Abstract
Background
Antibiotics are commonly prescribed to patients as they leave the hospital. We aimed to create a comprehensive metric to characterize antibiotic overuse after discharge among hospitalized patients treated for pneumonia or urinary tract infection (UTI), and to determine whether overuse varied across hospitals and conditions.
Methods
In a retrospective cohort study of hospitalized patients treated for pneumonia or UTI in 46 hospitals between 1 July 2017–30 July 2019, we quantified the proportion of patients discharged with antibiotic overuse, defined as unnecessary antibiotic use, excess antibiotic duration, or suboptimal fluoroquinolone use. Using linear regression, we assessed hospital-level associations between antibiotic overuse after discharge in patients treated for pneumonia versus a UTI.
Results
Of 21 825 patients treated for infection (12 445 with pneumonia; 9380 with a UTI), nearly half (49.1%) had antibiotic overuse after discharge (56.9% with pneumonia; 38.7% with a UTI). For pneumonia, 63.1% of overuse days after discharge were due to excess duration; for UTIs, 43.9% were due to treatment of asymptomatic bacteriuria. The percentage of patients discharged with antibiotic overuse varied 5-fold among hospitals (from 15.9% [95% confidence interval, 8.7%–24.6%] to 80.6% [95% confidence interval, 69.4%–88.1%]) and was strongly correlated between conditions (regression coefficient = 0.85; P < .001).
Conclusions
Antibiotic overuse after discharge was common and varied widely between hospitals. Antibiotic overuse after discharge was associated between conditions, suggesting that the prescribing culture, physician behavior, or organizational processes contribute to overprescribing at discharge. Multifaceted efforts focusing on all 3 types of overuse and multiple conditions should be considered to improve antibiotic prescribing at discharge.
Keywords: antibiotic stewardship, transitions of care, pneumonia, urinary tract infection, quality of care
Of 21 825 hospitalized patients with pneumonia or urinary tract infection in 46 hospitals, half (49.1%) had antibiotic overuse after discharge. Overuse varied 5-fold among hospitals (15.9%–80.6%) and was correlated between conditions. To improve prescribing, stewardship should include care transitions.
Though nearly all hospitals have antibiotic stewardship programs, fewer than 1 in 5 monitor antibiotic use after discharge [1]. Furthermore, the antibiotics prescribed at discharge, unlike inpatient antibiotics, are not tracked nationally [2]. Overlooking antibiotics at discharge may miss up to half of antibiotic days related to acute hospitalization [2–8]. Without data and benchmarking, stewardship programs may have difficulty improving prescribing at care transitions. For example, 1 multicenter study found that stewardship strategies targeting inpatient fluoroquinolone use had limited effects on fluoroquinolone use after discharge [4]. This may explain why inpatient fluoroquinolone use has been declining nationally while fluoroquinolone use at discharge has remained stable [9].
A difficulty in evaluating prescribing after discharge is the lack of a comprehensive metric to evaluate how much antibiotic overuse occurs during care transitions. Typically, studies have focused on a single disease or single type of overuse (eg, excess duration in pneumonia) [6–8, 10]. Small studies examining multiple types of overuse suggest that 41–53% of antibiotics prescribed after discharge could be improved [5, 11–13]. However, comprehensive antibiotic overuse after discharge has not been assessed across hospitals and conditions. Thus, we aimed to characterize antibiotic overuse more broadly after discharge. To do so, we created a hierarchical metric to assess antibiotic overuse in hospitalized patients who were treated for pneumonia or urinary tract infection (UTI), and applied it to 21 825 patients discharged from 46 Michigan hospitals [14].
METHODS
Study Setting and Participants
This retrospective cohort study included patients discharged between 1 July 2017 and 30 July 2019 from 46 diverse hospitals participating in the Michigan Hospital Medicine Safety (HMS) Consortium (a collaborative quality initiative funded by Blue Cross Blue Shield of Michigan). Hospital participation in HMS is voluntary and includes half of nongovernment hospitals in Michigan, including rural hospitals, community hospitals, and academic teaching hospitals [1]. Since 2017, HMS has collected data on patients treated for pneumonia or UTI. As part of its quality improvement efforts, HMS shares guidelines during in-person quarterly meetings, provides a toolkit to help hospitals improve [15], and sets pay-for-performance metrics targeting 3 critical areas for antibiotic stewardship: (1) unnecessary antibiotic treatment for asymptomatic bacteriuria; (2) reducing excessive duration (particularly in community-acquired pneumonia [CAP]) [16]; and (3) emphasizing use of nonfluoroquinolone therapy when safe to do so.
The sampling, inclusion, and exclusion criteria have been described previously [8, 17]. Briefly, we included non–critically ill, hospitalized, adult medical patients who were treated for either pneumonia or a UTI during hospitalization. To identify patients for inclusion, discharge lists at each hospital were consecutively screened by HMS abstractors until 2 patients met the inclusion criteria daily (including weekends). Abstractors (who are not involved in patient care) undergo in-person and online case-based training on data abstraction and eligibility criteria, and undergo random audits for quality assurance. Patients were excluded if they did not receive antibiotic therapy, were pregnant, were severely immune-compromised (ie, diagnosed with AIDS or neutropenia, had a history of transplant, or had received ≥2 immunosuppressive agents), had concomitant infections (eg, cellulitis), died during hospitalization, or were transferred to another hospital or to intensive care.
