultivation (Grabowski et al., 2018). Current ex vivo imaging methods for studying B. burgdorferi-tick interactions visualize GFP-expressing B. burgdorferi in fluorescent counter-stained dissected whole mount tick midguts and salivary glands at different stages of the tick blood meal and transmission, using epifluorescence or confocal time lapse microscopy, and confocal optical sectioning for three-dimensional z-series imaging (Figure 16) (Dunham-Ems et al., 2009; Dunham-Ems et al., 2012; Caimano et al., 2015).
Figure 16.
Live ex vivo 3D confocal microscopy z-series of dense, non-motile aggregates of GFP-expressing B. burgdorferi (green) in a hypertrophied region of the midgut epithelium of an infected feeding nymphal tick stained with FM4-64 membrane dye (red). Panel A depicts the composite (projection) image for all optical sections. Panels B-D show single slices obtained at different depths through the thickness of the midgut epithelial lining. xz and yz views of the composite stack are shown at far right/bottom. Panels E and F show similar regions from ticks infected with wild-type (WT) and ΔrpoS GFP-expressing B. burgdorferi. Images in A-D from (Dunham-Ems et al., 2009), reprinted with permission from the American Society for Clinical Investigation. Images in E-F from (Dunham-Ems et al., 2012).
In vitro live cell imaging of B. burgdorferi-mammalian host interactions
IVM visualization of B. burgdorferi in murine skin and the dermal microvasculature in turn enabled the development and validation of in vitro fluorescent live cell imaging models of host-pathogen interactions in the skin dermis (fluorescent bacteria moving in gelatin matrices) (Figure 12) and dermal microvasculature (fluorescent bacteria interacting with fluorescent counterstained primary human endothelial cells in blood-vessel mimicking flow chambers) (Figure 17) (Harman et al., 2012; Ebady et al., 2016; Salo et al., 2016; Kao et al., 2017; Niddam et al., 2017). These methods typically rely on GFP-expressing B. burgdorferi, although bacteria labelled with a non-toxic live cell imaging membrane dye can also be used (Salo et al., 2016).
Figure 17.
Trajectories of individual B. burgdorferi (different colours for each track) interacting with primary human endothelial cells in a flow chamber under postcapillary venule (PCV) shear stress conditions (IVT), and with a dermal PCV in a live mouse (PCV: IVM). Scale bars: IVT, 13 μm; PCV, 32 μm. Endothelial counterstain (red): plasma membrane dye (IVT), AlexaFluor 555-PECAM-1 antibody (PCV). Yellow and red arrows: B. burgdorferi interacting by one end only (yellow) and moving along edge of endothelial nucleus projecting above imaging plane (red). Image from (Ebady et al., 2016).
One of the biggest challenges for in vitro live imaging is keeping cells healthy and fully functional for the duration of experiments (Ettinger and Wittmann, 2014). Most live cell imaging experiments are performed in a medium that is hospitable to both bacteria and host cells (not always an easy task to find), and in a controlled physical environment where temperature, pH and sometimes humidity are stably maintained using specialized heaters, CO2 and humidity controllers. Flow chamber live cell imaging also requires syringe or peristaltic pumps capable of precise output at low flow rates. All equipment must be regularly calibrated since small linear changes in variables such as shear stress can have log-scale effects on host-pathogen interactions in settings such as flow chambers.
Real-time time lapse imaging can be performed using epifluorescence, spinning disk confocal or resonant scanner confocal microscopes equipped with electron multiplying charge-coupled device (EMCCD) cameras or high sensitivity photomultiplier tube (PMT) sensors. As for all live cell microscopy, including ex vivo imaging and IVM, phototoxicity and photobleaching can pose significant challenges for acquiring repeated high frame rate time courses at the same position, particularly for confocal laser light sources (Ettinger and Wittmann, 2014). Visualizing thin bacteria, such as B. burgdorferi, in fast-moving settings such as blood vessels and flow chambers requires prioritization of image acquisition speed over image brightness and “beauty”, especially for confocal microscopy, which excludes out of focus light. Prioritization of speed over beauty and brightness is especially important for measuring properties such as length and diameter of moving cells, which can appear larger when captured using EMCCD cameras and longer exposure times that produce more visually appealing, bright images (Jonkman et al., 2020). For this reason, accurate quantitative imaging for automated particle tracking and large-scale data analysis performed for fast-moving bacteria such as B. burgdorferi depends on non-saturated imaging conditions. For quantitative imaging, universal image acquisition conditions must also be pre-established and uniformly applied for all experimental conditions, and recalibrated over time as lasers age and become less powerful, and each time a new batch of fluorescent counterstain reagents is prepared or purchased (Jost and Waters, 2019).
