Abstract
Objective:
Prospective and longitudinal studies assessing the utility of SD-OCT to differentiate papilledema from pseudopapilledema are lacking. We studied the sensitivity and specificity of baseline and longitudinal change in SD-OCT parameters with 3D segmentation software to distinguish between papilledema and pseudopapilledema in a cohort of patients referred for evaluation of undiagnosed optic disc elevation.
Methods:
Fifty-two adult patients with optic disc elevation were enrolled in a prospective longitudinal study. A diagnosis of papilledema was made when there was change in the appearance of the optic disc elevation on fundus photographs as noted by an independent observer at or before six months. The degree of optic disc elevation was graded using the Frisen scale and patients with mild optic disc elevation (Frisen Grades 1 and 2) were separately analysed. SD-OCT parameters including peripapillary retinal nerve fiber layer (pRNFL), total retinal thickness (TRT), paracentral ganglion cell–to-inner plexiform layer (GCL-IPL) thickness, and optic nerve head volume (ONHV) at baseline and within six months of follow-up were measured.
Results:
Twenty-seven (52%) patients were diagnosed with papilledema and 25 (48%) with pseudopapilledema. Among patients with mild optic disc elevation (Frisen Grades 1 and 2), baseline pRNFL (110.1 μm vs 151.3 μm) and change in pRNFL (ΔpRNFL) (7.3 μm vs 52.3 μm) were greater among those with papilledema. Baseline and absolute change in TRT and ONHV were also significantly higher among patients with papilledema. Mean GCL-IPL thickness was similar at baseline but there was a small reduction in GCL-IPL thickness among patients with papilledema. Receiver operator curves (ROC) were generated; ΔpRNFL (0.93), ΔTRT (0.94), and ΔONHV(0.95) had the highest area under the curve (AUC).
Conclusions:
Mean baseline and absolute change in SD-OCT measurements (pRFNL, TRT, ONHV) were significantly greater among patients with papilledema, and remained significantly greater when patients with mild optic disc elevation were separately analyzed. ROC curves demonstrated that ΔpRNFL, ΔTRT, and ΔONHV have the highest AUC and are best able to differentiate between papilledema and pseudopapilledema.
Keywords: Optical Coherence Tomography, Papilledema, Pseudopapilledema
Introduction
Distinguishing papilledema from pseudopapilledema can be a challenging clinical dilemma when the degree of optic nerve elevation is mild. While patients with suspected papilledema need neuroimaging and lumbar puncture and treatment of increased intracranial pressure, pseudopapilledema is a benign condition that is rarely vision threatening and does not require invasive testing. Clinicians rely on multiple variables such as patient’s age, gender, physical characteristics, symptoms suggestive of raised intracranial pressure, ophthalmoscopic features of the elevated optic nerve head, and ancillary testing including ocular ultrasonography, fluorescein angiography, neuroimaging and lumbar puncture for diagnosis (1, 2).
While structural optic disc characteristics have been retrospectively studied using spectral-domain optical coherence tomography (SD-OCT), there are limited prospective and longitudinal studies which examine the value of change in SD-OCT parameters which may be diagnostically useful in differentiating papilledema from pseudopapilledema. (3) Visible optic disc drusen are clinically obvious and do not require additional confirmation. Buried optic disc drusen can be visualized with enhanced depth imaging on OCT (2). Increased thickness of the peripapillary retinal nerve fiber layer (pRNFL), total retinal thickness (TRT), peripapillary ring volume, the presence of peripapillary subretinal fluid, and subretinal hyporeflective spaces with smooth contour favor the diagnosis of papilledema (4) (5) (6) (7). In published reports of the idiopathic intracranial hypertension treatment trial (IIHTT), Frisen grading of optic disc swelling significantly correlated with mean circumpapillary pRNFL thickness (pRNFL), total retinal thickness (TRT), and optic nerve head volume (ONHV), suggesting that OCT parameters mirror the changes observed ophthalmoscopically in patients with papilledema (8) (9). The differences in the peripapillary capillary density between controls, patients with papilledema and pseudopapilledema have been recently described using OCT angiography (OCT-A). (10)
Our objective was to determine whether baseline and or longitudinal change in pRNFL, TRT, ONHV, and ganglion cell layer-inner plexiform layer (GCL-IPL) thickness, as measured by SD-OCT of the optic nerve and macula using 3D segmentation software, were significantly different between patients with papilledema and pseudopapilledema, and could therefore serve as diagnostic tests to distinguish between the two entities.
