Abstract
The COVID-19 pandemic surges on as vast research is produced to study the novel SARS-CoV-2 virus and the disease state it induces. Still, little is known about the impact of COVID-19-induced microscale damage in the lung on global lung dynamics. This review summarizes the key histological features of SARS-CoV-2 infected alveoli and links the findings to structural tissue changes and surfactant dysfunction affecting tissue mechanical behavior similar to changes seen in other lung injury. Along with typical findings of diffuse alveolar damage affecting the interstitium of the alveolar walls and blood-gas barrier in the alveolar airspace, COVID-19 can cause extensive microangiopathy in alveolar capillaries that further contribute to mechanical changes in the tissues and may differentiate it from previously studied infectious lung injury. Understanding microlevel damage impact on tissue mechanics allows for better understanding of macroscale respiratory dynamics. Knowledge gained from studies into the relationship between microscale and macroscale lung mechanics can allow for optimized treatments to improve patient outcomes in case of COVID-19 and future respiratory-spread pandemics.
Keywords: Coronavirus infection, Micromechanics, Diffuse alveolar damage, Fibrotic lesions, Surfactant dysfunction, Microangiopathy
Graphical abstract
1. Introduction
COVID-19 has now claimed the lives of hundreds of thousands of people around the world with millions of cases reported [1]. New information on SARS-CoV-2 is being published at a rapid rate, but there is still little information connecting the microlevel changes of the infected lung tissue with the functional changes seen at the macrolevel. Scientists and clinicians note progressive changes in macroscale lung dynamics in patients with COVID-19 acute respiratory distress syndrome (CARDS) that differ from patients with acute respiratory distress syndrome (ARDS) of other etiologies, questioning the validity of using ARDS specific ventilation protocols for treating COVID-19 patients [2,3]. As such, the macroscale mechanics of the lung at the whole organ level, or macromechanics, arise from structural changes at the tissue level making it imperative to understand the microlevel mechanics, or micromechanics, in gaining a complete picture of the COVID-19 lung to treat those that are severely affected. Histopathology studies have made great progress in determining the microlevel changes occurring in the lungs of COVID-19 patients [4]. These findings help elucidate the effects of the viral infection but must be linked to the mechanical properties to translate to the function and physiology of the lung.
In order to study the microlevel mechanics of COVID-19 infected lung, an understanding of mechanics in the healthy lung must first be obtained. With each breath in, the lung expands and fills with air, the ratio of the change in volume with respect to the change in pressure determines the lung compliance, defined by the ease in which the lung expands. The elastic and resistive properties of the lung can be seen in the sigmoidal shape of the pressure-volume (P-V) curve of the breathing cycle. The linear portion of the curve displays the elastic properties, mostly in the range of normal breathing, while the upper curve is indicative of increased lung stiffness and thus increased resistance [5]. As lung properties arise from micromechanical properties, it is seen that tissue properties are highly dependent on the surface tension, as well as the stress bearing elements of the tissues [6,7]. In the context of mechanics, stress refers to the forces acting on an area of tissue and is related to the stiffness of the tissue, defined as the amount of stress due to a unit change in strain. Strain is usually defined as the relative change in length, area or volume, and can be considered a measure of the relative change in deformation [8]. Stress and strain are important concepts in characterization of the mechanical behavior observed in the structure and function of the alveoli.
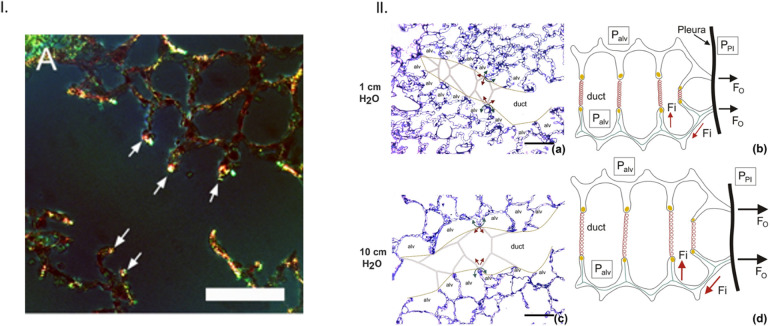
To better understand the role of alveolar components on lung function, a deeper look at the structure and function of the alveoli is needed. In fact, there are approximately 400 million alveoli in the adult human lung responsible for carrying out gas exchange. Densely packed within the parenchyma, the alveoli are grouped into sacs around alveolar ducts attached to the terminal bronchioles. The airway tree and alveolar structure have evolved to provide optimal gas exchange through matched components of gas transport [9]. Homogeneous ventilation of the gas-exchange units decreases the individual stress and strain in the alveolar walls [10]. The thin septa between the alveoli allow for a large surface area for gas exchange within the confines of the thoracic cavity and are made up of only a few cell layers, the thin interstitial layer and the capillary network [9]. Thin alveolar tissues must also be able to withstand the cyclic deformation imposed during the breathing cycle generating stress and strain within the tissue [11,12]. To support the thin alveolar membranes and protect against cell damage, a fiber network extends throughout the lungs made up of two main types of connective tissue fibers, elastin and collagen (Fig. 1 ). Axial fibers extend from the conducting airways into the parenchyma and peripheral fibers enter the parenchyma through the pleura, these fibers are connected by the septal fibers that extend through the alveolar septum and form the entrance rings of the alveolar ducts [9]. This well-developed alveolar structure provides optimal gas exchange function to the lung as a whole. Mechanical properties of the lung arise from the mechanics of individual components of the alveoli.
Fig. 1.
Collagen and elastin fiber network in the lung. I. A. Fluorescence microscopy of thin lung tissue sections stained with Sirius red collagen and green elastin staining concentrated at alveolar septal edges (arrows) (bar = 100 μm) (used with permission from [13]). II. Depiction of forces in the axial, septal and peripheral fiber systems, acting as the stress-bearing elements of the acinar airspaces, (a). Healthy rat lungs fixed at low pressure show narrow alveolar ducts with folded and pleated septal walls of alveoli. Fine dashed lines show the border between the duct and alveolar airspaces, while red and green arrows depict the direction of surface tension force (green) and the counteracting pull of axial system fibers at entrance rings (red). The axial network of collagen and elastin fibers is concentrated at the edges of alveolar septa, coil the ductal airspaces, and form the alveolar entrance rings (represented by the connecting thick gray lines), (c). Healthy rat lungs fixed at higher pressure, showing widening of alveolar ducts and stretching of alveolar septa, with the surface tension force counteracted by the tensile force of axial fiber system in the direction of the ductal lumen, (b, d). Schematic representation of the axial fiber network of collagen and elastin coiling the alveolar duct (depicted by red springs) and stabilizing the alveolar walls with changes in pressure. The axial fiber network is connected to the peripheral fiber system of the pleura (thick black line) through the alveolar septal fibers (green lines), which are found between the basal laminae of the alveolar epithelium and endothelium. Without these three fiber systems, the surface tension would cause the alveoli to collapse. During inflation, pressure gradient due to differences in the pleural pressure (PPl) and alveolar pressure (Palv) induce outward forces (FO) in the peripheral fiber system, which are transmitted into the fiber system of the septal wall (Fi) and eventually are counteracted by the tension in the axial fiber system of the ductal airspace (used from [11], Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The walls of the alveoli are composed of two types of epithelial cells. Type I cells form thin elongated cytoplasm branches that cover 95% of the alveolar surface. The cytoplasm of these cells forms tight junctions to control fluid accumulation in the alveolar space [9]. The junctions can undergo a coordinated repair process to avoid apoptosis if a relatively small area is damaged [14]. Type II cells are the progenitor cells for the alveolar epithelium thus functioning in repair and regulation of the alveolar epithelium. In addition to their regenerative functions, type II cells aid in regulating fluid in the alveolar space [15]. These type II cells also have indirect impact on alveolar mechanics by decreasing surface tension through secretion and recycling of surfactant components. The concentration of surfactant released is dependent on the cell signaling pathways activated by the stretch of alveolar walls in breathing [7]. On the opposing side of the alveolar wall, endothelial cells make up the wall of the capillary and exhibit a similar mosaic of two cell types as the alveolar epithelial cells. Aerocytes (aCap) cells, are specialized for gas exchange and can be found tightly fused to type I epithelial cells forming the thinnest part of the barrier membrane. General capillary (gCap) cells are similar to type II epithelial cells and act as progenitor cells to the endothelium and maintain homeostasis and vasomotor tone [16].
As stated above, type II cells impact alveolar mechanics through the secretion of the components that comprise the surfactant layer of the alveoli. Surfactant is a thin liquid layer that lines the inner wall of the alveoli modulating the surface tension inside the alveoli, therefore, protecting the wall from overextending at high volumes or collapsing at low volumes. At low volumes, areas of the alveolar wall are folded below the surfactant layer and unfold as volume rises. The surfactant works interdependently with the fiber network to maintain alveolar surface area through minimizing effects of surface tension on the edges of the septal walls allowing the septa to unfold with increased pressure (Fig. 1 (a, c)) [17]. Subsequently, under normal breathing, a 2-fold increase in volume only results in a 1.2-fold increase in alveolar surface area, greatly reducing the stress in the tissue [9]. In this way, expansion of alveolar walls is modulated by the surfactant maintaining optimal surface area without overstretching the axial fibers and smoothing the alveolar epithelial surface for efficient gas exchange [17]. Dysfunction or compromise of the surfactant layer can arise from damage to the type II epithelial cells and edematous fluid or other cellular components filling the alveolar space [6,11].
Within the alveolar septa is a thin interstitial layer comprised mostly of different cell types and the fiber network. Fibroblasts indirectly contribute to mechanical properties through forming and maintaining the fiber network system, impacting the nonlinear elastic properties of the alveolar wall [7]. Overall, cellular contribution to the mechanical properties is estimated to be small with the fiber network most significantly impacting tissue elasticity and stiffness [18]. The fiber network is the main stress bearing component and provides the main structural support for the lung, in particular the alveoli [19]. To protect the tissue from high stresses that can cause injury, the fiber network maintains the structure of the alveoli. Concentration of fibers throughout the alveolar wall is modulated by the surface tension. High positive surface tension at the free edge of the alveolar septum causes expansion and thus an increased concentration of fibers to stabilize the septum with the increased stress concentration (Fig. 1) [9]. The fiber network aids in controlling the degree of expansion and contraction of alveoli to protect tissues from damage and optimize gas exchange. Elastin is responsible for the elastic recoil of the lung and contributes mostly to the mechanical properties of the tissue at normal breathing volumes where strain levels are low. The elastin fibers exhibit linear elastic stress-strain relationship and can easily deform to two times in length [8]. The other main fiber component is collagen with fibers being composed mainly of variable amounts of type I and type III collagen, lending to different levels of stiffness among fibers [20]. Collagen fibers affect tissue properties at higher deformations and strain levels. Unlike elastin, collagen has a nonlinear elastic stress-strain relationship which leads to the apparent tissue stiffening at higher volumes [7]. At lower volumes, collagen is often seen as crimped or wavy in a heterogeneous network [21]. The crimping of the fibers allows for collagen to have greater effects on mechanical properties with larger expansion.
As a whole, mechanical properties of the alveolar tissue determine efficiency of gas exchange. Previous studies have determined how the behavior of alveolar tissue components, together with their arrangement, determine the mechanics of the whole lung [5]. In this review, we aim to determine the key histological features of the alveoli infected by the SARS-CoV-2 virus in relation to alveolar mechanical properties. Comparison to previously studied lung injury allows for drawing connections between the structural changes of the alveoli and the mechanical properties. This review begins by summarizing how the SARS-CoV-2 virus induces damage to the alveoli and its relation to previously established and known viruses affecting the lung. Next, histopathological findings in recent studies are reviewed which are then linked to changes in mechanical behavior. The findings are divided into sections based on areas affected: alveolar airspace, alveolar interstitium through fibrotic remodeling, and alveolar capillary. Finally, the microscale mechanics are related to the respiratory dynamics seen at the macroscale with COVID-19 infection. Quantifying and visualizing the microlevel changes in the COVID-19 lung can lead to a better understanding of their effects on the gas exchange function leading to better treatment protocols for patients and improved patient outcomes long term.
