Abstract
Currently, biological drug therapy for ocular angiogenesis treatment is based on the administration of anti‐VEGF agents via intravitreal route. The molecules approved with this purpose for ocular use include pegaptanib, ranibizumab, and aflibercept, whereas bevacizumab is commonly off‐label used in the clinical practice. The schedule dosage involves repeated intravitreal injections of anti‐VEGF agents to achieve and maintain effective concentrations in retina and choroids, which are administrated as solutions form. In this review article, we describe the features of different anti‐VEGF agents, major challenges for their ocular delivery and the nanoparticles in development as delivery system of them. In this way, several polymeric and lipid nanoparticles are explored to load anti‐VEGF agents with the aim of achieving sustained drug release and thus, minimize the number of intravitreal injections required. The main challenges were focused in the loading the molecules that maintain their bioactivity after their release from nanoparticulate system, followed the evaluation of them through studies of formulation stability, pharmacokinetic, and efficacy in in vitro and in vivo models. The analysis was based on the information published in peer‐reviewed published papers relevant to anti‐VEGF treatments and nanoparticles developed as ocular anti‐VEGF delivery system.
Keywords: anti‐VEGF agent, biological drugs, nanoparticles, ocular neovascularization
Higher concentrations of anti‐VEGF agents have been observed after ocular administration of nanoparticulate systems in the long‐term in comparison to those of solution formulations of the same agents. The improvements on therapy offered by these nanoparticulate systems have also been evidenced in the higher antiangiogenic properties reported in in vitro and in vivo efficacy models.
Abbreviations
- AMD
Age‐related macular degeneration
- BD
Biological drugs
- DME
Diabetic macular edema
- DR
Diabetic retinopathy
- FDA
Food and Drug Administration
- mAb
Monoclonal antibodies
- PEG
Polyethylene glycol
- PGF
Placental growth factor
- PLA
polylactic acid
- PLGA
poly (lactic‐co‐glycolic acid)
- RFP
Recombinant fusion proteins
- VEGF
Vascular endothelial growth factor
1. INTRODUCTION
Several ocular diseases are characterized for an excessive angiogenesis, which causes severe visual loss and ocular morbidity worldwide. The most common ones are age‐related macular degeneration (AMD), diabetic retinopathy (DR) and retinal vein occlusion. 1 These diseases present ocular neovascularization triggered by a pathologic angiogenesis process that involves degradation of the basement membrane, extracellular proteolysis and abnormal blood vessels growth. 2 , 3 AMD implies choroidal neovascularization as a consequence of hypoxia, ischemia, and inflammation, causing macular thickening and edema. 4 , 5 In DR, the hyperglycemia seems to have a structural and physiological effect: retinal capillaries become blocked and this results in early stages of the pathology where micro aneurism are formed. 6 , 7 Then, it usually progresses to hypoxia that stimulates the generation of new blood vessels. 7 , 8 Diabetic macular edema (DME) is the most common cause of vision loss in patients with DR. 1
Based on these facts, the angiogenesis control is the main focus for these diseases therapy. One of the key aspects of the angiogenesis therapy is targeting of the vascular endothelial growth factor (VEGF) whose family consists in five related glycoproteins 9 that play critical roles in the development, progression, and regularization of new blood vessels both in normal physiological and pathological conditions. 10 , 11 , 12 VEGF binding to its receptors, VEGFR1 and VEGFR2, on the surface of endothelial cells induces intracellular calcium increase and production of vasodilatory mediators like nitric oxide. 9 , 13 Thus, VEGF promotes endothelial cell proliferation, vascular leakage, and formation of new blood vessels. 14 Therefore, blockage of VEGF activity has become the main strategy in the treatment of neovascular ocular pathologies. 12 , 15
Conventional therapies targeting VEGF are based on biological drugs (BD) 16 also known as biological medicine or biotherapeutic agent. Its definition has changed over time and it may differ from one author to another. 17 However, in a broad sense, they are described as drugs obtained from living natural resources. 18 Moreover, it is also referred as biotechnological drugs when BD are obtained through living‐organism modification by taking advantage of the their biological mechanics; for example, using recombinant DNA techniques or the hybridoma technique. 19 , 20
Conventional chemically synthesized, small‐molecule drugs are subjected to safety and efficacy quality control by comparing them with a highly characterized material, known as a drug reference standard, which is usually the first drug to be patented. 18 , 21 , 22 , 23 However, this same comparison is not easily extrapolated for BD because they have little structural differences compared with the biological mechanics of the living organism used as source. 18 , 21 , 22 The BD spectrum of molecules can be quite broad: it can include therapies based on vaccines, antibiotics, interferons, recombinant fusion proteins (RFP), nucleic acids, and monoclonal antibodies (mAb), among others. 24 , 25 , 26 , 27
BD have had an increasingly important impact in ocular therapies, involving mainly mAb, proteins, peptides, and, most recently, DNA‐ and RNA‐based gene therapy. 15 , 28 , 29 , 30 Consequently, there has been a marked increase in the number of articles published about BD intended for ocular therapy in recent years (Figure 1).
FIGURE 1.
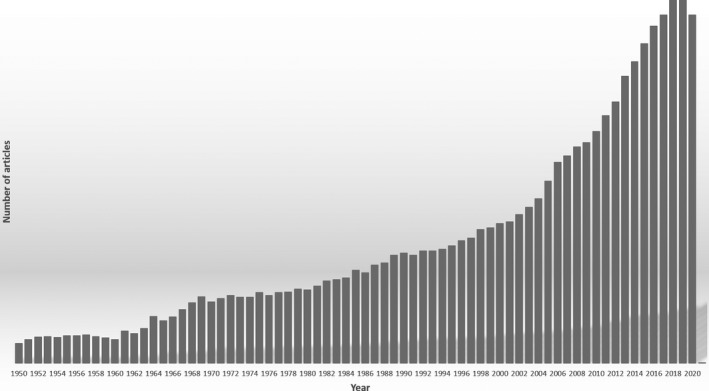
Peer‐reviewed published studies by year available on the PubMed database from advanced search of articles containing (((biological drugs) OR (biological medicine)) OR (biotherapeutic drug)) AND (((ocular) OR (eye)) OR (ophthalmic))
In fact, anti‐VEGF agents are BD used to treat ocular angiogenesis. They can inhibit activity of VEGF by different strategies such as binding or trapping. Thus, these agents prevent the interaction between VEGF and its receptors on the surface of endothelial cells and, thereby, angiogenesis. 14 The approved anti‐VEGF agents for human use in the eyes include pegaptanib, ranibizumab, and aflibercept. 31 , 32 However, bevacizumab is also widely used “off‐label” since there is no Food and Drug Administration (FDA) approval via intravitreal route for the treatment of wet AMD or DME. 33 These drugs present different structural features which impact in the pharmacokinetics, pharmacodynamics, and even in the clinical efficacy. 34 In addition to the continuous advancement of biotechnology, several strategies are under study to improve the performance of available anti‐VEGF drugs. This review article is based on information from peer‐reviewed published papers about BD used for treatment of ocular angiogenesis and related pathologies, focusing on the recent developments to overcome limitations of anti‐VEGF drugs as well as to minimize systemic exposure and adverse effects. Thus, different anti‐VEGF drugs, a wide range of nanotechnological tools for delivery of anti‐VEGF drugs, developing molecules, and novel therapies, are explored and described in the following sections.
2. BIOLOGICAL DRUG FEATURES
The target specificity and the potential lower off‐target toxicities could explain the success of BD as a therapy for several diseases and their continuous development in the pharmaceutical industry. Proteins represent the largest fraction of approved BD, which also include therapeutic proteins, growth factors, and mAb. 35
The first drug regarded as BD was human insulin, which the FDA approved in 1982 36 ; four years later, it also approved the first mAb used in clinical environments: “Muromonab‐CD3 ,” an immunosuppressant for organ transplantation. 37 The mAb are cloned immunoglobulins (Ig) of a single B lymphocyte, mostly produced by hybridoma technology. 28 , 38 They represent one of the oldest and most studied therapies to date in the BD therapeutic field: there is a wide spectrum of FDA‐approved drugs already used in clinical settings. 39 , 40 , 41 , 42 Currently, humanized mAb are the most used form to avoid immune responses, which was achieved by combining murine and human gene sequences. 39 , 40
In recent years, diverse classes of therapeutic proteins have gained traction as approved BD, including RFP and conjugated proteins. 35 RFP are the result of recombinant DNA expression (obtained by artificially combining preexisting DNA of different species) 43 where a mold DNA is used for the production of proteins through different vectors like plasmids, chromosomal integration, and viral vectors. 43 , 44 When two or more of these recombinant genes are translated together they express a RFP. 45 Finally, once proteins are expressed, post‐translational modification is a key factor on their activity, which will be influenced by the host cell used to produce the protein such as mammalian cells, 43 , 44 plant cells, bacteria, and other microorganisms. 25 , 46 , 47 The most representative molecule of the RFP is aflibercept whose mechanism is similar to mAb, 48 , 49 , 50 but has a higher degree of VEGF binding. 51
BD also include the small group of medicines based on nucleic acids (e.g., DNA, mRNA, and synthetic oligonucleotides) and they also include cell and gene‐based therapeutics, the latest and fastest growing class of BD. The DNA‐ and RNA‐based gene therapy can administered by either viral or non‐viral vectors and, more recently, by stem cells. 35 , 52 , 53 , 54 , 55 Gene therapy is based on the modification in genes associated with processes with therapeutic significance. 55 In this therapy, a modified gene sequence is introduced to a host cell to inherit the desired therapeutic effect using different vectors to accomplish this goal like viruses 24 or nanoparticles with lipid or polymer matrices. 56 These vectors must have good loading capacity and compatibility to incorporate the modified gene. 57 However, even with these BD tools in hand, an efficient ocular antiangiogenic therapy can be challenging, taking into account the anatomical features of human eye. 58 , 59
Regarding pathologies that present ocular neovascularization, BD—like mAb and RFP—are effectively being used 15 , 30 as pharmacological treatment, leaving almost obsolete pioneer treatments like laser photocoagulation and photodynamic therapy. 4 Moreover, these therapies appeared as an alternative to laser surgery and chronic corticosteroid use, which can induce raise of the intraocular pressure and cataracts. 60 , 61 Currently, corticosteroids are widely used as co‐treatment to antiangiogenic therapy for ocular angiogenesis disorders. 27 , 62
In the design and the development of a BD product, different factors should be taken into account due to their large size, high structural complexity, and limited stability. 35 BD are susceptible to physical and chemical degradation, which directly impact their structure and function and, consequently, their efficacy. The physical degradation of a formulation based on BD implies events such as unfolding, aggregation, particulate formation, adsorption at interfaces or morphological instabilities. Meanwhile, chemical degradation implies modifications of the covalent bonds, including oxidation, deamidation, isomerization, and disulfide bond shuffling, among others. Thus, BD should be designed with appropriate formulation components that keep drug efficacy and prevents potential physical and chemical degradation. In addition, BD should be administered through an appropriate route and using an optimal dosage form that considers the molecular stability, material compatibility, desired dose, dosage schedule, route of administration, and even patient compliance. Generally, BD products are designed as solutions for parenteral administration, since they can present limitations—enzymatic degradation, poor permeation, and low bioavailability—if they are delivered via non‐parenteral routes. Notwithstanding the therapeutic superiority of BD, all these factors should be considered when designing the optimal dosage form configuration.
In the following sections, we describe the BD developed as therapeutic approaches for neovascular ocular pathologies, including approved products, molecules in development, and different nanoparticulate systems studied to carry them.
