Abstract
Women undergo important changes in sex hormones throughout their lifetime that can impact cardiovascular disease risk. Whereas the traditional cardiovascular risk factors dominate in older age, there are several female-specific risk factors and inflammatory risk variables that influence a woman’s risk at younger and middle age. Hypertensive pregnancy disorders and gestational diabetes are associated with a higher risk in younger women. Menopause transition has an additional adverse effect to ageing that may demand specific attention to ensure optimal cardiovascular risk profile and quality of life. In this position paper, we provide an update of gynaecological and obstetric conditions that interact with cardiovascular risk in women. Practice points for clinical use are given according to the latest standards from various related disciplines (Figure 1).
Keywords: Coronary artery disease, Ischaemic heart disease, Menopausal hormone therapy, Female-specific risk factors, Hypertensive pregnancy disorders, Menopause, Transgender, Sexual health women
Abbreviations
ACOG, American College of Obstetricians and Gynaecologists
ADA, American Diabetes Association
AF, atrial fibrillation
BP, blood pressure
CAC, coronary artery calcium
CAD, coronary artery disease
CEE, conjugated equine oestrogens
CI, confidence interval
CIMT, carotid intima media thickness
CVD, cardiovascular disease
CT, computed tomography
ELITE, Early vs. Late Intervention trial With Estradiol
ESC, European Society of Cardiology
ESHRE, European Society of Human Reproduction and Embryology
HPD, hypertensive pregnancy disorders
HR, hazard ratio
HRT, hormone replacement therapy
IHD, ischaemic heart disease
MI, myocardial infarction
MINOCA, myocardial infarction with no obstructive coronary artery
MHT, menopausal hormone therapy
MPA, medroxyprogesterone acetate
NETA, norethisterone acetate
OCP, oral contraceptive pills
OGTT, oral glucose tolerance test
PCOS, polycystic ovarian syndrome
POC, progestin-only contraceptives
POI, premature ovarian insufficiency
PPCM, peripartum cardiomyopathy
PVD, peripheral vascular disease
RRSO, risk-reducing salpingo-oophorectomy
SCAD, spontaneous coronary artery dissection
TTS, Takotsubo syndrome
VTE, venous thromboembolism
WHI, Women’s Health Initiative
Preamble
This consensus document provides a summary of the views of an expert panel organized by the Task Force on Gender of the European Society of Cardiology (ESC) and an ad hoc multidisciplinary ESC working group on Women’s Health in Menopause. It is compiled in collaboration with experts from the International, European, British and Dutch Menopause Societies. Formal approval was provided by the ESC Clinical Practice Guidelines Committee. The writing task force members provide declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. This document provides guidance to the clinical community on diagnostic approach and the management of cardiovascular health during menopause transition, after pregnancy disorders, and other gynaecologic conditions based on existing evidence and the best available current practice.
Introduction
Menopause is an important stage in women’s lives, affecting many physical and social changes. The mean onset of menopause is 51 years, but there is substantial inter-individual variation, ranging between 40 and 60 years.1 Oestrogens regulate vascular reactivity, blood pressure (BP), endothelial function and cardiac remodelling.2–4 Alterations in oestrogen levels also affect the immune system, which is closely connected to vascular function and ageing.5 , 6 After menopause, traditional cardiovascular risk factors are adversely affected particularly hypertension.7–10
Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD).11 Our current knowledge of the typical patterns of ischaemic heart disease (IHD) in younger and middle-aged women helps to better diagnose and treat symptomatic women within this age group.12–15 In addition, the growing number of fertile women with stable and unstable IHD requires specific knowledge and attention from both the cardiology and gynaecology communities.
Although sex-specific risk variables related to hormonal and reproductive status are associated with CVD risk, the justified weighting of these variables remains to be elucidated. When considering all age groups together, they do not seem to alter 10-year risk estimation.16 , 17 However, when focusing on younger patients (<55 years), assessment of female-specific risk variables may help to identify women at premature higher risk.18 The strongest predictors are hypertensive disorders of pregnancy (HPD) and low birth weight, with a two-fold higher IHD risk, which is mediated by hypertension.19–21
Epidemiology of cardiovascular disease in women
Ischaemic heart disease is the most important cause of CVD mortality in women worldwide. The regions with the highest age-standardized prevalence of IHD are Eastern Europe, North Africa and Middle East, and Central Europe, while a lower risk of CVD is noted in Chinese and South Americans.22–25 Most recent European data show that IHD and stroke account for 82% of disability-adjusted life years due to CVD in ESC member countries.26 Although there are small declines in the age-standardized incidence and prevalence rates of IHD and stroke over the last 27 years, rates for peripheral vascular disease (PVD) and atrial fibrillation (AF) remain stable. As most IHD data are still largely derived from men, the true IHD incidence in women may be underestimated.15 Risk calculations are mostly based on mortality and not total IHD rates for which women tend to have higher rates of non-fatal events.27 In addition, women have a lower income and socio-economic status compared to men, which contributes to a lower health status in general.28
Although classic type 1 myocardial infarction (MI) occur three times more commonly in men than in (elderly) women, the number of women under 65 years with MI is gradually increasing.29 , 30 Especially, the number of type II MIs with no obstructive coronary arteries (MINOCAs) and spontaneous coronary artery dissections (SCADs) are more prevalent in younger women.31–33 It is estimated that up to 30% of MI in women <60 years are caused by a SCAD.32 In contrast, most women diagnosed with a Takotsubo syndrome (TTS) are post-menopausal and over sixty.34 Altered sex hormone levels, especially an oestradiol deficiency, have thus far not been identified as a risk factor for TTS35 Mental stress is more related to IHD caused by coronary vascular dysfunction and MINOCA than to obstructive coronary artery disease (CAD), which underscores important gender differences in coping with stress.36 , 37
Menopause, cardiovascular disease risk factors, and ischaemic heart disease
Obstructive CAD occurs 7–10 years later in women than in men, with women having fewer focal coronary artery stenoses at all ages.38 Women have a lower plaque burden, fewer vascular calcifications, a more diffuse pattern of atherosclerosis and, more often, soft plaques and erosive lesions compared to men.39–43 Coronary vasomotor disorders, such as coronary artery spasm and/or coronary microvascular dysfunction represent a major cause of IHD in middle-aged women.15 , 44–46 These can be present with or without non-obstructive CAD. In a sub-analysis of the ISCHEMIA trial, women have more frequent angina with less extensive CAD and less severe ischaemia than men.47 This was also shown in the large CorMICA trial.48 These findings confirm important sex differences in the complex relationships between angina, atherosclerosis, and ischaemia.49
Lower oestrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide-dependent vasodilation.8 , 9 , 50 , 51 Healthy endothelium is sensitive to the vasodilator properties of oestrogens, but this reverses when vascular stiffness and atherosclerotic disease develops over time.52 , 53 While CVD risk increases with the menopause, this cannot be distinguished from ageing.54 The Women’s Ischemia Syndrome Evaluation study found that the presence of cardiovascular risk factors accounted for comparable CAD lesions among pre- and post-menopausal women.55 A validated tool to measure CVD risk in middle-aged women is to assess the coronary artery calcium (CAC) score with computed tomography (CT) scanning, having a higher prognostic value than in men.43 It is recommended to assess the CAC score in symptomatic women and those at intermediate cardiovascular risk.43 , 47
The decline in endothelial function starts in early menopause even before signs of subclinical atherosclerosis are present.56 , 57 This mechanism may be involved in the pathophysiology of ‘undetermined’ chest pain and dyspnoea, which is often labelled as ‘stress’ or to ‘menopausal symptoms’. However, women with ‘undetermined’ chest pain syndromes have a two-fold increased risk of developing an IHD event in the following 5–7 years.58 , 59 The changing hormonal milieu is associated with alterations in body composition. Fat mass increases predominantly in the central and visceral regions, while lean mass decreases after menopause.60 Visceral adipose tissue secretes inflammatory cytokines such as tumour necrosis factor-α, interleukin-6, and retinol-binding protein-4. The efflux of free fatty acids to the liver generates reactive oxygen species. Chronic inflammation and oxidative stress respectively increase insulin resistance.61 Animal studies indicate that post-gonadectomy oestrogen decline is associated with an impairment of pancreatic β-cell function.62 In clinical practice, post-menopausal women have 2–3 times higher prevalence of metabolic syndrome, compared to similar aged premenopausal women.63
Menopause transition results in lipid profile changes, with a 10–15% higher LDL-cholesterol and triglyceride levels and slightly lower HDL cholesterol levels.64 The sharp rise in BP after menopause may be both a direct effect of hormonal changes on the vasculature and metabolic changes with ageing.65–70 Hypertension is a critically important risk factor that affects women in the early post-menopausal years and is often poorly managed.10 , 71 , 72 Recent data from Canada report a worsening of hypertension awareness and treatment over the past decade, especially in women.73 In all, 30–50% of women develop hypertension (BP >140/90 mmHg) before the age of 60 and the onset of hypertension can cause a variety of symptoms, such as palpitations, hot flushes, headaches, chest pain, pain between the shoulder blades, tiredness and sleeping disturbances, which are often attributed to menopause.74–76 Sodium sensitivity increases during menopausal transition, frequently leading to intermittent fluid retention (oedema of the legs, hands, and lower eyelids).77–80 Physicians should intensify the detection of hypertension in middle-aged women, especially after HPD and pre-eclampsia.81 , 82 Systolic BP is the most important arbiter of risk with ageing and results in greater vascular and myocardial stiffness in women than in men,83–85 an important factor in why heart failure with preserved ejection fraction dominates in older women.86 Sex differences in heart failure have been recently described, hence our focus on IHD.87 , 88
Immune reactivity increases in women during and after menopause transition.89 , 90 Autoimmune rheumatic and endocrine disorders such as rheumatic arthritis, systemic lupus erythematosus, antiphospholipid syndrome, Sjøgren-syndrome, and thyroid disorders are more prevalent in women than in men and are associated with an increased CVD risk.91–94 Patients with these disorders also have a higher clustering of traditional risk factors.95 These risk variables should be taken into consideration when assessing individual risk around menopause.