2. Methods
Due to the rapidly evolving nature of research on COVID-19, in addition to peer-reviewed research articles, we included preprints, editorials and letters to the editor in our search strategy. An optimized search strategy was used to find relevant literature for this narrative review. One author (KS) iteratively developed keywords in the domains of “lungs” and “biomechanics” based on the terms used in known, relevant studies, and combined keywords with controlled vocabulary (i.e., Medical Subject Heading [MeSH] terms) from PubMed/MEDLINE. These two search domains were combined with a slightly adapted version of the preset PubMed search strategy to identify COVID-19 studies. The search strategy is available (see Appendix 1). On October 9, 2020, one author (KS) mapped and implemented the search strategy in three databases (PubMed, Scopus, and BioRxiv). We did not have any language or geographic restrictions for our search of databases. A subsequent search was run on December 1, 2020 to capture newly published articles and preprints.
The combined search results from the three databases were deduplicated manually in EndNote and subsequently uploaded into Covidence. Covidence identified and removed additional duplicates not caught in the manual deduplication process.
Two authors (ED and AV) independently screened the titles and abstracts of articles in Covidence. Conflicts were resolved by discussion. Following title and abstract screening, the full text review of articles was performed independently by two authors (ED and AV). Conflicts were resolved by discussion.
3. Results
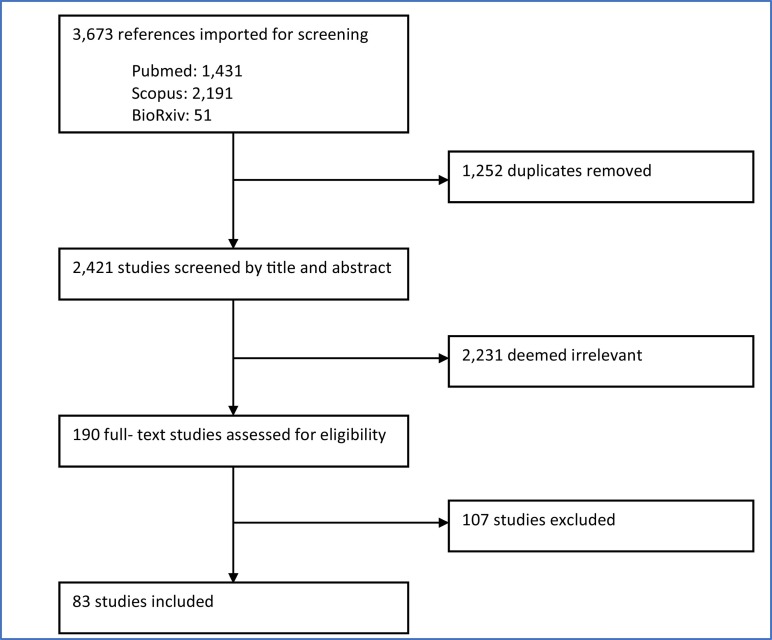
The original and updated searches retrieved 3673 records from the three databases queried (Fig. 2 ). After 1252 duplicates were removed, 2421 unique records remained for title and abstract screening. Following the title and abstract screening process, 2231 records were deemed irrelevant to the research questions and were excluded. The full text of the remaining 190 records was reviewed to determine full relevance and eligibility. This resulted in 83 eligible records included in this review.
Fig. 2.
Flow diagram of study selection.
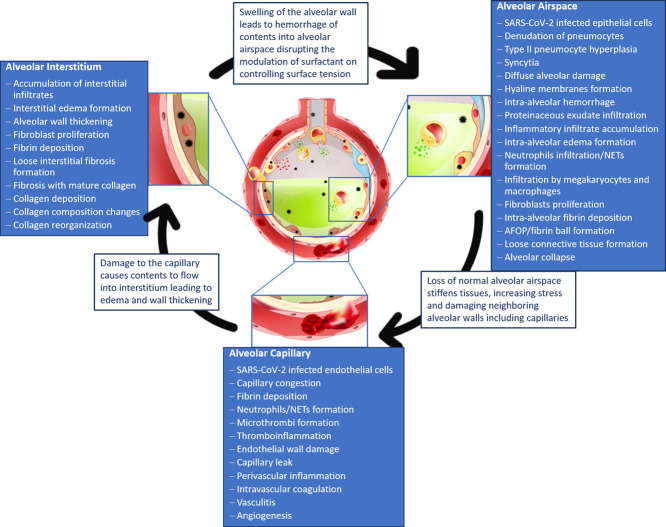
3.1. COVID-19 in the lung
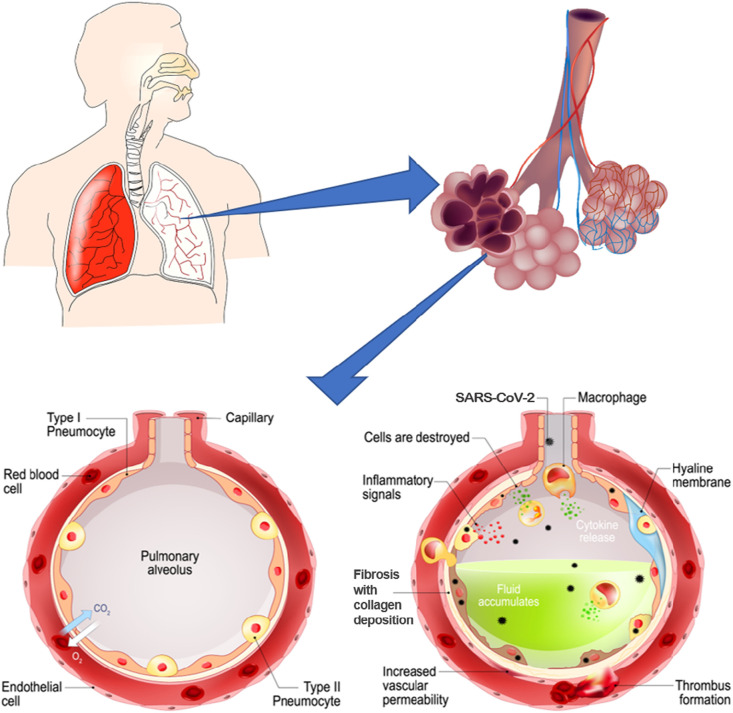
Information obtained from studies of COVID-19 infected lungs, points to lung injury that has epithelial, vascular, and fibrotic effects [4]. These injuries are very similar to those found in previous coronaviruses, SARS and MERS, as well as influenzas such as H1N1 with diffuse alveolar damage (DAD) and vascular injury as the main findings [[22], [23], [24], [25], [26]]. Even with so many similarities in histopathological findings, there is disagreement as to if the DAD in COVID-19 is typical or has novel differences that sets it apart from other pulmonary diseases, particularly with respect to the vascular findings [[27], [28], [29]]. Capillary and vascular changes were not initially studied in COVID-19 pathology but have since become a focal point for many studies [30]. As new information continues to be published on the structural effects of COVID-19 on the microscale lung, it is apparent that the viral infection induces tissue and cellular level changes that affect the dynamic function of the lung (Fig. 3 ).
Fig. 3.
SARS-CoV-2 infection induces alveolar level changes in the lung. The pulmonary alveolus is lined with type I and type II epithelial cells and surrounded by a meshwork of capillaries to facilitate gas exchange. As the healthy alveolus (left) becomes infected with the SARS-CoV-2 virus (right) damage occurs to the alveolar tissues and capillaries. The alveolar damage leads to build-up of debris and fluid inside the alveolus, affecting gas exchange function (images adapted and sourced from shutterstock.com).
Before looking at the specific damage induced by COVID-19 infection it is important to look at how the SARS-CoV-2 virus enters the cells. It is believed that SARS-CoV-2 enters the cells through the angiotensin converting enzyme 2 (ACE2) receptor after activation of the spike domain by transmembrane serine protease 2 (TMPRSS2) [31]. SARS-CoV-2 not only shows high binding affinity for ACE2 but may also be cleaved by the protease furin which in turn activates cell surface receptors, such as neuropilins, particularly Natriuretic Peptide Receptor 1 (NRP1), enhancing infectivity when co-expressed with ACE2 [[32], [33], [34]]. ACE2 is present on alveolar epithelial cells as well as endothelial cells of the alveolar capillaries. This is supported by findings of the virus in type I and II pneumocytes and endothelial cells [[35], [36], [37], [38]]. Viral RNA and capsid proteins are found colocalized with ACE2 receptors in tissues [39]. When the virus infects these cells, it is thought to damage the cells through direct viral impact and induce a host immune response causing further damage (Fig. 3). Higher viral loads are found in patients that died earlier in disease course and the virus is found in early stages of DAD while absent in later stages [[35], [36], [37], [38],40]. With the virus more prevalent in earlier stages of disease, the initial damage may be due to direct viral impact with later and continued damage due to the host immune response although this is hard to determine [36,41]. Many methods used to detect the virus in lung tissue rely on fresh tissue samples for examination, but a recent study has detected and sequenced the SARS-CoV-2 virus from formalin-fixed tissue samples allowing for viral detection and sequencing of previously studied samples to better track the virus [42].
Due to the ability for the virus to induce direct and indirect damage, the disease severity is dependent on the degree of damage to the alveoli, particularly the epithelium, microthrombi and other vascular effects that cause capillary damage [25,41,[43], [44], [45], [46]]. Current findings allow for preliminary understanding of how the microlevel changes of the alveoli affect the mechanics of the tissues in COVID-19 lungs.
3.2. Alveolar airspace deterioration
Within the alveoli of the lung, the SARS-CoV-2 virus is able to directly infect the type I and type II epithelial cells that line the alveolar wall. Infection leads to cell death with loss of the epithelial layer as well as DAD which can be triggered by the innate immune response that is activated by viral infection. The alveolar airspace can also be disrupted by capillary damage that causes fluid accumulation due to capillary leak and intra-alveolar hemorrhage [47,48]. Recent reviews of COVID-19 histopathology studies have found DAD as the main finding in the lung and similar to DAD of other etiologies [23,49,50]. DAD typically progresses through stages, the early acute or exudative phase, the proliferative or organizing phase, and less often the fibrotic phase [51]. The exudative and proliferative phases have the greatest impact on the alveolar space. The phases of DAD can be characterized by specific histopathology findings but there is often overlap of findings between phases [51]. Exudative DAD is characterized by hyaline membranes that normally appear early in infection [52]. Other typical findings in the acute phase are interstitial and alveolar edema, capillary congestion and microthrombi, inflammatory cell infiltrates, scattered fibroblasts, thickening of the alveolar septa, denudation of the alveolar epithelium and type II hyperplasia within a few days of infection [53]. The organizing phase occurs about a week later and is characterized by proliferation of fibroblasts in septa and alveolar spaces to begin tissue repair through organization of edema, disappearance of hyaline membranes, and atypia of pneumocytes [51,53]. Thromboemboli can also be seen in capillaries and pulmonary vessels. The organizing phase can progress into the fibrotic stage after about 3 to 4 weeks [53].
The most robust data on histopathology of the COVID-19 lung has come from postmortem biopsy and autopsy studies that have findings of heterogeneously distributed DAD in various stages [35,45,54] . These studies give insight into disease progression with relation to viral infection and damage to the alveoli [36,38]. Most studies find DAD in acute, organizing, or combination of acute and organizing stages with more severe findings after longer disease course [24,25,27,36,38,48,55]. Acute DAD was seen early in disease course typically within first 10 days of disease course [38,56]. An autopsy study on the first 12 deaths in a Hamburg hospital found acute DAD in 8 patients with prominent hyaline membranes, activated pneumocytes, capillary congestion, and protein-enriched edema [43]. Another large study of 20 patients found 14 with acute DAD and foci of organization [28]. Studies of smaller cohorts have also found acute DAD with little to no organization [26,42,57]. Organizing DAD begins to show up a week to 10 days after symptom onset and rarely within a few days of symptoms which may be due to subclinical phase or rapid disease progression [22,25,36,38]. A case report of a patient with unusual disease progression showed predominantly organizing DAD with intra-alveolar fibrin and fibroblastic tissue with type II hyperplasia and alveolar inflammation [58]. Other studies have also reported patients that show predominantly DAD in organizing phase [28,59]. Organizing DAD can also begin to show areas of loose interstitial fibrosis along with intra-alveolar fibrin and inflammatory infiltrates [60]. Few studies have been able to study the early effects of COVID-19 or effects of the disease not complicated by medical treatment, reporting similar microscale structural changes to those of patient cohorts with various comorbidities, medical treatments, and prolonged disease courses [[61], [62], [63], [64], [65], [66]].
