Abstract
A wide variety of mental disorders have been associated with resting-state functional network alterations, which are thought to contribute to the cognitive changes underlying mental illness. These observations appear to support theories postulating large-scale disruptions of brain systems in mental illness. However, existing approaches isolate differences in network organization without putting those differences in a broad, whole-brain perspective. Using a graph distance approach—connectome-wide similarity—we found that whole-brain resting-state functional network organization is highly similar across groups of individuals with and without a variety of mental diseases. This similarity was observed across autism spectrum disorder, attention-deficit hyperactivity disorder, and schizophrenia. Nonetheless, subtle differences in network graph distance were predictive of diagnosis, suggesting that while functional connectomes differ little across health and disease, those differences are informative. These results suggest a need to reevaluate neurocognitive theories of mental illness, with a role for subtle functional brain network changes in the production of an array of mental diseases. Such small network alterations suggest the possibility that small, well-targeted alterations to brain network organization may provide meaningful improvements for a variety of mental disorders.
Keywords: attention-deficit hyperactivity disorder, autism spectrum disorder, functional connectivity, resting state, schizophrenia
Introduction
Cognitive dysfunction (broadly construed to include perceptual, attentional, memory-based, emotional, and motor capabilities) is seen in a range of mental disorders and can significantly impact a patient’s well-being. Indeed, mental disorders are defined as harmful dysfunction (Wakefield 2007), such that all mental disorders involve cognitive dysfunction by definition. As a neural correlate of cognitive impairment (Greicius 2008; Zhang and Raichle 2010), abnormal resting-state functional connectivity (RSFC) has been used to identify neural mechanisms underlying mental illness (for review see Cole, Repovš, et al. 2014b). RSFC has strong potential for providing important insights in this area, given its relationship with a variety of cognitive abilities (Cole et al. 2011; Smith et al. 2015; Shen et al. 2017), its generalization to a variety of task states (Cole, Bassett, et al. 2014a; Krienen et al. 2014), and recent findings indicating that RSFC describes the routes of cognitive information flow during task performance (Cole et al. 2016; Ito et al. 2017). Indeed, this method has already provided promising results in the search for biomarkers in several psychiatric disorders. For example, combined with graph theory measures, machine learning approaches have successfully classified patient and control subjects for a range of disorders (Yahata et al. 2017).
Many theories postulate large-scale disruption of brain systems in psychiatric disorders (Uhlhaas and Singer 2011), typically driven by widespread neurotransmitter dysfunction (Olney et al. 1999; Risch et al. 2009; Nakic et al. 2010; Miller et al. 2013). However, such large-scale disruptions of brain systems have not been thoroughly tested. This is largely due to a fundamental aspect of methodology used in clinical studies, wherein only differences among patients and healthy controls are emphasized rather than both differences and similarities. Yet, taking similarities into account is essential for gaining perspective on the nature and severity of the neural changes underlying mental disorders. For instance, it is possible that a widespread neurotransmitter dysfunction causes a given disorder, but only by subtly altering the overall network organization. This would be important to know for understanding the underlying causes of the disorder and for developing treatments.
Here, we therefore take a comprehensive whole-connectome perspective using a simple graph distance approach—connectome-wide similarity (Schultz and Cole 2016)—that quantifies changes in all functional connections at once, providing information about the overall pattern of intrinsic network architecture. In this way, we were able to compare RSFC patterns across several psychiatric disorders and healthy control groups and derive measures of functional network pattern (dis)similarity. We initially developed this approach to compare task-evoked functional connectomes with resting-state functional connectomes (Cole, Bassett, et al. 2014a; Schultz and Cole 2016), but we apply it here to compare clinical connectomes with healthy control connectomes in the resting state. This provides a new way of quantifying brain function and neural system organization in psychiatric disorders.
We (and others) recently found that task-related changes to functional network organization are small relative to the overall functional network organization during rest and a variety of tasks (Cole, Bassett, et al. 2014a; Krienen et al. 2014). This suggests that meaningful changes in cognition (in this case task-related cognitive differences) correspond with small functional network changes. Further, the large majority of patients appear to maintain most basic cognitive abilities enjoyed by healthy individuals (e.g., the ability to recognize common objects, navigate a room, produce speech, and read simple sentences). Building on this logic, we hypothesized that the overall intrinsic functional network architecture—which appears to support these cognitive abilities via its particular network organization (Smith et al. 2009; Cole et al. 2016; Tavor et al. 2016; Ito et al. 2017)—would be similar across patients, and between patient and control subjects. We tested this idea across a range of patient datasets varying in diagnosis, age, and symptom severity. Importantly, even with high cross-group similarity, between-group differences can still indicate meaningful changes in functional network architecture. To test this, we predicted group membership (clinical vs. control) for each participant using a classification analysis based on RSFC pattern similarity to either group.
Testing these hypotheses about whole-brain intrinsic architecture pattern similarity across disorders—here including autism spectrum disorder (ASD), attention-deficit hyperactivity disorder (ADHD), and schizophrenia—would enable us to put disease-related RSFC alterations in perspective and possibly help shape future theories of the neural basis of cognitive deficits in these populations.
Materials and Methods
Datasets and Participants
Subjects from four different datasets were included in the present study to facilitate generalization of findings across mental disorders. ADHD subjects are part of the publicly available ADHD-200 dataset from NYU Langone Medical Center (http://fcon_1000.projects.nitrc.org/indi/adhd200/), which includes 123 patients diagnosed with ADHD and 99 healthy control subjects (presence or absence of an ADHD diagnosis based on evaluations with the K-SADS-PL (Kaufman et al. 1997) and the CPRS-LV (Gurley 2011). Subjects with low-quality resting-state or anatomical data, as determined by the ADHD-200 initiative’s quality assessment based on visual time series inspection, were excluded from our sample. Resting-state and anatomical data of the remaining 87 healthy control subjects and 93 ADHD subjects were preprocessed (age range 7–18 years). ASD subjects were part of the publicly available ABIDE dataset from NYU Langone Medical Center (http://fcon_1000.projects.nitrc.org/indi/abide/abide_I.html). Inclusion as a patient in the study required a clinician’s DSM-IV-TR diagnosis of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder Not-Otherwise-Specified, which was supported by review of available records, an Autism Diagnostic Observation Schedule, review of the participant’s history, and when possible, an Autism Diagnostic Interview-Revised. Following these inclusion criteria, 79 ASD and 105 control subjects were included for preprocessing (age range 6.5–39.1 years). The ADHD and ASD samples in this study were collected at the same site and could therefore be directly compared in our analyses without site differences as confounds.
In addition to these two samples with neurodevelopmental disorders, two schizophrenia samples were included in the present study. The first was collected at Yale University and consisted of 90 schizophrenia patients and 90 healthy control subjects (age range 17–65 years). Patients were identified through outpatient clinics and community mental health facilities (Anticevic et al. 2013). The second sample is the publicly available COBRE schizophrenia dataset, with 72 schizophrenia patients and 75 healthy control subjects (age range 18–65 years). Each patient completed the Structured Clinical Interview for DSM-IV Axis I disorders (First et al. 2002) to confirm their diagnosis (see http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html for exclusion criteria). The number of subjects in the final sample is displayed in Table 1.
Table 1.