Patients treated for pneumonia were identified by discharge diagnostic codes and receipt of antibiotic treatment by hospital Day 2 [18]. We included all patients treated for CAP [19], including those who (during the time frame of this study) would have been classified as having healthcare–associated pneumonia (HCAP) [20] and those who were treated for pneumonia but did not meet guideline-based diagnostic criteria for pneumonia. Patients with hospital-acquired or ventilator-associated pneumonia were excluded. Patients treated for a UTI (including both patients meeting the criteria for a UTI and those with asymptomatic bacteriuria) were identified based on any urine culture during the hospitalization that was flagged as positive (the criteria for “positive” varied across hospitals but was typically based on a colony-forming unit cutoff). Patients were also excluded if they had urological procedures during the hospitalization.
Data Collection
Detailed patient data—including diagnostic criteria (eg, symptoms, imaging, vital signs, and laboratory and culture results), comorbidities, and discharge antibiotic prescriptions—were collected from the medical record by abstractors for 30 days after discharge. Additional outcome data were obtained via a telephone follow-up 30 days after discharge using a standardized script; patients discharged on antibiotics were asked, “have you had any side effects from your prescribed antibiotic?”
Definition of Antibiotic Overuse After Discharge
The primary outcome of interest was the percentage of patients discharged with antibiotic overuse. Overuse is defined by the Institute of Medicine as healthcare where the “potential for harm exceeds the possible benefit.” [21] Thus, we defined antibiotic overuse as antibiotic use where the potential harm exceeds the potential benefit. We focused on 3 types of overuse that, based on prior studies [5, 9, 11], are common during care transitions: unnecessary antibiotic use, excess duration of use, and suboptimal use of fluoroquinolone therapy (Table 1). The criteria for overuse were determined using published studies and guidelines (detailed description available in the Supplementary Appendix) [8, 17, 22]. When evidence for appropriate treatment was conflicting, treatment was considered appropriate.
Table 1.
Characteristics of Patients With and Without Antibiotic Overuse at Hospital Discharge
| Category of overuse | Definition | Example in patient treated for pneumonia | Example in patient treated for urinary tract infection |
|---|---|---|---|
| Unnecessary antibiotic use | Antibiotic therapy prescribed for noninfectious or nonbacterial infections | Patient with a normal chest X-ray | Patient with asymptomatic bacteriuria |
| Excess antibiotic duration | Antibiotics prescribed beyond the indicated duration of therapy, absent any clinical reason for a lengthened course | Community-acquired pneumonia treated for longer than 5 days (despite being afebrile for 48 hours and clinically stable by Day 3 of hospitalization) | Uncomplicated urinary tract infection treated with nitrofurantoin for longer than 5 days |
| Suboptimal use of fluoroquinolones | Fluoroquinolone prescribed when safer alternative was available, after accounting for allergies, resistance, disease, and contraindications | Patient with community-acquired pneumonia who did not have a severe penicillin or cephalosporin allergy | Patient with uncomplicated cystitis who could use trimethoprim/sulfamethoxazole, fosfomycin, nitrofurantoin, or a cephalosporin |
“Unnecessary” antibiotic use is defined as antibiotic therapy prescribed for noninfectious or nonbacterial infections [23]. For this study, we defined unnecessary antibiotic use as antibiotic therapy prescribed for asymptomatic bacteriuria [17, 22] or in patients who did not meet guideline-based criteria for bacterial pneumonia [24] (ie, had negative chest imaging or lacked symptoms; see the Supplementary Appendix for definitions). Similarly, antibiotics prescribed “beyond the indicated duration of therapy absent any clinical reason for a lengthened course” [23] are considered unnecessary. To distinguish prolonged duration from the first type of unnecessary use, we referred to this as “excess duration.” Finally, suboptimal antibiotic therapy is defined as antibiotic use where the drug choice, route, or dose can be improved [23]. Because fluoroquinolones are the most common antibiotic prescribed at discharge [9], we focused on identifying suboptimal use of fluoroquinolones, defined as fluoroquinolones (levofloxacin, ciprofloxacin, or [for pneumonia only] moxifloxacin) prescribed when a safer alternative was available, after accounting for allergies, resistance, infectious condition, and contraindications (Supplementary Appendix).
Categories of antibiotic overuse were assessed hierarchically for each postdischarge antibiotic day, based on the anticipated risk-to-benefit ratio. Unnecessary antibiotic use was assessed first, as there is no benefit and only harm from prescribing antibiotics to patients without an infection. Next, we assessed for excess duration, followed by suboptimal use of fluoroquinolones. Because each day of postdischarge antibiotic use was assessed independently, a single postdischarge day could only be classified as 1 type of overuse. In contrast, a patient could have 2 types of antibiotic overuse after discharge (on different days). Examples are shown in Supplementary Figure 1.