| Practice points |
|---|
|
|
|
|
Healthy lifestyle in menopause
The loss of oestrogen has been associated with reduced energy expenditure.96 Lower oestrogen levels are associated with feeding behaviours and meal size, promoting hyperphagia and obesity.60 , 97 , 98 Obesity is also associated with depression, which enhances food intake and sleep deprivation and reduces physical activity.99 Effective management of vasomotor symptoms with menopausal hormone therapy (MHT) may reverse this.100–102 Regular physical exercise has a beneficial effect on vasomotor symptoms and quality of life.103–105 Although oestrogen therapy is not approved to treat perimenopausal depression, there is evidence that it has antidepressant effects and increases well-being in perimenopausal women.106
Improvement of quality of life enhances the ability to work. Women suffering from severe menopausal symptoms have an eight-fold increased risk of working disability, leading to lower productivity, more absenteeism, earlier termination of workforce participation, and a rise in employer and healthcare community costs.107 , 108
| Practice points |
|---|
|
|
Vasomotor symptoms and cardiovascular disease risk
Women with severe menopausal symptoms have an unfavourable cardiometabolic profile and overactivity of the sympathetic nervous system compared to asymptomatic women.109–115 Autonomic dysfunction enhances heart rate variability, which may result in symptoms of dyspnoea on exercise.50 Increased sympathetic activity with disabling vasomotor symptoms is more often present in women after HPD.116 , 117 In the Women’s Health Initiative (WHI) observational study, women with severe symptoms of hot flushes and night sweats had a 48% higher risk of incident diabetes at follow-up.118 They also have evidence of impaired endothelial function and increased subclinical atherosclerosis compared to women without vasomotor symptoms.119–121
| Practice points |
|---|
|
|
Use of menopausal hormone therapy since Women’s Health Initiative
Preliminary findings from the WHI reported a significant increase in IHD events with a combined MHT regimen of conjugated equine oestrogens (CEE) and medroxyprogesterone acetate (MPA) compared with placebo, but this was non-significant in the long-term follow-up.122–124 In contrast, MHT with CEE alone resulted in a non-significant decrease in coronary events compared with placebo, especially in those initiating treatment below 60 years of age.124 , 125 In a meta-analysis of 23 randomized clinical trials (RCTs) women initiating MHT treatment below 60 years of age or within 10 years of onset of menopause showed a significant reduction (>30%) of MI or cardiac deaths.126 In the Danish national registry wherein almost 700 000 women were included, about a quarter of whom were current or past MHT users.127 Overall, MI risk was not influenced by MHT use, but continuous combined oestrogen–progestogen appeared to increase the risk while a transdermal and vaginal oestrogen reduced the risk. The oestrogen used was almost universally oestradiol and vaginal oestrogen is 80% weaker than transdermal oestrogen. No differences in risk were seen between different progestogens, namely norethisterone acetate (NETA), MPA, or norgestrel. Further RCT data came from the Danish Osteoporosis Prevention Study (DOPS), which included over 1000 women in early post-menopause, randomised to oral MHT, oral oestradiol with or without NETA addition, or to no treatment.128 Menopausal hormone use was associated with a significant reduction in a composite endpoint of MI, death or admission to hospital with heart failure compared with placebo [hazard ratio (HR) 0.48; 95% confidence interval (CI) 0.26–0.87].
A more recent meta-analysis of RCTs and data from a Finnish register confirm that initiating MHT (oral/transdermal) within 10 years of the onset of menopause significantly reduces MI and death around 50%, whereas discontinuation of MHT resulted in a transient increase in coronary death.129–131 Thus, many studies following the initial WHI reports largely support a preventive effect of MHT on CVD. Recent MHT studies such as the Kronos Early Estrogen Prevention Study (KEEPS) and the Early vs. Late Intervention Trial with Estradiol (ELITE) have focused on recruiting mainly younger women (<6 years since menopause) using more favourable MHT regimens with surrogate cardiovascular endpoints.132 , 133 The ELITE trial demonstrated less progression in carotid intima media thickness (CIMT) in younger women randomized to MHT compared to older women who were more than 10 years post-menopause (P = 0.007 for the interaction).133 Possible mechanisms mediating the CVD benefit of MHT, especially transdermal, include increase in insulin sensitivity, improvement of the lipid profile and body composition, decrease in BP in case of drospirenone-containing regimens, and finally, a direct vasodilatory and anti-inflammatory effect.51 , 134 , 135
Breast cancer remains the main concern of MHT use. A recent meta-analysis of disparate studies with different entry criteria that included over 108 000 women diagnosed with breast cancer concluded that any MHT use would result in up to a two-fold increase in breast cancer risk.136 This study was dominated by the Million Women Study (MWS) data, a study widely criticized on a number of methodological issues.137 , 138 Few data were included from studies of modern MHT regimens with non-androgenic progestogens such as dydrogesterone and micronized progesterone.136 The French E3N cohort study was not included, but showed a lower breast cancer risk in users of micronized progesterone and dydrogesterone.139 , 140
Modern MHT regimens contain lower doses of systemic and vaginal oestrogens.101 Oral, but not transdermal, MHT increases the risk of venous thromboembolism (VTE).141 Current evidence is summarized in Table 1.
Table 1.
Benefits and risks of menopausal hormone therapy (MHT) for women with age at menopause >45 years and of hormone replacement therapy (HRT) for women with early menopause (<45 years) and women with premature ovarian insufficiency (POI, <40 years)
| Benefits | Risks |
|---|---|
|
|
| Practice points |
|---|
|
|
|
|
|
|
|
Premature ovarian insufficiency
Women with premature ovarian insufficiency (POI), defined as the loss of ovarian function before the age of 40, have a shorter life expectancy than women with a late menopause due to CVD and osteoporosis.149–151 A meta-analysis showed an increased risk of CVD for women with POI, early menopause (age 40–44 years), and relatively early menopause (age 45–49 years).152 Each year of early menopause was associated with a 3% increased risk of CVD.
Data regarding risk of stroke in early menopause and POI are conflicting.143 , 150 , 153 , 154 A recent meta-analysis demonstrated an increased risk of stroke in both POI and early menopause, but not in women with relatively early menopause.152 Adverse effects of POI and early menopause have been shown on lipid profile, body composition, systolic BP, insulin sensitivity, risk of metabolic syndrome, endothelial function, and inflammatory markers.155–162 Women with an early menopause have a 12% higher risk of developing diabetes compared to women who experienced menopause at a later age.163 , 164
Although in non-human primate studies premature atherosclerosis was found in animal models of POI, this was not replicated in human studies on subclinical atherosclerosis as assessed by CIMT and CAC.52 , 157 , 165 The lack of endogenous hormones after menopause and an underlying genetic predisposition to abnormal DNA repair may result in an accelerated general ageing phenotype, contributing to both early age at menopause and increased risk of CVD.166 Genetically impaired DNA repair also contributes to higher risk for cancer and cardiac damage of cancer therapy and to a higher risk for peripartum cardiomyopathy (PPCM).167 , 168
Management of premature ovarian insufficiency
Prospective randomized data are lacking on the effect of hormone replacement therapy (HRT) as it is termed in women with POI, although most available evidence suggests a beneficial effect on CVD.144 , 145 , 169 , 170 In women with POI, HRT is recommended until at least the average age of menopause.146 This is supported by a recent meta-analysis which showed that the largest reduction in CVD incidence was in women with POI or early menopause who used HRT for at least 10 years.152 Early initiation of HRT had the greatest reduction in CVD, highlighting the importance of timely diagnosis and treatment. Although combined oral contraceptive and HRT are both treatment options in women with POI, the use of HRT has a superior effect metabolically and on bone density.171 The risks and benefits of HRT in women with POI and early menopause are different from those using MHT in peri- and post-menopause, and accurate individual counselling is therefore vital.
| Practice points |
|---|
|
|
|
|
|
Pregnancy-related disorders and cardiovascular disease risk
Recurrent pregnancy loss
Recurrent miscarriage or recurrent pregnancy loss, the preferred term by the European Society of Human Reproduction and Embryology (ESHRE), includes all pregnancy losses from the time of conception until 24 weeks of gestation.172 Women with a history of two or more pregnancy losses, consecutive or not, appear to have an increased risk of IHD.173 , 174 Cardiovascular disease and recurrent pregnancy loss share common risk factors such as smoking, obesity, and alcohol intake.175 , 176 Moreover, endothelial dysfunction may be the underlying link between recurrent pregnancy loss, pre-eclampsia, intrauterine growth restriction, and future cardiovascular events.177 Most studies have not found any relationship between recurrent pregnancy loss and stroke. However, data from Danish registers have shown that women from families with manifest atherosclerotic disease may be predisposed to pregnancy losses which may induce a greater risk of IHD and stroke.178 Adjustment for antiphospholipid antibodies did not affect the estimates. A detailed family history for CVD and pregnancy history should therefore be an integral part of cardiovascular risk assessment in women.
Preterm delivery
Preterm delivery, defined as delivery before 37 weeks of gestation, affects about 10% of pregnancies in the US.179 Lower rates are found in Europe, around 5–6%.180 About 30–35% of preterm deliveries are medically indicated, most frequently due to pre-eclampsia and foetal growth restriction.181 In the Nurses’ Health Study II, preterm delivery was found to be independently predictive of CVD.182 Women with a history of preterm delivery appear to have a two-fold increased risk of CVD in later life.183 No specific follow-up for these women is recommended, except to optimize modifiable cardiovascular risk factors.184 Small-for-gestational age newborns also increase maternal CVD risk.185
Hypertensive pregnancy disorders
HPD affect 5–10% of pregnancies worldwide. These include pre-existing (chronic) hypertension, diagnosed before pregnancy or before 20 weeks of gestation, and gestational hypertension developing after 20 weeks of pregnancy. Pre-eclampsia is now defined as persistent hypertension that develops after 20 weeks of pregnancy or during the post-partum period, associated with proteinuria and/or other maternal organ dysfunction.186 Pre-existing hypertension is associated with increased risk of developing pre-eclampsia which may complicate up to 25% of cases. Pre-eclampsia is associated with a 4-fold increase in heart failure and hypertension and a 2-fold increased risk in IHD, stroke, and cardiovascular deaths.21 , 187 This finding is now endorsed by the 2018 American College of Cardiology/American Heart Association cholesterol guidelines using a history of pre-eclampsia to justify statin prescription in asymptomatic middle-aged women with an intermediate 10-year risk.188 Hypertensive complications in pregnancy are also a major risk factor for PPCM.189 , 190 The risk of developing pre-eclampsia can be substantially reduced by a low dose of aspirin, 100 mg up to 150 mg/day in high-risk women, initiated from week 12 and continued to weeks 36–37 of gestation.191 , 192
Thirty percent of previously pre-eclamptic women have signs of CAC around the age of 50 years compared with 18% in a reference group.193 Women with a history of HPD have increased risk of arterial stiffness and greater incidence of IHD, heart failure, aortic stenosis, and mitral regurgitation20 and a three-fold higher risk for vascular dementia later in life.194 Cardiovascular risk after HPD is largely, but not entirely, mediated by development of chronic hypertension.195 The severity, parity, and recurrence of these HPD increases the risk of subsequent cardiovascular events.196
Although women after HPD are recognized as a higher risk population in the 2018 ESC arterial hypertension guidelines, there is still a need to establish systematic follow-up recommendations aimed at timely detection and control of all major risk factors.23 , 197–199 Regular BP control is needed at least in the first post-partum months and use of eHealth technology with self-monitoring of BP with feedback to the primary care physician should be encouraged.184
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM), defined as the first development of glucose intolerance during pregnancy, occurs in about 7% of pregnancies.200 Although the carbohydrate intolerance of GDM frequently resolves after delivery, an estimated 10% of women with GDM will have diabetes mellitus soon after delivery with another at least 20% being affected by impaired glucose metabolism at post-partum screening. In the remaining women, 20–60% will develop type 2 diabetes mellitus later in life, often within 5–10 years after the index pregnancy.201 Gestational diabetes is associated with a two-fold risk of future CVD events, with the risk being apparent within ten years after pregnancy.202 There is also growing evidence that HPD are associated with increased risk of developing type 2 diabetes beyond sustained hypertension.196 It is recommended that all women with GDM have a screening oral glucose tolerance test (OGTT) test at 4–12 weeks post-partum. The American Diabetes Association (ADA) and American College of Obstetricians and Gynaecologists (ACOG) recommend repeat testing every 1–3 years for women who had GDM and normal post-partum test results.200 , 203
Pregnancy in women at increased risk for IHD
Due to an increasing maternal age of pregnancy, a greater number of women are at risk for stable or unstable IHD during pregnancy.192 , 204–206 In a large US cohort of 1.6 million pregnancies, HPD were associated with 1.4- to 7.6-fold higher risk of MI, heart failure, and stroke.207 Mortality data have been reported as high as 5–10% in elderly cohorts.208 , 209 In the European registry of pregnancy and cardiac disease (ROPAC), women with IHD accounted for about 4% of 5739 included pregnancies.210 Although these women were typically older and more often multiparous, no mortality was observed and in only 4%, heart failure was reported. Recent findings in a UK cohort of 79 women with pre-existing IHD reported only 6.6% adverse cardiac events without any maternal deaths.211 However, the rates of adverse obstetric and neonatal events were increased, with an occurrence rate of pre-eclampsia in 14%, preterm delivery in 25%, and small-for-gestational age in 25%. Foetal risk may therefore be higher than maternal risk in women with known IHD. In women with a prior SCAD, a new pregnancy seems to be well tolerated without evidence of an increased risk of SCAD recurrence.212
| Practice points |
|---|
|
|
|
|
Hormonal dysregulation and cardiovascular disease risk
Polycystic ovarian syndrome and cardiovascular disease risk
Polycystic ovarian syndrome (PCOS) affects 6–16% of women with marked ethnic variation.213 Central to the disorder are dysovulation, hyperandrogenism, and metabolic disturbances, particularly insulin resistance. Diagnosis is most commonly based on the Rotterdam criteria, requiring 2 out of 3 of oligo-or anovulation, clinical or biochemical evidence of hyperandrogenism, and polycystic ovary(-ies) on ultrasound.214 PCOS has been associated with many risk factors for CVD including impaired glucose tolerance, dyslipidaemia, hypertension, metabolic syndrome, type 2 diabetes and raised inflammatory markers.215–219 Young women with PCOS have evidence of endothelial dysfunction and subclinical atherosclerosis, as assessed by CIMT and CAC scores.220–223 Although most women are diagnosed in their 20s and 30s, long-term follow-up studies are limited. The natural progression of cardiovascular risk factors has been hampered by confounders like obesity and the heterogeneous criteria and various phenotypes of the disorder. Several cardiovascular risk factors associated with PCOS seem to ameliorate over time.224 In a meta-analysis performed for the development of the ESHRE/American Society for Reproductive Medicine guidelines on PCOS, and restricted to only higher quality studies, no increased risk of MI, stroke or CAD was found in women with PCOS compared to controls.225–227 Another meta-analysis confirmed that the risk of CVD was increased in women of reproductive age, but not in peri- or post-menopausal women.228 This may be related to timely modification of cardiovascular risk factors, a cardio-protective effect from a delayed menopause, or other unknown (genetic) factors.229–232
It is recommended that all women with PCOS should have an assessment of BP and OGTT, and a fasting lipid profile.227 , 233 Dietary and lifestyle education is recommended and as women with PCOS have increased risk of diabetes and HPD, they should be offered screening for GDM in pregnancy.
