Abstract
Meiosis is essential to the continuity of life in sexually-reproducing organisms through the formation of haploid gametes. Unlike somatic cells, the germ cells undergo two successive rounds of meiotic divisions after a single cycle of DNA replication, resulting in the decrease in ploidy. In humans, errors in meiotic progression can cause infertility and birth defects. Post-translational modifications, such as phosphorylation, ubiquitylation and sumoylation have emerged as important regulatory events in meiosis. There are dynamic equilibrium of protein phosphorylation and protein dephosphorylation in meiotic cell cycle process, regulated by a conservative series of protein kinases and protein phosphatases. Among these protein phosphatases, PP2A, PP4, and PP6 constitute the PP2A-like subfamily within the serine/threonine protein phosphatase family. Herein, we review recent discoveries and explore the role of PP2A-like protein phosphatases during meiotic progression.
Keywords: protein phosphorylation, meiosis, PP2A, PP4, PP6
Introduction
In eukaryotes, reversible phosphorylation and dephosphorylation of proteins represents an prominent type of post-translational modification that has an crucial effect on controlling some cellular processes and events (Hunter, 1995). The state of protein phosphorylation can be adjusted by some highly conserved protein kinases and protein phosphatases (Mumby and Walter, 1993). In general, it is necessary for a number of critical biological events to keep a proper balance between protein kinases and protein phosphatases (Cassimeris, 1999). Disruption of this equilibrium can contribute to many pathological circumstances and even diseases. This spatial and temporal regulation of protein phosphorylation occurs not only in mitotic program, but also in meiotic progression.
Meiosis is a peculiar type of division in which one single round of DNA replication is followed by two sequential rounds of chromosome segregation (meiosis I and meiosis II), which is an important procedure for gamogenesis. Through this progression, diploid parent cells give rise to haploid gametes with the correct number of chromosomes. Similar to mitosis, meiotic division occurs in all eukaryotes and is an intricate event that is needed to change the cell cycle (Wolgemuth and Roberts, 2010). DNA replication and chromosome segregation both occur in meiosis. However, there are some other particular events in meiosis, such as homologous chromosome pairing, synaptonemal complex formation, double-strand break (DSB) repairing, meiotic recombination and a reductional division (Berchowitz and Copenhaver, 2010). Prior to the meiotic divisions, changing maternal and paternal chromosome behaviors including pairing, synapsis, and recombination must occur in a highly adjusted manner during prophase (Sato-Carlton et al., 2014). Therefore, according to the different behaviors of chromosomes, the prophase I also can be divided into five stages which are named as leptotene, zygotene, pachytene, diplotene, diakinesis. These events result in the mutual exchange of DNA material between homologous chromosomes and increasing genetic diversity (Bishop and Zickler, 2004). During meiosis I, homologous chromosomes are segregated whereas sister chromatids are still interacted on each other. Then sister chromatids are fully segregated in the second meiotic division (Canela et al., 2003; Qi et al., 2013). Errors in any of these events attribute to failure of the gametogenesis. In this progress, human oocytes have an abnormally high chromosome error rate that significantly increases with age, with severe results for human fertility (Keating et al., 2020).
A battery of protein phosphorylation and protein dephosphorylation events, which are adjusted by protein kinases and protein phosphatases, are critical for meiotic process (Bornslaeger et al., 1986; Lu et al., 2001). Protein kinases shift a phosphoryl group from adenosine triphosphate (ATP) to the hydroxyl group of serine, threonine and tyrosine residues, while protein phosphatases dephosphorylate protein by phosphate group hydrolysis and thus oppose the actions of protein kinases (Lillo et al., 2014). Among the phosphorylation, almost 98% of protein phosphorylation occurs on serine and threonine residues (Pearlman et al., 2011; Hunter, 2014). In human genome, there are more than 500 protein kinases encoded that catalyze the phosphorylation (Subramani et al., 2013). These protein kinases can phosphorylate the specific sites of target proteins. Nevertheless, it is insufficient for protein kinases alone to control dynamic processes. Because the phosphorylation of serine and threonine sites is extraordinary stable, which has long half-life (Lad et al., 2003), protein phosphatases can ensure that protein phosphorylation is dynamic and reversible (Nilsson, 2019). For various reasons, compared with the rich knowledge on protein kinases, there is a relative lack of information about the functions of protein phosphatases (Afshar et al., 2016). Among these conserved phosphoprotein phosphatases, PP2A, PP4, and PP6 constitute the PP2A-like subfamily within the serine and threonine protein phosphatase family (Bielinski and Mumby, 2007). These phosphoprotein phosphatases play crucial roles in multiple series of fundamental cellular events. Recent studies have implicated that PP2A-like protein phosphatases play critical roles in regulating meiosis. In this review, we will summarize recent discoveries and explore the role of PP2A-like protein phosphatases during meiotic progression.