Methods
This study was approved by the site’s institutional review board and written informed consent was obtained from patients in compliance with the principles of the Declaration of Helsinki.
In this prospective study, patients referred for evaluation of newly diagnosed optic disc elevation were consecutively enrolled by neuro-ophthalmologists. Those that had received prior treatment for papilledema were excluded. Patients with any pre-existing retinal or macular disease, clinically visible superficial optic disc drusen, or those with other optic neuropathies causing optic disc elevation were excluded. All study participants were evaluated by the treating neuro-ophthalmologist who arranged further investigations and treatment based on their clinical impression of the optic disc swelling. The treating neuro-ophthalmologist’s diagnosis was not used for study purposes. Instead, a masked neuro-ophthalmologist, who was blinded to the patient’s clinical history, imaging results, or lumbar puncture findings, and had no influence over the patient’s treatment, separately evaluated the fundus photographs in order to provide an independent assessment of papilledema versus pseudopapilledema.
Diagnosis of Papilledema and Pseudo-Papilledema
A diagnosis of papilledema was made when the masked neuro-ophthalmologist identified a change in the appearance of optic nerve swelling when comparing the fundus photographs taken at baseline with fundus photographs taken at a follow up appointment at or before six months. Pseudopapilledema was diagnosed when no change was observed between two fundus photographs taken at baseline and at six months.
The treating neuro-ophthalmologist performed a baseline ophthalmic evaluation, including documentation of Frisen grading of optic disc swelling for each eye. SD-OCT scans of the optic disc and macula, fundus photography, and threshold 24–2 perimetry were performed for both eyes at the baseline visit. Results of other testing, including neuroimaging, lumbar puncture, and treatment rendered were recorded. Patients with pseudopapilledema were offered reassurance and monitored while those with papilledema from pseudotumor cerebri received treatment with weight loss, intracranial pressure lowering therapy, or surgery.
All patients underwent repeat fundus photography, SD-OCT testing at the six-month follow-up visit or earlier if a change in the optic nerve swelling was detected by the treating neuro-ophthalmologist. The patients were considered to have completed the study once both baseline and final study visits had occurred. Additional details on testing protocol are included below.
SD-OCT Measurements
Baseline and longitudinal change in OCT parameters were compared between patients diagnosed with papilledema and pseudopapilledema.
The OCT scan acquisition was performed with the Cirrus 5000 SD-OCT (8.1.0.117) modeled after the IIHTT protocol (8, 11). Three scans were performed: two 200×200 optic disc cube scans, two macular cube scans 200×200 centered around the fovea, and one high definition five-line raster scan horizontally oriented and separated by 0.5 mm imposed on the optic disc. The cube scans were generated from a 6 mm square grid composed of 200 horizontal lines containing 200 A-scans. Only the highest quality scans were chosen for analyses. OCT scans which had poor signal strength (<7), missing scans, poor alignment, or failure of the segmentation algorithm were discarded. Patients who did not have at least one scan of sufficient quality for each visit were not included in the study.
The IIHTT showed that proprietary OCT 2D segmentation software has a higher rate of retinal layer segmentation error in evaluating elevated optic nerves than a 3D segmentation algorithm developed at the University of Iowa. (11, 12, 13) The 3D segmentation algorithm that was used in this study uses neighboring image information from multiple B-scans which significantly reduces the rate of segmentation error, and was successfully utilized in the OCT arm of the IIHTT (see Figure 1). (8, 11) The original two-dimensional 200×200 macular cube and 200×200 optic disc cube scans were analyzed by the three-dimensional segmentation algorithm; pRNFL, TRT, ONHV were computed using the optic disc cube scan while the GCL+IPL thickness was derived using the macular cube scan. The mean pRNFL and TRT were computed using a circle around the optic nerve head with radius of 1.73 mm. The average GCL+IPL thickness were measured in an elliptical annulus centered around the fovea (with a vertical inner and outer radius of 0.5mm and 2.0mm respectively; and horizontal inner and outer radius of 0.6mm and 2.4 mm respectively) using the graph theoretic approach developed by the University of Iowa (12–14). The dimensions of the ring were chosen to exclude areas of GCL+IPL that are normally thin and difficult to measure.
