Abstract
Objective:
To report our ability to capture, grade reliably and analyze bedside macular optical coherence tomography (OCT) images from preterm infants and relate OCT findings to biological factors and retinopathy of prematurity (ROP) status at a single time window in the STudy of Eye imaging in Preterm infantS (BabySTEPS).
Design:
Prospective observational study
Participants:
Preterm infants eligible for ROP screening, with parental consent for research and a 36±1 weeks postmenstrual age (PMA) visit.
Methods:
We imaged both eyes of preterm infants with an investigational non-contact, handheld swept-source OCT at the time of clinical ROP examinations. Macular OCT features and layer thicknesses for untreated eyes of infants at 36±1 weeks PMA were compared to demographic data and clinical ROP examination performed by experts. Statistical analyses accounted for the use of both eyes of infants.
Main Outcome Measures:
Macular OCT features and layer thicknesses, gender, race/ethnicity, gestational age, birth weight, ROP stage, and plus disease.
Results:
We captured macular OCT from 169 eyes (one eye excluded for prior ROP treatment) at 36±1 weeks PMA. The quality of OCT volumes was excellent in 33 eyes (19%), acceptable in 112 (67%), poor in 24 (14%) and unusable in 0 (0%). Macular edema was present in 60% of eyes and bilateral in 82% of infants with edema. At the fovea, retinal thickness and inner nuclear layer (INL) thickness increased with edema severity: 183±36μm and 51±27μm in mild edema (16% of eyes), 308±57μm and 163±53μm in moderate (25%) and 460±76μm and 280±83μm in severe (12%), respectively. With an increase in ROP stage from 0 to 2, the mean (standard deviation) retinal thickness at fovea increased from 227(124) to 297(99)μm, p<0.001. The choroid was thinner 155(72) with pre-plus/plus disease versus without 236 (79), p=0.04, while retinal thickness did not vary.
Conclusion:
We demonstrate the reliability of methods and the prevalence of OCT findings in preterm infants enrolled in BabySTEPS, at a single time-point of 36±1 weeks PMA. The variations in layer thicknesses in infants at this time point may reflect abnormalities due to delay in foveal development that may be impacted by macular edema, ROP, or both.
Précis
We report: methods; rigor of investigational bedside optical coherence tomography (OCT) imaging, prevalence of OCT features; thickness measurements; and associations with severity of retinopathy of prematurity, from BabySTEPS (STudy of Eye imaging in Preterm infantS).
Retinal OCT imaging has revolutionized the diagnosis and management of retinal diseases in adults and older children. OCT imaging has had a far more limited role in the care of retinal disease in infants, especially preterm infants, predominantly because of the perceived difficulties in bringing an infant from the intensive care nursery (ICN) to the clinic for eye imaging, and in capturing images from a non-fixating infant. To acquire preterm infant OCT imaging before term equivalent age, some groups have used the “flying baby position” with the Optos system,1 while others have repositioned a tabletop OCT to image while pointing downwards (Optos Inc., Marlborough, MA) beside Optos system.2 Over the last decade, we have pioneered the use of bedside non-contact spectral-domain (SD) OCT imaging of the retina in very preterm infants from 30 weeks' postmenstrual age (PMA) onward that has revealed unique retinal information that may be relevant to retinopathy of prematurity (ROP) and eye-brain development.3-7 Findings from multiple groups have contributed to our understanding of preterm retinal development in-vivo and abnormalities associated with ROP.8-12 These have included correlations between SDOCT and histological structures9, 13, 14 and identification of novel infant features including macular edema,4, 8, 10-12, 15-17 delay in macular photoreceptor development,18, 19, differences in choroidal thickness20, 21 and retinal nerve fiber layer thickness.7 However, most of these studies were conducted in small subsets of infants or at varying age time points.
The Study of Eye Imaging in Preterm Infants (BabySTEPS), is a National Institutes of Health-funded prospective, longitudinal study that evaluated normal and abnormal microanatomic features of the developing retina and optic nerve using bedside handheld OCT in very preterm infants at risk of ROP. The study also determined the relationship of retinal and optic nerve head findings to brain injury, neurodevelopment, visual function and progression of ROP. Using investigational, handheld OCT imaging at the bedside, we propose to identify in vivo abnormalities in the retina, optic nerve head, and choroid that signal deviation from a healthy trajectory. Herein, we report our methods of capturing ROP data and bedside macular OCT images from a cross-sectional group of preterm infants in BabySTEPS. We examined and reported the reproducibility of thickness measures across handheld OCT systems, the quality of infant OCT images acquired, the prevalence and reproducibility of OCT features, retinal and choroidal thickness measurements, and their relationship to biological variables and ROP status at 36±1 weeks PMA.
Methods:
Study design and participants
The prospective, observational BabySTEPS was approved by the Duke University Health System Institutional Review Board and registered with clinicaltrials.gov (identifier, NCT02887157). The study adhered to the guidelines of the Health Insurance Portability and Accountability Act (HIPAA), and the tenets of the Declaration of Helsinki. Coordinators enrolled infants from a single ICN before the first ROP screening visit if they met the American Association of Pediatrics eligibility for ROP screening22 and the parent/legal guardian provided informed consent to study participation. Infants were excluded from the study if they had a health or an eye condition that precluded eye examination or imaging or a health condition other than prematurity which was expected to have a profound impact on brain development (intraventricular hemorrhages were not an exclusion). Of 118 infants enrolled, twelve were transferred out of the Duke ICN, and four died before any OCT imaging, leaving 102 infants with one or more OCT imaging sessions before 39 weeks PMA. For this cross-sectional analysis, we included infants who underwent an ROP visit at 36±1weeks PMA and had received no ROP treatment in at least one eye. Study enrollment and inclusion of infants are reported in Supplementary Figure 1. We chose this window to include the maximum number of infants with at least one ROP-treatment-free OCT imaging session available. For infants who underwent an imaging sessions at equal intervals from the midpoint, we randomly selected 1 visit for analysis. Eighty-four infants had an imaging session within this interval; we also included 1 infant who underwent a treatment-free imaging session one day outside of our chosen window. Seventeen infants either lacked imaging sessions within the interval because they had been discharged or transferred or had undergone treatment in both eyes (4 infants) for ROP.
