Summary
As the awareness among gastroenterologists regarding endoscopic features suggesting eosinophilic esophagitis is increasing, individuals without symptoms of esophageal dysfunction are increasingly being found to have esophageal eosinophilia on biopsies performed during upper gastrointestinal endoscopies. However, the course of disease and the management of these asymptomatic individuals with esophageal eosinophilia remain elusive.
In this review, we propose a definition of asymptomatic individuals with esophageal eosinophilia and discuss the prevalence, risk factors, and course of disease of this specific patient group. Furthermore, we have established a diagnostic and therapeutic pathway based on the most recent available data.
Keywords: eosinophilic esophagitis, eosinophilic gastrointestinal disorders, eosinophils
INTRODUCTION
Eosinophilic esophagitis (EoE) is a clearly defined chronic inflammatory esophageal disease with increasing incidence and prevalence worldwide.1 EoE is defined by symptoms related to esophageal dysfunction, an eosinophil-predominant inflammation of the esophageal mucosa with ≥15 eosinophils per high-power field (eos/hpf; or ≥60 eos/mm2) and exclusion of other causes of esophageal eosinophilia.2,3 It is key to understand that in patients without any symptoms, but an increased number of eosinophils in the esophageal tissue, a diagnosis of EoE cannot be established.2
In some cases, patients may demonstrate esophageal eosinophilia, even in the absence of esophageal symptoms. There are two clinical scenarios in which this may occur: (1) a routine esophageal biopsy is performed for a nonesophageal complaint; (2) an esophageal biopsy is obtained because of abnormal endoscopic findings. The significance of this asymptomatic esophageal eosinophilia (aEE) is still completely unclear. It is generally accepted that tissue eosinophilia represents a homeostatic response to barrier disruption appearing in various diseases. However, whether this barrier disruption in aEE rather represents a transient phenomenon, a precursor of EoE, a subtle subtype of EoE, or even another inflammatory disease of the esophagus currently remains unknown. Furthermore, data about prevalence, causes, disease course, management, and follow-up in these patients are scarce. Therefore, we performed a literature search using PubMed and Cochrane database with the keywords ‘asymptomatic eosinophilic esophagitis’ and ‘esophageal eosinophilia’. In this article, we evaluated available case series, observational studies, and peer-reviewed reviews. The aim of this review is to define aEE, distinguish it from EoE, and to provide an evidence/experience-based approach on how to address these patients.
DEFINITION OF ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
The definition of aEE implies the absence of any esophagus-related symptoms; therefore, it is crucial to only diagnose aEE after typical and atypical esophagus-related symptoms are excluded by a meticulous medical history. For EoE, a diagnostic cutoff value of 15 eos/hpf was arbitrarily fixed.2 As a healthy esophagus is devoid of eosinophils,4 it must be emphasized that even lower numbers of esophageal eosinophils might indicate pathology.5 Furthermore, current eosinophil thresholds do not definitively distinguish EoE from other diseases associated with esophageal eosinophilia.6–8 In contrast to EoE, for aEE it is currently not clear where we should set the histological cutoff value. However, for the clinician the threshold of 15 eos/hpf in a completely asymptomatic patient poses the greatest challenge, especially because of the critical distinction from EoE.
In this review article, we have therefore defined aEE as a condition with an esophageal eosinophilia with at least 15 eos/hpf without any typical or atypical esophagus-related symptoms. Of note, the term ‘asymptomatic EoE’ is misleading and for symptom-free patients previously diagnosed with EoE the term ‘clinical remission’ should be used.
PREVALENCE OF ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
The still rising prevalence1 of EoE is currently estimated at 10–79 cases/100 000 individuals in westernized areas.9 However, since no strict definition for the term ‘esophageal dysfunction’ is established, many studies confuse EoE with aEE and even with gastroesophageal reflux disease (GERD).10–12 The determination of the true prevalence of aEE, an even more undefined histopathologic entity, is therefore a challenge.
