Abstract
The world is currently under the threat of COVID pandemic and has focused every dimension of research in finding a cure to this novel disease. In this current situation, people are facing mental stress, agony, fear, depression and other associated symptoms which are taking a toll on their overall mental health. Nanoencapsulation of certain brain boosting polyphenols including quercetin, caffeine, cocoa flavanols and proteins like lectins can become new area of interest in the present scenario. Besides the brain boosting benefits, we have also highlighted the anti- viral activities of these compounds which we assume can play a possible role in combating COVID-19 given to their previous history of action against certain viruses. This review outlines the nanoencapsulation approaches of such synergistic compounds as a novel strategy to take the ongoing research a step ahead and also provides a new insight in bringing the role of nanotechnology in addressing the issues related to COVID pandemic.
Keywords: Lectins, Caffeine, Cocoa flavonoids, Nanoencapsulation, Quercetin
1. Introduction
As the current scenario of COVID-19 has left all the health workers in an adverse situation it becomes equally important for researchers to devise the strategy for formulation of drugs and therapies that have potential to alleviate the symptoms of emerging pandemic. The disease was first reported in Wuhan, in December 2019 and declared pandemic by World Health Organization (WHO) on March 11th 2020 labeling it as 5th registered pandemic after 1918 Spanish influenza. As of 27th September 2020, Covid-19 has already caused 32,925,668 positive cases and 995,352 deaths worldwide [1]. Some of the common behaviors observed in the prevailing situation are anxiety, confusion and fear which can take a toll on the mental health of people [2]. In a study, it was reported that COVID-19 situation triggered various mental issues like difficulty in sleep, social media distress and paranoia of acquiring this viral infection in 12.5%, 36.4% and 37.8% participants and 80% of participants needed mental healthcare [2]. This state of mind may result in the development of oxidative stress and loss of immunity which may further aggravate the symptoms. So we assume that if we exploit some naturally occurring anti-viral and brain-boosting compounds this may provide a new insight into the ongoing research to combat these inflictions of COVID-19. These brain boosting compounds if accompanied with the anti-viral activities can be a good area to dig into as no chemical therapeutic medicine is available to deal with this pandemic till date. In this review we have highlighted some brain boosting and anti-viral compounds derived naturally like quercitin, caffeine, lectins from banana (Banlec) and cocoa flavonoids which might prevent the neurons against apoptosis and oxidative stress (in case of quercitin) [3], provide anti-depressant effects (in case of caffeine & lectin) [4,5] and act as a neuro-protectant in case of cocoa flavonoid [6]. These compounds have reported anti-viral activities, like quercetin has inhibitory action against 3CLpro and PLpro protease of SARS and 3CLpro protease of Middle Eastern respiratory syndrome virus (MERS) [7], caffeine has anti-viral activity against human immunodeficiency virus type I (HIV-1) [8], lectin (Banlec) displayed anti-viral activity against influenza viruses [9] and flavonoids can inhibit the fusion of viral membrane with that of the lysosome [10]. By application of this synergistic approach alleviation of the associated symptoms of COVID-19 might be projected. To carry these compounds to their target sites, we foresee nanoencapsulation as a novel tool. The emerging field of nanotechnology has made a significant impact on the target delivery of nutraceuticals and therapeutics. The use of nanocarrier system presents an attractive strategy to provide for the target delivery of these health promoting compounds into the intracellular compartments. Moreover the application of nanotechnology in food and drug delivery systems has shown enhanced thermal stability, oral bioavailability and water solubility [11]. So in this review, we have summed up some of the brain boosting as well as anti-viral compounds and highlighted the means of nano-encapsulating these synergistic compounds which may pave a way in strategizing the formulation of therapeutics for combating the adverse conditions of COVID-19.
2. Synergistic approach (brain boosting and anti-viral target compounds and their mode of action)
The prospectus of anti-viral and brain boosting compounds derived from natural sources is more promising and some of these can grab a great deal of attention in coming years owing to their initial reports of anti-viral and brain stimulating effects. The domain of their health promoting factors is very wide which can have great scope amid this pandemic situation. The natural compounds including polyphenols and proteins have been studied over years to explore their anti-viral and brain stimulating effects. Summary of natural compounds with their anti-viral and brain boosting properties are given in Table 1, Table 2 .
Table 1.
Summary of some polyphenols with anti-viral properties, their possible mode of action and target virus.
| S. no | Antiviral polyphenols | Reported mechanism of action | Effective against virus |
|---|---|---|---|
| 1. | Quercetin | Inhibits viral entry and translation | Ebola virus [12] Polio virus [13] Hepatitis C [14] Coronavirus [15] Mango virus [16] Pseudorabies virus [17] |
| 2 | Resveratrol |
|
HSV [18] Influenza [19] Rhinovirus [20] MERS-CoV [21] |
| 3 | Fisetin, rutin | Induce inhibition of cytokine expression and synthesis | H1N1 [22] |
| 4 | Catechin Epicatechin Epicatechingallate |
Inhibits viral binding. | Sindabis virus [23] |
| 5 | Morin, galangin | Inhibits NLRP3 inflammasome | Poliovirus 1 HSV-1 & 2 RSV HCV CDV SARS-CoV [22] |
| 6 | Kaempferol | Inhibits secretion of many cytokines including IL-6 and IL-8. | Human cytomegalovirus Coxsackie B virus [24,25] |
| 7 | Myricetin | Inhibits ATPase activity | SARS-CoV [26] |
| 8 | Theaflavin | Inhibits RdRp activity | SARS-CoV-2 [26] |
| 9 | Caffeine | Promising inhibitors for 3-chymotrypsin-like protease of SARS-CoV-2 | SARS-CoV-2 [27] |
| 10 | Tingenone | Inhibits SARS-CoV 3CLpro | SARS-CoV [26] |
| 11 | Quercetin-3-β-galactoside | Competitively inhibits SARS-CoV 3CLpro | SARS-CoV [26] |
| 12 | Emodin | Blocks the binding of S protein to ACE2 | SARS-CoV [26] |
HSV-herpes simplex virus, MERS-CoV-Middle East respiratory syndrome coronavirus, H1N1-influenza A virus, RSV-respiratory syncytial virus, CA-Coxsackievirus, CDV-canine distemper virus, SARS–CoV severe acute respiratory syndrome coronavirus.
Table 2.
Summary of the natural compounds with brain boosting activity.
| S. no | Brain boosting compounds | Category | Reported mechanism of action |
|---|---|---|---|
| 1 | Epigallocatechin gallate (EGCG) | Polyphenol | Cross (BBB) model and protect neurons from oxidative-stress-induced cell death [28]8 |
| 2 | Resveratrol | Polyphenol | |
| 3 | Lectins
|
Protein |
|
| 4 | Chlorogenic acid (CGA) | Polyphenols | Reduce proinflammatory Mediators [33] |
| 5 | Caffeine | Polyphenols | Bring down oxidative Stress which plays role in depression & pathophysiology of anxiety [33] |
| 6 | Ferulic acid | Polyphenols |
|
BBB-human blood–brain barrier, Con A-concanavalin A, Dvl-Dioclea violacea.
2.1. Quercetin
(2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) is the most widely available flavonoid compound present in green tea and has been reported to prevent neurons against apoptosis and oxidative stress [3]. Over the years, flavonoids have been recognized as agents that affect the central nervous system in a beneficial manner including the stimulation of neuronal regeneration, enhancement of neuron functionality, ability to prevent the susceptible neurons and inducing neurogenesis [[34], [35], [36], [37]]. Quercetin also has the ability to prevent cell apoptosis in hippocampus which is considered as the centre of processing the spatial memory and presents a clear view that it can be used as a novel agent to treat oxidative stress [3,38]. It has anti-viral activity against RNA viruses due to its ability to inhibit reverse transcriptase of these viruses [39]. This feature can enable it to find great application in medicine as commonly used inhibitors including acyclovir and arabinosides have toxic nature and are not only lethal to viral cells but also damage the normal cells [39]. Apart from this, docking studies reveal that quercetin exhibits an inhibitory effect on Akt activity enabling cell survival, inhibits Cs2+-ATPase, ATPase of sarcoplasmic reticulum, protein disulfide isomerase and binds to CLUT1 thereby inhibiting glucose efflux [40]. Also quercetin (100 μg) has been employed as the main ingredient in Gene-Eden-VIR/Novirin which is a patented herbal broad-spectrum antiviral treatment and was subsequently studied for its anti-viral activity against betacoronavirus [41]. The samples of COVID-19 patients depicted that COVID-19 strain is a betacoronavirus having close resemblance to human severe acute respiratory syndrome (SARS-CoV) accounting to 79.5% sequence identity [42], 96% identical 3-chymotrypsine-like protease (3CLpro) amino acid sequences [41]. In a 2012, a study carried out by Nguyen et al. reported that quercetin in combination with epigallocatechin gallate displayed inhibitory effect against 3CLpro the main protease of SARS exhibiting an IC50 of 73 μM in vitro [43]. Quercetin has also shown its inhibitory action against 3CLpro and PLpro protease of SARS with IC50 value of 52.7 μM and 8.6 μM and 3CLpro protease of Middle Eastern respiratory syndrome virus (MERS) with IC50 value of 34.8 μM in vitro [7]. Further quercetin can regulate the unfolded protein response (UPR) of cells and as coronaviruses use UPR for the completion of their life cycle so administration of quercetin can have inhibitory effects on their activity via modulation of the mentioned pathway [44]. A generalized mechanism of action of polyphenols on entry of viral particles is given in Fig. 1 .
