Abstract
Effects of curcumin, a major ingredient of turmeric, were tested on the function of the α7-subunit of the human nicotinic acetylcholine (α7-nACh) receptor expressed in Xenopus oocytes and on nociception in mouse models of tonic and visceral pain. Curcumin caused a significant potentiation of currents induced by acetylcholine (ACh; 100 μM) with an EC50 value of 0.2 µM. The effect of curcumin was not dependent on the activation of G-proteins and protein kinases and did not involve Ca2+-dependent Cl− channels expressed endogenously in oocytes. Importantly, the extent of curcumin potentiation was enhanced significantly by decreasing ACh concentrations. Curcumin did not alter specific binding of [125I]α-bungarotoxin. In addition, curcumin attenuated nociceptive behavior in both tonic and visceral pain models without affecting motor and locomotor activity and without producing tolerance. Pharmacological and genetic approaches revealed that the antinociceptive effect of curcumin was mediated by α7-nACh receptors. Curcumin potentiated the antinociceptive effects of the α7-nACh receptor agonist N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide (PNU282987). Collectively, our results indicate that curcumin is a positive allosteric modulator of α7-nACh receptor and reverses nociception in mouse models of tonic and visceral pain.
Introduction
Turmeric, the rhizome of Curcuma longa L., has been used since ancient times as a spice, coloring, flavoring, and traditional medicine. Curcumin, a polyphenolic compound isolated from turmeric, has been shown to exhibit a wide range of pharmacological activities, including anti-inflammatory, anticancer, antioxidant, antiatherosclerotic, antimicrobial, and wound-healing effects (Kunnumakkara et al., 2017; Milani et al., 2017). Notably, in recent years, curcumin has been demonstrated to have beneficial effects in cognitive deficits and neurodegenerative disorders, such as Alzheimer and Parkinson diseases (Ji and Shen, 2014; Morales et al., 2014; Goozee et al., 2016; Spagnuolo et al., 2016). Although therapeutic effects of curcumin on a wide range of pathologic conditions have been reported, the precise mechanisms of these actions are poorly understood.
These diverse pharmacological activities of curcumin are based on its complex molecular structure and chemical features, as well as its ability to interact with multiple signaling molecules (Zhang et al., 2014). Many biologic molecules have been identified as targets of curcumin, including transcription factors, growth factors, inflammatory cytokines, protein kinases, enzymes, and ion channels (Zhou et al., 2011; Zhang et al., 2014; Kunnumakkara et al., 2017; Milani et al., 2017).
Nicotinic acetylcholine (nACh) receptors are important members of the ligand-gated ion channel family that includes GABAA and 5-HT3 receptors. Nicotinic receptors are divided into two groups: muscle nicotinic receptors, which are found at the skeletal muscle junction where they mediate neuromuscular transmission, and neuronal nicotinic receptors, which are found throughout the peripheral and central nervous systems where they are involved in fast synaptic transmission and in the modulation of transmitter release (Hogg et al., 2003; Albuquerque et al., 2009). The homomeric α7-nACh receptor subtype is abundantly expressed in the central nervous system and periphery (Albuquerque et al., 2009). Importantly, α7-nACh receptors, which have considerably high permeability to Ca2+, have been shown to be located on both glutamatergic and GABAergic nerve terminals, suggesting that both the excitatory and the inhibitory components of synaptic transmission can be modulated by the activity of these receptors (Hogg et al., 2003; Albuquerque et al., 2009). In fact, neuronal α7-nACh receptors are recognized targets for drug development in several preclinical models of cognitive and neurodegenerative disorders (Thomsen et al., 2010; Hone and McIntosh, 2017) and, more recently, in early human studies (Gee et al., 2017). Along with their well documented roles in cognition, activation of α7-nACh receptors produces analgesic effects in laboratory animal and human studies (Umana et al., 2013; Bagdas et al., 2017; Hone and McIntosh, 2017). Interestingly, curcumin also has been shown to attenuate pain and inflammation in various animal studies (Mittal et al., 2009; Liu et al., 2016). These findings may indirectly suggest the involvement of a common target between agonists of α7-nACh receptors and curcumin.
In the present study, we investigated the effects of curcumin on human α7-nACh receptors expressed in Xenopus oocytes and tested its effects on nociception in mouse models of tonic and visceral pain. Specifically, we provide evidence indicating that curcumin acts as a positive allosteric modulator of α7-nACh receptors in in vitro and in vivo conditions.
Materials and Methods
Recordings from Oocytes
Mature female Xenopus laevis frogs were purchased from Xenopus Express (Haute-Loire, France), housed in dechlorinated tap water at 19–21°C with a 12/12-hour light/dark cycle, and fed food pellets supplied by Xenopus Express. The procedures followed in this study were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD) and approved by the Institutional Animal Care and Use Committee at the College of Medicine and Health Sciences, United Arab Emirates University. Clusters of oocytes were removed surgically under benzocaine (Sigma-Aldrich, St. Louis, MO) local anesthesia (0.03% w/v), and individual oocytes were dissected away manually in a solution containing (in millimolar) NaCl, 88; KCl, 1; NaHCO3, 2.4; MgSO4, 0.8; and HEPES, 10 (pH 7.5) as described previously (Brauneis et al., 1996; Oz et al., 2004a). Dissected oocytes were then stored for 2–7 days in modified Barth’s solution containing (in millimolar) NaCl, 88; KCl, 1; NaHCO3, 2.4; CaCl2, 2; MgSO4, 0.8; and HEPES, 10 (pH 7.5), supplemented with 2 mM sodium pyruvate, 10,000 IU/l penicillin, 10 mg/l streptomycin, 50 mg/l gentamicin, and 0.5 mM theophylline. In brief, oocytes were placed in a 0.2-ml recording chamber and superfused at a rate of 3–4 ml/min. Under these conditions, solution exchange time was less than 100 ms. The bathing solution contained (in millimolar) NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 1, and HEPES, 5 (pH 7.5). The cells were impaled with two glass microelectrodes filled with a 3 M KCl (0.5–2 MΩ). The oocytes were routinely voltage clamped at a holding potential of −70 mV using a GeneClamp-500 amplifier (Axon Instruments Inc., Burlingame, CA), and current responses were recorded and stored digitally for further analysis.
Drugs were applied by gravity flow via a micropipette positioned about 2 mm from the oocyte. Some of the compounds were applied externally by addition to the superfusate (flow rate of 3–4 ml/min). Acetylcholine, GDPβS, methyllycaconitine (MLA), N-ethylmaleimide (NEM), α-bungarotoxin, pertussis toxin (PTX), and all chemicals used were obtained from Sigma-Aldrich. Procedures for the injections of 1,2-bis(o-aminophenoxy)ethane-N, N,N′,N′-tetraacetic acid (BAPTA; 50–100 nl, 100 mM) were performed as described previously (Oz et al., 1998). Stock solutions of curcumin used in this study were prepared in dimethylsulfoxide (DMSO) at a concentration of 100 mM. At the highest final concentrations used (0.1% v/v), DMSO did not have a significant effect on currents induced by acetylcholine (ACh; 100 µM) (n = 6).
The cDNA clone of human α7-nACh receptor was kindly provided by Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA). Capped cRNA transcripts were synthesized in vitro using an mMESSAGE mMACHINE kit from Ambion (Austin, TX) and analyzed on 1.2% formaldehyde agarose gel to check the size and quality of the transcripts. Approximately 3–5 ng of cRNA was injected into each oocyte. The cDNAs for human α4-, β2-, α3-, and β4-subunits were kindly provided by Dr. Isabel Bermudez (Oxford Brookes University, Oxford, UK). Subunit combinations (α- and β-subunits) were injected at a 1:1 ratio.
Radioligand Binding Studies
Oocytes were injected with 5 ng of human α7-nACh receptor cRNA, and the functional expression of the receptors was tested by electrophysiology on day 3. Isolation of oocyte membranes was carried out by modification of a method described previously (Oz et al., 2004b; Mahgoub et al., 2013). In brief, oocytes (200–300 oocytes per assay) were suspended (approximately 20 μl/oocyte) in a homogenization buffer containing 10 mM HEPES, 1 mM EDTA, 0.1 mM phenylmethane sulfonyl fluoride (PMSF), 0.02% NaN3, and 50 μg/ml bacitracin (pH 7.4) at 4°C on ice and homogenized using a motorized Teflon homogenizer (six strokes, 15 seconds each at high speed). The homogenate was centrifuged for 10 minutes at 800g. The supernatant was collected, and the pellet was resuspended in homogenization buffer and recentrifuged at 800g for 10 minutes. Supernatants were then combined and centrifuged for 1 hour at 36,000g. The membrane pellet was resuspended in homogenization buffer and used for the binding studies.