Along with findings indicative of DAD, intra-alveolar hemorrhage was also observed in COVID-19 patients [22,48,59,62,[66], [67], [68], [69], [70]]. Infiltration of the alveolar space with inflammatory cells as well as neutrophils, megakaryocytes, macrophages, extracellular matrix components, and other cells that disrupt function were also observed [22,25,43,71,72]. Neutrophils and NETs in the COVID-19 lung can occlude peripheral airways [73]. While most findings were that of typical DAD, acute fibrinous and organizing pneumonia (AFOP) was also seen in some studies which is thought to be a less studied and less well understood variant of DAD. In AFOP, fibrin balls form in the alveolar spaces instead of hyaline membranes which can block airflow into the alveoli [53,54,67,70,74,75]. A study of postmortem biopsies of 6 COVID-19 patients found 5 patients that died around 20 days after disease onset showed AFOP with fibrin balls in alveolar spaces, fibroblasts surrounding intra-alveolar fibrin with lymphocytes and type II hyperplasia along with loose connective tissue in alveolar ducts [29]. Fibrin balls can occlude airspaces while fibrin in the alveolar spaces can contribute to hyaline membrane formation, thickening the septa for gas exchange and to early granulation tissue organization that contributes to fibrosis [53,74]. The changes found in DAD and other types of alveolar damage in COVID-19 lungs are often accompanied by and lead to gas exchange function loss or severe impairment. This is seen in regions of alveolar collapse, atelectatic regions, and loss of airspace [66,69,76,77]. In addition to the primary viral infection by SARS-CoV-2, secondary infections such as bronchopneumonia and aspergillosis are seen in patients with longer durations of disease. It is important to distinguish findings of COVID-19 from those of secondary infections to determine the true impact of COVID-19 [24,44,68,75,78]. DAD can be further aggravated by these secondary infections as well as mechanical ventilation making it difficult to determine the extent of damage explicitly due to the virus.
Impact of DAD on microscale lung mechanics: DAD and other damage affect the tissue mechanics of the alveoli, thus impeding gas exchange function. Damage to the alveoli can cause an increase of the alveolar surface tension and the local forces surrounding areas of injury. The structural alveolar changes of DAD in COVID-19 are similar to those of DAD of other etiologies thus, previous studies of lung injury with DAD give valuable insight into mechanical effects of COVID-19 alveolar damage. Mechanical ventilation is also a factor in micromechanical changes in the lungs of COVID-19 patients. COVID-19 patients requiring mechanical ventilation may show higher incidence of secondary infection, as well as longer use of mechanical ventilation and increased incidence of ARDS compared to mechanically ventilated patients with influenza pneumonia or no viral infection [79]. Much of the damage that occurs in acute and organizing DAD, as seen in CARDS as well as typical ARDS, directly affects the function of the alveolar space through dysfunction of surfactant, alveolar edema, and atelectatic regions. As surfactant functions to regulate surface tension in alveoli, the loss or dysfunction of surfactant can increase surface tension, altering the compliance and increasing stress in the surrounding alveoli [6]. The loss of type II alveolar epithelial cells in COVID-19 leads to loss of surfactant regulation and production [80]. Although due to limited studies on the early pathogenesis of COVID-19, it is difficult to determine the cause of surfactant disruption. Loss of type I and type II epithelial cells allow the alveoli to become flooded with edema, blood, and cellular debris as ion channels regulating fluid in the alveoli are lost or impaired leading to further degradation of the surfactant layer and epithelial cells [6,80,81]. The surfactant can also be degraded by reactive oxygen species from the interstitial side. When surfactant is lost, the surface tension in the alveolar sacs is no longer regulated to protect against overdistention and further damage can occur.
With increase in surface tension in the alveolus, edema, hemorrhage, and atelectatic regions act as stiff regions that show little to no expansion with breathing which increases stress in surrounding alveoli [6,82]. Any occlusion of airspace, such as the fibrin balls in AFOP or neutrophil extracellular traps (NETs), can block airflow and act as stiff regions in otherwise homogenous lung. Just as fibrin balls can block alveolar airflow, NET formation can also restrict airflow from reaching peripheral airways as seen in infections caused by other respiratory viruses [83]. When alveoli become occluded with fluid due to edema and hemorrhage, the size of the alveoli decreases resulting in smaller surface area for gas exchange [6]. As the flooded alveolus decreases in size, the surrounding alveoli increase in volume and forces on the alveolar walls increase thus increasing the stress surrounding the foci of injury (Fig. 4B). It is not the flooding of the alveolus that affects the compliance of the region but rather the subsequent increase in stress of the surrounding area [82]. The continued increase in stress of the surrounding area due to mechanical ventilation may lead to further damage and increase the size of the injured area [84]. When surface tension is increased, the forces on the alveolar septa are increased which can cause injury and microatelectasis due to ventilation, often from mechanical ventilation but can also occur with spontaneous breathing [6,85,86]. As lung injury progresses, more alveoli are damaged leading to loss of aerated and recruitable alveoli and a decrease in lung compliance [82]. If the alveolar damage of COVID-19 continues to progress and is not correctly regulated, DAD can progress from the organizing phase to fibrotic remodeling.
Fig. 4.
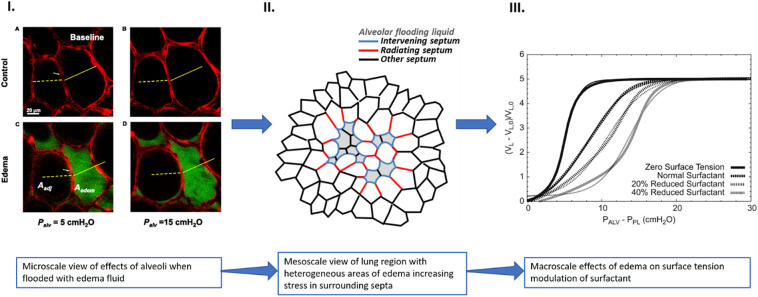
Microscale damage at the alveolar level leads to formation of stress concentrators affecting lung function at the macroscale. I.) optical sections of alveoli in isolated rat lung show edema formation in individual alveolus causing inflation differences between flooded and aerated alveoli (Aedem = flooded alveolus area, Aadj = aerated alveolus area) A. aerated alveoli at 5 cmH2O alveolar pressure B. inflation of aerated alveoli when alveolar pressure increased to 15 cmH2O C. fluid accumulation in single alveolus causes decrease in size with increase in size of neighboring aerated alveolus at 5 cmH2O alveolar pressure D. when alveolar pressure is increased to 15 cmH2O there is greater difference in inflation between aerated and flooded alveoli (Reprinted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. All rights reserved. Perlman et al. [131]. The American Journal of Respiratory Cell and Molecular Biology is an official journal of the American Thoracic Society [131]). II.) Areas of flooded alveoli may act as stress concentrators increasing stress in septa of surrounding alveoli. When alveoli become flooded and decrease in size, the forces on the walls of neighboring alveoli (radiating septum) increases leading to concentrated areas of increased stress in the lung (adapted from open access [6]). III.) As edema formation impacts the ability of surfactant to control surface tension in multiple focal areas of the lung, global lung function can become affected. The reduction in surface tension control at the microscale can cause a shift in the pressure-volume curve, as shown in silico modeling studies. The graph shows the sigmoid-shaped pressure-volume relationship for a block of 1241 alveoli computed for four different surfactant levels where PALV, PPL, VL and VL0 are the alveolar pressure, pleural pressure, total alveolar volume and total alveolar volume when all collagen and elastin fibers are at rest respectively. The computer model shows that as the amount of surfactant in the lung increases, the volume of alveoli at a fixed pressure increases as well (used with permission from [125]).
3.3. Fibrotic remodeling
Organizing DAD sometimes progresses to the fibrotic stage leading to thickening of the alveolar tissues and stiffening due to fibrosis. Before deposits of mature collagen are present in the alveoli, the alveolar septa thicken with fibroblast proliferation. Within the thickened walls of the alveoli the connective tissue begins to organize, there is interstitial inflammation with loose fibrosis and interstitial infiltrates and staining techniques show changes in collagen and elastin fiber content [27,36,38,60,63,66]. Organization leads to the final stage of fibrosis if repair of the epithelium is disorganized, there is repeated injury, or initial injury is severe [52,87].
Interstitial inflammation seen in COVID-19 shows the presence of neutrophils in acute DAD and chronic inflammatory infiltrates including lymphocytes and macrophages along with proliferating connective tissue [38]. The immune mechanism underlying the interstitial inflammation seen in COVID-19 seems to share many similarities to that of macrophage-activation syndrome with presence of helper CD4+ and cytotoxic CD8+ T lymphocytes [38,[88], [89], [90]]. Of particular interest is the role of regulatory T-cells, which are a subset of CD4+ T lymphocytes and have been shown to be involved in the maintenance of immune homeostasis and immunological self-tolerance. For example, a study by Ichikawa et al. [91] reported that in the inflamed lung, low integrin alpha E (CD103) expression by lung tissue-resident CD4+ T lymphocytes may define their pathogenic effector subpopulation promoting fibrotic response through production of interleukin 5 (IL-5) and interleukin 13 (IL-13). In contrast, it was shown that high CD103 expression can define the immunosuppressive tissue-resident cell subpopulation limiting fibrotic responses. These two functionally distinct CD4+ T cell populations thus may play a critical role in controlling alveolar fibrosis [91]. Readers interested in more detailed discussion of the role of T-cells in fibrosis are referred to a recent review by Zhang and Zhang [92].
The fibrotic phase is the most severe phase and leads to increased fibrotic tissue that impairs alveolar function and has been seen in some patients that died of COVID-19 [37,54,75,93]. A study by Y. Li et al. [94] used a minimally invasive autopsy technique utilizing ultrasound-guided postmortem core biopsies to look at the lungs of 30 patients and examine the progression of COVID-19 to the fibrotic stage. They found 12 patients with fibrosing DAD that was often in combination with other stages of DAD. The fibrosing DAD had dense interstitial fibrous tissue with collagen fibers as well as collagen duct fibrosis and collagen deposition in alveolar walls. Patients with fibrosing DAD not only had longer durations of illness but were also younger than those with less severe DAD [94]. Other studies using biopsy techniques have also found fibrosis in COVID-19 patients. In two samples by postmortem cryobiopsy, Barisione et al. [95] found late stage fibrosis with loss of the alveolar structure, honeycombing with structural remodeling with mature collagen and remaining alveoli with slit-like appearance. Aiolfi et al. [96] also studied biopsy samples from two patients antemortem that underwent thorascopies that showed alveolar damage with interstitial fibrosis and stained positive for collagen IV with immunohistochemistry staining. A study comparing patients with COVID-19, ARDS of other etiologies, pneumonia, influenza, and healthy lungs found more collagen type I in patients with lung pathologies with the most being seen in COVID-19 [97]. In one study, researchers used orcein and Masson trichrome staining to visualize the changes in collagen and elastin fibers, finding loss of fiber network in the alveolar walls with increase in reticulin (collagen III) fibers, in a patient that died after about 10 days of symptoms [66]. Further research is needed to better characterize the progression of alterations of collagen and elastin fibers in COVID-19. While damage in the lungs can be due to direct viral insult, the damage may continue after clearance of the virus from the tissues [36,38,76]. This was seen in the autopsy of a patient that died of pulmonary fibrosis after clearance of COVID-19 [98]. Some studies have looked at factors, other than DAD, that may contribute to the progression to fibrosis such as increased numbers of pulmonary megakaryocytes and endothelial to mesenchymal cell transition [99,100]. It is worth noting fibrosis is not specific to DAD, therefore it is important to account for health history of patients as it can be caused by other agents such as age, emphysema, and other previous exposures [45,55,70].
