Abstract
Purpose:
The primary aim of this study was to examine the pattern of associations among PD patient and caregiver sleep problems, caregiver burden, and caregiver life satisfaction. A secondary aim was to assess whether the pattern of associations differed between Mexican and U.S. caregivers.
Materials and methods:
Analyses were performed on data obtained from 253 caregivers (M age = 59.92). A composite score was produced for caregiver and patient sleep problems. The Zarit Burden Interview and Satisfaction with Life Scale measured caregiver burden and life satisfaction, respectively. A structural equation model with an invariance design was developed to examine and compare the pattern of associations.
Results:
The model was generally invariant across U.S. and Mexican caregivers. Three significant indirect effects were found: caregiver sleep problems were negatively associated with life satisfaction via caregiver burden (p = 0.003); PD patient sleep problems were positively related to caregiver burden via caregiver sleep problems (p = 0.005) and life satisfaction via caregiver burden and caregiver sleep problems (p = 0.002).
Conclusions:
PD patient sleep problems were associated with caregiver sleep problems, leading to increased burden in caregivers and poorer life satisfaction. The findings highlight a potential opportunity for empirically supported sleep interventions.
Keywords: Aging, burden, caregiving, life satisfaction, Parkinson’s disease, sleep
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative condition that affects approximately 680,000 people, according to 2010 estimates, and is expected to rise to 930,000 in 2020 [1]. Dopamine, a neurotransmitter responsible for body movement coordination, is slowly depleted over time in individuals with PD, which leads to the development of symptoms that impair functioning in several areas of life. The hallmark symptoms of PD, which include tremor at rest, rigidity, bradykinesia, and postural instability [2,3], contribute to subsequent psychological and socioeconomic difficulties for the patient [2]. Consequently, individuals with PD may require various amount of time, care, and support from others.
PD caregivers experience significant physical, psychological, and socioeconomic demands related to caring for individuals with PD [2]. Caregivers often assist individuals with maintaining personal hygiene, managing medication, mobility, transportation, and completing other daily physical activity routines [4]. As the disease progresses, caregivers may be tasked with greater physical demands that lead to feeling overwhelmed and stressed [2]. The needs of the individual with PD can contribute to financial strain for the caregiver who may reduce the amount of time dedicated for work to care for the individual with PD and have difficulty affording additional resources that may provide respite [2]. In terms of psychological demands, caregiving is associated with increased feelings of isolation and limited time for self-care [2]. Indeed, caregivers who dedicate more time to caregiving are more likely to have reduced contact with friends and engagement with hobbies and social events [5]. Consequently, caregivers may continue to provide care despite reaching physical, emotional, and financial limits, which contribute to burden for the caregiver [2].
Caregiver burden is the extent to which caregiving has had an adverse effect on the caregiver’s physical, emotional, financial, and spiritual functioning [6]. Greater caregiver burden is associated with lower quality of life, life satisfaction, and subjective well-being [7–9]. When evaluating their life in relation to caregiving activities, 65% of caregivers reported their social life suffered, 42% reported their health suffered, and 25% reported their relationship with other family members suffered [9]. Life satisfaction is an important outcome to monitor as it has been a reliable predictor of future physical and mental health problems [10,11].
Sleep disturbances are common among individuals with PD and can span the range of various sleep disorders such as insomnia, sleep-related movement disorders, and circadian rhythm disorders [12]. While the etiology of sleep disturbances in PD is complex, and appears to differ according to the type of sleep disturbance experienced, some evidence suggests motor symptoms such as tremor rigidity, dystonia, and tremors may contribute to difficulties with sleep-onset [13]. Moreover, motor symptoms such as bradykinesia and rigidity have been linked to difficulties with sleep maintenance [14]. The use of dopaminergic medication, and later discontinuation of the medication, has been shown to have negative effects on sleep disturbance for some individuals [13,14]. The metabolism and activation of dopamine is regulated by the circadian system, however, dopamine also exerts some influence on the circadian system via regulation of melatonin secretion, and therefore, delaying sleep onset [15,16]. Additionally, some evidence suggest that over the course of the degeneration process, changes in sleep macrostructure and microstructure occur which can lead to greater sleep fragmentation and limited sleep at certain stages [14,15].
Sleep disturbances among individuals with PD have been shown not only to be associated with greater caregiver distress and burden [17,18], but also caregiver sleep problems [17]. Sleep disturbances are frequently reported by PD caregivers and are an important factor to consider in relation to caregiver burden and life satisfaction [17,19]. Over 90% of PD caregivers reported sleep disturbance, 80% daytime dysfunction following sleep, and 65% poor subjective sleep quality [19]. Poor sleep among caregivers is associated with lower overall quality of life, including existential well-being, psychological symptoms, and support [20]. Furthermore, greater sleep disturbances and poorer sleep quality in caregivers of individuals with PD were associated with higher anxiety and depression in caregivers [19].
