Abstract
Background:
We have optimized a technique for cannulation of mesenteric lymph duct (MLD) in mice. Mice have low rates of intestinal lymph production, the MLDs are smaller and associated with fragile vasculature. Previous protocols for lymph collection based on the open lymph fistula model were associated with low success rates in mice. Bariatric surgery procedures worsen success rates due to post-operative adhesions and GI rearrangement. We have used this procedure to collect mesenteric lymph from mice undergoing bile diversion from gall bladder to ileum (GB-IL).
Hypothesis:
We hypothesize that peptide YY (PYY) levels in mesenteric lymph will increase following nutrient delivery in mice undergoing bile diversion from gall bladder to ileum (GB-IL).
Methods and Results:
We observe that cannulation of the MLD using a needled-catheter maintains lymph vessel integrity, prevents excessive lymph leakage, and is less traumatic leading to high success rates (>95%). PYY levels in mesenteric lymph after GB-IL were significantly higher post nutrient infusion. The procedure takes approximately 20 minutes; small rodent surgical experience and practice are required for success.
Conclusion:
Intestinal lymph can be collected from mice including those undergoing bariatric surgery procedure with high success rates by cannulation of the mesenteric lymph duct.
INTRODUCTION
This protocol describes the procedure for cannulation of the mesenteric lymph duct (MLD) in mice, and collection of mesenteric lymph over an extended period of time. The gastrointestinal lymphatic network is a one-directional vascular system (Figure 1) that plays critical roles in regulation of fluid homeostasis, mucosal immune function in acquired immunity, and transportation of dietary lipids. Conduits of this network return interstitial fluid and filtered tissue metabolites to the venous circulation to maintain fluid balance. Although short and medium chain fatty acids can be absorbed via the portal circulation, long chain fatty acids (>12C), including essential fatty acids must first be transported via chylomicrons to the intestinal lymphatic ducts (lacteals) before delivery to circulation. Recent reports suggest that lymph serves as a valuable tool for measuring incretins, GLP-1 and GIP that reach much higher concentrations in the intestinal lymph compared to peripheral blood because they are not diluted out by lymph from peripheral tissues.1,2 More importantly, the enzyme that proteolytically inactivates these hormones (DPP-IV, CD26) is absent in the lymph - t1/2 for incretins in blood is less than 2 minutes vs. t1/2 in the lymphatics 30–60 min3. Collection of lymph would therefore be vital to study incretin responses, and regulation of other metabolites in their nascent states before dilution and delivery to systemic circulation. More recently, gut peptides and hormones with established roles in hunger and satiety have been detected in the lymph, suggesting the possibility of unknown, alternate routes to transmission of sensory inputs to the brain.
Figure 1. Surgical Anatomy of gastrointestinal lymphatic network.
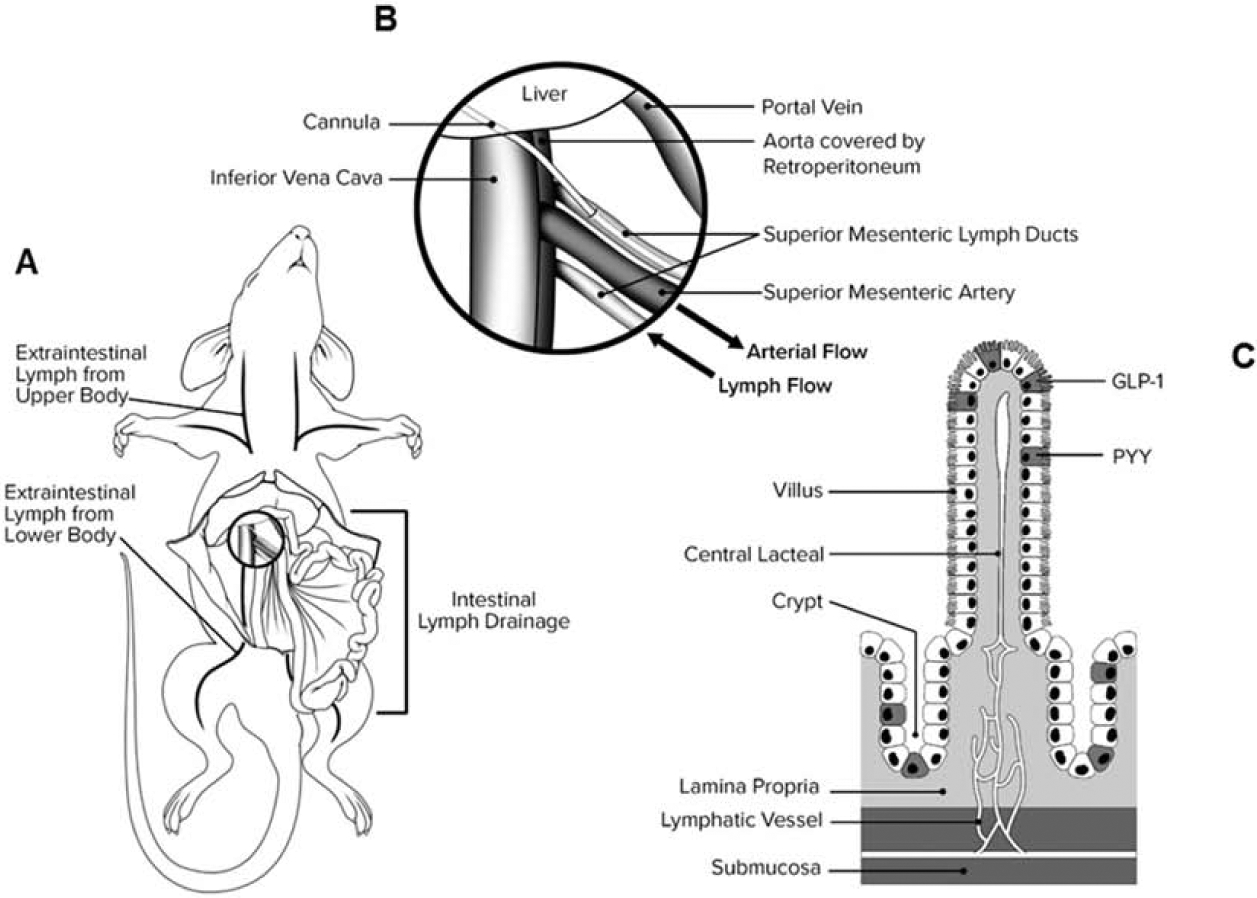
(A) MLD runs parallel to SMA (B) MLD is cannulated in the opposite direction of lymph flow so the lymph drains into the collection catheter. (C) Lymphatic capillaries (lacteals) are located below the absorptive epithelium of intestinal villi and form a sub-mucosal lymphatic network that drain into MLD.
Currently, procedures for collecting lymph in rats and mice are based on the lymph fistula model popularized by Tso and colleagues9. This protocol outlines the sampling of lymph from MLD of rats for up to 3 hours post nutrient bolus. When we applied the protocol to mice, the success rate dropped (50–60%). We speculate that this may be attributable to significant differences in size and anatomy of lymphatic ducts and lymph output between rats and mice. First, MLDs in mice are significantly smaller and adjoining blood vessels are more fragile. In the rat lymph fistula model, cannulation is done by inserting a catheter into the fistula. When same approach is applied to mice, the continuous outflow of lymph through the puncture site obscures the MLD opening making the cannulation difficult and time consuming. Moreover, leakage of lymph through the lymphotomy on the MLD reduces lymph output, which becomes critical when working with mice where lymph production is lower. In this communication, we describe key modifications to previous protocols3,4 that allows the procedure to be performed in mice with >95% success rate. Our technique uses a needled catheter to cannulate the MLD instead of puncturing it, which prevents lymph leakage, increases lymph flow, and is overall less traumatic. Since the lymph does not leak continuously outside of MLD, it remains clearly visible making cannulation easier. Moreover, lymph flow into the catheter can be visualized immediately following cannulation, such that the process can be repeated in case of unsuccessful cannulation.