Advantages
Ex vivo imaging in tick midguts and salivary glands after transmission to ticks or during transmission to mammals offers the only currently available method to directly observe B. burgdorferi-tick interactions at the single cell level. It has provided dynamic, localized insight into mechanisms of bacterial migration during tick transmission that would not be possible without direct observation at single cell resolution (Dunham-Ems et al., 2009; Dunham-Ems et al., 2012; Caimano et al., 2015).
Host-mimicking live cell imaging-based model systems support detailed, single cell resolution kinetic, biophysical, biochemical and genetic analysis of host-pathogen interaction mechanisms that are faster, less costly and more easily manipulated than IVM in animals (Harman et al., 2012; Ebady et al., 2016; Salo et al., 2016; Kao et al., 2017; Niddam et al., 2017). They facilitate interpretation of host-pathogen interaction data because immune clearance, which confounds IVM studies in blood vessels (Moriarty et al., 2012; Kumar et al., 2015), is largely absent. In vitro live cell imaging systems also support investigation of B. burgdorferi interactions with human cells, and rapid capture of large datasets (>10,000 datapoints) that can be analyzed by powerful segmentation and bioinformatics approaches.
Disadvantages
Ex vivo imaging in tick midguts and salivary glands requires delicate dissection, particularly if nymphal ticks are used, and separates these organs from the rest of the tick body, possibly disrupting some aspects of their physiology and function. Capturing optical sections through the full thickness of some tissues (e.g. late stage post-blood meal midguts) depends on physical sectioning or long working range objectives. Time-lapse imaging of live B. burgdorferi in tick midguts and salivary glands is performed for a maximum of 1.5 hours after dissection and fluorescent labeling of tick cells, necessitating the use of different ticks for each time point during the ~48-72 hour feeding period (Dunham-Ems et al., 2009).
In vitro live cell imaging model systems must be validated using in vivo methods such as whole body and IVM imaging, and/or qPCR and cultivation-based measurement of bacterial burden. In vitro live cell imaging depends on a stable, well-controlled physical environment. Quantitative live cell imaging also requires carefully controlled and calibrated imaging conditions, and substantial training and quality control monitoring. Efficient extraction of maximal amounts of information from high frame rate live cell imaging experiments can necessitate development and validation of automated methods for particle tracking and analyzing large volumes of data, and relies heavily on coding and bioinformatics skills that can be challenging for more conventionally trained biologists and microbiologists to quickly master if they are not working in collaborative teams.
Tick-B. burgdorferi interactions
Live ex vivo imaging methods developed for dissected tick guts and salivary glands (Dunham-Ems et al., 2009) have provided crucial insight into the processes of initial B. burgdorferi colonization of the tick midgut during mammalian host to tick transmission, and subsequent dissemination from the tick midgut to salivary glands during tick to mammalian host transmission. Live ex vivo imaging shows that these processes are controlled by the RpoS regulon (Dunham-Ems et al., 2012; Caimano et al., 2015). In B. burgdorferi-colonized unfed ticks, a mesh of non-motile spirochetes covers the epithelial lining of the tick midgut (Dunham-Ems et al., 2009). As colonized ticks feed, this mesh advances basally as the epithelium proliferates and differentiates in response to the blood meal. Basally positioned spirochetes then become motile and enter the hemocoel to migrate to and enter the salivary glands, from which they are transmitted to mammalian hosts (Dunham-Ems et al., 2009) (Figure 16). Spirochetes deficient in the RpoS regulon transform into a reversible round body morphotype as the blood meal progresses, and cannot migrate to salivary glands (Dunham-Ems et al., 2012). After naive ticks feed on infected mammalian hosts, gene regulation switches to an RpoS-off state that promotes formation of the non-motile mesh of bacteria on epithelial surfaces that is typical of unfed, midgut colonization (Caimano et al., 2015).