Binding assays were performed in 500 μl of 10 mM HEPES (pH 7.4) containing 50 μl of oocyte preparation and 0.1–5 nM [125I] α-bungarotoxin (2200 Ci/mmol; PerkinElmer, Inc., Waltham, MA). Nonspecific binding was determined using 10 μM α-bungarotoxin. Oocyte membranes were incubated with [125I]α-bungarotoxin in the absence and presence of drugs for 1 hour at room temperature (22–24°C). The radioligand was separated by rapid filtration onto GF/C filters presoaked in 0.2% polyethyleneimine. Filters were then washed with two 5-ml washes of ice-cold HEPES buffer, and the radioactivity was determined by counting samples in a Beckman Gamma-300 γ-counter (Beckman Coulter, Inc., Indianapolis, IN).
In Vivo Studies
Male ICR adult (8–10 weeks of age) mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice on C57BL/6J background null for the α7-subunits (B6.129S7-Chrna7tm1Bay/J-; Jackson Laboratory, Bar Harbor, ME) and their wild-type (WT) littermates were bred in an animal care facility at Virginia Commonwealth University. For all experiments, mice were backcrossed for ≥9–10 generations. Mutant and WT mice were obtained by crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–approved animal care facility. They were housed in groups of four and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7 AM). All experiments were performed during the light cycle (between 7 AM and 7 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Animals were sacrificed via CO2 following by cervical dislocation after the experiments were finished, unless noted otherwise.
Drugs
MLA citrate was purchased from RBI (Natick, MA). PNU282987 [N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide] was obtained from the National Institute on Drug Abuse supply program (Bethesda, MD). [9S-(9α,10β,11β,13α)]-N-(2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-1-oxo-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3′,2′,1′-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl)-N-methylbenzamide (PKC-412), 3-[1-[3-(Dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (Go-6983), (9R,10S,12S)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester (KT-5720), and 4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester (KN-62) were purchased from Tocris (Minneapolis, MN). They were dissolved in DMSO to 100 mM stock solutions. Curcumin was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX). In in vivo experiments, curcumin was dissolved in a mixture of 2:2:16 [2 volumes ethanol/2 volumes Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ)/18 volumes distilled water] and administered i.p. for systemic injections. Other drugs were dissolved in physiologic saline (0.9% sodium chloride) and injected s.c. at a total volume of 1 ml/100 g body weight, unless noted otherwise. All doses are expressed as the free base of the drug.
Behavioral Assessments.
Formalin test.
The formalin test was carried out in an open Plexiglas cage (29 × 19 × 13 cm each). Mice were allowed to acclimate for 15 minutes in the test cage prior to injection. Each animal was injected intraplantarly with 20 μl of (2.5%) formalin to the right hindpaw. Mice were observed from 0 to 5 minutes (phase I) and 20–45 minutes (phase II) postformalin injection. The amount of time spent licking the injected paw was recorded with a digital stopwatch. Paw diameter (see Measurement of Paw Edema) was also measured before and 1 hour after formalin injection.
Curcumin (3, 10, and 30 mg/kg) or vehicle were injected in male ICR mice i.p. 45 minutes before formalin injection. For the subchronic curcumin administration study, mice were given curcumin (30 mg/kg, i.p.) or vehicle for 6 days once daily and were challenged with curcumin (30 mg/kg, i.p.) on day 7 and tested in a formalin test 45 minutes after the injection. A vehicle control group, in which mice were exposed to 7 days of vehicle, was also included.
For the antagonist studies, α7 nicotinic antagonist MLA (10 mg/kg) or vehicle (saline) was injected s.c. 10 minutes before the curcumin (30 mg/kg, i.p.) or vehicle administration. In a separate group, curcumin (30 mg/kg, i.p.) effects in the formalin test were measured in α7 WT and knockout (KO) mice. To test possible potentiation of antinociceptive effects of curcumin on PNU282987 in the formalin test, curcumin (3 mg/kg, i.p.) was injected 45 minutes before PNU282987 (0.1 mg/kg, s.c.) administration. The formalin test was conducted 10 minutes after the last injection.
Measurement of paw edema.
The thickness of the formalin-treated paws was measured both before and after injections at the time points indicated earlier using a digital caliper (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ±0.01 mm and expressed as change in paw thickness (ΔPD = difference in the ipsilateral paw diameter before and after injection paw thickness).
Acetic acid–induced writhing test.
For the measurement of acetic acid–induced nociceptive behavior, each mouse was placed in a Plexiglas box and allowed to acclimate for 20 minutes. Then the mouse was given an i.p. injection of acetic acid (1.2%) or saline and then returned to the box. Counting of the number of typical writhing behaviors started immediately after acetic acid administration, and the number of stretches (a stretch was operationally defined as a contraction of the abdomen followed by an extension of the hind limbs) was recorded in 10-minute bins for a total of 40 minutes. Experiments were carried out by injecting the mice with either vehicle or curcumin (30 mg/kg, i.p.), and 45 minutes later, they received acetic acid and were tested as described earlier. For the antagonist studies, α7-nACh receptor (α7-nAChR) antagonist MLA (10 mg/kg) was injected s.c. 15 minutes before the curcumin (30 mg/kg, i.p.). Forty-five minutes after curcumin, mice received acetic acid or vehicle (saline) injection.
Motor coordination.
The effects of drugs on motor coordination were measured using the rotarod test (IITC Inc. Life Science, Woodland Hills, CA) as previously described (Freitas et al., 2013a, b). Mice were pretreated with either i.p. vehicle or curcumin (30 mg/kg, i.p.) 45 minutes before the test. Percentage impairment was calculated as follows: % impairment = (180 − test time)/(180 × 100).
Locomotor activity test.
Mice were placed into individual Omnitech (Columbus, OH) photocell activity cages (28 × 16.5 cm). Interruptions of the photocell beams (two banks of eight cells each) were recorded for the next 30 minutes. Mice were pretreated with either i.p. vehicle or curcumin (30 mg/kg, i.p.) 45 minutes before the test. Data are expressed as the number of photocell interruptions.
Statistical Analysis
In oocyte experiments, average values were calculated as the mean ± S.E.M. Statistical significance was analyzed using Student’s t test or analysis of variance (ANOVA) as indicated. Concentration-response curves were obtained by fitting the data to the logistic equation:
where x and y are concentration and response, respectively; Emax is the maximal response; EC50 is the half-maximal concentration; and n is the slope factor (apparent Hill coefficient).
In behavioral studies, the data obtained were analyzed using GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the one-way or two-way ANOVA test, followed by the post hoc Tukey’s test. Unpaired student t test was used for spontaneous activity and motor coordination. P values <0.05 were considered significant.
Results
Effects of Curcumin on α7-nACh Receptors.
Application of 100 μM ACh for 3–4 seconds activated fast inward currents that desensitized rapidly in oocytes injected with cRNA encoding the α7-subunit of human nACh receptor (Fig. 1A). In addition, ACh-induced currents were inhibited completely with 100 nM α-bungarotoxin (Supplemental Fig. 1, A and B), indicating that these currents are mediated by the activation of α7-nACh receptors. Bath application of curcumin (100 µM) for 5 minutes did not produce detectable currents in oocytes expressing α7-nACh receptors (n = 8 oocytes).
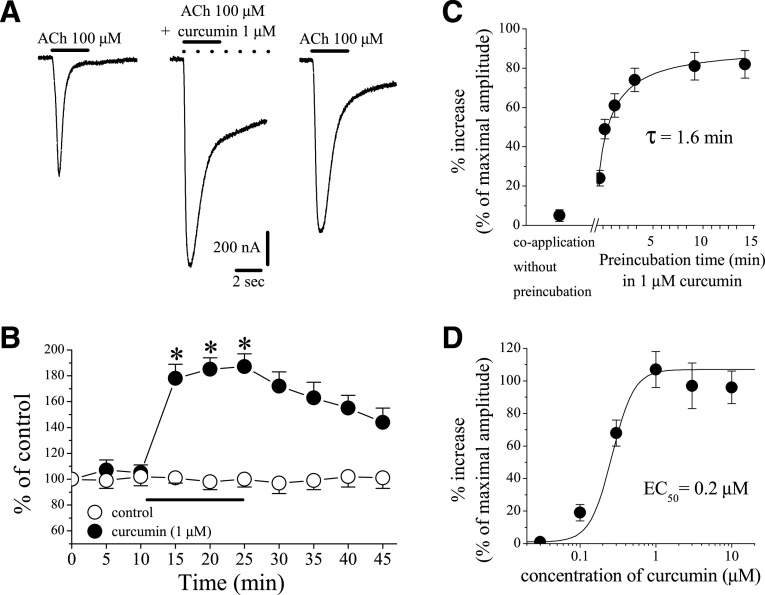
Fig. 1.