Applications of the method
Our MLD fistula model enables lymph sampling for up to 6 hours post nutrient bolus. Feasibility of lymph collection in mice will allow investigation of effects of innumerable genetic modifications on macronutrient metabolism, release of incretins and relevant gut peptides and hormones. Specifically, we have successfully used this model to collect lymph from bariatric surgical mouse models, including Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), bile diversion to ileum (GB-IL) Gastro paper, and bile diversion to proximal duodenum (GB-D) (unpublished work). The low success rate of previous procedures posed a formidable challenge to performing lymph studies in mice undergoing these intensive surgery procedures due to post-operative adhesions and GI tract rearrangement. Since the role of lymphatic system in metabolic improvements following bariatric surgery is yet to be investigated, this technique offers an additional avenue for exploring mechanisms underlying metabolic benefits of bariatric surgery.
Comparison with other models that use MLD cannulation
Since the introduction of lymphatic cannulation in the 1940s,5 few modifications have been made to the original protocol6, which relies on creating a fistula in the MLD by means of micro-scissors or micro-forceps, and then inserting a catheter into the lymph vessel. Despite its feasibility, we observed that this technique has high failure rates in mice especially in bariatric surgery models and has been an obstacle for further investigation. Mice have smaller MLDs with significantly lower lymph output. The open fistula model makes cannulation harder and is associated with reduced lymph output due to drainage into abdominal cavity. Use of needle to puncture the MLD in our protocol keeps it intact, making catheter insertion easier, and less traumatic. Also, since lymph does not continue flowing out there is no need for cleaning the layers of connective and adipose tissue surrounding the lymph duct that has been reported as being critically important7 to avoid the risk of lymph sticking to them. Representative data demonstrate how the improved cannulation model can be used to examine nutrient transport from the intestine via the mesenteric lymphatic network. We also describe challenges associated with establishing the protocol and explain the solution to those problems. Once established, this technique is a powerful tool to obtain samples from intestinal lymph and measure analytes of interest in mice.
Key points for successful implementation of the protocol
Several details and steps are critical for successful application of this technique:
Visualization of the lymphatic duct: The lymphatic ducts are readily visible in post-prandial mice because of the appearance of dietary lipids within the lymphatic system. For beginners we recommend the use of olive oil (maximal recommended dose of 17 μL/g body weight)8 via oral gavage 30–60min prior to cannulation. Exceeding the maximum allowable dose of olive oil will result in thickening and clotting of lymph in the catheter, blockage and ultimate catheter loss. Another alternative is to perform the cannulation procedure early in the post-prandial state, early in the morning, where lymph ducts are visible without use of olive oil.
Avoid simultaneous blood collection before or during lymph collection. Frequent blood collection during the cannulation procedure leads to significantly decreased lymph flow rates, likely secondary to splanchnic constriction of both mesenteric blood and lymphatic vessels. If needed, we recommend that blood collection be performed after completion of the lymphatic cannulation.
Catheter course and exterioration must parallel the lymphatic duct: Lymphatic vessels are highly tenuous and fragile. A catheter placed at any angle to the lymph duct increases the risk of vessel rupture due to tension on the vessel wall.
Short duration of surgery: We strongly recommend that the duration of surgery for lymph cannulation in mice remain under 30 minutes. We have observed that owing to low lymph flow rates in mice, longer operative time leads to stasis of the lymph inside the catheter and clot formation.
Experimental design
The experimental paradigm of our lymph cannulation protocol is shown in Figure 2. The study is completed over 2 days. On day 1, duodenal infusion of 5% dextrose in sterile saline is administered (via feeding catheter) following lymph cannulation and continued overnight. On day 2, three hours prior to lymph collection, the duodenal infusion is switched to saline (only) and allowed to equilibrate for an hour. Baseline lymph is then collected for a 2-hour period. A nutrient bolus is then administered via the feeding catheter (duodenal catheter placed at the time of the surgical procedure – see later comment) over a 5-minute period. Lymph is then collected for the next 3 hours for hormonal/nutrient analyses. Lymph collection tubes are kept on ice during the experiment and the amount of lymph produced is calculated from weight differences of the collection tubes before and after collection.
Figure 2. Representative study design and experimental paradigm.
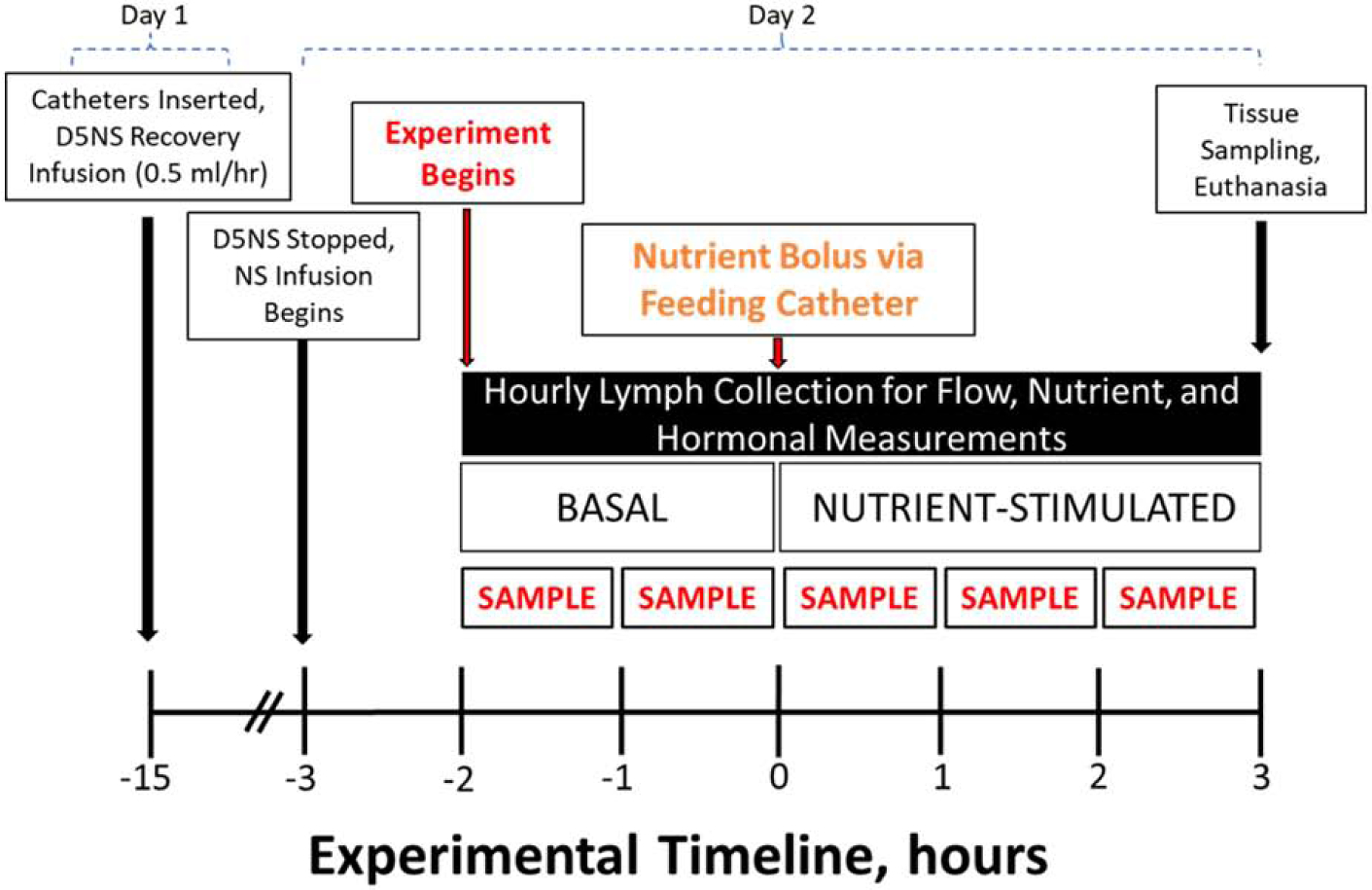
Cannulations are performed on day 1. Immediately following catheter cannulation, mice are placed in Bollman cages and maintained in a temperature and humidity control chamber. Mice receive 5% dextrose in normal saline (D5NS, 0.5mL/hr.) infusions overnight for nutrition and fluid/electrolyte replacement. At the start of day 2, D5NS infusion is discontinued and normal saline (NS) begun for fluid and electrolyte replacement. After a one-hour acclimation period, basal lymph sampling begins and continues for 2 hours, following which nutrient bolus is administered (time zero). Hourly lymph fractions are collected for up to 3 hours post nutrient bolus.