Poor sleep is associated with greater caregiver burden, and, in particular, both role and personal strain on the caregiver [17,21,22]. One study found this relationship remained after controlling for spousal age and gender [17]. Poor sleep among caregivers has not only been predicted by the severity of patient symptoms, poor sleep in patients, and higher frequency of caregiving [17], but has also been found to be an independent predictor of caregiver burden [21]. Therefore, sleep and caregiver burden may have a bi-directional relationship, whereby poor sleep leads to greater caregiver burden and greater caregiver burden promotes poor sleep.
Cultural differences in the relationship between caregiver burden and life satisfaction are likely to exist [23,24] and are important to consider. Familismo is a core, multifaceted cultural value within Latinx communities that emphasizes the importance and prioritization of family [25]. Familismo is characterized by beliefs of family interconnectedness, prioritization of family before the individual, family reciprocity, and family honor, and it manifests in behaviors that correspond to these beliefs [26]. Latino communities, including those in Mexico, contain large interconnected family networks that comprise not only immediate and extended family, but may also include close friends and members of the religious community [27,28]. Social support is typically expected and provided by members of the family network and may assist with overall burden for primary caregivers [27,29]. According to previous research, greater quality of family functioning has predicted better life satisfaction among Mexican caregivers of individuals with PD. Moreover, the relationship between family functioning and life satisfaction was mediated by caregiver distress [30]. An additional model further supported this finding, indicating caregiver burden mediated the relation between family cohesion and caregiver mental health-related quality of life [31]. Therefore, while familismo may an important cultural protective factor, limited work has explored caregiver burden, sleep problems, and life satisfaction in Latin America.
The proposed study aimed to examine the relationship among sleep problems in individuals with PD, caregiver sleep problems, caregiver burden, and caregiver life satisfaction in a sample of adult caregivers of individuals with PD from Mexico and the United States. Although several studies have indicated caregiver burden predicts caregiver sleep problems, the proposed study examined the hypothesized relationship whereby patient and caregiver sleep problems predict caregiver burden. It was hypothesized that greater patient sleep problems would be associated with greater caregiver sleep problems, which in turn would lead to higher caregiver burden, and finally, poorer caregiver life satisfaction. It was predicted that the hypothesized pattern of relationships would equally describe the experience of Mexican and U.S. caregivers.
Material and methods
Participants
Two hundred fifty-three informal caregivers of individuals with PD from Mexico (n = 148) and the United States (n = 105) participated in the study. Patients in Mexico were being seen at a PD clinic at the Hospital Civil Fray Antonio Alcalde in Guadalajara, Mexico, a hospital associated with the University of Guadalajara. Patients in the United States were being seen at the Parkinson’s and Movement Disorders Center at the Virginia Commonwealth University Medical Center in Richmond, Virginia. The two centers (one in each country) were chosen because they were PD referral specialty clinics situated in academic medical centers in the urban capital cities of their respective states with large metro catchment areas. To meet eligibility criteria, participants must have (1) identified as the primary caregiver for an individual with PD who was actively being seen in the PD clinics in Mexico or the United States, (2) been at least 18 years old, and (3) been fluent in either Spanish or English for either the Mexico or U.S. sites, respectively.
Procedures
The protocol was approved by the Institutional Review Boards at the Mexico and U.S. sites. Several methods were employed to recruit PD caregivers including at clinical visits, emails, phone calls, flyers, and direct contact. Caregivers who expressed interest were provided with information about the study when they accompanied the patient to an appointment. Individuals provided informed consent and completed all measures via paper and pen in the United States. Measures were administered orally to participants at the Mexico site due to higher rates of illiteracy. Following consent, participants provided information regarding their age, gender, education level, employment status, hours of care provided per week, and number of months providing care, as well as the age and gender of the patient. They then completed measures of their own sleep problems and that of the patient, as well as their own burden and life satisfaction.
Measures
Sleep problems:
A composite score was computed to produce a “sleep problems” score for patients and caregivers. The use of composite variables is a common procedure that may be performed using a simple averaging approach [32]. The caregiver sleep problems composite score was computed using item 3 of the Patient Health Questionnaire-9 (PHQ-9) [33], “Trouble falling or staying asleep, or sleeping too much,” item 4 of the PHQ-9, “Feeling tired or having little energy,” and item 24 of the Family Needs Questionnaire (FNQ) [34], “To get enough rest or sleep.” Responses to the PHQ-9 item range from “0” indicating “Not at all” to “3” indicating “Nearly every day.” On the FNQ, participants indicated whether their need has been met by responding “0” suggesting “Yes”, “1” suggesting “Partially”, or “2” suggesting “No.” The composite alpha for caregiver sleep problems was 0.57.
Patient sleep problems score was computed using item 1.7 of the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale(MDS-UPDRS) [35], “Sleep problems” and item 1.8, “Daytime Sleepiness.” For each of the items on this scale, caregivers rated the severity of the problem the patient had experienced within the past week. The composite alpha for patient sleep problems was 0.53.