Effects of curcumin on α7-nicotinic acetylcholine receptor. (A) Records of currents activated by ACh (100 μM) in control conditions (left), after 10-minute pretreatment with curcumin (1 µM) and coapplication of 1 μM curcumin and ACh (middle), and 15-minute washout (right). (B) Time-course of the effect of vehicle (0.1% DMSO; open circles) and curcumin (1 μM; filled circles) on the maximal amplitudes of the ACh-induced currents. Each data point represents the normalized mean ± S.E.M. of six to eight experiments. The horizontal bar indicates the duration of curcumin or vehicle application. (C) Effect of curcumin as a function of curcumin preapplication time. Each data point represents the mean ± S.E.M. of six to seven oocytes. (D) Curcumin potentiates α7-nACh receptor function in a concentration-dependent manner. Each data point represents the mean ± S.E.M. of six to nine oocytes. The curve is the best fit of the data to the logistic equation described in the Materials and Methods section.
The effect of curcumin was tested on ion currents induced with ACh (100 µM). An effect of 10-minute curcumin (1 μM) application on α7-nACh receptor–mediated currents is shown in Fig. 1A. Time courses of effects of curcumin or vehicle (0.1% DMSO) applications on the maximal amplitudes of ACh-induced currents are presented in Fig. 1B. Curcumin caused a significant potentiation of the current, which was partially reversed during a 10–15-minute washout. In the absence of curcumin, vehicle (0.1% DMSO) alone did not alter the amplitude of the ACh-induced current, further suggesting that curcumin acts on nACh receptors (Fig. 1B, controls vs. curcumin treatment group at 10 minutes of exposure, ANOVA, n = 5–7; P < 0.05).
The potentiating effect of curcumin was significantly dependent on the application mode. For example, without preincubation, coapplication of curcumin (1 μM) and ACh (100 μM) did not alter the amplitudes of maximal currents (Fig. 1C). However, when oocytes were preincubated with curcumin, the drug was found to potentiate maximal ACh-induced currents in a time-dependent manner, reaching a maximal level within 5 minutes with a half-time (τ1/2) of 1.6 minutes (Fig. 1C). Since the magnitude of the curcumin effect was time-dependent, 10-minute curcumin application time was used routinely to ensure equilibrium conditions. Curcumin was found to upregulate the function of α7-nACh receptor in a concentration-dependent manner with EC50 and slope values of 0.21 ± 0.14 µM and 1.6, respectively (Fig. 1D).
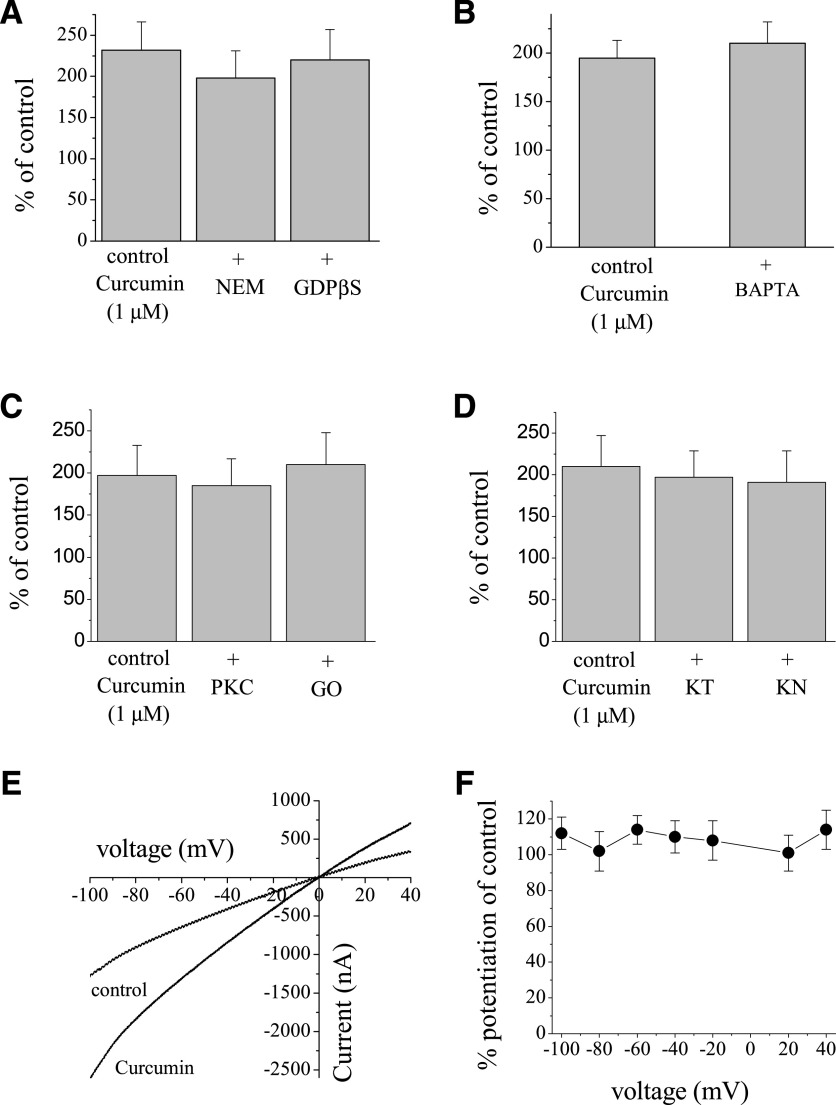
As shown in Fig. 1, B and C, regulation of α7-nACh receptor function by curcumin occurs gradually, reaching steady-state levels within a few minutes of curcumin application. Therefore, it is possible that activation of second messenger pathways by G-protein-coupled receptors (Pérez-Lara et al., 2011; Liu et al., 2013; Yang et al., 2015) is involved in curcumin regulation of α7-nACh receptors. Thus, we investigated the effects of pretreatments with NEM (10 mM, 50 nl, 30-minute preincubation time), a sulfhydryl-alkylating agent that blocks G-protein-effector interactions by alkylating α-subunits of PTX-sensitive GTP binding protein (Oz and Renaud, 2002), and GDPβS (10 mM, 50 nl, 30-minute preincubation time), an agent that inhibits binding of GTP to the α-subunit of G-proteins (Oz et al., 1998). Treatments with NEM and GDPβS did not alter the extent of curcumin potentiation of α7-nACh receptor (Fig. 2A).
Fig. 2.
Effects of curcumin on α7-nACh receptor are not mediated by G-proteins and protein kinases, and are not dependent on intracellular Ca2+ levels and changes in membrane potential. (A) Bar presentation of the effects of 1 µM curcumin application (10 minutes) on the maximal amplitudes of ACh (100 µM)-induced currents in oocytes injected with 50 nl of distilled water, controls (n = 16), or NEM (10 mM, 50 nl, n = 8) and GDPβS (10 mM, 50 nl, n = 7) 30 minutes before recordings. (B) Bar presentation of the effects of 1 µM curcumin application (10 minutes) on the maximal amplitudes of ACh (100 µM)-induced currents in oocytes injected with 50 nl distilled water, controls (n = 5), or BAPTA (200 mM, 50 nl, n = 7). (C) Bar presentation of the effects of 1 µM curcumin on α7-nACh receptor–mediated currents in oocytes pretreated with vehicle (0.01% DMSO, n = 5) or PKC-412 (PKC; 10 µM, 30-minute pretreatment, n = 7), or Go-6983 (GO; 10 µM, 30-minute pretreatment, n = 6). (D) Bar presentation of the effects of 1 µM curcumin on α7-nACh receptor–mediated currents in oocytes pretreated with vehicle (0.01% DMSO, n = 7) or KT-5720 (KT; 10 µM, 30-minute pretreatment, n = 7) and KN-62 (KN; 50 µM, 30-minute pretreatment, n = 6). (E) Current-voltage relationships of acetylcholine-activated currents in the absence and presence of curcumin. Normalized currents activated by 30 μM ACh before (control, filled circles) and after 10-minute treatment with 1 μM curcumin (open circles). Each data point presents the normalized means and S.E.M. of seven experiments. (F) Quantitative presentation of the effect of curcumin as percentage of controls at different voltages.