Figure 1:
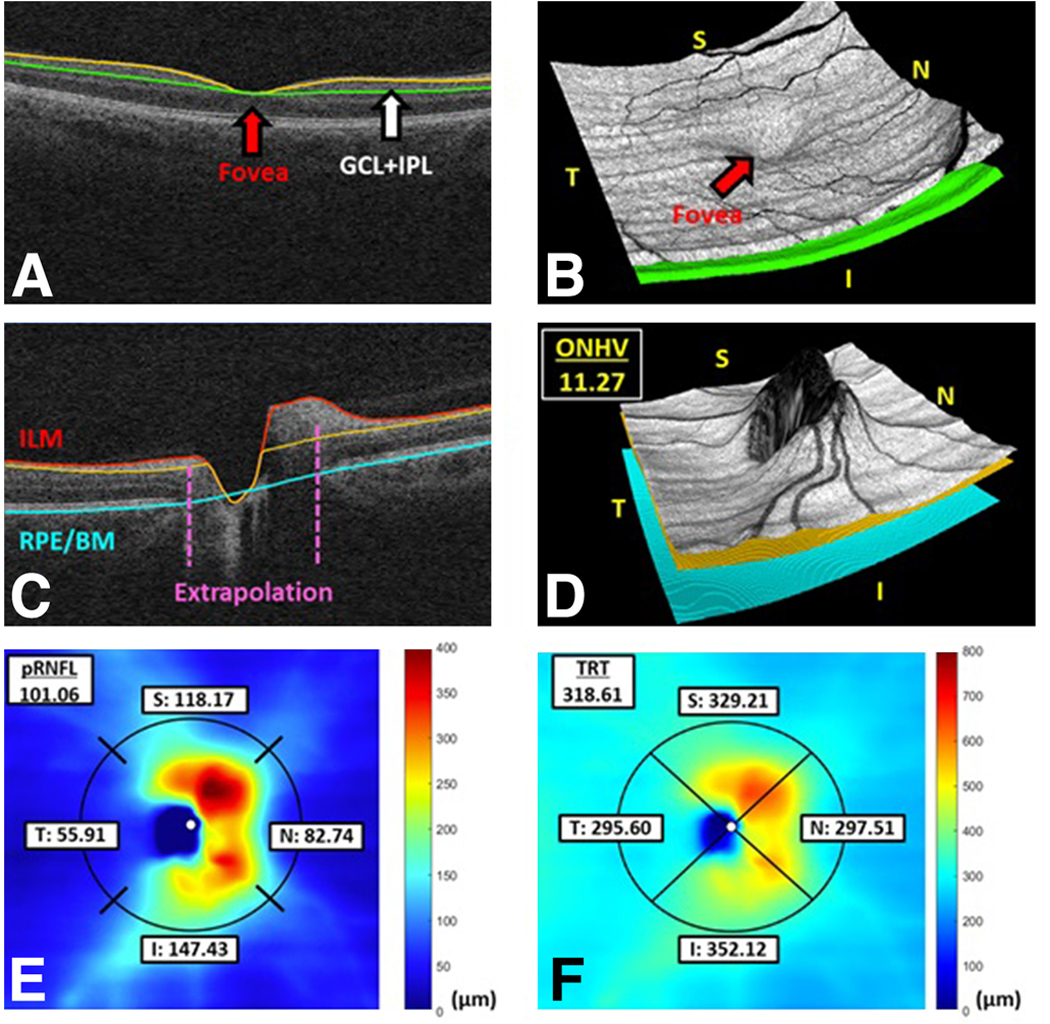
SD-OCT Measurements of pRNFL, TRT, ONHV, and GCL-IPL thickness using 3D segmentation software
Fundus Photography
Digital fundus photography testing was performed by a trained technician using the IIHTT protocol (11). The Zeiss FF450 was used with a 30 degree field angle. This fundus camera combined with Merge Healthcare software and 5 megapixel backing camera allowed for photos taken with a resolution of 2400 × 2048 pixels. The photographs were uploaded to a Windows 7 Professional SP1 PC and contained within the WinStation version 11.4 program provided by Merge Healthcare. The photographs contained an image centered on the optic disc at the retinal plane, on the plane of highest disc elevation, as well as the papillomacular area. Quality of images was assessed for appropriate quality, centration, and the presence of stereo by a trained technician. An independent neuro-ophthalmologist visualized the images under dim illumination with a stereoscope. All photographs were kept with the exception of those containing artifacts or capture errors such as poor focus, under or overexposure, patient movement, blinking, lashes, etc.
