Abstract
Background
Low-dose CT (LDCT) screening reduces lung cancer mortality by at least 20%. The COVID-19 pandemic required an unprecedented shutdown in our institutional LDCT program. The purpose of this study was to examine the impact of COVID-19 on lung cancer screening and subsequent cancer diagnosis.
Study Design
We analyzed our prospective institutional LDCT screening database, which began in 2012. In all, 2,153 patients have participated. Monthly mean number of LDCTs were compared between baseline (January 2017 to February 2020) and COVID-19 periods (March 2020 to July 2020).
Results
LDCT was suspended on March 13, 2020 and 818 screening visits were cancelled. Phased reopening began on May 5, 2020 and full opening on June 1, 2020. Total monthly mean ± SD LDCTs (146 ± 31 vs 39 ± 40; p < 0.01) and new patient monthly LDCTs (56 ± 14 vs 15 ± 17; p < 0.01) were significantly decreased during the COVID-19 period. New patient monthly LDCTs have remained low despite resuming full operations. Three- and 6-month interval follow-up LDCTs were prioritized and were significantly increased compared with baseline (11 ± 4 vs 30 ± 4; p < 0.01). The “no-show” rate was significantly increased from baseline (15% vs 40%; p < 0.04). Most concerning, the percentage of patients with lung nodules suspicious for malignancy (Lung-RADS 4) were significantly increased after screenings resumed (8% vs 29%; p < 0.01).
Conclusions
COVID-19 caused significant disruption in lung cancer screening, leading to a decrease in new patients screened and an increased proportion of nodules suspicious for malignancy once screening resumed. Using lung cancer and the LDCT screening program as a model, this early analysis showed the unrecognized consequences related to the pandemic for screening programs and cancer care.
Visual Abstract
Lung cancer is the leading cause of mortality among cancer diagnoses, and was responsible for 145,849 deaths in the US alone in 2017.1 Fortunately, during the last decade, lung cancer mortality has been in an accelerated decline1 due to successful public health campaigns, such as smoking cessation2 , 3 and therapeutic improvements.4 In addition, annual low-dose CT (LDCT) screening programs have been found to reduce mortality from lung cancer by at least 20% among high-risk patients in large clinical trials.5 , 6 In December 2013, the US Preventive Services Task Force published guidelines recommending LDCT for adults with the following criteria: aged 55 to 80 years, 30 pack-year smoking history, and currently smoking or quit within the last 15 years.7
The impact of the COVID-19 pandemic on the healthcare industry has been tremendous, with immediate consequences on resource use and wide-ranging long-term implications throughout medicine.8 Cancer care has not been spared, with difficult but necessary decisions to mitigate risk during the early phases of the pandemic.9 Cancer diagnoses in the US were decreased across 6 common malignancies,10 and The Netherlands Cancer Registry noted an almost 30% decrease in all primary cancer diagnoses.11
The impact of the COVID-19 pandemic on screening and subsequent cancer diagnosis is unknown. Early detection through effective screening programs saves lives in colorectal, breast, and lung cancer.6 , 12 , 13 Early in the COVID-19 pandemic, an expert panel from the American College of Chest Physicians recommended delaying initiation of screening for new individuals and delaying annual LDCT for patients in established lung cancer screening protocols.14 However, the short- and long-term outcomes after delaying screening in this population have not been studied. The purpose of this study was to examine the impact of COVID-19 on lung cancer screening and subsequent cancer at our institution.
Methods
We retrospectively reviewed our prospectively maintained database from January 1, 2012 to July 30, 2020. This study was approved by the IRB (2018-7564) with waiver of informed consent. The lung screening program at the University of Cincinnati was initiated in November 2012. It is composed of a multidisciplinary team of clinicians and 3 full-time staff members (2 registered nurses and 1 medical assistant). Data, such as demographic characteristics, medical comorbidities (eg COPD and pulmonary fibrosis), occupational exposures, CT screening results, interventions, and lung cancer diagnosis, are collected prospectively. Patient eligibility criteria include aged 55 to 80 years, at least 30 pack-year smoking history, and current smoker or former smoker. All patients who underwent screening were included in this investigation.