MATERIALS
REAGENTS
All equipment and reagents can be substituted with appropriate alternatives from other providers and manufacturers.
Mice: Male and female C57BL/6J mice (preferably at least 6 weeks old; 6–8 weeks were used in the following experiments) were housed at 23 °C on a 0700–1900-hour light cycle and were fed a standard chow diet (Jax Labs; Bar Harbor, ME). Mice are fed ad libitum, a high fat (60% kcal from fat) diet (Bio-Serv, Frenchtown, NJ) starting at 6 weeks of age for 12 weeks. All experiments and surgical preparations were performed according to protocols approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee (IACUC). Mice remained under the care of the Division of Animal Care (DAC) at Vanderbilt University in compliance with NIH guidelines, Principles of Laboratory Animal Care, and the Guide for the Care and Use of Laboratory Animals.
Ketoprofen (Patterson veterinary supply, cat. no. 07-803-7389). This compound can be stored at room temperature until the expiration date.
Isoflurane (Patterson veterinary supply, cat. no. 07-890-8115). This compound can be stored at room temperature until the expiration date. Caution: Isoflurane causes respiratory depression and thus animals must be monitored closely during the procedure.
Ophthalmic lubricant (Patterson veterinary supply, cat. no. 07-888-2572). This compound can be stored at room temperature until the expiration date.
Glue (cyanoacrylate glue, cat. no. KG585). This compound can be stored at room temperature until the expiration date.
Dextrose (5) in 0.9% sodium chloride (Baxter, cat. no. 2B1064X) can be stored at room temperature until the expiration date.
Sodium chloride, 0.9% (Baxter, cat. no. 2B1323Q) can be stored at room temperature until the expiration date.
Feeding bolus (Ensure®). This compound can be stored at room temperature until the expiration date.
Trasylol (DSM Pentapharm, cat. No. 073–80): This product needs to be stored at 4°C
DPP-IV inhibitor (Millipore, cat. no. DPP4–010). This product needs to be stored at 4°C upon arrival and at −20°C. for long-term use of more than 2 weeks.
Pefabloc (Roche, cat. no. 11 429 876 001): This product needs to be stored at −20°C.
Syringe 1mL (BD, cat. no. 329654). This compound can be stored at room temperature until the expiration date.
Syringe 20 mL (BD, cat. no. 302830).
Heparin (10,000 U/mL; McKesson, cat. no. 2020048). This compound can be stored at room temperature until the expiration date.
EDTA-coated microfuge tubes (Microvette, cat. no. 20.1341)
Hypodermic Needles, 30 gauge (BD, cat. no. 305106), 23 gauge (BD, cat. no. 305143) and 18 gauge (BD, cat. no. 305196).
EQUIPMENT
Infusion pump (Harvard Apparatus, cat. no.70-2000)
Incubator (Hill-Rom Air-Shields, Isolette Warmer Incubator C2000)
Bollman Cages (locally manufactured)
Surgical microscope (M690 Leica, Wild Heerbrug surgical operating microscope, Leica Microsystems, Wetzlar, Germany)
Heating pad (stryker)
Glove (Protexis, Ref. no. 2D72PT70X)
Micro scissors
Hair clippers
Cotton tipped applicators (Puritan, Ref. no. 826-WC)
Polyvinylchloride (PVC) catheters; ID 0.20mm × OD 0.50mm and ID 0.50mm × OD 0.80mm) (SteriHealth, cat. no. I-10320)
Reagent Setup
Inhibitor cocktail:
A mixture of three protease inhibitors is created by mixing the same volume of Pefabloc (100 mg/mL), DPP-IV (5 mM), and Trasylol (20,000 KIU). The final concentrations are Pefabloc (33 mg/mL), DPP-IV (1.6 mM), and Trasylol (6666 KIU). Add 6μL of the mixture to the EDTA collection tubes. Tubes are weighed prior to lymph collection. After adding the cocktail to the lymph collection samples, the tubes should be kept cold until the end of experiment when the samples have been aliquoted.
Equipment Setup
Surgical station:
All procedures should be performed under aseptic conditions. All surgical tools must be sterilized before use. Needled catheters should be prepared in mass and sterilized before implantation.
Post-operative animal housing:
Animals should be individually housed in Bollman restraint cages postoperatively as described6,10–13. We recommend that cages be placed in temperature controlled incubators before surgery to allow for temperature equilibration. Animal well-being must be monitored continuously according to approved protocols and institutional guidelines.
METHODS
Day 1
Preparation of lymphatic catheters and animals
-
1
First, 30-gauge needles are locked (approximately 4 mm distal to the tip) using a Castroviejo needle holder, under micro-surgical microscope. The needles are filed to create a brittle spot using micro-file, cut and inserted into poly-vinyl chloride (PVC) tubes (0.2 mm ID, 0.5 mm OD) (Fig. 3). Then, the needled catheters are inserted into larger PVC tubes (0.5 mm ID, 0.8 mm OD) to create a 15 cm catheter. Flush assembled catheters with heparin to prevent clotting of lymph inside the catheters.
-
2
Sterilize all necessary equipment.
-
3
Weigh the animal prior to surgery.
-
4
Shave abdominal fur from the femoral region to the xiphoid. Subsequently, clean the surgical site on the abdomen using povidone-iodine solution and 70% ethanol scrub.
-
5
Administer the preoperative analgesia by subcutaneous injection of Ketoprofen (5–10 mg/kg). We do not recommend using narcotics for analgesia due to their depressive effects on GI peristalsis.
-
6
Anesthetize the mouse by placing in the induction chamber with 800 mL/min oxygen flow and 2% isoflurane. Check for complete anesthesia by pinching the toe or tail. Once the mouse is appropriately anesthetized, place on the heating pad in dorsal recumbence position to maintain the body temperature at 37°C throughout the procedure. Apply the ophthalmic ointment to prevent corneal drying.
Figure 3. Lymphatic catheter preparation.