Statistical Analyses
Study data were collected and entered into Redcap electronic data capture tools (15). Differences in, pRNFL, TRT, ONHV, and GCL+IPL between patients with pseudopapilledema and papilledema were analyzed using analysis of variance for continuous characteristics and chi-square tests for categorical characteristics. All statistical analysis was performed with SAS 9.4 (SAS Inc, Cary, NC). The correlation between GCL+IPL and mean deviation of visual fields was assessed.
Results
Of the 52 patients enrolled in the study, 48 (92%) were female (Table 1). Twenty-seven patients (52%) were diagnosed with papilledema on the basis of a change in the appearance of optic disc elevation identified by the masked neuro-ophthalmologist at or before six months on fundus photography; 25 (48%) were diagnosed with pseudopapilledema. The average age and gender distribution were similar in both the pseudopapilledema and papilledema groups (see Table 1). The distribution of optic disc swelling as described by Frisen grade is shown in Table 1; higher grades of optic disc swelling were seen in patients with papilledema. Median baseline pRNFL thickness and median ΔpRNFL thickness were greater among patients who had higher Frisen grade of optic disc swelling (p<0.001).
Table 1:
Demographic characteristics and OCT measurements of patients with papilledema and pseudopapilledema (pRNFL: peripapillary retinal nerve fiber thickness; GCL-IPL: thickness of the ganglion cell layer-inner plexiform layer thickness; TRT: total retinal thickness; ONHV: optic nerve head volume)
| Pseudopapilledema | Papilledema | p- value | |
|---|---|---|---|
| Number of Patients | 25 | 27 | |
| Age (SD) | 37.5 (15.1) | 31.7 (10.5) | 0.11 |
| Sex (% Female) | 23 (92.0%) | 24 (88.9%) | 0.70 |
| Frisen Grade Optic Disc Swelling (%) | |||
| Frisen Grade 1 | 21 (84%) | 5 (19%) | |
| Frisen Grade 2 | 3 (12%) | 9 (33%) | |
| Frisen Grade 3 | 1 (4%) | 5 (19%) | |
| Frisen Grade ≥4 | 0 (0%) | 8 (30%) | |
| RNFL Thickness in Microns (SD) | |||
| Baseline pRNFL | 110.1μm (21.0) | 241.1 μm (136.5) | <0.001* |
| Baseline pRNFL (Frisen Grade ≤ 2) | 110.1μm (21.4) | 151.3 μm (54.6) | 0.002* |
| Absolute change in pRNFL | 9.1μm (8.7) | 116.1μm (110.1) | <0.001* |
| Absolute change in pRNFL (Frisen Grade ≤ 2) | 9.3μm (8.9) | 52.3μm (46.5) | <0.001* |
| Final RNFL | 104.1μm (19.7) | 120.1μm (34.3) | 0.051 |
| Total Retinal Thickness in Microns (SD) | |||
| Baseline TRT | 331.4μm (34.8) | 494.4μm (163.0) | <0.001* |
| Baseline TRT (Frisen Grade ≤ 2) | 330.8μm (35.4) | 386.6μm (71.3) | 0.002* |
| Absolute change in TRT | 12.1μm (11.5) | 145.1μm (131.7) | <0.001* |
| Absolute change in TRT (Frisen Grade ≤ 2) | 12.3μm (11.7) | 70.3μm (62.0) | <0.001* |
| Final TRT | 322.5μm (32.5) | 345μm (44.3) | 0.047* |
| Optic Nerve Head Volume (SD) | |||
| Baseline ONHV | 12.0μm3 (1.2) | 15.6μm3 (3.1) | <0.001* |
| Baseline ONHV (Frisen Grade ≤ 2) | 12.0μm3 (1.2) | 13.5μm3 (1.7) | 0.003* |
| Absolute change in ONHV | 0.5μm3 (0.4) | 3.2μm3 (2.2) | <0.001* |
| Absolute change in ONHV(Frisen Grade ≤ 2) | 0.5μm3 (0.4) | 2.1μm3 (1.4) | <0.001* |
| Final ONHV | 11.7μm3 (1.2) | 12.4μm3 (1.5) | 0.09 |
| GCL-IPL Thickness(SD) | |||
| Baseline GCL-IPL | 84.5μm (8.2) | 83.9μm (8.9) | 0.82 |
| Change in GCL-IPL | −0.8μm (1.4) | −2.1μm (2.0) | 0.02* |