LDCTs are reviewed by dedicated chest radiologists and managed according to the American College of Radiology Lung-RADS categorization criteria.15 All patients considered at high risk for malignancy, defined by the American College of Radiology category Lung-RADS 4, are reviewed at a multidisciplinary lung tumor board.15 Tumor board participants include lung cancer specialists from thoracic surgery, interventional pulmonology, medical and radiation oncology, pathology, and radiology. Recommendations include resuming annual screening, short-interval LDCT, PET, and/or referral to a thoracic surgeon or interventional pulmonologist for intervention. Invasive interventions included bronchoscopy, endobronchial ultrasound, percutaneous biopsy, and lung resection. After cancer diagnosis, treatment was based on the American Joint Committee on Cancer's cancer staging manual, 8th edition.16 Final treatment decisions are at the discretion of the physician and the patient.
Monthly mean LDCT screenings were compared between baseline (January 2017 to February 2020) and COVID-19 (March 2020 to July 30, 2020) periods. Rates of LDCT review at tumor board were compared between cohorts. For those presented at tumor board, records were reviewed to determine nodule size, presence of a new lung nodule, and time interval between LDCT in months. The indication for tumor board review was classified as either a new nodule or an enlarging nodule. Tumor board recommendations from January 1, 2020 and July 30, 2020 were analyzed to compare rates of referral for intervention vs repeat imaging. “No show” patients, defined as those that did not show up or reschedule their screening appointment, were identified from January 1, 2020 and July 30, 2020 and analyzed.
Data were described using mean ± SD and percentiles for continuous and categorical variables, respectively. Correlations between cohorts were evaluated using the 2-sample t-test, Pearson chi-square test or Fisher exact test, as appropriate. A p value <0.05 was considered significant. All analyses were conducted using JMP PRO, version 15 (SAS Institute Inc).
Results
From January 2012 to July 2020, a total of 2,153 unique patients underwent LDCT screening. Baseline demographic information and clinical characteristics are shown in Table 1 . The cohort's mean ± SD age was 63.8 ± 5.9 years; most were men and non-Hispanic White. Most patients screened were current smokers, with a mean ± SD number of pack-years of 53.8 ± 23.6. COPD (31.9%) and occupational exposures (23.4%) were also common. Since 2012, lung cancer was diagnosed within the LDCT cohort in 4.7% (n = 101) of patients.
Table 1.
Demographic and Clinical Characteristics of Patients in the Lung Cancer Screening Program
| Characteristic | Data (n = 2,153) |
|---|---|
| Age, y, mean ± SD | 63.8 ± 5.9 |
| Sex, f, n (%) | 866 (45.1) |
| Race, n (%) | |
| Non-Hispanic White | 1,118 (72.0) |
| African American | 409 (26.3) |
| Other | 25 (1.7) |
| Current smoker, n (%) | 1,003 (51.7) |
| Pack-years, mean ± SD | 53.8 ± 23.6 |
| COPD, n (%) | 618 (31.9) |
| Occupational exposure, n (%) | 285 (23.4) |
| Radon exposure, n (%) | 239 (12.3) |
| Pulmonary fibrosis, n (%) | 99 (5.1) |
| Personal cancer history, n (%) | 136 (7.0) |
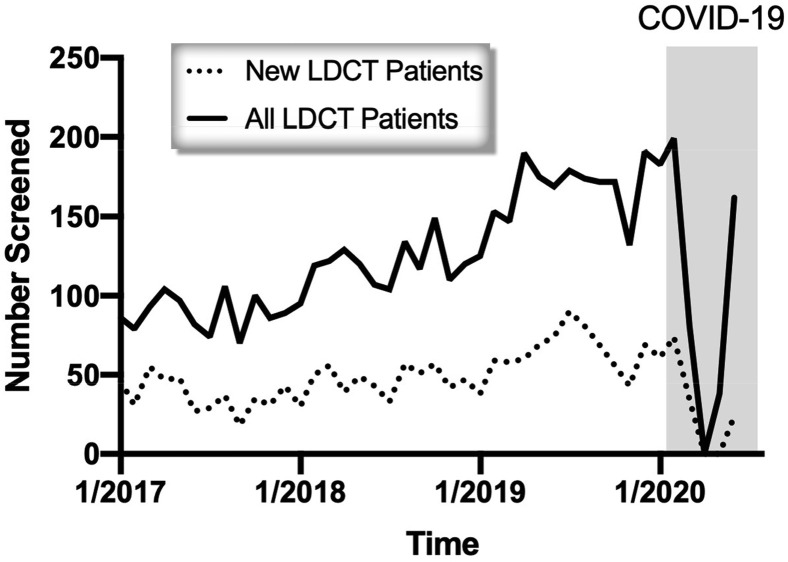
LDCT screening was suspended due to the COVID-19 pandemic on March 13, 2020 based on the direction of our hospital system and university. Phased reopening began on May 5, 2020 and full opening occurred on June 1, 2020. During this time period, 818 LDCTs were cancelled. Total monthly mean ± SD LDCTs (146 ± 31 vs 39 ± 40; p < 0.01) and new patient monthly LDCTs (56 ± 14 vs 15 ± 17; p < 0.01) were significantly decreased during the COVID-19 period (Fig. 1 ). On resuming operations, 3- and 6-month interval follow-up LDCTs were prioritized and were increased significantly from baseline (11 ± 4 vs 30 ± 4; p < 0.01). However, despite complete reopening of LDCT operations, new patient monthly LDCTs have remained low (Fig. 1).