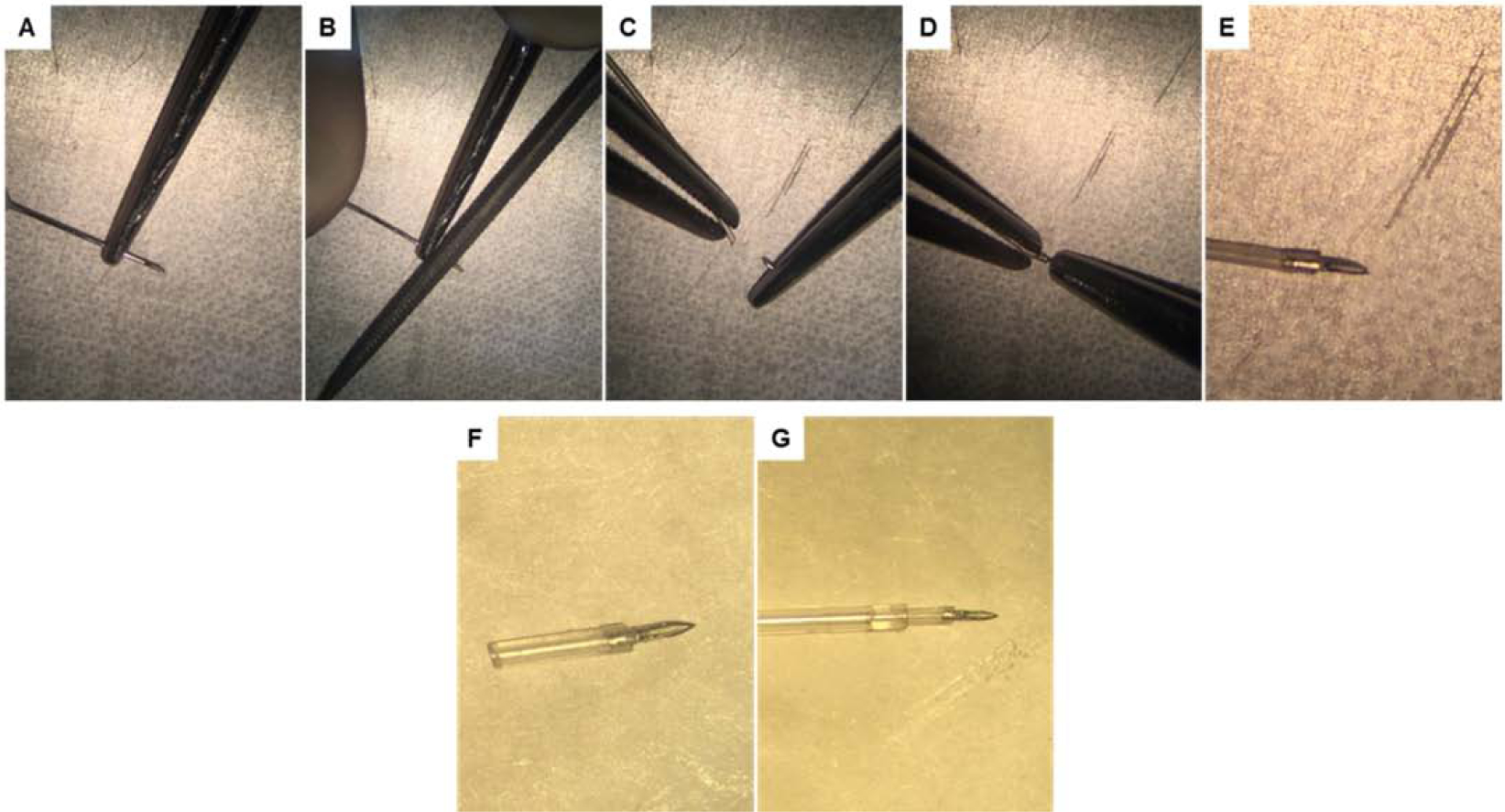
(A) The tip of a standard 30G needle is immobilized (approximately 4–6 mm proximal to the tip) using a Castroviejo needle holder. (B) Using a precision microfile, a brittle point is created circumferentially on the needle shaft. (C-D) After filing is complete, the tip of the needle is cut and inserted into the first catheter (0.2 mm ID, 0.5 mm OD); the overhanging portion is cut before insertion into the second catheter. (E-F) After the first catheter is needled its being cut with surgical scissors to reach 5–7 mm in length. (G) The first catheter with the needled end is then inserted to the second catheter (0.5 mm ID, 0.8 mm OD). The total length of assembled catheters is around 15 cm.
Surgical Procedures
Catheterization of MLD
-
7
Incise the abdomen on the midline. Retract the intestine and colon to the left side of the animal and cover with wet sterile gauze to protect against dryness. Locate the superior mesenteric artery (SMA) and the adjacent mesenteric lymph ducts (Fig. 4).
-
8
Dissect the retroperitoneal attachment of the liver (Fig. 5A). Pass an 18G needle from inside to the outside of the right abdominal wall while taking care that the needle is parallel to the SMA (Fig. 5B–C). Then, thread the needled catheter into the abdomen through the needle (Fig. 5D).
-
9
Rotate the needle so the beveled part of the needle becomes visible. Grab the needle shaft by micro-forceps and gently insert the needle into the MLD (Fig. 5E–F and 6A–B). Advance the needle for 2–3 millimeters and secure in place with a drop of cyanoacrylate glue (Fig. 5G and 6C–E).
-
10
Do not advance the needle too far into the MLD. This can lead to tearing of the lymph duct and leakage of lymph. Before proceeding it is crucial to ensure that the glue has dried. Even small undried parts of glue can adhere to other organs in the abdomen (e.g. intestine) and cause obstruction and failure of the experiment.
Figure 4. Vascular and lymphatic anatomy adjacent to the MLD.
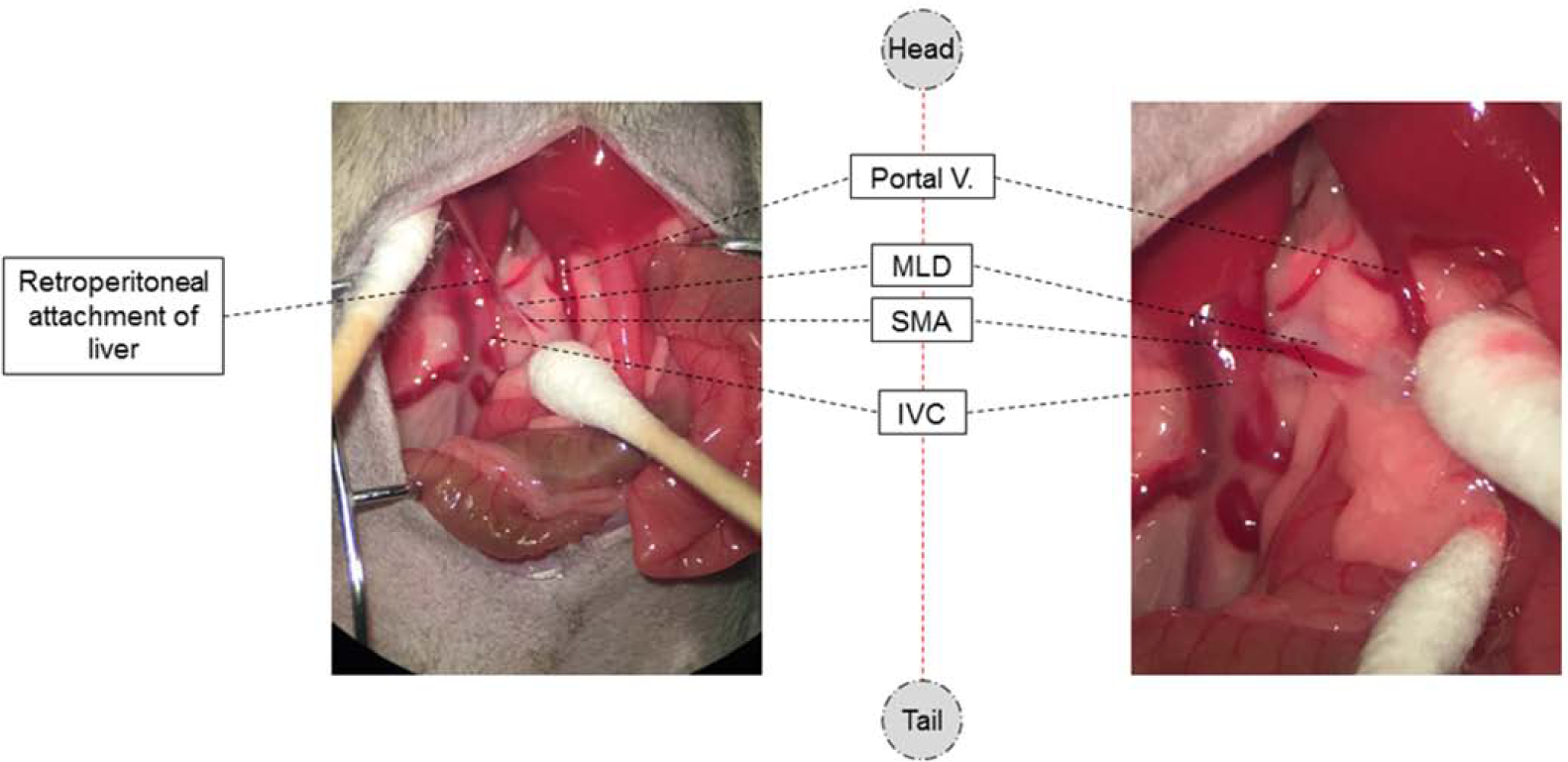
The blood and lymphatic vessels adjacent to the MLDs are composed of two small lymphatic branches that run parallel to SMA. These branches represent the confluence of the intestinal lymphatic system that drains the lamina propria. Aside from the MLDs, the SMA is easily visible, along with the portal vein and the inferior vena cava (IVC).