3. ANTI‐VEGF AGENTS FOR OCULAR NEOVASCULARIZATION
Anti‐VEGF therapy revolutionized the treatment of retinal disorders related to angiogenesis by reducing vascular permeability and decreasing the growth and leakage of new vessels involved in choroidal and retinal neovascularization. 63 The different approved anti‐VEGF agents are evidence of the continuous evolution of biotechnology. Anti‐VEGF agents comprise a range of molecular modalities (aptamers, mAb, and RFP) (Figure 2) which are formulated as injectable solutions and obtained by innovative biotechnological processes. 35 The approved anti‐VEGF agents are mainly applied by intravitreal route for ocular pathologies that present neovascularization as ADM and DR.
FIGURE 2.
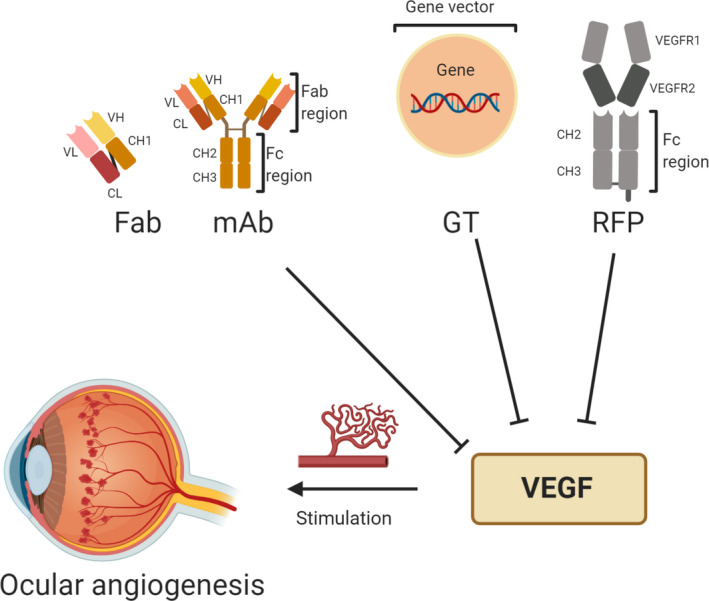
Representative molecular modalities of different anti‐VEGF agents to treatment of ocular angiogenesis.
Note: Image created with BioRender.com. Abbreviations: Fab, Fragment antigen binding; Fc, Crystallizable fragment; GT, Gene therapy; mAb, monoclonal antibody; RFP, Recombinant fusion proteins; VEGF, Vascular endothelial growth factor
Pegaptanib (Macugen) was the first anti‐VEGF drug approved by the FDA for the treatment of neovascular AMD. 64 This drug is a pegylated‐aptamer that recognizes and selectively binds to the isoform 165 of VEGF. 26 , 34 , 65 Structurally, it is a 28‐nucleotide ribonucleic acid with the VEGF‐binding sequence covalently linked to two ~20‐kDa polyethylene glycol (PEG) moieties to increase the half‐life of the drug. 65 Pegaptanib (~49 kDa) was superseded by other anti‐VEGF drugs that blocked all isoforms of VEGF as ranibizumab and bevacizumab that bind to all the VEGF‐A isoforms, and aflibercept that is able to trap VEGF‐A, VEGF‐B, and placental growth factor (PGF). 34 These remaining drugs nowadays used for the management ocular angiogenesis are based on mAb or RFP.
For mAb, both ranibizumab and bevacizumab are used as antiangiogenic therapy in ophthalmology. 26 , 28 Ranibizumab (Lucentis®) is a recombinant, humanized, IgG1κ‐isotype, monoclonal antibody fragment (Fab, 48 kDa), whereas bevacizumab (Avastin®) is a fully humanized, IgG1‐isotype, monoclonal antibody (149 kDa). Both agents bind to VEGF‐A and inhibit its activity. Ranibizumab was designed for intraocular use and approved by the FDA in 2006 for the treatment of patients with wet AMD. 14 , 26 As for bevacizumab, it was originally designed to be administered by intravenous infusion for the treatment of metastatic colon or rectal cancer, approved for FDA in 2004 33 , 66 ; however, it is also widely used off‐label by ophthalmologists to treat patients via ocular route for treatment of wet AMD or DR.
Although both molecules bind to VEGF and inhibit its action, 28 , 29 some authors mention that it could be a difference in affinity and potency between ranibizumab and bevacizumab, 67 whereas others researchers declared there is no enough statistical significance to prove it. 68 , 69 , 70 , 71 In turn, bevacizumab has demonstrated a comparable efficacy and a considerably lower cost than ranibizumab when both formulations are administered via intravitreal injections. 66 , 72
Aflibercept (Eylea®) is a RFP (115 kDa) obtained by combining the Fc portion of a full monoclonal antibody and the two highest affinity domains of VEGFR1 and VEGFR2 34 that binds strongly to VEGF and PGF. 73 This RPF was approved in 2011 by the FDA. It works as a VEGF trap molecule: it acts as decoy receptor for VEGF‐A isoforms that inhibits the binding and activation of the cognate VEGF receptors and, thereby, its pathway activation of new blood vessels formation. 50 , 74 Some in vitro studies showed that aflibercept has a greater blocking potency compared to that of either ranibizumab or bevacizumab, 71 whereas other studies showed very similar potencies between ranibizumab and aflibercept. 75
Among novel investigational anti‐VEGF agents in preclinical stage, there are conbercept (FP3/KH902), a soluble RFP derived from the extracellular domains of VEGFR1 and VEGFR2, and the Fc portion of human IgG1, which presents a very high affinity for all isoforms of VEGF‐A, VEGF‐B, VEGF‐C, and PGF. This RFP has a similar structure to aflibercept and it showed excellent efficacy in initial clinic studies and great promise as a future treatment option for DME. Other anti‐VEGF agents that are being pre‐clinically studied are KSI‐301, brolucizumab , and ziv‑aflibercept. KSI‐301 (Kodiak Sciences) is a novel anti‐VEGF antibody biopolymer conjugate (950 kDa) developed for the treatment of retinal vascular diseases; it has a large molecular structure to prolong the estimated intraocular anti‐VEGF effect. In contrast, brolucizumab (RTH258) is a single‐chain antibody fragment (scFv) with a small molecular weight (26 kDa) in the line of anti‐VEGF antibodies, which is capable of inhibiting all isoforms of VEGF‐A. 76 Ziv‐aflibercept (25 mg/mL, 1000 mOsm/kg) is an identical molecule to aflibercept, but formulated at a lower concentration and higher osmolarity than the human vitreous, resulting in hyperosmolarity. It was FDA‐approved for intravenous administration for treatment of colorectal carcinoma and it has been studied as alternative intravitreal treatment for retinal disorders despite no being approved for ocular use. 63
In addition, emerging therapies based on gene therapy are setting in as a novel, efficient, and specific antiangiogenic strategy treatment by inducing anti‐VEGF endogenous factor expression. 5 , 77 With this background, BD are being used in AMD or DR, 77 , 78 and recent efforts are focused on the gene therapy which can be substantially more effective by targeting the disease in its genetic origin and acting at a molecular level by introducing the therapeutic gene which stimulates or inhibits the expression of a protein of interest in the pathology. 52 , 79 These new therapies are described in following sections.
As it has been discussed, there are studies focusing on new and alternative therapies constantly in progress, as well as studies focusing on the effectiveness of approved anti‐VEGF agents based on mAb and RFP, which is related to their binding capacity with VEGF and their capacity to hold back the angiogenesis resulting in vision improvement. 4 , 26
4. MAJOR CHALLENGES OF ANTI‐VEGF DRUGS
The human eye is a globular structure divided in the anterior and posterior segments, according to their proximity to the outer part, and it possess specific biological barriers that protect it against foreign substances. 80 The anterior segment includes the cornea, iris, lenses, and aqueous humor, whereas the posterior segment is composed of vitreous, retina, choroids, and sclera. The cornea is the main biological barrier due to its anatomical structure made of several layers of epithelial cells and its charged surface that repels the majority of molecules. 16 , 81 , 82
Topical instillation is the most accepted route administration for the eye. However, drugs almost never reach a therapeutic concentration due to the eye characteristics, requiring large doses which can result in undesired adverse effects. 83 Ocular injections are required for the administration of anti‐VEGF agents intended to treat pathologies of the posterior segment of the eye such as AMD, DR, and DME, 84 being the intravitreal injection the most common route since can achieve therapeutic concentrations in retina and choroids. These pathologies are characterized by compromising vision in many patients, and both rapid establishment of treatment and maintenance of drug effective concentrations at the site of action for as long as possible are essential. To achieve the latter, the schedule dosage requires frequent injections of anti‐VEGF agents which can lead to serious adverse effects such as intravitreal hemorrhage, endophthalmitis, retinal detachment, raise of the intraocular pressure, retinal detachment, and cataracts, among others. 15 , 58 , 85
After an intravitreal drug injection and following its distribution in the vitreous humor the retina, the drug can egress into the systemic circulation via the choroidal vasculature and aqueous humor outflow. The exposure of the site of therapeutic action to anti‐VEGF agents could be dependent on their pharmacokinetic performance, since these drugs are generally uniformly distributed from the retina to the vitreous. 86 The pharmacokinetic performance of anti‐VEGF agents after intravitreal administration has been studied in experimental models as well as in patients with considerable variations with one another. 87 , 88 In rabbits, bevacizumab showed the longest vitreous half‐life (6.99 days) compared with aflibercept (3.92 days) and ranibizumab (2.51 days). However, a comparable vitreous half‐life for anti‐VEGF has been observed in patients with different ocular angiogenesis; anatomical and biological changes related to the pathology could influence the drug performance. 89 , 90
Animal pharmacokinetic studies of a variety of antibodies and antibody fragments showed that agents with a high molecular weight—like bevacizumab—could increase their ocular residence time when compared to agents with a lower molecular weight—such as ranibizumab. 86 On the other hand, agents containing a Fc have greater systemic exposures after intravitreal administration by binding to the neonatal Fc receptor (FcRn), expressed by endothelial cells in the blood retinal barrier; whereas agents devoid of Fc regions show a significant reduction in systemic exposures. Both molecular size and presence of the Fc function influence ocular residence time and also the systemic clearance. Agents with higher molecular size like complete mAb are not kidney‐excreted through urine, instead they are recycled though a FcRn‐mediated mechanism. 91 , 92 In line with this, different findings about pharmacokinetics of anti‐VEGF intravitreal administration suggest that bevacizumab presents longer systemic exposure levels than aflibercept and ranibizumab. Although only small amounts of anti‐VEGF are released from the eye into the systemic circulation, it should be taken into account due to the fact that it could suppress plasma‐free VEGF and lead to possible adverse events. 34 , 93
While approved anti‐VEGF agents are currently efficacious, they present certain limitations such as frequently needed injections, high treatment cost, and uneven response to treatment. 76 These challenges have led to an active search for more novel agents that may be able to overcome these limitations. Thus, biotechnological tools are continuously applied to improve the efficacy and safety profile of current therapeutic mAb and Fab, either by designing novel, more potent or multispecific inhibitors. On the other hand, nanotechnological tools are also being studied to extend BD half‐life in the vitreous and/or combine them with other drugs with different mechanisms of action with the aim of reduce the frequency of intravitreal injections and minimizing undesired systemic exposure.
Aflibercept shows better performance in pharmacokinetic and pharmacodynamics parameters than ranibizumab and bevacizumab thanks to advances in biotechnological tools. A comparison in terms of clinical efficacy between anti‐VEGF therapies with these agents is very complex to analyze since treatment schedule, ocular disorder, doses, and costs should be considered. However, different studies seem to indicate similar long‐term effects. Thus, no substantial difference between aflibercept and ranibizumab has been observed in patients with AMD 94 , 95 or even between bevacizumab and ranibizumab when both were administered with the same treatment schedule. 96 This leads us to think that the optimization of drug behavior after ocular administration could have a considerable impact on clinical efficacy. The use of strategies to agent control release, decreasing the intravitreal elimination rate or avoid the systemic absorption could lead to an improvement of therapeutic schedule. A less frequent treatment regimen may reduce treatment burden for patients and healthcare costs. 34
It is noteworthy that in addition to the advancement of biotechnology, there is also a constant development of drug delivery systems to overcome limitations associated with BD. In this sense, nanotechnological tools are widely applied as drug delivery systems and they are being explored as a means to transport BD. It must be taken into account that BD require special attention regarding the parameters used to obtain nanoparticles, such as temperature, pH, and that the excipients used must be compatible with the route of administration. With this on mind, the nanoparticulate systems developed in the field of preclinical anti‐VEGF research as alternatives to approved injectable solutions are detailed in the following section.