Study Procedures
Infant health and medical outcome data, including demographics, were extracted from the medical record consistent with data collected for the Generic Database (a registry of clinical information of very low birth weight infants born alive in Eunice Kennedy Shriver NICHD Neonatal Research Network centers [ClinicalTrials.gov: NCT00063063]). Coordinators obtained detailed eye examination data for the infants from the pediatric ophthalmologists during clinical examination for ROP (summary in Supplementary Table 1) and entered these and health data into the Research Electronic Data Capture (REDCap) software platform.23, 24
Infants were imaged with research OCT systems at the bedside in the Duke ICN or Duke Regional Hospital, usually on the same day as each clinical ROP examination, while pupils were still pharmacologically dilated as they had pharmacological pupil dilation for the clinical examination. When we captured OCT imaging at other times (without a corresponding clinical ROP screening examination), it was without pharmacologic dilation. We used pacifiers and oral sucrose at the ICN nurse’s preference at the time of imaging. The certified imager positioned the handheld system over the infant eye and held the lids open with fingertips. A second researcher operated the OCT computer software.
We used 2 noncontact, ultra-compact handheld probes: the UC2 (from September 13, 2016, through October 2, 2018), which used a 100-kHz swept-source (SS) laser, and the UC3 (from October 9, 2018, onward), which used a 200-kHz laser.25 The structural OCT scan protocol for UC2 was 6.93mm x 6.39mm with 512 A-scans per B-scan and 112 B-scans per volume at 0 degrees. For UC3 imaging sessions, we captured the structural OCT 10mm x 10mm or 13mm x13mm with 950 A-scans per B-scan and 256 B-scans per volume at 0 degrees; and every 2 B-scans were obtained in the same location for post-process averaging. The UC3 enabled a wider field of view, averaging of multiple locations, and OCT-angiography imaging26. The Leica SDOCT Envisu C2300 system (Buffalo Grove, IL) was used alternatively at the Duke ICN or the second nursery after the transfer, in 11 infants at 36±1 PMA according to an age-specific imaging protocol published by Maldonado et al.3 We assessed the repeatability and reproducibility of measurements of center foveal thickness for these three handheld OCT systems (Supplementary Table 2). For all infant imaging sessions, the goal was to capture the fovea, optic nerve, and papillomacular bundle. Herein we describe only the macular findings from OCT.
We excluded one infant eye that had undergone ROP treatment before imaging. Trained graders, masked to all clinical information except PMA, determined the quality of OCT scan volumes and graded as excellent, acceptable, poor, or unusable (definitions in Supplementary Table 3). We included one foveal volume from each eye in the analysis, and graders reviewed a single foveal scan from that volume for the presence or absence of pathology and key anatomical retinal layers at the foveal center (definitions in Supplementary Table 3). One trained grader evaluated for vitreous pathologic features, pre-retinal neovascularization, macular edema, and development of photoreceptor layers. We determined macular edema by the presence of cystoid changes usually in the inner nuclear layer (INL), and we scored the severity of edema as mild, moderate, or severe based on the deformation of the inner retinal contour at the foveal center (Figure 1). We graded for the presence of external limiting membrane (ELM) and ellipsoid zone (EZ) at the foveal center and central bulging of the photoreceptor layer. We also graded the images for visibility of the choroidal scleral junction (CSJ) and the presence of retinoschisis and detachment. Intra-retinal and vitreous hemorrhages were assessed based on the presence of pre-retinal or intra-retinal reflectivity and shadowing of retinal pigment epithelium and choroid. The primary outcomes for OCT grading were: macular edema, photoreceptor development, and choroidal thickness. Intragrader and intergrader reproducibility was performed at 36±1 weeks' PMA. Grading was carried out by a second grader (L.V.) masked to the primary grading findings for macular edema and photoreceptor development.
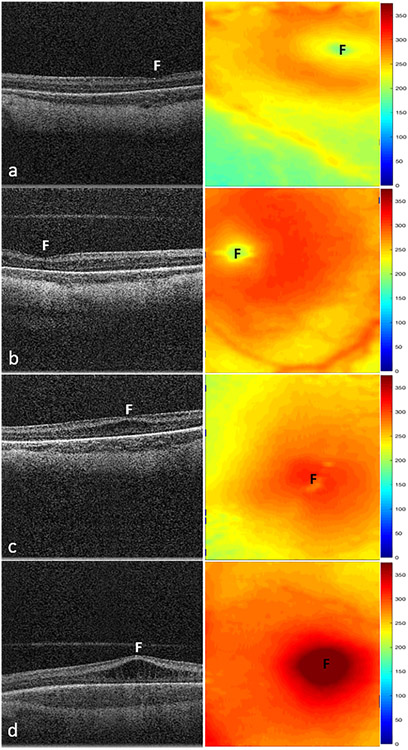
Figure 1.
Representative swept-source OCT B-scans (left column) and retinal thickness maps (right column) of macular edema severity in stage 2 ROP. A, Infant with no edema with a retinal thickness of 186 μm and inner nuclear layer (INL) thickness of 30 μm at the fovea (F). B, Infant with mild edema showing little to no deformation of the foveal contour with a retinal thickness of 212 μm and INL thickness of 52 μm. C, Infant with moderate edema showing flattening or slight upward bulging of the fovea and retina and INL thicknesses measuring 294 μm and 151 μm, respectively. D, Infant with severe edema showing severe upward bulging of the foveal contour with retinal and INL thicknesses of 433 μm and 286 μm, respectively. Mean retinal thickness at the fovea increased from 160 μm (standard deviation, 43 μm) without macular edema to 460 μm (standard deviation, 76 μm) with severe macular edema (P < 0.001).
Semiautomatic segmentation of retinal layers for thickness measurements in the central foveal frame across the entire scan was based on automated segmentation using the proprietary infant-specific software, the Duke OCT Retinal Analysis Program Marking Code Baby version 2.027 with manual correction by a trained grader. The retinal layers are diagrammed in Figure 2 and listed in Supplementary Table 3. We extracted and reported thicknesses at the foveal center and across 1mm centered on the fovea for 1) total retina, 2) combined RNFL, ganglion cell layer (GCL) and inner plexiform layer (IPL), 3) INL, 4) outer retina (from the inner border of the outer plexiform layer to the inner border of the retinal pigment epithelium), and 5) choroid. Because the contour of the outer border of the choroid varied across the outer choroidal vessels, we reported the choroidal thickness across the center 1mm as the primary outcome in addition to the thickness at the foveal center point. To test the reproducibility of the process of selecting the fovea, segmentation and correction of the retinal and choroidal layers we undertook the following steps in 20 eyes; the primary grader masked to segmentation, recorrected the scans that were originally chosen for analysis (intragrader reproducibility), a second grader masked to the segmentation findings, manually corrected the segmentation in the original scans (intergrader reproducibility). We picked an alternate scan at the same visit for the 20 eyes, which was corrected manually by both the primary and the second grader, to test intragrader reproducibility for both the graders and the intergrader reproducibility between the two graders.