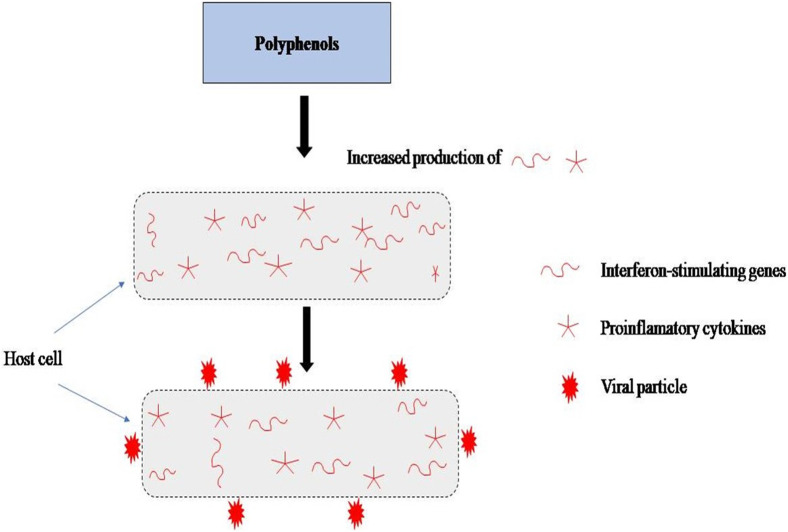
Fig. 1.
Mechanism of action of polyphenols in preventing entry of negative strand RNA into host cell.
2.2. Caffeine
The most widely available sources of caffeine include coffee, tea, chocolate bars, cocoa beverages and soft drinks etc. Caffeine (1, 3, 7-trimethylxanthine) is the major psychostimulant that has been studied for its antagonist reaction towards adenosine receptors and for inhibition of phosphodiesterase 3 (PDE3) [45]. With the result, caffeine has been associated with numerous mechanisms that describe the pathology of depression [33]. Recent years saw a great work highlighting the role of caffeine in affecting the neuroinflammatory hypotheses of depression including tryptophan catabolism, oxidative stress and inflammation [33]. These pharmacological effects of caffeine have made it a potent source of increasing alertness, as a stimulator of CNS and relaxant of smooth muscles [46]. In vivo studies have also revealed the anti-depressant effects of caffeine and it has been reported that the chronic administration of 8 mg/kg/day was able to reduce the symptoms of depression and the hippocampal secretion of serotonin and dopamine levels were comparable to that of the tricyclic deimipramine (10 mg/kg/day), which is the common anti-depressant [4]. Apart from these brain-boosting effects, caffeine has also displayed the anti-viral activity against human immunodeficiency virus type I (HIV-1) [8]. An essential step of replication for this virus involves (HIV-1) DNA integration and this stage is usually accompanied with damage to cellular DNA and therefore needs certain cellular repair proteins for its completion which includes ATR (ataxia telangiectasia and Rad3 related), DNA-PK (DNA-dependent protein kinase) and in some cases, ATM (ataxia telangiectasia mutated). Caffeine has shown to inhibit ATR and ATM kinases which form a potent target site for anti-HIV therapeutics [8]. This tendency of caffeine can reduce the replication rate of HIV-1 life-cycle. This property of the caffeine can make it an attractive agent to be included in various COVID drug formulations. The immunomodulatory effect of caffeine has been attributed to antagonist activity of its adenosine receptors and cAMP-phosphodiesterase which work in a dose dependent manner [47]. Also caffeine treatment lowered the growth rate of tumors which was possibly due to the antagonist activity of adenosine A2A receptor. The activation of A2A receptors of T cells by adenosine results in reduction of CTL proliferation, cytokines (TNF-α and IFN-γ) production and expression of programmed cell death [48]. Caffeine has also been studied for anti-HCV drugs and reported replication of HCV by preventing its replication. The mechanism of action has not been ascertained yet. Also the expression of certain proteins such as heat shock protein 90 (HSP90), COX-2 and Ras-ERK also reduced in cells resulting in inhibition of HCV replication [49]. The long term consumption of caffeine has also been observed to elevate IFN-γ [50]. Thus it can be considered as potential candidate for further investigations in SARS-COV-2 interventions [51].
2.3. Lectins from banana (Banlec)
Lectins are the category of proteins that have the ability to bind carbohydrates that contain at least one non-catalytic domain which can attach itself reversibly to a particular saccharide [52]. The lectin isolated from banana binds particularly to glucose and mannose. These are proposed to possess antiviral properties due to the ability to bind to different types of glycans (having high densities of glycoproteins) which are present on the surfaces of viruses (Fig. 2 ). Due to the presence of two key glycoproteins in influenza viruses namely, hemagglutinin (HA) and neuraminidase (NA), which play a major role in the replication cycle of influenza virus lectins have shown a potential anti-viral activity against influenza viruses. Furthermore, these viral glycoproteins serve the other functions such as binding the sialic acid-containing cell receptors and the viral membrane with the endosomal membrane, breaking the sialic acid entities resulting in release of virus and aggregation of the new virus particles [9]. The sensitivity of corona virus towards mannose specific lectins has been reported earlier in severe acute respiratory syndrome. The mechanism of their action towards corona virus has been attributed to their property to inhibit the viral attachment in early stages of replication and also decelerating the development of virus by binding towards the end of infection cycle [53]. But the use of lectins against the influenza viruses has limited applicability owing to their mutagenicity which results in inflammatory side effects [54]. In this context, an engineered banana lectin has been developed by inducing the mutation of single amino acid from histidine to threonine at position 84 called as (H84T BanLec, H84T) [9]. H84T when administered intranasally, is highly effective against avian influenza (in vitro), epidemic, pandemic, and fatal viral influenza infection (in vivo) [9]. However, the effects via other routes of administration and overall safety need an in-depth research. EC50 values of rBanLec H84T for H1NI and H3N2 virus were reported to be ranging from 1 to 4 μg/mL and 0.06 to 0.1 μg/mL and for HCV genotypes ranging from 8.8 to 20.8 nM [55]. Also in vitro studies have revealed that lectin from bananas can modulate the immune cells [56]. In a study carried in 2012 Dimitrijevic et al. reported that the oral administration of recombinant banana lectin in mice displayed a reaction with the mucosal membranes and triggered the formation of anti-bodies [58]. Also it has been reported that banana lectin through the medium of peripheral human cell cultures can produce IgG4 [59]. In a study carried in 2006, Allen et al. reported that a fructose binding lectin was isolated from Del Monte banana and it had the ability to induce cytokine interferon-gamma expression and inhibit the activity of HIV-1 reverse transcriptase and were subsequently suggested in anti-HIV formulation [60]. Since lectin has demonstrated a high anti-viral activity in the influenza type infections, focused and in depth study will provide more evidences in using these anti-viral compounds in drug formulations to combat COVID-19. In terms of brain boosting activity, lectins from marine alga Solieria filiformis lectin (SfL) reported anti-depressant effects in mice which were mostly due to its interaction with the dopaminergic system [61].
Fig. 2.
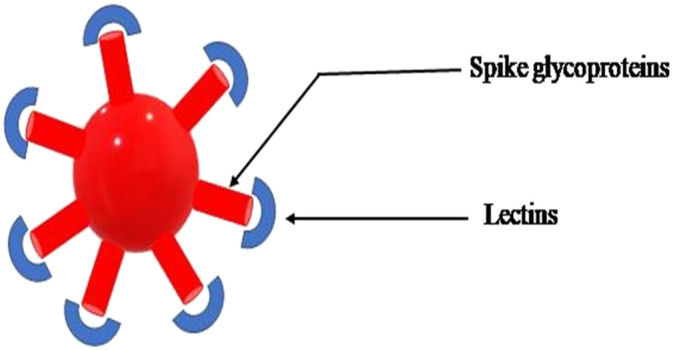
Binding of lectins (protein) to spike glycoprotein of corona virus.