Due to an existing nationwide screening program for gastric cancer, Japan has optimal prerequisites to investigate the population-based prevalence of aEE. Two different Japanese screening program studies demonstrated a prevalence of 60–80 aEE cases/100 000 persons.13,14 However, it is worth mentioning that middle-aged male individuals represent the population most sampled through the national gastric cancer screening program in Japan15 and the same individuals represent the highest risk group for EoE (and possibly aEE).16 As a result, these findings cannot be regarded as purely population-based data. An epidemiological study from China involving 1021 individuals showed a prevalence of 300 aEE cases/100 000 persons.12 This four-fold difference in the prevalence of aEE in the Chinese compared with the Japanese population is remarkable, in particular when considering that in the Chinese study even more women than men were included. Because in Western countries the prevalence of EoE is higher than in Asia,17 it is tempting to speculate that the prevalence for aEE could be higher as well. In a population-based Swedish study 1000 upper endoscopies were performed in the general population and the eosinophil count determined in the distal esophageal epithelium.18 In 1.1% of the participants, eosinophil counts exceeded ≥15 eos/hpf. However, these numbers do not estimate the genuine prevalence of aEE according to our previous definition due to several reasons. First, some of the individuals in this study had dysphagia and were subsequently diagnosed with EoE. Second, and more importantly, half of the patients suffered from troublesome reflux symptoms; consequently, a diagnosis of GERD may have been more appropriate. A smaller trial in a single center in the United States demonstrated similar results with a prevalence of aEE in 0.5% of patients having had an upper endoscopy.19 Taken together, aEE may be much more common than supposed, but accurate population-based data are still lacking.
DIAGNOSTIC STEPS FOR ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
Since individuals with aEE are by definition asymptomatic, the rationale for performing esophageal biopsies is typically routine esophageal biopsies performed for nonesophageal indications or incidental endoscopic findings suspicious for EoE. We therefore start the diagnostic algorithm not with the medical history but with the endoscopy.
First, despite the existence of established endoscopic features of EoE,20,21 these signs are neither pathognomonic for EoE nor for any tissue eosinophilia.22 Therefore, in the presence of typical endoscopic signs of EoE, esophageal biopsies should always be taken.23 Furthermore, in these patients, taking duodenal and gastric biopsies to exclude other causes of esophageal eosinophilia is mandatory.2
Second, a thorough medical history investigating potential swallowing difficulties to avoid misclassifying EoE as aEE is key. Dysphagia is typically a hidden symptom, as patients with dysphagia regularly develop adaptive behaviors. The acronym ‘IMPACT’ (Imbibe fluid with meals, Modify food, Prolong meal times, Avoid hard texture foods, Chew excessively, Turn away tablets/pills) summarizes these adaptive eating habits.24 An Australian study elegantly illustrates the importance of specifically asking the patients about dysphagia, demonstrating