While the use of well-known, validated sleep measures is preferable, their use in routine clinical care is limited due to the amount of time needed for completion and scoring. Therefore, single-item measures of sleep are often used to capture information pertaining to sleep. A few studies have investigated the use of single-item sleep measures, revealing that a single-item sleep question performed as well as other established sleep scales in cancer patients and individuals with insomnia and depression [36,37].
Caregiver burden:
The Zarit Burden Interview Short Version [38] is a 12-item scale designed to measure caregiver burden. The Zarit Burden Interview Short Version has demonstrated overall good internal consistency (α = 0.88). The measure demonstrated excellent reliability (α = 0.93) and acceptable criterion-related validity in PD caregivers [39], with good reliability in the current study (α = 0.89).
Life satisfaction:
The Satisfaction with Life Scale (SWLS) [40] is a 5-item scale that is used to measure life satisfaction. Several studies have examined its psychometric properties across a range of samples including caregivers of degenerative dementia, showing internal consistency ranging from 0.79 to 0.89 [41]. Reliability analyses for the current sample also indicated good reliability (α = 0.87).
Data analyses
A structural equation model (SEM) was developed using AMOS 26.0 [42]. SEM is a statistical analysis that is used to determine the strength and direction of theoretical effects among latent variables, variables not directly observed, but inferred based on the instruments used to measure them [43]. Sleep problems among PD patients were hypothesized to be positively associated with caregiver sleep problems, and in turn, contribute positively to caregiver burden and negatively to life satisfaction. Finally, caregiver burden was expected to be negatively associated with life satisfaction and serve as a mechanism for the relation between caregiver sleep problems and life satisfaction.
A two-step SEM strategy was used. The strategy involved the separate estimation of a measurement model prior to the simultaneous estimation of the measurement and structural models. The measurement model provided an initial assessment of how well items collectively captured the latent variables included in the model and fit with the observed data. The fit of the measurement and structural models were assessed using several different fit indices that are most commonly used and reported: root mean square residual (RMR), goodness of fit index (GFI), adjusted goodness of fit index (AGFI), normed fit index (NFI), Tucker-Lewis index (TLI), incremental fit index (IFI), comparative fit index (CFI), relative fit index (RFI), and root mean square error of approximation (RMSEA) [43]. A value of 0.05 or less on the RMR and a 0.08 or less on the RMSEA is suggestive of adequate fit between the model and the data. A value of at least 0.90 on the remaining indices indicate adequate fit [43]. The structural model examines the nature of the relationship among the variables of interest and how strongly each are associated. The measurement model in conjunction with the structural model allowed for a comprehensive assessment of the full SEM model.
An invariance analysis is statistical technique that is used to determine whether the pattern of relationships observed in the SEM are equivalent among two or more groups. In the current study, an invariance analysis was employed as a function of site to determine whether the SEM was similar for caregivers from Mexico and the United States. The invariance analysis was completed by examining the difference between an unconstrained model, a model which assumes the United States and Mexico yield different parameter values when the SEM is applied to the data, and a constrained model which assumes the United States and Mexico yield equivalent parameter values. Four sets of comparisons were examined: measurement weights, structural weights, structural residuals, and measurement residuals. Measurement weights describe the extent to which individual items collectively measure a latent variable. Structural weights determine the strength and direction of the relationship between two latent variables. Structural residuals and measurement residuals are parameters that indicate the degree of error associated with measuring latent variables and the items that make up the latent variables, respectively.
There were no missing data for caregiver sleep problems, caregiver burden, or life satisfaction. There were missing data for the two items that together produced patient sleep problems, therefore, expectation maximization was performed. Expectation maximization is an algorithm that, using a maximum-likelihood approach, calculates a regression model from the available data, and predicts the value of missing data. The inclusion of over 200 participants is consistent with sample size recommendations for SEM and other published studies using SEM [44].
Results
Demographic information regarding the sample is included in Table 1. A little more than half the sample were from Mexico (58.5%). On average, patients with PD (M = 68.14, SD = 10.18) were slightly older than their caregivers (M = 59.92, SD = 14.66). More than half of caregivers were female (73.1%); slightly more than half of patients were male (57.3%). The highest level of education reported most by caregivers were elementary school (34%) and a 4-year college degree (23.3%). In terms of employment status, caregivers were either retired (30.4%) or working part-time (20.2%).
Table 1.
Sample demographic information.