Although this seems remote, the sum of these two technologies may become a powerful strategy for the optimization of BD therapies in the future, taking into account that there are already available formulations based on liposomes, liposomes‐stealth, conjugates between drugs and mAb, and RPF conjugated to pegylated drugs, among others.
5. NANOPARTICULATE SYSTEMS FOR BD DELIVERY INTENDED FOR OCULAR ANGIOGENESIS TREATMENT
In order to overcome ocular therapies limitations, the development of novel delivery systems is constantly under study. In this way, nanotechnology has been exploited in ocular therapy with overall good results. 58 , 81 , 97 Nanomedicine is the use of nanotechnology in medicine. It is the science and technology used in the design and evaluation of systems at nanometric scale, made up of at least the active principle or biologically active molecule and the system itself, which generates a special applicability related to diagnosis, monitoring, treating, or prevention of a disease at the expense of its physical, chemical, and biological properties. 98 , 99 , 100
Nanoparticulate systems have sizes in the range of 1–1000 nm. In general, a bioactive molecule or a drug can be adsorbed, encapsulated, trapped or covalently bound to a matrix of nanoparticles 98 and, thus, its pharmacokinetics and pharmacodynamics can be modified. There is a wide variety of nanoparticulate systems with different properties, such as water‐soluble macromolecules, polymeric nanoparticles, dendritic structures, micelles, solid lipid nanoparticles, nanostructured lipids, lipid nanocapsules, and liposomes, among others. 99 Nanotechnological tools could allow–an improvement in the drug solubility or permeation resulting in better bioavailability. 101 Moreover, drug encapsulation in nanoparticles could make the drug less obvious for the immunological system and it could provide sustained release. 81 , 84 , 101 , 102 In relation to BD, this strategy is being exploited mainly on mAb 84 , 102 which have been used in the clinic for some time.
Anti‐VEGF agents are mainly administered through ocular injections. Therefore, improving injection parameters, for example, frequency and dose (that could result in a better pharmacological therapy), and even replacing injections with topical administration altogether are the main challenges to overcome. In this way, efforts are focused toward improving the pharmacokinetics of the drugs through nanotechnology. Several nanoparticle systems (Figure 3), both lipid and polymeric, have been studied for anti‐VEGF ocular delivery 30 , 103 which are summarized in Tables 1 and 2 and described in the following sections.
FIGURE 3.
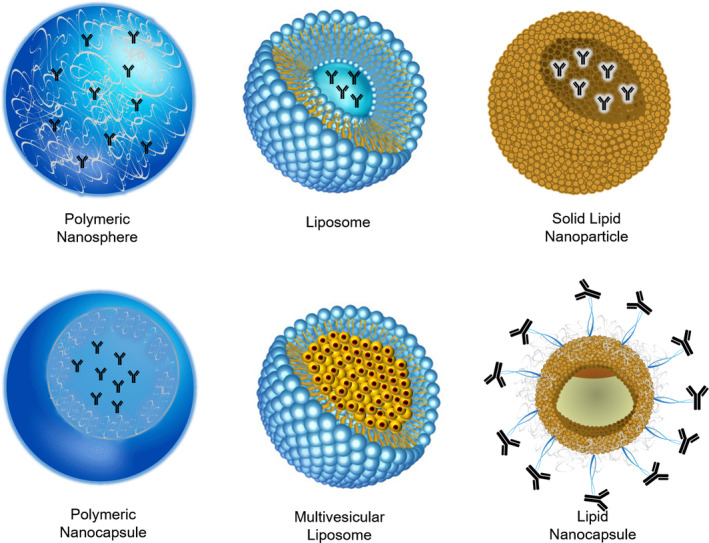
Representative nanoparticulate systems developed for delivery of anti‐VEGF agents.
Note: The figure schematizes nanoparticulate systems loaded with a mAb.
TABLE 1.
Polymeric nanoparticles for delivery of anti‐VEGF agents
| Active molecule | Main polymer | Nanoparticulate system | Components | Mean particle size (nm) | Main results in ocular delivery | Preparation method | Refs. |
|---|---|---|---|---|---|---|---|
| Bevacizumab and dexamethasone | PLGA | PLGA‐nanoparticles |
PLGA and polyethylenimine PVA |
190–222 |
|
Emulsion‐solvent evaporation | 107 |
| Bevacizumab | PLGA and Chitosan | Chitosan‐coated PLGA nanoparticles | PLGA, chitosan and |
222 |
|
Emulsion‐solvent evaporation | 108 |
| Bevacizumab | PLGA | PLGA‐nanoparticles | PLGA, ethyl acetate and PVA | 198 |
|
Double‐emulsion solvent evaporation | 103, 111 |
| Bevacizumab | PLGA | PLGA‐albumin nanoparticles | PLGA, PVA and albumin |
|
Double‐emulsion solvent evaporation | 106, 113 | |
| Bevacizumab | PLGA | PLGA‐nanoparticles | PLGA, PVA and Tween® 80 | 133 |
|
Double‐emulsion solvent evaporation | 109 |
| Bevacizumab | PLGA | Nanoparticles in porous microparticles | PLGA and PVA | 265 |
|
Emulsion‐solvent evaporation | 110 |
| Bevacizumab | Mesoporous silica | Mesoporous silica nanoparticles |
Tetraethyl orthosilicate, cetyltrimethylammonium chloride, triethanolamine,3‐aminopropyltriethoxysilane (3‐aminopropyl) triethoxysilane, and mPEG‐succinimidyl carboxymethyl ester |
140 |
|
Nanocasting | 114 |
| Ranibizumab | PLGA‐PEG copolymer | PLGA‐PEGylated magnetic nanoparticles | Iron oxide, PEG and PLGA | 5–10 |
|
Ring opening polymerization, following addition of iron oxide nanoparticles |
115 |
| Bevacizumab | Chitosan | Chitosan grafted‐polyethylene glycol) methacrylate nanoparticles |
Chitosan, polyethylene glycol methacrylate, Tween® 80 and Span® 80 |
200–900 |
|
Double cross‐linking (ionic and covalent) process in reverse emulsion | 118 |
| Bevacizumab | Chitosan | Chitosan nanoparticles | Chitosan and tripolyphosphate | 188 |
|
Ionic gelation | 119 |
| Bevacizumab | Chitosan | Chitosan nanoparticles | Unspecified | 88.9 |
|
Unspecified | 120 |
| Ranibizumab | PLGA and chitosan |
PLGA microparticles entrapping chitosan nanoparticles |
PLGA, chitosan, hyaluronic acid, among others. | 17–350 |
|
Chitosan crosslinking ‐modified double emulsion method | 122 |
| Bevacizumab | Human serum albumin | Human serum albumin nanoparticles | Human serum albumin and glutaraldehyde | 310 |
|
Desolvation followed by freeze‐drying | 123, 124 |
| Bevacizumab and suramin | Human serum albumin | Human serum albumin nanoparticles | Human serum albumin, glutaraldehyde and Gantrez® ES‐425 | 158–210 |
|
Desolvation | 125 |
Abbreviations: HUVEC, Human umbilical vein endothelial cells; PEG, polyethylene glycol; PLGA, poly(lactide‐co‐glycolic acid; PVA, polyvinyl alcohol; VEGF, vascular endothelial growth factor.
TABLE 2.
Nanoparticles based on lipids for delivery of anti‐VEGF agents
| Active molecule | Nanoparticulate system | Components | Mean particle size (nm) | Main results in ocular delivery | Preparation method | Referencs |
|---|---|---|---|---|---|---|
| Bevacizumab | Liposomes | Phospholipids and lipids of different chain size |
120–385 |
|
Lipid hydration and extrusion | 126 |
| Bevacizumab | Liposomes | Phospholipids and lipids of different chain size | Unspecified |
|
Dehydration‐rehydration | 127 |
| Bevacizumab | Multivesicular liposomes | Phospholipids and lipids of different chain size, albumin and PVA | 1190–4360 |
|
Double emulsification | 128 |
| Bevacizumab | Solid lipid nanoparticles | Solid lipid mix and stabilizers | 515–1213 |
|
Fatty‐acid coacervation | 129 |
| Bevacizumab and triamcinolone | Lipid nanocapsules | Lipid mix and stabilizers | 113–182 |
|
Phase inversion temperature | 131 |
Abbreviations: hCMEC/D3, primary human brain microvascular endothelial cell line; HUVEC, Human umbilical vein endothelial cells.
5.1. Nanoparticles based on polymers
Efforts have been mainly been focused in the development of nanoparticles as carriers of bevacizumab, since it has demonstrated a similar efficacy and a notable lower cost than ranibizumab when both formulations are administered via intravitreal injections. 72 , 104 Among polymeric nanoparticles (Table 1), those based on poly (lactic‐co‐glycolicacid) (PLGA) have been widely explored to transport this molecule, with promising results for ocular administration. PLGA is composed of lactic acid and glycolic acid, and it is widely used in drug delivery systems due to its biodegradability and biocompatibility. 105 Generally, bevacizumab‐loaded PLGA nanoparticles have a size around 200–300 nm 103 , 106 , 107 , 108 or even smaller 109 and they are prepared by different variants of double‐emulsion solvent evaporation procedure.