an increase of reported dysphagia from 29% to 89% after specifically inquiring patients.25 Furthermore, adult patients with EoE may present with atypical symptoms. Patients can complain about a burning feeling after ingestion of certain foods (food-induced rapid response of the esophagus),26 chest pain,10,25,27 heartburn,10,25 nausea/vomiting,10 or exercise-induced chest pain.28 Additionally, in patients with atypical symptoms other disorders with concomitant esophageal eosinophilia have to be excluded (see Fig. 1). Third, risk factors for aEE should be specifically assessed. Atopic diseases including asthma, aspirin-exacerbated respiratory disease, and IgE-mediated food allergy are associated with both EoE and aEE.29–31 A prospective cohort study in Brazil32 demonstrated 38.2% (34/89) of children with anaphylactic reaction to cow’s milk reaction had esophageal eosinophilia (≥15 eos/hpf). Of these children with esophageal eosinophilia, 29.4% were asymptomatic and 23.5% presented with a normal appearing esophagus. In a recently published study, 21 asymptomatic adult patients with IgE-mediated peanut allergy underwent upper endoscopy before initiation of oral immunotherapy (OIT) and 14% (5/21) of these patients had ≥15 eos/hpf (≥5 eos/hpf were observed in 24% of participants) in esophageal biopsies.33 This is particularly interesting due to the fact that OIT or sublingual immunotherapy (SLIT) may induce EoE in up to 5.3% of OIT subjects.34–36 A follow-up study of the same cohort aimed to characterize gastrointestinal eosinophil responses longitudinally during peanut OIT. OIT induced or exacerbated esophageal eosinophilia (>5 eos/hpf) in 86% (6/7) of the participants, and 57% (4/7) developed esophageal eosinophilia (≥15 eos/hpf) in biopsies 52 weeks after OIT initiation. Only one of these patients developed dysphagia and food impaction, meeting the clinicopathologic diagnostic criteria for EoE.37,38 These results suggest that in patients with IgE-mediated food allergies OIT may provoke aEE or even EoE in patients without tissue eosinophilia at baseline. Of note, most of the patients remained asymptomatic during OIT despite their esophageal eosinophilia, and, in general, the degree of esophageal infiltration was mild and resolved at the end of OIT. This is in line with previous studies indicating that in some cases OIT/SLIT-induced EoE may be transient or reversible.39–41
Fig. 1.
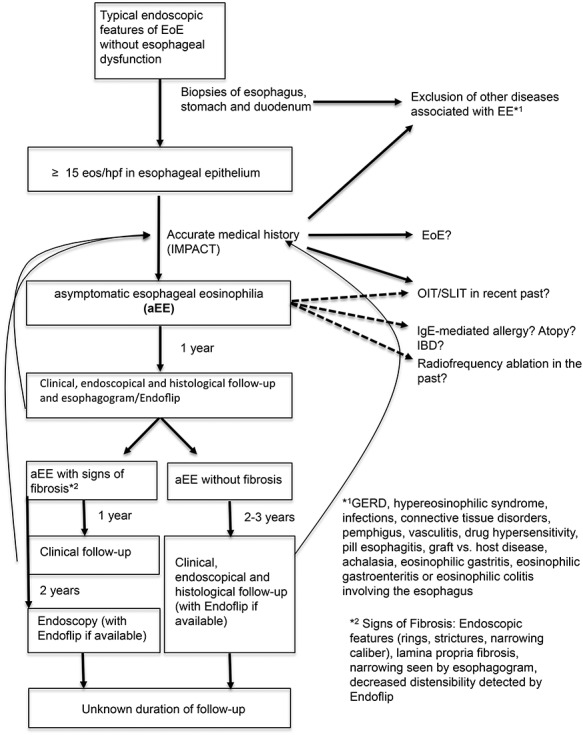
Management algorithm for asymptomatic esophageal eosinophilia.
A common thread connecting atopic disease, aEE, and EoE is the presence epithelial barrier dysfunction.37,42,43 Antigen exposure in this context elicits inflammatory responses that may result in persistent barrier defects. This is also true for other potential causes of esophageal eosinophilia including celiac disease, Crohn’s disease, or even patients presenting after endoscopic ablation of Barrett’s esophagus.44–48 Indeed, eosinophils may be recruited to the esophageal epithelium initially as a homeostatic response to barrier disruption and dysregulation of this response ultimately results in pathology.49 Importantly, the specificity of the eosinophilic response is not restricted to EoE. In summary, when evaluating an aEE a specific inquiry regarding subtle esophageal symptoms and the exclusion of differential diagnoses are key.