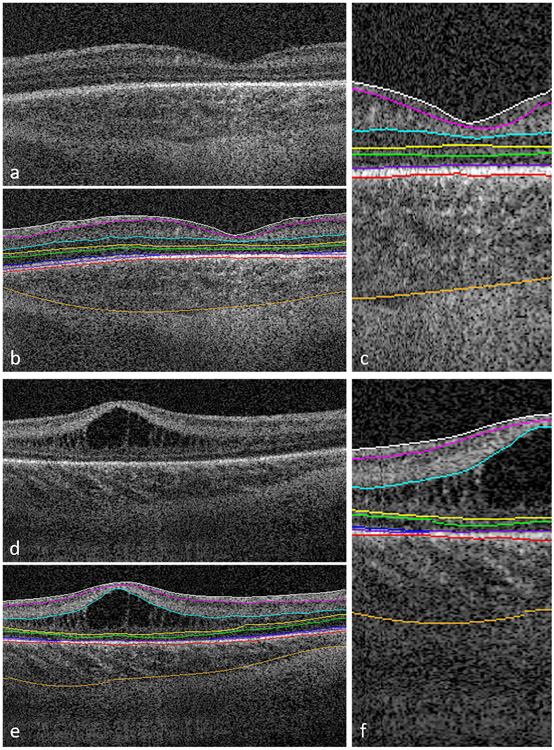
Figure 2.
Representative foveal B-scans from macular volumes from swept-source OCT systems in infants with (A, B, C) no macular edema and (D, E, F) with macular edema. B, C, E, F, Duke OCT Retinal Analysis Program Marking Code Baby version 2.0 semiautomated segmentation at the internal limiting membrane (white), outer borders of the nerve fiber layer (magenta), inner plexiform layer (aqua), inner nuclear layer (yellow), outer plexiform layer (green), ellipsoid zone (blue; not visualized in (C) and tapering at the foveal margin in (F)), retinal pigment epithelium (inner, purple; outer, pink), and choroid (orange).
Statistical Analysis
We performed all statistical analyses using R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). To assess the similarity of the retinal and choroidal layer thicknesses between the two eyes we used the paired t test and the mean absolute difference to describe the variability in thickness between eyes. To evaluate the differences in layer thicknesses between the temporal and the nasal side of the fovea, we performed the regression with generalized estimating equations standard errors. To determine the associations between the retinal layer thicknesses with macular edema severity and ROP stages and posterior pole vascular condition (plus, pre-plus or neither), we used regression analysis with generalized estimating equation standard errors. We log transformed retinal layer thicknesses (total retina, INL and outer retina) that were not normally distributed. For this cohort, because of the small numbers (n=3), we did not include stage 3 eyes for analysis of associations by ROP stage. For associations between qualitative OCT features and ROP stage and plus disease we performed a logistic regression analysis with generalized estimating equation standard errors. For associations between layer thickness measurements and biological variables such as gestational age and birth weight, we used Spearman rank correlation coefficients. For categorical variables like gender, race and ethnicity we used the Kruskal-Wallis rank sum test. To describe the intergrader and intragrader reproducibility of grading of OCT variables, we used the κ statistic. For OCT layer thicknesses, we used the interclass correlation coefficient and Bland-Altman plots to test intragrader and intergrader reproducibility. Interclass correlation coefficient values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9 and greater than 0.90 indicated poor, moderate, good and excellent reproducibility.28
Results
Demographics
For the 85 preterm infants who underwent OCT imaging attempted at 36 ± 1 weeks’ PMA, mean gestational age was 28 ± 2 weeks and 37 infants were born extremely preterm (<28 weeks’ gestational age), whereas 44 were very preterm (28–32 weeks’ gestational age). Four infants were at more than 32 weeks of gestation but were eligible for ROP screening because of low birth weight. The mean birth weight was 976±269gm. The demographic, systemic, and ROP status of the infants are in (Table 1). Eight infants (9%) went on to be treated for ROP in both eyes, by the completion of their hospital course.
Table 1:
Demographics, systemic and ocular health information of the preterm infants included in the study.
| Demographic information | Preterm N=85 |
||
|---|---|---|---|
| Gestational Age (wks), mean (SD) | 28 (2) | ||
| Birth Weight (gm), mean (SD) | 976 (269) | ||
| Age at Imaging (wks, PMA), mean(SD) | 36 (0.6) | ||
| Sex, n (%) | |||
| Male | 43 (51) | ||
| Race, n (%) | |||
| African-American | 38 (45) | ||
| Mixed | 5 (6) | ||
| Asian | 3 (3) | ||
| Caucasian | 39 (46) | ||
| Ethnicity, n (%) | |||
| Hispanic | 7 (8) | ||
| Non-Hispanic | 78 (92) | ||
| Systemic Health, n (%) | |||
| Maternal antenatal steroids | 78 (92) | ||
| Patent ductus arteriosus | 38 (45) | ||
| Treatment with Indomethacin | 13 (15) | ||
| Early onset sepsis | 2 (2) | ||
| Late onset sepsis | 11 (13) | ||
| H/O transfusion | 64 (75) | ||
| Intraventricular hemorrhage | 8 (9) | ||
| Periventricular leukomalacia | 5 (6) | ||
| Bronchopulmonary dysplasia | 11 (13) | ||
| Necrotizing enterocolitis | 5 (6) | ||
| Ocular health information of the preterm infants by eyes | |||
| OD (N=84)* | OS (N=85) | p value |
|
| ROP stage at the time of imaging, n (%) | 0.29 | ||
| Stage 0 | 47 (56) | 48 (57) | |
| Stage 1 | 14 (17) | 15 (18) | |
| Stage 2 | 22 (26) | 20 (23) | |
| Stage 3 | 1 (1) | 2 (2) | |
| Plus disease at the time of imaging, n (%) | 0.14 | ||
| None | 80 (95) | 80 (94) | |
| Pre-Plus | 1 (1) | 4 (5) | |
| Plus | 3 (4) | 1 (1) | |
| ROP zone at the time of imaging, n (%) | 0.77 | ||
| I | 8 (10) | 9 (11) | |
| II | 57 (68) | 58 (68) | |
| III | 16 (19) | 15 (18) | |
| Fully Vascularized | 3 (4) | 3 (3) | |
| Maximum ROP Stage, n (%) | 0.22 | ||
| Stage 0 | 36 (43) | 38 (45) | |
| Stage 1 | 14 (17) | 14 (16) | |
| Stage 2 | 24 (28) | 23 (27) | |
| Stage 3 | 10 (12) | 9 (11) | |
| stage 4 | 0 (0) | 1 (1) | |
| ROP Treatment*, n (%) | - | ||
| Bevacizumab & laser photocoagulation | 4 (5) | 5 (6) | |
| Laser photocoagulation | 3 (3) | 3 (3) | |
| No Therapy | 77 (92) | 77 (91) | |