Other chronic gynaecological conditions associated with cardiovascular disease risk
There is considerable overlap between gynaecologic conditions and chronic disease, particularly CVD. In addition to the gynae-endocrine disorders (e.g. PCOS, POI, hypogonado-trophic hypogonadism), endometriosis, uterine fibroids, and hysterectomy <50 years with ovarian conservation have all been associated with increased CVD risk.234–237 Endometriosis is associated with enhanced inflammation, oxidative stress, and an adverse lipid profile.238 Although causal relationships have not been proven, the gynaecological and reproductive history may provide important insights into potential long-term health risks in women for which a more systems-wide approach may be beneficial.
| Practice points |
|---|
|
|
|
Contraception in women at high cardiovascular disease risk
Combined oral contraceptive pills (OCP) carry an increased risk for venous thrombosis, MI, and stroke, which is significantly enhanced by cigarette smoking.239 , 240 OCPs containing high-dose ethinyl oestradiol have been associated with increased BP. This is due to increased production of angiotensinogen/angiotensin II and related to OCP formulation/dose. In the Danish Cohort Study, use of combined OCPs containing 20 μg of ethinyl oestradiol increased the relative risk of both thrombotic stroke and MI by 1.60 (95% CI 1.37–1.86) and 1.40 (95% CI 1.07–1.81), respectively, in comparison to non-OCP users.241 Thus, OCP’s containing ethinyl oestradiol should be avoided in women with a history of VTE, stroke, CVD, or any other PVD. The ACOG has developed guidelines for use of OCP in women at elevated cardiovascular risk.242 In healthy women below 35 years with pre-existing hypertension, OCP can be used. If BP remains stable after a few months, OCP may be continued.243 Use of OCP is contraindicated in women older than 35 years who smoke, have severe dyslipidaemia, or obesity.244 Progestin-only contraceptives (POCs) are not associated with increased vascular risk (arterial or venous), although evidence suggests that MPA use may be associated with a slightly increased risk.241 , 245 In women at CVD risk, POC administered by oral, sub-cutaneous, or intra-uterine routes can be prescribed.246 , 247
| Practice points |
|---|
|
|
|
Women with heart disease and abnormal uterine bleeding
With the rise in the number of premenopausal women in need of any kind of anticoagulant therapy and/or (dual) antiplatelet therapy including the growing number of young women with congenital heart disease, established IHD and AF, the prevalence of abnormal uterine bleeding is increasing.248 The levonorgestrel-releasing intra uterine system can be an effective and safe option in these women, both as a contraceptive and for treating heavy menstrual bleeding.247
| Practice point |
|---|
|
Cardiovascular disease risk in women with BRCA 1/2 mutations and after breast cancer
Breast cancer affects an estimated 2.1 million women worldwide each year.249 Early detection and improved treatment have increased survival rates; however, breast cancer remains the most common female cancer in Europe.250 , 251 The majority of hereditary breast cancers occur due to mutations in the BRCA 1 and 2 genes, which are also associated with ovarian cancer. Due to the lack of effective screening methods, a risk-reducing salpingo-oophorectomy (RRSO) is recommended at age 35–40 years in BRCA1 and age 40–45 years in BRCA2 mutation carriers.252 Women with BRCA1/2 mutations may be at increased risk for CVD by iatrogenic early menopause and a potentially elevated CVD risk as a result of abnormal ability of DNA repair.253–255 Moreover, BRCA1 is now considered as an important gatekeeper of cardiac function and survival after ischaemia and oxidative stress, making mutation carriers more susceptible for the occurrence of heart failure after an MI.167 Thus far, data on risk of BRCA ½ mutation carriers for cardiotoxicity after chemotherapy are conflicting.256–258
Hormone replacement therapy after risk-reducing salpingo-oophorectomy
Women who have an early or premature surgical menopause often have debilitating menopausal symptoms. Studies assessing the safety of HRT in this population are limited. A meta-analysis of 3 cohort studies with 1100 BRCA1/2 mutation carriers showed no increased risk of breast cancer with HRT after RRSO (HR 0.98, CI 0.63–1.52).147 BRCA2 mutation carriers have higher rates of oestrogen and progesterone-positive tumours and may have a different level of risk with HRT. Short-term (2.8–4.4 years) HRT appears to be safe with no increase in breast cancer risk.148 Current guidelines of the European Menopause and Andropause Society (EMAS) and International Gynaecologic Cancer Society (IGCS) recommend that BRCA1/2 mutation carriers who have had a RRSO should be offered HRT up until the natural age of menopause (51–52 years).259 As in the general menopause population, oestrogen therapy alone appears to have a different effect compared to combined oestrogen and progestogen therapy.147 In a prospective analysis of 872 BRCA1 mutation carriers, there was no overall difference in breast cancer risk between HRT users and non-users; however, the estimated 10-year risk of breast cancer differed significantly between women using oestrogen-only and combined oestrogen and progestin replacement therapy (12% vs. 22%; P = 0.04).260
MHT after breast cancer
Management of menopause symptoms should be individually tailored and carried out in close liaison with the oncologist. Lifestyle alterations and non-hormonal treatment options, such as clonidine, SSRIs, venlafaxine, gabapentin, and pregabalin are recommended first line in these women.142 , 261–263 Although these are effective for mild-to-moderate vasomotor symptoms, their use is often limited by side effects.262 SSRIs such as fluoxetine and paroxetine should be avoided in women on tamoxifen due to inhibition of the CYP2D6 enzyme pathway which may reduce its efficacy. Complementary therapies such as isoflavones, soy, red clover, and black cohosh are not recommended as they may have oestrogenic effects and there is a lack of data regarding safety and efficacy, although some may have SERM-type effects (NICE, NG101).
Data regarding the safety of MHT in breast cancer survivors are limited, as several studies were terminated early due to an increased risk of recurrence in the interim analysis.264 , 265 Current UK guidance suggests to reserve MHT for those with refractory symptoms after other non-hormonal treatments have been unsuccessful (NICE NG 101). Other guidelines advise against MHT in oestrogen receptor-positive breast cancer.142 , 266
| Practice points |
|---|
|
|
|
|
Sexual health, menopause, and cardiovascular disease
Sexual health concerns are common in patients with all types of CVD.267–270 Approximately 60–90% of patients with chronic heart failure report having sexual problems, but fewer than 15% have had a consultation with their physician in matters related to sex and intimacy.268 For women, the most frequently reported problems are diminished feelings of sexual arousal and enjoyment, leading to difficulties in experiencing orgasm, pain during intercourse, and, with sexual activity being less pleasurable and satisfactory, to decreased desire for sexual activity.267 Whereas in men CVD coexists with erectile dysfunction, for which endothelial dysfunction is the common underlying pathophysiological mechanism, a definitive pathophysiological link between sexual problems in women and CVD is less clear.271–274 However, endothelial dysfunction is unrelated to sexual problems in women with CVD.275 Sexual problems in women related to low sexual arousal are very common in healthy women.276 , 277 Theoretically, if the heart supplies less blood to the vaginal wall, labia, and clitoris during sexual stimulation, this may lead to reduced capacity to become genitally aroused, resulting in orgasm problems, dyspareunia, and decreased sexual desire. In the first large study investigating the impact of somatic and psychological comorbidities on sexual function in women, CVD was specifically related to lubrication difficulties.278 Psychological concerns about whether it is safe to be sexually active after a cardiac event may lead to avoidance of physical affection and intimacy.279 Other symptoms of CVD such as chest pain, shortness of breath, and fatigue may interfere with engaging in and enjoyment of sexual activities. Side effects of medication may also disrupt sexual arousal.
In recent years, a limited number of medical treatment options for women with sexual problems have become available. Flibanserin, a serotonin 5-HT-receptor agonist marketed for women with low sexual desire, is associated with considerable risk of syncope and hypotension, and is therefore unsuitable for women with CVD.280 Bremelanotide, a melanocortin receptor agonist for the treatment of premenopausal women with low sexual desire, was approved in 2019. However, efficacy and safety of the drug in women with CVD are unknown. Although not evidence-based, a recent position statement advises testosterone therapy in post-menopausal women with low sexual desire, supported by measurement of testosterone concentrations in blood to monitor treatment response to prevent overuse.281 Transdermal testosterone therapy is not associated with increases in BP, blood glucose, or HbA1c levels. However, its safety is not investigated in women at high CVD risk.
Figure 1.