Impact of fibrotic remodeling on microscale lung mechanics: The progression from septal thickening to fibrosis in the COVID-19 lung not only affects ventilation-perfusion but also stiffens the alveolar tissue further inhibiting gas exchange. Septal thickening with fibroblasts and connective tissue as seen in COVID-19, increases the barrier membrane thickness for gas exchange and affects the ability of gases to diffuse across it [9]. This is detailed in the capillary damage section below. It is important for there to be tight regulation of fibroblast proliferation and apoptosis, as fibroblasts function to lay down new matrix for healing which can also progress into fibrosis [101]. While increased collagen content increases the stiffness of the tissues, the interactions between collagen, elastin, and other extracellular matrix constituents also impact the mechanical properties of the alveolar tissues [7]. The collagen and elastin networks likely intermingle leading to behavior more complex than the properties of each fiber type. The stiffening of the tissues due to changes in fiber interactions and distribution in alveolar walls can cause contraction leading to increased strain on surrounding tissues, similar to the effects of other injury such as microatelectases [85]. Proteoglycans surrounding the connective tissue fibers may also affect tissue properties through osmolarity-dependent interactions, stabilizing the fiber network and influencing the ability of the collagen to fold and stretch under mechanical loading [21]. In the COVID-19 injured lung, the osmolarity of the interstitium could be altered by the accumulation of fluid from edema, capillary leak, and hemorrhage. Animal models of lung injury progressing to fibrosis have shown that there is relatively little change in the elastin content of the tissues but that increased collagen content can lead to changes in mechanical properties that affect the inflation of the alveoli such as increased elastance, resistance and hysteresivity [102]. Along with direct impact to the tissues, COVID-19 also affects the capillaries within the alveolar septa.
3.4. Capillary damage
While DAD of other etiologies is known to cause damage to the alveolar tissues accompanied by microthrombi in the alveolar capillaries, it is thought that vascular injury plays a greater role in COVID-19 disease progression than other lung injuries leading to ARDS [103]. Thromboinflammation and other vascular damage may contribute to the vascular injury seen in COVID-19 [104]. The co-localization of thrombosis with inflammation, termed thromboinflammation, in COVID-19 lung injury can lead to endothelial damage [105]. Endothelial and vascular damage can also arise from direct effects from the virus invading the cells through ACE2 receptors leading to perivascular inflammation and possible endothelial to mesenchymal transition disrupting the basement membrane [99,106]. A common histopathological finding in the alveolar capillaries of COVID-19 patients is capillary congestion [55,69]. A biopsy study of 10 patients showed no capillary microthrombi but areas of vascular congestion and hemorrhage were present [27]. Another autopsy study of 12 patients found microthrombi in small arteries and capillaries showed congestion leading to the belief that while patients died of pulmonary effects, coagulopathy is a common complication in COVID-19 [43]. In an autopsy study of 14 patients, capillary congestion was seen in areas of acute DAD. The study also identified 5 types of pulmonary thrombi within the cohort including capillary microthrombi, partially organized thrombi in mid-sized arteries with complete occlusion, non-organized thrombi in mid-sized arteries that did not completely occlude vessel, bone marrow emboli, and septic pulmonary thromboemboli, with the most common being capillary microthrombi in 11 of the 14 patients [44].
There is still some debate as to whether the incidence of microthrombi is greater in COVID-19 than in DAD of other etiologies, with some believing there is no difference [28,36,49], while others believe there is an increase in microthrombi and vascular changes in COVID-19 [25,35,40,45,65,70,96,[107], [108], [109], [110]]. Regardless of frequency, microthrombi may contribute to disease progression, with alveolar and interstitial inflammation, and are often seen despite use of anticoagulants and are not always found in conjunction with areas of DAD [35,88]. In a study that looked at 34 tissue samples from 16 patients, two immunopathological phenotypes were found based on interferon stimulated gene (ISG) expression. The low ISG group is thought to be indicative of later disease progression and has higher frequency of T and B lymphocytes as well as thromboembolic events or disseminated intravascular coagulation (DIC) when compared to the high ISG group [48]. ISG expression, such as interferon gamma-induced protein 10 (IP10), is associated with affecting the integrity of capillary endothelium through intra-alveolar hemorrhage as well as promoting lymphocyte adhesion to the endothelium [48,111]. Chemokines associated with compromised endothelial integrity were also found in the low ISG group [48]. Expression of proinflammatory cytokines in COVID-19 can lead to capillary damage. For example, increased release of tumor necrosis factor alpha (TNF-α) has been associated with viral binding of ACE2 [112]. The local release of TNF-α can impact vascular permeability while its global release can lead to DIC [113]. Readers interested in more detailed discussion of the gene expression and cytokine release in COVID-19 are referred to the recent reviews by Fung and Liu and Gustine and Jones [89,113]. Despite the use of anticoagulation drugs, microthrombi were found during autopsy of 10 COVID-19 patients where electron microscopy showed deposition of fibrin within the capillaries [68]. This study also found that DAD was less pronounced in patients that had microthrombi which was also seen in other studies [114]. Based on these findings, it is believed that the microthrombi are part of a COVID-19 associated coagulopathy and not typical DAD findings. A possible link to vascular damage is the finding of amyloidosis in COVID-19 patients [68,78]. Stepping beyond the traditional 2D view of histopathology, Li G. et al. used 3D images from high-resolution cleared tissue microscopy to gain a deeper look at autopsy findings. This allowed for visualization of the fibrin deposition and clot formation in COVID-19 further supporting the role of microangiopathy in pathogenesis of COVID-19 [115].
In addition to microangiopathy and microthrombi, endothelial damage in COVID-19 has been found to lead to angiogenesis [93,116]. In an in depth study by Ackermann and colleagues, autopsy findings from seven COVID-19 patients were compared with findings from H1N1 patients to study the vascular effects of COVID-19 [107]. They found that not only were capillary microthrombi nine times more likely in COVID-19 than H1N1 but that angiogenesis is also 2.7 times as likely with findings of structurally deformed capillaries and intussusceptive angiogenesis. To further explore these findings of angiogenesis, using samples previously studied by [68], Eckermann and colleagues used 3D images based on phase contrast x-ray tomography to visualize features of the samples in a more realistic fashion, allowing for the observation of vessel branching and the connection of hyaline membranes to the vessel wall. Others have also used pulmonary angiography and dual-energy computed tomography (CT) to study the perfusion of pulmonary vessels of COVID-19 patients [117]. The findings of impaired perfusion are suggestive of widespread pulmonary angiopathy and thrombosis in COVID-19 with the tree-in-bud pattern seen in CT images as a possible marker for immunothrombosis and angiogenesis seen at the microscale.
Along with thrombosis and direct injury to the capillaries, neutrophils may also play a role in vascular occlusion and clot formation. Neutrophils are found in capillaries of COVID-19 patients and can play a role in capillary damage through the formation of NETs with more NETs in CARDS than ARDS [37,45,73,110,118]. NETs are found to contribute to vascular occlusion in COVID-19 patients as clots in capillaries of the alveolar septa are high in neutrophils and exhibit nearby endothelial damage of the vessels wall [118]. A large study of 38 patients studying the immunothrombosis of COVID-19 found that microthrombi of platelets, fibrinogen, and neutrophils and increased NET formation were found in a larger percentage in COVID-19 lungs when compared to 24 non-COVID-19 control patients which is thought to lead to immunothrombosis in COVID-19 [110]. It is thought that neutrophils generate excess reactive oxygen species (ROS) leading to further lung damage, thrombosis, blood cell dysfunction, and systemic inflammatory response [47]. Other capillary components may contribute to pathogenesis and coagulopathy in COVID-19 such as pericyte damage, increased megakaryocytes, and damage to endothelial glycocalyx with increased hyaluronan [37,70,71,100,109,119,120].
Impact of capillary damage on microscale lung mechanics: Some studies have hinted that the damage to the alveolar capillaries contributes to poor oxygenation and decreased diffusion capacity [47,69,109,117,121]. Capillary effects of COVID-19 directly relate to the mechanics involved in alveolar expansion and blood flow in the alveolar capillaries. Comparisons of COVID-19 with similar viruses such as SARS and MERS show similar disease progression between the three but with increased vascular effects in COVID-19 [121]. There is heterogeneity in findings of vascular effects of COVID-19 but when observed, these findings at the alveolar level lead to poor oxygenation and decreased diffusion capacity.
It is necessary to continue to study the vascular effects of COVID-19 to gain a deeper understanding of the effects on the function of the alveoli and gas exchange particularly ventilation-perfusion mismatch and dynamics of blood flow. While the capillary structure does not contribute much to the stiffness of alveolar wall, the thickness of the alveolar-capillary barrier and mechanics of blood flow affect efficiency of gas-exchange, leading to hypoxia [9]. Diffusion of gases between the alveolar airspace and capillary blood is directly dependent on barrier thickness. When the endothelium is injured, as is seen in COVID-19, aerocytes become damaged and are regenerated by gCap cells during repair. When edema forms in the interstitium, liquid first accumulates in the thick portions of the alveolar septum to protect the thin areas and preserve gas exchange function [122]. As edema fluid continues to accumulate, it disrupts the thin portions and increases the barrier thickness for decreased diffusion [9]. Septal thickening also occurs with buildup of fibroblasts and deposition of collagen within the interstitial space as well as build-up of hyaline membranes and cellular debris within the alveolar space [36,94]. Congestion within the capillary with NETs and microthrombi disrupt perfusion and subsequently the ability of gases to diffuse across the barrier membrane. Optimization of ventilation and perfusion in the healthy lung allows for optimized gas exchange and is dependent on the amount of capillaries and the distribution of erythrocytes within the capillaries. When capillaries become occluded, as is seen in COVID-19, there is ventilation without perfusion, contributing to dead space in the lung [9]. This damage can lead to capillary hemorrhage which affects blood flow and decreases gas exchange. Animal studies have shown hemorrhage decreases the capillary surface area and reduces oxygen diffusion capacity [123]. Hemorrhage of capillaries and capillary leak allow for capillary contents to spread into the alveoli causing flooding of the airspace and interstitium. In this way, the damage to the capillaries, alveolar airspace, and alveolar walls are all connected and lead to progression of the others. Just as the microscale components of damage in COVID-19 are connected to one another, the microscale mechanical changes directly impact the function and dynamics of the lung at macroscale.
3.5. Importance of microlevel mechanical changes on whole lung dynamics
The microlevel changes seen in the COVID-19 lung at the alveolar level affect the function of the lung at macroscale through the formation of stress concentrators leading to heterogeneous ventilation of the alveoli [10,124]. Stress concentrators are areas of focal lung injury that can cause excessive and potentially harmful mechanical loading in the surrounding tissues (Fig. 4). Surfactant dysfunction, alveolar collapse, intra-alveolar edema, lung inflammation, and focal fibrotic remodeling all act as stress concentrators within the lung [11,82]. Stress concentrators are found in the COVID-19 lung at different stages of disease progression and can be found in mechanically ventilated lungs as well as due to self-inflicted lung injury [2]. These stress concentrators can alter mechanics and may become a trigger for injury progression (Fig. 4) [11]. Fig. 4 shows how flooding of the alveoli at the microlevel can lead to increased stress in septa of alveoli adjacent to areas of injury. As alveoli become flooded and decrease in size, the forces on the walls of neighboring alveoli increase leading to concentrated areas of increased stress in the lung. The microscale surface tension effects from loss of surfactant function with edema affect the P-V curve at the macroscale. Fig. 4 Part III., shows how lung compliance, determined by the slope of the P-V curve, decreases with a reduction in surfactant amount as shown by mathematical and in silico models. Fibrotic remodeling of the alveolar walls, as seen in COVID-19, would also affect the overall compliance of the lung [125]. Changes in overall compliance affect the ability of the lung to carry out gas exchange. This review has used the current literature on tissue level changes to assess the micromechanics of the COVID-19 lung. Previous studies have focused on the macroscale findings of the lung in COVID-19 patients [[126], [127], [128], [129], [130]]. Here, a brief look at the connection between microscale and macroscale is given as the microstructure of the lung largely determines whole lung dynamics.
Lung injury, including COVID-19, is often found with spatial heterogeneity where focal regions throughout the lung show damage surrounded by areas of seemingly healthy tissue [38]. These stress concentrators act as stiff regions that do not exhibit normal inflation during ventilation causing increased stress in neighboring, healthy alveoli (Fig. 4 Part II.). This increased stretch can lead to progression of injury in surrounding tissues. In this way, injured regions are thought to percolate and join together forming larger areas of injury, so what begins as few small areas of injury can quickly become a large area of injury affecting global lung function [5]. Fig. 4 illustrates how edema in the alveoli can increase stress in surrounding tissues, increasing the size of injured areas, and possibly percolating the areas of injury to induce changes seen at the macrolevel. This percolation could be a possible mechanism for seemingly rapid disease progression and can also be used to link microscale to macroscale as these larger areas of injury are visible on CT scans. COVID-19 studies have looked at macroscale images of the lung to characterize the microscale structural changes based on these larger areas of injury. Ultrasound findings of COVID-19 were able to characterize the progressive changes in pulmonary structure based off comparison of histology to ultrasound findings in a letter by Almeida Monteiro [132]. Another study looked at chronology of changes in the lung and related morphological changes seen in postmortem cryobiopsy samples correlation of histology to CT imagery findings [95].