Figure 5. Mesenteric lymph duct cannulation.
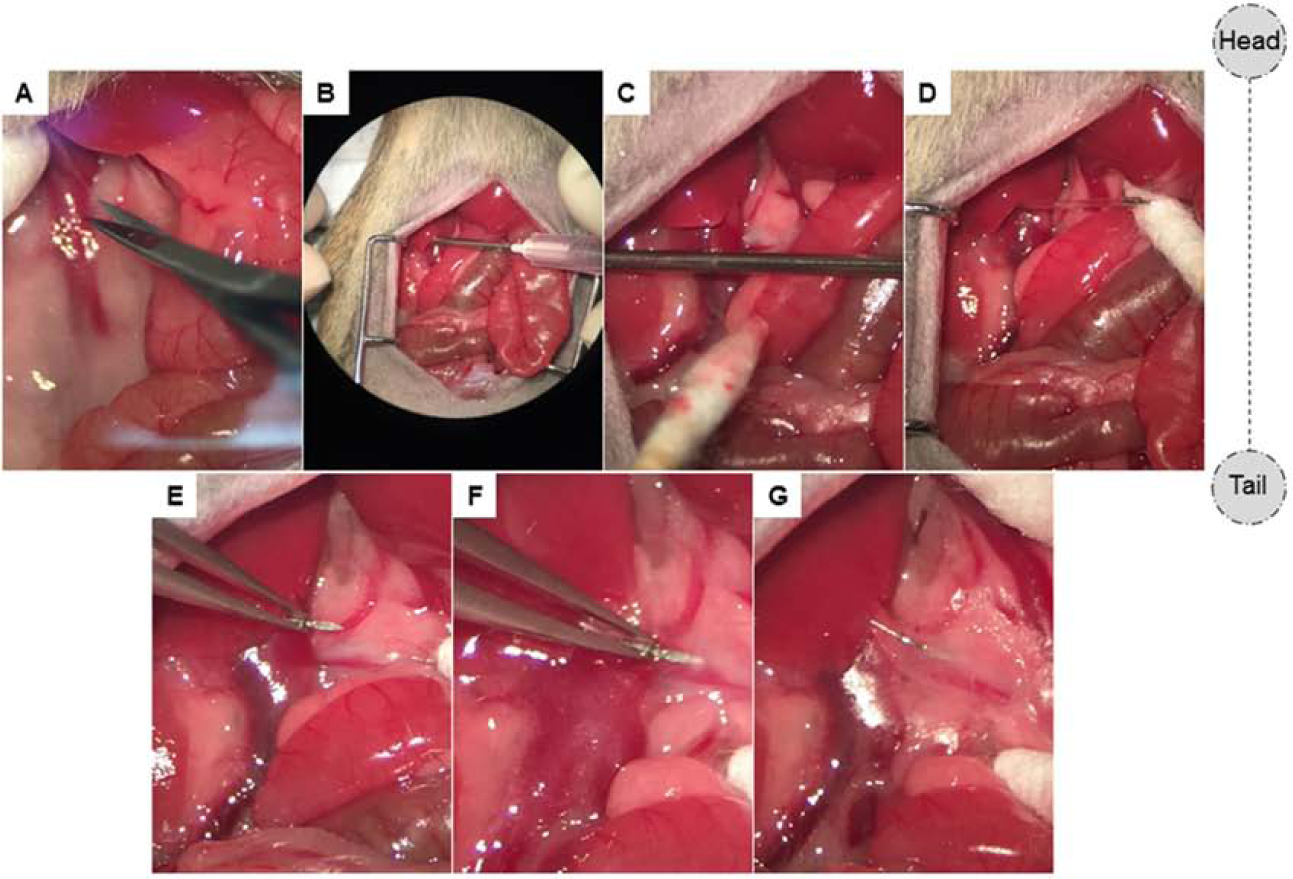
(A) Retroperitoneal attachment of the liver is released using micro-scissors in order to expose the MLDs sufficiently. (B-C) An 18G needle is passed through the abdominal wall from inside to out on the right side and inferior to the liver, parallel to SMA. (D) The lymphatic catheter is placed through the 18G needle and then the needle is removed leaving the catheter in place. (E-F) The tip of needled catheter is carefully inserted (~2–3mm) into the MLD. (G) Once the catheter is in place and lymph starts flowing, it is secured with a drop of cyanoacrylate glue.
Figure 6. Intestinal lymph flow starts immediately after correct placement of catheter.
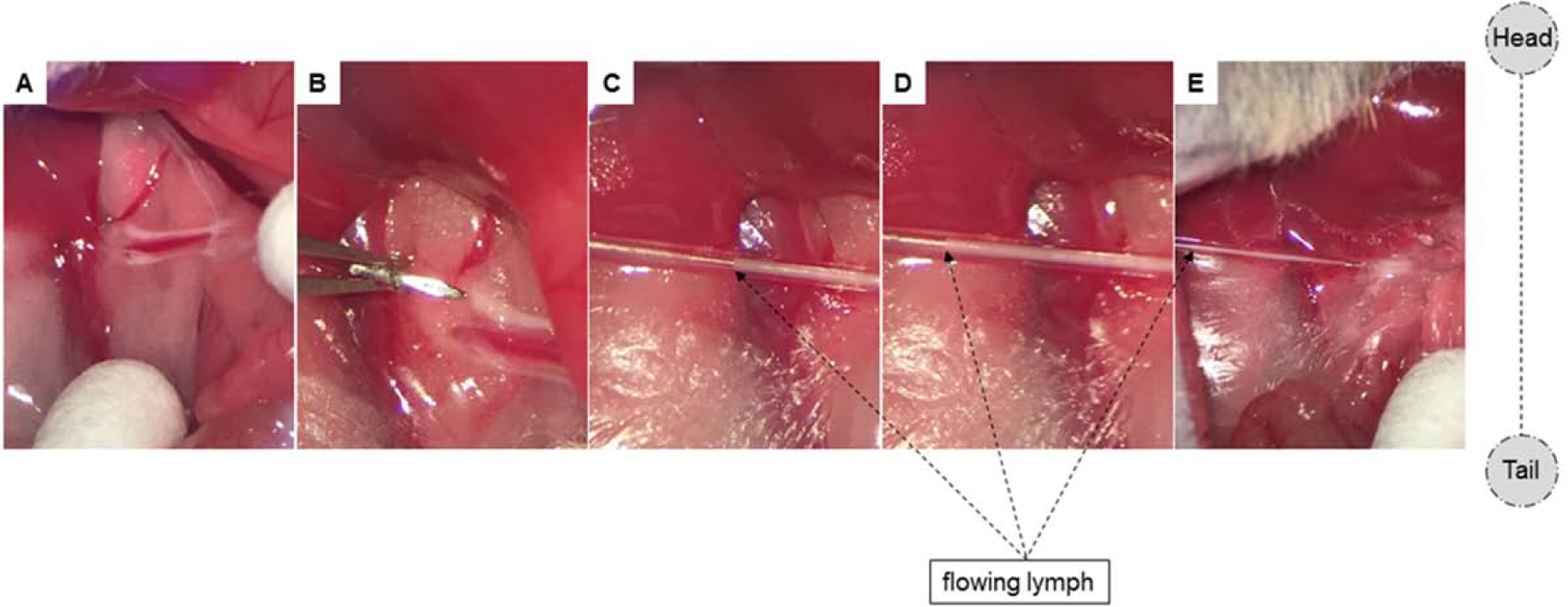
To augment the appearance of the lymph fluid for demonstrative purposes, commercially-available olive oil was administered 30min prior to cannulation. (A) Two MLDs are clearly visible with bright, white lymph fluid. (B-D) Immediately after lymphatic cannulation, lymph starts flowing. Pictures (C-E) were taken 3, 5, and 8 seconds after cannulation, respectively.