Clinical and demographic characteristics
| SCZ1 (Yale) | SCZ2 (COBRE) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | Control | Significance | Schizophrenia | Control | Significance | |||||||
| N | 87 | 90 | 59 | 70 | ||||||||
| M | SD | M | SD |
T-value /χ 2 |
P-value (Two-tailed) |
M | SD | M | SD |
T-value /χ 2 |
P-value (Two-tailed) |
|
| Age | 33.0 | 11.4 | 30.7 | 12.0 | −1.3 | 0.2 | 37.2 | 14.1 | 35.8 | 11.7 | −0.6 | 0.6 |
| Gender %male | 65.6 | 72.4 | 1.0 | 0.3 | 18.6 | 31.4 | 2.7 | 0.1 | ||||
| Education | 13.2 | 2.2 | 15.2 | 2.2 | 6.2 | <0.00001 | 4.0 | 1.4 | 4.7 | 1.3 | 2.7 | 0.008 |
| Educ. Mother | 13.4 | 2.9 | 14.0 | 2.8 | 1.3 | 0.2 | ||||||
| Educ. Father | 13.7 | 3.5 | 14.4 | 3.2 | 1.4 | 0.2 | ||||||
| Ed. Primary Caretaker |
4.3 4 |
2.2 | 4.7 | 1.9 | 1.1 | 0.3 | ||||||
| Hand. %right | 85.1 | 88.9 | 0.6 | 0.4 | 84.7 | 95.7 | 4.6 | 0.03 | ||||
| IQ missing |
97.8 2 |
15.3 | 106.8 7 |
8.9 | 4.6 | <0.0001 | ||||||
| CPZE missing |
228.3 0 |
197.0 | 352.8 1 |
307.7 | ||||||||
| Symptom score | 60.1 | 14.0 | 58.3 | 13.7 | ||||||||
| PANSS Pos | 15.7 | 4.8 | 14.5 | 4.8 | ||||||||
| PANSS Neg | 14.2 | 5.4 | 14.5 | 4.9 | ||||||||
| PANSS Gen | 30.2 | 7.1 | 29.2 | 8.2 | ||||||||
| ADHD-200 | ABIDE | |||||||||||
| ADHD | Control | Significance | ASD | Control | Significance | |||||||
| N | 91 | 84 | 79 | 105 | ||||||||
| M | SD | M | SD | T-value/χ 2 | P-value (Two-tailed) | M | SD | M | SD | T-value/χ2 | P-value (Two-tailed) | |
| Age | 11.5 | 2.7 | 12.6 | 3.1 | 2.3 | 0.02 | 14.5 | 7.0 | 15.8 | 6.3 | 1.3 | 0.19 |
| Gender %male | 75.6 | 50.0 | 12.2 | 0.0005 | 86.1 | 75.2 | 3.3 | 0.07 | ||||
| Education | ||||||||||||
| Hand. score | 65.8 | 25.9 | 62.0 | 24.6 | −1.0 | 0.33 | 41.1 | 50.4 | 65.2 | 27.6 | 4.1 | <0.00001 |
| IQ missing |
106.8 3 |
15.0 | 111.5 7 |
14.5 | 2.0 | 0.04 | 107.9 0 |
16.6 | 113.2 0 |
13.1 | 2.4 | 0.02 |
| on medication missing |
20 51 |
0 0 |
18 0 |
0 0 |
||||||||
| Symptom score missing | 71.6 2 |
8.9 | 45.1 2 |
6.1 | −22.5 | <.000001 | 11.3 0 |
4.1 | ||||
Clarification: IQ: WASI (Wechsler Abbreviated Scale of Intelligence) scores were available for ADHD and ASD, WAIS for the Yale dataset.
Education: Yale: education in years; COBRE: 1 = Grade 6 or less, 2 = Grade 7–11, 3 = high school graduate, 4 = attended college, 5 = graduated 2 years college, 6 = graduated 4 years college.
Symptom scores: ADHD index (ADHD patients and control subjects), ADOS total (ASD patients), PANSS total (Schizophrenia patients).
Hand: Handedness score for ADHD and ASD datasets, percentage right-handed subjects for SCZdata.
PANSS: Positive, negative, and general symptom scores for schizophrenia patients.
CPZE: Chlorpromazine equivalence; measure to compare dosage of different types of antipsychotics.
Missing: Number of subjects for which data are not available. Only mentioned when relevant.
The data were collected by multiple research groups and the type of clinical information collected was dependent on diagnosis (ADHD vs. ASD vs. schizophrenia). Consequently, diagnosis-specific symptom scores were available for each dataset: ADHD-index for ADHD patients, Autism Diagnostic Observation Schedule (ADOS) for ASD, and Positive and Negative Symptom Scale (PANSS) for Schizophrenia were included (see Table 1 for mean symptom scores). As expected, the mean ADHD index is higher in ADHD patients than control subjects (t(1,169) = −22.5, P < 0.00001, d = 3.2). No symptom scores were available for the other control groups. Comorbidity was reported for 30 ADHD patients and 41 ASD patients, and no comorbidity data were available for the schizophrenia groups. However, the COBRE dataset excludes all neurological disorders, substance abuse disorders (excluding nicotine), and intellectual impairment among schizophrenia patients and healthy controls. The COBRE dataset also excluded individuals using medications for ADHD, anxiety, or depression, or who have a “current or past psychiatric disorder” from the healthy control group.
Since our main focus in this study is on similarity of FC architecture across patients and healthy control subjects regardless of variation in phenotypic factors, groups were not specifically matched by age, gender, and IQ, although matching for age and gender was done at the time of collection for all available datasets. In the final sample, lower IQ scores were found in ADHD (t(1,169) = 2.0, P = 0.04, d = 0.32), ASD (t(1,182) = 2.4, P = 0.02, d = 0.35), and Yale schizophrenia patients (t(1,166) = 4.63, P < 0.0001, d = 0.72) than in control subjects of those groups. No IQ scores were available for the COBRE schizophrenia sample. However, education scores were significantly different between patients and control subjects (t(1,127) = 2.7, P = 0.008, d = 0.48) for that sample, as expected. ADHD subjects in the final sample for analysis were slightly younger than the controls (t(1,169) = 2.3, P = 0.02, d = 0.35), but there were no significant age differences in the other data samples. Note that lower IQ is associated with schizophrenia (Khandaker et al. 2011), ASD (Volkmar et al. 2004), and ADHD (Kuntsi et al. 2004), and so such differences in these samples were entirely expected.
Neuroimaging Acquisition
The resting-state fMRI data for ADHD and ASD patients and healthy control subjects were acquired on a Siemens Magnetom Allegra 3.0 Tesla MRI scanner. Participants were asked to remain still with their eyes closed but without falling asleep. For the resting state multiecho EPI images, 33 slices were acquired every 2000 ms (FOV = 240 x 192 mm, TE = 15 ms, flip angle = 90°, voxel size 3 x 3 x 4 mm3) with a total of 180 volumes per run.
Functional images for the SCZ1 (Yale) dataset were collected using a 3-T Siemens Allegra scanner. A total of 210 volumes were acquired for each participant in the Yale schizophrenia dataset, with a TR of 1500 ms, fov = 220 mm, TE = 27 ms, flip angle = 60°, and voxel size 3.43 x 3.43 x 4, eyes open (Anticevic et al. 2014).
For the SCZ2 (COBRE) dataset, a 3-T Siemens Trio Tim scanner was used to acquire 33 slices every 2000 ms (TE = 29 ms, flip angle = 75°, fov = 240 mm, voxel size 3 x 3 x 4 mm3) with a total of 150 volumes per run. Participants were asked to keep their eyes open. A T1-weighted MPRAGE image was collected for each participant for all datasets.
Preprocessing
Preprocessing was performed using Freesurfer (to identify ventricles, white matter, gray matter, and anatomical structures) (Destrieux et al. 2010), FSL’s FLIRT for brain image alignment (Smith et al. 2004), Matlab 2014b for bandpass filtering and nuisance regression, and AFNI (Cox 1996) for all other preprocessing steps. Volume analysis was performed on all datasets. Functional images underwent slice-time correction, alignment (with the anatomical image and MNI template), removal of first 6 s of data, motion scrubbing (censoring volumes with high motion based on framewise displacement with a 0.5 mm threshold, see Power et al. 2012), time series extraction, nuisance regression (removal of six motion estimates, ventricle and white matter signals, and their derivatives), bandpass filtering (0.008–0.09 Hz), and spatial smoothing (FWHM = 6 mm).
An additional cortical surface-based analysis was performed with the Yale schizophrenia sample (which was also preprocessed using the volume-based approach). The surface-based analysis involved using the HCP minimal preprocessing pipeline (Glasser et al. 2013), followed by removal of the first 6 s of data, motion scrubbing (using framewise displacement > 0.5 mm), time series extraction, nuisance regression, and temporal filtering. Note that obtaining similar results to the main analyses with this distinct preprocessing stream would indicate robustness of results to particular analysis choices.