Classification of Protein Phosphatases
In the past decades, there are numerous studies about the biological roles of protein phosphatases, especially in meiosis. Generally, eukaryotic protein phosphatases can be divided into four major gene families based on specific substrate, catalytic activity and inhibitor sensitivity (Lillo et al., 2014). These families are phosphoprotein phosphatases (PPP), Mg2+/Mn2+-dependent protein phosphatases (PPM), aspartate-based protein phosphatases, and phosphotyrosine phosphatases (PTP) (Kerk et al., 2008). Among these families, the PPPs are the most comparatively conservative members across the whole eukaryotic species from yeast to human, indicating their “housekeeping” importance (Brautigan, 2013). In eukaryotic cells, almost 80% of the protein phosphatase activity is regulated by PPP family (Janssens and Goris, 2001). The PPP family includes seven members, namely PP1, PP2A, PP2B (also known as PP3), PP4, PP5, PP6, and PP7.
The Structure of PP2A-Like Protein Phosphatases
Within the PPP family, PP2A, PP4, and PP6 come into being an independent cluster, whose catalytic subunits are most closely related, suggestive of a common origin (Chen et al., 2017). The catalytic subunits combine with scaffolding and/or regulatory subunits to form heterotrimeric or heterodimeric holoenzyme complexes (Brautigan and Shenolikar, 2018). Although their catalytic subunits have high sequence similarity, they have their own special structural compositions (Nasa and Kettenbach, 2020). PP2A is a heterotrimer holoenzyme complex consisting of a catalytic subunit, a scaffold subunit, and a regulatory subunit. The heterodimeric holoenzyme also named as core enzyme, composing of the catalytic and scaffold subunit, which is indispensable for the function of the holoenzyme (Price and Mumby, 2000). In higher eukaryotes, there are two isoforms in PP2A catalytic subunit (PPP2ACα and PPP2ACβ), which have 97% sequence similarity with each other. There are also two isoforms in PP2A scaffold subunit (PPP2R1α and PPP2R1β), which have abmost 87% sequence similarity (Saurin, 2018). The PP2A regulatory subunit has multiple members, which belong to four different families: B (B55), B′ (B56), B″ (B72), and B″′ (Striatin) family (Janssens and Goris, 2001). Therefore, the different combination of subunits results in various PP2A holoenzyme, differing in subcellular localization and distinct substrate specificity. For PP4, catalytic subunit combines with different regulatory subunits to form heterodimers or heterotrimers. The PP4 regulatory subunit has five isoforms: PPP4R1, PPP4R2, PPP4R3A, PPP4R3B, and PPP4R4 (Kloeker and Wadzinski, 1999; Cohen et al., 2005). Like other type 2A serine/threonine protein phosphatases, PP6 also works as a holoenzyme, consisting of a catalytic subunit, PPP6C, one of the three regulatory subunits including PPP6R1, PPP6R2 and PPP6R3, and one of the three ankyrin subunits including ARS-A, -B, -C (Stefansson and Brautigan, 2006; Stefansson et al., 2008).
PP2A-Like Protein Phosphatases in Regulation of Meiotic Progression
PP2A
Among type 2A protein phosphatases, PP2A is the most famous and is widely researched. For a long time, accumulating evidence revealed its cellular and molecular importance. Studies also suggest that PP2A is involved in multiple steps of meiosis. In mouse oocytes, up-regulation of PP2A activity results in the meiotic arrest phenotype (Su et al., 2012). In Oikopleura dioica, PP2A is also necessary for meiotic arrest and precaution of parthenogenesis by restraining the abnormal Ca2+ burst (Matsuo et al., 2020). These results indicate that the function of PP2A is highly conserved in different organisms.