These focal areas of damage, visualized in CT and ultrasound imagery in the lung lead to hypoxia through mismatch of ventilation and perfusion and decreased diffusion capacity [9,133]. When this hypoxia becomes severe, as is seen in severe cases of COVID-19, mechanical ventilation is often needed. Although mechanical ventilation is used to support patient respiration, it can cause hyperinflation of gas exchange units and further progression of lung injury through what is called ventilatory induced lung injury (VILI) [81]. Hyperinflation or over-distension of non-injured lung tissue occurs in areas surrounding the injury sites (Fig. 4) [6]. The injured area of the lung also exhibit reduced compliance, this then leads to reduced compliance of the whole lung [134]. The ability of mechanical ventilation to induce further injury and affect lung mechanics highlights the importance of using optimized ventilator settings to decrease VILI [82]. Adjusting ventilator settings to lower surface tension in the alveoli may decrease the septal stresses and reduce VILI, although hidden microatelectases in injured lungs may increase the probability of VILI even at safe mechanical ventilation settings [6,85]. Currently, there is not enough information from macroscale imaging to understand the mechanical changes that affect breathing and there is still a need to better understand the connection between the microscale damage and macroscale effects in the COVID-19 lung.
Differences are seen in the microscale examination of COVID-19 lung tissue compared to DAD of other etiologies, specifically the capillaries. At macroscale there have also been differences seen in the dynamics of the lung in the presentation of CARDS compared to ARDS of other etiologies as ARDS is the macroscale equivalent to DAD. Better understanding of these differences can lead to optimized treatment of COVID-19 including mechanical ventilation. Gattinoni and colleagues have proposed two different phenotypes in COVID-19 at the macroscale that account for the difference in disease progression seen in CARDS compared to those of a typical ARDS of other etiologies [2]. These phenotypes are based on a type L in which patients show near normal lung compliance with hypoxemia and a later type H that is similar to severe ARDS, both requiring different treatments including different mechanical ventilation protocols. It is hypothesized that the macroscale type L phenotype is due to the microscale vascular changes that occur in COVID-19 not decrease in lung volume, which could explain the “happy hypoxia” seen in some patients [2]. Studies have begun to look at these macroscale phenotypes in light of microscale damage and changes in COVID-19 lungs. An autopsy study of 6 patients found 1 patient correlated to the type L phenotype and died early in disease progression while the other 5 presented with type H phenotype and AFOP was the prominent histopathology finding along with vascular injury [29]. A study using dual-energy CT found that abnormal perfusion and vascular dysregulation in COVID-19 may not only be found in the early L phenotype as previously predicted [117]. These findings further support that COVID-19 is different than ARDS. Others have used CT images of COVID-19 patients to divide CARDS into three phenotypes: 1) multiple, focal, over perfused ground-glass opacities, 2) inhomogeneously distributed atelectatic regions, and 3) patchy ARDS-like pattern [128]. Further research is needed to understand possible phenotypes of COVID-19 that are seen through progression of the disease.
Another model that has been used to previously explain ARDS is the “baby lung” and it also seems to present differently in COVID-19 alongside the various phenotypes [135]. The “baby lung” is the concept that patients with ARDS have a decrease in the amount of aerated lung tissue reducing their lung to the size of a young child. It is believed that COVID-19 begins as a typical adult lung then transitions into a “baby lung” due to disease progression and possible exacerbation from improper mechanical ventilation. With COVID-19, it is important to look at not only the repeated high levels of stress and strain on the epithelial cells of the lung but also the endothelial cells [135]. The effects of COVID-19 on the lung structure and function has led clinicians to recommend personalized mechanical ventilation strategies as the macroscale mechanics are too heterogeneous to use ARDS protocols [128,129,136].
There are hypotheses into how the pathological changes of the COVID-19 lung lead to the different changes in function at the macroscale but visualization of these microscale changes in vivo is challenging due to inadequate imaging resolution and lack of validated testing protocols. Use of in silico modeling and simulation techniques could allow for visualization of lung micromechanics to better understand functional changes in COVID-19 [137,138]. There is currently great variation in procedures used in postmortem studies of COVID-19 pathology and in order to make better comparisons between studies, standardized procedures and classification systems would be beneficial [139,140].
4. Conclusion
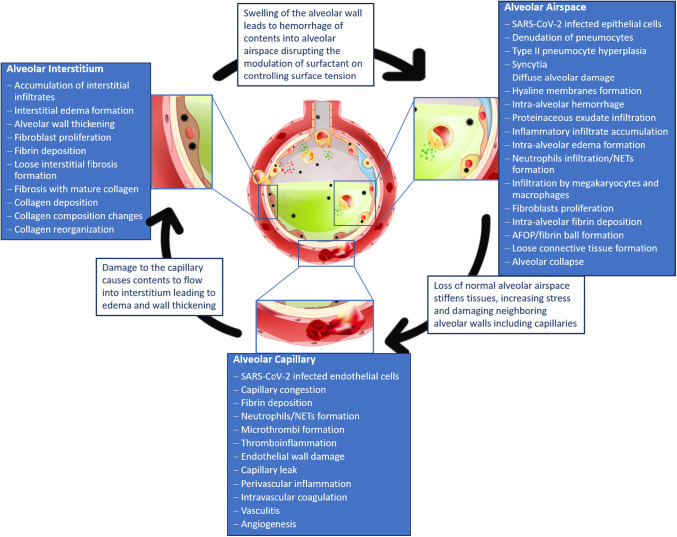
COVID-19 has highlighted the need to fully understand novel disease states, as they may initially present similar to previous diseases but later be found to induce damage specific to the virus that calls for different treatment methods. Fig. 5 provides an overview of the changes occurring in the COVID-19 lung with respect to different compartments of the alveolus and the interdependency of these changes in affecting lung micromechanics.
Fig. 5.
When the alveoli of the lung become infected with COVID-19, damage occurs that disrupts the alveolar airspace, interstitium and capillaries. Pathological findings that occur within each compartment of the alveoli are summarized. The pathological changes in each compartment disrupt the mechanical behavior of the alveoli which can induce further damage to neighboring areas. In this way, the impacts of COVID-19 on the airspace, interstitium, and capillaries are interconnected and lead to progression of one another (image adapted and sourced from shutterstock.com).
As more information comes to light on the pathophysiology of COVID-19, therapeutics and supplements can be implemented and tested to treat COVID-19 and decrease the severity of the disease as outlined by Morris et al. [141]. The ability of mesenchymal stem cells to modulate the immune response and repair damage to lungs make it a possible contender as a therapeutic for COVID-19 [142]. For treatment methods to be effective, the microscale mechanics must be considered to effectively treat patients without inducing further harm such as VILI. As this review focuses on microscale mechanics at the tissue level, future studies should also go deeper to look at the cellular lung mechanics, since structural changes that occur in the tissues induce changes to the niche of alveolar cells, imposing mechanical stress to the cells. Increased stress on the cells and strained cellular niches could lend to the pulmonary fibrosis seen in COVID-19 as fibrosis can be due to improper repair which is carried out by the cells [143]. Moving forward, it will be important to continue to study the tissue level changes occurring in the COVID-19 lung as well as the induced morphological changes in the alveolar cells. Recently, morphological changes have been viewed with light microscopy and 3D electron microscopy in the endoplasmic reticulum, mitochondria, Golgi apparatus, and cytoskeletal elements of SARS-COV-2 infected cells [144]. Such morphological and structural changes may contribute to viral replication and cell function affecting cellular mechanics within the alveolar niche [143,144]. Repair of the lung after injury is dependent on numerous factors including age and comorbidities as studies have found that near normal pulmonary function after ARDS can be regained within a year but there may be other lasting effects [9,101,145]. Overall, understanding the micromechanics of the COVID-19 induced lung damage can shed light on the effects of disease on the pulmonary ventilation dynamics and can provide mechanistic insights into optimized treatment options.
CRediT authorship contribution statement
ED, AV, and ZD conceptualized the study. KS implemented the search strategy and drafted the methodology. ED and AV reviewed and investigated relevant search results and drafted the manuscript. VM, JS, ZD, and SG reviewed and edited the manuscript. All authors revised and approved the manuscript. VM, JS, SG, and AV acquired funding. ED and AV have accessed verified the underlying data.
Declaration of competing interest
All authors declare no competing interest.
Acknowledgements
This material is based upon work supported by the National Science Foundation, Division of Civil, Mechanical, and Manufacturing Innovation, Directorate For Engineering under award number 2034964. The funding source had no role in writing the manuscript. All authors had full access to the full data in the study and accept responsibility to submit for publication.