Placement of Feeding Catheter
-
11
After making sure that the glue on the lymphatic catheter is dried, retract back the intestine and colon into the abdomen. Then, locate the stomach and fix in place by a cotton-tip applicator.
-
12
Pass an 18G needle from inside to the outside of the left abdominal wall. Then, internalize the feeding catheter (PVC tube, 0.5 mm inner diameter, 0.8 mm outer diameter) through the lumen of the needle (Fig. 7A–B).
-
13
Create a U-stich in the greater curvature of the stomach using 6–0 absorbable polyglactin suture (Fig. 7C–E). Puncture the stomach by a 20G needle and insert the feeding catheter into the stomach and advance through the pylorus into the duodenum (Fig. 7F–I). Then, tie the U-stich and seal with a drop of cyanoacrylate glue (Fig. 7J). Seal the openings of the catheters on the skin with a drop of glue.
-
14
Close the abdomen and skin with interrupted 6–0 absorbable polyglactin suture and 6–0 non-absorbable monofilament nylon sutures, respectively. Administer 0.5 mL of warm normal saline subcutaneously to compensate for fluid loss during operation.
-
15
Carefully place the mouse in the Bollman restraint cage ensuring adequate mobility to allow breathing comfortably. House the mouse in a temperature and humidity-regulated incubator. To compensate for fluid and electrolyte loss due to lymphatic drainage overnight, connect the feeding catheter to the infusion pump supplemented with 5% glucose in saline infusion (0.5 ml/h).
-
16
House the animal overnight in a temperature- and humidity-controlled incubator. Collect the lymph in a 25mL Erlenmeyer flask, ensuring that the tip of the catheter does not touch the walls of the flask to prevent clotting of the lymph overnight (Fig. 8A).
Figure 7. Placement of feeding catheter.
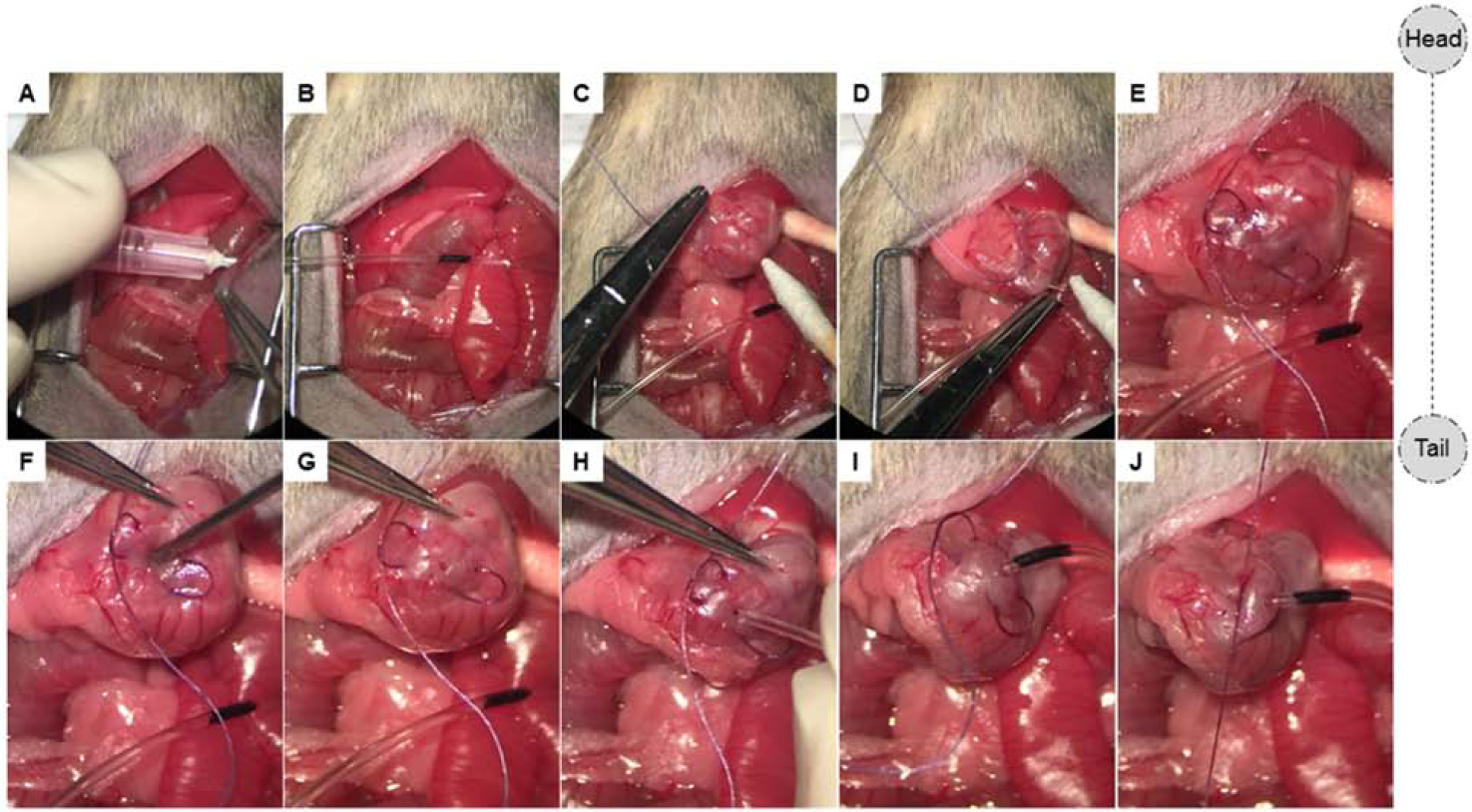
(A-B) An 18G needle is exteriorized on the left side and the feeding catheter placed through the needle. (C-E) A U-stich is made along the greater curvature of the stomach. (F-J) An opening is then made using a 20G hypodermic needle, the catheter is subsequently inserted and advanced through the pylorus into the first portion of the duodenum. To ensure that the catheter remains in place in the duodenum, a mark is made at a length sufficient to ensure the tip remains in the duodenum prior to securing. The suture is tied and a drop of cyanoacrylate glue is applied to further secure the catheter.
Figure 8. Lymph collection.