Another lectin ConBr obtained from Brazilian bean C. brasiliensis having affinity for mannose/glucose displayed an anti-depressant action by activating monoaminergic system in mice using forced swimming test [62]. Also it was shown that ConBr could prevent cell death, cause blocking of seizures, act as the inhibitor of N-methyl-d-aspartate (NMDA) receptor activation and can block the hippocampal neurotoxicity triggered by glutamate in vitro and quinolinic acid in vivo demonstrating the neuroprotective action via modulation of glutamatergic system [5,62]. So with the proven brain boosting activity of lectins, lectins from banana can also be looked into for similar effects and projected as a novel compound to combat the depressive and viral conditions of COVID-19. The summary of some anti-viral lectins with their EC50 values against SARS-CoV are given in Table 3 .
Table 3.
Summary of anti-viral lectins and their EC50 values against SARS-CoV in Vero and Crandell feline kidney cells.
| S. no | Lectins | EC50 (μg/ml) | CC50 (μg/ml) |
|---|---|---|---|
| 1 | GlcNAc-specific agglutinins | ||
| • PallGlcNac | ˃100 | ˃100 | |
| • Nictaba | 1.7 ± 0.3 | ˃100 | |
| 2 | Man/GalNAc-specific agglutinins | ||
| • TL C II | 38 ± 0 | >50 | |
| 3 | Mannose-specific agglutinins | ||
| • APA | 0.45 ± 0.08 | >100 | |
| • CA | 4.9 ± 0.8 | >100 | |
| • EHA | 1.8 ± 0.3 | >100 | |
| 4 | Gal-specific agglutinins | ||
| • Morniga G II | 50 ± 13 | >100 | |
| 5 | Man/Glc-specific agglutinins | ||
| • Cladistris | 7.4 ± 0.2 | >100 |
GlcNac: N-acetyl glucosamine, Man: mannose, Gal: galactose, GalNAc: N-acetyl galactosamine.
Adapted from the study of Keyaerts et al. [53] with permission from Elsevier.
2.4. Cocoa flavonoids
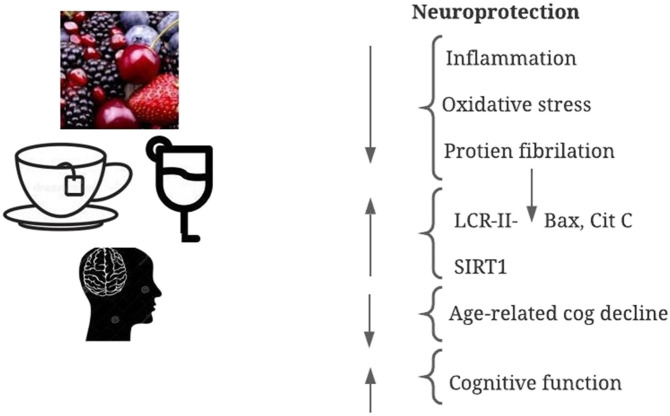
There is a high demand for cocoa and its derivatives worldwide given to their great sensory attributes. It is a rich source of polyphenols especially the flavonoids like (+)–catechin, (−)-epicatechin, and their dimmers like B1 (PB1) and procyanidins B2 (PB2) [63]. The daily intake of the cocoa flavanols have been reported to enhance sensitivity of insulin, decrease the blood pressure and fat oxidation and most importantly improve the brain function by increasing the flow of blood in the brain via spiked vasodilation [[64], [65], [66]]. The proper functioning of brain and the neuroprotection in mammals has been linked to the neurotrophic factors which play a key role in such regulation [67]. Also there are growing evidences that suggest the association between the neurotropic factors, brain and the polyphenolic compounds. Polyphenols are classified flavonoids, stilbenes, phenolic acids, and lignans. Their neuroprotective activities have been investigated in animal model studies indicating their ability to affect various pathological states of the nervous system including the modulation and protection against building up of ROS and oxidative stress, inflammation, dysfunctioning of mitochondria, accumulation of metals and all those processes that lead to the neurodegenerative diseases [6]. Furthermore, polyphenol and flavonoid rich diets have also been linked to alleviate the risks associated with neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Huntington's disease, stroke and multiple sclerosis [6,68]. The mechanism of their neuroprotective action and cognition activity is due to their modulatory effects on gene expression and cell death process via neuronal pathways. For instance, flavonoids inhibit MAP kinase cascades like p38 or signaling of ERK1/2 which affects the cytokine production and transcription factors like TNF-α, NF-κB and interleukins in iNOS that are the key determinants of the neuro-inflammatory response in the CNS [[69], [70], [71]]. The recent study on human Alzheimer's disease using an in vitro model, revealed that the polyphenolic extract of cocoa spiked the BDNF/TrkB signaling pathway thereby increasing neuroprotection [72]. Also its regular consumption has been linked to improved cognition and decrease in the risks associated with Alzheimer disease (AD) [46,73]. The principle flavanol present in cocoa is epi-catechin and plays the great role in regulating the vascular function such as regulation of blood pressure and vascular tone due to increase in the bioavailability of nitric oxide [74]. Also cocoa flavonols can accumulate in hippocampus which aids in improving the memory and learning [75]. There has been a growing research which provides great evidence that intake of the cocoa–derived products and chocolate consumption can aid in improving memory and attention in people with decrease in cognition thereby improving the neurocognitive and neuroprotective functions [74] (Fig. 3).
Fig. 3.
Neuroprotective functions of polyphenols.
In terms of the anti-viral activities, flavonoids can influence the IgM and IgG in blood circulation and many such polyphenolic extracts have been associated with the anti-viral activities against poxviruses, influenza, HIV-1 and herpes [76]. Although no in depth study has been made with respect to the anti-viral activity of polyphenols extracted from cocoa, but in general polyphenols, particularly flavonoids has been reported to inhibit the fusion of viral membrane with that of the lysosome [10]. The underlying mechanism of this anti-viral activity of the flavonoids is still unknown but it is assumed that flavonoids inhibit PG's that help in fusion of cell membranes [77]. Also, flavonoids have been reported to inhibit the proton pump which acidifies and activates lysosomes [78]. Another anti-viral property of flavonoids is the production of IFNs. These compounds exhibit their anti-viral effects through modifying the process of phosphorylation behavior of protein translation eIFs (eukaryotic initiation factors) resulting in stoppage of biosynthesis of all proteins including that of viral proteins, production of nucleases that attack the genome of virus and fortifying the membranes [79]. In vitro studies have shown that flavonoids also exhibit the anti-viral activity against human immune deficiency virus (HIV) [80].
3. Nano–based delivery systems previously used for target release of quercetin, caffeine, lectins and flavonoids
Nanotechnology has been widely used to carry out the target delivery of nutraceuticals and other therapeutics. The engineered nanoparticles possess high surface to volume ratio, good absorption properties and many bioactive components including resveratrol, curcumin, polyphenols, genistein, lycopene, anthocyanins and quercetin have been subjected to nanoencapsulation to combat the poor water solubility, low oral bioavailability and low taste profiles. Various techniques of encapsulating these target compounds have been explored including the designing of various nanocarrier systems including liposomes, nanosuspensions, nanoemulsions, nanodimensionsal lipid transporters, host matrices or micelles made up of polysaccharides, nanometer phytosomes proteins or their conjugates or complexes and solid lipid nanoparticles (SLNs) [81]. In addition to these, many inclusion complexes made up of amylose and cyclodextrins, nanotransporters like yeast cells, nanogels, nanofibres and nanosponges fabricated from polysaccharides and lipids have been employed for nanoencapsulation [82]. With the massive research going in the field of nanoscience, different carrier systems have been employed to carry forth the bioactive compounds. A novel approach for designing the nano-delivery system may be the structuring of colloidal delivery system [83]. A number of such delivery systems including starch nanocomposites and chitosan-coated liposomes have been used to encapsulate curcumin [84,85]. Similarly for encapsulating resveratrol soy protein isolates have been used as wall materials [86]. For quercetin delivery a novel strategy was employed by encapsulating the bioactive in nanostructures like nanotubes and conjugates [[87], [88], [89], [90]]. Also, superparamagnetic iron oxide nanoparticles (SPION) have been used as the carriers of quercetin owing to superparamagnetic nature, high ratio of spin polarization and elevated conductivity [91]. The use of this delivery system has added features like sensitivity to diagnostic tests, target delivery to amyloid beta (Aβ) in the brain arteries, inhibition of microglial cells, detection of DNA and magnetic resonance imaging (MRI) [[92], [93], [94], [95]]. Still the toxicity of SPION is a major issue in its rapid commercialization.