Appendix 1
PubMed:
("Coronavirus Infections"[Mesh] AND "Pandemics"[Mesh]) OR "severe acute respiratory syndrome coronavirus 2" [Supplementary Concept] OR "COVID-19" [Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[tiab] OR "ncov"[tiab] OR "2019 ncov"[tiab] OR "covid 19"[tiab] OR "sars cov 2"[tiab] OR (("coronavirus"[tiab] OR "cov"[tiab]) AND 2019/11/01:3000/12/31[Date - Publication])
AND ("Lung"[Mesh] OR Lung[tiab] OR Lungs[tiab] OR Alveol*[tiab] OR Pulmonary[tiab])
AND
(Biomechanics[tiab] OR Biomechanical[tiab] OR Mechanics[tiab] OR Dynamics[tiab] OR Stress[tiab] OR Strain[tiab] OR Histopathologic[tiab] OR Histopathological[tiab] OR Histopathology[tiab] OR Histologic[tiab] OR Morphologic[tiab] OR Morphology[tiab] OR morphological[tiab] OR Pathophysiologic[tiab] OR Pathophysiology[tiab] OR Pathophysiological[tiab] OR Physiopathology[tiab] OR Physiopathologic[tiab] OR Physiopathological[tiab] OR Model[tiab] OR Models[tiab] OR Modeling[tiab])
Scopus:
( TITLE-ABS-KEY ( "severe acute respiratory syndrome coronavirus 2" OR "ncov" OR "2019 ncov" OR "covid 19" OR "sars cov 2" ) )
AND
( TITLE-ABS-KEY ( lung OR lungs OR alveol* OR pulmonary ) )
AND
( TITLE-ABS-KEY ( biomechanics OR biomechanical OR mechanics OR dynamics OR stress OR strain OR histopathologic OR histopathological OR histopathology OR histologic OR morphologic OR morphology OR morphological OR pathophysiologic OR pathophysiology OR pathophysiological OR physiopathology OR physiopathologic OR physiopathological OR model OR models OR modeling ) ) AND ( TITLE-ABS-KEY ( lung OR lungs OR alveol* OR pulmonary ) )
BioRxiv:
("sars-cov2" OR "covid-19" OR "sars-cov-2" OR "covid-19") AND (alveoli) AND (histopathology OR biomechanic OR model)
Note: search run using the Advanced Search.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.F.M. Beloncle, B. Pavlovsky, C. Desprez, N. Fage, P.Y. Olivier, P. Asfar, J.C. Richard, A. Mercat, Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome, Ann. Intensive Care. 10 (2020). doi: 10.1186/s13613-020-00675-7. [DOI] [PMC free article] [PubMed]
- 4.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020 doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suki B., Bates J.H.T. Lung tissue mechanics as an emergent phenomenon. J. Appl. Physiol. 2011;110:1111–1118. doi: 10.1152/japplphysiol.01244.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlman C.E. The contribution of surface tension-dependent alveolar septal stress concentrations to ventilation-induced lung injury in the acute respiratory distress syndrome. Front. Physiol. 2020;11:26. doi: 10.3389/fphys.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suki B., Ito S., Stamenovic D., Lutchen K.R., Ingenito E.P., Ingenito Biomechanics E.P. Highlighted topic biomechanics and mechanotransduction in cells and tissues biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J. Appl. Physiol. 2005;98:1892–1899. doi: 10.1152/japplphysiol.01087.2004.-The. [DOI] [PubMed] [Google Scholar]
- 8.Fung Y.-C. Springer New York; New York, NY: 1993. Biomechanics. [DOI] [Google Scholar]
- 9.Hsia C.C.W., Hyde D.M., Weibel E.R. Lung structure and the intrinsic challenges of gas exchange. Compr. Physiol. 2016;6:827–895. doi: 10.1002/cphy.c150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead J., Takishima T., Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J. Appl. Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen L., Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018;150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredberg J.J., Kamm R.D. Stress transmission in the lung: pathways from organ to molecule. Annu. Rev. Physiol. 2006;68:507–548. doi: 10.1146/annurev.physiol.68.072304.114110. [DOI] [PubMed] [Google Scholar]
- 13.Wagner W., Bennett R.D., Ackermann M., Ysasi A.B., Belle J., Valenzuela C.D., Pabst A., Tsuda A., Konerding M.A., Mentzer S.J. Elastin cables define the axial connective tissue system in the murine lung. Anat. Rec. 2015;298:1960–1968. doi: 10.1002/ar.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cong X., Hubmayr R.D., Li C., Zhao X. Plasma membrane wounding and repair in pulmonary diseases. Am. J. Phys. Lung Cell. Mol. Phys. 2017;312:L371–L391. doi: 10.1152/ajplung.00486.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillich A., Zhang F., Farmer C.G., Travaglini K.J., Tan S.Y., Gu M., Zhou B., Feinstein J.A., Krasnow M.A., Metzger R.J. Capillary cell-type specialization in the alveolus. Nature. 2020;586:785–789. doi: 10.1038/s41586-020-2822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachofen H., Schürch S. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. Elsevier Inc.; 2001. Alveolar surface forces and lung architecture; pp. 183–193. [DOI] [PubMed] [Google Scholar]
- 18.Oeckler R.A., Hubmayr R.D. Cell wounding and repair in ventilator injured lungs. Respir. Physiol. Neurobiol. 2008;163:44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson T.A., Bachofen H. 1982. A Model for Mechanical Structure of the Alveolar Duct. [DOI] [PubMed] [Google Scholar]
- 20.Silver F.H., Horvath I., Foran D.J. Mechanical implications of the domain structure of fiber-forming collagens: comparison of the molecular and fibrillar flexibilities of the α1-chains found in types I-III collagen. J. Theor. Biol. 2002;216:243–254. doi: 10.1006/jtbi.2002.2542. [DOI] [PubMed] [Google Scholar]
- 21.Cavalcante F.S.A., Ito S., Brewer K., Sakai H., Alencar A.M., Almeida M.P., Andrade J.S., Majumdar A., Ingenito E.P., Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J. Appl. Physiol. 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 22.Beigmohammadi M.T., Jahanbin B., Safaei M., Amoozadeh L., Khoshavi M., Mehrtash V., Jafarzadeh B., Abdollahi A. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int. J. Surg. Pathol. 2020 doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.G. Mansueto, COVID-19: Brief check through the pathologist's eye (autopsy archive), Pathol. Res. Pract. 216 (2020). doi: 10.1016/j.prp.2020.153195. [DOI] [PMC free article] [PubMed]
- 24.Martines R.B., Ritter J.M., Matkovic E., Gary J., Bollweg B.C., Bullock H., Goldsmith C.S., Silva-Flannery L., Seixas J.N., Reagan-Steiner S., Uyeki T., Denison A., Bhatnagar J., Shieh W.J., Zaki S.R., Cole R., Lewis A., Fair P., Estetter L. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg. Infect. Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., Malheiros D.M.A.C., de Oliveira E.P., Theodoro-Filho J., Pinho J.R.R., Gomes-Gouvêa M.S., Salles A.P.M., de Oliveira I.R.S., Mauad T., Saldiva P.H.N., Dolhnikoff M. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., Xiao S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.B. Recalde-Zamacona, L. García-Tobar, A. Argueta, L. Álvarez, C.E. De Andrea, M. Fernández Alonso, A. Ezponda, F. Carmona Torre, C. Jordán Iborra, J.A. Quiroga, J.L. Del Pozo, J.J. Zulueta, G. Echarri, M.F. Landecho, M.D. Lozano, Histopathological findings in fatal COVID-19 severe acute respiratory syndrome: preliminary experience from a series of 10 Spanish patients, Thorax. (2020) thoraxjnl-2020-215577. doi: 10.1136/thoraxjnl-2020-215577. [DOI] [PubMed]
- 28.L. Prieto-Pérez, J. Fortes, C. Soto, Á. Vidal-González, M. Alonso-Riaño, M. Lafarga, M.J. Cortti, A. Lazaro-Garcia, R. Pérez-Tanoira, Á. Trascasa, A. Antonio, R. Córdoba, S.M. Rodríguez-Pinilla, O. Cedeño, G. Peces-Barba, I. Fernández-Ormaechea, M.J. Díez Medrano, M. López de Las Heras, A. Cabello, E. Petkova, B. Álvarez, I. Carrillo, A.M. Silva, M. Castellanos, S. Calpena, M. Valverde-Monge, D. Fresneda, R. Rubio-Martín, I. Cornejo, L. Astilleros Blanco de Cordova, S. de la Fuente, S. Recuero, M. Górgolas, M.A. Piris, Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection, Mod. Pathol. (2020). doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed]
- 29.M.C. Copin, E. Parmentier, T. Duburcq, J. Poissy, D. Mathieu, M. Caplan, N. Cousin, A. Durand, J. Goutay, A. El Kalioubie, R. Favory, P. Girardie, M. Houard, E. Jaillette, M. Jourdain, G. Ledoux, A.S. Moreau, C. Niles, S. Nseir, T. Onimus, S. Préau, L. Robriquet, A. Rouzé, A. Simonnet, S. Six, A. Toussaint, R. Dubois, J.B. Gibier, S. Humez, Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection, Intensive Care Med. 46 (2020) 1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed]
- 30.Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., von der Thüsen J., Timofeev S., Gorkiewicz G., Lunardi F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T.S. Schiergens, G. Herrler, N.H. Wu, A. Nitsche, M.A. Müller, C. Drosten, S. Pöhlmann, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell. 181 (2020) 271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 32.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.L. Cantuti-Castelvetri, R. Ojha, L.D. Pedro, M. Djannatian, J. Franz, S. Kuivanen, F. van der Meer, K. Kallio, T. Kaya, M. Anastasina, T. Smura, L. Levanov, L. Szirovicza, A. Tobi, H. Kallio-Kokko, P. Österlund, M. Joensuu, F.A. Meunier, S.J. Butcher, M.S. Winkler, B. Mollenhauer, A. Helenius, O. Gokce, T. Teesalu, J. Hepojoki, O. Vapalahti, C. Stadelmann, G. Balistreri, M. Simons, Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science (80-. ). 370 (2020) 856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed]
- 34.J.L. Daly, B. Simonetti, K. Klein, K.E. Chen, M.K. Williamson, C. Antón-Plágaro, D.K. Shoemark, L. Simón-Gracia, M. Bauer, R. Hollandi, U.F. Greber, P. Horvath, R.B. Sessions, A. Helenius, J.A. Hiscox, T. Teesalu, D.A. Matthews, A.D. Davidson, B.M. Collins, P.J. Cullen, Y. Yamauchi, Neuropilin-1 is a host factor for SARS-CoV-2 infection, Science (80-. ). 370 (2020) 861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed]
- 35.Borczuk A.C., Salvatore S.P., Seshan S.V., Patel S.S., Bussel J.B., Mostyka M., Elsoukkary S., He B., Del Vecchio C., Fortarezza F., Pezzuto F., Navalesi P., Crisanti A., Fowkes M.E., Bryce C.H., Calabrese F., Beasley M.B. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020 doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T., Yarid N., Marshall D.A. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.B. Schurink, E. Roos, T. Radonic, E. Barbe, C.S.C. Bouman, H.H. de Boer, G.J. de Bree, E.B. Bulle, E.M. Aronica, S. Florquin, J. Fronczek, L.M.A. Heunks, M.D. de Jong, L. Guo, R. du Long, R. Lutter, P.C.G. Molenaar, E.A. Neefjes-Borst, H.W.M. Niessen, C.J.M. van Noesel, J.J.T.H. Roelofs, E.J. Snijder, E.C. Soer, J. Verheij, A.P.J. Vlaar, W. Vos, N.N. van der Wel, A.C. van der Wal, P. van der Valk, M. Bugiani, Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study, The Lancet Microbe. (2020). doi: 10.1016/s2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed]
- 38.J.L. Sauter, M.K. Baine, K.J. Butnor, D.J. Buonocore, J.C. Chang, A.A. Jungbluth, M.J. Szabolcs, S. Morjaria, S.L. Mount, N. Rekhtman, E. Selbs, Z. Sheng, Y. Xiao, D.E. Kleiner, S. Pittaluga, J.K. Taubenberger, A. V. Rapkiewicz, W.D. Travis, Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies, Histopathology. (2020) his.14201. doi: 10.1111/his.14201. [DOI] [PMC free article] [PubMed]
- 39.Magro C.M., Mulvey J., Kubiak J., Mikhail S., Suster D., Crowson A.N., Laurence J., Nuovo G. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2020;50:151645. doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., Thursz M., Manousou P., Corbett R., Goldin R., Al-Sarraj S., Abdolrasouli A., Swann O.C., Baillon L., Penn R., Barclay W.S., Viola P., Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/s2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skok K., Stelzl E., Trauner M., Kessler H.H., Lax S.F. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Arch. 2020:1–11. doi: 10.1007/s00428-020-02903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.M. Sekulic, H. Harper, B.G. Nezami, D.L. Shen, S.P. Sekulic, A.T. Koeth, C. V Harding, H. Gilmore, N. Sadri, Molecular Detection of SARS-CoV-2 Infection in FFPE Samples and Histopathologic Findings in Fatal SARS-CoV-2 Cases, Am J Clin Pathol. 154 (2020) 190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed]
- 43.D. Wichmann, J.P. Sperhake, M. Lütgehetmann, S. Steurer, C. Edler, A. Heinemann, F. Heinrich, H. Mushumba, I. Kniep, A.S. Schröder, C. Burdelski, G. de Heer, A. Nierhaus, D. Frings, S. Pfefferle, H. Becker, H. Bredereke-Wiedling, A. de Weerth, H.R. Paschen, S. Sheikhzadeh-Eggers, A. Stang, S. Schmiedel, C. Bokemeyer, M.M. Addo, M. Aepfelbacher, K. Püschel, S. Kluge, Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study, Ann. Intern. Med. 173 (2020) 268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed]