CLINICAL FEATURES OF ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
Only one trial systematically investigated the differences of the clinical features in patients with aEE and EoE.50 The main difference was that patients with aEE were less likely to have concomitant allergic diseases than EoE patients (44 vs. 76%, P < 0.01). Interestingly, endoscopic findings were not different in aEE and EoE, underlining again that typical signs such as furrows, rings, and even exudates are not pathognomonic for EoE. However, there was a trend to significance regarding more rings in EoE patients (56 vs. 36%, P = 0.07). Furthermore, besides peak eosinophilic infiltration, pathologic features, such as basal cell hyperplasia, spongiosis, and lamina propria fibrosis, were not different in aEE compared with EoE patients.
In the aEE group, the eosinophilic inflammation responded better to proton pump inhibitor (PPI) therapy (90.5%) compared with patients with EoE (69.1%, P < 0.05). It can be speculated whether asymptomatic GERD may have been an important driver in a considerable fraction of these aEE individuals or whether aEE patients have a better histological response to PPI independent of concomitant GERD.
Altogether, aEE and EoE cannot be distinguished endoscopically or histologically. The question why aEE individuals respond better to PPI compared with patients with EoE remains open.
NATURAL COURSE OF ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
It is well established that untreated chronic inflammation in EoE may progress to fibrosis resulting in a structural and functional damage of the esophagus.51,52 However, today it remains unknown whether eosinophilic inflammation represents the driving force underlying the remodeling or if it is rather an interaction of other inflammatory cells via cytokines and the epithelial barrier disruption that contribute mainly to the progression of fibrosis.53 The fact that Ishimura et al50 found in three quarters of their aEE patients substantial lamina propria fibrosis would support the notion that eosinophilic inflammation alone without symptoms might be sufficient to provoke fibrosis and stricture formation. This point is further reinforced when considering that aEE patients have decreased compliance to esophageal distention.54 However, it is unknown whether aEE patients with fibrosis will have an unfavorable disease course with more complications.
In addition, it is currently unknown if patients with aEE have a higher risk to develop EoE than the general population. In the study of Ishibashi et al,14 a fifth of aEE patients developed symptomatic EoE during a follow-up of 7 years and 40% remained asymptomatic but showed more pronounced endoscopic findings such as diffuse distribution of linear furrowing. Younger age and mucosal edema were significant risk factors for progression from aEE to symptomatic EoE. However, we have to keep in mind that in Japan EoE generally has a milder course without fibrostenotic complications and a high rate of PPI response13,55; the findings of this study cannot therefore simply be generalized to Western patients. Further data in Western aEE individuals are eagerly awaited to clarify to question whether the natural course of aEE results in significant fibrosis.
MANAGEMENT AND FOLLOW-UP OF PATIENTS WITH ASYMPTOMATIC ESOPHAGEAL EOSINOPHILIA
Do patients with aEE need any treatment and how should we monitor these patients? Before beginning any treatment, it is essential to establish treatment goals. Unlike in EoE, in which the aim of treatment is an improvement in quality of life56 and prevention of fibrosis and its sequelae,52 in aEE the treatment endpoint is elusive. Due to the fact that aEE individuals are by definition asymptomatic, improvements in quality of life cannot be the goal. What remains is the goal to prevent fibrosis and its consequences. However, current data are too weak to conclude that aEE will progress to fibrosis. Hence, empiric treatment is not justified. Furthermore, one is faced with the question, even unanswered in EoE, which presently available medication (PPI or swallowed topical corticosteroids [STC]) would be best in achieving this specific goal of fibrosis prevention. Should we at least treat aEE individuals that present with endoscopic findings indicating fibrosis (rings or strictures) or histologically fibrosis in the lamina propria? Unfortunately, due to lack of data there is presently no suitable answer to this question either. Although the study by Ishimura et al50 demonstrated that in 90% of patients, eosinophil counts in the esophageal epithelium decreased significantly <15 eos/hpf after starting PPI therapy, there exists no evidence about regression of fibrosis. Additionally, long-term data on this topic are completely lacking. Hence, without more data regarding natural course of aEE, we do not support the idea of giving routinely PPI or STC in aEE patients. However, we emphasize that patients with minimal symptoms should be categorized as EoE and not aEE and should be treated accordingly.57,58