| Site (%) | |
| US | 41.5 |
| Mexico | 58.5 |
| Age (M, SD) | |
| Caregiver | 59.92 (14.66) |
| Patient | 68.14 (10.18) |
| Gender(%) | |
| Caregiver | |
| Male | 26.9 |
| Female | 73.1 |
| Patient | |
| Male | 57.3 |
| Female | 42.7 |
| Caregiver education (%) | |
| No formal schooling | 2.8 |
| Elementary School | 34.0 |
| High School/GED | 13.8 |
| 2-year College Degree | 12.6 |
| 4-year College Degree | 23.3 |
| Master’s Degree | 10.3 |
| Doctorate Degree | 3.2 |
| Employment Status (%) | |
| Full-time | 13.8 |
| Part-time | 20.2 |
| Unemployed | 15.4 |
| Student | 0.4 |
| Retired | 30.4 |
| Other | 11.5 |
| Homemaker (GDL Only) | 8.3 |
| Hours of care provided per week (M, SD) | 87.92 (66.85) |
| Number of months providing care (M, SD) | 50.12 (64.04) |
All fit indices for the SEM are included in Table 2. The Chi-square test for the measurement model was statistically significant, χ2 (48) = 98.91, p < 0.001 and the Chi-square-degrees of freedom ratio was 2.06. Given the sensitivity of these parameters to sample size, these parameters were accompanied by other indices of fit. RMR, GFI, and AGFI were all in the adequate range. The NFI and TLI were also in the adequate range. The IFI and CFI were in the good fit range; however, the RFI suggested less than adequate fit. Finally, the RMSEA suggested the model was in the adequate range. Collectively, these indices suggest that the measurement model fit the data adequately; therefore, no modifications were performed to improve the model. All manifest variables loaded highly (all beta-weights ≥ 0.50 and all p-values < 0.001) onto their latent constructs. Lastly, the correlations among the latent variables ranged in magnitude from 0.10 to 0.48, indicating that there was sufficient discrimination among the latent variables to proceed to the structural model.
Table 2.
Model fit summary.
| Fit indices | Measurement model | SEM # 1 | SEM # 2 |
|---|---|---|---|
| RMR | 0.05 | 0.07 | 0.06 |
| GFI | 0.94 | 0.93 | 0.94 |
| AGFI | 0.91 | 0.89 | 0.91 |
| NFI | 0.91 | 0.89 | 0.91 |
| RFI | 0.88 | 0.85 | 0.88 |
| IFI | 0.95 | 0.93 | 0.95 |
| TLI | 0.93 | 0.90 | 0.93 |
| CFI | 0.95 | 0.93 | 0.95 |
| RMSEA | 0.07 | 0.08 | 0.07 |
In the full SEM that included the structural model, greater patient sleep problems were significantly associated with greater caregiver sleep problems, β = 0.53, p < 0.001. Additionally, greater caregiver sleep problems were significantly associated with greater caregiver burden, β = 0.66, p < 0.001. Moreover, greater caregiver sleep problems (β = −0.55, p = 0.007) and caregiver burden (β = −0.36, p = 0.014) were significantly related to poorer caregiver life satisfaction. Patient sleep problems were not significantly related to caregiver life satisfaction (β = 0.13, p = 0.252). The fit indices for this model fit less adequately than observed in the measurement model; however, the fit indices indicated the structural model was still within adequate range. The RMR, AGFI, and NFI were slightly less than adequate in the structural model. Modification indices suggested the fit of the structural model would improve through the addition of a direct path from patient sleep problems to caregiver burden, a relationship that has received empirical support in the literature. Therefore, an additional path between patient sleep problems and caregiver burden was included and a new SEM was produced.
The revised SEM is included in Figure 1. In this SEM, the fit indices indicated the structural model fit the data better than the first without the additional path, and remained within the adequate range. All fit indices were within adequate range except the RFI. The relationships among the latent constructs remained similar to the first SEM. Greater patient sleep problems were significantly associated with greater caregiver sleep problems, β = 0.30, p = 0.013, and caregiver burden, β = 0.50, p < 0.001. Greater caregiver sleep problems were significantly and positively associated with caregiver burden, β = 0.38, p < 0.001. Moreover, caregiver sleep problems (β = −0.42, p < 0.001) and burden (β = −0.50, p = 0.003) were significantly and negatively related to caregiver life satisfaction. Similar to the previous model, patient sleep problems were not significantly related to caregiver life satisfaction (β = 0.08, p = 0.527). The SEM also indicated three significant indirect effects. Greater caregiver sleep problems were significantly related to poorer caregiver life satisfaction via caregiver burden, β = −0.19, p = 0.003, 95% CI [−0.39, −0.09]. Moreover, patient sleep problems were positively related to caregiver burden through caregiver sleep problems, β = 0.12, p = 0.005, 95% CI [0.05, 0.25], and caregiver life satisfaction via caregiver burden and caregiver sleep problems, β = −0.43, p = 0.002, 95% CI [−0.71, −0.24].
Figure 1.