Similarly, pretreatment with PTX (5 µg/ml, 50 nl, 30-minute preincubation time), a toxin that inhibits the α-subunit of Gi/o proteins, did not reverse the potentiating effect of curcumin (Supplemental Fig. 2). In addition, extent of curcumin-induced potentiation of currents activated by a low (30 µM) concentration of ACh was also not altered in the presence of NEM and GDPβS curcumin (Supplemental Fig. 2)
Activation of α7-nACh receptors allows sufficient Ca2+ entry to activate endogenous Ca2+-dependent Cl− channels in Xenopus oocytes (Sands et al., 1993; Uteshev, 2012). Therefore, it was important to determine whether the effect of curcumin was exerted on nACh receptor–mediated currents or on Cl− currents induced by Ca2+ entry. For this reason, we injected the Ca2+ chelator BAPTA into oocytes and replaced extracellular Ca2+ with Ba2+ since Ba2+ can pass through α7-nACh receptors but causes less activation of Ca2+-dependent Cl− channels (Sands et al., 1993). Under these conditions, we tested the effect of curcumin in a solution containing 2 mM Ba2+ in BAPTA-injected oocytes. Curcumin (1 µM) produced the same level of potentiation (195 ± 18 in controls vs. 210 ± 22 in BAPTA-injected oocytes; ANOVA, P > 0.05; n = 6 to 7) on ACh-induced currents in BAPTA-injected oocytes when currents were recorded in Ca2+-free solution containing 2 mM Ba2+ (Fig. 2B). It is important to mention that in the oocyte expression system, curcumin-induced changes in nicotinic receptor–mediated currents can be attributable to Ca2+-activated Cl− channels and concomitant alterations in the holding currents. However, in control experiments, curcumin (100 µM for 10 minutes) did not change the magnitudes of holding currents in oocytes voltage clamped at −70 mV (n = 7), indicating that intracellular Ca2+ levels were not altered by curcumin.
We also investigated the involvement protein kinases A and C, and Ca2+-calmodulin–dependent kinase (CaM-kinase) in curcumin potentiation of α7-nACh receptors. For this purpose, the effects of curcumin were tested in oocytes pretreated with PKC-412 (nonspecific kinase inhibitor, 10 µM for 30 minutes pretreatment), Go-6983 (specific protein kinase C inhibitor, 10 µM for 30 minutes), KT-5720 (specific protein kinase A inhibitor, 10 µM for 30 minutes), and KN-62 (specific inhibitor of CaM-kinase II, 50 µM for 30 minutes). Curcumin continued to upregulate nicotinic receptor–mediated currents in oocytes pretreated with kinase inhibitors (Fig. 2, C and D). Similarly, potentiating effects of curcumin remained unaltered by inhibitors of protein kinases A and C and CaM-kinase at low (30 µM) ACh concentrations (Supplemental Fig. 2).
In the next series of experiments, we examined if the extent of curcumin potentiation of the α7-nACh receptor–mediated current is altered by membrane potential. As indicated in Fig. 2E, the potentiation of ACh (30 µM)-induced currents by curcumin (1 µM) does not appear to be voltage-dependent. The extent of curcumin potentiation was similar at all tested membrane potentials from −100 to +40 mV. Evaluation of the current-voltage relationship (Fig. 2F) indicates that the extent of potentiation by curcumin does not change significantly at different test potentials (P > 0.05, n = 7, ANOVA).
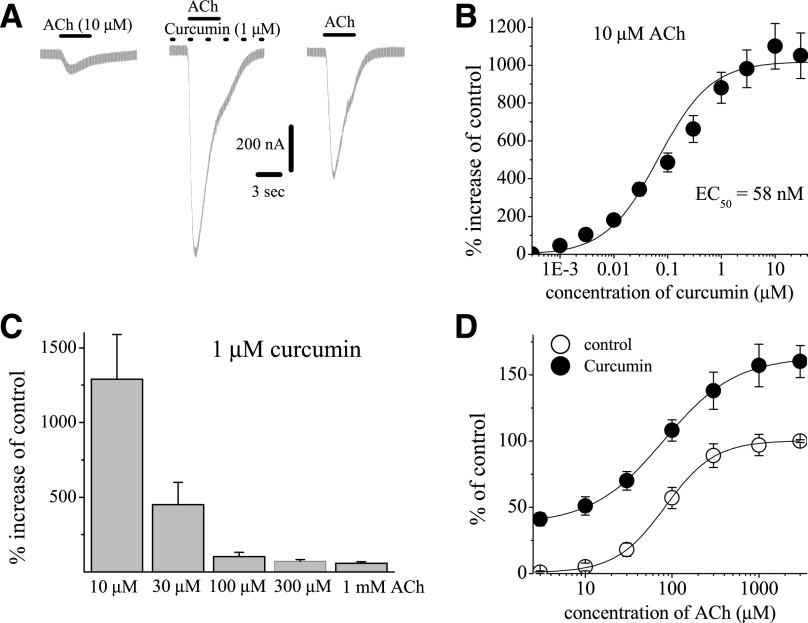
In the next series of experiments, we attempted to test the effects of curcumin at different ACh concentrations. Traces of low ACh (10 µM)-induced currents after 10-minute treatment with 1 µM curcumin are presented in Fig. 3A. At low ACh (10 µM) concentrations, curcumin caused an approximately 11- to 12-fold increase of ACh-induced currents with an EC50 of 58 nM (Fig. 3B). Further experiments indicated that the extent of curcumin potentiation decreased significantly with increasing concentrations of ACh (Fig. 3C). Concentration-response curves for ACh in the absence and presence of 1 µM curcumin are presented in Fig. 3D. In the presence of 1 µM curcumin, the maximal ACh response increased by 60%–70% of controls (n = 6–8). In the absence and presence of curcumin, the EC50 values for ACh were 107 ± 18 and 63 ± 16 μM, and slope values were 2.2 ± 0.4 and 1.9 ± 0.3, respectively (n = 6–7).
Fig. 3.
Effects of curcumin at different concentrations of acetylcholine. (A) Records of currents activated by ACh (10 μM) in control conditions (left), after 10-minute pretreatment with curcumin (1 µM) and coapplication of 1 μM curcumin and ACh (middle), and 10-minute washout (right). (B) Concentration-dependent effect of curcumin on α7-nACh receptors activated by low acetylcholine concentration. Each data point represents the mean ± S.E.M. of six to eight oocytes. The curve is the best fit of the data to the logistic equation described in the Materials and Methods section. (C) Bar presentation of the effect of curcumin at different acetylcholine concentrations. Bars represent the means ± S.E.M. of five to eight experiments. (D) Effect of curcumin on the ACh concentration-response relationship. Oocytes were voltage clamped at −70 mV, and currents were activated by applying ACh (1 μM to 3 mM). Oocytes were exposed to 10 µM curcumin for 10 minutes, and ACh was reapplied. Paired concentration-response curves were constructed and responses normalized to maximal response under control conditions. Data points obtained before (control) and after 10-minute treatment with curcumin (10 μM) are indicated by filled and open circles, respectively. Each data point presents the normalized means and S.E.M. of 7–11 experiments.