Secondary Outcomes
Patient outcomes, assessed via chart review at 30 days, included mortality, readmission, emergency department visits, Clostridioides difficile infection (CDI), provider-documented antibiotic-associated adverse events, and composite adverse outcomes. For patients discharged on antibiotics, we assessed patient-reported adverse events via a phone call at 30 days. Because patient-reported adverse events were not collected if patients were not discharged on antibiotics, patient-reported adverse events were not included in the composite outcome.
Data Analysis
Descriptive statistics were used to quantify how much antibiotic overuse after discharge was unnecessary or constituted an excess duration or suboptimal use of fluoroquinolones. Characteristics of patients with versus without antibiotic overuse at discharge were compared using 2-sided chi-squared or Wilcoxon rank-sum tests, as appropriate. Using linear regression, we assessed whether the percentage of patients treated for pneumonia who had antibiotic overuse after discharge at a hospital was associated with the percentage of patients treated for a UTI who had overuse at discharge. Using a previously described method [8], we report odds ratios to evaluate whether days of antibiotic overuse after discharge were associated with patient outcomes at 30 days. Briefly, odds ratios were derived from logit generalized estimating equations models adjusted for hospital clustering and with an inverse probability of treatment (antibiotic overuse after discharge), weighted by baseline covariates known to be associated with outcomes or that predicted overuse in a multivariable analysis model (see Supplementary Appendix for details).
There were minimal missing data; for the 2 variables with some missing data (insurance, 3.7% [798/21 825]; race, 0.4% [83/21 825]), values were imputed through a 10-fold multiple imputation procedure and combined using standard rules [25]. We conducted a sensitivity analysis without imputing missing data. Although evidence for fluoroquinolone avoidance is strong [26–31] and antibiotic stewardship guidelines recommend reducing fluoroquinolone use [32], there is controversy as to whether fluoroquinolones should be a first choice or an alternative choice for many conditions (eg, CAP) [19, 33, 34]. Therefore, we conducted a sensitivity analysis removing “suboptimal use of fluoroquinolones” as a type of antibiotic overuse after discharge. P values <.05 were considered significant. All tests were 2-sided. An analysis was completed using SAS version 9.4.s. We followed Enhancing the QUAlity and Transparency Of health Research (EQUATOR) reporting guidelines (STrengthening the Reporting of OBservational studies in Epidemiology [STROBE] checklist in the Supplementary Appendix).
Ethics Statement
As the purpose of HMS is to measure and improve the quality of existing care practices, it received a “not regulated” status by the University of Michigan institutional review board.
RESULTS
A total of 21 825 patients (12 445 treated for pneumonia and 9380 treated for UTI) were included. Many patients failed to meet the diagnostic criteria for infection, including 12.9% (1604/12 445) of patients treated for pneumonia and 28.6% (2687/9380) of patients treated for a UTI. The majority of patients (72.4%, 15 803/21 825) were prescribed an antibiotic at discharge. Fluoroquinolones were the most common antibiotic prescribed at discharge, accounting for 34.2% of prescriptions. Fluoroquinolones were prescribed at discharge for 22.4% (359/1604) of patients treated for pneumonia who did not meet the diagnostic criteria for pneumonia and 17.6% (473/2687) of patients treated for a UTI who had asymptomatic bacteriuria. Patient characteristics are shown in Table 2.
Table 2.
Characteristics of Patients With and Without Antibiotic Overuse at Hospital Discharge
| Variable | Antibiotic overuse after discharge, n = 10 709 | No antibiotic overuse after discharge, n = 11 116 | P value |
|---|---|---|---|
| Race, White,a n/N (%) | 8186/10 661 (76.8%) | 8409/11 081 (75.9%) | .11 |
| Sex, female, n (%) | 5940 (55.5%) | 6994 (62.9%) | <.001 |
| Age, median (IQR) | 72 (59–82) | 74 (61–84) | <.001 |
| Charlson Comorbidity Index, median (IQR) | 3 (1–4) | 3 (1–5) | <.001 |
| Any sepsis,b n (%) | |||
| Sepsis | 6882 (64.3%) | 6812 (61.3%) | <.001 |
| Severe sepsis | 1986 (18.5%) | 2356 (21.2%) | <.001 |