PP2A is essential for chromosome segregation during meiosis (Kerr et al., 2016). Several in vitro experiments have indicated that PP2A can associate with shugoshins and hold back the phosphorylation of Rec8 at the centromeres, a member of the cohesin complex, and finally stop split of Rec8 and keep the cohesion of chromatids in meiosis I (Kitajima et al., 2006; Lee et al., 2008; Rattani et al., 2013). In addition, Sororin and Shugoshin-PP2A collaborates in the regulation of centromeric cohesion during meiosis (Gomez et al., 2016). In Drosophila meiosis, the Shugoshin MEI-S332 and PP2A reciprocally promote localization of the other to centromeres and together they thus function to ensure accurate segregation (Pinto and Orr-Weaver, 2017). Also, a new study indicated that SCF (Skp1–Cul1–F box) -Fbxo42 down-regulates the protein level of the PP2A-B56 during synaptonemal complex assembly and maintenance (Barbosa et al., 2021). In mice spermatocytes, Previato de Almeida et al. found that Sgo2 is essential to protect centromere pairing by recruiting PP2A, while Sgo1 regulates non-exchange segregation by recruiting PP2A to centromeres (Previato de Almeida et al., 2019). In meiosis II, sister chromatids disjoin upon cleavage of centromeric Rec8. One assumption is that PP2A is separated from Rec8 by bipolar spindle forces in metaphase II. A recent experiment suggested that PP2A is removed from centromeres by the ubiquitin-ligase APC/CCdc20, which can decrease the activity of Sgo1 and kinase Mps1 (Arguello-Miranda et al., 2017; Jonak et al., 2017).
Exact kinetochores-microtubule (KT-MT) is essential for correct chromosome segregation. In mitosis, correct KT-MT attachments are stabilized by inner sister KT stretching and the phosphorylation level of the KT. However, because of inherent property of the MI chromosomes, there is a difference between meiosis I and mitosis. This may explain the high incidence of KT-MT attachment errors in oocytes. In meiosis, PP2A-B56, which is regulated by the BubR1, is essential to determine the stability of KT-MT attachments independently of bivalent stretching (Yoshida et al., 2015). Overall, PP2A is targeted by Shugoshin and BubR1 to protect centromeric cohesion and stabilize KT-MT attachments in yeast and mouse meiosis. In C. elegans meiosis, the function of PP2A remains unclear. A recent study found that PP2A is necessary for female meiotic progression, such as spindle assembly and chromosome segregation. The mechanism is that BUB-1 targets PP2A-B′56 via a conserved LxxIxE motif and this regulation is necessary for correct meiotic progression (Bel Borja et al., 2020).
In addition, treatment with okadaic acid (OA) or calyculin-A (CL-A), which inhibits PP2A, caused an absence of microtubule polymerization and spindles. These studies have also showed that PP2A participated in microtubule organization and spindle formation (Lu et al., 2002). Protein phosphatase 2A regulatory subunit B55α (PP2A-B55α) is encoded by Ppp2r2a. Liang et al. found that PP2A-B55α was an important regulator of oocyte asymmetric division, chromosome congression, DNA damage response and spindle dynamics by RNA interference (Liang et al., 2017). In Xenopus oocytes, protein phosphatase 2A regulatory subunit B′56 (PP2A-B′56) and calcineurin (CaN) jointly contributes to APC/CCdc20 activation by inhibiting phosphorylation of XErp1 (Heim et al., 2018). Two studies suggested that PP2A might be controlled by two distinct mechanisms in mouse oocytes. One is a post-translational modification by which MASTL (microtubule associated serine/threonine kinase-like), inhibit PP2A activity to promote anaphase (Adhikari et al., 2014). The other is CRL4-mediated degradation of the PP2A scaffold subunit, which reduces PP2A activity to facilitate non-reversible meiotic progression (Yu et al., 2015). These two regulation mechanisms of PP2A activity in conjunction with other meiotic regulators ensure precise meiotic progression in oocytes.