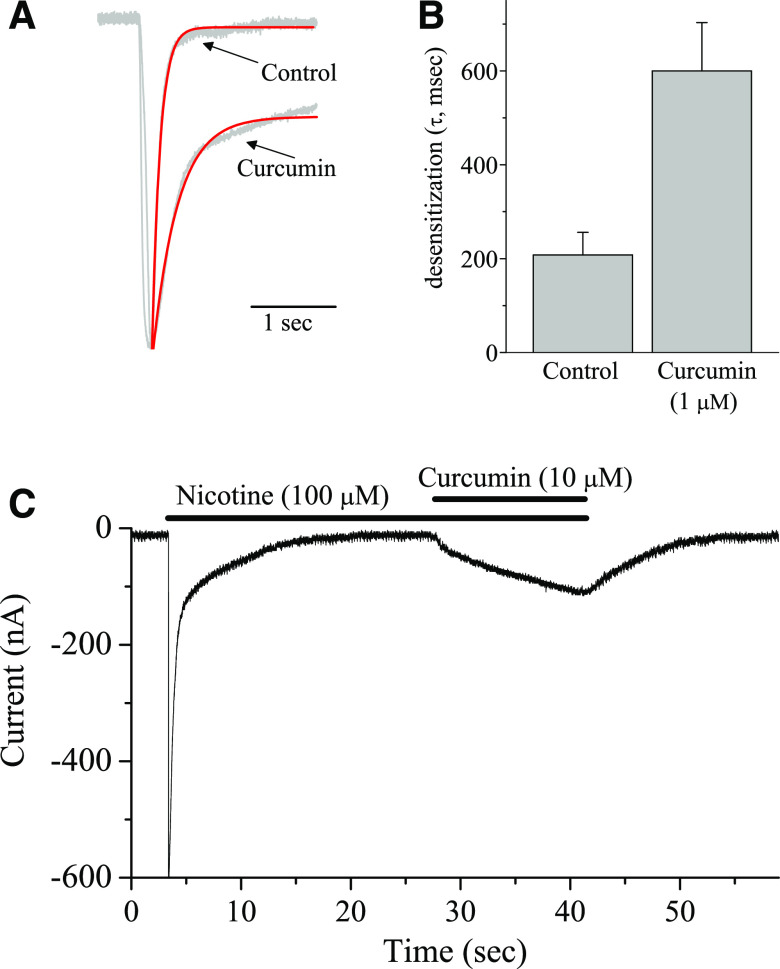
The results of functional studies indicate that curcumin significantly decreases desensitization of currents mediated by the activation of α7-nACh receptors. Normalized and superimposed current traces in the absence and presence of 1 μM curcumin are presented in Fig. 4A. A summary of the results showing the effect of curcumin (1 μM) on the half decay times of the α7-nACh receptor–mediated currents is shown in Fig. 4B. In the absence and presence of curcumin, means of half decay times were 208 ± 42 and 643 ± 85 seconds, respectively (paired t test; n = 8; P ˂ 0.01). These findings suggest that curcumin caused a significant decrease of α7-nACh receptor desensitization. We further investigated whether curcumin can convert α7-nACh receptors that were already desensitized by a concentration of an agonist back to conducting state. We tested the effect of a bath application of curcumin (10 µM) on the α7-nACh receptors that was desensitized by 100 µM nicotine application for 25–30 seconds (n = 6). As illustrated in Fig. 4C, subsequent addition of curcumin resulted in activation of a sustained inward current that was reversed during washout.
Fig. 4.
Effect of curcumin on the desensitization of nicotinic receptors. (A) Normalized current traces in control (100 µM ACh) and in the presence of 1 µM curcumin. (B) Bar presentation of the effect of curcumin (1 µM) on mean desensitization half-times of nicotinic receptors activated by 100 µM ACh. Bars represent the means ± S.E.M. of eight experiments. (C) Effect of curcumin (10 µM) on α7-nicotinic receptors desensitized by bath application of 100 µM nicotine (n = 6).
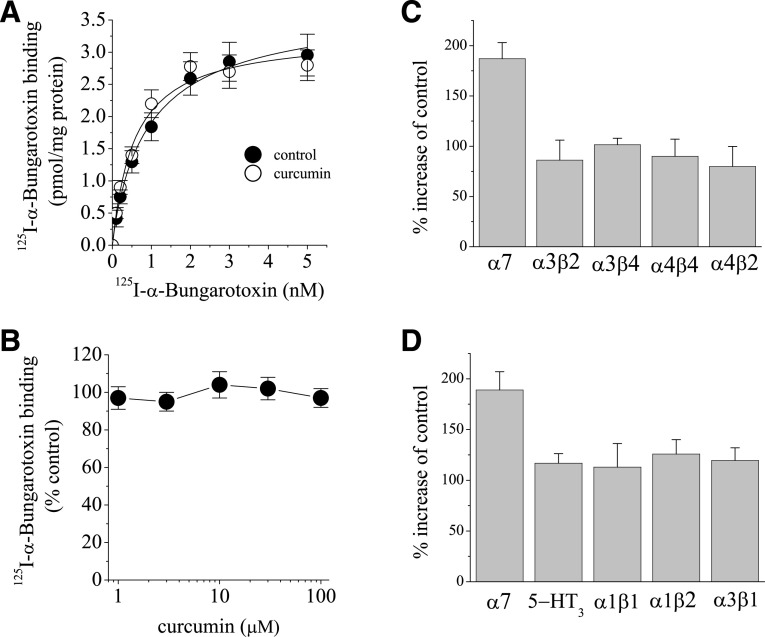
[125I]α-bungarotoxin competes with ACh, an endogenous activator of α7-nACh receptors, by binding to the ACh binding site on the receptor (Albuquerque et al., 2009). For this reason, the effect of curcumin was investigated on the specific binding of [125I]α-bungarotoxin. Equilibrium curves for the binding of [125I]α-bungarotoxin in the presence and absence (controls) of curcumin are presented in Fig. 5A. Maximum binding activities (Bmax) of [125I]α-bungarotoxin were 3.61 ± 0.37 and 3.47 ± 0.41 pM/mg (means ± S.E.M.) for controls and curcumin-treated preparations, respectively. The apparent affinities (KD) of the receptor for [125I]α-bungarotoxin were 0.87 ± 0.26 and 0.67 ± 0.23 pM for controls and curcumin, respectively. There was no statistically significant difference between controls and curcumin-treated groups with respect to KD and Bmax values (P > 0.05, ANOVA, n = 6–7), suggesting that curcumin does not compete with α-bungarotoxin at the same binding site. Curcumin up to a concentration of 100 μM did not cause a significant change on the specific binding of [125I]α-bungarotoxin (Fig. 5B). The effect of curcumin on the functional properties of other neuronal nACh receptor subtypes was also examined. Application of curcumin (10 µM for 10 minutes) did not cause alterations of ACh (100 µM)-induced currents mediated by different subtype combinations of nicotinic receptors expressed in oocytes (Fig. 5C). Similarly, curcumin (10 µM for 10 minutes) did not cause significant changes on the amplitudes of currents mediated by 5-HT3A (1 µM 5-HT) subunit and glycine receptors (30 µM glycine; mediated by α1β1, α2β1, and α3β1 subunit combinations) (Fig. 5D).
Fig. 5.
Effects of curcumin on the specific binding of [125I]α-bungarotoxin and on the currents mediated by different nicotinic receptor subunits. (A) The effects of curcumin on the specific binding of [125I]α-bungarotoxin to oocyte membrane preparations. In the presence and absence of curcumin, specific binding as a function of the concentration of [125I]α-bungarotoxin is presented. Data points for controls and curcumin (10 μM) are indicated by filled and open circles, respectively. Data points are the means of four independent experiments carried out in triplicate. (B) The effects of increasing concentrations of curcumin on the specific binding of [125I]α-bungarotoxin. Each data point represents the normalized means and S.E.M. of five to seven experiments. (C) Comparison of the effect of 10 µM curcumin on ACh (100 µM)-induced currents mediated by α7-, α4β2-, α3β4-, α3β2-, and α4β4-subunit combinations of nicotinic receptors expressed in oocytes. Bars represent the mean inhibition ± S.E.M. from six to eight experiments. (D) Comparison of the effects of 10 µM curcumin on 5-HT3 receptors and α1β1, α1β2, and α3β1 glycine receptor subunits expressed in oocytes. Bars represent the mean effect ± S.E.M. from five to seven experiments.
Effects of Curcumin Treatment in Formalin-Induced Pain Responses.