It should be taken into account that the preparation process, the excipients, and the formation of aggregates could affect the activity of the antibody; therefore, stability of the nanoparticulate system and antibody structure is usually studied. A dramatic reduction in bevacizumab concentration and activity was observed after it was encapsulated in PLGA‐nanoparticles through a modified double‐emulsion solvent evaporation procedure. However, different additives were evaluated to protect bevacizumab performance. Albumin was the most successful one: it allowed to obtain a formulation without neither detectable bevacizumab aggregates or structural fractures that had a size of 197 nm, narrow size distribution, negative zeta potential, and high encapsulation efficiency of bevacizumab (82.4%). 106 In this way, it was observed that VEGF‐165 binding activity of bevacizumab and its physical and chemical stability of bevacizumab were maintained at 37 °C during the 4‐month study after bevacizumab‐coated polylactic acid (PLA) nanoparticles (265 nm) were encapsulated inside the porous PLGA microparticles (11.61 µm). Moreover, no visible aggregates were present in released bevacizumab, until the end of the study. 110
In other work, bevacizumab structure was studied after encapsulation in PLGA‐nanoparticles and after release from PLGA‐nanoparticles. The secondary structure of bevacizumab is dominated by β‐sheet (characteristic of IgG) which could be caused by the lyophilization process used in the preparation methods of PLGA‐nanoparticles, that still allows the formation of intermolecular β‐sheets due to water removal. It has been observed that although there were bevacizumab conformational changes while encapsulated into PLGA‐nanoparticles, a refolding of bevacizumab structure could occur after its release, as suggested by the circular dichroism spectrum of released bevacizumab, which is similar to the native bevacizumab spectrum. 103 In this way, the co‐encapsulation of trehalose and bevacizumab was explored to achieve long‐term bevacizumab stability in PLGA nanoparticles using lyophilization. The nanoparticles with an external 10% (w/v) of trehalose, allowed to maintain the physical–chemical characteristics of the nanoparticles and the secondary and tertiary structure of bevacizumab. In addition, the antiangiogenic activity of bevacizumab was kept over 6 months of storage. 111
In relation to bevacizumab release, these nanoparticulate systems allowed a controlled release of the drug. An in vitro study showed a pH‐dependent bevacizumab release profile during 168 hours, 103 with a bevacizumab sustained release at around 14% in a buffer, pH = 7.4. Other study showed that bevacizumab experienced an initial burst release with more than 40% of bevacizumab released from bevacizumab‐encapsulated PLGA in the first 2 hours in phosphate‐buffered saline, another sustained release (40%) in the next 7 days, followed by a slow drug release for up to 21 days. 109 Also, a very slow and consistent drug release was obtained with chitosan‐coated PLGA nanoparticles of bevacizumab that did not achieve complete release until 72 hours (maximum 25%). 108 In diluted rabbit vitreous (ex vivo), bevacizumab‐loaded PLGA nanoparticles showed an initial burst release of about 10.3% of the encapsulated dose and then, a slow rate of drug release. 106 Also, bevacizumab‐coated PLA nanoparticles encapsulated inside the porous PLGA microparticles have showed a sustained release of bevacizumab in vitro with a cumulative total release between 67% and 81% at 4 months when prepared with bevacizumab PLA nanoparticles between 5% and 15% w/w. 110 Beyond the fact that aflibercept is the most recently used drug in clinic practice, PLGA nanoparticles have also been studied as a carrier system for this RFP. The obtained spherical nanoparticles showed high encapsulation efficiency (75%) and a sustained release of drug reaching 75% of drug release at 7 days, whereas an aflibercept solution released the full payload in 24 hours. 112
The ocular performance efficacy of bevacizumab‐loaded PLGA nanoparticles has also been studied. The maximum bevacizumab concentration in vitreous was obtained a week after the bevacizumab‐loaded PLGA‐nanoparticles injection, whereas the maximum bevacizumab concentration for Avastin® was observed immediately after the injection. 113 There was no evidence of ocular toxicity after the ocular administration of these nanoparticles, whereas the distribution in posterior segment tissues such as retina, choroid and sclera was observed, along with a concentration decrease 7–56 days after administration. 113 Similar results were observed with other nanoparticles in which the maximum concentration of bevacizumab in the vitreous body and aqueous humor was observed at about 6 days after bevacizumab‐loaded PLGA‐nanoparticles injection, which showed a significantly greater half‐life than the bevacizumab solution. 109 Comparable results were observed for bevacizumab‐loaded nanoparticles based on mesoporous silica, in which the maximum concentration of bevacizumab in the vitreous and aqueous humor was observed at about 7 days after the formulation injection. 114
Also, a more sustained delivery of bevacizumab from bevacizumab‐coated PLA nanoparticles encapsulated inside the porous PLGA was observed in rats after intravitreal injection of the system compared to bevacizumab solution, with a concentration of 21.1 μg/mL of bevacizumab in the vitreous on day 1 and 13.96 μg/mL on day 45. Moreover, bevacizumab was present in sclera, choroid‐retinal pigment epithelium, vitreous, and lens tissue 2 months after dose administration, suggesting sustained release. 110
in vitro studies have shown that endothelial cell proliferation significantly decreased after incubation with either bevacizumab‐loaded PLGA‐nanoparticles 103 or bevacizumab, whereas others studies showed that the nanoparticle formulation was more effective than the antibody solution in inhibiting VEGF‐mediated endothelial cell proliferation, migration, and tube formation. 109 The latest bevacizumab‐encapsulated PLGA had no significant cytotoxicity and tissue toxicity effect in vitro and in vivo. Also, in vivo studies showed that they enhanced the anti‐angiogenic efficiency of bevacizumab in treating corneal neovascularization and retinal neovascularization in a model of oxygen‐induced retinal angiogenesis. Thus, authors concluded that this formulation of bevacizumab‐loaded PLGA nanoparticles could increase the bioavailability and decrease the toxicity of bevacizumab during ocular angiogenesis therapy. In the same way, the aforementioned nanoparticles based on mesoporous silica also presented high antiangiogenic effect in in vitro assays of VEGF‐induced endothelial cell proliferation, migration, and tube formation and, also, showed sustained inhibitory effects on corneal neovascularization and retinal neovascularization in vivo. 114
On the other hand, electrostatically conjugated bevacizumab in dexamethasone‐loaded poly (D,L‐lactide‐co‐glycolide)/polyethylenimine nanoparticles showed a good anti‐angiogenic effect in apoptosis, migration, invasion, and tube formation assays, greatly reducing the amount of blood vessels in in vivo chick embryo chorioallantoic membrane assay and decreasing the leakage area in laser‐induced rabbit choroidal neovascularization model. 107
Collectively, the encapsulation of bevacizumab in PLGA‐nanoparticles seems to be a good alternative for ocular administration of this antibody. It allows a sustained release, increases the half‐time of bevacizumab, and it might potentially offset some of the treatment drawbacks.
In addition to the advantages offered by PLGA‐nanoparticles as an anti‐VEGF delivery system, it has been explored combining them with other nanoparticles or materials. Thus, nanoparticles based on conjugated iron oxide (Fe3O4)/polyethylene glycol‐ poly lactide‐co‐glycolide (PEG‐PLGA) have shown an efficient inhibition of the tube formation in the matrigel‐based assay method by using human umbilical vein endothelial cells. 115 Besides, the surface of PLGA nanoparticles can be modified to enhance its ocular performance. In fact, a formulation of chitosan‐coated PLGA nanoparticles loaded with bevacizumab showed high mucoadhesive strength with pig mucin suspension, which resulted in an increase in scleral permeation. The nanometric size and chitosan‐coating of the nanoparticles can explain the enhanced permeation due to the interaction of a positively charged amino group of chitosan with negatively charged scleral surface. In addition, these nanoparticles resulted non‐irritant and well tolerated by the chorioallantoic membrane, suggesting that they may be used for ocular administration. 108
Chitosan is considered to be a mucoadhesive cationic polymer as a result of the ability of its positively charged amino groups to develop molecular attraction forces by electrostatic interactions with the negative charges of the mucous layer. This polymer is a biocompatible and biodegradable material that does not present any risk of accumulation or retention in the body, therefore it could be very useful in the preparation of ocular nanoparticles. 116 , 117
In addition to being used to coat nanoparticles, chitosan can form a matrix of polymeric nanoparticles. Accordingly, chitosan nanoparticles have been studied as bevacizumab carriers. Thus, polymeric nanocarriers based on the chitosan grafted poly (ethylene glycol) methacrylate with high water solubility were studied as a drug delivery system of bevacizumab. This system retained the maximum amount of drug (0.327 mg bevacizumab/mg nanoparticles) after 72 hours, with an encapsulation efficiency of 39%. It also showed a controlled drug release for several days, related to the swelling behavior in an aqueous environment. Moreover, it resulted practically non‐toxic, hemocompatible and it had moderate efficacy in the treatment of inflammation induced by lipopolysaccharide and central retinal vein occlusion models in rabbits and high efficacy as an anti‐angiogenic treatment in a model of diabetes in rabbits. 118
In other study, bevacizumab‐loaded chitosan nanoparticles with a diameter around 188 nm, showed an in vitro release for 3 weeks in the case of nanoparticles that had a 38% loading efficiency. Furthermore, this nanoparticulate system could penetrate through the sclera, did not produce any allergic or inflammatory reaction after ocular administration and reached high intravitreal concentrations of bevacizumab after subtenon injection. 119 In the same way, the effect of bevacizumab‐chitosan nanoparticles of around 90 nm prepared by an emulsification‐evaporation method, was evaluated regarding the pathological morphology of the retina, and the expression of VEGF protein and VEGF mRNA in the retina of diabetic rats. The results showed that the intravitreal injection of bevacizumab inhibits VEGF expression in retina, and bevacizumab‐chitosan nanoparticles have a longer duration of action compared with a solution formulation. 120 In addition, the preparation of ocular biodegradable implants containing bevacizumab‐loaded nanoparticles has been explored to design a sustained release formulation combining different polymers. Thus, bevacizumab‐loaded chitosan nanoparticles of 78.5 nm and an entrapment‐efficiency of 67.6%, prepared by ionic gelation method and inserted in a matrix of hyaluronic acid and zinc sulfate, showed an appropriate extended release of bevacizumab from the carrier over 60 days. These results can be associated with the cationic character of chitosan that improves bioadhesion 16 ; moreover, hyaluronic acid showed improvements in mucoadhesion and drug release. 121 In other study, different PLGA microparticles incorporating chitosan‐based nanoparticles (17–350 nm) were explored as ranibizumab carrier. These systems presented a drug entrapment efficiency up to 69% and slow drug release in nanoparticles containing chitosan‐N‐acetyl‐L‐cysteine. Conversely, the incorporation of tripolyphosphate to this formulation increased the rate of protein release and reduced entrapment efficiency. Furthermore, ranibizumab released from all preparations maintained its structural integrity and in vitro activity. 122
Additionally, nanoparticles based on human serum albumin were explored as a bevacizumab delivery system. Human serum albumin is the most abundant protein in human blood and is a natural and suitable material for the preparation of nanoparticles intended for drug delivery. Bevacizumab‐loaded nanoparticles were prepared by a desolvation process followed by freeze‐drying, without any chemical, physical or enzymatic cross‐linkage. These nanoparticles showed a mean size of 310 nm, a high payload and a bevacizumab release profile characterized by an initial burst effect, proceeded by a continuous release of bevacizumab at a rate of 6 μg/hour. No alteration has been observed after their incubation with retinal pigment epithelium cells. Besides, they showed a significantly higher capability to inhibit the formation of new vessels than that of free bevacizumab in a rat model of corneal neovascularization after topical administration once a day. Reduced inflammation, fibroblast activity and edema were observed when animals were treated with this nanoparticulate system. Despite the bioadhesive properties of polymer PEG, the surface modification in these nanoparticles with this polymer (“pegylation”) did not offer any improvement to the antiangiogenic effect of bevacizumab. 123 On the other hand, the preparation of human serum albumin nanoparticles to load bevacizumab with crosslinking agents as stabilizers, was explored. While human serum albumin nanoparticles crosslinked with glutaraldehyde did not resulted suitable to load bevacizumab due to the inactivation of the antibody after reacting with this crosslinking, 124 human serum albumin nanoparticles stabilized by Gantrez® ES‐425 presented important payloads (97 μg/mg nanoparticle) of the active form of the antibody and a release profile characterized by a small burst effect followed by a sustained release rate. 125
As it is well‐known, polymeric nanoparticles represent a versatile and effective system for drug delivery. In turn, these nanoparticulate systems prove to be an auspicious carrier of anti‐VEGF agents, which can lead to an optimized ocular therapy taking into account the results reported for these systems in relation with the improvements in permeation, controlled drug release, and efficacy.
5.2. Nanoparticles based on lipids
Lipid‐based nanoparticles as system carrier for anti‐VEGF agents compared with polymeric nanoparticles have shown a lower degree of development. This can be explained by the fact that lipid components can limit the loading of hydrophilic molecules such as anti‐VEGF agents. However, the advantages of lipid‐based systems are greater than the challenge and some progress has been possible. Thus, liposomes, solid lipid nanoparticles and lipid nanocapsules as bevacizumab nanocarriers have been developed.