The second key question is whether aEE individuals should have regular follow-up or not. Based on the limited data currently available, we recommend monitoring aEE patients for the following two reasons: (1) we do not understand the condition of aEE well enough, so that a clinical monitoring including a thorough medical history focusing on eating behavior is important in order to detect an early form of EoE; (2) it is known that if untreated EoE may lead to esophageal remodeling with stricture formation.51,52 We further know that symptoms correlate poorly with histological activity in EoE patients.59 Endoscopic monitoring is therefore standard of care for EoE patients.60 However, in contrast to monitoring in EoE, in which, beyond the endoscopic inspection, a histologic evaluation is the gold standard, in aEE the clinically relevant determinants are unknown. For aEE, no eosinophilic threshold exists for determining whether the individual is at risk for developing fibrosis. If we focus on evaluation of fibrosis, next to endoscopic features and histological evaluation of lamina propria, patients may have surveillance with esophagogram in order to search for strictures61 or with a newer option, an endoluminal functional lumen imaging probe (EndoFLIP)62 to evaluate the distensibility of the esophagus. However, the repeated exposure to radiation required for esophagrams and the expertise, cost, and lack of long-term data associated with EndoFLIP, limit the utility of these surveillance modalities in daily practice. In other words, currently we do not have safe, readily available, evidence-based assessment tools to monitor for the potential development of fibrosis.
We propose the following treatment algorithm for managing patients who are incidentally found to have aEE (see Fig. 1 and Table 1 for special situations). We emphasize that these recommendations are based on expert opinion only. After diagnosing aEE, we recommend in general no empiric treatment but a regular follow-up. As long as the patient remains asymptomatic, we suggest scheduling the first clinical and endoscopic follow-up within 1 year regardless of the presence of fibrosis. However, if any new symptoms will appear, an earlier endoscopy is necessary. If available, an esophagram or EndoFLIP to evaluate potential fibrosis should be performed. If fibrosis is present (endoscopic signs, such as rings or strictures; histological fibrosis of lamina propria; esophageal strictures identified by esophagram; or decreased esophageal distensibility by EndoFLIP) clinical follow-up should be performed annually. If tissue eosinophilia persists, we recommend these patients have further endoscopic follow-up evaluations at least every 2 years to monitor for worsening fibrosis, preferably with EndoFLIP. In aEE patients without fibrosis, the interval of the follow-up visits can be extended to every 2–3 years. However, patients must be made aware that any new symptoms have to be reported to their physician immediately. Discontinuation of surveillance should be evaluated on a case-by-case basis.
Table 1.
Specific situations
| Specific situation | Management |
|---|---|
| Normal esophageal endoscopic finding with aEE |
|
| aEE with stricture at index endoscopy |
|
| aEE with risk factors for EoE (positive family history, atopic disease) |
|
| aEE with esophageal eosinophilia limited to the distal esophagus |
|
aEE, asymptomatic esophageal eosinophilia; EndoFLIP, endoluminal functional lumen imaging probe; EoE, eosinophilic esophagitis; GERD, gastroesophageal reflux disease.
CONCLUSION
Increased knowledge of typical EoE features among endoscopists will likely lead to increased detection of aEE. Since some patients have long-standing disease resulting in behavioral adaptations, the most important step is a detailed history focusing on subtle swallowing problems and gastrointestinal symptoms that may have been missed. After exclusion of alternative diagnoses, a diagnosis of aEE can be established. Although no long-term therapy is necessary, the possibility of a progressive disease with fibrosis formation must be kept in mind, so that regular monitoring, both clinically and endoscopically, seems justified. Finally we underscore that more studies on aEE are needed to shed light on the natural course of this condition and to put management and potential treatments strategies on a more robust base.
Specific author contributions
Substantial contributions to the conception or design of the work, drafting the work, approved the final version: Philipp Schreiner.