Shared human and mouse B. burgdorferi-endothelial interaction mechanisms
Live cell imaging of B. burgdorferi interactions with primary human umbilical cord vein endothelial cells in flow chambers under postcapillary venule shear stress conditions reveals that basic interaction features and properties observed in dermal postcapillary venules (PCVs) of mice by IVM (Moriarty et al., 2008; Norman et al., 2008) are recapitulated with human endothelial cells in vitro (Ebady et al., 2016; Kao et al., 2017; Niddam et al., 2017). In both settings, B. burgdorferi exhibits tethering, dragging and stationary adhesion, tethering and dragging depend on Fn, GAGs, the vascular adhesin BBK32 and at least one additional unidentified vascular adhesin, and bacterial strain-specific differences in interaction properties are conserved. Conversely, B. burgdorferi-endothelial interaction mechanisms first identified in flow chambers, such as transfer of mechanical load along a series of single adhesion complexes, were subsequently confirmed in vivo (Ebady et al., 2016). Identification of a T. pallidum adhesin supporting human endothelial interactions in flow chambers (pallilysin) led to confirmation of a role for this adhesin in dermal PCV interactions in mice (Kao et al., 2017). Thus, data derived from flow chamber model systems and human cells are useful for dissecting and predicting mouse vascular interaction mechanisms in vivo.
Biomechanics of B. burgdorferi-endothelial interactions
High speed particle tracking performed using large flow chamber and IVM datasets reveals that B. burgdorferi interactions with murine skin microvessels and human endothelial cells in flow chambers occur by transfer of mechanical load along a series of single or tightly clustered adhesion complexes (Ebady et al., 2016), similar to the interaction mechanism used by leukocytes and platelets rolling along endothelial surfaces in PCVs (Begandt et al., 2017; Coenen et al., 2017) (Figure 17).
Leukocyte and platelet rolling interactions are stabilized by tethers, which are extendible membranous or filamentous structures that distribute (reduce) force imposed on load-bearing adhesion complexes (Begandt et al., 2017; Coenen et al., 2017). Particle tracking of B. burgdorferi-endothelial interactions in flow chambers and PCVs shows that these interactions are also stabilized by tethers, although the composition of these tethers remains unknown. These tethered interactions correspond to the faster-moving transient interactions referred to as “tethering” in prior observational IVM studies (Figure 18) (Ebady et al., 2016).
Figure 18.
Under PCV shear stress conditions, slower moving “dragging” (untethered) B. burgdorferi is oriented edge-on to the endothelial surface and moves in a stepwise fashion, by transferring mechanical load from a single or coordinated, tightly clustered adhesion complex at the peak of the bacterial sine wave to another adhesion complex on the next sine wave peak. This process is facilitated by rotation of bacteria driven by flow-induced torque or bacterial motility. Faster-moving interactions are stabilized by tethers of unknown composition anchoring adhesion complexes and B. burgdorferi to the endothelial surface. Interactions are also stabilized by BBK32-dependent catch bonds, which are bonds that become longer lived as pulling force on the bond increases. Image from (Ebady et al., 2016).
The slow interaction type identified as “dragging” in early IVM studies in fact corresponds to untethered bacteria that are oriented with their sine wave edge-on to endothelial surfaces, projecting up into flow (Figure 18) (Ebady et al., 2016). In untethered interactions, mechanical load is transferred from peak to peak of the sine wave, resulting in slow-moving step-wise incremental displacements equal to the distance between sine wave peaks (~2.83 μm).
Load-bearing bonds supporting untethered interactions are subjected to considerably more pulling force, due to the projection of bacteria up into flow. For human umbilical vein endothelial cells and murine dermal PCVs, the adhesin BBK32 stabilizes higher force interactions by a catch bond mechanism. Catch bonds are adhesive bonds that become longer-lived (more stable) under increasing pulling force, and are important for stabilization of other interaction types in the vasculature, including leukocyte and platelet rolling (Begandt et al., 2017; Coenen et al., 2017).
Flow chamber and particle-tracking approaches also show that the B. burgdorferi-endothelial catch bond interaction mechanism depends on soluble Fn recruited from blood plasma (plasma Fn, pFn), and not on insoluble Fn found on endothelial surfaces (Figure 19) (Niddam et al., 2017). BBK32-expressing bacteria induce pFn polymerization to form pFn caps positioned at the mechanical load-bearing ends of B. burgdorferi, stimulating formation of adhesion complexes with receptors on endothelial surfaces, and slowing dissociation of these complexes by a catch bond mechanism.
Figure 19.