One eye of one infant excluded for analysis due to early treatment for ROP
Subsequent to current exam visit; PMA: postmenstrual age; SD: standard deviation; OD: right eye, OS: left eye
OCT capture, prevalence and reproducibility of features
We were able to capture foveal OCT successfully in both eyes for all 85 infant OCT bedside imaging sessions at 36±1 weeks PMA. Imaging was carried out with the novel handheld SSOCT systems in 74 infants (50 infants with UC2, 24 infants with UC3) and with the Leica Envisu 2300 system in 11 infants. The quality of OCT volumes of the fovea for these 169 eyes was excellent in 33 eyes (19%), acceptable in an additional 112 eyes (67%), and poor (but useful for some grading) in 24 eyes (14%) and unusable in 0 eyes. More than 60% of scans were of acceptable quality, with each of the 3 handheld OCT systems (UC2, 62/100 eyes [62%]; UC3, 32/47 eyes [68%]; Leica, 18/22 eyes [82%]). The frequency of excellent-quality scans across the systems was: UC2, 29 of 100 eyes (29%); UC3, 3 of 47 eyes (6%); and Leica SD OCT imaging system, 1 of 22 eyes (4%). We imaged twenty-one eyes (12%) without pharmacologic pupil dilation, of which 6 (29%) were of poor image quality. We could ascertain primary outcome features such as the severity of macular edema, presence of photoreceptor sub-layers, and choroidal thickness, in 96%, 96%, and 94% (159 eyes), respectively. Good to moderate intragrader and intergrader agreement (range, 0.71–0.92) was found for the presence of OCT features, except for external limiting membrane presence at the fovea at 36 ± 1 weeks’ PMA of 0.41 (Supplementary Table 4).
Of the 169 gradable scans from 85 infants, we found vitreous pathologies such as vitreous separation or opacities in 28 eyes of 19 infants (23%). These vitreous pathologic features were bilateral in nine infants. Seventy-nine percent of eyes (22 eyes) with vitreous pathologic features also had macular edema (p=0.01). OCT images documented macular edema in 91 eyes of 50 infants (60%). In eighty-two percent (41 infants) of the infants, edema was bilateral. When edema was unilateral, it was always of mild severity. The maximum grade of macular edema across both eyes was mild in 22% of infants, moderate in 23% of infants, and severe in 16% of infants. External limiting membrane was visible at the foveal center in both eyes of 2 infants and in 1 eye each of 2 infants (5%), and both eyes of 1 infant (1%) showed ellipsoid zone at the fovea (Table 2). We did not find intra-retinal hemorrhage or retinal detachment in any eyes.
Table 2:
Prevalence of qualitative optical coherence tomography features in preterm infants at 36 weeks postmenstrual age
| Feature# | N= 85 infants | Laterality among infants with the feature in at least one eye ** |
||
|---|---|---|---|---|
| Not Present N (%) |
Present in at least one eye N (%) |
Bilateral N (%) |
Unilateral N (%) |
|
| Vitreous pathologies | 65 (76.5%) | 19 (22.6 %) | 9 (47.4 %) | 10 (52.6 %) |
| Pre-retinal neovascularization | 84 (98.8%) | 1 (1.2 %) | 1 (100 %) | 0 (0 %) |
| Macular Edema | 33 (39.8%) | 50 (60.2 %) | 41 (82 %) | 9 (18 %) |
| Mild | 18 (21.7 %) | 9 (50 %) | 9 (50 %) | |
| Moderate | 19 (22.9 %) | 18 (94.7 %) | 1 (5.3 %) | |
| Severe | 13 (15.7 %) | 8 (61.5 %) | 5 (38.5 %) | |
| Elongated cystoid spaces | 54 (65.9%) | 28 (34.1 %) | 17 (60.7 %) | 11 (39.3 %) |
| ELM at foveal center | 79 (95.2%) | 4 (4.8 %) | 2 (50 %) | 2 (50 %) |
| EZ band at foveal center | 84 (98.8%) | 1 (1.2 %) | 1 (100 %) | 0 (0 %) |
| Photoreceptor bulging | 55 (64.7%) | 30 (35.3 %) | 22 (73.3 %) | 8 (26.7 %) |
| Retinoschisis | 84 (98.8%) | 1 (1.2 %) | 1 (100 %) | 0 (0 %) |
| Retinal detachment | 85 (100.0%) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
ELM: external limiting membrane; EZ: ellipsoid zone
one subject with only one pre-treatment eye removed from laterality analysis
Some features could not be determined or graded in the following number of eyes: 4 (vitreous pathologies), 6 (macular edema), 12 (elongated cystoid spaces), 6 (ELM), 6 (photoreceptor bulging), 1 (retinal detachment)
Retinal and choroidal layer thicknesses
The thicknesses of all retinal layers and the choroid were symmetric between left and right eyes of the same infant, both at the foveal center and across the center 1mm, as evidenced by the small mean absolute difference between eyes relative to the standard deviation of the distribution of layer thickness in Table 3. The layer thicknesses varied widely among infants at 36±1 weeks' PMA. Mean retinal thickness at the foveal center was 239±115μm, and ranged from 92-634μm. The mean RNFL+GCL+IPL was 50±21μm (range, 12–125 μm), with almost all eyes showing some degree of persistence of these layers at the foveal center (Figure 2). The mean INL thickness at the foveal center was 104±98μm, and this layer showed the greatest thickness range across infant retinal layers (range, 0–476 μm). The mean outer retinal thickness at the foveal center was 85±31μm (range, 48–262 μm). Mean choroidal thickness at the foveal center was 232±81μm and ranged from 61-459μm. Good intragrader and intergrader agreement was found between graders for the segmentation of both retinal and choroidal layers (Supplementary Figure 2 & Table 5).