By using genetically modified mouse models, we further studied the functions of PP2A in oocyte meiosis We employed the conditional knockout method by using growth differentiation factor 9 (Gdf9)-Cre mice to gain mutant mice with depletion of PPP2R1α in oocytes in order to research its function in female meiosis. The results indicated that oocyte-specific depletion of PPP2R1α resulted in female subfertility because of production of aneuploid oocytes came from wrong separation of sister chromatids, but did not affect folliculogenesis, ovulation and spindle formation during meiosis II (Hu et al., 2014). Interestingly, another report generated conditional knockout mice by crossing Ppp2caf/f and (or) Ppp2cbf/f with Zp3-Cre mice to study PP2A in female meiosis. They found that single knockout PPP2ACα females or PPP2ACβ females were fertile, indicating the paralogs were functionally redundant. Only the deficiency of both PPP2ACα and PPP2ACβ in oocytes finally resulted in female infertility (Tang et al., 2016). In this study, they also found that the PP2A can regulate chromosome behavior and bipolar spindle formation in meiosis I. PP2A counteracts Aurora kinase B/C to ensure bivalent stretching and KT-MT attachment stability (Tang et al., 2016). In contrast, PP2A is also essential for spermatogenesis, especially meiosis (Pan et al., 2015). However, the study is descriptive only, with a lack on mechanistic insight. It will be fascinating to reveal the deeply regulatory mechanism of PP2A in male meiosis.
PP4
PP4 has been widely studied over the past decade. However, there is a relative lack of information about PP4 in meiosis. In C. elegans, PP4 is indispensable for spindle formation during male meiosis, but it is not essential for female meiosis (Sumiyoshi et al., 2002). Moreover, at least four critical events in prophase require PP4, such as synapsis-independent chromosome pairing, prevention of non-homologous chromosome synapsis, DSB initiation, and crossover formation. The failure of these series of events eventually results in the failure of chiasmata formation (Sato-Carlton et al., 2014). In yeast, PP4 seems to be highly active during the whole meiotic progression. PP4 has an important role in single-end invasions, synaptonemal complex assembly, spindle formation and centromere pairing (Falk et al., 2010). To clarify whether PP4 has conserved functions in meiosis in mammalian species, we generated its catalytic subunit gene Ppp4c conditional knockout (Ppp4cf/f) mouse strain using CRISPR/Cas9 technology, and showed that loss of PPP4C did not affect male germ cell meiosis, acrosome formation, nuclear condensation and elongation, but caused the defect of cytoplasm removal, which in turn leads to the failure of spermiogenesis completion and male infertility (Han et al., 2020). Hence, the physiological roles and regulatory mechanism of PP4 in other organisms remain to be further studied.
PP6
Like other type 2A serine/threonine protein phosphatases, PP6 is also ubiquitously expressed in cells. However, PP6 has suffered less notice than its near relative PP2A and PP4, especially in meiosis. Until now, there only three papers about the functions of PP6 in meiosis. We showed that knockout of PP6 in oocytes from primary follicle stage resulted in female subfertility by disturbing MII spindle formation and MII exit after fertilization, indicating that PP6 can act as antagonizer to oocyte aneuploidy. But it is dispensable in oocyte meiotic maturation, follicle growth and ovulation (Hu et al., 2015). However, we showed that knockout of PP6 in oocytes from primordial follicle stage resulted in complete infertility of female mice. Deletion of PP6 caused meiotic prophase oocyte loss and abnormal folliculogenesis because of aberrant phosphorylation level of H2AX, which then led to lots of oocyte disappearance and eventually premature ovarian failure (POF). These results indicated that PP6 can also safeguard oocyte genomic integrity and regulate folliculogenesis during the long prophase I arrest (Hu et al., 2016). In male meiosis, our recent study by crossing Ppp6cf/f mice with Stra8-Cre mice to obtain genetically mutant mice with specific malformation of the Ppp6c in male germ cells. We discovered that the mutant mice were male infertile and male germ cells were blocked at the pachytene stage during meiosis. Further study found that the loss of PP6 in male germ cells affected chromatin relaxation owing to abnormal MAPK pathway activity, thus stopping the recruitment of DSB repair factors to the appropriate sites on chromosomes (Lei et al., 2020).