Conventional liposomes with DPPC:DPPE:DPPG:cholesterol compositions of 60:10:0:30, 65:5:5:25, and 60:5:5:30, and stealth liposomes were developed as bevacizumab drug delivery systems. All formulations were able to extend the release time and prevent protein degradation until release. Accelerated stability studies showed that presence of adjuvants like trehalose and beta carotene seemed to maintain the stability of the antibody in a liposomal solution. Additionally, an in vitro cytotoxic assay with retinal pigment epithelium cells showed that the adjuvants maintained cell viability after incubation of liposomes encapsulating bevacizumab at varying concentrations, whereas cell viability decreased after incubation with a bevacizumab solution at the same concentrations. Also, in vivo studies showed that bevacizumab minimum therapeutic concentration in the vitreous was maintained up to 22 weeks after a single intravitreal administration of bevacizumab encapsulated within the liposomes, whereas bevacizumab solution was eliminated before 6 weeks. Thus, these liposomal formulations result interesting alternatives to reach a slow release of bevacizumab and to retain its anti‐VEGF activity. 126 In line with this results, another bevacizumab‐loaded liposomal formulation showed a higher drug concentration in vitreous (48 µg/mL) 28 days after the intravitreal injection in comparison to the concentration observed for an injection of bevacizumab solution (28 µg/mL) after the same time. Also, the clearance of bevacizumab in the vitreous from liposomal formulations was slower than that of the soluble form. 127
On the other hand, multivesicular liposome formulation was developed to improve bevacizumab bioavailability by a double emulsification method. First, several additives were evaluated regarding their preservation of the bioactivity of bevacizumab during emulsification. It was found that the use of 10% of human serum albumin helped to preserve the antibody activity. Bevacizumab multivesicular liposomes achieved a high encapsulation efficiency (80%) of the antibody and showed a sustained release of bevacizumab in different mediums in vitro. Also, antibody structural integrity was retained after its release. in vivo imaging studies in rats after intravitreal formulations injection confirmed the sustained delivery properties of liposomal formulation due to a slower elimination rate of bevacizumab‐loaded multivesicular liposomes compared to a bevacizumab solution (both labeled to a hydrophilic fluorescence dye ester). Moreover, a pharmacokinetics assessment showed higher concentrations and half‐life of bevacizumab in the vitreous after a bevacizumab‐loaded multivesicular liposomes injection in comparison to those observed after the administration of antibody solution. The area under the curve of bevacizumab‐loaded multivesicular liposomes was twice greater than antibody solution. Finally, bevacizumab‐loaded multivesicular liposomes could effectively inhibit the thickness of choroidal neovascularization lesion in an animal model. 128
In addition to liposomal formulations, other lipid‐based nanoparticles have been studied as a bevacizumab nanocarrier, such as solid lipid nanoparticles. Thus, bevacizumab entrapped in solid lipid nanoparticles has been prepared by the fatty‐acid coacervation technique using a hydrophobic ion pair between bevacizumab and sodium dioctyl sulfosuccinate to increase lipophilicity of antibody and facilitate its incorporation into this nanoparticulate system, thanks to the hydrophilic molecule. The obtained formulation was homogeneous and presented a bevacizumab entrapment efficiency of around 30%. In physiologic pH conditions, no bevacizumab release from the solid lipid nanoparticles was observed at 48 hours, whereas the nanoparticulate system significantly inhibited cell migration of endothelial cells at concentrations that were 100–50 times lower than those of free bevacizumab. Also, they inhibit endothelial tube formation, being 100–200 times more active than the free drug. Although this nanoparticulate system was developed as therapeutic alternative to glioblastoma treatment, in vitro results may suggest that it could evaluated for ocular angiogenesis treatment. 129
Recently, a novel hybrid formulation based on lipid nanocapsules containing bevacizumab on the surface and triamcinolone acetonide in the inner core was developed to improve ocular therapy; taking into account the strong need to develop complementary and synergistic therapies to target other biomarkers involved in angiogenesis and the high efficacy presented by triamcinolone‐loaded lipid nanocapsules in the endotoxin‐induced uveitis rabbit model. 130 For this purpose, a phase inversion‐insertion one‐step method was developed for drug loading and surface modification in nanocapsules by post‐inserting a bifunctional polymer, followed by antibody coupling using “click” chemistry. The novel hybrid lipid nanocapsule formulation presented nanometric size (102 nm), negative surface potential, and exhibited 56% of corticosteroid drug in the lipid core. Moreover, it tended to prevent endothelial cell migration and significantly prevented in vitro VEGF‐induced capillary formation, proving that the antibody maintained its bioactivity after nanocapsule conjugation. Thus, it is a promising alternative to improve the therapy for eye disorders that occur with inflammation and/or neovascularization. 131
As discussed earlier, lipid nanoparticles represent an efficient system for drug delivery considering their easy formulation and the possibility of post‐formulation modifications that allow improves their characteristics. Also, the lipids used are generally recognized as safe and they have good biocompatibility. Despite the major challenge about hydrophilic molecules loading in lipid‐based nanoparticles, an important progress has been observed in this research field with encouraging results about the in vitro and in vivo effect of bevacizumab loaded in them.
6. DEVELOPING MOLECULES AND THERAPIES FOR TREATMENT OF OCULAR ANGIOGENESIS
Novel molecules are continually being developed as alternative to anti‐VEGF agents for ocular angiogenesis treatment including, primarily, RFP and peptides that act directly on the VEGF signaling pathways 132 , 133 and also inhibit ocular angiogenesis by other mechanisms not VEGF‐related, like fibroblast growth factor, 134 , 135 matrix metalloproteinases, 136 and certain genes expression cascade. 137 , 138
Among this, it has been reported an insulin‐like growth factor called CW‐703, which showed suppression of endothelial cell migration, tube formation and proliferation, both in in vitro and in vivo studies by a down‐regulation process on VEGF. 137 A recombinant adinopectin (cytokine) had an inhibiting effect on basal tube formation and migration comparable to the effect of bevacizumab in different endothelial cells by down regulating VEGF. 133 Moreover, a novel molecule named RC‐28‐E, which works as a VEGF and basic fibroblast growth factor dual decoy receptor that inhibits not only the VEGF cascade but also basic fibroblast growth factor (bFGF, which stimulates VEGF expression), showed 3‐day sustained release in primate models. 134 , 135
In the same way, developed RFP (DAVP2 and DAVP3) showed suppression of endothelial cell migration and proliferation and, inhibition of angiogenesis in a laser‐induced choroidal neovascularization mouse model after intravitreal injections by inhibiting not only VEGF but also platelet‐derived growth factor through suppression of the phosphorylation activation processes in the metabolic cascade. 138 Also, a collagen IV‐derived peptide that acted by coupling to receptors of VEGF and platelet‐derived growth factor, named “AXT 107”, has been developed. It showed suppression of subretinal and retinal neovascularization in different in vivo models. 139 Other small peptides based on pigment epithelium‐derived (endogenous inhibitor of angiogenesis) displayed a significant decrease in choroidal neovascularization in a mouse model, whereas a nanoparticle‐conjugate prodrug of one peptide showed longer intraocular residence and extended efficacy. 140
While the novel molecules based on RFP and peptides are developed for ocular angiogenesis treatment that not only targets VEGF, gene therapy seems to be also an interesting alternative. Most of the gene therapies act by regulating the expression of proangiogenic and antiangiogenic mediators and represent the most recent group of biological molecules being researched, 141 mainly because their variety of action and their adaptability according to the needs of patients. 142 Thus, there are DNA/RNA type molecules like a plasmidic DNA for uveitis treatment, 143 a small no coding RNA for retinopathies, 144 a small interference RNA for glaucoma, 145 and a supplemental DNA for retinopathies. 146 Furthermore, there have been molecules developed that act as antagonists for specific proteins related to phosphorylation processes or ocular angiogenesis 147 and also, a transposon inhibiting the human pigment epithelium‐derived factor expression. 148
On the other hand, there are pluripotent stem cells which act as a self‐reparative stimuli for damaged eye tissues like human retinal progenitor cells for retinopathies treatment 149 and bone marrow‐derived stem cells for AMD treatment 150 that are being studied. Finally, there are also aptamers that are single‐stranded oligonucleotides capable of specifically binding the target with high affinity recognition such as those that acting by inhibition of bFGF 151 and nucleolin, an inside nucleus protein associated with corneal neovascularization. 152 In comparison with the other groups of molecular modalities, the gene‐based therapies are new in the therapeutics field, so all efforts are toward new molecules development. The main molecules in development and therapies for the treatment of ocular angiogenesis are summarized in Table 3.
TABLE 3.
Developing molecules and therapies for treatment of ocular angiogenesis
| Active molecule | Molecular features | Target pathology | Main results | References |
|---|---|---|---|---|
| CW‐703 | Peptide from human insulin‐like growth factor‐2 | Retinal angiogenesis |
|
137 |
| rAPN | Recombinant adinopectin | Choroidal neovascularization |
|
133 |
| RC‐28‐E | VEGF/bFGF dual decoy receptor (IgG1 Fc‐fusion protein) | Choroidal neovascularization |
|
134, 135 |
| DAVP2 and DAVP3 |
Recombinant fusion protein containing the ligand‐binding domains of VEGFR1, VEGFR2 and PDGFRβ |
Ocular angiogenesis |
|
138 |
| AXT 107 | Mimetic peptide derived from collagen IV | Ocular angiogenesis |
|
139 |
| PEDF 335, 8‐mer and PEDF 336, 9‐mer | Pigment epithelium‐derived factor peptides | Choroidal angiogenesis |
|
140 |
| AGX51 | Antagonist molecule of Id helix‐loop‐helix proteins | Ocular angiogenesis |
|
147 |
| SB100X/PEDF |
Transposon SB100x as a vehicle for the human pigment epithelium‐derived factor |
Age‐related macular degeneration |
|
148 |
| Human retinal progenitor cells (hRPCs) | Pluripotent stem cells | Retinopathies |
|
149 |
| RBM‐007 | Anti‐ bFGF aptamer | Retinopathies |
|
151 |
| AS1411 | Nucleolin‐binding DNA aptamer | Corneal angiogenesis |
|
152 |
Abbreviations: bFGF, Basic fibroblast growth factor; hRPCs, Human retinal progenitor cells; HUVEC, Human umbilical vein endothelial cells; NVAMD, neovascular age‐related macular degeneration; PDGFRβ, platelet‐derived growth factor receptor beta; PDR, proliferative diabetic retinopathy; VEGF, vascular endothelial growth factor; VEGFR1, vascular endothelial growth factor receptor 1; VEGFR2, vascular endothelial growth factor receptor 2.
7. CONCLUSION
In this review, we have summarized the different anti‐VEGF agents approved by FDA, their major challenges for ocular administration and the nanoparticulate systems developed so far for improving their ocular performance. While novel anti‐VEGF molecules are in continuous development to enhance the affinity and efficacy of binding to VEGF, nanotechnological tools are also applied to overcome the limitations of ocular administration of already approved agents.
Several nanoparticulate systems have been reported as carriers of ranibizumab, aflibercept and, mainly, bevacizumab. Polymeric nanoparticles are widely explored for this purpose, offering high loading and sustained release of anti‐VEGF agents, and enhanced permeation after surface modification with a cationic polymer. In relation to lipid nanoparticles, the reported systems demonstrate that they could also be considered as an alternative for ocular controlled release of anti‐VEGF agents. Finally, both nanoparticles based on polymers and those based on lipids have presented a small size, preserved antibody bioactivity and controlled drug release. In turn, higher concentrations of anti‐VEGF agents have been observed after ocular administration of nanoparticulate systems in the long‐term, in comparison with drug concentration from solution formulations of the same agents. The improvements on therapy offered by these nanoparticulate systems have also been evidenced in the greater antiangiogenic properties reported in in vitro and in vivo efficacy models.
Advancing research in this field could bring significant improvements in the clinical efficacy of VEGF agents. The use of nanoparticulate systems with a better pharmacokinetic performance of these molecules could lead to the modification in therapeutic schedules by minimizing intravitreal injections and thus, decreasing both patient morbidity and healthcare costs.
Furthermore, it is noteworthy that new biological molecules developed to inhibit ocular angiogenesis have shown promising results in in vitro and in vivo studies. In the same way, gene‐based therapies began to show some advances in neovascular ocular pathologies. Collectively, the different fields of study related to BD for ocular angiogenesis are making significant progress and their complementarity may become a powerful strategy in the future.
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest to disclose.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 153 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 154
ACKNOWLEDGEMENTS
The authors are grateful for the funding assistance of Fondo para la Investigación Científica y Tecnológica de la República Argentina (FONCYT) (Argentina, ID: PICT 2018‐1834) and postdoctoral and doctoral fellowships from Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET) for María Lina Formica and Hamoudi Ghassan Awde Alfonso, respectively.