For caffeine, which is an amphiphilic drug having considerable lipolytic activity, many host compounds have been used as wall materials including liposomes, concentrated protein hydrogels, poly- (epsilon-caprolactone) polymer, cyclodextrins, niosomes, PLGA-mPEG copolymers and silica [96]. Encapsulation controls the release of caffeine and ensures its stability from rapid degradation, enables its target delivery and masks the bitter taste of caffeine [46]. In 2014, Bagheri et al. prepared the nano-particles of caffeine (300 nm) loaded in bioactive peptides (whey proteins) which were further encapsulated in alginate microparticles resulting in a more stable system and accounted for the slow release of caffeine in the gastric fluid [97]. A copolymerized temperature and pH- sensitive nanogel termed as poly (NIPAM-co-AAc) comprising of poly (N-isopropylacrylamide) (polyNIPAM) and 5% acrylic acid (AAc) was used as a drug carrier system for delivery of caffeine [98]. It was shown that in vitro diffusion of caffeine loaded in poly (NIPAM-co-AAc) at 2–4 °C enhanced the delivery of caffeine by the magnitude of 3.5 as compared to the saturated solution of caffeine. Since the nanogels were stimuli-responsive, so the release of encapsulant (caffeine) was directed by the changes in its temperature which was the major stimuli for release of caffeine. Nanostructures like electrospun nanofibres have also been a recent area of interest in drug delivery systems and many polymers have been investigated for their applicability to form nano fibres like fibrinogen, collagen, poly (lactic acid), chitosan and poly (d,l-lactide-co-glycolide) [99]. Electrospun poly (lactic-co-glycolic acid) fibre meshes have been employed as the carrier system to release retinoic acid [100], electrospun poly (dl-lactide) nanofibres have been used for controlled release of paracetamol [101] and electrospun nanofibres made from poly- caprolactone have been used for the drug metronidazole benzoate for treatment of periodontal diseases [102]. A similar attempt with respect to these electrospun nanofibres as medium to act delivery systems have been put forth [99]. The workers employed electrospun polyvinyl-alcohol nanofibres as oral fast-dissolving delivery system and the results displayed that caffeine was released in a burst about 100% within 60 s from such nanofibres.
The latest nano carrier system has been produced using the gold nanoparticles (Au NPs), which serve as the affinity probes for the trapping lectins like BanLec [103] Con A [[104], [105], [106]], and ricin B [[107], [108], [109]]. In a study carried out by Selvaprakash & Chen functionalized gold nanoparticles (AuNPs) were generated from chicken egg white (cew) proteins. The obtained Au@cew nanoparticles were then encapsulated within ovalbumin, whose surface was enriched with hybrid mannose and Galβ (1 → 4) GlcNAc terminated glycan ligands. Thus the complex generated from such fabrication i.e. Au@cew NPs encapsulating hybrid mannose and Galβ (1 → 4) GlcNAc were used to bind the specific lectins. The workers used various model lectins including banana lectin, concanavalin A and ricin B that possess certain binding moieties towards particular sugars like mannose, glucose, and β-lactose. The results revealed that Au@cew nanoparticles can bind lectins and selectively release them using specific sugar moieties like glucose, mannose and β-lactose as the releasing agents from Au@cew NP-lectin conjugates [110].
For flavanols, which belongs to the category of polyphenols, low water solubility due to high molecular weight may be a constraint for their bioavailability [111]. Also their stability in the gastro intestinal tract is very poor and degrades easily in acidic medium. In a study carried out by McGhie and Walton, at a pH of intestinal tract, anthocyanins displayed poor stability [112]. Similarly, for polyphenol like EGCG, pH below 1.5 resulted in poor stability whereas nanoencapsulation resulted in the doubling of stability at pH 1.2 [113]. In general encapsulation of polyphenols in generated nanoparticles increases their water solubility as shown by encapsulation of curcumin in the chitosan or (poly-lactic-co-glycolic acid) nanoparticles [[114], [115], [116], [117], [118], [119]]. Since the site of absorption of polyphenols is the small intestine and because the epithelia of small intestine have no specific receptors, so the polyphenols are transported via passive diffusion [120,121]. Nanoparticles encapsulating polyphenols can improve the transportation by inducing paracellular or transcellular mechanism [122]. Also nanoparticles can disrupt the tight junctions of the epithelial cells which favours the entry of these polyphenols. Overall nanoencapsulation of these synergistic compounds may become a good area of research in the coming times due to role of these bioactives as an anti viral and brain boosting system.
4. Can nano-technology based approaches work?
The delivery of anti-viral drugs into the upper respiratory tract is often exposed to a number of hurdles including lower flow of blood, small surface area, presence of a mucus layer trapping the inhaled entities and filtration of foreign bodies [123]. The ideal environment for the effective drug delivery might be the presence of large surface area and ciliated cells which are present in the lower respiratory tract but even these pose certain challenges including branched alveolar macrophages and presence of phospholipids, proteins and mucins which acts as surfactants and decrease drug efficiency [124]. To address these issues, nano delivery system can be employed due to their enhanced features. A novel strategy of applying nanotechnology has been demonstrated recently in which Novochizol, a potential COVID-19 drug has been formulated using an advanced delivery system. The drug delivery system is comprised of a nanoparticle-based aerosol in which chitosan nanoparticles (forming the polysaccharide delivery system) adheres to the wall of lung epithelia and provides for sustained release [125].
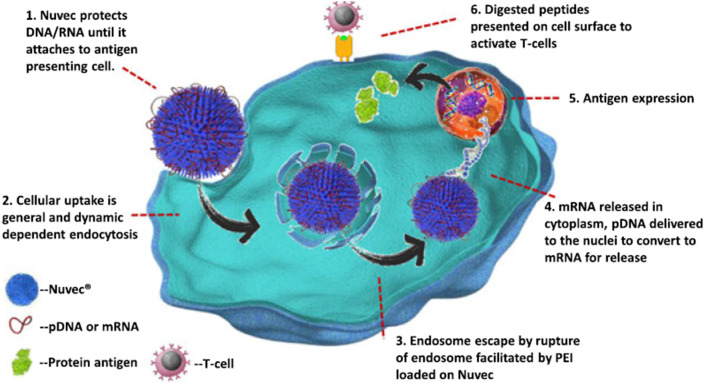
Similarly silica nanoparticles having exceptionally good chemical stability and biocompatibility are good candidates for the encapsulating nucleic acids. The silica based nanoparticles can be modified to bind specific oligonucleotides with varying dimensions including DNA, RNA, and siRNA [126]. The recent development in this regard has been observed in case of an anti-viral vaccine named, Nuvec® proposed by a pharma company. The company proposes to develop the nano-delivery system for vaccines and medicines employing novel silica-nanoparticles having irregular surfaces functionalized with polyethylenimine for carrying nucleic acids. This functionalized surface binds nucleic acids such as mRNA/pDNA and protects these from the nucleic enzymes. Besides this Nuvec® will neither not pose any harm to the cell membrane as compared to the lipid based delivery systems nor inflict any inflammatory response at the site of injection. The mechanism of action of functionalized silica nanoparticles is elucidated in Fig. 4 .
Fig. 4.
Functionalized silica nanoparticles as an effective delivery system to deliver DNA/RNA in anti-viral vaccines.
Adapted from study of Theobald [127] with permission from Elsevier.
Talking about plant based therapeutics, the herbal metal nanoparticles obtained by green technology presents a novel strategy for combating the chronic respiratory disorders due to the minimal toxicity, physiological compatibility, scalability and cost effectiveness [128]. The metal nanoparticles derived from plant source offers an ability to block the entry of viral particles inside the healthy cells which prevents the propagation of these viral particles [129]. Apart from these benefits, the unique magnetic, physicochemical and optical properties of the metal nanoparticles enable them to be modified into a sensor to detect the presence of virus or evaluate the buildup of metabolites generated by the virus infection.
A latest entry in application of nanotechnology in combating COVID-19 comes from the development of nanotheranostics which involves the delivery of theranostic nanoparticles that carry the therapeutic drug. The system provides an efficient approach of preventing the transmission of COVID-19 virus as the drug can be delivered via alternative routes apart from being delivered via intranasal delivery route. The system caters to the needs of the challenges faced by mucosal routes, maintains an effective concentration of drug at the point of infection without any side effects on the healthy cells [130]. The agents under this delivery system have been classified as inorganic, organic and virus like self-assembling protein nanoparticles. Quantum dots have also been proposed to act as antiviral agents. “Quantum dots (QDs),” also called as “semiconductor nanomaterials,” are based on longterm fluorescence imaging of cellular processes. Their size ranges from 1 to 10 nm with tunable optical wavelength. As such these can be used as a novel probes for molecular imaging [131]. The use of QDs in combating SARS-CoV-2 infections can become a great area of research owing to its ability to be traceable under specific wavelength of light, to be tunable to desired range of sizes (1–10 nm) and shape. Owing to their nano size and shape, QDs can easily penetrate SARS-CoV-2 with size ranging from 60 and 140 nm [132]. QDs can also not only sequester the S protein of SARS-CoV-2 due to their positive surface charge but their cationic surface charge can also interact with negative RNA strand of the virus, creating reactive oxygen species within SARS-CoV-2. [133]. In a study reported by Du et al., reported the antiviral activity of carbon dots (CDs) against respiratory syndrome virus, pseudorabies virus and porcine reproductive virus [134]. CDs can activate interferonstimulated genes in particular interferon-α which can suppress viral replication. In a recent study by Loczechin et al., CDs were prepared by hydrothermal conjugation and carbonization with boronic acid (carbon quantum dots-3) and were observed to show anti-viral activity against pathogenic human coronavirus in a dose-dependent manner [135].