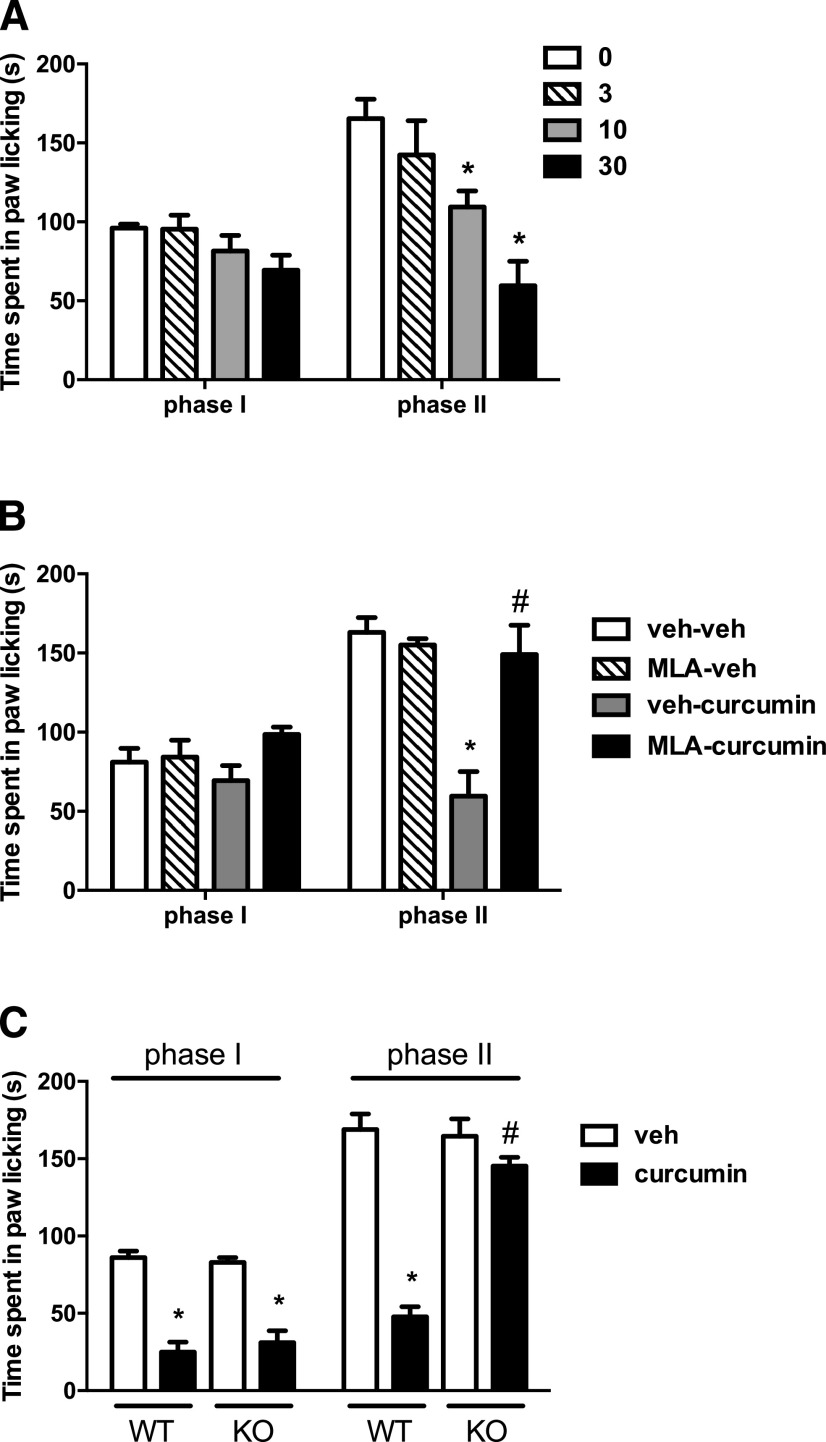
To determine the effect of acute curcumin treatment in the formalin-induced pain model, mice were given an i.p. injection of curcumin (3, 10, and 30 mg/kg) or vehicle 45 minutes before intraplantar formalin injection. Ordinary two-way ANOVA revealed significant effects for phase of formalin test [Fphase(1,40) = 14.54, P < 0.001] and dose of curcumin [Fdose(3,40) = 11.04, P < 0.001]. As seen in Fig. 6A, curcumin failed to show a significant antinociceptive effect in phase I of the test (Tukey post hoc, P > 0.05). However, it dose-dependently attenuated nociceptive behavior in phase II (Tukey post hoc, P < 0.001).
Fig. 6.
The antinociceptive effects of acute curcumin in the formalin test. (A) The effect after i.p. administration of curcumin (3, 10, and 30 mg/kg) on formalin-induced pain behavior in ICR mice. Mice were treated with i.p. curcumin 45 minutes prior to formalin (2.5%, 20 μl) injection into the plantar region of the right hind paw. The cumulative pain response of time of licking was measured during the period of 0–5 (first phase) and 20–45 minutes (second phase). (B) Blockade of the antinociceptive effect of curcumin in the second phase of the formalin test by the α7 antagonist MLA citrate. MLA (10 mg/kg, s.c.) was given 10 minutes before curcumin (30 mg/kg, i.p.) or vehicle (veh) in ICR mice. After 45 minutes, formalin test was performed. (C) Antinociceptive effects of curcumin (30 mg/kg, i.p.) in the formalin test in the α7 WT and KO mice on C57BL/67 background. Data are given as the mean ± S.E.M. of six animals for each group. *P < 0.05, significantly different from its vehicle group; #P < 0.05, significantly different from its corresponding control group.
Role of α7-nACh Receptors in the Effects of Curcumin.
We explored the possible role of α7-nAChRs in the effect of curcumin in the formalin test. We first investigated if the α7-nAChR antagonist MLA would block curcumin’s effects in phase II of the test. Ordinary two-way ANOVA revealed significant effects for phase of formalin test [Fphase(1,40) = 37.61, P < 0.001] and treatment [Fdose(3,40) = 13.29, P < 0.001; Fig. 6B]. A post hoc Tukey test showed that while the α7-nAChR antagonist MLA (10 mg/kg, s.c.) given alone did not alter formalin-induced pain responses (P < 0.05), it completely blocked the antinociceptive effect of curcumin (P < 0.001). We then tested curcumin in the α7 WT and KO mice. Interestingly, curcumin significantly reduced the phase I nociceptive responses in α7 WT mice. Surprisingly, the antinociceptive effect of curcumin was also preserved in transgenic KO mice. On the other hand, whereas curcumin reduced formalin-induced paw licking in WT mice, the effect vanished in α7 KO mice in the phase II of the test [Fphase(1,44) = 215.8, P < 0.001] and genotype [Fgenotype(3,44) = 75.87, P < 0.001; Fig. 6C].
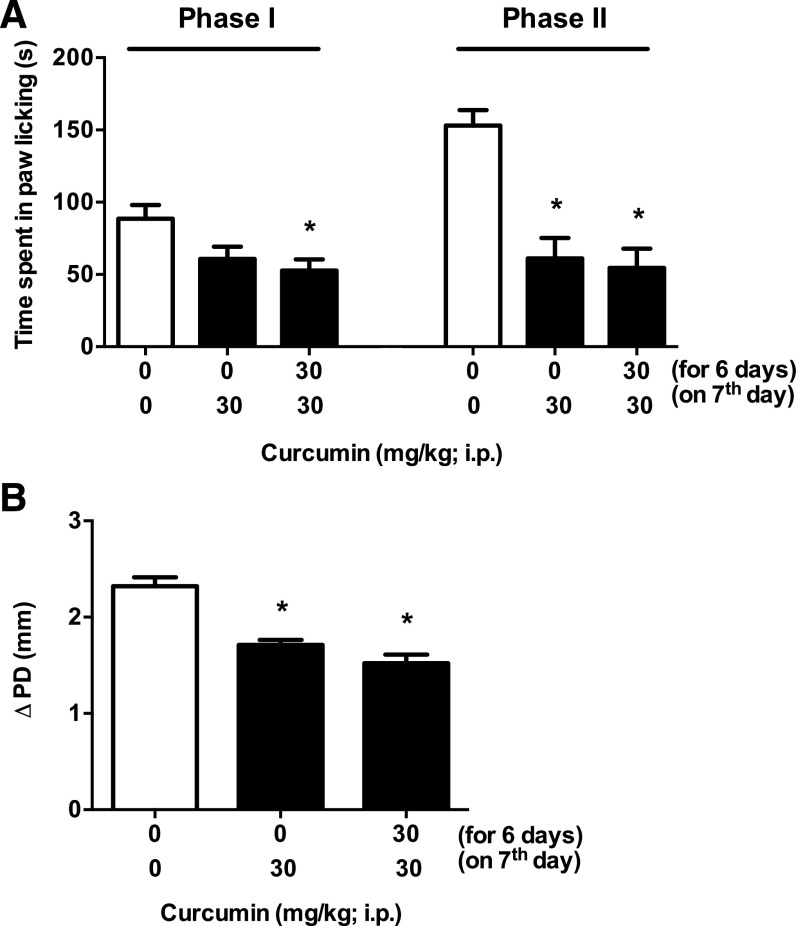
Subchronic Curcumin Treatment Attenuates Formalin-Induced Pain Responses, without Producing Tolerance.
In the next experiment, we investigated whether the antinociceptive effects of curcumin in the formalin test would undergo tolerance after subchronic administration. Curcumin produced significant effects in phase I [one-way ANOVA, Tukey post hoc, F(2,15) = 4.896, P < 0.05] and phase 2 [F(2,15) = 18.24, P < 0.001; Fig. 7A]. Subchronic curcumin attenuated pain responses at both phase I and phase II without producing tolerance. Moreover, curcumin also reduced the formalin-induced paw edema without producing tolerance [one-way ANOVA, Tukey post hoc, F(2,15) = 26.74, P < 0.001; Fig. 7B].