Alternative Motion Correction
As an alternative preprocessing approach correcting for motion artifacts, we adopted the “36P” nuisance regression method as evaluated in Ciric et al. (2017), also see (Satterthwaite et al. 2013) in a follow-up (volume-based) analysis. This method was previously found to be especially effective in limiting the impact of motion artifacts on functional connectivity data. In addition to removal of six motion estimates, ventricle and white matter signals, and their derivatives, this method involves removal of global signal, all derivatives, quadratic terms, and squares of derivatives (resulting in a total of 36 parameters). If mean relative root-mean-squared (RMS) displacement exceeded 0.25, then volumes were flagged for spike regression (Ciric et al. 2017). Flagged time points were set to a BOLD value of 0 and therefore did not contribute to the model fit. Functional images underwent slice-time correction, alignment (with the anatomical image and MNI template), removal of first 6 s of data, time series extraction, bandpass filtering (0.01–0.08 Hz), and nuisance regression as described above. In this follow-up analysis, Matlab 2014b was used for bandpass filtering, Python 2.7 for nuisance regression, and AFNI for all other preprocessing steps. The main results of the similarity analyses (see below) were calculated using this version of the preprocessed data, as noted (using the term "36P" or "36 parameters").
To further address motion in this analysis of clinical groups with high levels of head motion, we used RMS displacement to identify high-motion participants for removal. We excluded subjects with less than 4 min of “unflagged” resting-fMRI data to increase the likely reliability of the functional connectivity estimates in our sample. This resulted in 6 control subjects and 2 patients being removed for analysis from the ADHD dataset, 0 control subjects and 0 patients from the ASD dataset, 0 control subjects and 3 schizophrenia subjects from the Yale dataset, and 5 control subjects and 13 schizophrenia subjects from the COBRE dataset. Hence, subject retention rates (subjects with more than 4 min of good quality resting-state data) after nuisance regression with spike filtering for patients and control subjects were respectively 98% and 97% for ADHD, 100% and 100% for ASD, 97% and 100% for the Yale schizophrenia data, and 82% and 93% for the COBRE schizophreniadata.
Statistical Analysis
For our main analysis including all datasets, we sampled data for each individual from a set of 264 independently identified functional regions (Power et al. 2011) to provide results at the region, system, and whole-brain level. In an additional analysis—including only schizophrenia patients from the Yale study—we used another set of regions with 360 parcels (Glasser et al. 2016) to test the robustness of our results, see Replication of architecture similarity with a different set of ROIs. For both analyses, time courses were extracted from each region and averaged across voxels/vertices for use in all subsequent analyses, which were performed in MATLAB 2014b (The Mathworks). Pearson correlations were calculated between all ROIs for each subject, and the resulting functional connectivity (FC) matrix was Fisher’s Z-transformed to normalize the distribution of values. These values were then used on all subsequent statistical tests.
To determine similarity of whole-brain RSFC between two groups, FC patterns were compared by taking the upper triangle of the RSFC matrices to be compared (thereby excluding self-connections and redundant connections), vectorizing the Fisher’s Z-transformed FC values, averaging across subjects within each group, and computing Spearman rank correlations (and, separately, Pearson correlations) on the resulting vectors. Note that Pearson correlation is a standard pattern distance approach, with a simple transform (1 – r) switching the measure from similarity to dissimilarity. We chose to use this distance measure in terms of similarity rather than dissimilarity (despite their one-to-one correspondence) in order to facilitate intuitive understanding of the results. Further, using the original r values (and rho values in the case of Spearman correlations) allowed us to use Fisher’s z-transform in order to make better statistical inferences at the group level.
We used the Mantel permutation test approach (Mantel 1967; Nummenmaa et al. 2012) to calculate similarity P-values. This nonparametric test accounts for nonindependence of the values in similarity/distance matrices such as FC matrices. Such nonindependence arises from the same source time series in a given row of an RSFC matrix being compared with all other time series, such that any idiosyncrasies in the source time series would affect all such comparisons in that row. Implementing the Mantel permutation test involved random shuffling of region identity (as opposed to individual connections) in the RSFC matrices followed by comparison of the matrices (using Pearson and Spearman correlation). This was repeated 10 000 times to create a null distribution of similarity/distance values. The resulting null distribution was converted into a probability distribution function (using MATLAB function ksdensity) before calculating a P-value. All reported P-values for RSFC matrix comparison used a Mantel permutation test. Note that we report P-values lower than 0.000001 as “P < 0.000001” because of the unrealistic level of precision inherent in numbers smaller thanthis.
We also used a more strict version of the Mantel test that takes region clusters (functional networks) into account, addressing potential concerns that higher order organization could also bias similarity/distance matrix comparisons (Guillot and Rousset 2013). Note that a strict null hypothesis would be that network organization is completely different between groups, such that the original Mantel permutation test would be sufficient. However, a less strict null hypothesis is that regions are similarly clustered into networks but that those networks are differently organized. The network-level Mantel test uses this latter null hypothesis. This test was identical to the Mantel test described above except that the Power et al. (2011) networks were permuted in order rather than the individual regions. The resulting P-values for RSFC matrix comparison using the network-level Mantel permutation test were identical to those for the original Mantel test (all P < 0.000001).
Since region-level differences have been found in RSFC between the clinical populations studied here and healthy controls, we also compared individual connections between groups. T-tests were used to compare RSFC values for each of the 34 716 connections. Due to the large number of statistical tests, we report false discovery rate (FDR)-corrected P-values (Genovese et al. 2002). We also compared the resulting t-statistic connectivity matrices by running a Spearman correlation test on the upper triangle across pairs of unthresholded t-statistic connectivity matrices, aswas done for the RSFC matrix comparisons in the prior analyses.
Classification Analysis
We also ran a classification analysis (Yahata et al. 2017) to further investigate if existing differences in resting-state network architecture can be used to distinguish patients from healthy controls. In order to investigate whether we could accurately predict group membership based on RSFC patterns, we conducted a leave-two-out classification analysis for each dataset separately to classify each subject. We balanced datasets by randomly selecting the same number of patients and control subjects per dataset (based on the number of subjects in the smallest group). Such balancing is essential to reduce classifier bias (e.g., always predicting “patient” for a dataset with more patients than controls). For each cross-validation fold, we compared one patient and one healthy control subjects’ RSFC patterns (test set) with the average patient and average control RSFC pattern (training set, excluding the held-out control and patient). In addition, we tested across ADHD and ASD, and across schizophrenia datasets by training on one dataset and testing on the other dataset, and vice versa. Similarity based on Pearson’s correlation of a subject’s RSFC pattern to the average RSFC pattern of the other dataset’s groups was used to assign a label “patient” or “control” to a subject in the test set. Prediction accuracy, sensitivity, and specificity—based on a classification with 10 000 randomly initialized iterations—are reported in Results. P-values were calculated based on standard binomial tests from the classification outcomes.
Results
Large Overlap in RSFC Patterns in ADHD, ASD Patients, and Healthy Controls
We used fMRI to examine RSFC in patients with various mental disorders. We sought to identify common functional network patterns (compared with matched healthy control subjects) for multiple mental disorders with diverse pathophysiology, symptomatology, and etiology. We started by analyzing publicly available resting-state fMRI data from ADHD and ASD patients and healthy control subjects. We extracted time series from 264 regions of interest covering all major systems of the brain (Power et al. 2011, see Fig. 1a) and calculated all pairwise correlations, which were normalized with a Fisher’s z-transformation for all subsequent statistical tests. RSFC matrices ordered by large-scale functional network for ADHD and ASD are displayed in Figure 1b (we discuss the results with schizophrenia in the following section).
Figure 1.