Perspectives
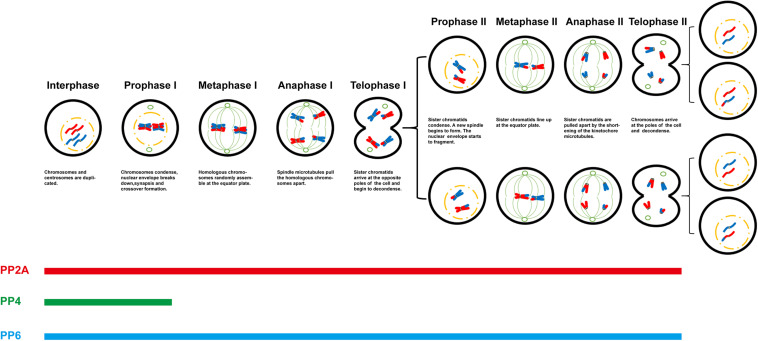
Undoubtedly, protein phosphorylation is one of the most significant post-translational modifications during meiotic progression. The phosphorylation state of a special protein is regulated by protein kinases and protein phosphatases. As a member of PPP family, these new researches on PP2A-like protein phosphatases reported in past decades enriched the list of functions in meiosis, especially by employing conditional knockout mice (Table 1). Nonetheless, the most studies are descriptive only, with a lack of deep mechanistic insight. The special substrates of the different PP2A-like protein phosphatases are still a remaining impediment. In meiosis, it is not hard to notice that all three members can play the same role in special stages or special biological events (Figure 1). Are they functionally redundant? In the future, these unanswered questions remain to be further studied. Quantitative mass spectrometry-based proteomic and phosphoprotoemic approaches maybe provide a solution for understanding regulatory functional mechanism of PP2A-like protein phosphatases in meiotic progress. In addition, the progress of short linear motifs (SLiM) also provides a method to study their biological functions and distinct substrates. These will fill the gaps in the regulation networks of phosphorylation.
TABLE 1.
Consequences of deletions of PP2A-like protein phosphatases in mouse germ cells.
| PP2A-like protein phosphatases | Subunit deleted | Cre recombinase | Phenotype | References |
| PP2A | PPP2R1α | Gdf9 | Subfertile | Hu et al., 2014 |
| PPP2ACα | Zp3 | Fertile | Tang et al., 2016 | |
| PPP2ACβ | Zp3 | Fertile | Tang et al., 2016 | |
| PPP2ACα& PPP2ACβ | Zp3 | Infertile | Tang et al., 2016 | |
| PPP2ACα | DDx4 | Infertile | Pan et al., 2015 | |
| PP4 | PPP4C | Stra8 | Infertile | Han et al., 2020 |
| PP6 | PPP6C | Zp3 | Subfertile | Hu et al., 2015 |
| PPP6C | Gdf9 | Infertile | Hu et al., 2016 | |
| PPP6C | Stra8 | Infertile | Lei et al., 2020 |
FIGURE 1.
Role of PP2A-like protein phosphatases in meiotic progression.
Author Contributions
W-LL collected the data, drew the picture and tables, and wrote the manuscript. W-PQ and Q-YS revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from the National Natural Science Foundation of China (31530049) and the Guangdong Basic and Applied Basic Research Foundation (2020A1515011414).
References
- Adhikari D., Diril M. K., Busayavalasa K., Risal S., Nakagawa S., Lindkvist R., et al. (2014). Mastl is required for timely activation of APC/C in meiosis I and Cdk1 reactivation in meiosis II. J. Cell Biol. 206 843–853. 10.1083/jcb.201406033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar K., Werner M. E., Tse Y. C., Glotzer M., Gönczy P. (2016). Regulation of cortical contractility and spindle positioning by the protein phosphatase 6 PPH-6 in one-cell stage C. elegans embryos. Development 143:2689. 10.1242/dev.141515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello-Miranda O., Zagoriy I., Mengoli V., Rojas J., Jonak K., Oz T., et al. (2017). Casein Kinase 1 Coordinates Cohesin Cleavage. Gametogenesis, and Exit from M Phase in Meiosis II. Dev. Cell 40 37–52. 10.1016/j.devcel.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Barbosa P., Zhaunova L., Debilio S., Steccanella V., Kelly V., Ly T., et al. (2021). SCF-Fbxo42 promotes synaptonemal complex assembly by downregulating PP2A-B56. J. Cell Biol. 220:202009167. 10.1083/jcb.202009167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel Borja L., Soubigou F., Taylor S. J. P., Fraguas Bringas C., Budrewicz J., Lara-Gonzalez P., et al. (2020). BUB-1 targets PP2A:B56 to regulate chromosome congression during meiosis I in C. elegans oocytes. eLife 9:65307. 10.7554/eLife.65307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchowitz L. E., Copenhaver G. P. (2010). Genetic interference: don’t stand so close to me. Curr. Genomics 11 91–102. 10.2174/138920210790886835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinski V. A., Mumby M. C. (2007). Functional analysis of the PP2A subfamily of protein phosphatases in regulating Drosophila S6 kinase. Exp. Cell Res. 313 3117–3126. 10.1016/j.yexcr.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Zickler D. (2004). Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117 9–15. 10.1016/s0092-8674(04)00297-1 [DOI] [PubMed] [Google Scholar]