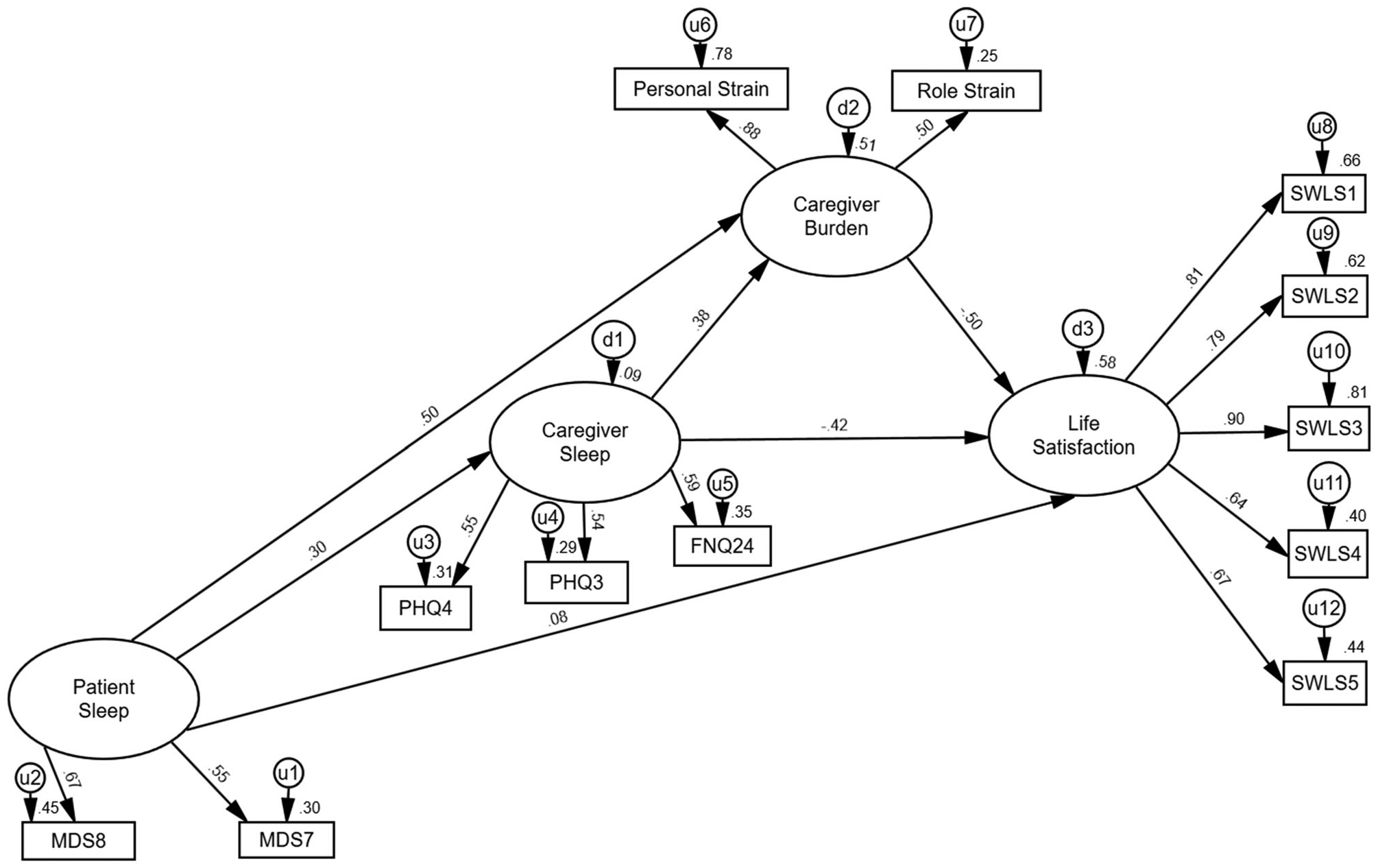
Final structural equation model. Strength and direction are shown of the relationships among latent variables.
The invariance analyses indicated that all sets of omnibus comparisons between Mexican and U.S. caregivers were significant: measurement weights (loadings of measured variables on latent variables; p = 0.003), structural weights (strength and direction of the association; p = 0.004), structural residuals (error terms of latent variables; p = 0.004), and measurement residuals (error terms of measured variables; p < 0.001). Bonferroni-corrected post-hoc comparisons were performed to limit inflated risk of type I error and identify which parameters United States and Mexican caregivers differed. Once Bonferroni correction was applied, the vast majority of comparisons were not statistically significant. The only comparisons that remained significantly different between United States and Mexican caregivers were the error term for the Role Strain subscale of the Zarit Burden Inventory (z = 2.92) and item 1 of the Satisfaction with Life Scale (z = 3.37). The error term on Role Strain was smaller in the United States (0.49) than in Mexico (0.89), suggesting that Role Strain was a better index of burden in the United States than Mexico. The error term on item 1 of the Satisfaction with Life Scale was smaller in the United States (0.38) than in Mexico (0.86), similarly suggesting that item 1 was a better index of life satisfaction in the United States. Despite these two caveats, the overall SEM was generally similar between Mexico and the United States.
Discussion
The current study examined the relationships among PD patient and caregiver sleep problems, caregiver burden, and caregiver life satisfaction in sample of caregivers from Mexico and the United States. The findings robustly supported the hypothesized outcomes. Caregiver sleep problems were negatively related to poorer caregiver life satisfaction via caregiver burden and patient sleep problems were positively related to caregiver burden through caregiver sleep problems. While patient sleep problems were not directly related to caregiver life satisfaction, it was because the effect was mediated by caregiver sleep problems and caregiver burden. An additional aim of the study was to assess whether the pattern of relationships among these variables differed between Mexican and U.S. caregivers. The pattern of relationships generally did not differ between the two groups; however, there were slight degrees of differences in the measurement of these constructs between groups.