- 44.Grosse C., Grosse A., Salzer H.J.F., Dünser M.W., Motz R., Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc. Pathol. 2020;49:107263. doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., Vander K., Bargfrieder U., Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Nemer A. Histopathologic and autopsy findings in patients diagnosed with coronavirus disease 2019 (COVID-19): what we know so far based on correlation with clinical, morphologic and pathobiological aspects. Adv. Anat. Pathol. 2020;27:363–370. doi: 10.1097/pap.000000000000027610.1097/PAP.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 47.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nienhold R., Ciani Y., Koelzer V.H., Tzankov A., Haslbauer J.D., Menter T., Schwab N., Henkel M., Frank A., Zsikla V., Willi N., Kempf W., Hoyler T., Barbareschi M., Moch H., Tolnay M., Cathomas G., Demichelis F., Junt T., Mertz K.D. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konopka K.E., Nguyen T., Jentzen J.M., Rayes O., Schmidt C.J., Wilson A.M., Farver C.F., Myers J.L. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020;77:570–578. doi: 10.1111/his.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.W.O. Vasquez-Bonilla, R. Orozco, A. Md, M. Sierra, L.I. Zambrano, F. Muñoz-Lara, D. Salomón López-Molina, K. Arteaga-Livias, Z. Grimes, C. Bryce, A. Paniz-Mondolfi, A.J. Rodríguez-Morales, A.J. Co, ) Rodríguez-Morales, A review of the main histopathological findings in coronavirus disease 2019 *, Hum. Pathol. xx (2020) 1–10. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed]
- 51.Tomashefski J.F. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000;21:435–466. doi: 10.1016/S0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 52.Ware L.B., Belperio J.A., Matthay M.A., Semin Respir F.C.C.P. Pathophysiology of acute lung injury and the acute respiratory distress syndrome acute lung injury and acute respiratory distress syndrome. Crit. Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 53.Castro C.Y. ARDS and diffuse alveolar damage: a pathologist’s perspective. Semin. Thorac. Cardiovasc. Surg. 2006;18:13–19. doi: 10.1053/j.semtcvs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan L., Mir M., Sanchez P., Beg M., Peters J., Enriquez O., Gilbert A. COVID-19 in a hispanic woman: autopsy report with clinical-pathologic correlation. Arch. Pathol. Lab. Med. 2020;144:1041–1047. doi: 10.5858/arpa.2020-0217-SA. [DOI] [PubMed] [Google Scholar]
- 56.Heinrich F., Sperhake J.P., Heinemann A., Mushumba H., Lennartz M., Nörz D., Glatzel M., Lütgehetmann M., Püschel K. Germany’s first COVID-19 deceased: a 59-year-old man presenting with diffuse alveolar damage due to SARS-CoV-2 infection. Virchows Arch. 2020;477:335–339. doi: 10.1007/s00428-020-02872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs W., Lammens M., Kerckhofs A., Voets E., Van San E., Van Coillie S., Peleman C., Mergeay M., Sirimsi S., Matheeussen V., Jansens H., Baar I., Vanden Berghe T., Jorens P.G. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020 doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harkin T.J., Rurak K.M., Martins J., Eber C., Szporn A.H., Beasley M.B. Delayed diagnosis of COVID-19 in a 34-year-old man with atypical presentation. Lancet Respir. Med. 2020;8:644–646. doi: 10.1016/S2213-2600(20)30232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conde P.N., Monraval P.A., Medina Medina C., Jiménez Sánchez A., Carlos J., Teruel A., Ferrando Marco J., Santos P., Aranda M. Autopsy findings from the first known death from Severe Acute Respiratory Syndrome SARS-CoV-2 in Spain. Rev. Esp. Patol. 2020;53:188–192. doi: 10.1016/j.patol.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., Zhang S., Cao T., Yang C., Li M., Guo G., Chen X., Chen Y., Lei M., Liu H., Zhao J., Peng P., Wang C.-Y., Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pernazza A., Mancini M., Rullo E., Bassi M., De Giacomo T., Della Rocca C., d’Amati G. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020;1 doi: 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.R. Scendoni, F. Marchesani, N. Cannovo, P. Fedeli, M. Cingolani, Histopathology of COVID-19 pneumonia in two non-oncological, non-hospitalised cases as a reliable diagnostic benchmark, Diagn. Pathol. 15 (2020). doi: 10.1186/s13000-020-00990-4. [DOI] [PMC free article] [PubMed]
- 64.Puzović V., Baković M., Bubalo P., Mayer D. Accidental death from a fall from height at workplace turned out to be a COVID-19 death. Forensic Sci. Int. Rep. 2020;2:100139. doi: 10.1016/j.fsir.2020.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suess C., Hausmann R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int. J. Legal Med. 2020;134:1285–1290. doi: 10.1007/s00414-020-02319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A. Cîrstea, R. Lucian Buzulică, D. Pirici, M. Constantin Ceauşu, R. Vasile Iman, O. Gheorghe, S. Daniela Neamţu, L. Stanca, R. Ene, S. Kumar-singh, L. Mogoantă, Histopathological findings in the advanced natural evolution of the SARS-CoV-2 infection, Rom J Morphol Embryol. 61 (2020) 209–218. doi:10.47162/RJME.61.1.23. [DOI] [PMC free article] [PubMed]
- 67.Buja L.M., Wolf D., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., Aisenberg G.M., Madjid M., Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., Frank S., Turek D., Willi N., Pargger H., Bassetti S., Leuppi J.D., Cathomas G., Tolnay M., Mertz K.D., Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oprinca G.C., Muja L.A. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int. J. Legal Med. 2020 doi: 10.1007/s00414-020-02406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.F. Sadegh Beigee, M. Pourabdollah Toutkaboni, N. Khalili, S.A. Nadji, A. Dorudinia, M. Rezaei, E. Askari, B. Farzanegan, M. Marjani, A. Rafiezadeh, Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients, Pathol. Res. Pract. 216 (2020). doi: 10.1016/j.prp.2020.153228. [DOI] [PMC free article] [PubMed]
- 71.G. Kaber, M.J. Kratochvil, E.B. Burgener, E.L. Peltan, G. Barlow, S. Yang, M.R. Nicolls, V.A. de Jesus Perez, J. Rosser, A.J. Wardle, A. Kalinowski, M.G. Ozawa, D.P. Regula, N. Nagy, S.C. Heilshorn, C.E. Milla, A.J. Rogers, P.L. Bollyky, P. Bollyky, Hyaluronan is abundant in COVID-19 respiratory secretions, MedRxiv. (2020) 2020.09.11.20191692. doi: 10.1101/2020.09.11.20191692. [DOI]
- 72.Narasaraju T. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;173:323–324. doi: 10.7326/L20-0894. [DOI] [PubMed] [Google Scholar]
- 73.C. Radermecker, N. Detrembleur, J. Guiot, E. Cavalier, M. Henket, C. d'Emal, C. Vanwinge, D. Cataldo, C. Oury, P. Delvenne, T. Marichal, Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19, J. Exp. Med. 217 (2020). doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed]
- 74.Beasley M.B., Franks T.J., Galvin J.R., Gochuico B., Travis W.D. Allen Press; 2002. Acute Fibrinous and Organizing Pneumonia a Histologic Pattern of Lung Injury and Possible Variant of Diffuse Alveolar Damage. [DOI] [PubMed] [Google Scholar]
- 75.Flikweert A.W., Grootenboers M.J.J.H., Yick D.C.Y., du Mée A.W.F., van der Meer N.J.M., Rettig T.C.D., Kant M.K.M. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J. Crit. Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu J., Yu J., Zhou S., Zhang J., Xu J., Niu C., Qu G., Han B., Hu J., Dong L. What can we learn from a COVID-19 lung biopsy? Int. J. Infect. Dis. 2020;99:410–413. doi: 10.1016/j.ijid.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.X.-X. Wang, C. Shao, X.-J. Huang, L. Sun, L.-J. Meng, H. Liu, S.-J. Zhang, H.-J. Li, F.-D. Lv, Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19, J. Clin. Pathol. 0 (2020) 1–6. doi: 10.1136/jclinpath-2020-206623. [DOI] [PMC free article] [PubMed]
- 78.Roden A.C., Bois M.C., Johnson T.F., Aubry M.C., Alexander M.P., Hagen C.E., Lin P.T., Quinton R.A., Maleszewski J.J., Boland J.M. The spectrum of histopathologic findings in lungs of patients with fatal COVID-19 infection. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2020-0491-sa. [DOI] [PubMed] [Google Scholar]
- 79.A. Rouzé, I. Martin-Loeches, P. Povoa, D. Makris, A. Artigas, M. Bouchereau, F. Lambiotte, M. Metzelard, P. Cuchet, C. Boulle Geronimi, M. Labruyere, F. Tamion, M. Nyunga, C.E. Luyt, J. Labreuche, O. Pouly, J. Bardin, A. Saade, P. Asfar, J.L. Baudel, A. Beurton, D. Garot, I. Ioannidou, L. Kreitmann, J.F. Llitjos, E. Magira, B. Mégarbane, D. Meguerditchian, E. Moglia, A. Mekontso-Dessap, J. Reignier, M. Turpin, A. Pierre, G. Plantefeve, C. Vinsonneau, P.E. Floch, N. Weiss, A. Ceccato, A. Torres, A. Duhamel, S. Nseir, R. Favory, S. Preau, M. Jourdain, J. Poissy, C. Bouras, P. Saint Leger, H. Fodil, F. Aptel, T. Van Der Linden, A.W. Thille, E. Azoulay, F. Pène, K. Razazi, F. Bagate, D. Contou, G. Voiriot, D. Thevenin, B. Guidet, L. Le Guennec, A. Kouatchet, S. Ehrmann, G. Brunin, E. Morawiec, A. Boyer, L. Argaud, S. Voicu, A. Nieszkowska, B. Kowalski, G. Goma, E. Diaz, L. Morales, V. Tsolaki, G. Gtavriilidis, S.D. Mentzelopoulos, D. Nora, S. Boyd, L. Coelho, J. Maizel, D. Du Cheyron, M. Imouloudene, J.P. Quenot, A. Guilbert, C. Cilloniz, Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study, Intensive Care Med. (2021). doi: 10.1007/s00134-020-06323-9. [DOI]
- 80.Mason R.J. Thoughts on the alveolar phase of COVID-19. Am. J. Physiol. Cell Mol. Physiol. 2020;319:L115–L120. doi: 10.1152/ajplung.00126.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mac Sweeney R., McAuley D.F. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knudsen L., Lopez-Rodriguez E., Berndt L., Steffen L., Ruppert C., Bates J.H.T., Ochs M., Smith B.J. Alveolar micromechanics in bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2018;59:757–769. doi: 10.1165/rcmb.2018-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cortjens B., De Boer O.J., De Jong R., Antonis A.F.G., Sabogal Piñeros Y.S., Lutter R., Van Woensel J.B.M., Bem R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016;238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 84.Cereda M., Xin Y., Hamedani H., Bellani G., Kadlecek S., Clapp J., Guerra L., Meeder N., Rajaei J., Tustison N.J., Gee J.C., Kavanagh B.P., Rizi R.R. Tidal changes on CT and progression of ARDS. Thorax. 2017;72:981–989. doi: 10.1136/thoraxjnl-2016-209833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.K. Albert, J.M. Krischer, A. Pfaffenroth, S. Wilde, E. Lopez-Rodriguez, A. Braun, B.J. Smith, L. Knudsen, Hidden Microatelectases Increase Vulnerability to Ventilation-Induced Lung Injury, Front. Physiol. 11 (2020). doi: 10.3389/fphys.2020.530485. [DOI] [PMC free article] [PubMed]
- 86.Albert R.K., Smith B., Perlman C.E., Schwartz D.A. Is progression of pulmonary fibrosis due to ventilation-induced lung injury? Am. J. Respir. Crit. Care Med. 2019;200:140–151. doi: 10.1164/rccm.201903-0497PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bitterman P.B. Pathogenesis of fibrosis in acute lung injury. Am. J. Med. 1992;92:S39–S43. doi: 10.1016/0002-9343(92)90606-C. [DOI] [PubMed] [Google Scholar]
- 88.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gustine J.N., Jones D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 91.Ichikawa T., Hirahara K., Kokubo K., Kiuchi M., Aoki A., Morimoto Y., Kumagai J., Onodera A., Mato N., Tumes D.J., Goto Y., Hagiwara K., Inagaki Y., Sparwasser T., Tobe K., Nakayama T. CD103hi Treg cells constrain lung fibrosis induced by CD103lo tissue-resident pathogenic CD4 T cells. Nat. Immunol. 2019;20:1469–1480. doi: 10.1038/s41590-019-0494-y. [DOI] [PubMed] [Google Scholar]