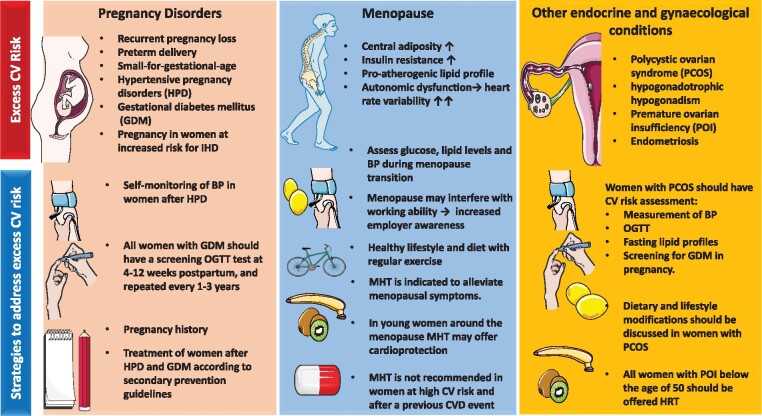
Female-specific risk factors and strategies for prevention. BP, blood pressure; CVD, cardiovascular disease; GDM, gestational diabetes mellitus; HPD, hypertensive pregnancy disorders; IHD, ischaemic heart disease; MHT, menopausal hormone therapy; OGTT, oral glucose tolerance test; PCOS, polycystic ovarian syndrome; POI, premature ovarian insufficiency.
| Practice points |
|---|
|
|
Cardiovascular disease risks for cross-sex therapy in female transgender persons
Currently, the prevalence of transgender persons is 0.6% in adulthood.282 , 283 Evidence-based recommendations for cardiovascular risk prevention are lacking, as treatment regimens vary globally.284 , 285 Gender-affirming therapy, including sex hormones, enables a life in congruence with a personal gender identity, which significantly improves quality of life. Until recently, only VTE risk has been evaluated in transgender women (meaning persons assigned male at birth) undergoing oestrogen treatment. Other appearances of CVD have only been considered within the range of the cisgender population whose gender identity matches the sex that they were assigned at birth.286–289 Current evidence in the ageing transgender population suggests that both transgender men and transgender women are more at risk for various manifestations of CVD compared to others.290–294
Transfeminine hormone therapy
In the late 1990s, reports showed an increase up to 20-fold in VTE with oral ethinyloestradiol and this led to cessation of the use of this medication in this context.287 Since then, oestradiol has become the preferred oestrogen for the transfeminine treatment. The hyper-coagulable effect of oestrogen may be one of the mediators of the increased CVD risk in transgender women.295 Transdermal oestradiol is therefore preferred in transgender females over 40–50 years to avoid the increased risk of a prothrombotic state by oral intake.296 The VTE risk in transgender women, however, is different compared to cisgender women in whom this risk is mainly present in the first year of use and thereafter reduces over time.141 In contrast, VTE risk in transgender women increases over time, with a 2-year and 8-year risk of 4.1 (95% CI 1.6–6.7) and 16.7 (95% CI 6.4–27.5) per 1000 person-years, respectively, compared to the reference population of men 3.4 (95% CI 1.1–5.6) and women 13.7 (95% CI 4.1–22.7).293 Concomitant treatment of transgender women with androgen-lowering agents (e.g. spironolactone or cyproterone acetate) allows for administration of lower doses of exogenous oestrogen.285 , 297
Transgender women receiving hormonal therapy have no increased risk of MI but are at increased risk of stroke (127 per 100 000 person-years) and VTE (320 per 100 000 person-years). This is respectively 80% higher and 355% higher than in cisgender men.293 In addition, concomitant use of tobacco and other negative lifestyle factors in transgender persons are disproportionally present.298 Ischaemic stroke appears most pronounced after 6 years of continuous oestrogen use and continues to rise thereafter.
Cessation of cross-sex hormones is not an option for transgender persons. Therefore, they should always be encouraged to reduce modifiable lifestyle risks. The psychosocial benefits of hormone therapy with an improved body image may result in healthier lifestyle choices.
| Practice points |
|---|
|
|
|
Conflict of interest: AHEMM none; GR none; RC none; AC reports personal fees from Abbott, personal fees from Abiomed, personal fees from Cardinal Health , personal fees from Biosensor, personal fees from Magenta, outside the submitted work; DvD none; HH none; VK none; EL none; IL none; KM none; NP reports to have lectured and advised for pharma companies which produce MHT products. JCS reports grants and personal fees from Abbott, personal fees from Mitsubishi Tanabe, grants and personal fees from Mylan, grants and personal fees from Pfizer, personal fees from Bayer, personal fees from Gedeon Richter, outside the submitted work; MvT none; PC reports speaking honoraria from Menarini, outside the submitted work.
Contributor Information
Angela H E M Maas, Department of Cardiology, Director Women’s Cardiac Health Program, Radboud University Medical Center, Geert Grooteplein-Zuid 10, Route 616, 6525GA Nijmegen, The Netherlands.
Giuseppe Rosano, Centre for Clinical and Basic Research, Department of Medical Sciences, IRCCS San Raffaele Pisana, Rome, Italy.
Renata Cifkova, Center for Cardiovascular Prevention, Charles University in Prague, First Faculty of Medicine and Thomayer Hospital, Vídeňská 800, 140 59 Prague 4, Czech Republic; Department of Internal Cardiovascular Medicine, First Medical Faculty, Charles University in Prague and General University Hospital in Prague, U Nemocnice 2, 128 08 Prague 2, Czech Republic.
Alaide Chieffo, Interventional Cardiology Unit, IRCCS San Raffaele Hospital, Olgettina Street, 60 - 20132 Milan (Milan), Italy.
Dorenda van Dijken, Department of Obstetrics and Gynaecology, OLVG location West, Jan Tooropstraat 164, 1061 AE Amsterdam, The Netherlands.
Haitham Hamoda, Department Gynaecology, King's College Hospital, Denmark Hill, London SE5 9RS, UK.
Vijay Kunadian, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University and Cardiothoracic Centre, Freeman Hospital, Newcastle upon Tyne NHS Foundation Trust, M4:146 4th Floor William Leech Building, Newcastle upon Tyne NE2 4HH, UK.
Ellen Laan, Department of Sexology and Psychosomatic Gynaecology, Amsterdam University Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
Irene Lambrinoudaki, Menopause Clinic, 2nd Department of Obstetrics and Gynecology, National and Kapodistrian University of Athens, Aretaieio Hospital, 30 Panepistimiou Str., 10679 Athens, Greece.
Kate Maclaran, Department Gynaecology, Chelsea and Westminster Hospital, NHS Foundation Trust, 69 Fulham Road London SW10 9NH, UK.
Nick Panay, Department of Gynaecology, Queen Charlotte's & Chelsea and Westminster Hospitals, Imperial College, Du Cane Road, London W12 0HS, UK.
John C Stevenson, Department of Cardiology, National Heart & Lung Institute, Imperial College London, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK.
Mick van Trotsenburg, Bureau Gender PRO Vienna and Department of Obstetrics and Gynaecology, University Hospital St. Poelten-Lilienfeld, Probst Führer Straße 4 · 3100 St. Pölten, Austria.
Peter Collins, Department of Cardiology, National Heart & Lung Institute, Imperial College London, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK.
References
- 1. Morabia A, Costanza MC. International variability in ages at menarche, first livebirth, and menopause. World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Am J Epidemiol 1998;148:1195–1205. [DOI] [PubMed] [Google Scholar]
- 2. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 2008;60:210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res 2016;118:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 2006;27:575–605. [DOI] [PubMed] [Google Scholar]
- 5. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015;294:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 7. Schunkert H, Danser AHJ, Hense H-W, Derkx FHM, KüRzinger S, Riegger G¨N. AJ. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation 1997;95:39–45. [DOI] [PubMed] [Google Scholar]
- 8. Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the renin-angiotensin system. Hypertension 2010;56:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 2014;29:8–15. [DOI] [PubMed] [Google Scholar]
- 10.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins P, Rosano G, Casey C, Daly C, Gambacciani M, Hadji P, Kaaja R, Mikkola T, Palacios S, Preston R, Simon T, Stevenson J, Stramba-Badiale M. Management of cardiovascular risk in the peri-menopausal woman: a consensus statement of European cardiologists and gynaecologists. Eur Heart J 2007;28:2028–2040. [DOI] [PubMed] [Google Scholar]
- 12. Bullock-Palmer RP, Shaw LJ, Gulati M. Emerging misunderstood presentations of cardiovascular disease in young women. Clin Cardiol 2019;42:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humphries KH, Izadnegahdar M, Sedlak T, Saw J, Johnston N, Schenck-Gustafsson K, Shah RU, Regitz-Zagrosek V, Grewal J, Vaccarino V, Wei J, Bairey Merz CN. Sex differences in cardiovascular disease—impact on care and outcomes. Front Neuroendocrinol 2017;46:46–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EUGenMed Cardiovascular Clinical Study Group, Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Maas AH, Kautzky-Willer A, Knappe-Wegner D, Kintscher U, Ladwig KH, Schenck-Gustafsson K, Stangl V. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2016;37:24–34. [DOI] [PubMed] [Google Scholar]
- 15. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, Louise Buchanan G, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Meer MG, van der Graaf Y, Schuit E, Peelen LM, Verschuren WM, Boer JM, Moons KG, Nathoe HM, Appelman Y, van der Schouw YT. Added value of female-specific factors beyond traditional predictors for future cardiovascular disease. J Am Coll Cardiol 2016;67:2084–2086. [DOI] [PubMed] [Google Scholar]
- 17. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, Waring ME, Schenken RS, Johnson KC, Edstedt-Bonamy AK, Allison MA, Howard BV. Reproductive risk factors and coronary heart disease in the Women's Health Initiative Observational Study. Circulation 2016;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi J, Daskalopoulou SS, Thanassoulis G, Karp I, Pelletier R, Behlouli H, Pilote L; GENESIS-PRAXY Investigators. Sex- and gender-related risk factor burden in patients with premature acute coronary syndrome. Can J Cardiol 2014;30:109–117. [DOI] [PubMed] [Google Scholar]
- 19. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, Selmer R, Iversen AC, Daltveit AK. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol 2019;282:81–87. [DOI] [PubMed] [Google Scholar]
- 20. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sondergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park K, Salmoirago-Blotcher E, Sattari M, Sealy-Jefferson S, Shadyab AH, Valdiviezo C, Manson JE, Parikh NI. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol 2020;5:1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cainzos-Achirica M, Fedeli U, Sattar N, Agyemang C, Jenum AK, McEvoy JW, Murphy JD, Brotons C, Elosua R, Bilal U, Kanaya AM, Kandula NR, Martinez-Amezcua P, Comin-Colet J, Pinto X. Epidemiology, risk factors, and opportunities for prevention of cardiovascular disease in individuals of South Asian ethnicity living in Europe. Atherosclerosis 2019;286:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P; European Society of Cardiology. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 27. Jorstad HT, Colkesen EB, Boekholdt SM, Tijssen JG, Wareham NJ, Khaw KT, Peters RJ. Estimated 10-year cardiovascular mortality seriously underestimates overall cardiovascular risk. Heart 2016;102:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, Ricceri F, d'Errico A, Barros H, Bochud M, Chadeau-Hyam M, Clavel-Chapelon F, Costa G, Delpierre C, Fraga S, Goldberg M, Giles GG, Krogh V, Kelly-Irving M, Layte R, Lasserre AM, Marmot MG, Preisig M, Shipley MJ, Vollenweider P, Zins M, Kawachi I, Steptoe A, Mackenbach JP, Vineis P, Kivimäki M; LIFEPATH consortium. Socioeconomic status and the 25 x 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017;389:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gabet A, Danchin N, Juilliere Y, Olie V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004-14. Eur Heart J 2017;38:1060–1065. [DOI] [PubMed] [Google Scholar]
- 30. Chieffo A, Buchanan GL, Mehilli J, Capodanno D, Kunadian V, Petronio AS, Mikhail GW, Capranzano P, Gonzal N, Karam N, Manzo-Silberman S, Schupke S, Byrne RA, Capretti G, Appelman Y, Morice MC, Presbitero P, Radu M, Mauri J. Percutaneous coronary and structural interventions in women: a position statement from the EAPCI Women Committee. EuroIntervention 2018;14:e1227–e35. [DOI] [PubMed] [Google Scholar]