Differences in OCT Parameters between patients with Papilledema and Pseudopapilledema
As shown in Table 1, mean baseline pRNFL (p<0.001), ΔpRNFL (p<0.001), baseline TRT (p<0.001), ΔTRT (p<0.001), final TRT (p=0.047), baseline ONHV (p<0.001), ΔONHV (p<0.001) were significantly higher among patients with papilledema. ΔGCL-IPL was also significantly lower in patients with papilledema (p=0.02) though the amplitude of change (−2.1um) was small. Patients with mild optic disc swelling (Frisen Grades 1 and 2) were separately analyzed to better represent the population of patients in whom the diagnosis of papilledema versus pseudopapilledema is more challenging. Among patients with mild optic disc swelling, baseline pRNFL (p=0.002), ΔpRNFL (p<0.001), baseline TRT (p=0.002), ΔTRT (p<0.001), baseline ONHV (p<0.003), ΔONHV (p<0.001) remained significantly higher among patients with papilledema versus pseudopapilledema.
The final pRNFL thickness (p=0.051), final ONHV (p=0.09), and baseline GCL+IPL (p=0.82) did not differ significantly between groups. No significant correlation was found when comparing initial, final, or change in GCL+IPL thickness to HVF mean deviation.
Receiver Operator Curves for OCT Parameters in Detecting Papilledema
Receiver operator curves were generated to assess each variable’s ability to differentiate between papilledema and pseudopapilledema. (see Table 2). ΔpRNFL, ΔTRT, and ΔONHV had the highest area under the curve. Examples of data thresholds which balanced sensitivity and specificity were selected from the ROC curves and are shown (see Table 2).
Table 2:
Area Under the Curve (AUC)_ and Sample Sensitivity and Specificity of OCT Measurements for Detecting Papilledema
| AUC | Measurement | Sensitivity | Specificity | |
|---|---|---|---|---|
| Baseline pRNFL Thickness | 0.88 (0.77, 0.98) | 119.1 | 91.3% | 85.7% |
| Change in pRNFL Thickness | 0.93 (0.84, 1.00) | 13.5 | 88.5% | 87.5% |
| Baseline TRT | 0.88 (0.78, 0.98) | 355.6 | 81.5% | 84.0% |
| Change in TRT | 0.94 (0.87, 1.00) | 0.0644 | 88.5% | 91.7% |
| Baseline ONHV | 0.87 (0.76, 0.97) | 12.7 | 88.9% | 76.0% |
| Change in ONHV | 0.95 (0.90, 1.00) | 0.0774 | 88.5% | 87.5% |
OCT Change in the Absence of Fundus Photographic Change
A change in pRNFL thickness of ≥10 um was detected in seven patients who had a stable appearance on fundus photography and were therefore classified in the study as having pseudopapilledema. Results of ancillary testing such as MRI signs of increased intracranial pressure and opening pressure on lumbar puncture were reviewed for these seven patients. Three of the seven patients had evidence of raised intracranial pressure on MRI and lumbar puncture, with an opening pressure >25cm of H2O. One patient had a normal MRI but elevated opening pressure. Two patients had a normal MRI and borderline lumbar puncture opening pressures (24cmH2O and 23cmH2O), and one patient did not receive a lumbar puncture. Post-hoc analysis of these seven patients revealed that five of them were eventually diagnosed with papilledema by the treating neuro-ophthalmologist despite being classified as pseudopapilledema in the study.
Although the optic nerve swelling did not appreciably change on fundus photographs in the seven patients that showed change in OCT RNFL thickness, five of the seven were diagnosed with true papilledema based on ancillary testing.