Fig. 7.
The antinociceptive effects of subchronic curcumin in the formalin test. (A) The effect of subchronic curcumin administration on formalin-induced pain behavior was measured. Mice were treated with curcumin (30 mg/kg, i.p.) or vehicle for 6 days once daily and were challenged with curcumin (30 mg/kg, i.p.) on day 7 and tested in a formalin (2.5%) test. A vehicle control group, in which mice were exposed to 7 days of vehicle, was also included. (B) The antiedema effect of formalin injection of curcumin, measured by the difference in the ipsilateral paw diameter before and after formalin injection (ΔPD), was assessed 1 hour after formalin injection. Data are given as the mean ± S.E.M. of six animals for each group. *P < 0.05, significantly different from its vehicle group.
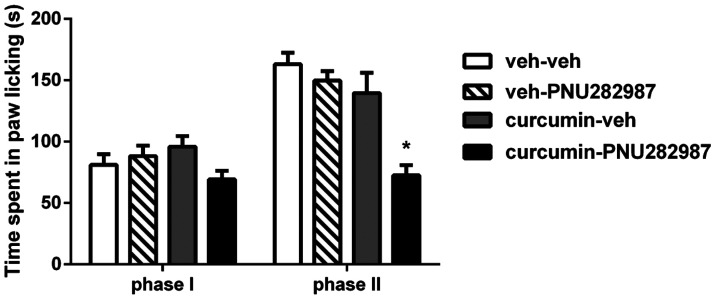
Potentiation of the Antinociceptive Effects of the α7-Nicotinic Agonist PNU282987 by Curcumin.
We determined the effects of curcumin on the PNU282987-evoked antinociceptive effects in the formalin test. Data were analyzed using two-way ordinary ANOVA followed by Tukey post hoc test. Analysis revealed significant effects for phase of formalin test [Fphase(1,40) = 47.55, P < 0.001] and treatment [Fdose(3,40) = 12.51, P < 0.001; Fig. 8]. Pretreatment with a low dose of curcumin (3 mg/kg, i.p.) or PNU282987 (0.1 mg/kg, s.c.) failed to attenuate formalin-induced pain responses when tested alone (P > 0.05). However, combination of curcumin and PNU282987 significantly reversed pain behavior in phase II (P < 0.001) but not phase I of the test (P > 0.05).
Fig. 8.
Antinociceptive effects of curcumin plus PNU282987 combination in the formalin test. Curcumin (3 mg/kg, i.p.) was injected 45 minutes before PNU282987 (0.1 mg/kg, s.c.) injection. The formalin test was conducted 10 minutes after PNU282987 administration in ICR mice. Data are given as the mean ± S.E.M. of six animals *P < 0.05, significantly different from vehicle (veh) group.
Effects of Curcumin Treatment in Acetic Acid–Induced Stretching.
Acetic acid significantly evoked stretching behavior as a nociceptive behavior outcome [Facetic acid(1,56) = 813.1, P < 0.0001; Fig. 9]. Curcumin (30 mg/kg) significantly reduced acetic acid–induced nociceptive stretching behaviors, and MLA blocked this antinociceptive effect of curcumin [F(3,56) = 20.79, P < 0.0001; Fig. 9].
Fig. 9.
Effects of curcumin on acetic acid-induced writhing. ICR mice were treated with i.p. vehicle or curcumin (10 or 30 mg/kg) 45 min prior to i.p. acetic acid (1.2%) injection. Animals were observed for 40 minutes for the number of typical stretching behaviors. To test blockade of the antinociceptive effect of curcumin in the writhing test, the α7 antagonist methyllycaconitine citrate (MLA, 10 mg/kg, s.c.) was given 10 min before curcumin (30 mg/kg, i.p.) or vehicle. After 45 min, acetic acid writhing test was performed. Data are given as the mean ± S.E.M. of 8 animals for each group.
Effects of Curcumin on Motor Activity and Coordination.
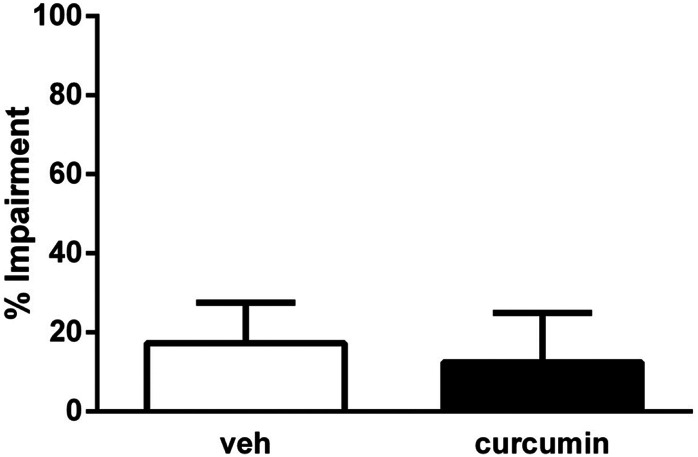
As seen in Table 1, curcumin at a dose of 30 mg/kg (i.p.), the highest active dose used in our study, did not affect 1) motor performance or 2) spontaneous activity of mice after acute injection (t test, t = 0.2988, df = 8, P > 0.05 and t = 0.5841, df = 10, P > 0.05, respectively).
TABLE 1 .
Effects of curcumin on motor activity and coordination of mice
ICR mice were placed into photocell activity cages for 30 minutes or placed on the rotarod for 3 minutes after 45-minute i.p. administration of curcumin (30 mg/kg). Data are presented as the mean ± S.E.M. as the number of photocell interruptions and time to fall in percentage impairment for each group, respectively (5–6).
| Treatment | Spontaneous Activity |
Rotarod Activity |
|---|---|---|
| # Interrupts/30 min | % Impairment | |
| Vehicle | 1018 ± 146.7 | 17.3 ± 10.3 |
| Curcumin | 1125 ± 111.5 | 12.5 ± 12.5 |
Discussion
In the present study, using electrophysiological, biochemical, and behavioral methods, we provide evidence that curcumin upregulates the function of human α7-nACh receptors expressed in Xenopus oocytes and reverses nociception in mouse models of tonic and visceral pain through an α7-nACh receptor mechanism. The enhancement of α7-nACh receptor function by curcumin is reversible and occurs in a time- and concentration-dependent manner, but is independent of G-protein activation, protein kinase activity, intracellular Ca2+ levels, and membrane potential.
A relatively slow time course of curcumin effect and the results of earlier studies on curcumin modulation of various second messenger pathways and kinases (Mahmmoud, 2007; Takikawa et al., 2013) suggest that activation of G-protein-coupled receptors and/or kinase-mediated phosphorylation is involved in curcumin-induced upregulation of α7-nACh receptors. However, neither treatments with established kinase inhibitors nor pharmacological disruption of G-protein activity reversed curcumin potentiation of α7-nACh receptors, suggesting that curcumin acts directly on ion channel-receptor complex.
In Xenopus oocytes, activation of α7-nACh receptors, due to their high Ca2+ permeability, allows sufficient Ca2+ entry to activate endogenous Ca2+-dependent Cl− channels (Sands et al., 1993; Hartzell et al., 2005). Therefore, the direct actions of curcumin on Ca2+-dependent Cl− channels may contribute to the observed effects of curcumin on ACh-activated currents in this expression system. In oocytes injected with BAPTA and recorded in a solution containing 2 mM Ba2+, curcumin continued to potentiate α7-nACh receptor–mediated ion currents, suggesting that Ca2+-dependent Cl− channels were not involved in curcumin potentiation of nicotinic responses. In addition, the reversal potential in solutions containing Ba2+ was not altered in the presence of curcumin, suggesting that the potentiation by curcumin is not due to alterations in the Ca2+ permeability of the α7-nACh receptor-channel complex.
Curcumin has been reported to alter Ca2+ homeostasis in various cell types (Dyer et al., 2002; Ibrahim et al., 2011; Wang et al., 2012; Moustapha et al., 2015). However, in Xenopus oocytes, Ca2+-activated Cl− channels are highly sensitive to intracellular Ca2+ levels [KD of Ca2+-activated Cl− channels for Ca2+ is less than 1 µM; for a review, Hartzell et al., (2005)], and alterations in intracellular Ca2+ levels would be reflected by changes in the holding current under voltage-clamp conditions. In our experiments, application of curcumin (1–100 μM) did not cause alterations in baseline or holding currents, and curcumin continued to potentiate nicotinic receptors after the chelation of intracellular Ca2+ by BAPTA, suggesting that alterations in intracellular Ca2+ concentrations does not play a role in curcumin’s effect on nicotinic receptors.