Figure 1.
New patient and all patient monthly low-dose CT (LDCT) screening visits. Highlighted box identifies the COVID-19 period.
The no-show rate increased significantly from baseline during COVID-19 (40% vs 15%; p < 0.04). Of the 139 no-shows during the COVID-19 period, most were for annual examinations (n = 112 [80.6%]), the remaining were for baseline scan (n = 12 [8.7%]), 3-month (n = 6 [4.3%]), and 6-month (n = 9 [6.5%]) follow-up appointments. Compared with the entire LDCT screening cohort, patients who “no-showed” to their appointments in 2020 were more likely to be younger, female, African American, and current smokers (Table 2 ).
Table 2.
Patient Demographic Characteristics of Our Low-Dose CT Screening Program Compared with the No-Show Population in 2020
| Characteristic | Lung cancer screening program (n = 1,939) | No-show population (n = 214) | p Value |
|---|---|---|---|
| Age, y, mean ± SD | 63.8 ± 5.9 | 61.8 ± 4.8 | <0.01 |
| Sex, f, n (%) | 866 (45.1) | 123 (58.0) | <0.01 |
| Race, n (%) | <0.01 | ||
| Non-Hispanic White | 1,118 (72.1) | 94 (48.5) | |
| African American | 433 (27.9) | 100 (51.6) | |
| Current smoker, n (%) | 1,003 (51.7) | 154 (72.3) | <0.01 |
| Pack-years, mean ± SD | 53.8 ± 23.6 | 45.5 ± 23.9 | <0.01 |
| COPD, n (%) | 139 (31.9) | 69 (33.2) | 0.70 |
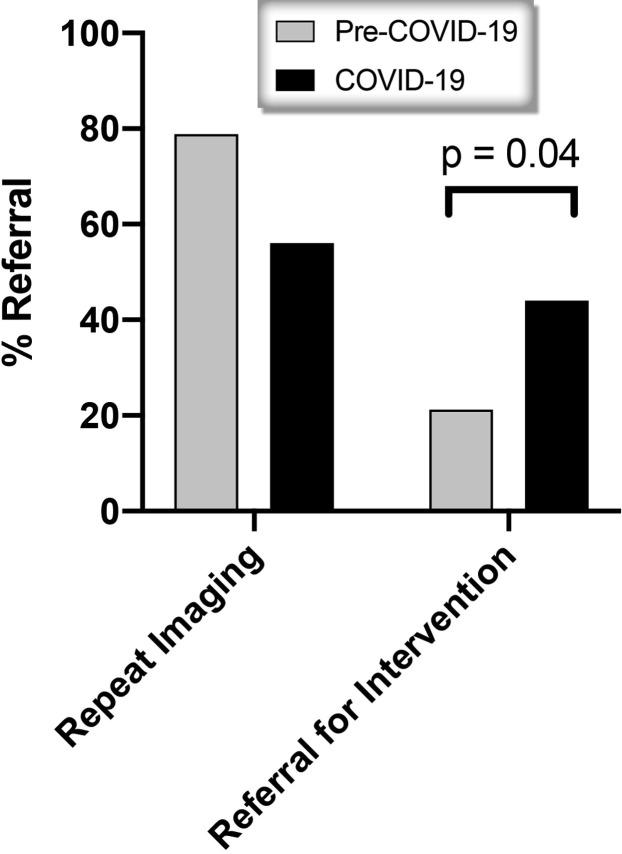
Most concerning, the percentage of patients with lung nodules suspicious for malignancy (Lung-RADS 4) was increased significantly after screening operations resumed (29% vs 8%; p < 0.01). Clinical details about size of nodule, interval between scans, indication for tumor board evaluation, and tumor board recommendation were compared between the pre COVID-19 and COVID-19 cohorts (Table 3 ) Among patients with Lung-RADS 4 lesions reviewed at tumor board, patients were less likely to have new nodules (69.8% vs 38.5%; p < 0.01). However, there was no significant differences in the mean ± SD size of nodule (14.5 ± 16.4 mm vs 10.5 ± 7.1 mm; p = 0.56) or interval from previous scan (13.6 ± 13.2 months vs 11.7 ± 14.1 months; p = 0.36). There was also a significant