Post Hoc Analysis
Twenty-five patients were classified as having pseudopapilledema using the study definition. The study diagnosis was compared with the treating neuro-ophthalmologist’s final diagnosis for these patients. Fourteen of these patients were diagnosed with pseudopapilledema by the treating neuroophthalmologist (56%) while eleven were diagnosed with papilledema (44%).
The ancillary study results of eleven patients with conflicting diagnosis (i.e. a study diagnosis of pseudopapilledema but a diagnosis of papilledema by the treating neuro-ophthalmologist) were separately analysed (see Figure 2).
Figure 2:
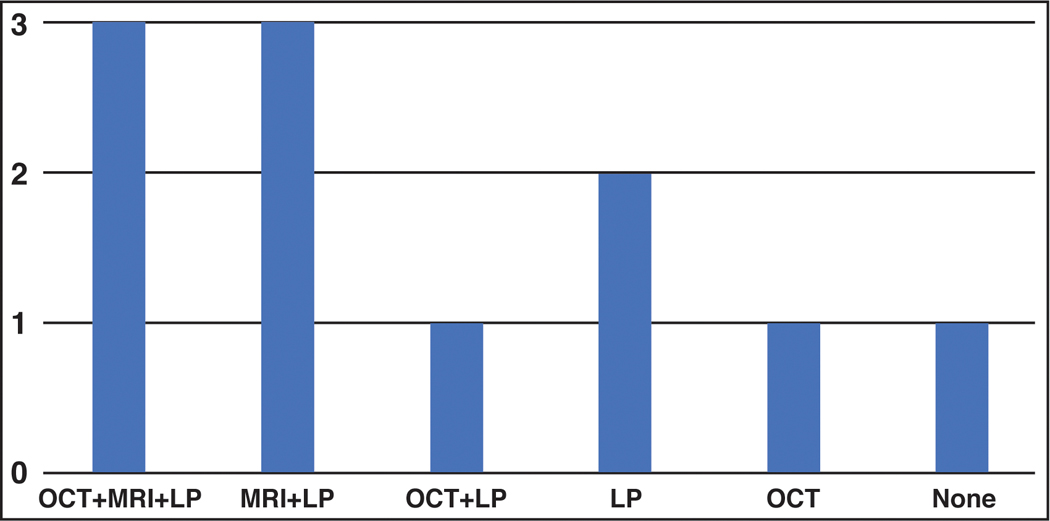
Results of ancillary studies for patients who fulfilled study criteria for pseudopapilledema but were diagnosed with papilledema by the treating neuro-ophthalmologist
Discussion
A gold standard diagnostic test that can distinguish between papilledema and pseudopapilledema does not exist. The diagnosis of papilledema is made clinically based on the patient’s symptoms, signs of raised intracranial pressure, and ancillary tests such as MRI, lumbar puncture, and ultrasonography. The present study prospectively examined the utility of baseline and longitudinal OCT parameters in differentiating papilledema from pseudopapilledema among patients with optic disc elevation. SD-OCT has shown much promise by providing an objective assessment of the optic nerve head and macular thickness; however, proprietary software present in the OCT machine is prone to errors in reproducibility and variability. By analyzing data using 3D segmentation software, we were able to obtain accurate measurements of various optic nerve head parameters at baseline and follow up.
We found that baseline pRNFL, TRT, and ONHV thicknesses were significantly greater among patients with papilledema even among patients with mild optic disc swelling. These findings largely concur with previously published data. In a retrospective case control series, Bassie et al found that mean pRNFL thickness and sectoral measurements of TRT were higher among patients with papilledema (185.4um) versus pseudopapilledema (122.3um) and controls (91.6um), but this study included both patients with buried and superficial optic disc drusen (16). A retrospective study by Fard et al found that patients with mild papilledema had greater mean pRNFL thickness (156.3um) than those with pseudopapilledema (113.8um), and a pRNFL thickness greater than 127um had 73% sensitivity and specificity for papilledema (4). In Fard et al’s prospective study using OCT and OCT angiography, pRNFL thickness was greater among patients with papilledema vs pseudopapilledema and healthy controls, though the ganglion cell complex thickness was not different between groups (10). Other studies comparing patients with papilledema to normal patients observed greater sensitivity of TRT over pRNFL in detecting mild papilledema (5, 17), though our study found that pRNFL and TRT had similar sensitivity (4).