REFERENCES
- 1. Fogli S, Mogavero S, Egan CG, Del Re M, Danesi R. Pathophysiology and pharmacological targets of VEGF in diabetic macular edema. Pharmacol Res. 2016;103:149‐157. 10.1016/j.phrs.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43(3):245‐269. 10.1016/S0039-6257(98)00035-6 [DOI] [PubMed] [Google Scholar]
- 3. Bressler SB. Introduction: understanding the role of angiogenesis and antiangiogenic agents in age‐related macular degeneration. Ophthalmology. 2009;116(10):S1‐S7. 10.1016/j.ophtha.2009.06.045 [DOI] [PubMed] [Google Scholar]
- 4. Mehta S. Age‐related macular degeneration. Prim Care ‐ Clin Off Pract. 2015;42(3):377‐391. 10.1016/j.pop.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 5. Muñoz‐Ramón PV, Hernández Martínez P, Muñoz‐Negrete FJ. New therapeutic targets in the treatment of age‐related macular degeneration. Arch la Soc Española Oftalmol. 2020;95(2):75‐83. 10.1016/j.oftale.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 6. Crawford T, Alfaro D III, Kerrison J, Jablon E. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8‐13. 10.2174/157339909787314149 [DOI] [PubMed] [Google Scholar]
- 7. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343‐358. 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Supplement 1):S84‐S87. 10.2337/diacare.27.2007.s84 [DOI] [PubMed] [Google Scholar]
- 9. Pandey AK, Singhi EK, Arroyo JP, et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor‐associated hypertension and vascular disease. Hypertension. 2018;71(2):E1‐E8. 10.1161/HYPERTENSIONAHA.117.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubio RG, Adamis AP. Ocular angiogenesis: vascular endothelial growth factor and other factors. Dev Ophthalmol. 2015;55:28‐37. 10.1159/000431129 [DOI] [PubMed] [Google Scholar]
- 11. Tong JP, Yao YF. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006;39(3):267‐276. 10.1016/j.clinbiochem.2005.11.013 [DOI] [PubMed] [Google Scholar]
- 12. Amadio M, Govoni S, Pascale A. Targeting VEGF in eye neovascularization: what’s new?: a comprehensive review on current therapies and oligonucleotide‐based interventions under development. Pharmacol Res. 2016;103:253‐269. 10.1016/j.phrs.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 13. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) signaling in angiogenesis: a crucial target for anti‐ and pro‐angiogenic therapies. Genes and Cancer. 2011;2(12):1097‐1105. 10.1177/1947601911423031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narayanan R, Kuppermann BD, Jones C, Kirkpatrick P. Ranibizumab. Nat Rev Drug Discov. 2006;5(10):815‐816. 10.1038/nrd2157 [DOI] [PubMed] [Google Scholar]
- 15. Joseph M, Trinh HM, Cholkar K, Pal D, Mitra AK. Recent perspectives on the delivery of biologics to back of the eye. Expert Opin Drug Deliv. 2017;14(5):631‐645. 10.1080/17425247.2016.1227783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnaswami V, Kandasamy R, Alagarsamy S, Palanisamy R, Natesan S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int J Biol Macromol. 2018;110:7‐16. 10.1016/j.ijbiomac.2018.01.120 [DOI] [PubMed] [Google Scholar]
- 17. U.S. Food & Drug Administration . Biological product definitions. Published online. 2018;1‐2.
- 18. Rodriguez Cumplido D, Asensio OC. Biological and biosimilar drugs: clarifying concepts. Aten Primaria. 2018;50(6):323‐324. 10.1016/j.aprim.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metcalf B, Rappuoli R. Pharmaceutical biotechnology. Curr Opin Biotechnol. 2003;14(6):618‐620. 10.1016/j.copbio.2003.10.008 [DOI] [Google Scholar]
- 20. Deb PK, Al‐Attraqchi OHA, Stanslas J, Al‐Aboudi A, Al‐Attraqchi N, Tekade RK. Biotechnology‐based pharmaceutical products. 2019. 10.1016/B978-0-12-814427-5.00005-6 [DOI]
- 21. Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies. Drugs. 2011;71(12):1527‐1536. 10.2165/11593730-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 22. Sharma A, Reddy P, Kuppermann BD, Bandello F, Lowenstein A. Biosimilars in ophthalmology: “is there a big change on the horizon?”. Clin Ophthalmol. 2018;12:2137‐2143. 10.2147/OPTH.S180393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Culbert PA, Johnson BD. Reference standards. Sep Sci Technol. 2004;5(C):119‐143. 10.1016/S0149-6395(03)80008-9 [DOI] [Google Scholar]
- 24. De Haan P, Van Diemen FR, Toscano MG. Viral gene delivery vectors: the next generation medicines for immune‐related diseases. Hum Vaccin Immunother. 2020:1‐8. 10.1080/21645515.2020.1757989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Overton TW. Recombinant protein production in bacterial hosts. Drug Discov Today. 2014;19(5):590‐601. 10.1016/j.drudis.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 26. Supuran CT. Agents for the prevention and treatment of age‐related macular degeneration and macular edema: a literature and patent review. Expert Opin Ther Pat. 2019;29(10):761‐767. 10.1080/13543776.2019.1671353 [DOI] [PubMed] [Google Scholar]
- 27. Kaya C, Zandi S, Pfister IB, Gerhardt C, Garweg JG. Adding a corticosteroid or switching to another anti‐VEGF in insufficiently responsive wet age‐related macular degeneration. Clin Ophthalmol. 2019;13:2403‐2409. 10.2147/OPTH.S224456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodrigues EB, Farah ME, Maia M, et al. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28(2):117‐144. 10.1016/j.preteyeres.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 29. Quinteros DA, Bermúdez JM, Ravetti S, Cid A, Allemandi DA, Palma SD. Therapeutic Use of Monoclonal Antibodies: General Aspects and Challenges for Drug Delivery. Nanostructures for Drug Delivery. Amsterdam, Netherlands: Elsevier Inc.; 2017. 10.1016/b978-0-323-46143-6.00025-7 [DOI] [Google Scholar]
- 30. Mandal A, Pal D, Agrahari V, Trinh HM, Joseph M, Mitra AK. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67‐95. 10.1016/j.addr.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biagi C, Conti V, Montanaro N, Melis M, Buccellato E. Comparative safety profiles of intravitreal bevacizumab, ranibizumab and pegaptanib: the analysis of the WHO database of adverse drug reactions. Eur J Clin Pharmacol. 2014;70:1505‐1512. 10.1007/s00228-014-1755-1 [DOI] [PubMed] [Google Scholar]
- 32. Korobelnik JF, Do DV, Schmidt‐Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247‐2254. 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 33. Kovach JL, Schwartz SG, Flynn HW, Scott IU. Anti‐VEGF treatment strategies for wet AMD. J Ophthalmol. 2012;2012:1‐7. 10.1155/2012/786870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti‐VEGF drugs. Eye (Lond). 2018;32(6):1010‐1020. 10.1038/s41433-018-0021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muralidhara BK, Wong M. Critical considerations in the formulation development of parenteral biologic drugs. Drug Discov Today. 2020;25(3):574‐581. 10.1016/j.drudis.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 36. Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984;7:188‐199. [DOI] [PubMed] [Google Scholar]
- 37. Smith SL. Ten years of orthoclone OKT3 (Muromonab‐CD3): a review. J Transpl Coord. 1996;6(3):109‐121. 10.1177/090591999600600304 [DOI] [PubMed] [Google Scholar]
- 38. Parray HA, Shukla S, Samal S, Shrivastava T, Ahmed S. International Immunopharmacology Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int Immunopharmacol. 2020;85:106639. 10.1016/j.intimp.2020.106639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Volz C, Pauly D. Antibody therapies and their challenges in the treatment of age‐related macular degeneration. Eur J Pharm Biopharm. 2015;95:158‐172. 10.1016/j.ejpb.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 40. Banker DD. Monoclonal antibodies: a review . Indian J Med Sci. 2001;55(12):651‐654. 10.2174/1574884712666170809124728 [DOI] [PubMed] [Google Scholar]
- 41. Patil R, Wong CW, Michelet F, Teo K, Ting D. Angiogenesis‐Based Therapies for Eye Diseases. In: Mehta J., Mathur P., Dhalla N., eds. Advances in Biochemistry in Health and Disease. cham: Springer; 2017. 10.1007/978-3-319-61115-0_12 [DOI] [Google Scholar]
- 42. Chen S, Zhou M, Wang W, et al. Levels of angiogenesis‐related vascular endothelial growth factor family in neovascular glaucoma eyes. Acta Ophthalmol. 2015;93(7):e556‐e560. 10.1111/aos.12624 [DOI] [PubMed] [Google Scholar]
- 43. Sanchez S, Demain A. Special issue on the production of recombinant proteins. Biotechnol Adv. 2012;30(5):1100‐1101. 10.1016/j.biotechadv.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 44. Bertolini LR, Meade H, Lazzarotto CR, et al. The transgenic animal platform for biopharmaceutical production. Transgenic Res. 2016;25(3):329‐343. 10.1007/s11248-016-9933-9 [DOI] [PubMed] [Google Scholar]
- 45. Yadav DK, Yadav N, Yadav S, Haque S, Tuteja N. An insight into fusion technology aiding efficient recombinant protein production for functional proteomics. Arch Biochem Biophys. 2016;612:57‐77. 10.1016/j.abb.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto T, Hoshikawa K, Ezura K, et al. Improvement of the transient expression system for production of recombinant proteins in plants. Sci Rep. 2018;8(1):1‐10. 10.1038/s41598-018-23024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tripathi NK. Production and purification of recombinant proteins from Escherichia coli . ChemBioEng Rev. 2016;3(3):116‐133. 10.1002/cben.201600002 [DOI] [Google Scholar]
- 48. Klimešová YM, Penčák M, Straňák Z, Lalinská L. One‐year follow‐up outcomes of treatment of wet age‐related macular degeneration with aflibercept. Czech Slovak Ophthalmol. 2018;75:47‐52. 10.31348/2018/1/1-2-2018 [DOI] [PubMed] [Google Scholar]
- 49. Anzidei R, Brent AJ, Konidaris VE, de la Mata G, Tsaousis KT. Real‐world results of switching treatment from ranibizumab to aflibercept in macular oedema secondary to branch retinal vein occlusion. Ophthalmol Ther. 2018;7(2):387‐395. 10.1007/s40123-018-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Unsal E, Cubuk MO. The results of aflibercept therapy as a first line treatment of age‐related macular degeneration. J Curr Ophthalmol. 2019;31(1):66‐71. 10.1016/j.joco.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chong V, Kaiser PK, Mitchell P. Posterior segment age‐related macular degeneration safety and efficacy of ranibizumab and bevacizumab for the treatment of neovascular age‐related macular degeneration. 2012;34‐42. 10.17925/EOR.2012.06.01.34 [DOI]
- 52. Solinís MÁ, Pozo‐Rodríguez A, Apaolaza PS. Treatment of ocular disorders by gene therapy. Eur J Pharm Biopharm. 2014;95(Part B):331–342. 10.1016/j.ejpb.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 53. Fermin G, Rampersad S, Tennant P. Chapter 12 ‐ Viruses as Tools of Biotechnology: Therapeutic Agents, Carriers of Therapeutic Agents and Genes, Nanomaterials, and More. In: Viruses Molecular Biology, Host Interactions and Applications to Biotechnology. London, UK: Elsevier Inc.; 2018. 10.1016/B978-0-12-811257-1.00012-7 [DOI] [Google Scholar]
- 54. Maeda A, Mandai M, Takahashi M. Gene and induced pluripotent stem cell therapy for retinal diseases. Ann Rev Genomics Hum Genet. 2019;20:1‐16. [DOI] [PubMed] [Google Scholar]