| Length of stay, days, median (IQR) | 4 (4–6) | 5 (4–7) | <.001 |
| Discharged to postacute care facilityc | 1979 (18.5%) | 2840 (25.5%) | <.001 |
| Disease state | |||
| Hospitalized patients treated for urinary tract infection, n (%) | 3633 (33.9%) | 5747 (51.7%) | <.001 |
| Asymptomatic bacteriuriad | 1431 (13.4%) | 1256 (11.3%) | |
| Complicated urinary tract infection | 1580 (14.8%) | 2486 (22.4%) | |
| Other urinary tract infection | 622 (5.8%) | 2005 (18.0%) | |
| Hospitalized patients treated for community-onset pneumonia, n (%) | 7076 (66.1%) | 5369 (48.3%) | <.001 |
| Not meeting criteriae | 1015 (9.5%) | 589 (5.3%) | |
| Community-acquired pneumonia | 4553 (42.5%) | 3052 (27.5%) | |
| Healthcare–associated pneumonia | 1508 (14.1%) | 1728 (15.5%) | |
| Antibiotic treatment and documentation | |||
| Prescribed an antibiotic after discharge, n (%) | 10709 (100%) | 5094 (45.8%) | <.001 |
| Prescribed a fluoroquinolone after discharge, n (%) | 4473 (41.8%) | 934 (8.4%) | <.001 |
| Total antibiotic duration, days, median (IQR) | 9 (8–11) | 6 (4–8) | <.001 |
| Antibiotic duration after discharge, days, median (IQR) | 5 (4–7) | 0 (0–3) | <.001 |
| Antibiotic overuse after discharge, days, median (IQR) | 4 (2–6) | N/A | N/A |
| Antibiotic duration documented in discharge summary, n (%) | 3113 (29.1%) | 3311 (29.8%) | .25 |
| Hospital characteristics, self-reported | |||
| Hospital bed size, median (IQR) | 310 (186–443) | 327 (202–520) | <.001 |
| Hospital profit type, n (%) | <.001 | ||
| For-profit | 864 (8.1%) | 691 (6.2%) | |
| Nonprofit | 9845 (91.9%) | 10 425 (93.8%) | |
| Academic hospital, n (%) | 9223 (86.1%) | 10 112 (91.0%) | <.001 |
The table shows the characteristics of patients who had antibiotic overuse after discharge, compared to those who did not. P values are shown for comparisons using a 2-sided chi-squared or Wilcoxon rank-sum test, as appropriate. P < .05 is significant.
Abbreviations: IQR, interquartile range; NA, not applicable; SIRS, systemic inflammatory response syndrome.
aThere were 83 patients missing race data (0.4%).
bSepsis was defined as 2 or more SIRS criteria. Severe sepsis was defined as sepsis plus evidence of organ dysfunction.
cIncludes long-term acute care hospitals, skilled nursing facilities, inpatient rehabilitation, and subacute rehabilitation.
dPatients treated for a urinary tract infection (ie, urine culture with bacterial growth) but without symptoms attributable to a urinary tract infection were considered to have asymptomatic bacteriuria. Uncomplicated urinary tract infection consisted of women without a urinary catheter or comorbid conditions associated with complicated urinary tract infection (see Supplementary Appendix for details).
ePatients with a discharge diagnosis of pneumonia who lacked signs or symptoms of pneumonia on hospital Day 1 or 2 or who had normal imaging tests were considered not to meet the criteria for pneumonia (see Supplementary Appendix for details).
Nearly half (49.1%, 10 709/21 825) of patients treated for pneumonia or a UTI experienced antibiotic overuse after discharge, including 56.9% (7076/12 445) of patients treated for pneumonia and 38.7% (3633/9380) of patients treated for a UTI. The median duration of antibiotic overuse after discharge was 4 days (interquartile range, 2–6). There were 2397 days of antibiotic overuse after discharge per 1000 hospitalized patients treated for pneumonia and 1834 days of antibiotic overuse after discharge per 1000 hospitalized patients treated for a UTI. Differences between patients with versus without antibiotic overuse were largely an artifact of classification (eg, patients prescribed a fluoroquinolone were more likely to have overuse; Table 2).
The most common type of antibiotic overuse after discharge differed by condition. In patients treated for pneumonia, 63.1% of overuse days after discharge were due to an excess antibiotic duration (19.5% suboptimal use of fluoroquinolones; 17.4% unnecessary therapy). In patients treated for a UTI, 43.9% of overuse days were due to unnecessary antibiotic treatment of asymptomatic bacteriuria (37.3% excessive therapy; 18.7% suboptimal use of fluoroquinolones). For both diseases, the majority of fluoroquinolone overuse after discharge was due to a combination of unnecessary and excessive therapy (59.0% treated for pneumonia; 57.9% treated for a UTI) rather than suboptimal use of fluoroquinolones.