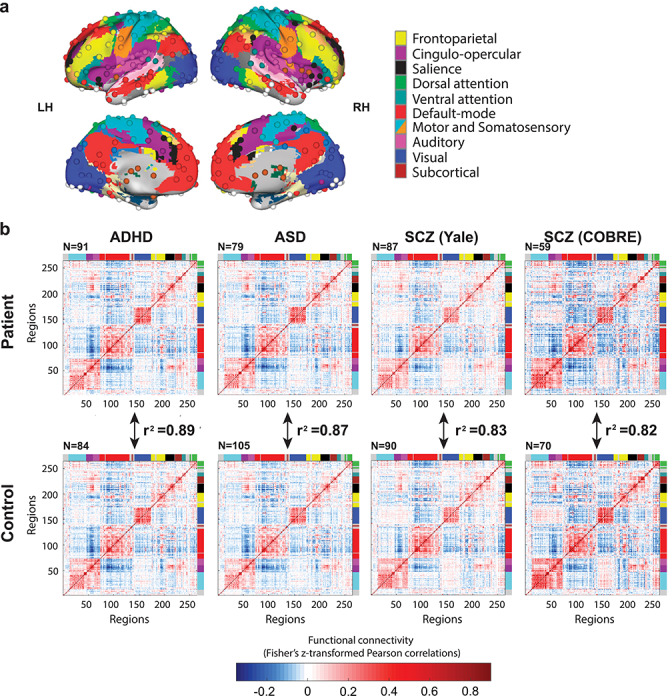
Whole-brain functional network organization is highly similar between patient and healthy control groups across a variety of mental diseases. (a) Functionally defined cortical and subcortical regions (nodes), along with a standard network partition based on healthy young adults (Power et al. 2011) used for whole-brain analyses. (b) RSFC matrices for ADHD, ASD, and schizophrenia patients show highly similar resting-state network architectures with healthy individuals. Colors along the edges of the matrices indicate previously identified functional networks, matching the colors in (a). The red blocks along the diagonal indicate stronger RSFC within relative to between networks. The r2 values can be interpreted as the percentage of z-normalized linear variance shared (e.g., 0.89 = 89% shared linear variance). Note that these r2 values can easily be converted into correlation distance (1 – r) values, such that these results also imply low distance in network state space (Schultz and Cole 2016) between groups. The results visualized here involved the “36P” approach for greater motion-related confound removal, but the results were highly similar when using a more typical preprocessing approach.
For both clinical studies, we calculated the similarity of RSFC patterns between patients and control subjects using the 264 x 264 RSFC matrices with pairwise connections. Each individual’s FC matrix consisted of a minimum of 4 min of resting-state fMRI data (after removing high-motion volumes; see Methods). In addition to a (parametric) Pearson correlation, a (nonparametric) Spearman rank correlation (rho) for the whole-brain RSFC configuration was used to compare patients’ RSFC pattern with that of control subjects. Mantel permutation tests were used to calculate P-values, accounting for the nonindependence of RSFC values for a given region within the RSFC matrices (Diniz-Filho et al. 2013). We also used a more strict version of the Mantel permutation test that takes region clusters (functional networks) into account, addressing potential concerns that higher order organization could also bias similarity/distance matrix comparisons (Guillot and Rousset 2013). All P-values were slightly higher than the original Mantel test results (as expected), but all remained P < 0.000001. See Methods for more details.
We found that for both clinical groups, RSFC patterns exhibited complex network organizations (apparent in Fig. 1b) that were highly similar to the control RSFC patterns. ADHD & control: Pearson’s r2 was 0.89, P < 0.000001 for the ADHD and healthy control groups and 0.87, P < 0.000001 for the ASD and healthy control groups. These results suggest a largely common intrinsic network architecture in healthy individuals and patients, with 89% and 87% of the linear variance shared between patients and controls on average for these groups. Results were similar when using Spearman correlations: rho = 0.89, P < 0.000001 for the ADHD groups and rho = 0.86, P < 0.000001 for the ASD groups. Note that the results in Figure 1 were generated using the “36P” preprocessing method, but that results from the less stringent motion correction method were not significantly different. By focusing on the whole-brain RSFC pattern—using a connectome-wide comparison measure—we found that intrinsic network architecture is highly similar across patients and healthy control subjects in these two clinical groups, as hypothesized.
Large Overlap in RSFC Patterns in Schizophrenia and Healthy Controls
Since the ADHD-200 NYU and ABIDE NYU data were collected at the same site, in the same age group, and with the same MRI parameters, we decided to test the generalizability of our conclusions with a distinct mental disorder and distinct datasets. These datasets included schizophrenia patients and matched healthy controls collected at two separate sites, in a distinct age group, and with distinct MRI parameters from the previous datasets. Whereas comorbidity exists between schizophrenia and ASD, and between schizophrenia and ADHD, there is much less overlap in symptoms than between ASD and ADHD (note that a dual diagnosis of these disorders is only possible since the introduction of DSM-V). Moreover, in this group of patients and control subjects only adults were included, in contrast to the ADHD (only children) and ASD (mostly children, but also adults) groups.
Our connectome-wide distance analysis again indicated a highly similar RSFC configuration pattern between schizophrenia patients and healthy control subjects with r2 of 0.83 and 0.82 (respectively, Yale schizophrenia & control: Pearson’s r2 = 0.83, P < 0.00001; COBRE schizophrenia & control: Pearson’s r2 = 0.82, P < 0.00001, see Fig. 1b), demonstrating that the previous finding of RSFC similarity generalizes to other mental disorders (and other age groups and MRI parameters). Results were similar when Spearman correlation was used as the similarity/distance measure (Yale schizophrenia & control: rho = 0.84, P < 0.00001; COBRE schizophrenia & control: rho = 0.84, P < 0.00001).
A combined analysis of the two schizophrenia datasets resulted in even higher similarity between patient and control groups with an r2 of 0.88 (P < 0.00001), and Spearman’s rho of 0.89 (P < 0.00001).
RSFC Pattern Similarity Across Disorders
We next sought to determine the general similarity between patients and healthy controls across all three mental disorders. The whole-brain RSFC matrices were averaged across all patients and, separately, across all healthy controls. These matrices showed a strong correlation between patients and controls. The r2 was 0.95, suggesting 95% of the linear variance was shared between patients and controls on average. Spearman rho was 0.94, P < 0.00001. Similarity between patient groups was high as well (ADHD/ASD: r2 = 0.87, P < 0.00001, rho = 0.87, P < 0.00001; ADHD/schizophrenia: r2 = 0.77, P < 0.00001, rho = 0.80, P < 0.00001; ASD/schizophrenia: r2 = 0.74, P < 0.00001, rho = 0.86, P < 0.00001) and between control groups (ADHD/ASD: r2 = 0.93, P < 0.00001, rho = 0.93, P < 0.00001; ADHD/schizophrenia: r2 = 0.80, P < 0.00001, rho = 0.83, P < 0.00001; ASD/schizophrenia: r2 = 0.80, P < 0.00001, rho = 0.90, P < 0.00001).
Replication of Architecture Similarity with a Different Set of ROIs
To test the robustness of our RSFC similarity findings, we sought to replicate them with a different set of brain regions (with the Yale schizophrenia dataset). We used a parcellation of functionally defined regions that was recently developed by Glasser et al. (2016). This cortical parcellation with 360 regions was constructed using convergence across multiple neuroimaging techniques (resting-state and task fMRI, myelin maps and cortical thickness), and is believed to be more accurate than previous parcellations because of the consistency of areal borders between data from different imaging modalities (Glasser et al. 2016). Networks were defined in a separate study using community detection analysis with RSFC data (see Methods – Alternative Motion Correction) (Ji et al. 2019). Similar to the analysis with volume (Power) regions, data from surface regions were extracted, RSFC was estimated, and Spearman correlations between RSFC matrices were calculated. Similarity analysis of RSFC matrices of schizophrenia patients and control subjects returned to r2 = 0.85 for the Yale dataset (rho = 0.92, P < 0.000001, see Fig. 2; RSFC similarity was r2 = 0.79 with the volume ROIs), again supporting the existence of a largely similar intrinsic functional network architecture across patients and controls.
Figure 2.
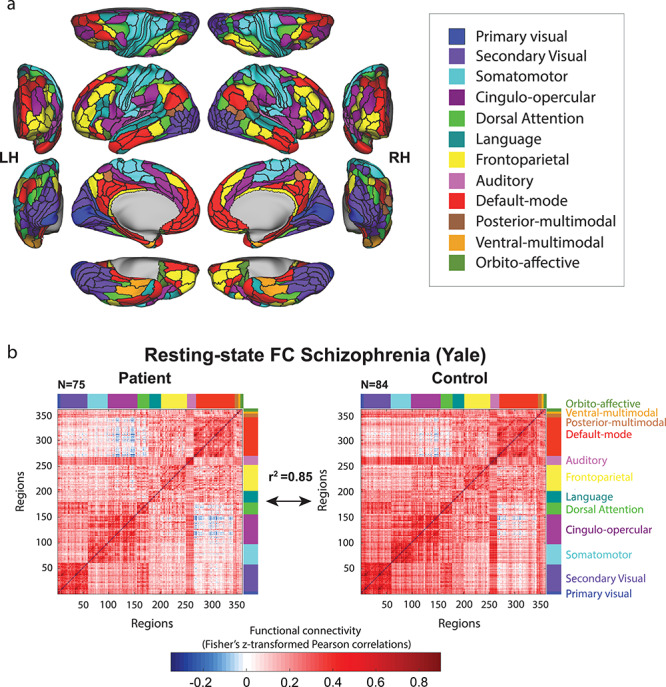
Testing for generalization to a distinct set of regions, network definition. (a) Network partition based on the multimodal parcellation by Glasser et al. (2016). The main parcellation (Fig. 1) used regions defined in terms of voxels, whereas this parcellation is defined in terms of surface vertices. Functional networks were assigned based on the General Louvain method for community detection with resting-state data in healthy adults (Ji et al. 2019). (b) RSFC matrices based on this alternative set of regions and networks were used to replicate the previously found similarity between schizophrenia patients and healthy control subjects. This suggests that the particular choice of regions and networks used for the main analyses did not substantially influence results—that the results are generalizable.