Our study offers an important finding that longitudinal change in pRNFL, TRT, and ONHV had greater diagnostic value than baseline SD-OCT parameters to differentiate papilledema from pseudopapilledema. Analysis of receiver operator curves revealed that ΔpRNFL, ΔTRT, and ΔONHV had the highest area under the curves, and therefore were best able to differentiate between papilledema and pseudopapilledema versus baseline SD-OCT parameters. It is important note that the effects of lifestyle modifications such as weight loss and intracranial pressure lowering agents were not accounted for in the study and almost certainly influenced the amplitude of change in OCT parameters among treated patients with papilledema. SD-OCT parameters such as change in pRNFL thickness likely captures subclinical change in peripapillary dimensions, and when detected, should prompt a reassessment of the likelihood of raised intracranial pressure.
We acknowledge that the criteria utilized for this study (i.e. a change in the appearance of optic disc swelling between initial and follow up visits on fundus photography) is not a perfect method to diagnose papilledema. Identifying a change in optic disc appearance in the setting of mild papilledema may be challenging, and the six-month interval that was chosen for study purposes may have been too short to detect a change. We acknowledge that there can be inherent variability in interpretation of optic disc edema, even by experienced neuro-ophthalmologists (6). Moreover, we acknowledge that clinical symptoms, MRI findings, and measurement of CSF opening pressure may guide the diagnosis when the fundus appearance is equivocal, though test results may conflict. In our study, 44% of patients diagnosed with pseudopapilledema based on the study definition were actually diagnosed with papilledema by the treating neuro-ophthalmologist. Such patients, who had an undetectable change in disc swelling on fundus photography, could have been misclassified, especially when MRI findings of intracranial hypertension were present, lumbar puncture demonstrated elevate opening pressure, or OCT demonstrated a change in pRNFL ≥10um. Future studies will need to determine more stringent definitions of pseudopapilledema and true papilledema.
Our study offers several strengths. Foremost, this was a prospective study which included all patients referred with optic disc elevation, making the study population representative of undifferentiated patients referred to neuro-ophthalmic practices. Other studies included patients with pseudopapilledema only if they demonstrated buried optic disc drusen on ultrasound; however, the present study was inclusive of all forms of pseudopapilledema. We separately analyzed patients with mild optic disc elevation to isolate the population of patients whose diagnosis is most in question. The six months of longitudinal follow-up enabled analysis of longitudinal change in SD-OCT parameters. The use of SD-OCT with 3D segmentation software provided more accurate retinal layer segmentation than would be expected from proprietary 2D segmentation software (11).
Conclusion
This prospective longitudinal study confirms the high sensitivity and specificity of baseline SD-OCT parameters such as pRNFL thickness, TRT, and ONHV. We demonstrate the greater diagnostic utility of longitudinal change in these parameters in differentiating patients with papilledema from those with pseudopapilledema.
Examples of retinal layer segmentations using 3D graph-based algorithm. (a, b) A central B-scan from a 200×200 macular cube scan with indicated segmentation of the GCL+IPL (yellow and green lines) and the 3D visualization. (c, d) A central B-scan of the corresponding 200×200 optic disc cube scan with segmentation of the RNFL (red and orange lines) and lower bonding surface of the RPE complex (blue line) and the 3D visualization; the ONH volume (ONHV) was defined by the total volumetric space between the ILM and RPE/BM. (e) The corresponding thickness map of the RNFL with regional peripapillary RNFL measurements, where the radius of the peripapillary circle is 1.73 mm. (f) The corresponding thickness map of the total retina (between the ILM and the RPE/BM) with regional peripapillary TRT measurements using the same peripapillary circle.
Results of ancillary studies are shown for the eleven patients who met the study criteria for pseudopapilledema but were diagnosed with papilledema by the treating neuro-ophthalmologist. Abbreviations are as follows: “MRI” - signs of elevated opening pressure were present on MRI; “LP” - elevated opening pressure of >25cmH2O; “OCT” ≥ 10um change of pRNFL thickness from baseline visit to study completion.
Acknowledgments
Funding support: This study was supported by a P30-EY01583–26 federal grant
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to disclose.
Contributor Information
Imran Jivraj, Department Ophthalmology, University of Alberta.