Proposed mechanism of plasma fibronectin (pFn)-dependent B. burgdorferi-vascular interactions under physiological shear stress. Conformational changes and polymerization of pFn exposed to a BBK32-expressing bacterium (A) exposes pFn binding sites for endothelial receptors and increases bond formation rate (Kon) (B). As bacteria are pushed/pulled by shear stress, stretching of the load-bearing adhesion complex induces additional force-induced conformational changes in adhesion complex components (BBK32, pFn, and endothelial receptors) to promote formation of longer-lived catch bonds and slower adhesion complex dissociation. Image from (Niddam et al., 2017).
Interestingly, multiple flow chamber and IVM studies have found that adhesive bond forces supporting bacterial-endothelial interactions under PCV shear stress are sufficiently small that propulsive forces generated by B. burgdorferi motility should be large enough to modulate the forces imposed on these bonds, or even to break them (Ebady et al., 2016; Kao et al., 2017; Niddam et al., 2017). It is also possible that B. burgdorferi motility contributes to maintaining untethered interactions in an edge-on position relative to endothelial surfaces or bacterial rotation during interactions (Figure 18), or could contribute to the pulling forces driving pFn polymerization to the load-bearing ends of cells (Figure 19).
Acknowledgements
The authors would like to thank Alexia Belperron and Linda Bockenstedt for providing the image for Figure 10 and Björn Petri and Xi Tan for the image for Figure 15. Work in the Chaconas aboratory is supported by the Canadian Institutes of Health Research (PJT-153336) and the National Institute of Health (R01AI18799) and work in the Moriarty laboratory is supported by the Canadian Institutes of Health Research (PJT-159466) and the Natural Sciences and Engineering Research Council of Canada (RGPIN-401938-17). Research in the Skare laboratory is currently funded by NIAID Public Health Service grants AI131656 and AI146930 and research in the Hyde laboratory is currently funded by NIAID Public Health Service grants AI101740 and AI131656.
References
- Adams PP, Flores Avile C, and Jewett MW (2017). A Dual Luciferase Reporter System for B. burgdorferi Measures Transcriptional Activity during Tick-Pathogen Interactions. Frontiers in cellular and infection microbiology 7, 225. 10.3389/fcimb.2017.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PP, Flores Avile C, Popitsch N, Bilusic I, Schroeder R, Lybecker M, and Jewett MW (2016). In vivo expression technology and 5' end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Res. 10.1093/nar/gkw1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu N, Zelmer A, and Wiles S (2011). Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol Rev 35, 360–394. 10.1111/j.1574-6976.2010.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Sidman CL, and Smith AL (1992). Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. The American journal of tropical medicine and hygiene 47, 605–613. [DOI] [PubMed] [Google Scholar]
- Begandt D, Thome S, Sperandio M, and Walzog B (2017). How neutrophils resist shear stress at blood vessel walls: molecular mechanisms, subcellular structures, and cell-cell interactions. J Leukoc Biol 102, 699–709. 10.1189/jlb.3MR0117-026RR [DOI] [PubMed] [Google Scholar]
- Belperron AA, Mao J, and Bockenstedt LK (2018). Two Photon Intravital Microscopy of Lyme Borrelia in Mice. Methods Mol Biol 1690, 279–290. 10.1007/978-1-4939-7383-5_20 [DOI] [PubMed] [Google Scholar]
- Blevins JS, Hagman KE, and Norgard MV (2008). Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC microbiology 8, 82. 10.1186/1471-2180-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JS, Revel AT, Smith AH, Bachlani GN, and Norgard MV (2007). Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi. Appl Environ Microbiol 73, 1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, Gonzalez D, Mao J, Li M, Belperron AA, and Haberman A (2014). What ticks do under your skin: two-photon intravital imaging of Ixodes scapularis feeding in the presence of the Lyme disease spirochete. Yale J Biol Med 87, 3–13. [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, Gonzalez DG, Haberman AM, and Belperron AA (2012). Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 122, 2652–2660. h 10.1172/JCI58813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz DD, Sundsbak RS, Ma Y, Akira S, Kirschning CJ, Zachary JF, Weis JH, and Weis JJ (2004). MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol 173, 2003–2010. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, and Radolf JD (2015). Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun 83, 3043–3060. 10.1128/IAI.00315-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Hazlett KR, and Radolf JD (2004). RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun 72, 6433–6445. 10.1128/IAI.72.11.6433-6445.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Alter L, Barthold SW, and Parveen N (2015). Disruption of bbe02 by Insertion of a Luciferase Gene Increases Transformation Efficiency of Borrelia burgdorferi and Allows Live Imaging in Lyme Disease Susceptible C3H Mice. PLoS One 10, e0129532. 10.1371/journal.pone.0129532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen DM, Mastenbroek TG, and Cosemans J (2017). Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood 130, 2819–2828. 10.1182/blood-2017-04-780825 [DOI] [PubMed] [Google Scholar]