Direct relationships were found among patient sleep problems, caregiver sleep problems, caregiver burden, and caregiver life satisfaction, with the exception of a direct relation between patient sleep problems and caregiver life satisfaction. This suggests that patient sleep problems alone may not be sufficient for caregiver life satisfaction to be negatively affected until it begins to negatively impact caregiver sleep and contribute greater caregiver burden. This finding aligns with previous empirical research indicating patient sleep problems are negatively related to caregiver burden and sleep problems [2,17]. Furthermore, the global indirect association between patient sleep problems and caregiver life satisfaction indicates patient sleep problems may be a pervasive factor that negatively affects other caregiver factors simultaneously on its path to caregiver life satisfaction. Therefore, greater attention is needed to address patient sleep problems in order to prevent downstream effects on caregivers.
Two additional indirect associations were found indicating that sleep problems were a source of subsequent caregiver burden and poorer caregiver life satisfaction. In addition to the current findings, studies have shown patient sleep problems may be a root source through which other caregiving experiences are negatively affected [2,17]; however, caregiver sleep problems warrant greater attention. Caregiver sleep problems were the factor linking all other factors together in the structural model. Caregiver sleep problems not only served as a mechanism through which patient sleep problems led to caregiver burden, but were also indirectly linked to poor caregiver life satisfaction through caregiver burden. This finding aligns with other empirical studies that found independent associations between caregiver sleep problems and both caregiver burden [8,21] and caregiver life satisfaction [19,20]. Moreover, in contrast to previous work indicating that caregiver burden impacts sleep [17,22], this study lends initial support to the bi-directional nature of caregiver sleep and burden whereby sleep also impacts caregiver burden. While caregiver burden may initially lead to difficulty sleeping, continued sleep difficulties may contribute to the maintenance of caregiver burden over time due to impaired cognitive and emotional functioning, factors sleep are known to impact and are needed to provide care for individuals with PD [45,46]. As such, patient and caregiver sleep may be worth targeting as it produces downstream negative effects on other caregiver experiences.
There was no evidence supporting a difference in the pattern of relationships between Mexican and U.S. caregivers, suggesting that the theoretical model largely operated similarly cross-culturally. The theoretical model was not necessarily expected to differ between Mexico and the United States, although cultural differences surrounding family interconnectedness, prioritization, and honor [25] may influence the conceptualization of caregiver burden. Indeed, Role Strain was a better index of burden in the United States than in Mexico, suggesting that caregiver burden may in fact be conceptualized somewhat differently in the two cultures, although patient and caregiver sleep problems contribute largely and similarly to caregiver burden and life satisfaction. Caregiver burden and distress have served as mechanisms linking family functioning and life satisfaction in prior studies with Mexican families [30,31]. Therefore, while the impact of sleep on caregiver burden and life satisfaction are similar for both communities, the pathways to those outcomes may differ when accounting for family functioning and cohesion. As such, future research may benefit from theoretical models that include measures of family functioning to assess whether it functions as a link between sleep problems and caregiver burden in diverse Latinx communities.
The study contains several limitations. First, sleep problems were not measured using validated sleep scales and were constructed using composite scores derived from different measures. Although the construction of the variables in this manner did not influence model fit or interrelationships, sleep may be better captured using scales that provide greater breadth and depth of sleep information. Second, patient sleep problems were measured from the perspective of the caregiver, therefore, providing some measurement bias. In future studies, the inclusion of patient self-reported sleep problems may help further clarify current findings. Third, future research should consider potential measurement differences across countries. For example, educational systems may vary across the United States and Mexico, and, therefore, the educational categories included the current study may not be fully equivalent for both countries. Depending on the specific aims of a study, future researchers could consider making adjustments such as asking participants to report on formal of years of education or other indices that may better account for country-level differences. Fourth, although study data were examined in latent space, the study utilizes cross-sectional data which preclude statements of temporality and causality regarding the relationship among variables. Fifth, although the invariance design detected significant differences between Mexican and U.S. caregivers initially, the invariance design was slightly underpowered and may have limited the detection of small differences between groups. Sixth, the study did not collect physician-rated information pertaining to the physical impairment level of the person with PD (such as a Hoehn and Yahr score), which may also have an impact on sleep for both patients and caregivers. While the primary objective of the study was to examine whether patient sleep problems were related to caregiver sleep problems and quality of life, regardless of source, it is important that additional research determine the potential multitude of sources of patient sleep problems including physical impairment level. Future research may build upon the initial evidence provided in this study by investigating the role of physical impairment level and other potential sources within the theoretical model. Finally, information was not collected on potential sources of cultural differences related to sleep, caregiver burden, and life satisfaction. While the cultural value familismo is implicated as potential contributing factor in study hypotheses, no formal measurement of this construct was included in the study. This and other cultural variables would be very important in future research.