- Bornslaeger E. A., Mattei P., Schultz R. M. (1986). Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev. Biol. 114 453–462. 10.1016/0012-1606(86)90209-5 [DOI] [PubMed] [Google Scholar]
- Brautigan D. L. (2013). Protein Ser/Thr phosphatases–the ugly ducklings of cell signalling. Febs J. 280 324–345. 10.1111/j.1742-4658.2012.08609.x [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Shenolikar S. (2018). Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Annu Rev Biochem 87 921–964. 10.1146/annurev-biochem-062917-012332 [DOI] [PubMed] [Google Scholar]
- Canela N., Rodriguez-Vilarrupla A., Estanyol J. M., Diaz C., Pujol M. J., Agell N., et al. (2003). The SET protein regulates G2/M transition by modulating cyclin B-cyclin-dependent kinase 1 activity. J. Biol. Chem. 278 1158–1164. 10.1074/jbc.M207497200 [DOI] [PubMed] [Google Scholar]
- Cassimeris L. (1999). Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 11 134–141. 10.1016/s0955-0674(99)80017-9 [DOI] [PubMed] [Google Scholar]
- Chen M. J., Dixon J. E., Manning G. (2017). Genomics and evolution of protein phosphatases. Sci. Signal. 10:aag1796. 10.1126/scisignal.aag1796 [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Philp A., Vazquez-Martin C. (2005). Protein phosphatase 4–from obscurity to vital functions. FEBS Lett. 579 3278–3286. 10.1016/j.febslet.2005.04.070 [DOI] [PubMed] [Google Scholar]
- Falk J. E., Chan A. C., Hoffmann E., Hochwagen A. (2010). A Mec1- and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Dev. Cell 19 599–611. 10.1016/j.devcel.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Gomez R., Felipe-Medina N., Ruiz-Torres M., Berenguer I., Viera A., Perez S., et al. (2016). Sororin loads to the synaptonemal complex central region independently of meiotic cohesin complexes. EMBO Rep. 17 695–707. 10.15252/embr.201541060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Dong M. Z., Lei W. L., Xu Z. L., Gao F., Schatten H., et al. (2020). Oligoasthenoteratospermia and sperm tail bending in PPP4C-deficient mice. Mol. Hum. Reprod 2020:gaaa083. 10.1093/molehr/gaaa083 [DOI] [PubMed] [Google Scholar]
- Heim A., Tischer T., Mayer T. U. (2018). Calcineurin promotes APC/C activation at meiotic exit by acting on both XErp1 and Cdc20. EMBO Rep. 19:201846433. 10.15252/embr.201846433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. W., Meng T. G., Jiang Z. Z., Dong M. Z., Schatten H., Xu X., et al. (2016). Protein Phosphatase 6 Protects Prophase I-Arrested Oocytes by Safeguarding Genomic Integrity. PLoS Genet. 12:e1006513. 10.1371/journal.pgen.1006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. W., Wang Z. B., Jiang Z. Z., Qi S. T., Huang L., Liang Q. X., et al. (2014). Scaffold subunit Aalpha of PP2A is essential for female meiosis and fertility in mice. Biol. Reprod 91:19. 10.1095/biolreprod.114.120220 [DOI] [PubMed] [Google Scholar]
- Hu M. W., Wang Z. B., Teng Y., Jiang Z. Z., Ma X. S., Hou N., et al. (2015). Loss of protein phosphatase 6 in oocytes causes failure of meiosis II exit and impaired female fertility. J. Cell Sci. 128 3769–3780. 10.1242/jcs.173179 [DOI] [PubMed] [Google Scholar]
- Hunter T. (1995). Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80 225–236. 10.1016/0092-8674(95)90405-0 [DOI] [PubMed] [Google Scholar]