- 55. Gonçalves GA, Paiva RD. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). 2017;15(3):369‐375. 10.1590/S1679-45082017RB4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foldvari M, Chen DW, Nafissi N, Calderon D, Narsineni L, Rafiee A. Non‐viral gene therapy: gains and challenges of non‐invasive administration methods. J Control Release. 2016;240:165‐190. 10.1016/j.jconrel.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 57. Athanasopoulos T, Munye MM, Yáñez‐Muñoz RJ. Nonintegrating gene therapy vectors. Hematol Oncol Clin North Am. 2017;31(5):753‐770. 10.1016/j.hoc.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 58. Agarwal P, Huang D, Thakur SS, Rupenthal ID. Nanotechnology for ocular drug delivery. In: Design of Nanostructures for Versatile Therapeutic Applications. Oxford, UK: Elsevier Inc.; 2018:137–188. 10.1016/B978-0-12-813667-6.00004-8 [DOI] [Google Scholar]
- 59. Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Curr Drug Deliv. 2006;3(2):207‐217. 10.2174/156720106776359186 [DOI] [PubMed] [Google Scholar]
- 60. Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti‐VEGF treatments, adopting corticosteroid‐based treatments, and combination therapy. Expert Opin Biol Ther. 2016;16(3):365‐374. 10.1517/14712598.2016.1131265 [DOI] [PubMed] [Google Scholar]
- 61. Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of corticosteroids for diabetic macular edema. Investig Ophthalmol Vis Sci. 2018;59(1):1‐12. 10.1167/iovs.17-22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vakalis N, Echiadis G, Deligiannis I, Giannikakis S, Papaefthymiou I. Comparison of combined bevacizumab plus dexamethasone vs. ranibizumab monotherapy as first‐line therapy in patients with treatment naive neovascular age‐related macular degeneration in real‐life clinical practice: a retrospective case‐series analysis. J Clin Exp Ophthalmol. 2017;08(2): 10.4172/2155-9570.1000644 [DOI] [Google Scholar]
- 63. de Lima FJ, Sano R, Maugéri IML, et al. Evaluation of aflibercept and ziv‐aflibercept binding affinity to vascular endothelial growth factor, stability and sterility after compounding. Int J Retin Vitr. 2018;4:39. 10.1186/s40942-018-0143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mansour SE, Browning DJ, Wong K, Flynn HWJ, Bhavsar AR. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;14:653‐678. 10.2147/OPTH.S236637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fine SL, Martin DF, Kirkpatrick P. Pegaptanib sodium. Nat Rev Drug Discov. 2005;4(3):187‐188. 10.1038/nrd1677 [DOI] [PubMed] [Google Scholar]
- 66. Steinbrook R. The Price of Sight — Ranibizumab, Bevacizumab, and the Treatment of Macular Degeneration. New England Journal of Medicine. 2006;355(14):1409–1412. 10.1056/nejmp068185. [DOI] [PubMed] [Google Scholar]
- 67. Lowe J, Araujo J, Yang J, et al. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85(4):425‐430. 10.1016/j.exer.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 68. Peyman M, Peyman A, Lansingh VC, Orandi A. Science Direct Intravitreal bevacizumab versus ranibizumab: effects on the vessels of the fellow non‐treated eye. J Curr Ophthalmol. 2020;31(1):55‐60. 10.1016/j.joco.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vader MJC, Schauwvlieghe ASME, Verbraak FD, et al. Comparing the efficacy of Bevacizumab and Ranibizumab in Patients with Diabetic Macular Edema (BRDME): the BRDME Study, a randomized trial. Ophthalmol Retin. 2020;4(8):777‐788. 10.1016/j.oret.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 70. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: two‐year results. Ophthalmology. 2020;127(4):S135‐S145. 10.1016/j.ophtha.2020.01.029 [DOI] [PubMed] [Google Scholar]
- 71. Papadopoulos N, Martin J, Ruan Q, Rafique A, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171‐185. 10.1007/s10456-011-9249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hykin P, Prevost AT, Vasconcelos JC, et al. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 2019;137(11):1256. 10.1001/jamaophthalmol.2019.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nat Rev Drug Discov. 2012;11(4):269‐270. 10.1038/nrd3700 [DOI] [PubMed] [Google Scholar]
- 74. Ciombor KK, Berlin J. Aflibercept ‐ a decoy VEGF receptor topical collection on evolving therapies. Curr Oncol Rep. 2014;16(2):368. 10.1007/s11912-013-0368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu L, Liang XH, Ferrara N. Biochemical and biophysical research communications comparing protein VEGF inhibitors: in vitro biological studies. Biochem Biophys Res Commun. 2011;408(2):276‐281. 10.1016/j.bbrc.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 76. Sadiq MA, Halim MS, Hassan M, et al. Pharmacological agents in development for diabetic macular edema. Int J Retin Vitr. 2020;6:29. 10.1186/s40942-020-00234-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77. Sacconi R, Giuffrè C, Corbelli E, Borrelli E, Querques G, Bandello F. Emerging therapies in the management of macular edema: a review. F1000Res. 2019;8:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iyer S, Radwan AE, Hafezi‐moghadam A, Malyala P, Amiji M. Long‐acting intraocular Delivery strategies for biological therapy of age‐ related macular degeneration. J Control Release. 2019;296(January):140‐149. 10.1016/j.jconrel.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 79. Ludwig PE, Freeman SC, Janot AC. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int J Retin Vitr. 2019;5(1):1‐14. 10.1186/s40942-019-0158-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nayak K, Misra M. A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother. 2018;107(February):1564‐1582. 10.1016/j.biopha.2018.08.138 [DOI] [PubMed] [Google Scholar]
- 81. Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology‐based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281‐291. 10.1016/j.apsb.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Subrizi A, del Amo EM, Korzhikov‐Vlakh V, Tennikova T, Ruponen M, Urtti A. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov Today. 2019;24(8):1446‐1457. 10.1016/j.drudis.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 83. Jumelle C, Gholizadeh S, Annabi N, Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J Control Release. 2020;321:1‐22. 10.1016/j.jconrel.2020.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jiang S, Franco YL, Zhou Y, Chen J. Nanotechnology in retinal drug delivery. Int J Ophthalmol. 2018;11(6):1038‐1044. 10.18240/ijo.2018.06.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang D, Chen YS, Rupenthal ID. Overcoming ocular drug delivery barriers through the use of physical forces. Adv Drug Deliv Rev. 2018;126:96‐112. 10.1016/j.addr.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 86. Gadkar K, Pastuskovas CV, Le Couter JE, et al. Design and pharmacokinetic characterization of novel antibody formats for ocular therapeutics. Invest Ophthalmol Vis Sci. 2015;56(9):5390‐5400. 10.1167/iovs.15-17108 [DOI] [PubMed] [Google Scholar]
- 87. Yu Y, Lau LC, Lo AC, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6‐month in vivo study. Transl Vis Sci Technol. 2015;4:5. 10.1167/tvst.4.2.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Park SJ, Choi Y, Na YM, et al. Intraocular pharmacokinetics of intravitreal aflibercept (Eylea) in a rabbit model. Investig Opthalmol Vis Sci. 2016;57(6):2612‐2617. 10.1167/iovs.16-19204 [DOI] [PubMed] [Google Scholar]
- 89. Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age‐related macular degeneration: a population approach. Investig Ophthalmol Vis Sci. 2013;54(3):1616‐1624. 10.1167/iovs.12-10260 [DOI] [PubMed] [Google Scholar]
- 90. Krohne TIMU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012;154(4):682‐686.e2. 10.1016/j.ajo.2012.03.047 [DOI] [PubMed] [Google Scholar]
- 91. Keizer RJ, Huitema ADR, Schellens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493‐507. [DOI] [PubMed] [Google Scholar]
- 92. Sarav M, Wang Y, Hack BK, et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol. 2009;1941‐1952. 10.1681/ASN.2008090976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or a fl ibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98:1636‐1641. 10.1136/bjophthalmol-2014-305252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brown DM, Regillo CD. Anti‐VEGF agents in the treatment of neovascular age‐related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144(4):627‐637. 10.1016/j.ajo.2007.06.039 [DOI] [PubMed] [Google Scholar]
- 95. Schmid MK, Bachmann LM, Fäs L, Kessels AG, Job OM, Thiel MA. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age‐related macular degeneration: a trade‐off analysis. Br J Ophthalmol. 2015;99(2):141‐146. 10.1136/bjophthalmol-2014-305149 [DOI] [PubMed] [Google Scholar]
- 96. Rosenfeld PJ. Intravitreal avastin: the low cost alternative to lucentis? Am J Ophthalmol. 2006;142(1):141‐143. 10.1016/j.ajo.2006.03.036 [DOI] [PubMed] [Google Scholar]
- 97. Petrovic N, Petrovic MJ, Sreckovic S, Jovanovic S, Todorovic D, Vulovic TS. Nanotechnology in Ophthalmology. In: Brabazon D. et al. eds. Commercialization of Nanotechnologies–A Case Study Approach. cham: Springer; 2018. 10.1007/978-3-319-56979-6_11 [DOI] [Google Scholar]
- 98. Torchilin V. Introduction. Nanocarriers for drug delivery: Needs and requirements. In: Nanoparticulates as Drug Carriers. PUBLISHED BY IMPERIAL COLLEGE PRESS AND DISTRIBUTED BY WORLD SCIENTIFIC PUBLISHING CO.; 2006:1‐8. 10.1142/9781860949074_0001 [DOI]
- 99. Fornaguera C, Garcia‐Celma MJ. Personalized nanomedicine: a revolution at the nanoscale. J Pers Med. 2017;7(4):12. 10.3390/jpm7040012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery: a mini review. Int Nano Lett. 2014;4(1):94. 10.1007/s40089-014-0094-7 [DOI] [Google Scholar]
- 101. Pakzad Y, Fathi M, Omidi Y, Zamanian A, Mozafari M. Nanotechnology for ocular and optic drug delivery and targeting. In: Nanoengineered Biomaterials for Advanced Drug Delivery. Amsterdam, The Netherlands: Elsevier Ltd.; 2020:499–523. 10.1016/b978-0-08-102985-5.00021-8 [DOI] [Google Scholar]
- 102. Lin TC, Hung KH, Peng CH, et al. Nanotechnology‐based drug delivery treatments and specific targeting therapy for age‐related macular degeneration. J Chinese Med Assoc. 2015;78(11):635‐641. 10.1016/j.jcma.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 103. Sousa F, Cruz A, Fonte P, Pinto IM, Neves‐Petersen MT, Sarmento B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci Rep. 2017;7(1):1‐13. 10.1038/s41598-017-03959-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Real JP, Luna JD, Urrets‐Zavalia JA, De Santis MO, Palma SD, Granero GE. Accessibility as a conditioning factor in treatment for exudative age‐related macular degeneration. Eur J Ophthalmol. 2013;23(6):857‐864. 10.5301/ejo.5000299 [DOI] [PubMed] [Google Scholar]
- 105. Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide‐co‐glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475‐2490. 10.1016/S0142-9612(00)00115-0 [DOI] [PubMed] [Google Scholar]
- 106. Varshochian R, Jeddi‐Tehrani M, Mahmoudi AR, et al. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur J Pharm Sci. 2013;50(3‐4):341‐352. 10.1016/j.ejps.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 107. Liu J, Zhang X, Li G, et al. Anti‐angiogenic activity of bevacizumab‐bearing dexamethasone‐loaded PLGA nanoparticles for potential intravitreal applications. Int J Nanomedicine. 2019;14:8819‐8834. 10.2147/IJN.S217038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pandit J, Sultana Y, Aqil M. Chitosan‐coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif Cells, Nanomed Biotechnol. 2017;45(7):1397‐1407. 10.1080/21691401.2016.1243545 [DOI] [PubMed] [Google Scholar]