In summary, the current study contributes three key theoretical findings to the literature. First, it adds additional empirical support of the independent relationships among patient sleep problems, caregiver sleep problems, caregiver burden, and caregiver life satisfaction. Second, it provides a theoretical model of how these factors relate to one another and may lead to poorer life satisfaction among caregivers. Lastly, the study supplements the literature with initial evidence suggesting caregiver sleep problems leads to caregiver burden, and is not just predicted by caregiver burden. Patient and caregiver sleep problems were key factors contributing to poorer caregiver burden and life satisfaction, therefore a highlighting a potential point of intervention, especially when effective interventions for sleep problems in older adults exist [47,48]. Poor sleep is common in individuals with PD and their caregivers, and in addition to caregiver burden and life satisfaction, is associated with a variety of poor health outcomes [49]. Therefore, improving sleep may not only be beneficial for improving current functioning, but reduce risk of potential problems in the future.
IMPLICATIONS FOR EHABILITATION.
Parkinson’s disease is a progressive neurological condition that impacts patient and caregiver quality of life.
Patient sleep problems contribute to greater caregiver burden, sleep problems, and reduced life satisfaction.
The findings suggest patient and caregiver sleep may be a worthwhile target for intervention in order to reduce risk of caregiver burden and improve life satisfaction.
Funding
This work was supported in part by the National Institute on Aging (1K23AG049955 to J. M. D.).
Footnotes
Disclosure statement
The authors report no conflicts of interest.
Research presented at the Annual Meeting of the Associated Professional Sleep Societies (APSS). Philadelphia, PA (June, 2020).
References
- [1].Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Park Dis. 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bhimani R Understanding the burden on caregivers of people with Parkinson’s: a scoping review of the literature. Rehabil Res Pract. 2014;2014:718527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. [DOI] [PubMed] [Google Scholar]
- [4].McLaughlin D, Hasson F, Kernohan WG, et al. Living and coping with Parkinson’s disease: perceptions of in formal carers. Palliat Med. 2011;25(2):177–182. [DOI] [PubMed] [Google Scholar]
- [5].McCabe MP, Roberts C, Firth L. Work and recreational changes among people with neurological illness and their caregivers. Disabil Rehabil. 2008;30(8):600–610. [DOI] [PubMed] [Google Scholar]
- [6].Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: a longitudinal study. Gerontologist. 1986;26(3):260–266. [DOI] [PubMed] [Google Scholar]
- [7].Chappell NL, Reid RC. Burden and well-being among caregivers: examining the distinction. Gerontologist. 2002;42(6): 772–780. [DOI] [PubMed] [Google Scholar]
- [8].Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ. Quality of life and burden in caregivers for patients with Parkinson’s disease: concepts, assessment and related factors. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):221–230. [DOI] [PubMed] [Google Scholar]
- [9].Schrag A, Hovris A, Morley D, et al. Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35–41. [DOI] [PubMed] [Google Scholar]
- [10].Fergusson DM, McLeod GFH, Horwood LJ, et al. Life satisfaction and mental health problems (18 to 35 years). Psychol Med. 2015;45(11):2427–2436. [DOI] [PubMed] [Google Scholar]
- [11].Strine TW, Chapman DP, Balluz LS, et al. The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among US community-dwelling adults. J Community Health. 2008;33(1):40–50. [DOI] [PubMed] [Google Scholar]
- [12].Dhawan V, Healy DG, Pal S, et al. Sleep-related problems of Parkinson’s disease. Age Ageing. 2006;35(3):220–228. [DOI] [PubMed] [Google Scholar]
- [13].Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stefani A, Högl B. Sleep in Parkinson’s disease. Neuropsychopharmacology. 2020;45(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mantovani S, Smith SS, Gordon R, et al. An overview of sleep and circadian dysfunction in Parkinson’s disease. J Sleep Res. 2018;27(3):e12673. [DOI] [PubMed] [Google Scholar]
- [16].Videnovic A, Lazar AS, Barker RA, et al. ‘The clocks that time us’-circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Happe S, Berger K. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson’s disease. Age Ageing. 2002;31(5):349–354. [DOI] [PubMed] [Google Scholar]
- [18].Lau K-M, Au A. Correlates of informal caregiver distress in Parkinson’s disease: a meta-analysis. Clin Gerontol. 2011;34(2):117–131. [Google Scholar]
- [19].Pal PK, Thennarasu K, Fleming J, et al. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson’s disease and in their caregivers. Parkinsonism Relat Disord. 2004;10(3):157–168. [DOI] [PubMed] [Google Scholar]