increase in referrals for intervention by thoracic surgery or interventional pulmonology among tumor board patients in the COVID-19 era (44.0% vs 21.2%; p = 0.04) (Fig. 2 ). During the COVID-19 period, 4 patients were referred to interventional pulmonology. Three patients underwent endobronchial ultrasound and 1 patient underwent repeat imaging. Seven patients were referred to thoracic surgery and 3 patients underwent minimally invasive diagnostic wedge resection to confirm malignancy, followed by lobectomy and mediastinal lymph node dissection. One patient was offered lung resection but refused and was treated with stereotactic body radiation therapy. Three patients underwent repeat imaging.
Table 3.
Details of Patients Referred for Tumor Board Evaluation and Tumor Board Recommendation, January 1, 2020 to July 30, 2020
| Variable | Pre COVID-19 period (n = 53) | COVID-19 period (n = 34) | p Value |
|---|---|---|---|
| Nodule size, mm, mean ± SD | 14.5 ± 16.4 | 10.5 ± 7.1 | 0.56 |
| New nodule, size | 16.5 ± 19.0 | 8.1 ± 4.0 | 0.11 |
| Follow-up, Δ | 2.1 ± 1.9 | 2.5 ± 2.8 | 0.84 |
| Interval scan, mo, mean ± SD | 13.6 ± 13.2 | 11.7 ± 14.1 | 0.36 |
| Indication for tumor board, n (%) | <0.01 | ||
| New nodule | 37 (69.8) | 10 (38.5) | |
| Enlarging nodule | 16 (30.2) | 16 (61.5) | |
| Tumor board recommendation, n (%) | 0.04 | ||
| Repeat imaging | 41 (78.9) | 14 (56.0) | |
| Intervention | 11 (21.2) | 11 (44.0) |
Δ, change.
Figure 2.
Multidisciplinary tumor board recommendation for patients in 2020.
Discussion
We have reported the impact of the COVID-19 pandemic on our institutional lung cancer screening program. There was decreased total and new patients screened during the COVID-19 period, even after screening operations resumed. Patients were also more likely to be a no-show for their LDCT after screening resumed. A higher proportion of Lung-RADS 4 nodules were identified and there were increased referrals for intervention. These findings have important implications for lung cancer screening and subsequent lung cancer diagnosis with the ongoing COVID-19 pandemic.