Interpretation of data for the work, revising it critically for important intellectual content, approved the final version: Luc Biedermann.
Interpretation of data for the work, revising it critically for important intellectual content, approved the final version: Thomas Greuter.
Interpretation of data for the work, revising it critically for important intellectual content, approved the final version: Benjamin L Wright.
Substantial contributions to the conception or design of the work, drafting the work, approved the final version: Alex Straumann.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Philipp Schreiner, Department of Gastroenterology & Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Luc Biedermann, Department of Gastroenterology & Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Thomas Greuter, Department of Gastroenterology & Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Benjamin L Wright, Division of Allergy, Asthma, and Clinical Immunology, Department of Medicine, Mayo Clinic Arizona, Scottsdale, AZ, USA; Division of Pulmonology, Phoenix Children's Hospital, Phoenix, AZ, USA.
Alex Straumann, Department of Gastroenterology & Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
References
- 1. Navarro P, Arias A, Arias-Gonzalez L et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019; 49: 1116–25. [DOI] [PubMed] [Google Scholar]
- 2. Lucendo A J, Molina-Infante J, Arias A et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017; 5: 335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dellon E S, Liacouras C A, Molina-Infante J et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the agree conference. Gastroenterology 2018; 155: 1022–33 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kato M, Kephart G M, Talley N J et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 1998; 252: 418–25. [DOI] [PubMed] [Google Scholar]
- 5. Reed C C, Wolf W A, Cotton C C et al. Optimal histologic cutpoints for treatment response in patients with eosinophilic esophagitis: analysis of data from a prospective cohort study. Clin Gastroenterol Hepatol 2018; 16: 226–33 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dellon E S, Gibbs W B, Fritchie K J et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009; 7: 1305–13 quiz 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodrigo S, Abboud G, Oh D et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103: 435–42. [DOI] [PubMed] [Google Scholar]
- 8. Dellon E S, Aderoju A, Woosley J T, Sandler R S, Shaheen N J. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol 2007; 102: 2300–13. [DOI] [PubMed] [Google Scholar]
- 9. Limketkai B N, Shah S C, Hirano I, Bellaguarda E, Colombel J F. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut 2019; 68: 2152–60. [DOI] [PubMed] [Google Scholar]
- 10. Kapel R C, Miller J K, Torres C, Aksoy S, Lash R, Katzka D A. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134: 1316–21. [DOI] [PubMed] [Google Scholar]
- 11. Veerappan G R, Perry J L, Duncan T J et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7: 420–6 6 e1-2. [DOI] [PubMed] [Google Scholar]
- 12. Ma X, Xu Q, Zheng Y et al. Prevalence of esophageal eosinophilia and eosinophilic esophagitis in adults: a population-based endoscopic study in Shanghai, China. Dig Dis Sci 2015; 60: 1716–23. [DOI] [PubMed] [Google Scholar]
- 13. Sato H, Honma T, Nozawa Y et al. Eosinophilic esophagitis in Japanese patients: a mild and slow-progressing disorder. PLoS One 2018; 13: e0206621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishibashi F, Fukushima K, Onizuka R, Tanaka R. Risk of progression to eosinophilic esophagitis in patients with asymptomatic esophageal eosinophilia: a retrospective pilot study. JGH Open 2020; 4: 422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato N, Ito Y, Ioka A, Tanaka M, Tsukuma H. Gender differences in stomach cancer survival in Osaka, Japan: analyses using relative survival model. Jpn J Clin Oncol 2009; 39: 690–4. [DOI] [PubMed] [Google Scholar]
- 16. Arias A, Perez-Martinez I, Tenias J M, Lucendo A J. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016; 43: 3–15. [DOI] [PubMed] [Google Scholar]