- [20].Cupidi C, Realmuto S, Coco GL, et al. Sleep quality in caregivers of patients with Alzheimer’s disease and Parkinson’s disease and its relationship to quality of life. Int Psychogeriatr. 2012;24(11):1827–1835. [DOI] [PubMed] [Google Scholar]
- [21].Carod-Artal FJ, Mesquita HM, Ziomkowski S, et al. Burden and health-related quality of life among caregivers of Brazilian Parkinson’s disease patients. Parkinsonism Relat Disord. 2013;19(11):943–948. [DOI] [PubMed] [Google Scholar]
- [22].Cifu DX, Carne W, Brown R, et al. Caregiver distress in parkinsonism. J Rehabil Res Dev. 2006;43(4):499–508. [DOI] [PubMed] [Google Scholar]
- [23].Matsushita M, Pai M-C, Jhou C-Y, et al. Cross-cultural study of caregiver burden for Alzheimer’s disease in Japan and Taiwan: result from Dementia Research in Kumamoto and Tainan (DeReKaT). Int Psychogeriatr. 2016;28(7):1125–1132. [DOI] [PubMed] [Google Scholar]
- [24].Salguero RH, Kohn R, Salguero LF, et al. Caregivers of persons with Alzheimer’s disease: cultural differences in perceived caregiver burden in Guatemala and Rhode Island. J Cross Cult Gerontol. 1998;13(3):229–240. [DOI] [PubMed] [Google Scholar]
- [25].Keefe SE. Real and ideal extended familism among Mexican Americans and Anglo Americans: on the meaning of “close” family ties. Hum Organ. 1984;43(1):65–70. [Google Scholar]
- [26].Steidel AGL, Contreras JM. A new familism scale for use with Latino populations. Hisp J Behav Sci. 2003;25(3): 312–330. [Google Scholar]
- [27].Landale NS, Oropesa RS, Bradatan C. Hispanic families in the United States: family structure and process in an era of family change. In: Tienda M, Mitchell F, editors. Hispanics and the Future of America. Washington, D.C. (USA): National Academies Press; 2006. p. 138–178. DOI: 10.17226/11539 [DOI] [Google Scholar]
- [28].Garcia C What do we mean by extended family? A closer look at hispanic multigenerational families. J Cross Cult Gerontol. 1993;8(2):137–146. [DOI] [PubMed] [Google Scholar]
- [29].Katiria Perez G, Cruess D. The impact of familism on physical and mental health among Hispanics in the United States. Health Psychol Rev. 2014;8(1):95–127. [DOI] [PubMed] [Google Scholar]
- [30].Trapp S, MacKenzie J, Gonzalez-Arredondo S, et al. Mediating role of caregiver burden among family caregivers of patients with Parkinson’s disease in Mexico. Int J Psychiatry Med. 2019;54(3):203–216. [DOI] [PubMed] [Google Scholar]
- [31].Trapp SK, Ertl MM, Gonzalez-Arredondo S, et al. Family cohesion, burden, and health-related quality of life among Parkinson’s disease caregivers in Mexico. Int Psychogeriatr. 2019;31(07):1039–1045. [DOI] [PubMed] [Google Scholar]
- [32].Song M-K, Lin F-C, Ward SE, et al. Composite variables: when and how. Nurs Res. 2013;62(1):45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kreutzer JS, Serio CD, Bergquist S. Family needs after brain injury: a quantitative analysis. J Head Trauma Rehabil. 1994;9(3):104–115. [Google Scholar]
- [35].Goetz CG, Tilley BC, Shaftman SR, Movement Disorder Society UPDRS Revision Task Force, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- [36].Snyder E, Cai B, DeMuro C, et al. A new Single-Item Sleep Quality Scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hofmeister D, Schulte T, Hinz A. Sleep problems in cancer patients: a comparison between the Jenkins Sleep Scale and the single-item sleep scale of the EORTC QLQ-C30. Sleep Med. 2020;71:59–65. [DOI] [PubMed] [Google Scholar]
- [38].Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652–657. [DOI] [PubMed] [Google Scholar]
- [39].Hagell P, Alvariza A, Westergren A, et al. Assessment of burden among family caregivers of people with Parkinson’s disease using the Zarit burden interview. J Pain Symptom Manage. 2017;53(2):272–278. [DOI] [PubMed] [Google Scholar]
- [40].Diener ED, Emmons RA, Larsen RJ, et al. The satisfaction with life scale. J Pers Assess. 1985;49(1):71–75. [DOI] [PubMed] [Google Scholar]
- [41].Pavot W, Diener E. Review of the satisfaction with life scale. Psychol Assess. 1993;5(2):164–172. [Google Scholar]
- [42].Arbuckle JL. Amos. Chicago: IBM SPSS; 2019. [Google Scholar]
- [43].Meyers LS, Gamst G, Guarino AJ. Applied multivariate research: design and interpretation. Thousand Oaks (CA): Sage Publications; 2017. [Google Scholar]
- [44].Kline R. Principles and practice of structural equation modeling. 3rd ed. New York (NY): Guilford Press; 2011. [Google Scholar]
- [45].Lowe CJ, Safati A, Hall PA. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci Biobehav Rev. 2017;80:586–604. [DOI] [PubMed] [Google Scholar]
- [46].Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- [47].Dzierzewski JM, Griffin SC, Ravyts S, et al. Psychological interventions for late-life insomnia: current and emerging science. Curr Sleep Med Rep. 2018;4(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- [49].Itani O, Jike M, Watanabe N, et al. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]