Small-Scale RSFC Deviation in Mental Disorders
Importantly, similar RSFC patterns between patient and control groups do not necessarily mean that all functional connections are normal in patients with mental disorders. Indeed, many results in the literature demonstrate statistically significant RSFC differences between patients and healthy controls, even after controlling for between-group motion confounds as we have here. To test for consistency with prior studies, we also compared individual connections, that is, tested for between-group differences on every unique connection pair in the matrix (34 716 connections). In this way, in addition to testing the overall pattern with Spearman correlations, we examined smaller-scale (individual connection-level) FC alterations in the clinical groups. The resulting patterns of t-values (patient vs. control for each connection, on “36P” preprocessed data) are displayed in Figure 3a, in which t-values have been controlled for covariates. For ADHD and ASD, after regressing out age and gender, 4.35% and 6.43% of connections were changed (uncorrected). After regressing out age, gender and education in the schizophrenia datasets, 6.46% (Yale) (for age, gender, mother, and father education: 8.06%) and 8.29% (COBRE) of connections showed changes. When correcting these results for multiple comparisons, however, only when the Yale and COBRE schizophrenia datasets were combined, results showed 0.10% changed connections (see Fig. 3b) (uncorrected this was 10.76%). It should be noted that regressing out education for schizophrenia is especially problematic since lower educational attainment appears to be part and parcel of the illness expression itself (Kahn and Keefe 2013).
Figure 3.
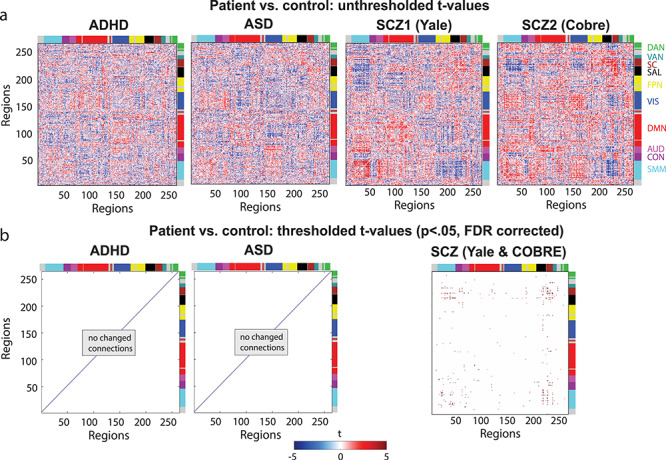
Patient versus control group comparisons yield minimal differences (especially after controlling for motion confounds). RSFC differences were present between patients and controls but only for a small set of connections that were only statistically significant for the schizophrenia group (P < 0.05, FDR corrected). The “36P” results with additional motion confound removal are shown. (a) The t-value patterns resulting from patient-control comparison of individual connections. (b) FDR-corrected t-values (p < 0.05) resulting from the same patient-control comparison of individual connections as in (a). Note that data from the COBRE dataset (33 patients and 45 controls) and the Yale dataset (75 patients and 84 controls) are combined given their similar RSFC patterns. A small set of statistically significant results were identified in the ADHD versus control comparison (but not the ASD comparison) when the “36P” approach for removing additional motion confounds was not used (see the main text).
Altered Connections After Removal of 6 Motion Estimates, Ventricle and White Matter Signals, and Their Derivatives: Similar Results Across Datasets
Our initial preprocessing pipeline resulted in patterns of t-values that show similarity across the datasets with a significant correlation between (unthresholded) t-value patterns of ADHD and ASD datasets (rho = 0.24, P < 0.000001) and the two schizophrenia datasets (rho = 0.29, P < 0.000001). Between ADHD/ASD and schizophrenia datasets, correlations were weaker but also significant (ADHD & Yale: rho = −0.04, P < 0.000001, ASD & Yale: rho = 0.14, P < 0.000001, ADHD & COBRE: rho = 0.04, P < 0.000001, ASD & COBRE: rho = 0.09, P < 0.000001).
After thresholding (P < 0.05) and FDR correcting the t-value patterns for multiple comparisons on this preprocessed data with less nuisance regression, we found several connections that had significantly altered RSFC connectivity in ADHD and schizophrenia patients compared with healthy control groups: 0.34% of connections for ADHD, 4.71% for Yale and 0.02% for COBRE schizophrenia data were changed. When the two schizophrenia datasets were combined, 8.13% of connections were altered in patients compared with control subjects (FDR-corrected).
Weaker Results for Altered Connections After Stringent “36P” Nuisance Regression
When nuisance regression with 36 parameters was applied, similarities across datasets became weaker, but a significant correlation remained between t-value patterns of ADHD and ASD datasets (rho = 0.21, P < 0.000001) and the two schizophrenia datasets (rho = 0.20, P < 0.000001). Between ADHD/ASD and schizophrenia datasets, some correlations were also significant (ADHD & Yale: rho = −0.03, P < 0.000001, ASD & Yale: rho = 0.09, P < 0.000001, ADHD & COBRE: rho = 0.01, P = 0.06, ASD & COBRE: rho = 0.05, P < 0.000001).
After correcting for multiple comparisons, however, only the schizophrenia groups had significantly changed connections (0.003% for the Yale dataset and 0.05% for the COBRE dataset). When we also regressed out age and gender from the connectivity data, similarity was still rho = 0.19, P < 0.00001 for the ADHD and ASD datasets. Similarly, for the Yale and COBRE datasets, when age, gender and education were controlled for, similarity was rho = 0.21, P < 0.00001. Between ADHD/ASD and schizophrenia datasets correlations were low, and only significant in some cases (ADHD & Yale: rho = −0.004, P = 0.41, ASD & Yale: rho = 0.03, P < 0.00001, ADHD & COBRE: rho = 0.03, P < 0.00001, ASD & COBRE: rho = −0.0009, P = 0.86).
Note the high similarity between unthresholded RSFC patterns between the schizophrenia datasets in Figure 3a, which helps justify combining them into a single analysis.
What Exactly are the Aberrant Functional Connections?
Aside from knowing the percentage of aberrant functional connections, it would also be useful to know their identity. For the ADHD comparisons, no connections reached the FDR threshold of significance, but 1834 were significant before correction, with the large majority implicating the default mode network. The 10 most common types of aberrant connections (with exact number in parentheses) were: sensory/somatomotor hand and default mode (100 connections), default mode and visual (100), default mode and frontoparietal task control (100), default mode and default mode (88), default mode and uncertain (64), default mode and salience (58), visual and frontoparietal task control (58), uncertain and default mode (50), sensory/somatomotor hand and frontoparietal task control (43), and default mode and subcortical (43).
For the ASD comparisons, no connections reached the FDR threshold of significance, but 2241 were significant before correction, with the large majority again related to the default mode network. The ten most common types of aberrant functional connections were: default mode and visual (140), default mode and default mode (132), default mode and frontoparietal task control (116), sensory/somatomotor hand and default mode (91), default mode and salience (78), default mode and uncertain (67), uncertain and default mode (63), default mode and ventral attention (59), cingulo-opercular task control and default mode (58), visual and frontoparietal task control (52).
For the combined SZ group, 194 connections reached the FDR threshold and these primarily were associated with somatomotor and subcortical regions, most notably: sensory/somatomotor hand and salience (18), sensory/somatomotor hand & subcortical (17), sensory/somatomotor hand and sensory/somatomotor hand (15), subcortical and subcortical (14), salience and subcortical (13), cingulo-opercular task control and subcortical (11), sensory/somatomotor mouth and salience (7), sensory/somatomotor hand and auditory (7), default mode and subcortical (6), and uncertain and subcortical (6).