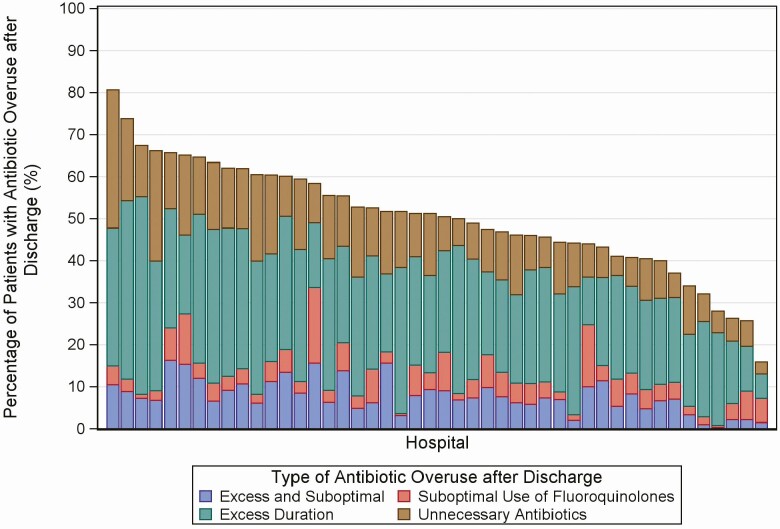
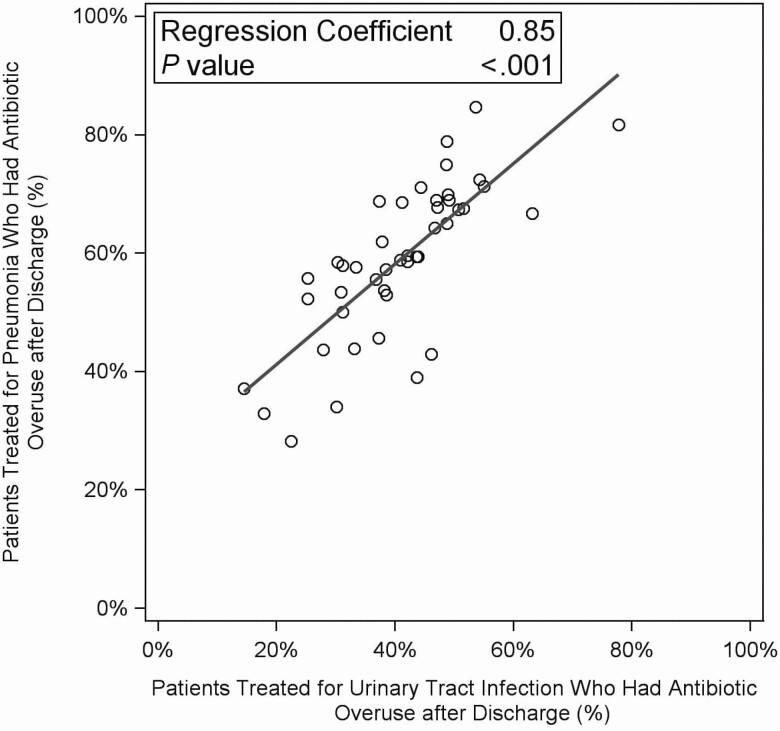
The percentage of patients discharged with antibiotic overuse varied 5-fold among hospitals, from 15.9% (95% confidence interval [CI], 8.7%–24.6%) to 80.6% (95% CI, 69.4%–88.1%). The distribution of categories of antibiotic overuse also varied (see Figure 1). Antibiotic overuse after discharge for a UTI was associated with antibiotic overuse after discharge for pneumonia: for every 10% increase in patients at a hospital treated for a UTI who had overuse after discharge, there was an 8.5% (95% CI, 6.3–10.6) increase in patients treated for pneumonia who had overuse after discharge (Figure 2).
Figure 1.
Antibiotic overuse after discharge in patients treated for pneumonia or urinary tract infection, by hospital (n = 46 hospitals; n = 21 825 patients). Each bar represents 1 hospital. Each postdischarge day was classified as only 1 type of overuse; however, 1 patient could have both excess duration and suboptimal use of fluoroquinolones on different days.
Figure 2.
Antibiotic overuse after discharge in patients treated for a urinary tract infection versus patients treated for pneumonia, by hospital (n = 44 hospitals; n = 21 506 patients). We excluded 2 hospitals due to low numbers (fewer than 10 patients treated for a urinary tract infection).
Secondary Outcomes
After adjustments, antibiotic overuse after discharge was not associated with mortality, readmission, emergency department visits, CDI, or antibiotic-associated adverse events (Table 3). Antibiotic overuse after discharge was not associated with patient-reported antibiotic-associated adverse events (odds ratio, 1.02 per day of antibiotic overuse at discharge; 95% CI, .99–1.05; P = .20).
Table 3.
Association of Antibiotic Overuse After Discharge With 30-Day Adverse Outcomes
| Outcomes at 30 days | Patients with antibiotic overuse after discharge, n = 10 709, n (%) | Patients without antibiotic overuse after discharge, n = 11 116, n (%) | Unadjusted OR per day of antibiotic overuse (95% CI) | Unadjusted P value | Adjusted OR per day of antibiotic overuse (95% CI) | Adjusted P value |
|---|---|---|---|---|---|---|
| Composite adverse outcome | 2485 (23.2) | 2938 (26.4) | .98 (.97–.99) | <.001 | .99 (.97–1.00) | .15 |
| Mortality | 224 (2.1) | 365 (3.3) | .94 (.90–.97) | <.001 | .99 (.94–1.05) | .83 |
| Readmission | 1333 (12.4) | 1654 (14.9) | .97 (.95–.98) | <.001 | .98 (.96–1.01) | .21 |
| Emergency department visit | 1269 (11.8) | 13 988 (12.5) | .99 (.98–1.01) | .28 | .99 (.97–1.01) | .28 |
| Clostridioides difficile infection | 45 (.4) | 58 (.5) | .96 (.89–1.05) | .38 | 1.01 (.94–1.08) | .79 |
| Provider-documented adverse events | 167 (1.6) | 176 (1.6) | 1.01 (.98–1.04) | .71 | 1.01 (.98–1.04) | .60 |
| Patient-reported adverse eventsa | 167/5302 (3.1) | 90/2585 (3.5) | 1.02 (.99–1.05) | .18 | 1.02 (.99–1.05) | .20 |
Outcomes collected via the medical record and a follow-up telephone call at 30 days, and their associations with number of days of antibiotic overuse after discharge are shown. n = 21 825. P values are shown for unadjusted or odds ratios adjusted for hospital clustering, inverse probability of treatment, and known predictors of the outcome of interest (see Supplementary Appendix for details). P values <.05 are significant.