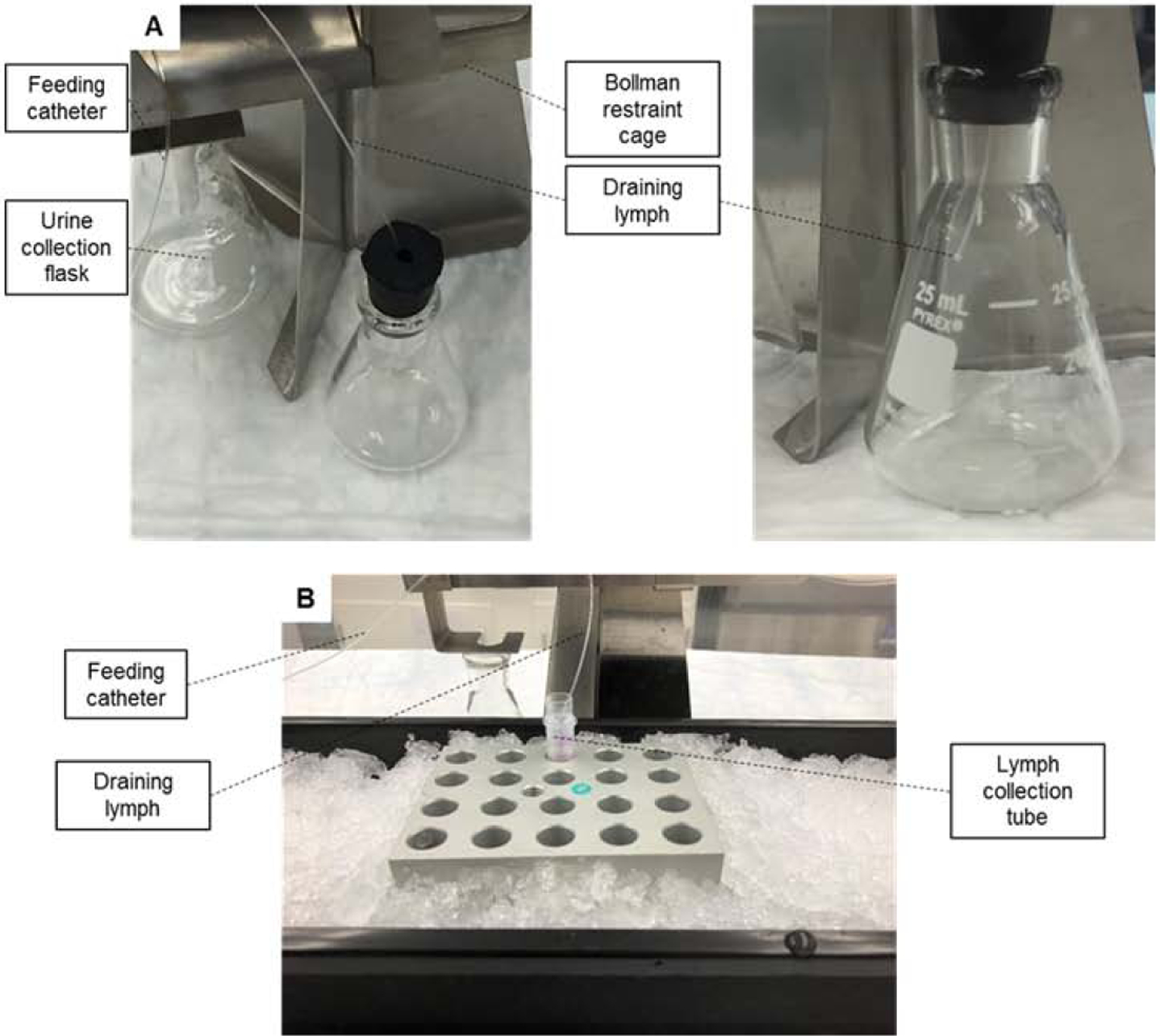
(A) The tip of the lymph catheter is inserted into an Erlenmeyer flask (day 1). The collected lymph will be discarded and will not be used for analytical purposes. (B) During the actual lymph collection experiments, the collection tube is kept at 0–4° C (day 2).
Day 2
Preparation for the experiment
-
17
Stop the infusion pump and unhook the feeding catheter from the pump. Change the infusion solution to normal saline (no glucose) and flush the line. Hook on the feeding catheter to the infusion pump and restart the infusion with same flow rate.
-
18
After one hour of saline infusion, start the lymph collection experiment. Place the lymphatic catheter into the EDTA tubes loaded with inhibitor cocktail. Collect hourly baseline samples for two hours (Fig. 8B).
-
19
Unhook the feeding catheter and hook it to a feeding syringe loaded with 400 μL nutrient bolus. Gently push the syringe to deliver the bolus gradually over 3–5 minutes. Rapid infusion of the nutrient bolus can cause expansion and tearing of intestinal wall. After the animal has received the bolus, unhook the feeding catheter from the syringe and hook it back to the saline infusion catheter. Continue cumulative hourly sample collections for three hours post-nutrient bolus.
RESULTS AND DISCUSSION
Approximately 50 animals were used in the development of the technique. After successful development and establishment of the protocol, our research team conducted 225 mice with an overall success rate of 96%. Typical factors associated with failure included the following: increasing age, pre-procedural blood collection or bleeding during catheter placement, and low body weight (<12g)
We first validated that samples collected were enriched in lymph. We measured complete blood counts and found that collected lymph samples were consistently enriched with lymphocytes (Fig. 9A). Lymph collected using this protocol was more than 200μL/hr (baseline) and about 400μL/hr post-prandially (Fig. 9B). Glucose and triglyceride outputs increased up to 1 hour following nutrient bolus and decreased subsequently (Figs. 9C, D, respectively).
Figure 9. Basal and post-prandial lymph volume and nutrient levels in intestinal lymph.
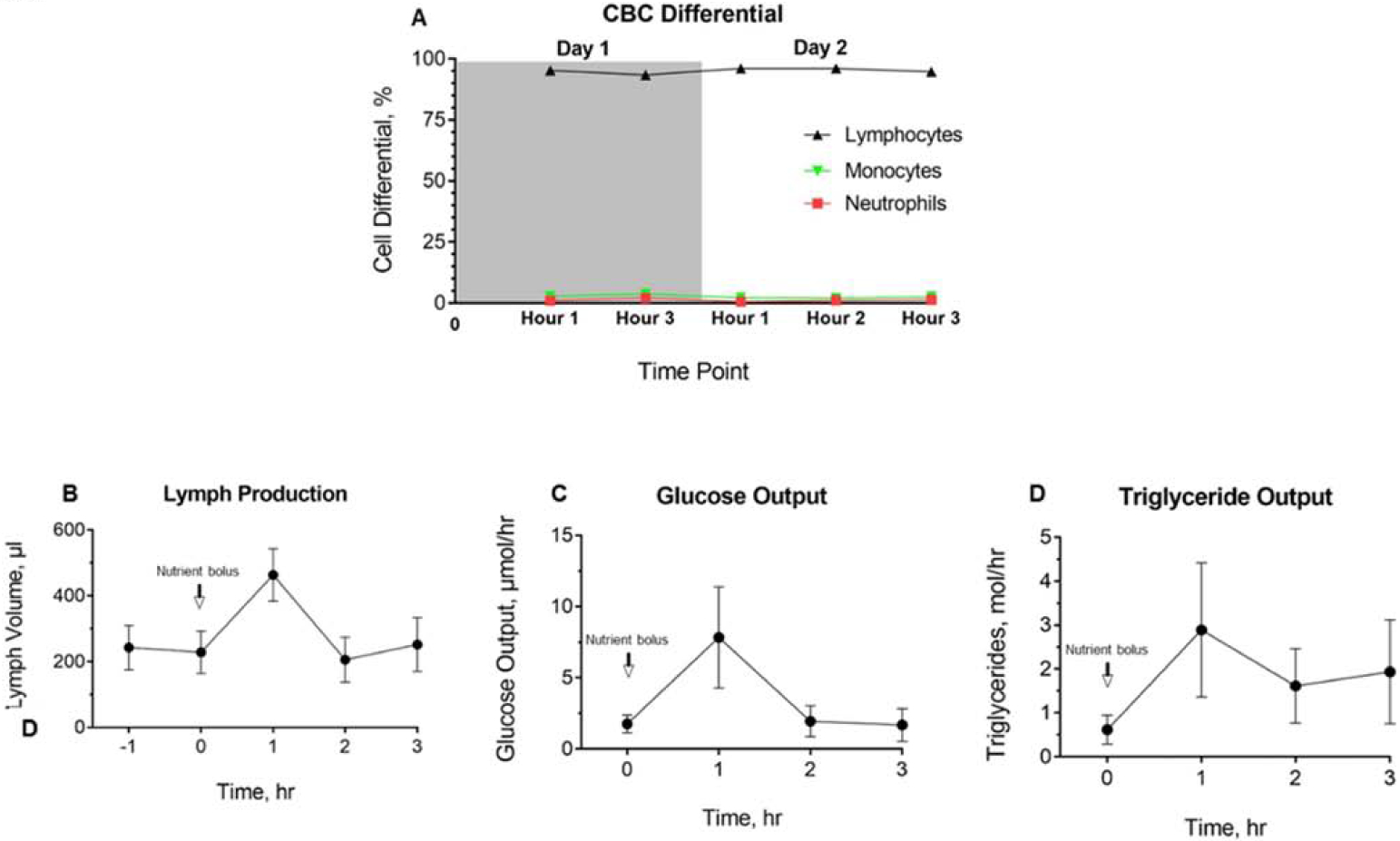
(A) Complete blood count with differential analysis of lymph collected on day 1 and day 2 (n=6). (B) Baseline and post-prandial lymph volume, (C) glucose output, and (D) triglyceride output on day 2 are shown (n=5). Data are represented as the mean ± standard error.