COVID-19 has dramatically impacted many aspects of medicine, including the field of oncology. Cross-sectional analysis of diagnosis codes demonstrated a 46% decrease in new cancer diagnoses in the US during March and April 2020 among 6 common malignancies, including lung and esophageal cancer.10 The Netherlands Cancer Registry noted an almost 30% decrease in new cancer diagnoses in all primary cancer sites; new lung cancer diagnoses had a similar decrease.11 New cancer diagnoses remained low, despite a national public awareness campaign to increase cancer diagnoses by encouraging patients to discuss new symptoms with their primary care provider, encouraging primary care providers to refer patients to oncology specialist, and resuming cancer screening operations.11 In our study, we observed a decrease in total and new patient monthly LDCTs during the COVID-19 period. Similar decreases in cancer screening have been reported for breast17 and colon cancer.18 , 19 National use of LDCT is low at baseline, so any additional decreases has potential negative consequences both in terms of cancer-related mortality and future use of lung cancer screening.20, 21, 22
Fear related to contracting COVID-19 has caused patients to be more apprehensive to seek medical care for routine or emergent issues.23 , 24 Diagnoses for many acute conditions, such as appendicitis, MI, stroke, and aortic dissection have decreased during the COVID-19 pandemic.25, 26, 27, 28 Similarly, in our study, new-patient monthly LDCTs remained low despite resuming full lung cancer screening operations, and the no-show rate was increased significantly. We observed that patients who were younger, female, African American, and current smokers were more likely to no-show for LDCT. This aspect of lung cancer screening should be studied further, as it could worsen existing disparities in lung cancer survival. African Americans already have worse overall survival29 and are less likely to undergo curative lung resection.30
Risk mitigation from COVID-19 is important in cancer patients, with reports of 13% mortality in cancer patients affected with COVID-19.31 Lung cancer patients are particularly at risk due to underlying comorbidities, such as smoking and pulmonary disease. Patients with thoracic malignancies infected with COVID-19 have a reported 76% hospitalization rate and 33% mortality rate.32 Several societies and expert panels have released recommendations for the management of malignancy during the COVID-19 pandemic.33 Specifically for lung cancer, expert panels from the Thoracic Surgery Outcomes Research Network recommended deferring operations for 3 months if hospital resources were limited due to COVID-19. If hospital resources were normal with only a few COVID-19 cases, they recommended operations for node-positive lung cancer, tumors ≥2 cm, or for those who received neoadjuvant therapy only.34 Previous reports have found that these delays can increase perioperative morbidity and negatively impact overall survival.35 Recent evidence from the UK estimates a 4.8% to 5.3% increase in lung cancer mortality due to diagnostic delay, highlighting the critical stakes at hand.24 We observed increased lung nodules suspicious for malignancy (Lung-RADS 4) after screening operations resumed. Patients were more likely to be referred to tumor board for enlarging nodules and there was a significant increase in referrals for intervention among tumor board patients in the COVID-19 period. There is also a backlog of new patients who are awaiting initial screening, as we are still rescheduling cancelled appointments.
The COVID-19 pandemic is ongoing and the US and Europe experienced substantial increases in new cases during the fall and winter of 2020. Our results provide a framework for future decisions amid the ongoing COVID-19 pandemic. Lung cancer screening operations should be prioritized and continued to prevent negative consequences, such as delay in diagnosis, which could lead to increased cancer-specific mortality. The COVID-19 pandemic also highlights the following important areas of improvement needed in lung cancer screening: refinement of selection criteria for LDCT and risk stratification of identified pulmonary nodules.36 , 37 These improvements could help prioritize which LDCTs are performed, which would help reduce risk of exposure during current and future pandemics.
There are several limitations to consider. We report our single-center institutional experience and our results might not be externally generalizable. Our initial experience with COVID-19 in spring of 2020 was a relatively low number of cases in the region and our LDCT screenings were shut down for a relatively short time period. Analysis was performed using historical data to make comparisons with the COVID-19 period. It is possible that observed associations are due to other unmeasured variables aside from the COVID-19 pandemic, as cause and effect could not be established by our methods. Due to the limited follow-up period, not all suspicious lung nodules were confirmed as lung malignancy. Instead, we measured variables, such as referral for intervention.
Conclusions
COVID-19 has caused considerable disruption in lung cancer screening. There were fewer new patients screened, more patients were apprehensive to visit the healthcare system, and an increased proportion of nodules suspicious for malignancy. This early analysis represents possible unintended consequences of the pandemic in regard to lung cancer and possibly other malignancies. It can provide a framework for future decisions amid the ongoing COVID-19 pandemic.
Author Contributions
Study conception and design: Van Haren, Delman, Turner, Shah, Starnes
Acquisition of data: Van Haren, Delman, Turner, Waits, Hemingway, Shah, Starnes
Analysis and interpretation of data: Van Haren, Delman, Turner, Waits, Hemingway, Shah, Starnes
Drafting of manuscript: Van Haren, Delman, Turner, Waits, Hemingway, Shah, Starnes
Critical revision: Van Haren, Delman, Turner, Waits, Hemingway, Shah, Starnes
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Van Haren is a paid consultant to Intuitive Surgical, Inc. Other authors have nothing to disclose.