- 17. Ito J, Fujiwara T, Kojima R, Nomura I. Racial differences in eosinophilic gastrointestinal disorders among Caucasian and Asian. Allergol Int 2015; 64: 253–9. [DOI] [PubMed] [Google Scholar]
- 18. Ronkainen J, Talley N J, Aro P et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56: 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sealock R J, Kramer J R, Verstovsek G et al. The prevalence of oesophageal eosinophilia and eosinophilic oesophagitis: a prospective study in unselected patients presenting to endoscopy. Aliment Pharmacol Ther 2013; 37: 825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dellon E S, Cotton C C, Gebhart J H et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol 2016; 14: 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wechsler J B, Bolton S M, Amsden K et al. Eosinophilic esophagitis reference score accurately identifies disease activity and treatment effects in children. Clin Gastroenterol Hepatol 2018; 16: 1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hori K, Watari J, Fukui H et al. Do endoscopic features suggesting eosinophilic esophagitis represent histological eosinophilia? Dig Endosc 2014; 26: 156–63. [DOI] [PubMed] [Google Scholar]
- 23. Kim H P, Vance R B, Shaheen N J, Dellon E S. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 988–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirano I, Furuta G T. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Gastroenterology 2020; 158: 840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Croese J, Fairley S K, Masson J W et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc 2003; 58: 516–22. [DOI] [PubMed] [Google Scholar]
- 26. Biedermann L, Holbreich M, Atkins D et al. Food-induced immediate response of the esophagus - a newly identified syndrome in patients with eosinophilic esophagitis. Allergy 2020. [DOI] [PubMed] [Google Scholar]
- 27. Remedios M, Campbell C, Jones D M, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006; 63: 3–12. [DOI] [PubMed] [Google Scholar]
- 28. Kahn J, Bussmann C, Beglinger C, Straumann A, Hruz P. Exercise-induced chest pain: an atypical manifestation of eosinophilic esophagitis. Am J Med 2015; 128: 196–9. [DOI] [PubMed] [Google Scholar]
- 29. Capucilli P, Cianferoni A, Grundmeier R W, Spergel J M. Comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large population. Ann Allergy Asthma Immunol 2018; 121: 711–6. [DOI] [PubMed] [Google Scholar]
- 30. Erkman J, Vaynblat A, Thomas K et al. Airway and esophageal eosinophils in children with severe uncontrolled asthma. Pediatr Pulmonol 2018; 53: 1598–603. [DOI] [PubMed] [Google Scholar]
- 31. Eid R C, Palumbo M L, Laidlaw T M, Buchheit K M, Cahill K N. A retrospective analysis of esophageal eosinophilia in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019; 7: 1338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbosa A C, Castro F M, Meireles P R et al. Eosinophilic esophagitis: latent disease in patients with anaphylactic reaction to cow's milk. J Allergy Clin Immunol Pract 2018; 6: 451–6 e1. [DOI] [PubMed] [Google Scholar]
- 33. Wright B L, Fernandez-Becker N Q, Kambham N et al. Baseline gastrointestinal eosinophilia is common in oral immunotherapy subjects with IGE-mediated peanut allergy. Front Immunol 2018; 9: 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petroni D, Spergel J M. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol 2018; 120: 237–40 e4. [DOI] [PubMed] [Google Scholar]
- 35. Miehlke S, Alpan O, Schroder S, Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol 2013; 7: 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucendo A J, Arias A, Tenias J M. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014; 113: 624–9. [DOI] [PubMed] [Google Scholar]
- 37. Wright B L, Fernandez-Becker N Q, Kambham N et al. Gastrointestinal eosinophil responses in a longitudinal, randomized trial of peanut oral immunotherapy. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chinthrajah R S, Purington N, Andorf S et al. Sustained outcomes in oral immunotherapy for peanut allergy (poised study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019; 394: 1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cafone J, Capucilli P, Hill D A, Spergel J M. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol 2019; 19: 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez M J, Ibanez M D. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol 2012; 129: 1155–7. [DOI] [PubMed] [Google Scholar]