- 31. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 32. Adlam D, Alfonso F, Maas A, Vrints C, Al-Hussaini A, Bueno H, Capranzano P, Gevaert S, Hoole SP, Johnson T, Lettieri C, Maeder MT, Motreff P, Ong P, Persu A, Rickli H, Schiele F, Sheppard MN, Swahn E; Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Juni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;doi:10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 34. Ghadri J-R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y.-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moller C, Stiermaier T, Brabant G, Graf T, Thiele H, Eitel I. Comprehensive assessment of sex hormones in Takotsubo syndrome. Int J Cardiol 2018;250:11–15. [DOI] [PubMed] [Google Scholar]
- 36. Konst RE, Elias-Smale SE, Lier A, Bode C, Maas AH. Different cardiovascular risk factors and psychosocial burden in symptomatic women with and without obstructive coronary artery disease. Eur J Prev Cardiol 2019;26:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnston N, Schenck-Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J 2011;32:1331–1336. [DOI] [PubMed] [Google Scholar]
- 39. Qureshi W, Blaha MJ, Nasir K, Al-Mallah MH. Gender differences in coronary plaque composition and burden detected in symptomatic patients referred for coronary computed tomographic angiography. Int J Cardiovasc Imaging 2013;29:463–469. [DOI] [PubMed] [Google Scholar]
- 40. Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J 2011;161:681–688. [DOI] [PubMed] [Google Scholar]
- 41. Frink RJ. Gender gap, inflammation and acute coronary disease: are women resistant to atheroma growth? Observations at autopsy. J Invasive Cardiol 2009;21:270–277. [PubMed] [Google Scholar]
- 42. Han SH, Bae JH, Holmes DR Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 2008;29:1359–1369. [DOI] [PubMed] [Google Scholar]
- 43. Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al-Mallah MH, Budoff MJ, Blaha MJ. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, Kaski JC, Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: why, how, and when. JACC Cardiovasc Interv 2020;13:1847–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Padro T, Manfrini O, Bugiardini R, Canty J, Cenko E, De Luca G, Duncker DJ, Eringa EC, Koller A, Tousoulis D, Trifunovic D, Vavlukis M, de Wit C, Badimon L. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc Res 2020;116:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ, Bairey Merz CN. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J 2013;166:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, Lopes RD, Lopez-Sendon JL, Held C, Szwed H, Senior R, Gosselin G, Nair RG, Elghamaz A, Bockeria O, Chen J, Chernyavskiy AM, Bhargava B, Newman JD, Hinic SB, Jaroch J, Hoye A, Berger J, Boden WE, O’Brien SM, Maron DJ, Hochman JS; ISCHEMIA Research Group. Association of sex with severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2020;5:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 49. Haider A, Bengs S, Luu J, Osto E, Siller-Matula JM, Muka T, Gebhard C. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur Heart J 2020;41:1328–1336. [DOI] [PubMed] [Google Scholar]
- 50. Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin Reprod Med 2009;27:338–345. [DOI] [PubMed] [Google Scholar]
- 51. Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, Santoro N, Simoncini T. Menopause. Nat Rev Dis Primers 2015;1:15004. [DOI] [PubMed] [Google Scholar]
- 52. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 2007;14(3 Pt 1):373–384. [DOI] [PubMed] [Google Scholar]
- 53. Collins P, Maas A, Prasad M, Schierbeck L, Lerman A. Endothelial vascular function as a surrogate of vascular risk and aging in women. Mayo Clin Proc 2020;95:541–553. [DOI] [PubMed] [Google Scholar]
- 54. O'Keeffe LM, Kuh D, Fraser A, Howe LD, Lawlor D, Hardy R. Age at period cessation and trajectories of cardiovascular risk factors across mid and later life. Heart 2020;106:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gierach GL, Johnson BD, Bairey Merz CN, Kelsey SF, Bittner V, Olson MB, Shaw LJ, Mankad S, Pepine CJ, Reis SE, Rogers WJ, Sharaf BL, Sopko G; WISE Study Group. Hypertension, menopause, and coronary artery disease risk in the Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol 2006;47:S50–S58. [DOI] [PubMed] [Google Scholar]
- 56. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 2012;97:4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, Chrousos GP, Michalis LK. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab 2010;95:1199–1206. [DOI] [PubMed] [Google Scholar]
- 58. Robinson JG, Wallace R, Limacher M, Ren H, Cochrane B, Wassertheil-Smoller S, Ockene JK, Blanchette PL, Ko MG. Cardiovascular risk in women with non-specific chest pain (from the Women's Health Initiative Hormone Trials). Am J Cardiol 2008;102:693–699. [DOI] [PubMed] [Google Scholar]
- 59. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update 2017;23:300–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv Clin Chem 2015;72:1–75. [DOI] [PubMed] [Google Scholar]
- 62. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev 2017;38:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, Qorbani M, Pakzad R, Hasanzadeh A, Sullman MJM, Safiri S. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause 2018;25:1155–1164. [DOI] [PubMed] [Google Scholar]
- 64. Choi Y, Chang Y, Kim BK, Kang D, Kwon MJ, Kim CW, Jeong C, Ahn Y, Park HY, Ryu S, Cho J. Menopausal stages and serum lipid and lipoprotein abnormalities in middle-aged women. Maturitas 2015;80:399–405. [DOI] [PubMed] [Google Scholar]
- 65. Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, Thomson LE, Berman DS, Shaw LJ, Petersen JW, Brown GH, Anderson RD, Shuster JJ, Cook-Wiens G, Rogatko A, Pepine CJ. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 67. Anagnostis P, Theocharis P, Lallas K, Konstantis G, Mastrogiannis K, Bosdou JK, Lambrinoudaki I, Stevenson JC, Goulis DG. Early menopause is associated with increased risk of arterial hypertension: a systematic review and meta-analysis. Maturitas 2020;135:74–79. [DOI] [PubMed] [Google Scholar]
- 68. Cadeddu C, Franconi F, Cassisa L, Campesi I, Pepe A, Cugusi L, Maffei S, Gallina S, Sciomer S, Mercuro G; Working Group of Gender Medicine of Italian Society of Cardiology. Arterial hypertension in the female world: pathophysiology and therapy. J Cardiovasc Med (Hagerstown) 2016;17:229–236. [DOI] [PubMed] [Google Scholar]
- 69. Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension 2005;45:170–174. [DOI] [PubMed] [Google Scholar]
- 70. Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension 2008;51:952–959. [DOI] [PubMed] [Google Scholar]
- 71. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 72. Hage FG, Mansur SJ, Xing D, Oparil S. Hypertension in women. Kidney Int Suppl (2011) 2013;3:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leung AA, Williams JVA, McAlister FA, Campbell NRC, Padwal RS, Tran K, Tsuyuki R, McAlister FA, Campbell NRC, Khan N, Padwal R, Quan H, Leung AA; Hypertension Canada’s Research and Evaluation Committee. Worsening hypertension awareness, treatment, and control rates in Canadian women between 2007 and 2017. Can J Cardiol 2020;36:732–739. [DOI] [PubMed] [Google Scholar]
- 74. Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension 1995;26:60–69. [DOI] [PubMed] [Google Scholar]
- 75. Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension 2000;36:780–789. [DOI] [PubMed] [Google Scholar]
- 76. Jackson EA, El Khoudary SR, Crawford SL, Matthews K, Joffe H, Chae C, Thurston RC. Hot flash frequency and blood pressure: data from the Study of Women's Health Across the Nation. J Womens Health (Larchmt) 2016;25:1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pechere-Bertschi A, Burnier M. Gonadal steroids, salt-sensitivity and renal function. Curr Opin Nephrol Hypertens 2007;16:16–21. [DOI] [PubMed] [Google Scholar]
- 78. Tominaga T, Suzuki H, Ogata Y, Matsukawa S, Saruta T. The role of sex hormones and sodium intake in postmenopausal hypertension. J Hum Hypertens 1991;5:495–500. [PubMed] [Google Scholar]
- 79. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020;5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens 2004;17:994–1001. [DOI] [PubMed] [Google Scholar]
- 81. Mancia G. Blood pressure control in the hypertensive population. Is the trend favourable? J Hypertens 2013;31:1094–1095. [DOI] [PubMed] [Google Scholar]
- 82. Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, Maas AH. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol 2012;19:1138–1144. [DOI] [PubMed] [Google Scholar]
- 83. Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol 2012;59:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis 2007;49:241–251. [DOI] [PubMed] [Google Scholar]
- 85. Coutinho T, Bailey KR, Turner ST, Kullo IJ. Arterial stiffness is associated with increase in blood pressure over time in treated hypertensives. J Am Soc Hypertens 2014;8:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 87. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868. c. [DOI] [PubMed] [Google Scholar]
- 88. Pepine CJ, Merz CNB, El Hajj S, Ferdinand KC, Hamilton MA, Lindley KJ, Nelson MD, Quesada O, Wenger NK, Fleg JL. Heart failure with preserved ejection fraction: similarities and differences between women and men. Int J Cardiol 2020;304:101–108. [DOI] [PubMed] [Google Scholar]
- 89. Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol 2014;8:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020;396:565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lasrado N, Jia T, Massilamany C, Franco R, Illes Z, Reddy J. Mechanisms of sex hormones in autoimmunity: focus on EAE. Biol Sex Differ 2020;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Amaya-Amaya J, Montoya-Sánchez L, Rojas-Villarraga A. Cardiovascular involvement in autoimmune diseases. Biomed Res Int 2014;2014:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Del Buono M, Abbate A, Toldo S. Interplay of inflammation, oxidative stress and cardiovascular disease in rheumatoid arthritis. Heart 2018;104:1991–1992. [DOI] [PubMed] [Google Scholar]
- 95. Agca R, Heslinga SC, van Halm VP, Nurmohamed MT. Atherosclerotic cardiovascular disease in patients with chronic inflammatory joint disorders. Heart 2016;102:790–795. [DOI] [PubMed] [Google Scholar]
- 96. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 2006;361:1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 2010;122:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers M, Randolph JF Jr. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). J Affect Disord 2007;103:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, Davis SR, Gompel AA, Henderson VW, Langer R, Lobo RA, Plu-Bureau G, Sturdee DW; on behalf of the International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013;16:316–337. [DOI] [PubMed] [Google Scholar]
- 101. Armeni E, Lambrinoudaki I, Ceausu I, Depypere H, Mueck A, Pérez-López FR, Schouw YT, Senturk LM, Simoncini T, Stevenson JC, Stute P, Rees M. Maintaining postreproductive health: a care pathway from the European Menopause and Andropause Society (EMAS). Maturitas 2016;89:63–72. [DOI] [PubMed] [Google Scholar]
- 102. Lumsden MA, Davies M, Sarri G; Guideline Development Group for Menopause: Diagnosis and Management (NICE Clinical Guideline No. 23). Diagnosis and Management of Menopause: the National Institute of Health and Care Excellence (NICE) guideline. JAMA Intern Med 2016;176:1205–1206. [DOI] [PubMed] [Google Scholar]
- 103. Neves ECM, Birkhauser M, Samsioe G, Lambrinoudaki I, Palacios S, Borrego RS, Llaneza P, Ceausu I, Depypere H, Erel CT, Perez-Lopez FR, Schenck-Gustafsson K, van der Schouw YT, Simoncini T, Tremollieres F, Rees M. EMAS position statement: the ten point guide to the integral management of menopausal health. Maturitas 2015;81:88–92. [DOI] [PubMed] [Google Scholar]
- 104. Berin E, Hammar M, Lindblom H, Lindh-Åstrand L, Rubér M, Spetz Holm A-C. Resistance training for hot flushes in postmenopausal women: a randomised controlled trial. Maturitas 2019;126:55–60. [DOI] [PubMed] [Google Scholar]
- 105. Eigendorf J, Melk A, Haufe S, Boethig D, Berliner D, Kerling A, Kueck M, Stenner H, Bara C, Stiesch M, Schippert C, Hilfiker A, Falk C, Bauersachs J, Thum T, Lichtinghagen R, Haverich A, Hilfiker-Kleiner D, Tegtbur U. Effects of personalized endurance training on cellular age and vascular function in middle-aged sedentary women. Eur J Prev Cardiol 2019;26:1903–1906. [DOI] [PubMed] [Google Scholar]
- 106. Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, Bobo WV, Rubin LH, Koleva HK, Cohen LS, Soares CN; on behalf of the Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Womens Health (Larchmt) 2019;28:117–134. [DOI] [PubMed] [Google Scholar]