Overall nanoparticles can deliver range of anti-viral moieties and target both the adaptive as well as innate immune system. The nanodimensions and the flexibility are the key features for developing a vaccine in future. Nanoparticles can not only be administered orally but intranasal, subcutaneous and intramuscular routes are also an option. The potential of nanotechnology in fighting this deadly disease has not only been realized in context of developing a nano-vaccine but by delivering the nano-based anti-viral agents. In case of former, scientists have proposed the designing of the virus-like nanoparticles i.e. NANOparticles (1c-SApNPs) a self assembling protein bearing SARSCoV-like 2 protein spikes (StatNano.com). As reported the system can stimulate the immune system to generate anti-bodies which can be detrimental to coronavirus and ultimately offer the first hand protection against real SARS-CoV-2 virus [136]. The system exploits the characteristic properties of high surface area to volume ratio of nanoparticles in association of the virus surface proteins to evoke an immune response. The system has certain shortcomings as well, particularly in terms of manufacturing and cost effectiveness of the process. To overcome these short comings, novel vaccines based on messenger RNA (mRNA) have been suggested. These produce viral proteins through the action of host body on synthetic mRNA of the virus. The development of such nanovaccines using nanotechnology has a tremendous potential of bringing forth the possibilities of eliminating this deadly virus. For instance, angiotensin-converting enzyme 2 (ACE2) receptor is a known receptor for SARS-CoV and related human respiratory coronavirus NL63 present in the lung parenchyma and epithelium of air passage [137]. So a strategy of developing the SARS-CoV nanovaccine will lie in the delivery of the oral synthetic mRNA which will target the interaction sites between SARS-CoV and ACE2 and thus uplift the immunostimulatory effect of vaccine. Apart from these nanovaccines, which seems to the reliable possibility, other immune boosting anti-viral agents can also be targeted for inhibiting the either the replication or entry of virus. For this purpose nano based vehicles have already entered in line for instance, nanoparticles attached to ligands have shown the ability to act as a barrier on the cell wall to prevent the entry of virus. Another preventive strategy has shown that polyphenols have intrinsic anti-viral properties which can enhance the synthesis of pro-inflammatory cytokines and interferon-stimulating genes (ISGs) by the host cells and these can retard the production of negative-strand RNA of the virus and prevent the entry of viral particles inside the cell membrane [138,139]. Since the virus are structurally different and phylogenetically apart, so the vaccines which will be developed in future will be virus specific. In such a situation, a promising strategy will be to develop broad spectrum anti-viral nanoparticles which will help in prevention and treatment of COVID-19. So reviewing the novel anti-viral agents and their nano based delivery systems might generate an approach of devising the treatment to combat COVID-19.
5. Conclusions
The overall scenario created by this pandemic has put a tremendous pressure on the workers to look into every possibility to come with the anti-virals and associated compounds that can alleviate the COVID symptoms. The mental agony and trauma inflicted upon the COVID patients and general masses has surpassed like never before. So it becomes the need of the hour to focus the research towards those naturally occurring substances which can help to reduce these complications as no chemical therapeutic is available till date. In order to provide comprehensive information of such compounds we have reviewed some naturally occurring compounds including quercitin, lectin, caffeine and cocoa flavonoids for their brain stimulating and anti-viral activities. Further we have summed up few nano-carrier systems for such synergistic compounds. Nanoencapsulation of these target compounds may provide a new area of research to derive the benefits and modify the pharmacologically active part of these compounds.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
Dr Adil Gani is thankful to DST, INSPIRE programme, Government of India for the award of Junior Research Fellowship in favor of Miss Nairah Noor under grant number No: DST/INSPIRE Fellowship/[IF180189]
References
- 1.C. Wang, P. W, Horby, F. G. Hayden, G. F. Gao, A novel coronavirus outbreak of global health concern, Lancet. 395 (2020), 470–473. [DOI] [PMC free article] [PubMed]
- 2.Roya D., Kara S.K., Sharma N., Verma S.K., Kaushal V. Study of knowledge, attitude, anxiety & perceived mental healthcare need in Indian population during COVID-19 pandemic. Asian J. Psychiatr. 2020;51:102083. doi: 10.1016/j.ajp.2020.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn T.B., Jeon B.S. The role of quercetin on the survival of neuron-like PC12 cells and the expression of –synuclein. Neural Regen. Res. 2015;10:1113. doi: 10.4103/1673-5374.160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pechlivanova D.M., Tchekalarova J.D., Alova L.H., Petkov V.V., Nikolov R.P., Yakimova K.S. Effect of long-term caffeine administration on depressive-like behavior in rats exposed to chronic unpredictable stress. Behav. Pharmacol. 2012;23:339–347. doi: 10.1097/FBP.0b013e3283564dd9. [DOI] [PubMed] [Google Scholar]
- 5.Jacques A.V., Rieger D.K., Maestri M., Lopes M.W., Peres T.V., Gonçalves F.M., Pedro D.Z., Tasca C.I., Lopez M.G., Egea J., Nascimento K.S., Cavada B.S., Leal R.B. Lectin from Canavalia brasiliensis (ConBr) protects hippocampal slices against glutamate neurotoxicity in a manner dependent of PI3K/Akt pathway. Neurochem. Int. 2013;62:836–842. doi: 10.1016/j.neuint.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Bhullar K.S., Rupasinghe H.P. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013;1-18:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.G. Nunnari, E. Argyris, J. Fang, K. E. Mehlman, R. J. Pomerantz T, R. Daniel, Inhibition of HIV-1 replication by caffeine and caffeine-related methylxanthine, Virol. J. 335 (2005) 177–184. [DOI] [PubMed]
- 9.Coves-Datson E.M., King S.R., Legendre M., Gupta A., Chan S.M., Gitlin E., Kulkarni V.V., García J.P., Smee D.F., Lipka E., Evans S.E., Tarbet E.B., Ono A., Markovitzc D.M. Molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. PNAS. 2020;117:2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.J. D. Lambert, S. Sang, C. S. Yang, Possible controversy over dietary polyphenols: benefits vs risks Chem. Res, Toxicol. 20 (2007) 583–585. [DOI] [PubMed]
- 11.Ezhilarasi P.N., Karthik P., Chhanwal N., Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2013;6:628–647. [Google Scholar]
- 12.Qio X., Kroeker A., He S., Kozak R., Audet J., Mbikayr M., Chretein M. Prophylactic efficacy of quercetin 3-0-glucoside against Ebola virus infection. Antimicrob. Agents Chemother. 2016;60:5158–5188. doi: 10.1128/AAC.00307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed E.F. Antiviral properties of garlic cloves juice compared with onion bulbs juice against potato virus (pvy) J Am Sci. 2010;6:302–310. [Google Scholar]
- 14.Gpnzalez O., Fontanes V., Raychaudhuri S., Loo R., Loo J., Arumugaswami V., French S.W. The heat shock protein inhibitor quercetin attenuates hepatitis C virus production. J. Hepatol. 2009;50:1756–5029. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H.C., Chou T.W., Cheng L.H., Ho C.W. In vitro anti adenoviral activity of five Allium plants. J Taiwan Inst Chem E. 2011;42:228–232. [Google Scholar]
- 16.A. Silva, S. M. Morais, M. M. M. Marques, D. M. Lima, S. C. C. Santos, R. R. Almeida, I. G. P, Vieira, M. I. F. I, Guedes, Antiviral activities of extracts and phenolic components of two Spondias species against dengue virus, J. Venom. Anim. Toxins incl. Trop. Dis., 17 (2011) 406–413.
- 17.A. M. Balde, L. V. Hoof, L. A. Pieters, D. A. B. Vanden, A. J Vlietinck, Plant antiviral agents-VII, antiviral and antibacterial proanthocyanidins from the bark of Pavetta owariensis, Phytother Res. 4 (2011) 182–8.
- 18.R. T. Deca, I. J. Gouzalez, T. M. V. Mactinez, J. Moreno, S. C. A. Romo, Soil Bio. In vitro antifungal activity of some flavonoids and their metabolites, Biochem.19 (1987) 223–31.