Table 3:
Mean retinal and choroidal thickness measurements and the mean absolute difference of the layer thickness between eyes at foveal center and across the center 1mm in preterm infants at 36 weeks' postmenstrual age
| Layer | Location | Layer Thickness (μm) | Mean Absolute difference |
p-value | |
|---|---|---|---|---|---|
| OD (N=84 eyes) |
OS (N=85 eyes) |
||||
| Mean (SD) | Mean (SD) | (μm) | |||
| Total retina* | Foveal center | 240 (115) | 238 (115) | 16 | 0.91 |
| Across center 1 mm | 261 (93) | 257 (94) | 12 | 0.15 | |
| RNFL+GCL+IPL | Foveal center | 50 (21) | 51 (21) | 12 | 0.85 |
| Across center 1 mm | 79 (17) | 77 (18) | 7 | 0.24 | |
| Inner nuclear layer | Foveal center | 105 (99) | 103 (97) | 17 | 0.88 |
| Across center 1 mm | 107 (85) | 105 (83) | 11 | 0.67 | |
| Outer retina | Foveal center | 85 (30) | 85 (33) | 13 | 0.90 |
| Across center 1 mm | 75 (14) | 75 (14) | 6 | 0.59 | |
| Choroid | Foveal center | 237 (81) | 226 (81) | 37 | 0.22 |
| Across center 1 mm | 237 (80) | 227 (80) | 36 | 0.26 | |
Does not include choroid, RNFL: Retinal nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer, OD: right eye, OS: left eye
Table 5:
Pairwise difference in retinal and choroidal thickness measurements between temporal and nasal side at 500μm distance from the foveal center in preterm infants at 36 weeks' postmenstrual age
| Layer thickness (μm) | Nasal (N=166 eyes) |
Temporal (N=166 eyes) |
p-value |
|---|---|---|---|
| Total retina*, mean(SD) | |||
| 284 (69) | 278 (65) | < 0.001 | |
| RNFL+GCL+IPL, mean(SD) | |||
| 111 (17) | 106 (16) | <0.001 | |
| Inner nuclear layer, mean(SD) | |||
| 102 (61) | 99 (60) | 0.013 | |
| Outer retina, mean(SD) | |||
| 71 (10) | 73 (10) | 0.006 | |
| Choroid, mean(SD) | |||
| 237 (83) | 224 (77) | 0.001 |
does not include choroid; RNFL: Retinal nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer
Macular edema caused the most substantial amount of variance in the retinal thickness and appeared to affect all retinal layers except the choroid (Table 4). Retinal thickness and INL thickness were greater with increasing macular edema severity (P < 0.001 for each, respectively): mean retinal thickness at the fovea with no macular edema was 160 ± 43 μm, mild retinal thickness was 183 ± 36 μm, moderate retinal thickness was 308 ± 57 μm, and severe retinal thickness was 460 ± 76 μm. These same measures for INL were 38 ± 23 μm, 51 ± 27 μm, 163 ± 53μm, and 292 ± 83 μm, respectively. The outer retinal thickness was also greater with increasing macular edema severity (p< 0.001). Variation in the retinal thicknesses at the foveal center with macular edema was also evident for all of these layers across the center 1mm as well. The RNFL+GCL+IPL thickness across the center 1mm (but not at the foveal center) varied significantly with edema severity (p=0.04). Unlike retinal thickness, choroidal thickness did not vary with the presence or severity of macular edema.
Table 4:
Associations between severity of macular edema and retinal thickness measurements at the foveal center and across the foveal center 1mm in preterm infants at 36 weeks' postmenstrual age
| Layer thickness (μm) | None (N=72 eyes) |
Mild (N=28 eyes) |
Moderate (N=48 eyes) |
Severe (N=21 eyes) |
p-value |
|---|---|---|---|---|---|
| Total retina*, mean(SD) | |||||
| Foveal center | 160 (43) | 183 (36) | 308 (57) | 460 (76) | < 0.001 |
| Across center 1 mm | 192 (34) | 222 (28) | 311 (47) | 441 (67) | < 0.001 |
| RNFL+GCL+IPL, mean(SD) | |||||
| Foveal center | 47 (22) | 52 (17) | 52 (21) | 56 (23) | 0.17 |
| Across center 1 mm | 73 (20) | 83 (15) | 82 (14) | 81 (15) | 0.04 |
| Inner nuclear layer, mean(SD) | |||||
| Foveal center | 38 (23) | 51 (27) | 163 (53) | 280 (83) | < 0.001 |
| Across center 1 mm | 48 (17) | 67 (20) | 151 (39) | 275 (69) | < 0.001 |
| Outer retina, mean(SD) | |||||
| Foveal center | 74 (16) | 80 (16) | 94 (37) | 112 (54) | <0.001 |
| Across center 1 mm | 71 (11) | 73 (10) | 78 (15) | 85 (19) | 0.001 |
| Choroid, mean(SD) | |||||
| Foveal center | 236 (84) | 249 (81) | 212 (74) | 240 (70) | 0.59 |
| Across center 1 mm | 235 (83) | 249 (79) | 213 (74) | 240 (69) | 0.63 |
does not include choroid; RNFL: Retinal nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer
For the 166 eyes in which we had measurements at 500μm from the foveal center on both the nasal and the temporal sides, we found a significant difference between thicknesses of all layers of the nasal and temporal retina and choroid across the macula, with the nasal side always found to be thicker than the temporal side in all the layers except the outer retina (Table 5).
Associations with ROP and biological variables
As ROP stage ranged from 0 to 2, the prevalence of edema increased from 50% to 71%, although not significant (p=0.08). An increase in retinal thickness was associated with higher ROP stages, both at the foveal center (p= 0.003) and across the center 1mm (p=0.01); this was also true for RNFL+GCL+IPL at both locations (p<0.001 for both). The INL appeared thicker at the foveal center with a higher ROP stage (p=0.03) but not across the center 1mm (Figure 3). Outer retinal thickness and choroidal thickness were not associated with ROP stage. The retinal thickness at the fovea for the three stage 3 eyes excluded from this analysis ranged from 149-268μm, and two of the three eyes had moderate severity of macular edema.