- 107. Geukes M, van Aalst MP, Robroek SJ, Laven JS, Oosterhof H. The impact of menopause on work ability in women with severe menopausal symptoms. Maturitas 2016;90:3–8. [DOI] [PubMed] [Google Scholar]
- 108. Griffiths A, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Perez-Lopez FR, van der Schouw YT, Senturk LM, Simoncini T, Stevenson JC, Stute P, Rees M. EMAS recommendations for conditions in the workplace for menopausal women. Maturitas 2016;85:79–81. [DOI] [PubMed] [Google Scholar]
- 109. van der Schouw YT, Grobbee DE. Menopausal complaints, oestrogens, and heart disease risk: an explanation for discrepant findings on the benefits of post-menopausal hormone therapy. Eur Heart J 2005;26:1358–1361. [DOI] [PubMed] [Google Scholar]
- 110. Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension 2008;51:1492–1498. [DOI] [PubMed] [Google Scholar]
- 111. Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, Wijnands-van Gent CJ, van der Schouw YT. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause 2011;18:146–151. [DOI] [PubMed] [Google Scholar]
- 112. Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M, Franco OH. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PLoS One 2016;11:e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol 2014;142:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension 2014;63:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tuomikoski P, Savolainen-Peltonen H. Vasomotor symptoms and metabolic syndrome. Maturitas 2017;97:61–65. [DOI] [PubMed] [Google Scholar]
- 116. Collen AC, Manhem K, Sverrisdottir YB. Sympathetic nerve activity in women 40 years after a hypertensive pregnancy. J Hypertens 2012;30:1203–1210. [DOI] [PubMed] [Google Scholar]
- 117. Drost JT, van der Schouw YT, Herber-Gast GC, Maas AH. More vasomotor symptoms in menopause among women with a history of hypertensive pregnancy diseases compared with women with normotensive pregnancies. Menopause 2013;20:1006–1011. [DOI] [PubMed] [Google Scholar]
- 118. Gray KE, Katon JG, LeBlanc ES, Woods NF, Bastian LA, Reiber GE, Weitlauf JC, Nelson KM, LaCroix AZ. Vasomotor symptom characteristics: are they risk factors for incident diabetes? Menopause 2018;25:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thurston RC, Chang Y, Barinas-Mitchell E, Jennings JR, von Kanel R, Landsittel DP, Matthews KA. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause 2018;25:1354–1361. [DOI] [PubMed] [Google Scholar]
- 120. Biglia N, Cagnacci A, Gambacciani M, Lello S, Maffei S, Nappi RE. Vasomotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric 2017;20:306–312. [DOI] [PubMed] [Google Scholar]
- 121. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation 2008;118:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 123. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M; Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 124. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S; Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 126. Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006;21:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lokkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard O. Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J 2008;29:2660–2668. [DOI] [PubMed] [Google Scholar]
- 128. Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Kober L, Jensen JE. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345:e6409–e6409. [DOI] [PubMed] [Google Scholar]
- 129. Boardman H, Hartley L, Eisinga A, Main C, Figuls MR. Cochrane corner: oral hormone therapy and cardiovascular outcomes in post-menopausal women. Heart 2016;102:9–11. [DOI] [PubMed] [Google Scholar]
- 130. Tuomikoski P, Lyytinen H, Korhonen P, Hoti F, Vattulainen P, Gissler M, Ylikorkala O, Mikkola TS. Coronary heart disease mortality and hormone therapy before and after the Women's Health Initiative. Obstet Gynecol 2014;124:947–953. [DOI] [PubMed] [Google Scholar]
- 131. Mikkola TS, Tuomikoski P, Lyytinen H, Korhonen P, Hoti F, Vattulainen P, Gissler M, Ylikorkala O. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab 2015;100:4588–4594. [DOI] [PubMed] [Google Scholar]
- 132. Miller VM, Jenkins GD, Biernacka JM, Heit JA, Huggins GS, Hodis HN, Budoff MJ, Lobo RA, Taylor HS, Manson JE, Black DM, Naftolin F, Harman SM, de Andrade M. Pharmacogenomics of estrogens on changes in carotid artery intima-medial thickness and coronary arterial calcification: kronos Early Estrogen Prevention Study. Physiol Genomics 2016;48:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hirschberg AL, Tani E, Brismar K, Lundström E. Effects of drospirenone and norethisterone acetate combined with estradiol on mammographic density and proliferation of breast epithelial cells-A prospective randomized trial. Maturitas 2019;126:18–24. [DOI] [PubMed] [Google Scholar]
- 135. Santen RJ. Use of cardiovascular age for assessing risks and benefits of menopausal hormone therapy. Menopause 2017;24:589–595. [DOI] [PubMed] [Google Scholar]
- 136.Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019;394:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:419–427. [DOI] [PubMed] [Google Scholar]
- 138. Stevenson JC, Farmer RDT. HRT and breast cancer: a million women ride again. Climacteric 2020; 23:226–223. [DOI] [PubMed] [Google Scholar]
- 139. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat 2007;107:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric 2018;21:111–122. [DOI] [PubMed] [Google Scholar]
- 141. Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ 2008;336:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 143. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 2011;1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Christ JP, Gunning MN, Palla G, Eijkemans MJC, Lambalk CB, Laven JSE, Fauser B. Estrogen deprivation and cardiovascular disease risk in primary ovarian insufficiency. Fertil Steril 2018;109:594–600.e1. [DOI] [PubMed] [Google Scholar]
- 146. European Society For Human R, Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F, Liao L, Vlaisavljevic V, Zillikens C, Vermeulen N; Embryology Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- 147. Marchetti C, De Felice F, Boccia S, Sassu C, Di Donato V, Perniola G, Palaia I, Monti M, Muzii L, Tombolini V, Benedetti Panici P. Hormone replacement therapy after prophylactic risk-reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018;132:111–115. [DOI] [PubMed] [Google Scholar]
- 148. Vermeulen RFM, Korse CM, Kenter GG, Brood-van Zanten MMA, Beurden MV. Safety of hormone replacement therapy following risk-reducing salpingo-oophorectomy: systematic review of literature and guidelines. Climacteric 2019;22:352–360. [DOI] [PubMed] [Google Scholar]
- 149. Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–562. [DOI] [PubMed] [Google Scholar]
- 150. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- 151. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–279. [DOI] [PubMed] [Google Scholar]
- 152. Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, Gold EB, Derby CA, Matthews KA, Cade JE, Greenwood DC, Demakakos P, Brown DE, Sievert LL, Anderson D, Hayashi K, Lee JS, Mizunuma H, Tillin T, Simonsen MK, Adami HO, Weiderpass E, Mishra GD. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tao XY, Zuo AZ, Wang JQ, Tao FB. Effect of primary ovarian insufficiency and early natural menopause on mortality: a meta-analysis. Climacteric 2016;19:27–36. [DOI] [PubMed] [Google Scholar]
- 154. Roeters van Lennep JE, Heida KY, Bots ML, Hoek A; collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:178–186. [DOI] [PubMed] [Google Scholar]
- 155. Lip GY, Blann AD, Jones AF, Beevers DG. Effects of hormone-replacement therapy on hemostatic factors, lipid factors, and endothelial function in women undergoing surgical menopause: implications for prevention of atherosclerosis. Am Heart J 1997;134:764–771. [DOI] [PubMed] [Google Scholar]
- 156. Knauff EA, Westerveld HE, Goverde AJ, Eijkemans MJ, Valkenburg O, van Santbrink EJ, Fauser BC, van der Schouw YT. Lipid profile of women with premature ovarian failure. Menopause 2008;15:919–923. [DOI] [PubMed] [Google Scholar]
- 157. Daan NM, Muka T, Koster MP, Roeters van Lennep JE, Lambalk CB, Laven JS, Fauser CG, Meun C, de Rijke YB, Boersma E, Franco OH, Kavousi M, Fauser BC. Cardiovascular risk in women with premature ovarian insufficiency compared to premenopausal women at middle age. J Clin Endocrinol Metab 2016;101:3306–3315. [DOI] [PubMed] [Google Scholar]
- 158. Gunning MN, van Rijn BB, Bekker MN, de Wilde MA, Eijkemans MJC, Fauser B. Associations of preconception Body Mass Index in women with PCOS and BMI and blood pressure of their offspring. Gynecol Endocrinol 2019;35:673–678. [DOI] [PubMed] [Google Scholar]
- 159. Corrigan EC, Nelson LM, Bakalov VK, Yanovski JA, Vanderhoof VH, Yanoff LB, Bondy CA. Effects of ovarian failure and X-chromosome deletion on body composition and insulin sensitivity in young women. Menopause 2006;13:911–916. [DOI] [PubMed] [Google Scholar]
- 160. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab 2004;89:3907–3913. [DOI] [PubMed] [Google Scholar]
- 161. Eshtiaghi R, Esteghamati A, Nakhjavani M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010;65:262–266. [DOI] [PubMed] [Google Scholar]
- 162. Daan NM, Koster MP, de Wilde MA, Dalmeijer GW, Evelein AM, Fauser BC, de Jager W. Biomarker profiles in women with PCOS and PCOS offspring; a Pilot study. PLoS One 2016;11:e0165033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Anagnostis P, Christou K, Artzouchaltzi AM, Gkekas NK, Kosmidou N, Siolos P, Paschou SA, Potoupnis M, Kenanidis E, Tsiridis E, Lambrinoudaki I, Stevenson JC, Goulis DG. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol 2019;180:41–50. [DOI] [PubMed] [Google Scholar]
- 164. Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A, Meczekalski B, Zozulinska-Ziolkiewicz D, Jaremek JD, Cano A, Chedraui P, Goulis DG, Lopes P, Mishra G, Mueck A, Rees M, Senturk LM, Simoncini T, Stevenson JC, Stute P, Tuomikoski P, Paschou SA, Anagnostis P, Lambrinoudaki I. Menopause and diabetes: EMAS clinical guide. Maturitas 2018;117:6–10. [DOI] [PubMed] [Google Scholar]
- 165. Gunning MN, Meun C, van Rijn BB, Maas AHEM, Benschop L, Franx A, Boersma E, Budde RPJ, Appelman Y, Lambalk CB, Eijkemans MJC, Velthuis BK, Laven JSE, Fauser BCJM; On behalf of the CREW‐consortium. Coronary artery calcification in middle-aged women with premature ovarian insufficiency. Clin Endocrinol (Oxf) 2019;91:314–322. [DOI] [PubMed] [Google Scholar]
- 166. Laven JSE, Visser JA, Uitterlinden AG, Vermeij WP, Hoeijmakers JHJ. Menopause: genome stability as new paradigm. Maturitas 2016;92:15–23. [DOI] [PubMed] [Google Scholar]
- 167. Shukla PC, Singh KK, Quan A, Al-Omran M, Teoh H, Lovren F, Cao L, Rovira II, Pan Y, Brezden-Masley C, Yanagawa B, Gupta A, Deng CX, Coles JG, Leong-Poi H, Stanford WL, Parker TG, Schneider MD, Finkel T, Verma S. BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nat Commun 2011;2:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley-Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J 3rd, McNamara DM, Seidman CE, Seidman JG, Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821–828. [DOI] [PubMed] [Google Scholar]
- 170. Lokkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of Hormone Therapy. Maturitas 2006;53:226–233. [DOI] [PubMed] [Google Scholar]
- 171. Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, Critchley HO, Newby DE, Wallace WH. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 2009;53:805–811. [DOI] [PubMed] [Google Scholar]
- 172.ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open 2018;2018:hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Oliver-Williams CT, Heydon EE, Smith GC, Wood AM. Miscarriage and future maternal cardiovascular disease: a systematic review and meta-analysis. Heart 2013;99:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wagner MM, Bhattacharya S, Visser J, Hannaford PC, Bloemenkamp KW. Association between miscarriage and cardiovascular disease in a Scottish cohort. Heart 2015;101:1954–1960. [DOI] [PubMed] [Google Scholar]
- 175. Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015;241:211–218. [DOI] [PubMed] [Google Scholar]
- 176. Garcı’a-Enguı’danos A, Calle ME, Valero J, Luna S, Domı’nguez-Rojas V, Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol 2002;102:111–119. [DOI] [PubMed] [Google Scholar]