Abbreviations: CI, confidence interval; OR, odds ratio.
a Data were only collected on patient-reported adverse events if the patient was discharged on antibiotics. The proportions shown include only eligible patients who were able to be reached by telephone.
Sensitivity Analyses
When missing data were not imputed, antibiotic overuse after discharge remained not associated with patient outcomes (eTable 1). When suboptimal use of fluoroquinolones was not considered antibiotic overuse after discharge, slightly fewer patients had antibiotic overuse after discharge (51.6% [6419/12 445] of patients treated for pneumonia; 33.8% [3171/9380] of patients treated for a UTI). Fluoroquinolones were still the most common antibiotic prescribed after discharge in patients with antibiotic overuse (35.0%, 3354/9 590). Without suboptimal use of fluoroquinolones, the percentage of patients discharged with antibiotic overuse varied nearly 8-fold among hospitals, from 10.1% (95% CI, 4.3%–18.1%) to 76.1% (95% CI, 64.2%–83.6%). The percentage of patients treated for a UTI who were discharged with antibiotic overuse remained associated with the percentage of patients with pneumonia discharged with antibiotic overuse (regression coefficient = 1.01, P < .001).
DISCUSSION
Using a novel, comprehensive metric to assess antibiotic overuse at discharge for hospitalized patients treated for pneumonia or a UTI, we found that half of 21 825 patients discharged from 46 hospitals experienced antibiotic overuse after discharge. The most common types of antibiotic overuse after discharge varied by condition and included an excess duration for pneumonia and unnecessary treatment of asymptomatic bacteriuria. Antibiotic overuse after discharge varied 5-fold among hospitals, and an association was demonstrated between 2 disparate conditions, suggesting the prescribing culture, physician behavior, or organizational processes play a role in overprescribing after discharge.
Our findings add to the growing body of literature suggesting that antibiotic overuse after discharge needs to be addressed through antibiotic stewardship programs [3–8, 11]. By creating a metric that incorporates 3 common types of overuse (unnecessary use, excess duration use, and suboptimal use of fluoroquinolones), we anticipate that future studies can evaluate the comprehensive effect of stewardship interventions on antibiotic overuse at discharge. Though we found that targets at the highest need for improving discharge therapy vary by condition (excess duration for pneumonia and asymptomatic bacteriuria), our findings also point to the need for a more comprehensive intervention at discharge that evaluates antibiotic use in its entirety. For example, we found that the majority (58.6%) of fluoroquinolone overuse after discharge was due to a combination of unnecessary and excessive therapy rather than suboptimal use of fluoroquinolones. Thus, there was more potential to reduce fluoroquinolone overuse by reducing unnecessary therapy and excessive duration, rather than by emphasizing fluoroquinolone avoidance alone. This finding may explain previous findings that stewardship interventions targeting inpatient fluoroquinolone use had limited effects on fluoroquinolone prescribing after discharge [4] and that, unlike inpatient fluoroquinolone prescribing, fluoroquinolone prescribing at discharge has not decreased over time [9]. Moving forward, disease-based or discharge-based interventions (eg, timeouts or audit and feedback) may be more effective for reducing fluoroquinolone overuse and improving antibiotic use at discharge [35, 36].
The fact that antibiotic overuse after discharge for 1 disease was strongly correlated with antibiotic overuse after discharge for another suggests that the prescribing culture, physician behavior, or organizational processes may strongly affect prescribing after discharge. For example, a hospital culture that is more tolerant of diagnostic uncertainty may influence clinicians to avoid antibiotics for patients with vague or nonspecific symptoms. Similarly, a culture that believes “shorter is better” for antibiotic therapy may be more likely to avoid excess durations regardless of condition. Prior studies have found that better teamwork improves preparation for discharge [35, 37]; similarly, better collaboration with pharmacists or antibiotic stewards could improve prescribing practices regardless of condition. Hospitals with low antibiotic overuse after discharge may also be more likely to monitor antibiotics prescribed at discharge, intervening as necessary. Given the wide variation between hospitals, further quantitative and qualitative studies are needed to assess organizational cultures and identify current discharge and stewardship practices at high-performing hospitals.