- Comstock LE, and Thomas DD (1989). Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun 57, 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock LE, and Thomas DD (1991). Characterization of Borrelia burgdorferi invasion of cultured endothelial cells. Microb Pathog 10, 137–148. [DOI] [PubMed] [Google Scholar]
- Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, and Benaron DA (1995). Photonic detection of bacterial pathogens in living hosts. Mol Microbiol 18, 593–603. 10.1111/j.1365-2958.1995.mmi_18040593.x [DOI] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Eggers CH, and Radolf JD (2012). Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog 8, e1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, and Radolf JD (2009). Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest 119, 3652–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebady R, Niddam AF, Boczula AE, Kim YR, Gupta N, Tang TT, Odisho T, Zhi H, Simmons CA, Skare JT, et al. (2016). Biomechanics of Borrelia burgdorferi Vascular Interactions. Cell Rep. 10.1016/j.celrep.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TC, Jain S, Linowski AK, Rike K, Bestor A, Rosa PA, Halpern M, Kurhanewicz S, and Jewett MW (2013). In vivo expression technology identifies a novel virulence factor critical for Borrelia burgdorferi persistence in mice. PLoS Pathog 9, e1003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi MD (2019). Neutrophil transendothelial migration: updates and new perspectives. Blood. 10.1182/blood-2018-12-844605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski JM, Offerdahl DK, and Bloom ME (2018). The Use of Ex Vivo Organ Cultures in Tick-Borne Virus Research. ACS Infect Dis 4, 247–256. 10.1021/acsinfecdis.7b00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, and Rosa PA (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101, 3142–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer B, Moter A, Kahl O, Alberti G, and Gobel UB (2001). Visualization of Borrelia burgdorferi sensu lato by fluorescence in situ hybridization (FISH) on whole-body sections of Ixodes ricinus ticks and gerbil skin biopsies. Microbiology 147, 1425–1436. 10.1099/00221287-147-6-1425 [DOI] [PubMed] [Google Scholar]
- Harman MW, Dunham-Ems SM, Caimano MJ, Belperron AA, Bockenstedt LK, Fu HC, Radolf JD, and Wolgemuth CW (2012). The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proc Natl Acad Sci U S A 109, 3059–3064. 10.1073/pnas.1114362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, and Norgard MV (2001). Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98, 12724–12729. 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens M, and Luker GD (2007). Applications of bioluminescence imaging to the study of infectious diseases. Cell Microbiol 9, 2315–2322. 10.1111/j.1462-5822.2007.00995.X [DOI] [PubMed] [Google Scholar]
- Hyde JA, and Skare JT (2018). Detection of Bioluminescent Borrelia burgdorferi from In Vitro Cultivation and During Murine Infection. Methods Mol Biol 1690, 241–257. 10.1007/978-1-4939-7383-5_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Hook M, Cirillo JD, and Skare JT (2011). Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol 82, 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J, Purchio T, and Contag PR. (2003). Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis 20, 733–744. 10.1023/b:clin.0000006815.49932.98 [DOI] [PubMed] [Google Scholar]
- Jost AP, and Waters JC (2019). Designing a rigorous microscopy experiment: Validating methods and avoiding bias. J Cell Biol 218, 1452–1466. 10.1083/jcb.201812109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WA, Petrosova H, Ebady R, Lithgow KV, Rojas P, Zhang Y, Kim YE, Kim YR, Odisho T, Gupta N, et al. (2017). Identification of Tp0751 (Pallilysin) as a Treponema pallidum Vascular Adhesin by Heterologous Expression in the Lyme disease Spirochete. Sci Rep 7, 1538. 10.1038/s41598-017-01589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Ristow LC, Shi M, Mukherjee P, Caine JA, Lee WY, Kubes P, Coburn J, and Chaconas G (2015). Intravital Imaging of Vascular Transmigration by the Lyme Spirochete: Requirement for the Integrin Binding Residues of the B. burgdorferi P66 Protein. PLoS Pathog 11, e1005333. 10.1371/journal.ppat.1005333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AM (2011). Multiphoton microscopy. Nature Photonics 5, 1. [Google Scholar]