- Hunter T. (2014). The genesis of tyrosine phosphorylation. Cold Spring Harb. Perspect. Biol. 6:a020644. 10.1101/cshperspect.a020644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V., Goris J. (2001). Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 353(Pt 3), 417–439. 10.1042/0264-6021:3530417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak K., Zagoriy I., Oz T., Graf P., Rojas J., Mengoli V., et al. (2017). APC/C-Cdc20 mediates deprotection of centromeric cohesin at meiosis II in yeast. Cell Cycle 16 1145–1152. 10.1080/15384101.2017.1320628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating L., Touati S. A., Wassmann K. (2020). A PP2A-B56-Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I. Cells 9:9020390. 10.3390/cells9020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk D., Templeton G., Moorhead G. B. (2008). Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 146 351–367. 10.1104/pp.107.111393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr G. W., Wong J. H., Arumugam P. (2016). PP2A(Cdc55)’s role in reductional chromosome segregation during achiasmate meiosis in budding yeast is independent of its FEAR function. Sci. Rep. 6:30397. 10.1038/srep30397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S., Sakuno T., Ishiguro K., Iemura S., Natsume T., Kawashima S. A., et al. (2006). Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441 46–52. 10.1038/nature04663 [DOI] [PubMed] [Google Scholar]
- Kloeker S., Wadzinski B. E. (1999). Purification and identification of a novel subunit of protein serine/threonine phosphatase 4. J. Biol. Chem. 274 5339–5347. 10.1074/jbc.274.9.5339 [DOI] [PubMed] [Google Scholar]
- Lad C., Williams N. H., Wolfenden R. (2003). The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases. Proc. Natl. Acad. Sci. U S A 100 5607–5610. 10.1073/pnas.0631607100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kitajima T. S., Tanno Y., Yoshida K., Morita T., Miyano T., et al. (2008). Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10 42–52. 10.1038/ncb1667 [DOI] [PubMed] [Google Scholar]
- Lei W. L., Han F., Hu M. W., Liang Q. X., Meng T. G., Zhou Q., et al. (2020). Protein phosphatase 6 is a key factor regulating spermatogenesis. Cell Death Differ 27 1952–1964. 10.1038/s41418-019-0472-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Guo J., Choi J. W., Shin K. T., Wang H. Y., Jo Y. J., et al. (2017). Protein phosphatase 2A regulatory subunit B55alpha functions in mouse oocyte maturation and early embryonic development. Oncotarget 8 26979–26991. 10.18632/oncotarget.15927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C., Kataya A. R., Heidari B., Creighton M. T., Nemie-Feyissa D., Ginbot Z., et al. (2014). Protein phosphatases PP2A, PP4 and PP6: mediators and regulators in development and responses to environmental cues. Plant. Cell Environ. 37 2631–2648. 10.1111/pce.12364 [DOI] [PubMed] [Google Scholar]
- Lu Q., Dunn R. L., Angeles R., Smith G. D. (2002). Regulation of spindle formation by active mitogen-activated protein kinase and protein phosphatase 2A during mouse oocyte meiosis. Biol. Reprod. 66 29–37. 10.1095/biolreprod66.1.29 [DOI] [PubMed] [Google Scholar]
- Lu Q., Smith G. D., Chen D. Y., Yang Z., Han Z. M., Schatten H., et al. (2001). Phosphorylation of mitogen-activated protein kinase is regulated by protein kinase C, cyclic 3′,5′-adenosine monophosphate, and protein phosphatase modulators during meiosis resumption in rat oocytes. Biol. Reprod 64 1444–1450. 10.1095/biolreprod64.5.1444 [DOI] [PubMed] [Google Scholar]
- Matsuo M., Onuma T. A., Omotezako T., Nishida H. (2020). Protein phosphatase 2A is essential to maintain meiotic arrest, and to prevent Ca(2+) burst at spawning and eventual parthenogenesis in the larvacean Oikopleura dioica. Dev. Biol. 460 155–163. 10.1016/j.ydbio.2019.12.005 [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. (1993). Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol. Rev. 73 673–699. 10.1152/physrev.1993.73.4.673 [DOI] [PubMed] [Google Scholar]
- Nasa I., Kettenbach A. N. (2020). Effects of carboxyl-terminal methylation on holoenzyme function of the PP2A subfamily. Biochem. Soc. Transac. 48 2015–2027. 10.1042/bst20200177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J. (2019). Protein phosphatases in the regulation of mitosis. J. Cell Biol. 218 395–409. 10.1083/jcb.201809138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Chen X., Tong X., Tang C., Li J. (2015). Ppp2ca knockout in mice spermatogenesis. Reproduction 149 385–391. 10.1530/REP-14-0231 [DOI] [PubMed] [Google Scholar]