- 19.Yu C., Yan Y., Wu X., Zhang B., Wanga W., Wu Q. Anti-influenza virus effects of the aqueous extract from Mosla scabra. J. Ethnopharmacol. 2010;127:280–285. doi: 10.1016/j.jep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Tahara S., Hashidoka Y., Mizutani J. Flavonoids as medicines. Agri BioI Chem. 1987;51:1039–1045. [Google Scholar]
- 21.Tripathi V.D., Rastogi R.P. In vitro anti-HIV activity of flavonoids isolated from Garcinia multifolia. J Sci Indian Res. 1981;40:116–121. [Google Scholar]
- 22.Mouffouk C., Mouffouk S., Mouffouk S., Hambaba H. Hamada, Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2) Eur. J. Pharmacol. 2021;891:173759. doi: 10.1016/j.ejphar.2020.173759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J. W Selway. Antiviral activity of flavones and flavans. Prog Clin Biol Res. 213 (1986) 521–36. PMID: 3012583. [PubMed]
- 24.Kamboj A., Saluja A.K., Kumar M., Atri P. Antiviral activity of plant polyphenols. J. Pharm. Res. 2012;5:2402–2412. [Google Scholar]
- 25.Grenier D., Chen H., Lagha A.B., Fournier-Larente J., Morin M.P. Dual action of myricetin on Porphyromonas gingivalis and the inflammatory response of host cells: a promising therapeutic molecule for periodontal diseases. PLoS One. 2015;10(2015) doi: 10.1371/journal.pone.0131758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm. Sin. B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elzupir Amin O. Elzupir (2020): Caffeine and caffeine-containing pharmaceuticals as promising inhibitors for 3-chymotrypsin-like protease of SARS-CoV-2, J. Biomol. Struct. Dyn., DOI: 10.1080/07391102.2020.1835732. [DOI] [PMC free article] [PubMed]
- 28.Pogacnik L., Pirc K., Palmela I., Skrt M., Kim K.S., Brites D., Brito M.A., Ulrih N.P., Silva R.F. Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Res. 2016;1651:17–26. doi: 10.1016/j.brainres.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Cao W., Dou Y., Li A. Resveratrol boosts cognitive function by targeting SIRT1. Neurochem. Res. 2018;43:1705–1713. doi: 10.1007/s11064-018-2586-8. [DOI] [PubMed] [Google Scholar]
- 30.Sun A.Y., Simonyi A., Sun G.Y. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic. Biol. Med. 2002;32:314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 31.Menard C., Bastianetto S., Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front. Cell. Neurosci. 2013;7:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A., Behura A., Mawatwal S., Kumar A., Naik L., Mohanty S.S., Mann D., Dokania P., Mishra A., Patra S.K., Dhiman R. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019;134:110827. doi: 10.1016/j.fct.2019.110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall S., Desbrowa B., Anoopkumar-Dukie S., Davey A.K., Arora D., McDermott C., Schubert M.M., Perkins A.V., Kiefel M.J., Grant G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015;76:626–636. doi: 10.1016/j.foodres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., Lius Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Y. H. Jin, L. Cai, Z. S. Cheng, H. Cheng, T. Deng, Y. P. Fan, C. Fang, D. Huang, L. Q. Huang, Q. Huang, Y. Han, B. Hu, F. Hu, B. H. Li, Y. R. Li, K. Liang, L. K. Lin, L. S. Luo, J. Ma, L. L. Ma, Z. Y. Peng, Y. B. Pan, Z. Y. Pan, X. Q. Ren, H. M. Sun, Y. Wang, Y. Y. Wang, H. Weng, C. J. Wei, D. F. Wu, J. Xia, Y. Xiong, H. B. Xu, X. M. Yao, Y. F. Yuan, T. S. Ye, X. C. Zhang, Y. W. Zhang, Y. G. Zhang, H. M. Zhang, Y. Zhao, M. J. Zhao, H. Zi, X. T. Zeng, Y. Y. Wang, X. H. Wang, A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res. 7(2020) 4. [DOI] [PMC free article] [PubMed]
- 36.Norman K., Pichard C., Lochs H., Pirlich M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., Kutz A., Tribolet P., Bregenzer T., Braun N., Hoess C., Pavlicek V., Schmid S., Bilz S., Sigrist S., Brändle M., Benz C., Henzen C., Mattmann S., Thomann R., Brand C., Rutishauser J., Aujesky D., Rodondi N., Donze J., Stanga Z., Mueller B. Individualized nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira F.J., Santos H.O., Howell S.L., Pimentel G.D. Whey protein in cancer therapy: a narrative review. Pharmacol. Res. 2019;144:245–256. doi: 10.1016/j.phrs.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad A., Kaleem M., Ahmed Z., Shafiq H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections - a review. Food Res. Int. 2015;77:221–235. [Google Scholar]
- 40.Amanzadeh E., Esmaeili A., Enteshari R., Abadi N., Kazemipour N., Pahlevanneshan Z., Beheshti S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019;9:6876. doi: 10.1038/s41598-019-43345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polansky H., Lori G. Coronavirus (COVID-19), first indication of efficacy of gene-eden-VIR/Novirin in SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55:7105971. doi: 10.1016/j.ijantimicag.2020.105971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu R., Zhao X., Li J., Niu P., Yang B. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:391–393. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen T.Y.H., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W., Ahn S.A., Xia Y., Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.S. Nabirotchkin, A. Peluffo, J. Bouaziz, D. Cohen, Focusing on the unfolded protein response and autophagy related pathways to reposition common approved drugs against COVID-19, Preprints. (2020) 2020030302 (doi: 10.20944/preprints202003.0302.v1).
- 45.Daly J.W. Caffeine analogs: biomedical impact. Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.N. Noor, A. Shah, A, Gani, A. Gani, F. A. Masoodi, Microencapsulation of caffeine loaded in polysaccharide based delivery systems, Food Hydrocoll. 82 (2018), 312–321.
- 47.Horrigan L.A., Kelly J.P., Connor T.J. Immunomodulatory effects of caffeine: friend or foe? Pharmacol. Ther. 2006;111:877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Tej G.N.V.C., Neogi K., Verma S.S., Gupta S.C., Nayak P.K. Caffeine-enhanced anti-tumor immune response through decreased expression of PD1 on infiltrated cytotoxic T lymphocytes. Eur. J. Pharmacol. 2019;859:172538. doi: 10.1016/j.ejphar.2019.172538. [DOI] [PubMed] [Google Scholar]
- 49.Batista M.N., Carneiro B.M., Braga A.C.S., Rahal P. Caffeine inhibits hepatitis C virus replication in vitro. Arch. Virol. 2015;160:399–407. doi: 10.1007/s00705-014-2302-1. [DOI] [PubMed] [Google Scholar]
- 50.Sheth C.C., Lopez-Pedrajas R.M., Jovani-Sancho M.D.M., Gonzalez-Martinez R., Veses V. Modulation of salivary cytokines in response to alcohol, tobacco and caffeine consumption: a pilot study. Sci. Rep. 2018;8:1–7. doi: 10.1038/s41598-018-35094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagheri A., Moezzi S.M.I., Mosaddeghi P., Parashkouhi S.N., Hoseini S.M.F., Badakhshan F., Negahdaripour M. Interferon-inducer antivirals: potential candidates to combat COVID-19. Int. Immunopharmacol. 2021;91:107245. doi: 10.1016/j.intimp.2020.107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peumans W.J., VanDamme E.J. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keyaerts E., Vijgen L., Pannecouque C., Damme E.V., Peumans W., Egberink H., Balzarini J., Ranst M.V. Plant lectins are potent inhibitors of corona viruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huskens D., Vermeire K., Vandemeulebroucke E., Balzarini J., Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Cell Biol. 2008;40:2802–2814. doi: 10.1016/j.biocel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 55.M. D. Swanson, D. M. Boudreaux, L. Salmon, J. Chugh, H. C. Winter, J. L. Meagher, S. Andre, P. V. Murphy, S. Oscarson, R. Roy, S. King, M. H. Kalpan, I. J. Goldstein, E. B. Tarbet, B. L. Hurst, D. F. Smee, F. De La, H. H. Hoffmann, Y. Xue, C. M. Rice, D. Schols, J. V. Garcia, J. A. Stuckey, H. J Gabius, H. M. Al-Hashimi, D. M. Markovitz, Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity, Cell 163 (2015) 746–758. [DOI] [PMC free article] [PubMed]