- Laschke MW, Kerdudou S, Herrmann M, and Menger M (2005). Intravital fluorescence microscopy: a novel tool for the study of the interaction of Staphylococcus aureus with the microvascular endothelium in vivo. J Infect Dis 191, 435–443. 10.1086/427193 [DOI] [PubMed] [Google Scholar]
- Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, and Kubes P (2010). An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol 11, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sanz MJ, Wong CH, Hardy PO, Salman-Dilgimen A, Moriarty TJ, Chaconas G, Marques A, Krawetz R, Mody CH, et al. (2014). Invariant natural killer T cells act as an extravascular cytotoxic barrier for joint-invading Lyme Borrelia. Proc Natl Acad Sci U S A 111, 13936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Beck DS, Kantor FS, and Fikrig E (2006). Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun 74, 3305–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienenklaus S, Cornitescu M, Zietara N, Lyszkiewicz M, Gekara N, Jablonska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, et al. (2009). Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol 183, 3229–3236. 10.4049/jimmunol.0804277 [DOI] [PubMed] [Google Scholar]
- Lin YP, Tan X, Caine JA, Castellanos M, Chaconas G, Coburn J, and Leong JM (2020). Strain-specific joint invasion and colonization by Lyme disease spirochetes is promoted by outer surface protein C. PLoS Pathog 16, e1008516. 10.1371/journal.ppat.1008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Montgomery RR, Barthold SW, and Bockenstedt LK (2004). Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect Immun 72, 3195–3203. 10.1128/IAI.72.6.3195-3203.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masedunskas A, Milberg O, Porat-Shliom N, Sramkova M, Wigand T, Amornphimoltham P, and Weigert R (2012). Intravital microscopy: a practical guide on imaging intracellular structures in live animals. Bioarchitecture 2, 143–157. 10.4161/bioa.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Perez DN, Wager B, Troy E, Gao L, Norris SJ, Lin T, Hu L, Hyde JA, Lybecker M, and Skare JT (2020). The intergenic small non-coding RNA ittA is required for optimal infectivity and tissue tropism in Borrelia burgdorferi. PLoS Pathog 16, e1008423. 10.1371/journal.ppat.1008423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, and Chaconas G (2008). Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog 4, e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, Odisho T, Hardy PO, Salman-Dilgimen A, Wu J, Weening EH, et al. (2012). Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol 86, 1116–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Liu J, and Wooten RM (2015). Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Current Opinion in Microbiology 28, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam AF, Ebady R, Bansal A, Koehler A, Hinz B, and Moriarty TJ (2017). Plasma fibronectin stabilizes Borrelia burgdorferi-endothelial interactions under vascular shear stress by a catch-bond mechanism. Proc Natl Acad Sci U S A 114, E3490–E3498. 10.1073/pnas.1615007114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, and Chaconas G (2008). Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog 4, e1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak EA, Sekar P, Xu H, Moon KH, Manne A, Wooten RM, and Motaleb MA (2016). The Borrelia burgdorferi CheY3 Response Regulator is Essential for Chemotaxis and Completion of its Natural Infection Cycle. Cell Microbiol. 10.1111/cmi.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreopoulos J, Berman R, and Browne M (2014). Spinning-disk confocal microscopy: present technology and future trends. Methods in Cell Biology 123, 153–175. 10.1016/B978-0-12-420138-5.00009-4. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, and Norgard MV (2012). Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC microbiology 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD and Samuels DS (2021). Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis (Norfolk, UK: Caister Academic Press; ). 10.21775/9781913652616 [DOI] [Google Scholar]
- Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, and Ross BD (2000). Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2, 491–495. 10.1038/sj.neo.7900121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot RT, Wudel LJ, Jansen DE, Debelak JP, Yull FE, Christman JW, Blackwell TS, and Chapman WC (2002). Hepatic cryoablation-induced multisystem injury: bioluminescent detection of NF-kappaB activation in a transgenic mouse model. J Gastrointest Surg 6, 264–270. 10.1016/s1091-255x(01)00064-6 [DOI] [PubMed] [Google Scholar]