Classification of Individuals Based on Multivariate Connectome-Wide Similarity
Although we found functional network architecture in patients and control subjects to be highly similar, the altered connections that were found when comparing individual connections (see Fig. 3) suggested that connectome-wide similarity might still be informative with regard to diagnosis. Further, it might be the case that multivariate patterns of RSFC values distinguish individual patients from healthy controls where connection-specific t-tests did not. We tested these ideas with a multivariate classification analysis. In our classification model we used RSFC pattern similarity as a predictor variable, and tested whether an individual’s functional architecture was more similar to that of the average patient or control by using a leave-two-out approach (one patient and one healthy control subject as test set for each cross-validation fold). This is a form of minimum-distance classification (Mur et al. 2009). While future research will likely find more effective classification approaches, there were multiple advantages of this approach relative to more standard classification approaches (e.g., support vector machines). First and most importantly, this approach directly relates to the connectome-wide similarity analyses reported earlier, expanding connectome-wide similarity to the individual subject level. Second, unlike most classification approaches (e.g., the “C” parameter in support vector machines), this approach does not require parameter choices. Third, this approach reflects the fact that multivariate correlations are now well-validated similarity/distance measures for neuroscientific data (Walther et al. 2016). Fourth, this approach is highly intuitive, since it simply measures which group an individual is closer to in multivariate feature space. Note that individual-level connectome-wide similarity tended to be smaller than the group-level results, likely because group-level averaging increases the signal-to-noise ratio (from more data) and smoothes across individual differences that are idiosyncratic with regard to group membership. Such increased signal-to-noise ratio is a clear advantage, and smoothing across individual differences is not problematic under the straightforward assumption that there is some common set of features across most individuals of each group. Some of these advantages were still retained in the classification analyses, since each individual was compared with group averages.
Classification with “Conventional” Motion Corrected Data
Results from the classifications with our initial preprocessing pipeline are shown in Figure 4 (chance = 50%). This analysis—based on whole-brain RSFC patterns—resulted in 58% prediction accuracy for ADHD (P = 0.03, 57% sensitivity, 58% specificity, N = 77 per class), and 64% prediction accuracy for ASD (P = 0.0005, 66% sensitivity, 63% specificity, N = 70 per class). Note that sensitivity and specificity are directly related to false negative and false positive rates, as well as other measures of classifier performance (Florkowski 2008). For the two schizophrenia groups, we could accurately predict group membership for 75% (Yale, P < 0.00001, 81% sensitivity, 68% specificity, N = 75 per class) and 68% (COBRE, P = 0.002, 79% sensitivity, 58% specificity, N = 33 per class) of subjects.
Figure 4.
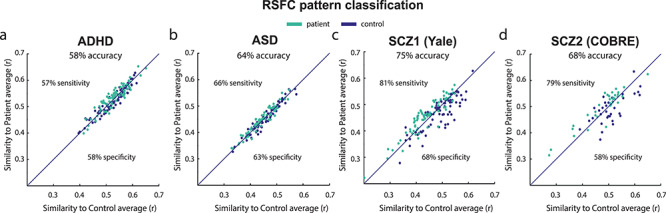
Individual patient-control multivariate classification based on connectome-wide resting-state functional connectivity patterns. Class labels were predicted above chance using a simple classification method in (a) ADHD, (b) ASD and the schizophrenia, (c) Yale, and (d) COBRE datasets. Classifying a subject involved computing that subject’s connectome-wide similarity with the patient-group average and the control-group average, with that subject being assigned to the group with higher similarity. This demonstrated the potential informativeness of whole-brain RSFC similarity in diagnosing individuals, despite overall high similarity between patients and controls at the group level. Note that more complex classifiers might provide better classifications but that straightforward inferences regarding connectome-wide similarity would not be possible with such approaches.
We next sought to test for generalization of connectome-wide RSFC patterns across datasets, assessing the ability to predict diagnoses based on cross-dataset RSFC alterations. This would suggest robustness of the RSFC alterations despite their small effect on connectome-wide similarities, and despite the data being collected at different locations (with potential scanner-specific influences on RSFC patterns). Demonstrating robust generalization, we were able to accurately predict group membership (patient vs. control) above chance across the ADHD and ASD datasets, and across the two schizophrenia datasets. For this analysis, we trained a classifier on one dataset and tested subjects in the other dataset, and vice versa, comparing each subject’s RSFC pattern with the average control and patient FC architecture. A group label was assigned to each subject in the test dataset based on highest similarity (to either patient or to control average in the training dataset). Binomial tests showed 64% prediction accuracy (P < 0.000001) for ADHD and ASD datasets (N = 147), with 52% sensitivity and 77% specificity. The same analysis for Yale and COBRE schizophrenia datasets (N = 108) resulted in 65% prediction accuracy (P < 0.0001), with 68% sensitivity and 59% specificity.
Classification After Stringent (36 Parameter) Motion Correction
To evaluate whether our alternative preprocessing approach would affect the above classification results, we performed the same classification analysis for the functional connectivity data of the four groups preprocessed using “36P” nuisance regression (see Methods for details). Overall, results for the groups looked consistent with previous findings. TheADHD classification resulted in 53% prediction accuracy for ADHD, a 5% decrease compared with the initial results (with 58% sensitivity and 49% specificity). The ASD analysis resulted in 64% accuracy (48.7% sensitivity and 79.8% specificity), the same accuracy as before, but with a lower ability to correctly classify ASD patients as such. The Yale schizophrenia data showed 72% accuracy (sensitivity 70.1% and specificity 74.1%), that is, a 3% decrease in accuracy compared with our previous classification anasysis, and a 67% accuracy for the COBRE schizophrenia dataset (64.4% sensitivity, 69.5% specificity), a 1% difference.
Discussion
The clinical neuroscience literature typically focuses on disease alterations in the functional connectome without putting those alterations in their overall context. We took a more comprehensive view in the present study by comparing RSFC patterns across the whole connectome, taking all connections into account at once when comparing patients and healthy controls across multiple mental disorders. By adopting such a whole-brain view of functional network architecture, we aim to provide informative new constraints on psychiatric theory, emphasizing the role of small brain network changes in the negative life-altering effects of mental illness. Supporting this new perspective, we used a connectome-wide similarity analysis to demonstrate that—while being predictive of diagnosis—connectome-wide RSFC patterns are very similar across patients and controls in a range of mental disorders and even across patient groups.
Large Overlap in Network Patterns Across Health and Disease has Implications for Neurocognitive Theories of Psychiatric Illness
The clinical RSFC literature to date has been so focused on showing only cross-group differences that the results illustrated in Figure 1—showing high cross-group similarity between patients and healthy controls in ADHD, ASD, and schizophrenia—initially appear to run counter to most publications in this area of research. Critically, however, there is technically nothing contradictory about these and prior results. These results suggest that by ignoring or understating the baseline similarity between groups, prior studies have been inadvertently presenting a biased view of psychiatric differences in brain network organization.
The observed high similarity between patients and healthy individuals has important implications for neurocognitive theories of mental illness. This is principally due to the emphasis on large-scale neurotransmitter disruptions in most psychiatric theories. For instance, current theories suggest that schizophrenia may be caused by large-scale disruptions to the dopamine system (Howes and Kapur 2009) or large-scale disruptions to the glutamate system (Moghaddam and Javitt 2012). There are similar theories with ASD involving glutamate (Purcell et al. 2001), acetylcholine (Perry et al. 2001), and serotonin (Jr et al. 1997). With ADHD, there are also similar theories involving dopamine (Kirley et al. 2002), norepinephrine (Zimmer 2009), and serotonin (Zepf et al. 2010). All of these neurotransmitter systems are extremely widespread and critical to brain network functionality (especially glutamate), such that these theories most directly predict widespread alterations to functional brain network organization.
The current results do not rule these theories out completely, however. Rather, these results constrain these theories in important ways. It is possible that, for example, glutamatergic functionality is disrupted widely in schizophrenia, but that the disruption is minimal at each synapse. As evidence, for each clinical group, even though the vast majority of connections failed to individually differ from controls, the totality of differences (t-values) did differ from controls. Alternatively, the disruption could substantially alter very specific functionality (such as timing of neurotransmitter binding) massively, with minimal effect on functional network organization but pervasive effects on behavior. We leave full explanations of how the present results might be compatible with observations supporting widespread neurotransmitter dysfunction in these mental disorders to future work. For now, we emphasize the need for future studies to shift their hypotheses to account for such small functional network changes.