Selected for the 2020 Southern Surgical Association Program.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Chronic Disease Prevention and Health Promotion . Centers for Disease Control and Prevention; Atlanta, GA: 2014. Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. [Google Scholar]
- 3.Moolgavkar S.H., Holford T.R., Levy D.T. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975-2000. J Natl Cancer Inst. 2012;104:541–548. doi: 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N., Forjaz G., Mooradian M.J. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church T.R., Black W.C., Aberle D.R. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning H.J., van der Aalst C.M., de Jong P.A. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 7.Blackmon S.H., Feinglass S.R. The United States Preventive Services Task Force recommendations for lung cancer screening. Thorac Surg Clin. 2015;25:199–203. doi: 10.1016/j.thorsurg.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Emanuel E.J., Persad G., Upshur R. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 9.van de Haar J., Hoes L.R., Coles C.E. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26:665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinmohamed A.G., Visser O., Verhoeven R.H.A. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zauber A.G., Winawer S.J., O'Brien M.J. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry D.A., Cronin K.A., Plevritis S.K. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone P.J., Gould M.K., Arenberg D.A. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST Expert Panel Report. Chest. 2020;158:406–415. doi: 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Radiology Lung-RADS®. Version 1.1. Assessment categories. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf Available at: Released 2019. Accessed October 28, 2020.
- 16.Amin M.B., Edge S., Greene F., editors. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; New York: 2017. [Google Scholar]
- 17.Tsai H.Y., Chang Y.L., Shen C.T. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast. 2020;54:52–55. doi: 10.1016/j.breast.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Ovidio V., Lucidi C., Bruno G. Impact of COVID-19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer. 2020 Jul 30 doi: 10.1016/j.clcc.2020.07.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter M.D., Brookes M., Lee T.J. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2020 July 20 doi: 10.1136/gutjnl-2020-322179. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Coughlin J.M., Zang Y., Terranella S. Understanding barriers to lung cancer screening in primary care. J Thorac Dis. 2020;12:2536–2544. doi: 10.21037/jtd.2020.03.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ersek J.L., Eberth J.M., McDonnell K.K. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122:2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 23.Jones D., Neal R.D., Duffy S.R.G. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tankel J., Keinan A., Blich O. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. 2020;44:2458–2463. doi: 10.1007/s00268-020-05599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diegoli H., Magalhaes P.S.C., Martins S.C.O. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020;51:2315–2321. doi: 10.1161/STROKEAHA.120.030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bugger H., Gollmer J., Pregartner G. Complications and mortality of cardiovascular emergency admissions during COVID-19 associated restrictive measures. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach P.B., Cramer L.D., Warren J.L., Begg C.B. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 30.Sineshaw H.M., Wu X.C., Flanders W.D. Variations in receipt of curative-intent surgery for early-stage non-small cell lung cancer (NSCLC) by state. J Thorac Oncol. 2016;11:880–889. doi: 10.1016/j.jtho.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garassino M.C., Whisenant J.G., Huang L.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Surgeons COVID-19 guidelines for triage of cancer surgery patients 2020. https://www.facs.org/covid-19/clinical-guidance/elective-case/cancer-surgery Available at:
- 34.Thoracic Surgery Outcomes Research Network Inc. Antonoff M., Backhus L. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. J Thorac Cardiovasc Surg. 2020;160:601–605. doi: 10.1016/j.jtcvs.2020.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinsky N.C., Wima K., Morris M.C. Outcome of delayed versus timely esophagectomy after chemoradiation for esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2020;159:2555–2566. doi: 10.1016/j.jtcvs.2019.09.169. [DOI] [PubMed] [Google Scholar]
- 36.Walter J.E., Heuvelmans M.A., Yousaf-Khan U. New subsolid pulmonary nodules in lung cancer screening: the NELSON Trial. J Thorac Oncol. 2018;13:1410–1414. doi: 10.1016/j.jtho.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Heuvelmans M.A., Walter J.E., Oudkerk M. Management of baseline and new sub-solid nodules in CT lung cancer screening. Expert Rev Respir Med. 2018;12:1–3. doi: 10.1080/17476348.2018.1398087. [DOI] [PubMed] [Google Scholar]