- Salo J, Pietikainen A, Soderstrom M, Auvinen K, Salmi M, Ebady R, Moriarty TJ, Viljanen MK, and Hytonen J (2016). Flow-Tolerant Adhesion of a Bacterial Pathogen to Human Endothelial Cells Through Interaction With Biglycan. J Infect Dis 213, 1623–1631. 10.1093/infdis/jiw003 [DOI] [PubMed] [Google Scholar]
- Saputra EP, Trzeciakowski JP, and Hyde JA (2020). Borrelia burgdorferi spatiotemporal regulation of transcriptional regulator bosR and decorin binding protein during murine infection. Sci Rep 10, 12534. 10.1038/s41598-020-69212-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secklehner J, Lo Celso C, and Carlin LM (2017). Intravital microscopy in historic and contemporary immunology. Immunol Cell Biol 95, 506–513. 10.1038/icb.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, and Skare JT (2006). Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol 59, 1591–1601. [DOI] [PubMed] [Google Scholar]
- Shi Y, Xu Q, McShan K, and Liang FT (2008). Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun 76, 1239–1246. 10.1128/IAI.00897-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Shaw DK, Trzeciakowski JP, and Hyde JA (2016). In Vivo Imaging Demonstrates That Borrelia burgdorferi ospC Is Uniquely Expressed Temporally and Spatially throughout Experimental Infection. PLoS One 11, e0162501. 10.1371/journal.pone.0162501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, and Motaleb MA (2013). Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun 81, 2012–2021. 10.1128/IAI.01228-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Sekar P, Zhao X, Manne A, Liu J, Wooten RM, and Motaleb MA (2015). Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect Immun 83, 1765–1777. 10.1128/iai.03097-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski A, Furie M, Benach J, Lane B, and Fleit H (1990). Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest 85, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs CN, Kloos ZA, Scott M, Rosa PA, and Jacobs-Wagner C (2018). Fluorescent Proteins, Promoters, and Selectable Markers for Applications in the Lyme Disease Spirochete Borrelia burgdorferi. Appl Environ Microbiol 84. 10.1128/AEM.01824-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Petri B, and Chaconas G (2020). The Lyme disease spirochete hijacks the host immune system to extravasate through the microvasculature. Manuscript submitted. [DOI] [PubMed]
- Tilly K, Bestor A, Dutebohn DP, and Rosa PA. (2009). OspC-independent infection and dissemination by host-adapted Borrelia burgdorferi. Infect Immun 77, 2672–2682. 10.1128/IAI.01193-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, et al. (2006). Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian Infection. Infect Immun 74, 3554–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager B, Shaw DK, Groshong AM, Blevins JS, and Skare JT (2015). BB0744 Affects Tissue Tropism and Spatial Distribution of Borrelia burgdorferi. Infect Immun 83, 3693–3703. 10.1128/IAI.00828-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidmann MS, Bleichrodt FS, Laslo T, and Riedel CU (2011). Bacterial luciferase reporters: the Swiss army knife of molecular biology. Bioeng Bugs 2, 8–16. 10.4161/bbug.2.1.13566 [DOI] [PubMed] [Google Scholar]
- Wang G, Ma Y, Buyuk A, McClain S, Weis JJ, and Schwartz I (2004). Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol Lett 231, 219–225. 10.1016/S0378-1097(03)00960-1 [DOI] [PubMed] [Google Scholar]
- Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Hook M, and Skare JT (2008). Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun 76, 5694–5705. 10.1128/IAI.00690-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP (2006). Hematogenous dissemination in early Lyme disease. Wien Klin Wochenschr 118, 634–637. [DOI] [PubMed] [Google Scholar]
- Xu T, Close D, Handagama W, Marr E, Sayler G, and Ripp S (2016). The Expanding Toolbox of In Vivo Bioluminescent Imaging. Front Oncol 6, 150. 10.3389/fonc.2016.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, Samuels DS, and Norgard MV (2005). Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol 187, 4822–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Contag PR, Madan A, Stevenson DK, and Contag CH (1999). Bioluminescence for biological sensing in living mammals. Advances in experimental medicine and biology 471, 775–784. 10.1007/978-1-4615-4717-4_89 [DOI] [PubMed] [Google Scholar]
- Zhi H, Weening EH, Barbu EM, Hyde JA, Hook M, and Skare JT (2015). The BBA33 lipoprotein binds collagen and impacts Borrelia burgdorferi pathogenesis. Mol Microbiol 96, 68–83. 10.1111/mmi.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
