The present results are consistent with a recent study that also found high similarity in brain network organization between schizophrenia patients and healthy controls (Lerman-Sinkoff and Barch 2016). That study used community detection to categorize each brain region with a community label, then used several measures of categorical assignment similarity to assess cross-group similarity. While useful in many circumstances, community detection technically reduces information by converting continuous weighted graph values into discrete categories. Further, community detection algorithms come with parameters and assumptions that can alter results substantially. For these reasons, we focused on similarity measures that retained weighted graph information and involved minimal parameters, facilitating a more comprehensive and principled assessment of network organization similarity. Despite these differences, the same conclusion was reached: There are only minimal differences in large-scale functional network organization between schizophrenia patients and healthy controls.
In accounting for the small functional network changes observed here, three possibilities seem prominent: (1) Most patients actually have very similar cognition/behavior to healthy individuals in aggregate (similar routines, basic abilities like language, etc.), with high brain network similarity reflecting how similar the cognition/behavior implemented by these networks actually is. The possibility is supported by the common observation of similar behavior across patients and healthy individuals (relative to random behaviors). This conclusion is also supported by the exclusion of, for example, the most severe schizophrenia patients in fMRI studies (since they cannot comply with experiment instructions). This predicts that including severe cases of psychiatric illness would reduce the similarity between patients and controls observed here. Nonetheless, insofar as mental disorders are a matter of classification (with the mildest positive cases defining the threshold between positive and negative diagnoses), inferences based on mild cases of a disorder are likely valid. Further, less than 10% of schizophrenia patients are severe enough to require institutionalization (Uggerby et al. 2011), suggesting the number of patients unable to take part in these kinds of studies due to psychosis severity is likely small. (2) Large changes in cognition/behavior arise from small changes in functional network organization. This idea is supported by the small differences in functional network organization across highly distinct cognitive/behavioral states. This high connectome-wide similarity was observed in healthy young adults across a wide variety of highly distinct tasks and resting state (Cole, Bassett, et al. 2014a; Krienen et al. 2014). (3) RSFC does not capture the effects of large-scale network disruption. This possibility is unlikely, given the widespread RSFC changes observed with pharmacological studies that experimentally manipulate neurotransmitter functionality (Anticevic et al. 2012; Klumpers et al. 2012; Scheidegger et al. 2012). It will nonetheless be important to test this possibility using the connectome-wide similarity approach used here. Notably, if connectome-wide similarity remains high in such studies, this would also have strong implications for neurocognitive theories of mental illness, since it would suggest that the brain can exhibit small functional network changes from widespread neurotransmitter functional alteration. It is also worth considering that the primary methodological issues with fMRI and RSFC are their lack of specificity (e.g., whether a signal originated from excitatory or inhibitory neurons) rather than lack of sensitivity (Logothetis 2008; van den Heuvel and Hulshoff Pol 2010; Ma et al. 2016; Grandjean et al. 2017), suggesting that widespread functional network disruption would very likely be detected with RSFC withfMRI.
High Similarity in Resting-State Functional Connectivity Patterns Between Mental Disorders
We also used connectome-wide similarity to compare connectivity between clinical groups, finding that they were very similar. The highest pattern similarity was found between ADHD and ASD groups (with a shared variance of 92%). This result is likely due in part to the data being collected at the same site, though even when comparing schizophrenia patients with either ADHD or ASD patients shared variance was high at 83%. Note that comparable similarity was also found for the cross-diagnostic control subjects. However, the polygenic nature of many mental disorders may also have contributed to highly similar RSFC patterns in patients. A recent study found an association between certain cross-disorder polygenic risk factors for mental disorders (ADHD, ASD, schizophrenia, bipolar disorder, and major depressive disorder) and alterations in RSFC (Wang et al. 2017). Furthermore, Sprooten et al. (2016) showed that cortical regions implicated in several psychiatric disorders (based on task fMRI studies) are very similar across diagnoses and are thus largely diagnostic-general. The present results support this possibility, extending it to include RSFC patterns shared between psychiatric disorders.
Functionally Meaningful Alterations in Intrinsic Functional Architecture of Patients
The RSFC literature has clearly described alterations in FC in a variety of mental disorders, including those disorders covered in the current study. For example, schizophrenia—which is characterized by severe cognitive impairment (Kahn and Keefe 2013)—has been shown to involve global disruption of prefrontal cortex RSFC (Zhou et al. 2007; Cole et al. 2011; Anticevic et al. 2015). Overall, less integrated intrinsic brain networks and connections with prefrontal cortex have been found in schizophrenia patients (for a recent review of RSFC studies and links to cognition in schizophrenia, see Sheffield and Barch 2016).
In ADHD, RSFC changes in the frontoarietal network (FPN) have been identified and associated with cognitive symptoms like attentional control deficits, response inhibition deficits, and impulsivity (Lin et al. 2015) (for a review of RSFC alterations in ADHD see Posner et al. 2014). Further supporting the involvement of FPN RSFC in ADHD, one study was able to predict individual IQ scores (a reduction in IQ is strongly associated with ADHD) based on FPN connectivity measures in children and adolescents with ADHD (Park et al. 2016). Dysfunctional connectivity of cognitive control networks such as FPN to the default-mode network has also been implicated in ADHD (Castellanos et al. 2008; Sun et al. 2012; Hoekzema et al. 2014). The extent to which these changes represent fundamental alterations to brain system organization remains unclear, however.
Patients with ASD show cognitive impairments in several domains such as social cognition, language, attention, executive function, and working memory (Baron-Cohen et al. 1985; Rogers and Pennington 1991; Charman et al. 2011). RSFC studies have indicated a variety of functional network changes associated with ASD, with these changes including both overconnectivity and underconnectivity in areas associated with the cognitive impairments seen in ASD (for a review see Hull et al. 2017).
These RSFC alterations identified by previous studies might seem to contradict our results indicating little differences between patients and healthy controls. We therefore performed a series of analyses to test whether the overall theme of prior results (that there are reliable RSFC differences between patients and control subjects) could be replicated in the data used here. The results indicated that the whole-brain FC pattern can be largely similar across patients and control subjects while, simultaneously, there can be small but reliable RSFC alterations consistent with the existing literature.
When testing all individual connections, alterations survived correction for multiple comparisons—and with two different motion correction methods—for the combined schizophrenia groups, and thus showed the existence of deviant RSFC architecture in the current study. Interestingly, t-statistic pattern correlations (Fig. 3a, connectome-wide patterns of unthresholded t-test results) between mental disorders were significantly above chance, indicating there is a shared component in the deviant functional connections between disorders. Correlations were largest between ADHD and ASD, and between the Yale and Cobre schizophrenia datasets, which is likely a combined effect of data collection site and diagnosis (note that there is high comorbidity between ADHD and ASD (Leitner 2014)). Importantly, these results remained significant after regressing out several covariates (age, gender, and education when available) and were robust enough to withstand conventional and more strict motion correction procedures.
Further reconciling the present results with the existing clinical neuroscience literature, we used machine learning to show that the small connectome-wide RSFC differences between patients and healthy controls were nonetheless predictive of clinical status. Specifically, we found above-chance classifications of patients versus healthy controls in all three clinical groups, based on connectome-wide similarity of RSFC values. This resulted in 58% clinical status prediction accuracy for ADHD, 64% for ASD, 75% for the Yale schizophrenia dataset, and 68% for the COBRE schizophrenia dataset. These percentages were slightly (1–5%) lower for each dataset than the above-mentioned percentages but still above chance when we applied our machine learning approach on the “36P” motion corrected data, showing again the robustness of these results. Notably, the connectome-wide similarity differences used to make these predictions were quite small (see Fig. 4), consistent with the small cross-group connectome-wide similarity differences identified in the main results.
Together, these results further reconcile the current results with the rest of the clinical RSFC literature, demonstrating the utility of RSFC pattern changes despite the small overall connectome-wide differences associated with the psychiatric diseases investigatedhere.
Notes
We acknowledge the contributors to (and funders of) the publicly available ADHD-200, ABIDE, and COBRE datasets used in this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. Conflict of Interest: None declared.