Figure 3:
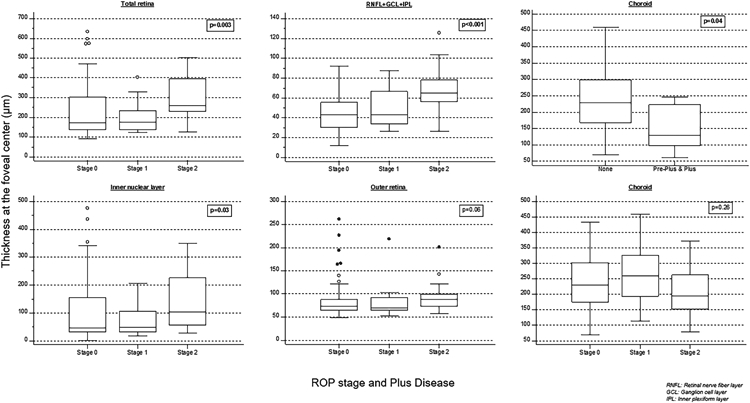
Box-whisker plots showing retinal layer and choroidal thicknesses across retinopathy of prematurity stages and choroidal thickness association with pre-plus and plus disease at the fovea center. Thicker total retina, retinal nerve fiber layer (RNFL)+ganglion cell layer (GCL)+inner plexiform layer (IPL), and inner nuclear layer were associated with a higher ROP stage, and the choroid was thinner in infants with preplus and plus disease.
We combined eyes with preplus or plus disease for analysis (n = 9) because of the small numbers. The outer retina at the foveal center was thicker in eyes with preplus or plus disease (p=0.01) than in 160 eyes with normal posterior pole vessels. The choroid was thinner at the foveal center and across the foveal 1mm in eyes with preplus or plus disease than in those with normal posterior pole vessels (p= 0.04 and 0.02, respectively) (Figure 3). None of the other retinal thicknesses were associated with preplus or plus disease.
Retinal thickness at the foveal center at 36 weeks' PMA was associated inversely with gestational age (R2=−0.34, p=0.001) and birth weight (R2= −0.21, p=0.04), as were thicknesses of RNFL+GCL+IPL (R2=−0.61, p=<0.001; R2=−0.34, p=0.001) and outer retina (R2=−0.45, p=<0.001; R2= −0.37, p=<0.001), respectively. Choroidal thickness at the foveal center was directly associated with both gestational age (R2=0.27, p=0.01) and birth weight (R2= 0.44, p=<0.001). We found that the total retina, RNFL+GCL+IPL, and the INL were thicker in White versus non-White infants (p=0.04). No significant associations between OCT layer thicknesses and gender or ethnicity. Macular edema and other OCT retinal features did not vary by race, gender, or ethnicity.
Discussion:
A goal of BabySTEPS was to investigate the efficacy of a new methodology for SSOCT-based bedside ocular neurovascular imaging of preterm infants being screened for ROP. This work addressed an increasing need to develop new strategies for improving ROP outcomes, which include robust testing of new tools capable of providing potentially better information while documenting structural retina changes over time during acute-phase ROP.29 This study also addressed the limitations of previous OCT imaging studies in preterm infants that have not sought to generate serial images within specified periods or to measure the sub-layer thicknesses of the infant retina in the developing fovea (Table 6).8, 10-12
Table 6:
Comparison of macular edema and retinopathy of prematurity findings in preterm infant macular optical coherence tomography studies
| Number of infants |
Gestational age Mean (SD) (weeks) |
Birth weight Mean (SD) (gm) |
Age at Imaging (weeks PMA) |
Macular Edema (%) |
ROP Stage | Association between Macular edema & ROP |
|
|---|---|---|---|---|---|---|---|
| Current Study | 85 infants (169 eyes) | 28 (2) | 976 (269) | 36 (0.6) | 60% | 0: 95 eyes 1: 29 eyes 2: 42 eyes 3: 3 eyes* |
Prevalence of macular edema increased with higher stages of ROP |
| Zepeda et al, 201830 | 65 infants | 28 (2.7) | 997 (286) | 34 (3) | 40% | 0: 43 eyes 1: 8 eyes 2: 4 eyes 3: 10 eyes |
Association between macular edema and ROP not tested in this study. |
| Erol et al, 201411 | 179 infants (358 eyes) | 30.9 (2.7) | 1609 (477) | 38.2 (3.9) | 38% | 1: 82 eyes 2: 28 eyes 3: 16 eyes |
The presence of macular edema increased with increasing ROP stage |
| Dubis et al, 201312 | 46 infants | 27 (3) | 914 (358) | 30-57 | 56% | 0: 17 infants 1: 4 infants 2: 8 infants 3: 12 infants 4A: 2 infants |
No association between the most severe ROP stages and the presence of macular edema |
| Maldonado et al, 20128 | 42 infants | 26 (1.8) (median) | 760 (272) (median) | 34 (1.6) (median) | 50% | 0: 13 infants 1: 6 infants 2: 17 infants 3: 6 infants |
Increased severity of edema was associated with plus disease, higher ROP stage and subsequent laser treatment |
| Vinekar et al, 201110 | 74 infants (146 eyes) | 31.2 (2.32) | 1282 | 37 (2.7) | 29% of stage 2 | 1: 27 eyes 2: 79 eyes |
Macular edema was present only in Stage 2 eyes |
SD: Standard deviation; PMA: postmenstrual age; ROP: retinopathy of prematurity
Stage 3 not included in the analysis due to small numbers
The Study of Eye Imaging in Preterm Infants was a carefully designed and rigorously implemented infant study. The early enrollment of infants to the study by nonophthalmic ICN coordinators ensured unbiased inclusion of preterm infants who would develop varying levels of ROP severity. Our study population was equally distributed between the two genders and was diverse, with an equal number of Black and White infants. The cohort included a fairly even spread across extremely preterm and very preterm infants. With the use of the investigational, handheld SSOCT system in a prospective, longitudinal study, we acquired OCT volumes in awake preterm infants at the bedside, and as reported previously30, we were also able to image through a non-dilated pupil when necessary. Most of the captured volumes were of excellent or acceptable quality; poor-quality scans were still useful for grading. Graders were able to ascertain all qualitative OCT features with moderate to good reproducibility. We were also able to visualize the full depth of the choroid across the foveal center 1mm in 94% of the infants without enhanced depth imaging techniques. The quality of OCT volumes allowed for the successful automatic segmentation of the foveal frames, with manual correction, to generate reproducible layer-thickness measurements. These outcomes strongly suggest our method for OCT imaging is sufficiently robust to support future longitudinal reports from BabySTEPS.