- 109. Zhang XP, Sun JG, Yao J, et al. Effect of nanoencapsulation using poly (lactide‐co‐glycolide) (PLGA) on anti‐angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed Pharmacother. 2018;107(August):1056‐1063. 10.1016/j.biopha.2018.08.092 [DOI] [PubMed] [Google Scholar]
- 110. Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol Pharm. 2013;10(12):4676‐4686. 10.1021/mp400487f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sousa F, Cruz A, Pinto IM, Sarmento B. Nanoparticles provide long‐term stability of bevacizumab preserving its antiangiogenic activity. Acta Biomater. 2018;78:285‐295. 10.1016/j.actbio.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 112. Kelly SJ, Hirani A, Shahidadpury V, et al. Aflibercept nanoformulation inhibits VEGF expression in ocular in vitro model: a preliminary report. Biomedicines. 2018;6(3):92. 10.3390/biomedicines6030092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Varshochian R, Riazi Esfahani M, Jeddi‐Tehrani M, et al. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J Biomed Mater Res Part A. 2015;103(10):3148‐3156. 10.1002/jbm.a.35446 [DOI] [PubMed] [Google Scholar]
- 114. Sun J‐G, Jiang Q, Zhang X‐P, et al. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int J Nanomed. 2019;14:1489‐1501. 10.2147/IJN.S195504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yan J, Peng X, Cai Y, Cong W. Development of facile drug delivery platform of ranibizumab fabricated PLGA‐PEGylated magnetic nanoparticles for age‐related macular degeneration therapy. J Photochem Photobiol B. 2018;183:133‐136. 10.1016/j.jphotobiol.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 116. Başaran E, Yenilmez E, Berkman MS, Büyükköroğlu G, Yazan Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J Microencapsul. 2013;2048:1‐9. 10.3109/02652048.2013.805839 [DOI] [PubMed] [Google Scholar]
- 117. Formica ML, Calles JA, Palma SD. Polysaccharide‐based nanocarriers for ocular drug delivery. Curr Pharm Des. 2015;21(33):4851‐4868. [DOI] [PubMed] [Google Scholar]
- 118. Savin C‐L, Popa M, Delaite C, Costuleanu M, Costin D, Peptu CA. Chitosan grafted‐poly(ethylene glycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater Sci Eng C Mater Biol Appl. 2019;98:843‐860. 10.1016/j.msec.2019.01.036 [DOI] [PubMed] [Google Scholar]
- 119. Ugˇurlu N, Aşık MD, Çakmak HB, et al. Transscleral delivery of bevacizumab‐loaded chitosan nanoparticles. J Biomed Nanotechnol. 2019;15(4):830‐838. 10.1166/jbn.2019.2716 [DOI] [PubMed] [Google Scholar]
- 120. Lu Y, Zhou N, Huang X, et al. Effect of intravitreal injection of bevacizumab‐chitosan nanoparticles on retina of diabetic rats. Int J Ophthalmol. 2014;7(1):1‐7. 10.3980/j.issn.2222-3959.2014.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Widjaja LK, Bora M, Chan PNPH, Lipik V, Wong TTL, Venkatraman SS. Hyaluronic acid‐based nanocomposite hydrogels for ocular drug delivery applications. J Biomed Mater Res ‐ Part A. 2014;102(9):3056‐3065. 10.1002/jbm.a.34976 [DOI] [PubMed] [Google Scholar]
- 122. Elsaid N, Jackson TL, Elsaid Z, Alqathama A, Somavarapu S. PLGA microparticles entrapping chitosan‐based nanoparticles for the ocular delivery of ranibizumab. Mol Pharm. 2016;13(9):2923‐2940. 10.1021/acs.molpharmaceut.6b00335 [DOI] [PubMed] [Google Scholar]
- 123. Luis de Redín I, Boiero C, Recalde S, et al. In vivo effect of bevacizumab‐loaded albumin nanoparticles in the treatment of corneal neovascularization. Exp Eye Res. 2019;185:107697. 10.1016/j.exer.2019.107697 [DOI] [PubMed] [Google Scholar]
- 124. Luis de Redín I, Boiero C, Martínez‐Ohárriz MC, et al. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int J Pharm. 2018;541(1‐2):214‐223. 10.1016/j.ijpharm.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 125. Llabot JM, Luis de Redin I, Agüeros M, et al. In vitro characterization of new stabilizing albumin nanoparticles as a potential topical drug delivery system in the treatment of corneal neovascularization (CNV). J Drug Deliv Sci Technol. 2019;52:379‐385. 10.1016/j.jddst.2019.04.042 [DOI] [Google Scholar]
- 126. Karumanchi DK, Skrypai Y, Thomas A, Gaillard ER. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J Drug Deliv Sci Technol. 2018;47(May):275‐282. 10.1016/j.jddst.2018.07.003 [DOI] [Google Scholar]
- 127. Abrishami M, Zarei‐Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh‐Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes‐encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29(5):699‐703. 10.1097/IAE.0b013e3181a2f42a [DOI] [PubMed] [Google Scholar]
- 128. Mu H, Wang Y, Chu Y, et al. Multivesicular liposomes for sustained release of bevacizumab in treating laser‐induced choroidal neovascularization. Drug Deliv. 2018;25(1):1372‐1383. 10.1080/10717544.2018.1474967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Battaglia L, Gallarate M, Peira E, et al. Bevacizumab loaded solid lipid nanoparticles prepared by the coacervation technique: preliminary in vitro studies. Nanotechnology. 2015;26(25):255102. 10.1088/0957-4484/26/25/255102 [DOI] [PubMed] [Google Scholar]
- 130. Formica ML, Ullio Gamboa GV, Tártara LI, Luna JD, Benoit JP, Palma SD. Triamcinolone acetonide‐loaded lipid nanocapsules for ophthalmic applications. Int J Pharm. 2020;573:118795. 10.1016/j.ijpharm.2019.118795 [DOI] [PubMed] [Google Scholar]
- 131. Formica ML, Legeay S, Bejaud J, et al. Novel hybrid lipid nanocapsules loaded with a therapeutic monoclonal antibody – Bevacizumab – and Triamcinolone acetonide for combined therapy in neovascular ocular pathologies. Mater Sci Eng C. 2021;119:111398. 10.1016/j.msec.2020.111398 [DOI] [PubMed] [Google Scholar]
- 132. Wen S, Zhang K, Li Y, et al. A self‐assembling peptide targeting VEGF receptors to inhibit angiogenesis. Chinese Chem Lett. 2020;2019:1‐5. 10.1016/j.cclet.2020.03.077. [DOI] [Google Scholar]
- 133. Palanisamy K, Nareshkumar RN, Sivagurunathan S, Raman R, Sulochana KN, Chidambaram S. Anti‐angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvasc Res. 2019;122:136‐145. 10.1016/j.mvr.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 134. Jiang J, Wang L, Kou X, et al. In vivo characterization of RC28‐E, a fusion protein targeting VEGF and bFGF: pharmacokinetics and ocular distribution in primates. Exp Eye Res. 2020;190:107823. 10.1016/j.exer.2019.107823 [DOI] [PubMed] [Google Scholar]
- 135. Jiang J, Xu K, Wang L, et al. Pharmacology study of a chimeric decoy receptor trap fusion protein on retina neovascularization by dual blockage of VEGF and FGF‐2. Eur J Pharm Sci. 2018;121:251‐259. 10.1016/j.ejps.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 136. Gao F, Sun M, Gong Y, Wang H, Wang Y, Hou H. MicroRNA‐195a‐3p inhibits angiogenesis by targeting Mmp2 in murine mesenchymal stem cells. Mol Reprod Dev. 2016;83(5):413‐423. 10.1002/mrd.22638 [DOI] [PubMed] [Google Scholar]
- 137. Zheng Y, Sun Q, Xu X, Wang W. Novel peptide derived from IGF‐2 displays anti‐angiogenic activity in vitro and inhibits retinal angiogenesis in a model of oxygen‐induced retinopathy. Clinical & Experimental Ophthalmology. 2020;48(9):1261–1275. 10.1111/ceo.13864. [DOI] [PubMed] [Google Scholar]
- 138. Yin X, Lin X, Ren X, et al. Novel multi‐targeted inhibitors suppress ocular neovascularization by regulating unique gene sets. Pharmacol Res. 2019;146(April):104277. 10.1016/j.phrs.2019.104277 [DOI] [PubMed] [Google Scholar]
- 139. Lima e Silva R, Kanan Y, Mirando AC, et al. Tyrosine kinase blocking collagen IV‐derived peptide suppresses ocular neovascularization and vascular leakage. Sci Transl Med. 2017;9(373):1‐12. 10.1126/scitranslmed.aai8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sheibani N, Wang S, Darjatmoko SR, et al. Novel anti‐angiogenic PEDF‐derived small peptides mitigate choroidal neovascularization. Exp Eye Res. 2019;188(June):107798. 10.1016/j.exer.2019.107798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bainbridge JWB, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13(16):1191‐1197. 10.1038/sj.gt.3302812 [DOI] [PubMed] [Google Scholar]
- 142. Powell AB, Williams K, Cruz CRY. Gene‐modified, cell‐based therapies—an overview. Cytotherapy. 2016;18(11):1351‐1359. 10.1016/j.jcyt.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 143. Touchard E, Benard R, Bigot K, et al. Non‐viral ocular gene therapy, pEYS606, for the treatment of non‐infectious uveitis: preclinical evaluation of the medicinal product. J Control Release. 2018;285(April):244‐251. 10.1016/j.jconrel.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 144. Shi Z, Li SK, Charoenputtakun P, Liu CY, Jasinski D, Guo P. RNA nanoparticle distribution and clearance in the eye after subconjunctival injection with and without thermosensitive hydrogels. J Control Release. 2018;270:14‐22. 10.1016/j.jconrel.2017.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Martínez T, González MV, Roehl I, Wright N, Pañeda C, Jiménez AI. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol Ther. 2014;22(1):81‐91. 10.1038/mt.2013.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral‐eye administration of AAV2 gene therapy in patients with childhood‐onset blindness caused by RPE65 mutations: a follow‐on phase 1 trial. Lancet. 2016;388(10045):661‐672. 10.1016/S0140-6736(16)30371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wojnarowicz PM, Lima e Silva R, Ohnaka M, et al. A small‐molecule Pan‐Id antagonist inhibits pathologic ocular neovascularization. Cell Rep. 2019;29(1):62‐75.e7. 10.1016/j.celrep.2019.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hernandez M, Recalde S, Garcia‐Garcia L, et al. Preclinical evaluation of a cell‐based gene therapy using the sleeping beauty transposon system in choroidal neovascularization. Mol Ther ‐ Methods Clin Dev. 2019;15(December):403‐417. 10.1016/j.omtm.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang Z, Gao F, Zhang M, et al. Intravitreal injection of human retinal progenitor cells for treatment of retinal degeneration. Med Sci Monit. 2020;26:e921184. 10.12659/MSM.921184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Weiss JN, Levy S. Stem cell Ophthalmology Treatment Study (SCOTS): bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig. 2019;6(September):31. 10.21037/sci.2019.08.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Matsuda Y, Nonaka Y, Futakawa S, et al. Anti‐angiogenic and anti‐scarring dual action of an anti‐fibroblast growth factor 2 aptamer in animal models of retinal disease. Mol Ther ‐ Nucleic Acids. 2019;17(September):819‐828. 10.1016/j.omtn.2019.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vivanco‐Rojas O, García‐Bermúdez MY, Iturriaga‐Goyon E, et al. Corneal neovascularization is inhibited with nucleolin‐binding aptamer, AS1411. Exp Eye Res. 2020;193:107977. 10.1016/j.exer.2020.107977 [DOI] [PubMed] [Google Scholar]
- 153. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Alexander SPH, Fabbro D, Kelly E, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: catalytic receptors. Br J Pharmacol. 2019;176(S1):S247‐S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]