- Pearlman S. M., Serber Z., Ferrell J. E., Jr. (2011). A mechanism for the evolution of phosphorylation sites. Cell 147 934–946. 10.1016/j.cell.2011.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto B. S., Orr-Weaver T. L. (2017). Drosophila protein phosphatases 2A B’ Wdb and Wrd regulate meiotic centromere localization and function of the MEI-S332 Shugoshin. Proc. Natl. Acad. Sci. U S A 114 12988–12993. 10.1073/pnas.1718450114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato de Almeida L., Evatt J. M., Chuong H. H., Kurdzo E. L., Eyster C. A., et al. (2019). Shugoshin protects centromere pairing and promotes segregation of nonexchange partner chromosomes in meiosis. Proc. Natl. Acad. Sci. U S A 116 9417–9422. 10.1073/pnas.1902526116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. E., Mumby M. C. (2000). Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry 39 11312–11318. 10.1021/bi0008478 [DOI] [PubMed] [Google Scholar]
- Qi S. T., Wang Z. B., Ouyang Y. C., Zhang Q. H., Hu M. W., Huang X., et al. (2013). Overexpression of SETbeta, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocytes. J. Cell Sci. 126(Pt 7), 1595–1603. 10.1242/jcs.116541 [DOI] [PubMed] [Google Scholar]
- Rattani A., Wolna M., Ploquin M., Helmhart W., Morrone S., Mayer B., et al. (2013). Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. Elife 2:e01133. 10.7554/eLife.01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Carlton A., Li X., Crawley O., Testori S., Martinez-Perez E., Sugimoto A., et al. (2014). Protein phosphatase 4 promotes chromosome pairing and synapsis, and contributes to maintaining crossover competence with increasing age. PLoS Genet. 10:e1004638. 10.1371/journal.pgen.1004638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin A. T. (2018). Kinase and Phosphatase Cross-Talk at the Kinetochore. Front. Cell Dev. Biol. 6:62. 10.3389/fcell.2018.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson B., Brautigan D. L. (2006). Protein phosphatase 6 subunit with conserved Sit4-associated protein domain targets IkappaBepsilon. J. Biol. Chem. 281 22624–22634. 10.1074/jbc.M601772200 [DOI] [PubMed] [Google Scholar]
- Stefansson B., Ohama T., Daugherty A. E., Brautigan D. L. (2008). Protein phosphatase 6 regulatory subunits composed of ankyrin repeat domains. Biochemistry 47 1442–1451. 10.1021/bi7022877 [DOI] [PubMed] [Google Scholar]
- Su Y. Q., Sugiura K., Sun F., Pendola J. K., Cox G. A., Handel M. A., et al. (2012). MARF1 regulates essential oogenic processes in mice. Science 335 1496–1499. 10.1126/science.1214680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Jayapalan S., Kalpana R., Natarajan J. (2013). HomoKinase: A Curated Database of Human Protein Kinases. ISRN Computational. Biol. 2013:417634. 10.1155/2013/417634 [DOI] [Google Scholar]
- Sumiyoshi E., Sugimoto A., Yamamoto M. (2002). Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J. Cell Sci. 115(Pt 7), 1403–1410. [DOI] [PubMed] [Google Scholar]
- Tang A., Shi P., Song A., Zou D., Zhou Y., Gu P., et al. (2016). PP2A regulates kinetochore-microtubule attachment during meiosis I in oocyte. Cell Cycle 15 1450–1461. 10.1080/15384101.2016.1175256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Roberts S. S. (2010). Regulating mitosis and meiosis in the male germ line: critical functions for cyclins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365 1653–1662. 10.1098/rstb.2009.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kaido M., Kitajima T. S. (2015). Inherent Instability of Correct Kinetochore-Microtubule Attachments during Meiosis I in Oocytes. Dev. Cell 33 589–602. 10.1016/j.devcel.2015.04.020 [DOI] [PubMed] [Google Scholar]
- Yu C., Ji S. Y., Sha Q. Q., Sun Q. Y., Fan H. Y. (2015). CRL4-DCAF1 ubiquitin E3 ligase directs protein phosphatase 2A degradation to control oocyte meiotic maturation. Nat. Commun. 6:8017. 10.1038/ncomms9017 [DOI] [PMC free article] [PubMed] [Google Scholar]