- 92.Zhang M., Zhang S. T cells in fibrosis and fibrotic diseases. Front. Immunol. 2020;11:1142. doi: 10.3389/fimmu.2020.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li K., Yu Y., Li D., Luan C., Hu L., Wang J., Ding J., Yu Y., Yang H., Chen J., Chen F., Su C. The histopathological features of the explanted lungs From an end-stage COVID-19 patient. Forensic Sci. Res. 2020 doi: 10.1080/20961790.2020.1807128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y., Wu J., Wang S., Li X., Zhou J., Huang B., Luo D., Cao Q., Chen Y., Chen S., Ma L., Peng L., Pan H., Travis W.D., Nie X. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2020 doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barisione E., Grillo F., Ball L., Bianchi R., Grosso M., Morbini P., Pelosi P., Patroniti N.A., De Lucia A., Orengo G., Gratarola A., Verda M., Cittadini G., Mastracci L., Fiocca R. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2020 doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.A. Aiolfi, B. Bruni, T. Biraghi, A. Montisci, A. Miceli, B. Baronio, D. Khor, S. Cirri, F. Donatelli, C. Clemente, D. Bona, Late histological findings in symptomatic COVID-19 patients: A case report, Medicine (Baltimore). 99 (2020) e21046. doi: 10.1097/MD.0000000000021046. [DOI] [PMC free article] [PubMed]
- 97.A. Figueiredo Rendeiro, H. Ravichandran, Y. Bram, S. Salvatore, A. Borczuk, O. Elemento, R. Edward Schwartz, The spatio-temporal landscape of lung pathology in SARS-CoV-2 infection, MedRxiv. (2020) 2020.10.26.20219584. doi: 10.1101/2020.10.26.20219584. [DOI]
- 98.Schwensen H.F., Borreschmidt L.K., Storgaard M., Redsted S., Christensen S., Madsen L.B. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 99.Eapen M.S., Lu W., Gaikwad A.V., Bhattarai P., Chia C., Hardikar A., Haug G., Sohal S.S. Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur. Respir. J. 2020;56:2003167. doi: 10.1183/13993003.03167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valdivia-Mazeyra M.F., Salas C., Nieves-Alonso J.M., Martín-Fragueiro L., Bárcena C., Muñoz-Hernández P., Villar-Zarra K., Martín-López J., Ramasco-Rueda F., Fraga J., Jiménez-Heffernan J.A. Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature. Virchows Arch. 2020:1–10. doi: 10.1007/s00428-020-02926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Horowitz J.C., Naba A., Ambalavanan N., Atabai K., Balestrini J., Bitterman P.B., Corley R.A., Sen Ding B., Engler A.J., Hansen K.C., Hagood J.S., Kheradmand F., Lin Q.S., Neptune E., Niklason L., Ortiz L.A., Parks W.C., Tschumperlin D.J., White E.S., Chapman H.A., Thannickal V.J. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018;73:77–104. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.P.R.M. Rocco, E.M. Negri, P.M. Kurtz, F.P. Vasconcellos, G.H. Silva, V.L. Capelozzi, P. V Romero, W.A. Zin, Lung Tissue Mechanics and Extracellular Matrix Remodeling in Acute Lung Injury, Thoracic Society, 2001. www.atsjournals.org (accessed September 30, 2020). [DOI] [PubMed]
- 103.Mangalmurti N.S., Reilly J.P., Cines D.B., Meyer N.J. Correspondence COVID-19-associated acute respiratory distress syndrome clarified: a vascular Endotype? Am. J. Respir. Crit. Care Med. 2020;202:750–753. doi: 10.1164/rccm.202006-2598LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020 doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitchell W.B. Thromboinflammation in COVID-19 acute lung injury. Paediatr. Respir. Rev. 2020;35:20–24. doi: 10.1016/j.prrv.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.A. Huertas, D. Montani, L. Savale, J. Pichon, L. Tu, F. Parent, C. Guignabert, M. Humbert, Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)?, Eur. Respir. J. 56 (2020). doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed]
- 107.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grimes Z., Bryce C., Sordillo E.M., Gordon R.E., Reidy J., Paniz Mondolfi A.E., Fowkes M. Fatal pulmonary thromboembolism in SARS-CoV-2-infection. Cardiovasc. Pathol. 2020;48:107227. doi: 10.1016/j.carpath.2020.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.A. V. Rapkiewicz, X. Mai, S.E. Carsons, S. Pittaluga, D.E. Kleiner, J.S. Berger, S. Thomas, N.M. Adler, D.M. Charytan, B. Gasmi, J.S. Hochman, H.R. Reynolds, Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series, EClinicalMedicine. 24 (2020). doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed]
- 110.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., Scherer C., Rudelius M., Zoller M., Höchter D., Keppler O., Teupser D., Zwißler B., von Bergwelt-Baildon M., Kääb S., Massberg S., Pekayvaz K., Stark K. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petrey A.C., Qeadan F., Middleton E.A., Pinchuk I.V., Campbell R.A., Beswick E.J. Cytokine release syndrome in COVID-19: innate immune, vascular, and platelet pathogenic factors differ in severity of disease and sex. J. Leukoc. Biol. 2021;109:55–66. doi: 10.1002/JLB.3COVA0820-410RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fung T.S., Liu D.X. 2021. Annual Review of Microbiology Similarities and Dissimilarities of COVID-19 and Other Coronavirus Diseases. [DOI] [PubMed] [Google Scholar]
- 114.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.G. Li, S. Fox, B. Summa, B. Hu, C. Wenk, A. Akmatbekov, J. Harbert, R. Vander Heide, J.Q. Brown, Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy, BioRxiv. (2020) 2020.04.11.037473. doi: 10.1101/2020.04.11.037473. [DOI]
- 116.L. Cipolloni, F. Sessa, G. Bertozzi, B. Baldari, S. Cantatore, R. Testi, S. D'Errico, G. Di Mizio, A. Asmundo, S. Castorina, M. Salerno, C. Pomara, Preliminary post-mortem COVID-19 evidence of endothelial injury and factor VIII hyperexpression, Diagnostics. 10 (2020). doi: 10.3390/diagnostics10080575. [DOI] [PMC free article] [PubMed]
- 117.B. V Patel, D.J. Arachchillage, C.A. Ridge, P. Bianchi, J.F. Doyle, B. Garfield, S. Ledot, C. Morgan, M. Passariello, S. Price, S. Singh, L. Thakuria, S. Trenfield, R. Trimlett, C. Weaver, S.J. Wort, T. Xu, S.P.G. Padley, A. Devaraj, S.R. Desai, S.R.D. Literature, S.R.D. Data, S.R.D. Statistical, Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations A list of members of the Severe Acute Respiratory Failure Service and the Departments of Anesthesia and Critical Care is provided in the online supplement. Author Contributions: Study concepts and design, Am. J. Respir. Crit. Care Med. 202 (2020) 690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed]
- 118.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Stürzl M., Staats L., Mahajan A., Schauer C., Kremer A.N., Völkl S., Amann K., Evert K., Falkeis C., Wehrfritz A., Rieker R.J., Hartmann A., Kremer A.E., Neurath M.F., Muñoz L.E., Schett G., Herrmann M. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cardot-Leccia N., Hubiche T., Dellamonica J., Burel-Vandenbos F., Passeron T. Pericyte alteration sheds light on micro-vasculopathy in COVID-19 infection. Intensive Care Med. 2020;46:1777–1778. doi: 10.1007/s00134-020-06147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamaoka-Tojo M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biom. J. 2020 doi: 10.1016/j.bj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.J. von der Thüsen, M. van der Eerden, Histopathology and genetic susceptibility in COVID-19 pneumonia, Eur. J. Clin. Invest. 50 (2020). doi: 10.1111/eci.13259. [DOI] [PMC free article] [PubMed]
- 122.Szidon J., Pietra G., Fishman A. The alveolar-capillary membrane and pulmonary edema. N. Engl. J. Med. 1972;286:1200–1204. doi: 10.1056/NEJM197206012862208. [DOI] [PubMed] [Google Scholar]
- 123.Vock R., Weibel E.R. Massive hemorrhage causes changes in morphometric parameters of lung capillaries and concentration of leukocytes in microvasculature. Exp. Lung Res. 1993;19:559–577. doi: 10.3109/01902149309031728. [DOI] [PubMed] [Google Scholar]
- 124.Makiyama A.M., Gibson L.J., Harris R.S., Venegas J.G. Stress concentration around an atelectatic region: a finite element model. Respir. Physiol. Neurobiol. 2014;201:101–110. doi: 10.1016/j.resp.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 125.Fujioka H., Halpern D., Gaver D.P. A model of surfactant-induced surface tension effects on the parenchymal tethering of pulmonary airways. J. Biomech. 2013;46:319–328. doi: 10.1016/j.jbiomech.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 126.Haudebourg A.F., Perier F., Tuffet S., De Prost N., Razazi K., Dessap A.M., Carteaux G. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grieco D.L., Bongiovanni F., Chen L., Menga L.S., Cutuli S.L., Pintaudi G., Carelli S., Michi T., Torrini F., Lombardi G., Anzellotti G.M., De Pascale G., Urbani A., Bocci M.G., Tanzarella E.S., Bello G., Dell’Anna A.M., Maggiore S.M., Brochard L., Antonelli M. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit. Care. 2020;24:529. doi: 10.1186/s13054-020-03253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., Vena A., Giacobbe D.R., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020;279:103455. doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haouzi P., Zamir A., Villarreal-Fernandez E., Stauffer D., Ventola L., Ahmad D., Dewaters A., Khalid M., Wojnar M. Mechanics of breathing and gas exchange in mechanically ventilated patients with COVID-19-associated respiratory failure. Am. J. Respir. Crit. Care Med. 2020;202:626–628. doi: 10.1164/rccm.202004-1041LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am. J. Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 131.Perlman C.E., Lederer D.J., Bhattacharya J. Micromechanics of alveolar edema. Am. J. Respir. Cell Mol. Biol. 2010;44:34–39. doi: 10.1165/rcmb.2009-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.R.A. Almeida Monteiro, E.P. de Oliveira, P.H. Nascimento Saldiva, M. Dolhnikoff, A.N. Duarte-Neto, L.F.F. da Silva, T. Mauad, G.A.B. dos Santos, T.L.L.F. Leite, C.S. de Moura, J.T. Filho, K.C.S. Bispo, A.B.G. dos Santos, S. de M. Fernezlian, R.S. do Nascimento, Histological–ultrasonographical correlation of pulmonary involvement in severe COVID-19, Intensive Care Med. 46 (2020) 1766–1768. doi: 10.1007/s00134-020-06125-z. [DOI] [PMC free article] [PubMed]
- 133.Rodriguez-Roisin R., Wagner P.D. Clinical relevance of ventilation-perfusion inequality determined by inert gas elimination. Eur. Respir. J. 1990;3:469–482. [PubMed] [Google Scholar]
- 134.Kharge A.B., Wu Y., Perlman C.E. Surface tension in situ in flooded alveolus unaltered by albumin. J. Appl. Physiol. 2014;117:440–451. doi: 10.1152/japplphysiol.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gattinoni L., Meissner K., Marini J.J. The baby lung and the COVID-19 era. Intensive Care Med. 2020;46:1438–1440. doi: 10.1007/s00134-020-06103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not Lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burrowes K.S., Iravani A., Kang W. Integrated lung tissue mechanics one piece at a time: computational modeling across the scales of biology. Clin. Biomech. 2019;66:20–31. doi: 10.1016/j.clinbiomech.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 138.J. Herrmann, V. Mori, J.H.T. Bates, B. Suki, Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia, Nat. Commun. 11 (2020). doi: 10.1038/s41467-020-18672-6. [DOI] [PMC free article] [PubMed]
- 139.Maiese A., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Frati P., Fineschi V. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci. Med. Pathol. 2020 doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parmar D., Ponnampalam J., Seligmann G., Gandhi T. Emerging pulmonary histopathological findings in COVID-19 patients - a letter to the editor in response to Grosse et al. 2020. Cardiovasc. Pathol. 2020;51:107302. doi: 10.1016/j.carpath.2020.107302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O’Neil A., Athan E., Carvalho A., Maes M., Walder K., Berk M. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yadav P., Vats R., Bano A., Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci. 2020;263:118588. doi: 10.1016/j.lfs.2020.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J., Pan X., Wang L., Yu G. Alveolar cells under mechanical stressed niche: critical contributors to pulmonary fibrosis. Mol. Med. 2020;26:95. doi: 10.1186/s10020-020-00223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.M. Cortese, J.-Y. Lee, B. Cerikan, C.J. Neufeldt, V.M.J. Oorschot, S. Köhrer, J. Hennies, N.L. Schieber, P. Ronchi, G. Mizzon, I. Romero-Brey, R. Santarella-Mellwig, M. Schorb, M. Boermel, K. Mocaer, M.S. Beckwith, R.M. Templin, V. Gross, C. Pape, C. Tischer, J. Frankish, N.K. Horvat, V. Laketa, M. Stanifer, S. Boulant, A. Ruggieri, L. Chatel-Chaix, Y. Schwab, R. Bartenschlager, Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies, Cell Host Microbe. (2020). doi: 10.1016/j.chom.2020.11.003. [DOI] [PMC free article] [PubMed]
- 145.Herridge M.S. Recovery and long-term outcome in acute respiratory distress syndrome. Crit. Care Clin. 2011;27:685–704. doi: 10.1016/j.ccc.2011.04.003. [DOI] [PubMed] [Google Scholar]