- 177. Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdés G. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007;49:90–95. [DOI] [PubMed] [Google Scholar]
- 178. Ranthe MF, Diaz LJ, Behrens I, Bundgaard H, Simonsen J, Melbye M, Boyd HA. Association between pregnancy losses in women and risk of atherosclerotic disease in their relatives: a nationwide cohort studydagger. Eur Heart J 2016;37:900–907. [DOI] [PubMed] [Google Scholar]
- 179. Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2014. Natl Vital Stat Rep 2015;64:1–64. [PubMed] [Google Scholar]
- 180. Zeitlin J, Szamotulska K, Drewniak N, Mohangoo AD, Chalmers J, Sakkeus L, Irgens L, Gatt M, Gissler M, Blondel B; The Euro‐Peristat Preterm Study Group. Preterm birth time trends in Europe: a study of 19 countries. BJOG 2013;120:1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ, Rich-Edwards JW. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Heida KY, Velthuis BK, Oudijk MA, Reitsma JB, Bots ML, Franx A, van Dunné FM; Dutch Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:253–263. [DOI] [PubMed] [Google Scholar]
- 184. Heida KY, Bots ML, de Groot CJ, van Dunne FM, Hammoud NM, Hoek A, Laven JS, Maas AH, Roeters van Lennep JE, Velthuis BK, Franx A. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol 2016;23:1863–1879. [DOI] [PubMed] [Google Scholar]
- 185. Nilsson PM, Li X, Sundquist J, Sundquist K. Maternal cardiovascular disease risk in relation to the number of offspring born small for gestational age: national, multi-generational study of 2.7 million births. Acta Paediatr 2009;98:985–989. [DOI] [PubMed] [Google Scholar]
- 186. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S; International Society for the Study of Hypertension in Pregnancy (ISSHP). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- 187. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 188. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 189. Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J. Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J 2015;36:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gammill HS, Chettier R, Brewer A, Roberts JM, Shree R, Tsigas E, Ward K. Cardiomyopathy and preeclampsia. Circulation 2018;138:2359–2366. [DOI] [PubMed] [Google Scholar]
- 191. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 192. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA; ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 193. Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, van der Graaf Y, de Groot CJM, Maas AHEM, Roeters van Lennep JE, Steegers EAP, Visseren FL, van Rijn BB, Velthuis BK, Franx A; CREW Consortium. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45- to 55-year-old women with a history of preeclampsia. Circulation 2018;137:877–879. [DOI] [PubMed] [Google Scholar]
- 194. Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, Vatten LJ, Romundstad PR, Rich-Edwards JW, Åsvold BO. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord-Trondelag Health Study. JAMA Cardiol 2019;4:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–951. [DOI] [PubMed] [Google Scholar]
- 197. Cifkova R. Cardiovascular sequels of hypertension in pregnancy. J Am Heart Assoc 2018;7:e009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Smith GN, Louis JM, Saade GR. Pregnancy and the postpartum period as an opportunity for cardiovascular risk identification and management. Obstet Gynecol 2019;134:851–862. [DOI] [PubMed] [Google Scholar]
- 199. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 200.Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 2018;131:e49–e64. [DOI] [PubMed] [Google Scholar]
- 201. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905–914. [DOI] [PubMed] [Google Scholar]
- 203.American Diabetes Association. 16. Diabetes Advocacy: standards of Medical Care in Diabetes-2019. Diabetes Care 2020;43(Suppl 1):S203–S204. [DOI] [PubMed] [Google Scholar]
- 204. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–1036. [DOI] [PubMed] [Google Scholar]
- 205. Fryearson J, Adamson DL. Heart disease in pregnancy: ischaemic heart disease. Best Pract Res Clin Obstet Gynaecol 2014;28:551–562. [DOI] [PubMed] [Google Scholar]
- 206. Ramlakhan KP, Johnson MR, Roos-Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol 2020;17:718–731. [DOI] [PubMed] [Google Scholar]
- 207. Arnaout R, Nah G, Marcus G, Tseng Z, Foster E, Harris IS, Divanji P, Klein L, Gonzalez J, Parikh N. Pregnancy complications and premature cardiovascular events among 1.6 million California pregnancies. Open Heart 2019;6:e000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation 2006;113:1564–1571. [DOI] [PubMed] [Google Scholar]
- 209. Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol 2008;52:171–180. [DOI] [PubMed] [Google Scholar]
- 210. Roos-Hesselink J, Baris L, Johnson M, De Backer J, Otto C, Marelli A, Jondeau G, Budts W, Grewal J, Sliwa K, Parsonage W, Maggioni AP, van Hagen I, Vahanian A, Tavazzi L, Elkayam U, Boersma E, Hall R. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019;40:3848–3855. [DOI] [PubMed] [Google Scholar]
- 211. Cauldwell M, Steer PJ, von Klemperer K, Kaler M, Grixti S, Hale J, O'Heney J, Warriner D, Curtis S, Mohan AR, Dockree S, Mackillop L, Head CEG, Sterrenberg M, Wallace S, Freeman LJ, Patridge G, Baalman JH, McAuliffe FM, Simpson M, Walker N, Girling J, Siddiqui F, Bolger AP, Bredaki F, Walker F, Vause S, Gatzoulis MA, Johnson MR, Roberts A. Maternal and neonatal outcomes in women with history of coronary artery disease. Heart 2020;106:380–386. [DOI] [PubMed] [Google Scholar]
- 212. Tweet MS, Young KA, Best PJM, Hyun M, Gulati R, Rose CH, Hayes SN. Association of pregnancy with recurrence of spontaneous coronary artery dissection among women with prior coronary artery dissection. JAMA Netw Open 2020;3:e2018170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351–96358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 215. Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 2018;24:455–467. [DOI] [PubMed] [Google Scholar]
- 216. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16:347–363. [DOI] [PubMed] [Google Scholar]
- 217. Wild RA. Dyslipidemia in PCOS. Steroids 2012;77:295–299. [DOI] [PubMed] [Google Scholar]
- 218. Lim S, Smith CA, Costello MF, MacMillan F, Moran L, Ee C. Barriers and facilitators to weight management in overweight and obese women living in Australia with PCOS: a qualitative study. BMC Endocr Disord 2019;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001;86:2453–2455. [DOI] [PubMed] [Google Scholar]
- 220. Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJ, Aziz N, Green DJ, Cable NT, Jones H. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta-analysis of the observational studies. Clin Endocrinol (Oxf) 2013;78:438–446. [DOI] [PubMed] [Google Scholar]
- 221. Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012;18:112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Calderon-Margalit R, Siscovick D, Merkin SS, Wang E, Daviglus ML, Schreiner PJ, Sternfeld B, Williams OD, Lewis CE, Azziz R, Schwartz SM, Wellons MF. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: the Coronary Artery Risk Development in Young Adults Women's study. Arterioscler Thromb Vasc Biol 2014;34:2688–2694. [DOI] [PubMed] [Google Scholar]
- 223. Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab 2007;92:4609–4614. [DOI] [PubMed] [Google Scholar]
- 224. Meun C, Gunning MN, Louwers YV, Peters H, Roos‐Hesselink J, Roeters van Lennep J, Rueda Ochoa O‐L, Appelman Y, Lambalk N, Boersma E, Kavousi M, Fauser BC, Laven JS, Baart S, Benschop L, Brouwers L, Budde R, Cannegieter S, Dam V, Eijkemans R, Ferrari M, Franx A, de Groot C, Hoek A, Koffijberg E, Koster W, Kruit M, Lagerweij G, Linstra K, van der Lugt A, Maas A, Maassen van den Brink A, Middeldorp S, Moons KG, van Rijn B, Scheres L, van der Schouw YT, Steegers E, Steegers R, Terwindt G, Velthuis B, Wermer M, Zick B, Zoet G; on behalf of the CREW consortium. The cardiovascular risk profile of middle-aged women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2020;92:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update 2011;17:495–500. [DOI] [PubMed] [Google Scholar]
- 226. Zhao L, Zhu Z, Lou H, Zhu G, Huang W, Zhang S, Liu F. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 2016;7:33715–33721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, Hart R, Johnson L, Jordan C, Legro RS, Norman RJ, Mocanu E, Qiao J, Rodgers RJ, Rombauts L, Tassone EC, Thangaratinam S, Vanky E, Teede HJ; International PCOS Network. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open 2019;2019:hoy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol 2020;36:12–23. [DOI] [PubMed] [Google Scholar]
- 229. Minooee S, Ramezani Tehrani F, Rahmati M, Mansournia MA, Azizi F. Prediction of age at menopause in women with polycystic ovary syndrome. Climacteric 2018;21:29–34. [DOI] [PubMed] [Google Scholar]
- 230. Khatibi A, Agardh CD, Shakir YA, Nerbrand C, Nyberg P, Lidfeldt J, Samsioe G. Could androgens protect middle-aged women from cardiovascular events? A population-based study of Swedish women: the Women's Health in the Lund Area (WHILA) Study. Climacteric 2007;10:386–392. [DOI] [PubMed] [Google Scholar]
- 231. Carmina E, Fruzzetti F, Lobo RA. Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol Endocrinol 2018;34:301–304. [DOI] [PubMed] [Google Scholar]
- 232. Li J, Eriksson M, Czene K, Hall P, Rodriguez-Wallberg KA. Common diseases as determinants of menopausal age. Hum Reprod 2016;31:2856–2864. [DOI] [PubMed] [Google Scholar]
- 233. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine S. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Tan J, Taskin O, Iews M, Lee AJ, Kan A, Rowe T, Bedaiwy MA. Atherosclerotic cardiovascular disease in women with endometriosis: a systematic review of risk factors and prospects for early surveillance. Reprod Biomed Online 2019;39:1007–1016. [DOI] [PubMed] [Google Scholar]
- 235. Uimari O, Auvinen J, Jokelainen J, Puukka K, Ruokonen A, Järvelin M-R, Piltonen T, Keinänen-Kiukaanniemi S, Zondervan K, Järvelä I, Ryynänen M, Martikainen H. Uterine fibroids and cardiovascular risk. Hum Reprod 2016;31:2689–2703. [DOI] [PubMed] [Google Scholar]
- 236. Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2018;25:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011;32:745–750. [DOI] [PubMed] [Google Scholar]
- 238. Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes 2016;9:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Plu-Bureau G, Hugon-Rodin J, Maitrot-Mantelet L, Canonico M. Hormonal contraceptives and arterial disease: an epidemiological update. Best Pract Res Clin Endocrinol Metab 2013;27:35–45. [DOI] [PubMed] [Google Scholar]
- 240. Plu-Bureau G, Maitrot-Mantelet L, Hugon-Rodin J, Canonico M. Hormonal contraceptives and venous thromboembolism: an epidemiological update. Best Pract Res Clin Endocrinol Metab 2013;27:25–34. [DOI] [PubMed] [Google Scholar]
- 241. Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012;366:2257–2266. [DOI] [PubMed] [Google Scholar]
- 242.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2019;133:e128–e150. [DOI] [PubMed] [Google Scholar]
- 243. Shufelt C, LeVee A. Hormonal contraception in women with hypertension. JAMA 2020;324:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 2009;53:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health (Lond) 2015;11:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Plu-Bureau G, Sabbagh E, Hugon-Rodin J. [Hormonal contraception and vascular risk: CNGOF Contraception Guidelines]. Gynecol Obstet Fertil Senol 2018;46:823–833. [DOI] [PubMed] [Google Scholar]
- 247. Ueda Y, Kamiya CA, Horiuchi C, Miyoshi T, Hazama R, Tsuritani M, Iwanaga N, Neki R, Ikeda T, Yoshimatsu J. Safety and efficacy of a 52-mg levonorgestrel-releasing intrauterine system in women with cardiovascular disease. J Obstet Gynaecol Res 2019;45:382–388. [DOI] [PubMed] [Google Scholar]
- 248. Maas AH, Euler M, Bongers MY, Rolden HJ, Grutters JP, Ulrich L, Schenck-Gustafsson K. Practice points in gynecardiology: abnormal uterine bleeding in premenopausal women taking oral anticoagulant or antiplatelet therapy. Maturitas 2015;82:355–359. [DOI] [PubMed] [Google Scholar]
- 249.WHO. Breast cancer. https://www.who.int/cancer/detection/breastcancer/en/ (8 January 2021).