Our study has limitations. First, overuse is difficult to define and quantify. When evidence was insufficient, we considered antibiotic use to be appropriate. Furthermore, because guidelines changed in October 2019 (after the study cohort), we considered many patients treated with a 7-day antibiotic duration for HCAP to have received an “appropriate” duration when newer guidelines would consider this to be excess for most of this cohort [19]. We also did not include all potential types of suboptimal antibiotic therapy (eg, prescribing broad-spectrum antibiotics when narrow therapy would suffice). Rather, we focused on fluoroquinolones, as they are the most common antibiotic prescribed after discharge and are high-value targets for stewardship programs, given their association with adverse events, CDI, and antibiotic resistance. For these reasons, we likely underestimate antibiotic overuse after discharge and bias our estimates of the effect of overuse on outcomes to the null. Additionally, because of the ordering of our hierarchical assessment of overuse, we underestimate suboptimal fluoroquinolone use. Second, our study is observational, making it susceptible to confounding. We attempted to account for as many factors as possible during our assessment of overuse, including antibiotic allergies, resistance, disease, and contraindications to certain medications; however, we could not account for all factors. Third, we focused on the 2 most common inpatient bacterial infections, though these account for just under half of all inpatient antibiotic use. Fourth, patients who were not prescribed an antibiotic on discharge were not asked about side effects (and thus were not included in the assessment of outcomes); not including patients at low risk for side effects likely biased our assessment of the effect of overuse on patient-reported adverse events to the null. Similarly, we were likely underpowered to detect an effect of antibiotic overuse on rare outcomes (eg, CDI, death). Finally, we did not have physician-level data; thus, our findings could not discern whether physician or organizational factors are responsible for the correlation in overuse across diseases.
This is the first large-scale, multicenter study to comprehensively quantify antibiotic overuse after discharge. We hope the methodology used here can be applied to other studies to assess the effectiveness of interventions at improving antibiotic use at discharge. Given the ubiquity of overuse after discharge, it is imperative that stewardship programs enact interventions to improve prescribing—which often means stopping antibiotics—at care transitions. As of 2019, both the Centers for Disease Control and Prevention and the Joint Commission recommend stewardship activities include review of discharge antibiotics [38, 39]. Currently, however, discharge prescriptions are difficult for hospitals to monitor, as they are often filled by external pharmacies and are not easy to count electronically. Moving forward, we need easier methods to track antibiotic prescriptions at discharge, which could enable more complete national measures of antibiotic use.
In summary, we created a novel, comprehensive metric to evaluate antibiotic overuse at discharge for 2 conditions. We found that antibiotic overuse after discharge was common and varied widely between hospitals. Targets to improve antibiotic prescribing during care transitions vary by condition and include excess duration of use for pneumonia and stopping unnecessary treatment of asymptomatic bacteriuria. Multifaceted efforts focusing on all types of overuse could be more effective at reducing fluoroquinolone prescribing than efforts focusing on antibiotic selection alone. Finally, hospital-level antibiotic overuse after discharge was associated between conditions, suggesting that the prescribing culture, physician behavior, or organizational processes contribute to overprescribing at discharge.
Supplementary Material
Notes
Author contributions. V. M. V. and D. R. had full access to all the data in the study and final responsibility for the decision to submit for publication.
Disclaimer. No funder had a role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Financial support. This work was supported by the Agency for Healthcare Research and Quality (grant number K08HS026530 to V. M. V.); by Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program, which supported data collection at each participating site and funded the data coordinating center; and by the Society for Healthcare Epidemiology of America, which funded the data analysis.
Potential conflicts of interest. V. M. V. is supported by a career development award from the Agency for Healthcare Research and Quality (1-K08- HS26530-01) and received a Society for Healthcare Epidemiology of America Epi Project during the conduct of the study. S. A. F. reports personal fees from Expert Testimony and Wiley Publishing, outside the submitted work; and grants from Blue Cross and Blue Shield of Michigan and the Agency for Healthcare Research, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Vaughn VM, Petty LA, Flanders SA, et al. . A deeper dive into antibiotic stewardship needs: a multihospital survey. Open Forum Infect Dis 2020;7:ofaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis 2014; 58:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dyer AP, Dodds Ashley E, Anderson DJ, et al. . Total duration of antimicrobial therapy resulting from inpatient hospitalization. Infect Control Hosp Epidemiol 2019; 40:847–54. [DOI] [PubMed] [Google Scholar]
- 4. Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in Michigan hospitals: a multi-hospital cohort study. Clin Infect Dis 2019; 69:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yogo N, Haas MK, Knepper BC, Burman WJ, Mehler PS, Jenkins TC. Antibiotic prescribing at the transition from hospitalization to discharge: a target for antibiotic stewardship. Infect Control Hosp Epidemiol 2015; 36:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yi SH, Hatfield KM, Baggs J, et al. . Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2018; 66:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madaras-Kelly KJ, Burk M, Caplinger C, et al. ; Pneumonia Duration of Therapy Medication Utilization Evaluation Group . Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: results of a national medication utilization evaluation. J Hosp Med 2016; 11:832–9. [DOI] [PubMed] [Google Scholar]