- 56.Peumans W.J., Zhang W., Barre A., Astoul C.H., Balint-Kurti P.J., Rovira P., Rouge P., May G.D., Van Leuven F., Truffa-Bachi P., Van Damme E.J. Fruit-specific lectins from banana and plantain. Planta. 2000;211:546–554. doi: 10.1007/s004250000307. [DOI] [PubMed] [Google Scholar]
- 58.Dimitrijevic R., Stojanovic M., Micic M., Dimitrijevic L., Gavrovic-Jankulovic M. Recombinant banana lectin as mucosal immunostimulator. J. Funct. Foods. 2012;4:636–641. [Google Scholar]
- 59.Koshte V.L., Aalbers M., Calkhoven P.G., Aalberse R.C. The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int.Arch. Allergy Immunol. 1992;97:17–24. doi: 10.1159/000236090. [DOI] [PubMed] [Google Scholar]
- 60.H. K. Allen, J. H. Cheung, T. B. Ng. Wong, Musa acuminata (Del Montebanana) lectin is a fructose-binding lectin with cytokine-inducing activity, Phytomedicine. 16 (2009). [DOI] [PubMed]
- 61.Abreu T.M., Monteiro V.S., Martins A.B.S., Teles F.B., Rivanor R.L.C., Mota E.F., Macedoc D.S., Vasconcelos S.M.M., Junior J.E.R.H., Benevides N.M.B. Involvement of the dopaminergic systemin the antidepressant-like effect of the lectin isolated from the red marine alga Solieria filiformis in mice. Int. J. Biol. Macromol. 2018;111:534–541. doi: 10.1016/j.ijbiomac.2017.12.132. [DOI] [PubMed] [Google Scholar]
- 62.Barauna S.C., Kaster M.P., Heckert B.T., Nascimento K.S., Rossi F.M., Teixeira E.H., Cavada B.S., Rodrigues A.L., Leal R.B. Antidepressant-like effect of lectin from Canavalia brasiliensis (ConBr) administered centrally in mice. Pharmacol. Biochem. Behav. 2006;85:160–169. doi: 10.1016/j.pbb.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 63.Rabaneda S., Jauregui O., Casals I., Andres-Lacueva C., Izquierdo-Pulido M., Lamuela-Raventos R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) J. Mass Spectrom. 2003;38:35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 64.D. Grassi, G. Desideri, S. Necozione, C. Lippi, R. Casale, G. Properzi, Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate, J. Nutr. 138 (2008)1671–6. [DOI] [PubMed]
- 65.Sorond F.A., Hurwitz S., Salat D.H., Greve D.N., Fisher N.D. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.J. I. Dower, J. M. Geleijnse, L. Gijsbers, P. L. Zock, D. Kromhout, P. C. Hollman, Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial, Am J Clin Nutr. 101 (2015), 914–921. [DOI] [PubMed]
- 67.Namiki J., Kojima A., Tator C.H. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J. Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- 68.Basil A., Soulet S., Chaher N., Merillon J.M., Chibane M., Monti J.P., Richard T. Wine polyphenols: potential agents in neuroprotection. Oxidative Med. Cell. Longev. 2012;2012:805762. doi: 10.1155/2012/805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong A.N., Yu R., Chen C., Mandlekar S., Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000;23:1–16. doi: 10.1007/BF02976458. [DOI] [PubMed] [Google Scholar]
- 70.Spencer J.P., Rice-Evans C., Williams R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003;278:34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]
- 71.Wiseman S., Mulder T., Rietveld A. Tea flavonoids: bioavailability in vivo and effects on cell signaling pathways in vitro. Antioxid. Redox Signal. 2001;3:1009–1021. doi: 10.1089/152308601317203549. [DOI] [PubMed] [Google Scholar]
- 72.Cimini A., Gentile R.D., D’Angelo B., Benedetti E., Cristiano L., Avantaggiat M., Giordano A., Ferri C., Desideri G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013;114:2209–2220. doi: 10.1002/jcb.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crichton G.E., Elias M.F., Alkerwi A. A chocolate intake is associated with better cognitive function: the Maine-Syracuse Longitudinal Study. Appetite. 2016;100:126–132. doi: 10.1016/j.appet.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Socci V., Tempesta D., Desideri G., Gennaro L., Ferrara M. Enhancing human cognition with cocoa flavonoids. Front Nutr. 2017;4:19. doi: 10.3389/fnut.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sokolov A.N., Pavolva M.A., Klosterhalfen S., Enck S. Chocolate and the brain: neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013;37:2445–2453. doi: 10.1016/j.neubiorev.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Su X., Sangster M.Y., D’Souza D.H. Time-dependent effects of pomegranate juice and pomegranate polyphenols on foodborne viral reduction. Foodborne Pathog. Dis. 2011;8:117783. doi: 10.1089/fpd.2011.0873. [DOI] [PubMed] [Google Scholar]
- 77.Prasain J., Carlson S., Wyss J. Flavonoids and age-related disease: risk, benefits and critical windows. Maturitas. 2010;66:163–171. doi: 10.1016/j.maturitas.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corcoran M.P., McKay D.L., Blumberg J.B. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J Nutr Gerontol Geriatr. 2012;31:176–189. doi: 10.1080/21551197.2012.698219. [DOI] [PubMed] [Google Scholar]
- 79.Cliver D.O. Capsid and infectivity in virus detection. Food Environ Virol. 2009;1:123–128. doi: 10.1007/s12560-009-9020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DellAica I., Dona M., Tonella F., Piris A., Mock M., Montecucco C., Garbisa S. Potent inhibitors of anthrax lethal factor from green tea. EMBO Rep. 2004;5:418–422. doi: 10.1038/sj.embor.7400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jafari S.M. Nanoencapsulation of food bioactive ingredients: principles and applications. Academic Press. 2017:1–62. [Google Scholar]
- 82.Rezaei A., Fathi M., Jafari S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019;88:146–162. [Google Scholar]
- 83.M. Mehanny, R. M. Hathout, A. S. Geneidi, S. Mansour, Exploring the use of nanocarrier systems to deliver the magical molecule; curcumin and its derivatives. J. Control, Release. 225 (2016) 1–30. [DOI] [PubMed]
- 84.Liu Y., Liu D., Zhu L., Gan Q., Le X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015;74:97–105. doi: 10.1016/j.foodres.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 85.Li J., Shin G.H., Lee I.W., Chen X., Park H.J. Soluble starch formulated nanocomposite increases water solubility and stability of curcumin. Food Hydrocoll. 2016;56:41–49. [Google Scholar]
- 86.Zhang L., Zhang F., Fang Y., Wang S. Alginate-shelled SPI nanoparticle for encapsulation of resveratrol with enhanced colloidal and chemical stability. Food Hydrocoll. 2019;90:313–320. [Google Scholar]
- 87.Singh A., Dutta P.K., Kumar H., Kureel A.K., Rai A.K. Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr. Polym. 2018;193:99–107. doi: 10.1016/j.carbpol.2018.03.092. [DOI] [PubMed] [Google Scholar]
- 88.Aghapour F., Moghadamnia A.A., Nicolini A., Kani S.N.M., Barari L., Morakabati P., Rezazadeh L., Kazemi S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018;500:860–865. doi: 10.1016/j.bbrc.2018.04.174. [DOI] [PubMed] [Google Scholar]
- 89.Kumar M., Sharma G., Misra C., Kumar R., Singh B., Katare O.P., Raza K. N-desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: a synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C. 2018;89:274–282. doi: 10.1016/j.msec.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 90.Yamina A.M., Fizir M., Itatahine M.A., He H., Dramou P. Preparation of multifunctional PEG-graft-halloysite nanotubes for controlled drug release, tumor cell targeting, and bio-imaging. Colloids Surf. B. 2018;170:322–329. doi: 10.1016/j.colsurfb.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 91.Mehta V., Parashar A., Malairaman U. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017;171:69–78. doi: 10.1016/j.physbeh.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 92.A. C. H. Barreto, V. R. Santiago, S. E. Mazzetto, J. C. Denardin, R. Lavin, Giuseppe Mele, M. E. N. P. Ribeiro, Icaro G. P. Vieira, Tamara Gonçalves, N. M. P. S. Ricardo, P. B. A. Fechine, Magnetic nanoparticles for a new drug delivery system to control quercetin releasing for cancer chemotherapy, Journal of J Nanopart Res. 13 (2011) 6545–6553.