We reported earlier that GIP and GLP-1 levels in mesenteric lymph obtained from mice undergoing GB-IL were significantly higher post nutrient infusion (GASTRO Paper). Incretin hormones GIP and GLP-1 are rapidly degraded in the plasma (in less than 2 minutes) due to the presence of the proteolytic enzyme dipeptidyl peptidase IV (DPPIV). DPPIV is absent in the lymph, hence incretins stay intact. Given their rapid degradation in blood, metabolic studies designed to measure plasma incretin levels have posed technical challenges. Lymph collected using our method overcomes this challenge. In this study we measured levels of satiety hormone PYY in the lymph. PYY is secreted in response to nutrient entry by L cells that are abundant in the distal small intestine. We hypothesized that like blood levels, lymph PYY levels will be elevated following nutrient infusion. As shown in Figure 10, lymph PYY levels in control mice (with no surgical manipulations) were 10 fold-higher at 1 hour post nutrient infusion and dropped at the end of 3 hours. Mice that underwent GB-IL showed the same trend. Baseline PYY levels were higher and nutrient infusion led to approximately 3 fold increase in PYY levels that dropped by the end of 3 hours. Thus, intestinal lymph can be collected from mice successfully by the cannulation method described above. This technique can also be used in mice undergoing bariatric surgery procedures.
Figure 10. Basal and post-prandial PYY levels in intestinal lymph from mice undergoing no or GB-IL surgeries.
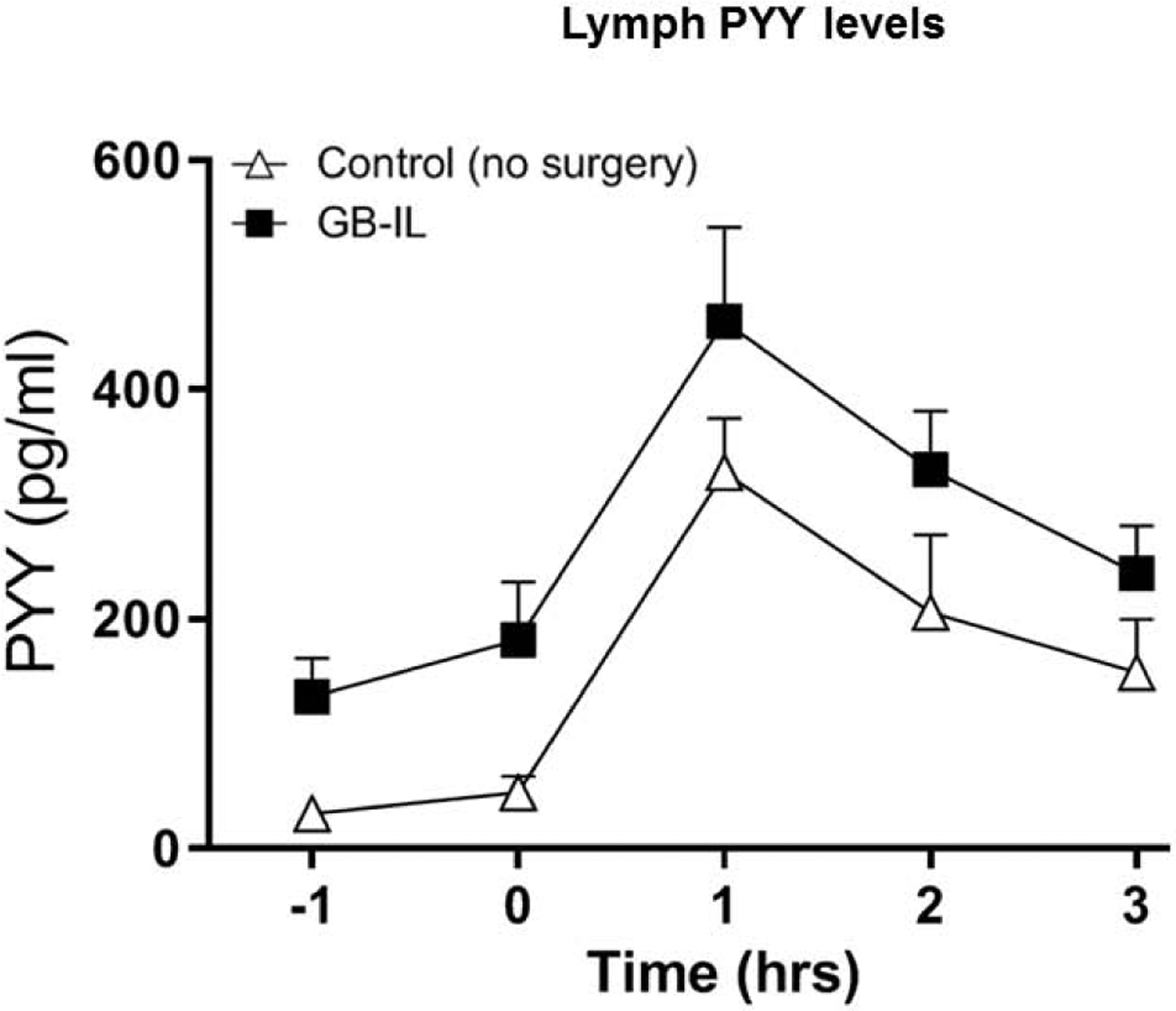
Baseline and post-prandial lymph PYY levels (on day 2) are shown (n=8 for control; 6 for GB-IL). Data are represented as the mean ± standard error.
CONCLUSION
Lymph collection in mice has remained challenging due to size and fragility of vascular and lymphatic vasculature. Our technique outlines how to overcome this challenge; its use in mice enables investigation of role of the genetic landscape in fat transport, enteroendocrine hormone regulation of fat metabolism, and lymph in metabolic diseases including obesity and type 2 diabetes.
Supplementary Material
Acknowledgments:
We thank Professor Phillip Williams at Vanderbilt University Medical Center for his invaluable support. The study was funded by F32 DK0113712-02 to BB, F32 DK103474-01A1 and DK076169 to VA, R01 DK105847-01 to CRF and NNA. O.S. is a Howard Hughes Medical Institute Medical Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement: The authors declare no conflict of interest.
References
- 1.Tong J et al. The intestinal lymph fistula model—a novel approach to study ghrelin secretion. AJP Gastrointest. Liver Physiol 298, G474–G480 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu WJ et al. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. AJP Gastrointest. Liver Physiol 294, G1130–G1138 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Ohlsson L, Kohan AB, Tso P & Ahrén B GLP-1 released to the mesenteric lymph duct in mice: Effects of glucose and fat. Regul. Pept 189, 40–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohan AB et al. Apolipoprotein A-IV regulates chylomicron metabolism–mechanism and function. AJP Gastrointest. Liver Physiol 302, G628–G636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollman JL, Cain JC & Grindlay JH Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J. Lab. Clin. Med 33, 1349–52 (1948). [PubMed] [Google Scholar]
- 6.Zhang LS et al. Apolipoprotein A-V deficiency enhances chylomicron production in lymph fistula mice. AJP Gastrointest. Liver Physiol 308, G634–G642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevaskis NL, Hu L, Caliph SM, Han S & Porter CJH The Mesenteric Lymph Duct Cannulated Rat Model: Application to the Assessment of Intestinal Lymphatic Drug Transport. Journal of Visualized. J. Vis. Exp e52389–e52389 (2015). doi: 10.3791/52389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval JC et al. Molecular mechanisms of ezetimibe-induced attenuation of postprandial hypertriglyceridemia. J. Atheroscler. Thromb 17, 914–924 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Donnelly RF, Raj Singh TR & Woolfson AD Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 17, 187–207 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu WJ et al. Chylomicron Formation and Secretion is Required for Lipid-Stimulated Release of Incretins GLP-1 and GIP. Lipids 47, 571–580 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohan AB, Yoder SM & Tso P Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. November 30, 82–88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F et al. The role of apolipoprotein A-IV in regulating glucagon-like peptide-1 secretion. Am. J. Physiol. Gastrointest. Liver Physiol 309, G680–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nauli AM et al. CD36 Is Important for Chylomicron Formation and Secretion and May Mediate Cholesterol Uptake in the Proximal Intestine. Gastroenterology 131, 1197–1207 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