- 41. Goldberg M R, Nachshon L, Levy M B, Elizur A, Katz Y. Risk factors and treatment outcomes for oral immunotherapy-induced gastrointestinal symptoms and eosinophilic responses (OITIGER). J Allergy Clin Immunol Pract 2020; 8: 125–31. [DOI] [PubMed] [Google Scholar]
- 42. Hellings P W, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 2020; 145: 1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rochman M, Azouz N P, Rothenberg M E. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol 2018; 142: 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugita K, Kabashima K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J Leukoc Biol 2020; 107: 749–62. [DOI] [PubMed] [Google Scholar]
- 45. Schumann M, Siegmund B, Schulzke J D, Fromm M. Celiac disease: role of the epithelial barrier. Cell Mol Gastroenterol Hepatol 2017; 3: 150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chelakkot C, Ghim J, Ryu S H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018; 50: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Owens V L, Katzka D A, Lutzke L S, Wang K K, Smyrk T C. Endoscopic ablative therapy for Barrett's esophagus: a potential cause of eosinophilic esophagitis. Dis Esophagus 2012; 25: 33–9. [DOI] [PubMed] [Google Scholar]
- 48. Halsey K D, Arora M, Bulsiewicz W J et al. Eosinophilic infiltration of the esophagus following endoscopic ablation of Barrett's neoplasia. Dis Esophagus 2013; 26: 113–6. [DOI] [PubMed] [Google Scholar]
- 49. Lee J J, Jacobsen E A, McGarry M P, Schleimer R P, Lee N A. Eosinophils in health and disease: the liar hypothesis. Clin Exp Allergy 2010; 40: 563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishimura N, Sumi S, Okada M et al. Is asymptomatic esophageal eosinophilia the same disease entity as eosinophilic esophagitis? Clin Gastroenterol Hepatol 2019; 17: 1405–7. [DOI] [PubMed] [Google Scholar]
- 51. Schoepfer A M, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145: 1230–6 e2. [DOI] [PubMed] [Google Scholar]
- 52. Dellon E S, Kim H P, Sperry S L et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79: 577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muir A B, Wang J X, Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol 2019; 54: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kinoshita Y, Oouchi S, Fujisawa T. Eosinophilic gastrointestinal diseases - pathogenesis, diagnosis, and treatment. Allergol Int 2019; 68: 420–9. [DOI] [PubMed] [Google Scholar]
- 55. Abe Y, Iijima K, Ohara S et al. A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol 2011; 46: 25–30. [DOI] [PubMed] [Google Scholar]
- 56. Safroneeva E, Balsiger L, Hafner D et al. Adults with eosinophilic oesophagitis identify symptoms and quality of life as the most important outcomes. Aliment Pharmacol Ther 2018; 48: 1082–90. [DOI] [PubMed] [Google Scholar]
- 57. Lieberman J A. Minimally symptomatic patients with eosinophilic esophagitis should still be actively treated-con. Ann Allergy Asthma Immunol 2019; 122: 574–5. [DOI] [PubMed] [Google Scholar]
- 58. Muir A, Moore H, Spergel J M. Minimally symptomatic patients with eosinophilic esophagitis should still be actively treated-pro. Ann Allergy Asthma Immunol 2019; 122: 572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Safroneeva E, Straumann A, Coslovsky M et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150: 581–90 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Godwin B, Wilkins B, Muir A B. Eoe disease monitoring: where we are and where we are going. Ann Allergy Asthma Immunol 2020; 124: 240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Menard-Katcher C, Swerdlow M P, Mehta P, Furuta G T, Fenton L Z. Contribution of esophagram to the evaluation of complicated pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2015; 61: 541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hirano I, Pandolfino J E, Boeckxstaens G E. Functional lumen imaging probe for the management of esophageal disorders: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol 2017; 15: 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