In BabySTEPS, we acquired images at each ROP examination, which enabled the extraction of data for the majority of infants at specified time windows such as 36±1weeks' PMA. We will analyze the data for multiple time windows across the entire nursery stay in future reports. To relate our findings, we investigated how our 36±1 weeks' PMA data aligned with those drawn from previous small-scale studies. We found rates of vitreous opacities that were comparable to Zepeda et al,31 23% versus 37%. We also found a significant association between the presence of abnormal vitreous morphologic features and macular edema in this cohort; Zepeda et al31 also reported that association from SDOCT in similar infants, but with a sample that spanned a wide range of ages (Table 6). Also, in this cohort, our data indicated that macular edema was present and mostly bilateral in at least 60 percent of infants. The prevalence of macular edema in our current study was similar to previous reports from the United States8, 12 but was higher than those reported in India10 and Turkey11 (Table 6). We found lower rates of bilateral edema8, 10, 11, but all eyes with unilateral edema showed mild severity; the asymmetry of edema in these few cases may be the result of to the presence of microcysts in the other eye that may not have reached the level for a grade of mild edema. We found the presence of external limiting membrane and ellipsoid zone in only a few eyes. This result is consistent with what we know about preterm birth, wherein continual, progressive maturation of photoreceptors occurs from 40 PMA onwards9, 18 and over several years after birth, even in term-born infants, as shown by Lee et al32 in a report on healthy foveal development in term infants. In our cohort, the photoreceptor layers would still be under development at 36±1 weeks' PMA. Therefore, analysis of photoreceptor development will necessarily involve additional imaging at older PMA. As noted above, the general agreement between our findings 36 ± 1 weeks’ PMA and those at multiple time points from earlier reports at sites in multiple different countries suggest that the data are applicable to additional communities.
Retinal layer thicknesses (useful measures for macular edema), both at the fovea and across the center 1 mm, varied widely at this age. There was a progressive eight-fold increase in the retinal layer thicknesses, especially of the INL at the foveal center and across the center 1 mm, which was parallel to the severity of macular edema (Table 4). This supports the use of retinal thickness at the foveal center and especially INL thickness at this age as continuous objective measures of macular edema. The change in thickness of NFL+GCL+IPL with edema was more evident across the center 1 mm than focally at the foveal center. We believe this is the result of the distribution of small cystoid spaces in these layers in the parafovea, although this could also be the result of a delay in foveation (fusion of inner layers) associated with macular edema.9, 18 Previous studies on macular edema in preterm infants have reported the use of central foveal thickness and foveal-to-parafoveal ratio for macular edema assessment.8, 10, 11 However, because the cystoid spaces are limited to the INL, and the central foveal thickness and foveal-to-parafoveal ratio may be influenced by ongoing foveal development, it would be most acceptable to use INL thickness as an objective marker of edema at 36±1 weeks' PMA. The BabySTEPS longitudinal analysis will assess whether this measure remains useful for the course of edema.
A known developmental morphology in ROP and after preterm birth is the persistence of the inner retinal layers.18 This persistence is characterized by the presence of GCL, IPL, and INL as distinct measurable layers at the foveal center.18 Our data suggest an association between a thicker retina at the fovea and RNFL+GCL+IPL with higher ROP stages, lower birth weights, and lower gestational ages, which is indicative of this persistence and possible delayed foveation. A known interrelationship exists among these factors: low gestational age and low birth weight are major risk factors for ROP, and both are related to the extent of the immaturity of retinal neural and vascular development at birth and, therefore, the retinal vulnerability to insult.33 In contrast to stage of ROP, we did not find significant associations between retinal layer thicknesses and plus disease.
Finally, at 36±1 weeks' PMA, thinner choroid was associated with preplus or plus disease and with lower gestational age and lower birth weight. Erol et al21 in a study of 80 of 100 infants in whom they could visualize the choroid with SDOCT between 36-42 weeks' PMA, found a similar association with gestational age and birth weight. We did not find any association between choroidal thickness and the stage of ROP. In contrast to our results, Erol et al21 reported that the choroid was thinner at higher ROP stages; however, they combined stage 2 and 3 for analysis, which we did not, to did not look for associations with plus disease. Our pilot SDOCT study showed a thinner choroid in preterm infants relative to that of term infants.20 Our data provides the first step in establishing associations between choroid thickness and plus disease. Zhao and Overbeek34, report that vascular endothelial growth factor expression in the retinal pigment epithelium in rats increases from embryonic to postnatal stages and could play a critical role in vascular development in the choroid. We hypothesize that there may be an alteration in the vascular endothelial growth factor levels and other factors due to the impact of preterm birth and plus disease that could lead to this choroidal thinning.
The global epidemic of ROP has increased the need for an improved grading system for ROP stages and plus disease and for incorporating OCT-based indicators, when validated.29 This report of OCT layer associations with ROP and plus disease can help begin validating potential objective structural markers for ROP. Obtaining location-specific retinal layer thicknesses at a single time point also provides a baseline value that may be extrapolated to longitudinal studies that monitor the trajectory of retina development and disease in preterm infants. Documenting these subclinical features will also be useful for monitoring the effects of ROP treatment.35
A notable limitation in this study is the small number of eyes with advanced ROP severity and the exclusion of stage-three eyes from the analysis for layer thickness associations with ROP stages. Another limitation is that our investigational OCT system and software limits replicability and clinical usefulness. Standard measurements across different handheld OCT devices did not include infant data. Further research is needed to establish the effect of severe ROP and its treatment on foveal development. We also recognize that a combination of systemic, neurological, and ocular health factors may affect retina development in preterm infants, and we will attempt to address these factors in future reports.
This study establishes the potential usefulness of OCT as an adjunct to routine clinical screening for ROP. Our data from this first report focuses on the quality and feasibility of the OCT image capture of awake preterm infants at the bedside and identifies and documents retinal micro-anatomical findings, not routinely identified on clinical screening, in these infants at risk for ROP. The data could expand our understanding, provide new perspectives, and help document age-appropriate macular development associated with preterm birth and ROP. The rigor of the data capture is valuable and supports the knowledge-based applications of BabySTEPS by future ophthalmologists in attempts to advance research and for clinical application. Because the retina and choroid are rapidly changing before term age, demonstrating OCT findings in a treatment-free cohort of infants at a given PMA may contribute to our understanding of maldevelopment related to preterm birth and the relationships among retinal microanatomic features, advanced ROP, and (with data from future vision testing) vision loss. Although this study provides a consistent report of findings, at 36±1 weeks' PMA and before ROP treatment, of vitreous pathology, macular edema, photoreceptor development, and retinal and choroidal layer thicknesses, future work using this methodology at other time points could help enhance our understanding of retinal development, disease progression, and treatment outcomes over time.