- 250.ECIS. https://ecis.jrc.ec.europa.eu (8 January 2021).
- 251. Dafni U, Tsourti Z, Alatsathianos I. Breast Cancer Statistics in the European Union: incidence and survival across European countries. Breast Care (Basel) 2019;14:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Daly MJ. https://www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf (8 January 2021).
- 253. Arts-de Jong M, Maas AH, Massuger LF, Hoogerbrugge N, de Hullu JA. BRCA1/2 mutation carriers are potentially at higher cardiovascular risk. Crit Rev Oncol Hematol 2014;91:159–171. [DOI] [PubMed] [Google Scholar]
- 254. van Westerop LL, Arts-de Jong M, Hoogerbrugge N, de Hullu JA, Maas AH. Cardiovascular risk of BRCA1/2 mutation carriers: a review. Maturitas 2016;91:135–139. [DOI] [PubMed] [Google Scholar]
- 255. Gast KC, Viscuse PV, Nowsheen S, Haddad TC, Mutter RW, Wahner Hendrickson AE, Couch FJ, Ruddy KJ. Cardiovascular concerns in BRCA1 and BRCA2 mutation carriers. Curr Treat Options Cardiovasc Med 2018;20:18. [DOI] [PubMed] [Google Scholar]
- 256. Sajjad M, Fradley M, Sun W, Kim J, Zhao X, Pal T, Ismail-Khan R. An exploratory study to determine whether BRCA1 and BRCA2 mutation carriers have higher risk of cardiac toxicity. Genes (Basel) 2017;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Pearson EJ, Nair A, Daoud Y, Blum JL. The incidence of cardiomyopathy in BRCA1 and BRCA2 mutation carriers after anthracycline-based adjuvant chemotherapy. Breast Cancer Res Treat 2017;162:59–67. [DOI] [PubMed] [Google Scholar]
- 258. Barac A, Lynce F, Smith KL, Mete M, Shara NM, Asch FM, Nardacci MP, Wray L, Herbolsheimer P, Nunes RA, Swain SM, Warren R, Peshkin BN, Isaacs C. Cardiac function in BRCA1/2 mutation carriers with history of breast cancer treated with anthracyclines. Breast Cancer Research and Treatment 2016;155:285–293. [DOI] [PubMed] [Google Scholar]
- 259. Rees M, Angioli R, Coleman RL, Glasspool R, Plotti F, Simoncini T, Terranova C. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) position statement on managing the menopause after gynecological cancer: focus on menopausal symptoms and osteoporosis. Maturitas 2020;134:56–61. [DOI] [PubMed] [Google Scholar]
- 260. Kotsopoulos J, Gronwald J, Karlan BY, Huzarski T, Tung N, Moller P, Armel S, Lynch HT, Senter L, Eisen A, Singer CF, Foulkes WD, Jacobson MR, Sun P, Lubinski J, Narod SA; for the Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018;4:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Marsden J, Marsh M, Rigg A; British Menopause Society. British Menopause Society consensus statement on the management of estrogen deficiency symptoms, arthralgia and menopause diagnosis in women treated for early breast cancer. Post Reprod Health 2019;25:21–32. [DOI] [PubMed] [Google Scholar]
- 262. Rada G, Capurro D, Pantoja T, Corbalan J, Moreno G, Letelier LM, Vera C. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev 2010;9:CD004923. [DOI] [PubMed] [Google Scholar]
- 263. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–635. [DOI] [PubMed] [Google Scholar]
- 264. Holmberg L, Iversen O-E, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, Jassem J, Dobaczewska D, Fjosne HE, Peralta O, Arriagada R, Holmqvist M, Maenpa J; HABITS Study Group. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 2008;100:475–482. [DOI] [PubMed] [Google Scholar]
- 265. Fahlen M, Fornander T, Johansson H, Johansson U, Rutqvist LE, Wilking N, von Schoultz E. Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer 2013;49:52–59. [DOI] [PubMed] [Google Scholar]
- 266.Committee on Practice Bulletins-Gynecology.. ACOG Practice Bulletin No. 126: management of gynecologic issues in women with breast cancer. Obstet Gynecol 2012;119:666–682. [DOI] [PubMed] [Google Scholar]
- 267. Levine GN, Steinke EE, Bakaeen FG, Bozkurt B, Cheitlin MD, Conti JB, Foster E, Jaarsma T, Kloner RA, Lange RA, Lindau ST, Maron BJ, Moser DK, Ohman EM, Seftel AD, Stewart WJ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Quality of Care and Outcomes Research. Sexual activity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2012;125:1058–1072. [DOI] [PubMed] [Google Scholar]
- 268. Schwarz ER, Kapur V, Bionat S, Rastogi S, Gupta R, Rosanio S. The prevalence and clinical relevance of sexual dysfunction in women and men with chronic heart failure. Int J Impot Res 2008;20:85–91. [DOI] [PubMed] [Google Scholar]
- 269. Vazquez LD, Sears SF, Shea JB, Vazquez PM. Sexual health for patients with an implantable cardioverter defibrillator. Circulation 2010;122:e465–e467. [DOI] [PubMed] [Google Scholar]
- 270. Mosack V, Steinke EE. Trends in sexual concerns after myocardial infarction. J Cardiovasc Nurs 2009;24:162–170. [DOI] [PubMed] [Google Scholar]
- 271. Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet 1985;325: 181–184. [DOI] [PubMed] [Google Scholar]
- 272. Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes 2013;6:99–109. [DOI] [PubMed] [Google Scholar]
- 273. Jackson G, Boon N, Eardley I, Kirby M, Dean J, Hackett G, Montorsi P, Montorsi F, Vlachopoulos C, Kloner R, Sharlip I, Miner M. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract 2010;64:848–857. [DOI] [PubMed] [Google Scholar]
- 274. Roushias S, Ossei-Gerning N. Sexual function and cardiovascular disease: what the general cardiologist needs to know. Heart 2019;105:160–168. [DOI] [PubMed] [Google Scholar]
- 275. Eyada M, Atwa M. Sexual function in female patients with unstable angina or non-ST-elevation myocardial infarction. J Sex Med 2007;4:1373–1380. [DOI] [PubMed] [Google Scholar]
- 276. Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract 1998;15:519–524. [DOI] [PubMed] [Google Scholar]
- 277. Brotto L, Laan ET. Problems of sexual desire and arousal. In: Wiley KR (ed). ABC of Sexual Health. 3rd ed. UK: Wiley-Blackwell; 2015. p59–67. [Google Scholar]
- 278. Polland AR, Davis M, Zeymo A, Iglesia CB. Association between comorbidities and female sexual dysfunction: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int Urogynecol J 2019;30:377–383. [DOI] [PubMed] [Google Scholar]
- 279. Rosman L, Cahill JM, McCammon SL, Sears SF. Sexual health concerns in patients with cardiovascular disease. Circulation 2014;129:e313–e316. [DOI] [PubMed] [Google Scholar]
- 280. Jaspers L, Feys F, Bramer WM, Franco OH, Leusink P, Laan ET. Efficacy and safety of flibanserin for the treatment of hypoactive sexual desire disorder in women: a systematic review and meta-analysis. JAMA Intern Med 2016;176:453–462. [DOI] [PubMed] [Google Scholar]
- 281. Davis SR, Baber R, Panay N, Bitzer J, Perez SC, Islam RM, Kaunitz AM, Kingsberg SA, Lambrinoudaki I, Liu J, Parish SJ, Pinkerton J, Rymer J, Simon JA, Vignozzi L, Wierman ME. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab 2019;104:4660–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry 2015;30:807–815. [DOI] [PubMed] [Google Scholar]
- 283. Kuyper L, Wijsen C. Gender identities and gender dysphoria in the Netherlands. Arch Sex Behav 2014;43:377–385. [DOI] [PubMed] [Google Scholar]
- 284. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- 285. Safer JD, Tangpricha V. Care of transgender persons. N Engl J Med 2019;381:2451–2460. [DOI] [PubMed] [Google Scholar]
- 286. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LA. long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 2011;164:635–642. [DOI] [PubMed] [Google Scholar]
- 287. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism 1989;38:869–873. [DOI] [PubMed] [Google Scholar]
- 288. van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf) 1997;47:337–342. [DOI] [PubMed] [Google Scholar]
- 289. Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T'Sjoen G. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012;9:2641–2651. [DOI] [PubMed] [Google Scholar]
- 290. Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol 2016;4:943–956. [DOI] [PubMed] [Google Scholar]
- 291. Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, Cohen AT, Handelsman DJ. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ 2016;355:i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation 2019;139:1461–1462. [DOI] [PubMed] [Google Scholar]
- 294. Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med 2018;169:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Renoux C, Dell'aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ 2010;340:c2519. [DOI] [PubMed] [Google Scholar]
- 296. Olie V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol 2010;17:457–463. [DOI] [PubMed] [Google Scholar]
- 297. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab 2003;88:3467–3473. [DOI] [PubMed] [Google Scholar]
- 298. LaHue SC, Torres D, Rosendale N, Singh V. Stroke characteristics, risk factors, and outcomes in transgender adults: a case series. Neurologist 2019;24:66–70. [DOI] [PubMed] [Google Scholar]