- 93.Hasany S., Ahmed I., Rajan J., Rehman A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. J. Nanosci. Nanotechnol. 2012;2:148–158. [Google Scholar]
- 94.Poduslo J.F. Targeting vascular amyloid in arterioles of Alzheimer disease transgenic mice with amyloid β protein antibodycoated nanoparticles. J. Neuropathol. Exp. Neurol. 2011;70:653–661. doi: 10.1097/NEN.0b013e318225038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glat M., Skaat H., Menkes-Caspi N., Margel S., Stern E.A. Age-dependent effects of microglial inhibition in vivo on Alzheimer’s disease neuropathology using bioactive-conjugated iron oxide nanoparticles. J. Nanobiotechnology. 2013;11:32. doi: 10.1186/1477-3155-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liedana N., Galve A., Rubio C., Tellez C., Coronas J. CAF@ZIF-8: one-step encapsulation of caffeine in MOF. Appl. Mater. Interface. 2012;4:5016–5021. doi: 10.1021/am301365h. [DOI] [PubMed] [Google Scholar]
- 97.Bagheri L., Madadlou A., Yarmand M., Mousavi M.E. Potentially bioactive and caffeine-loaded peptidic sub-micron and nanoscalar particles. J. Funct. Foods. 2014;6:462–469. [Google Scholar]
- 98.Abu Samah N.H., Heard C.M. Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc) Int. J. Pharm. 2013;453:630–640. doi: 10.1016/j.ijpharm.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 99.Li X., Kanjwala M.A., Lind L., Chronakisa I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B. 2013;103:182–188. doi: 10.1016/j.colsurfb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Puppi D., Piras A.M., Detta N., Dinucci D., Chiellini F. Poly (lactic-co-glycolic acid) electrospun fibrous meshes for the controlled release of retinoic acid. Acta Biomater. 2010;6:1258. doi: 10.1016/j.actbio.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 101.W. Cui, X. Li, X. Zhu, G. Yu, S. Zhou, J. Weng, Investigation of drug release and matrix degradation of electrospun poly(dl-lactide) fibers with paracetanol inoculation, Biomacromolecules. 7 (2006)1623–1629. [DOI] [PubMed]
- 102.Zamani M., Morshed M., Varshosaz J., Jannesari M. Controlled release of metronidazole benzoate from poly ε-caprolactone electrospun nanofibers for periodontal diseases. Eur. J. Biopharm. 2010;75:179–185. doi: 10.1016/j.ejpb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Nakamura-Tsuruta S., Kishimoto Y., Nishimura T., Suda Y. One-step purification of lectins from banana pulp using sugar-immobilized gold nano-particles. J. Biochem. 2008;143:833–839. doi: 10.1093/jb/mvn038. [DOI] [PubMed] [Google Scholar]
- 104.Lin C.C., Yeh Y.C., Yang C.Y., Chen G.F., Chen Y.C., Wu Y.C., Chen C.C. Quantitative analysis of multivalent interactions of carbohydrate-encapsulated gold nanoparticles with concanavalin. A. Chem. Commun. 2003;23:2920–2921. doi: 10.1039/b308995a. [DOI] [PubMed] [Google Scholar]
- 105.Lim K.R., Ahn K.S., Lee W.Y. Detection of concanavalin A based on attenuated fluorescence resonance energy transfer between quantum dots and mannose-stabilized gold nanoparticles. Anal. Methods. 2013;5:64–67. [Google Scholar]
- 106.Jiang X., Housni A., Gody G., Boullanger P., Charreyre M.T., Delair T., Narain R. Synthesis of biotinylated α-D-mannoside or N-acetyl β-D-glucosaminoside decorated gold nanoparticles: study of their biomolecular recognition with Con A and WGA lectins. Bioconjug. Chem. 2010;21:21521–21530. doi: 10.1021/bc900431p. [DOI] [PubMed] [Google Scholar]
- 107.Schofield C.L., Mukhopadhyay B., Hardy S.M., McDonnell M.B., Field R.A., Russell D.A. Colorimetric detection of Ricinus communis Agglutinin 120 using optimally presented carbohydrate-stabilised gold nanoparticles. Analyst. 2008;133:626–634. doi: 10.1039/b715250g. [DOI] [PubMed] [Google Scholar]
- 108.Yin H.Q., Jia M.X., Yang S., Wang S.Q., Zhang J.G. A nanoparticle-based bio-barcode assay for ultrasensitive detection of ricin toxin. Toxicon. 2012;39:12–16. doi: 10.1016/j.toxicon.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 109.Nagatsuka T., Uzawa H., Sato K., Kondo S., Izumi M., Yokoyama K., Nishida Y. Localized surface plasmon resonance detection of biological toxins using cell surface oligosaccharides on glyco chips. ACS Appl. Mater. Interfaces. 2013;5:4173–4180. doi: 10.1021/am4002937. [DOI] [PubMed] [Google Scholar]
- 110.Selvaprakash K., Chen Y. Functionalized gold nanoparticles as affinity nanoprobes for multiple lectins. Colloids and Surfaces Colloids Surf. B. 2018;162:60–68. doi: 10.1016/j.colsurfb.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 111.Gao S., Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Med. Chem. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGhie T.K., Walton M.C. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 113.Onoue S., Ochi M., Yamada S. Development of (−)-epigallocatechin-3-gallate (EGCG)-loaded enteric microparticles with intestinal mucoadhesive property. Int. J. Pharm. 2011;10:111–113. doi: 10.1016/j.ijpharm.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 114.Duan J., Zhang Y., Chen Y., Li B., Liao M. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010;400:211–220. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 115.Luz P. Curcumin-loaded into PLGA nanoparticles. Parasitol. Res. 2012;110:593–598. doi: 10.1007/s00436-011-2527-9. [DOI] [PubMed] [Google Scholar]
- 116.Teng Z., Luo Y., Wang Q. Nanoparticles synthesized from soy protein: preparation characterization and application for nutraceutical encapsulation. J. Agric. Food Chem. 2012;60:2712–2720. doi: 10.1021/jf205238x. [DOI] [PubMed] [Google Scholar]
- 117.Anand P., Nair H.B., Sung B., Kunnumakkara A.B., Yadav V.R., Tekmal R.R., Aggarwal B.B. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Kim T.H., Jiang H.H., Youn Y.S., Park C.W., Tak K.K., Lee S., Kim H., Jon S., Chen X., Lee K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011;403:285–291. doi: 10.1016/j.ijpharm.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 119.Das R.K., Kasoju N., Bora U. Encapsulation of curcumin in alginate chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. 2010;6:153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 120.Kahle K., Kraus M., Scheppach W., Richling E. Colonic availability of apple polyphenolsea study in ileostomy subjects. Mol. Nutr. Food Res. 2005;49:1143–1150. doi: 10.1002/mnfr.200500132. [DOI] [PubMed] [Google Scholar]
- 121.Barrington R., Williamson G., Bennett R.N., Davis B.D., Brodbelt J.S., Kroon P.A. Absorption conjugation and efflux of the flavonoids kaempferol and galangin using the intestinal CaCo-2/TC7 cell model. J. Funct. Foods. 2009;1:74–87. doi: 10.1016/j.jff.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Z., Jiang H., Xu C., Gu L.A. Review: using nanoparticles to enhance absorption and bioavailability of phenolics phytochemicals. Food Hydrocoll. 2015;43:153–164. [Google Scholar]
- 123.Clarke D., McMillan N. Targeted drug delivery to the virus-infected airway; complications and remedies. Curr. Drug Deliv. 2015;12:86–97. doi: 10.2174/1567201811666140918114528. [DOI] [PubMed] [Google Scholar]
- 124.Duncan J.E., Whitsett J.A., Horowitz A.D. Pulmonary surfactant inhibits cationic liposome-mediated gene delivery to respiratory epithelial cells in vitro. Hum. Gene Ther. 1997;8:431–438. doi: 10.1089/hum.1997.8.4-431. [DOI] [PubMed] [Google Scholar]
- 125.S. R Bonam, N. G. Kotla, R. A. Bohara, Y. Rochev, T. J. Webster, J. Bayry, Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections, Nano Today.36 (2020) 101051. [DOI] [PMC free article] [PubMed]
- 126.D. Tarn, C. E. Ashley, M. Xue, E. C. Carnes , J. I. Zink, C. J. J. Brinker. Mesoporous silica nanoparticle nanocarriers, biofunctionality and biocompatibility. 406 (2013) 792–801. [DOI] [PMC free article] [PubMed]
- 127.Theobald N. Emerging vaccine delivery systems for COVID-19: functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov. Today. 2020;25:1556–1558. doi: 10.1016/j.drudis.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nandi D.K.M.M. Herbal gold nanoparticles for attenuating pandemic infection of COVID-19 virus. J Nanomed Nanotechnol. 2020;11:547. [Google Scholar]
- 129.X. X. Yang, Li. CM, CZJN. Huang. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 8 (2016) 3040–3048. [DOI] [PubMed]
- 130.[102] R. Itani, M. Tobaiqy, AJT Al Faraj, Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patient. 10 (2020) 5932. [DOI] [PMC free article] [PubMed]
- 131.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 132.Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: a systematic review. Indian J. Pharm. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dong X., Moyer M.M., Yang F., Sun Y.P., Yang L. Carbon dots’ antiviral functions against noroviruses. Sci. Rep. 2017;27:519. doi: 10.1038/s41598-017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Du T., Liang J., Dong N., Liu L., Fang L., Xiao S., Han H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. [Google Scholar]
- 135.Loczechin A., Seron K., Barras A., Giovanelli E., Belouzard S., Chen Y.T., Metzler-Nolte N., Boukherroub R., Dubuisson J., Szunerits S. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces. 2019;11:42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Talebian S., Conde J. Why go NANO on COVID-19 pandemic. Matter- Cell press. 2020;3:598–601. doi: 10.1016/j.matt.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., Jr., McCray P.B. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Applied NanoMater. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 139.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2008;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]