Funding
This work was supported by the National Institutes of Health (grant numbers K99-R00 MH096801, R01 AG055556, R01 MH109520, and K01 H108783).
References
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW et al. 2013. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. 2014. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH et al. 2012. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 109:16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, Cole MW, Savic A, Yang GJ, Repovs G et al. 2015. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 35:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. 1985. Does the autistic child have a “theory of mind” ? Cognition. 21:37–46. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal BS et al. 2008. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry, Impulse Control: Aggression, Addiction, and Attention Deficits. 63:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. 2011. IQ in children with autism spectrum disorders: data from the special needs and autism project (SNAP). Psychol Med. 41:619–627. [DOI] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C et al. 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. 2011. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry, CNTRICS II: Developing Imaging Biomarkers for Schizophrenia. 70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. 2014a. Intrinsic and task-evoked network architectures of the human brain. Neuron. 83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Ito T, Bassett DS, Schultz DH. 2016. Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci. 19:1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repovš G, Anticevic A. 2014b. The frontoparietal control system: a central role in mental health. Neuroscientist. 20:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz-Filho JAF, Soares TN, Lima JS, Dobrovolski R, Landeiro VL, de Campos Telles MP, Rangel TF, Bini LM. 2013. Mantel test in population genetics. Genet Mol Biol. 36:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Florkowski CM. 2008. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev. 29(Suppl 1):S83–S87. [PMC free article] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15:870–878. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR et al. 2013. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J, Zerbi V, Balsters JH, Wenderoth N, Rudin M. 2017. Structural basis of large-scale functional connectivity in the mouse. J Neurosci. 37:8092–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. 2008. Resting-state functional connectivity in neuropsychiatric disorders. [Miscellaneous article]. Curr Opin Neurol. 21:424–430. [DOI] [PubMed] [Google Scholar]
- Guillot G, Rousset F. 2013. Dismantling the mantel tests. Methods Ecol Evol, Handbooks of Modern Statistical Methods. 4:336–344. [Google Scholar]
- Gurley JR. 2011. Conners’ parent rating scales – revised. In: Goldstein, S Naglieri, JA editors. Encyclopedia of child behavior and development. Boston, MA: Springer, pp. 404–405. [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, Rovira M, Bulbena A, Tobeña A, Casas M et al. 2014. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp. 35:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. 2009. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. 2017. Resting-state functional connectivity in autism spectrum disorders: a review. Front Psychiatry. 7: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kulkarni KR, Schultz DH, Mill RD, Chen RH, Solomyak LI, Cole MW. 2017. Cognitive task information is transferred between brain regions via resting-state network topology. Nat Commun. 8:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JL, Spronk M, Kulkarni K, Repovš G, Anticevic A, Cole MW. 2019. Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage. 185:35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jr EHC, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. 1997. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 2:247. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RSE. 2013. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 70:1107–1112. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Barnett JH, White IR, Jones PB. 2011. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 132:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, Waldman I, Fitzgerald M, Gill M. 2002. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 27:607–619. [DOI] [PubMed] [Google Scholar]
- Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SARB, van Gerven JMA. 2012. Manipulating brain connectivity with δ9-tetrahydrocannabinol: a pharmacological resting state FMRI study. Neuroimage. 63:1701–1711. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Yeo BTT, Buckner RL. 2014. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philos Trans R Soc Lond B Biol Sci. 369:20130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, Moffitt TE. 2004. Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Genet B Neuropsychiatr Genet. 124:41–47. [DOI] [PubMed] [Google Scholar]
- Leitner Y. 2014. The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci. 8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman-Sinkoff DB, Barch DM. 2016. Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage Clin. 10:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-Y, Tseng W-YI, Lai M-C, Matsuo K, Gau SS-F. 2015. Altered resting-state Frontoparietal control network in children with attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 21:271–284. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. 2008. What we can do and what we cannot do with fMRI. Nature. 453:869–878. [DOI] [PubMed] [Google Scholar]
- Mantel N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res. 27:209–220. [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kozberg MG, Kim SH, Portes JP, Timerman D, Hillman EMC. 2016. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc Natl Acad Sci USA. 113:E8463–E8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EM, Thomas TC, Gerhardt GA, Glaser PE. 2013. Dopamine and glutamate interactions in ADHD: implications for the future neuropharmacology of ADHD. In: Banerjee S, editor. Attention deficit hyperactivity disorder in children and adolescents. IntechOpen. [Google Scholar]
- Moghaddam B, Javitt D. 2012. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur M, Bandettini PA, Kriegeskorte N. 2009. Revealing representational content with pattern-information fMRI--an introductory guide. Soc Cogn Affect Neurosci. 4:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakic M, Krystal JH, Bhagwagar Z. 2010. Neurotransmitter systems in bipolar disorder. In: Yatham, LN Maj, M, editors. Bipolar disorder. John Wiley & Sons, Ltd, pp. 210–227. [Google Scholar]
- Nummenmaa L, Glerean E, Viinikainen M, Jääskeläinen IP, Hari R, Sams M. 2012. Emotions promote social interaction by synchronizing brain activity across individuals. Proc Natl Acad Sci USA. 109:9599–9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. 1999. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 33:523–533. [DOI] [PubMed] [Google Scholar]
- Park B-Y, Hong J, Lee S-H, Park H. 2016. Functional connectivity of child and adolescent attention deficit hyperactivity disorder patients: correlation with IQ. Front Hum Neurosci. 10: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH et al. 2001. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 158:1058–1066. [DOI] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z. 2014. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 24:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL et al. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. 2001. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 57:1618–1628. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. 2009. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 301:2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. 1991. A theoretical approach to the deficits in infantile autism. Dev Psychopathol. 3:137–162. [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E. 2012. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 7:e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DH, Cole MW. 2016. Higher intelligence is associated with less task-related brain network reconfiguration. J Neurosci. 36:8551–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Barch DM. 2016. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 61:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT. 2017. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 12:506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR et al. 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci. 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TEJ, Glasser MF, Ugurbil K, Barch DM, Van Essen DC, Miller KL. 2015. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 18:1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Rasgon A, Goodman M, Carlin A, Leibu E, Lee WH, Frangou S. 2016. Addressing reverse inference in psychiatric neuroimaging: meta-analyses of task-related brain activation in common mental disorders. Hum Brain Mapp. 38:1846–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, Zuo X, An L, Song Y, Zang Y et al. 2012. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiat Res: Neuroimag. 201:120–127. [DOI] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S. 2016. Task-free MRI predicts individual differences in brain activity during task performance. Science. 352:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggerby P, Nielsen RE, Correll CU, Nielsen J. 2011. Characteristics and predictors of long-term institutionalization in patients with schizophrenia. Schizophr Res. 131:120–126. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2011. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 37:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 20:519–534. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. 2004. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 45:135–170. [DOI] [PubMed] [Google Scholar]
- Wakefield JC. 2007. The concept of mental disorder: diagnostic implications of the harmful dysfunction analysis. World Psychiatry. 6:149–156. [PMC free article] [PubMed] [Google Scholar]
- Walther A, Nili H, Ejaz N, Alink A, Kriegeskorte N, Diedrichsen J. 2016. Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage. 137:188–200. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang X, Li A, Zhu M, Liu S, Qin W, Li J, Yu C, Jiang T, Liu B. 2017. Polygenic risk for five psychiatric disorders and cross-disorder and disorder-specific neural connectivity in two independent populations. Neuroimage Clin. 14:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Kasai K, Kawato M. 2017. Computational neuroscience approach to biomarkers and treatments for mental disorders. Psychiatry Clin Neurosci. 71:215–237. [DOI] [PubMed] [Google Scholar]
- Zepf FD, Gaber TJ, Baurmann D, Bubenzer S, Konrad K, Herpertz-Dahlmann B, Stadler C, Poustka F, Wöckel L. 2010. Serotonergic neurotransmission and lapses of attention in children and adolescents with attention deficit hyperactivity disorder: availability of tryptophan influences attentional performance. Int J Neuropsychopharmacol. 13:933–941. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. 2010. Disease and the brain’s dark energy. Nat Rev Neurol. 6:15–28. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. 2007. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 417:297–302. [DOI] [PubMed] [Google Scholar]
- Zimmer L. 2009. Positron emission tomography neuroimaging for a better understanding of the biology of ADHD. Neuropharmacology. 57:601–607. [DOI] [PubMed] [Google Scholar]