- 8. Vaughn VM, Flanders SA, Snyder A, et al. . Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 9. Vaughn VM, Seelye SM, Wang XQ, Wiitala WL, Rubin MA, Prescott HC. Inpatient and discharge fluoroquinolone prescribing in Veterans Affairs hospitals between 2014 and 2017. Open Forum Infect Dis 2020;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aliberti S, Blasi F, Zanaboni AM, et al. . Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J 2010; 36:128–34. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki H, Perencevich EN, Alexander B, et al. . Inpatient fluoroquinolone stewardship improves the quantity and quality of fluoroquinolone-prescribing at hospital discharge: a retrospective analysis among 122 Veterans Health Administration Hospitals. Clin Infect Dis 2020; 71:1232–9. [DOI] [PubMed] [Google Scholar]
- 12. Scarpato SJ, Timko DR, Cluzet VC, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program. An evaluation of antibiotic prescribing practices upon hospital discharge. Infect Control Hosp Epidemiol 2017; 38:353–5. [DOI] [PubMed] [Google Scholar]
- 13. Jenkins TC, Stella SA, Cervantes L, et al. . Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with national guideline recommendations. Infection 2013; 41:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magill SS, Edwards JR, Beldavs ZG, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Prevalence of antimicrobial use in US acute care hospitals, May–September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michigan Hospital Medicine Safety Consortium. HMS antimicrobial toolkit. Available at: https://mi-hms.org/resources/hms-quality-initiative-toolkits/hms-antimicrobial-toolkit. Access 8 July, 2020.
- 16. Gandhi TN, Vaughn VM, Petty LA, et al. . 2893. The Michigan Hospital Medicine Safety Consortium: improving patient care by reducing excessive antibiotic use in patients hospitalized with community-acquired pneumonia. Open Forum Infect Dis 2019;6(Supp 2):S80–S81. [Google Scholar]
- 17. Petty LA, Vaughn VM, Flanders SA, et al. . Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med 2019;179:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drahos J, Vanwormer JJ, Greenlee RT, Landgren O, Koshiol J. Accuracy of ICD-9-CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol 2013; 23:291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 21. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine national roundtable on health care quality. JAMA 1998; 280:1000–5. [DOI] [PubMed] [Google Scholar]
- 22. Nicolle LE, Gupta K, Bradley SF, et al. . Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68:e83–e110. [DOI] [PubMed] [Google Scholar]
- 23. Spivak ES, Cosgrove SE, Srinivasan A. Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 2016; 63:1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin DB. Multiple imputations in sample surveys—a phenomenological Bayesian approach to nonresponse. Alexandria, VA: American Statistical Association; 1978; 20–8. Available at: http://www.asasrms.org/Proceedings/y1978f.html. [Google Scholar]
- 26. Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis 2003; 9:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frankel WC, Trautner BW, Spiegelman A, Grigoryan L, LeMaire SA. Patients at risk for aortic rupture often exposed to fluoroquinolones during hospitalization. Antimicrob Agents Chemother 2019;63:e01712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewardson AJ, Vervoort J, Adriaenssens N, et al. ; SATURN WP1 Study Group; SATURN WP3 Study Group. Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect 2018; 24:972–9. [DOI] [PubMed] [Google Scholar]
- 29. Low M, Neuberger A, Hooton TM, et al. . Association between urinary community-acquired fluoroquinolone-resistant Escherichia coli and neighbourhood antibiotic consumption: a population-based case-control study. Lancet Infect Dis 2019; 19:419–28. [DOI] [PubMed] [Google Scholar]
- 30. Dancer SJ, Kirkpatrick P, Corcoran DS, Christison F, Farmer D, Robertson C. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum β-lactamase–producing coliforms and methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 2013; 41:137–42. [DOI] [PubMed] [Google Scholar]
- 31. Dingle KE, Didelot X, Quan TP, et al. ; Modernising Medical Microbiology Informatics Group . Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hooton TM, Bradley SF, Cardenas DD, et al. ; Infectious Diseases Society of America . Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:625–63. [DOI] [PubMed] [Google Scholar]
- 34. Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 35. Haas MK, Dalton K, Knepper BC, et al. . Effects of a syndrome-specific antibiotic stewardship intervention for inpatient community-acquired pneumonia. Open Forum Infect Dis 2016; 3:ofw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yogo N, Shihadeh K, Young H, et al. . Intervention to reduce broad-spectrum antibiotics and treatment durations prescribed at the time of hospital discharge: a novel stewardship approach. Infect Control Hosp Epidemiol 2017; 38:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manges K, Groves PS, Farag A, Peterson R, Harton J, Greysen SR. A mixed methods study examining teamwork shared mental models of interprofessional teams during hospital discharge. BMJ Qual Saf 2020; 29:499–508. [DOI] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. Atlanta, Georgia:US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. Available at: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. [Google Scholar]
- 39. Baker DW, Hyun D, Neuhauser MM, Bhatt J, Srinivasan A. Leading practices in antimicrobial stewardship: conference summary. Jt Comm J Qual Patient Saf 2019; 45:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.