Cesar Alfaro Cruz, Perelman School of Medicine at the University of Pennsylvania.
Maxwell Pistilli, Center for Preventative Ophthalmology and Biostatistics at the University of Pennsylvania.
Anita A. Kohli, Department of Ophthalmology and Visual Science, Yale School of Medicine.
Grant T. Liu, Division of Neuro-ophthalmology, Departments of Ophthalmology and Neurology, Scheie Eye Institute at the University of Pennsylvania.
Kenneth S. Shindler, Division of Neuro-ophthalmology, Departments of Ophthalmology and Neurology, Scheie Eye Institute at the University of Pennsylvania.
Robert A. Avery, Division of Neuro-ophthalmology, Departments of Ophthalmology and Neurology, Scheie Eye Institute at the University of Pennsylvania.
Mona K. Garvin, Center for the Prevention and Treatment of Visual Loss, VA Health Care System, Iowa City, IA, USA and Department of Electrical and Computer Engineering, the University of Iowa, Iowa City, IA, USA..
Jui-Kai Wang, Center for the Prevention and Treatment of Visual Loss, VA Health Care System, Iowa City, IA, USA and Department of Electrical and Computer Engineering, the University of Iowa, Iowa City, IA, USA..
Ahmara Ross, Division of Neuro-ophthalmology, Departments of Ophthalmology and Neurology, Scheie Eye Institute at the University of Pennsylvania.
Madhura A Tamhankar, Division of Neuro-ophthalmology, Departments of Ophthalmology and Neurology, Scheie Eye Institute at the University of Pennsylvania.
References
- 1.Heidary G, Rizzo JF 3rd. Use of optical coherence tomography to evaluate papilledema and pseudopapilledema. Semin Ophthalmol. 2010;25(5–6):198–205. [DOI] [PubMed] [Google Scholar]
- 2.Rebolleda G, Kawasaki A, de Juan V, Oblanca N, Munoz-Negrete FJ. Optical Coherence Tomography to Differentiate Papilledema from Pseudopapilledema. Curr Neurol Neurosci Rep. 2017;17(10):74. [DOI] [PubMed] [Google Scholar]
- 3.Kardon R. Optical coherence tomography in papilledema: what am I missing? J Neuroophthalmol. 2014;34 Suppl:S10–7. [DOI] [PubMed] [Google Scholar]
- 4.Fard MA, Fakhree S, Abdi P, Hassanpoor N, Subramanian PS. Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol. 2014;158(1):136–43. [DOI] [PubMed] [Google Scholar]
- 5.Skau M, Yri H, Sander B, Gerds TA, Milea D, Jensen R. Diagnostic value of optical coherence tomography for intracranial pressure in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):567–74. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45–9. [DOI] [PubMed] [Google Scholar]
- 7.Lee KM, Woo SJ, Hwang JM. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(5):971–7. [DOI] [PubMed] [Google Scholar]
- 8.Group OCTS-SCfNIIHS, Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014;55(12):8173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Optical Coherence Tomography Substudy C, Group NIIHS. Papilledema Outcomes from the Optical Coherence Tomography Substudy of the Idiopathic Intracranial Hypertension Treatment Trial. Ophthalmology. 2015;122(9):1939–45 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fard MA, Sahraiyan A, Jalili J, Hejazi M, Suwan Y, Ritch R, et al. Optical Coherence Tomography Angiography in Papilledema Compared With Pseudopapilledema. Invest Ophthalmol Vis Sci. 2019;60(1):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group OCTS-SCfNIIHS, Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci. 2014;55(12):8180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvin MK, Abramoff MD, Wu X, Russell SR, Burns TL, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging. 2009;28(9):1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JK, Kardon RH, Kupersmith MJ, Garvin MK. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):4069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvin MK, Abramoff MD, Kardon R, Russell SR, Wu X, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008;27(10):1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassi ST, Mohana KP. Optical coherence tomography in papilledema and pseudopapilledema with and without optic nerve head drusen. Indian J Ophthalmol. 2014;62(12):1146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vartin CV, Nguyen AM, Balmitgere T, Bernard M, Tilikete C, Vighetto A. Detection of mild papilloedema using spectral domain optical coherence tomography. Br J Ophthalmol. 2012;96(3):375–9. [DOI] [PubMed] [Google Scholar]