Supplementary Material
Supplementary Figure 1: Enrollment, inclusion and exclusion of infants for the current study.
Supplementary Figure 2: Bland-Altman plots showing inter-grader reproducibility of retinal and choroidal layer thicknesses at the foveal center and across the center 1 mm.
Acknowledgments
Financial Support: Grants RO1 EY025009 and P30 EY005722 from the National Eye Institute (NEI); K23 EY02827. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NEI, or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Financial Disclosures: Dr. Toth receives royalties through her university from Alcon and Hemosonics. Dr. Toth has unlicensed and pending patents related to the investigational device and imaging in this study. Drs. Viehland, Izatt and Toth have a patent application pending on the novel handheld probe (investigational handheld UC3 system) described in this manuscript. Dr. Izatt and Duke University have licensed technology to and have a financial interest (including royalty/milestone payments) in Bioptigen/Leica Microsystems Inc., which manufactures handheld and intrasurgical OCT systems. Dr. Vajzovic has received research funding from Heidelberg Engineering Inc., Orbit Biomedical Inc., Novartis and Second Sight Inc. and has served as a consultant to AERI, Alcon, Alimera Sciences, Allergan, Baush and Lomb, DORC, Genentech, Guidepoint, Janssen Pharmaceutical, Orbit Biomedical and Second Sight. No other authors have related conflict of interest. No authors have a proprietary interest in the current study.
References:
- 1.Patel CK. Optical coherence tomography in the management of acute retinopathy of prematurity. Am J Ophthalmol 2006;141(3):582–4. [DOI] [PubMed] [Google Scholar]
- 2.Vinekar A, Sivakumar M, Shetty R, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT+HRA) to image supine non-anaesthetized infants: utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond) 2010;24(2):379–82. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci 2010;51(5):2678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina 2011;31(8):1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology 2009;116(12):2448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothman AL, Tran-Viet D, Gustafson KE, et al. Poorer neurodevelopmental outcomes associated with cystoid macular edema identified in preterm infants in the intensive care nursery. Ophthalmology 2015;122(3):610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman AL, Sevilla MB, Mangalesh S, et al. Thinner Retinal Nerve Fiber Layer in Very Preterm Versus Term Infants and Relationship to Brain Anatomy and Neurodevelopment. Am J Ophthalmol 2015;160(6):1296–308 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado RS, O'Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol 2012;130(5):569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vajzovic L, Hendrickson AE, O'Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol 2012;154(5):779–89 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011;52(8):5183–8. [DOI] [PubMed] [Google Scholar]
- 11.Erol MK, Ozdemir O, Turgut Coban D, et al. Macular findings obtained by spectral domain optical coherence tomography in retinopathy of prematurity. J Ophthalmol 2014;2014:468653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubis AM, Subramaniam CD, Godara P, et al. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 2013;120(8):1665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol 2012;154(5):767–78 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubis AM, Costakos DM, Subramaniam CD, et al. Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol 2012;130(10):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinekar A, Avadhani K, Sivakumar M, et al. Macular edema in premature infants. Ophthalmology 2012;119(6):1288–9 e1; author reply 9–90 e1. [DOI] [PubMed] [Google Scholar]
- 16.Vinekar A, Mangalesh S, Jayadev C, et al. Macular edema in Asian Indian premature infants with retinopathy of prematurity: Impact on visual acuity and refractive status after 1-year. Indian J Ophthalmol 2015;63(5):432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gursoy H, Bilgec MD, Erol N, et al. The macular findings on spectral-domain optical coherence tomography in premature infants with or without retinopathy of prematurity. Int Ophthalmol 2016;36(4):591–600. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado RS, O'Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology 2011;118(12):2315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajzovic L, Rothman AL, Tran-Viet D, et al. Delay in retinal photoreceptor development in very preterm compared to term infants. Invest Ophthalmol Vis Sci 2015;56(2):908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno TA, O'Connell RV, Chiu SJ, et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci 2013;54(6):4140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erol MK, Coban DT, Ozdemir O, et al. Choroidal Thickness in Infants with Retinopathy of Prematurity. Retina 2016;36(6):1191–8. [DOI] [PubMed] [Google Scholar]
- 22.Fierson WM. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaRocca F, Nankivil D, Keller B, et al. Ultra-compact swept-source optical coherence tomography handheld probe with motorized focus adjustment SPIE BiOS, (International Society for Optics and Photonics; )2017. [Google Scholar]
- 26.Viehland C, Chen X, Tran-Viet D, et al. Ergonomic handheld OCT angiography probe optimized for pediatric and supine imaging. Biomed Opt Express 2019;10(5):2623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu SJ, Li XT, Nicholas P, et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express 2010;18(18):19413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LEH, Hellstrom A, Stahl A, et al. Development of a Retinopathy of Prematurity Activity Scale and Clinical Outcome Measures for Use in Clinical Trials. JAMA Ophthalmol 2019;137(3):305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran-Viet D, Wong BM, Mangalesh S, et al. HANDHELD SPECTRAL DOMAIN OPTICAL COHERENCE TOMOGRAPHY IMAGING THROUGH THE UNDILATED PUPIL IN INFANTS BORN PRETERM OR WITH HYPOXIC INJURY OR HYDROCEPHALUS. Retina 2018;38(8):1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zepeda EM, Shariff A, Gillette TB, et al. Vitreous Bands Identified by Handheld Spectral-Domain Optical Coherence Tomography Among Premature Infants. JAMA Ophthalmol 2018;136(7):753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Purohit R, Patel A, et al. In Vivo Foveal Development Using Optical Coherence Tomography. Invest Ophthalmol Vis Sci 2015;56(8):4537–45. [DOI] [PubMed] [Google Scholar]
- 33.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382(9902):1445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis 2001;7:277–82. [PubMed] [Google Scholar]
- 35.Hartnett ME, Toth CA. Experimental Evidence Behind Clinical Trial Outcomes in Retinopathy of Prematurity. Ophthalmic Surg Lasers Imaging Retina 2019;50(4):228–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Enrollment, inclusion and exclusion of infants for the current study.
Supplementary Figure 2: Bland-Altman plots showing inter-grader reproducibility of retinal and choroidal layer thicknesses at the foveal center and across the center 1 mm.