Previous studies have demonstrated that curcumin acts on several integral membrane proteins, including enzymes, transporters, and ion channels (Zhang et al., 2014; Li et al., 2017), T-type Ca2+ channels in bovine adrenal cells (Enyeart et al., 2009; IC50 = 10–20 µM), L-type Ca2+ channels in hippocampal neurons (Liu et al., 2013; IC50 ≈ 10 µM), TREK-1 K+ channels (Enyeart et al., 2008; IC50 = 0.9 µM), Kv1.4 K+ channels (Liu et al., 2006) in bovine adrenal cells, Kv1.4. K+ channels (Lian et al., 2013; IC50 = 4.2 µM) in human T-lymphocytes, ERG K+ channels (Hu et al., 2012, IC50 = 5.5 µM; Choi et al., 2013, IC50 = 10.6 µM; Banderali et al., 2011, IC50 = 2 µM), and K+ channels in rabbit coronary arterial smooth muscle cells (Hong et al., 2013; IC50 = 1.1 µM). In addition to voltage-dependent conductances, curcumin has also been shown to act on transient-receptor potential receptors (Yeon et al., 2010; Zhi et al., 2013).
In this study, curcumin was applied in the concentration range of 1 nM to 100 µM, and it was found that it can enhance the effects of ACh on the function of α7-nACh receptors in a concentration-dependent manner, with EC50 values ranging from 58 nM to several micromolars. The concentration of curcumin in plasma and its ability to pass the blood-brain barrier following oral and intravenous administration have been studied previously (Anand et al., 2007). When curcumin was given orally at a dose of 2 g/kg to rats, a maximum serum concentration of 1.35 µg/ml (3.5 µM) was attained (Shoba et al., 1998). Since curcumin is a highly lipophilic compound with a logP (octanol–water partition coefficient) value of 3.3 (https://pubchem.ncbi.nlm.nih.gov/compound/curcumin#section=Top), its membrane concentration is expected to be considerably higher than blood levels. Therefore, the functional modulation of α7-nACh receptors demonstrated in this study can be pharmacologically relevant.
As was mentioned earlier, the roles of G-proteins, kinases, and intracellular Ca2+ levels in curcumin actions were excluded in our functional and pharmacological studies. Radioligand binding experiments suggested that curcumin does not interact with ACh binding to receptors. Further analysis of the curcumin effect indicated that curcumin significantly (more than 3-fold) decreased desensitization of the receptor and converted the already-desensitized nACh receptor back to conducting state (Fig. 4C). Importantly, binding of α-bungarotoxin, a competitive antagonist of ACh, was not altered in the presence of curcumin, suggesting that curcumin does not interact with the ACh binding site in the receptor. It is plausible that curcumin acts as an allosteric modulator for various receptors and ion channels at the lipid membrane, accounting for some of its pharmacological actions in animal studies (Zhang et al., 2014). Allosteric modulators alter the functional properties of ligand-gated ion channels by interacting with sites that are topographically distinct from the ligand binding sites [for a review, Onaran and Costa (2009)]. Two different types of positive allosteric modulator (PAM) have been postulated (Uteshev, 2014; Chatzidaki and Millar, 2015). Whereas type I enhances agonist-induced currents without affecting macroscopic current kinetics, type II PAMs also delay desensitization and reactivate desensitized receptors. Results of our experiments indicate that curcumin significantly decreases desensitization (Fig. 4, A and B) and reactivates completely desensitized nicotinic receptors (Fig. 4C), suggesting that curcumin acts as a type II PAM.
It is likely that curcumin, a highly lipophilic agent, first dissolves into the lipid membrane and then diffuses into a nonannular lipid space to potentiate the function of the ion channel-receptor complex. Consistent with this idea, the effect of curcumin on α7-nACh receptor reached a maximal level within 5–10 minutes of application, suggesting that the binding site(s) for these allosteric modifiers is located inside the lipid membrane and requires a relatively slow (in minutes) time course to modulate the function of the receptor. It is likely that these hydrophobic agents affect the energy requirements for gating-related conformational changes in ligand-gated ion channels (Spivak et al., 2007).
Our in vitro data suggested that curcumin is a selective PAM of α7-nACh receptors. We therefore tested this possibility after systemic in vivo administration in mice. α7-nAChRs are present in supraspinal and spinal pain-transmission pathways as well as on immune and nonimmune cytokine-producing cells, such as macrophages and microglia. α7-nAChR expressed on immune cells are involved in the initiation, maintenance, and resolution of inflammation, and modulate inflammation process. In addition, α7-nAChRs expressed on microglia regulate inflammatory factors (Bagdas et al., 2017). Since α7-nACh receptor PAMs have been reported to be active in animal models of tonic and chronic pain (Munro et al., 2012; Freitas et al., 2013a,b; Bagdas et al., 2015), we evaluated the antinociceptive and anti-inflammatory effects of curcumin in the mouse formalin test, a model of tonic and persistent pain (Hunskaar and Hole, 1987). The formalin test consists of two distinct phases. The first phase (immediately after formalin injection) seems to be caused by the direct effect of formalin on sensory C-fibers. The second phase (starting later after formalin injection), known as the inflammatory phase, is associated with the development of a delayed inflammatory response and spinal dorsal horn sensitization (Abbott et al., 1995; Davidson and Carlton, 1998). In the outbred ICR mice, curcumin attenuated pain behaviors dose-dependently in the late (inflammatory) but not the early phase of the formalin test. Importantly, no changes were seen in motor locomotion or coordination with antinociceptive doses of curcumin in mice (Table 1). Using both pharmacological (i.e., the selective α7-nACh antagonist MLA) and genetic approaches (i.e., α7 KO mice), we confirmed that curcumin’s effect in the late phase of formalin is mediated by α7-nACh receptors. The effects of curcumin in the early phase of formalin is strain-dependent since, in contrast to ICR mice, curcumin at a dose of 30 mg/kg significantly attenuated pain behaviors in phase I in the α7 WT (C57BL/6J strain) mice. Interestingly, the effects of curcumin in phase I were not eliminated in the α7 KO mice, suggesting the involvement of non–α7-nACh receptor mechanisms in curcumin’s effects.
Furthermore, our results show that tolerance did not develop following subchronic exposure to the antinociceptive and anti-inflammatory effects of curcumin. PAMs are compounds that facilitate endogenous neurotransmission and/or enhance the efficacy and potency of exogenous agonists, without directly stimulating the agonist binding sites. Supporting this possibility, curcumin enhanced the effects of subactive dose of PNU282987, a full α7-nACh receptor agonist, in the formalin test.
Our vivo data extend curcumin behavioral effects reported in several animal pain models, including acetic acid–induced visceral nociception (Tajik et al., 2008) and formalin-induced orofacial pain (Mittal et al., 2009), and suggest a new mechanism for curcumin-induced antinociception. In addition, the results obtained from this study are in agreement with previous studies, which show anti-inflammatory and antinociceptive actions of PAMs for α7-nACh receptors in the tested mouse models (Freitas et al., 2013a; Bagdas et al., 2015, 2016). In conclusion, using both in vitro and in vivo approaches, this study establishes that curcumin acts as a PAM of the α7-nACh receptors and provides evidence for a new mechanism for the analgesic-like properties of curcumin.
Acknowledgments
We gratefully acknowledge Dr. Jon Lindstrom (University of Pennsylvania) for providing cDNA clones of the human α7-nACh receptor subunit, and Dr. Isabel Bermudez-Diaz (Oxford Brookes University) for human α2, α3, α4, β2, and β4 subunits.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N, N,N′,N′-tetraacetic acid
- CaM-kinase
Ca2+-calmodulin–dependent kinase
- DMSO
dimethylsulfoxide
- Go-6983
XXX
- KN-62
XXX
- KO
knockout
- KT-5720
XXX
- MLA
methyllycaconitine
- nACh
nicotinic acetylcholine
- nAChR
nACh receptor
- NEM
N-ethylmaleimide
- PAM
positive allosteric modulator
- PKC-412
XXX
- PNU282987
N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide
- PTX
pertussis toxin
- WT
wild-type
Authorship Contributions
Participated in research design: Yang, Howarth, Damaj, Oz.
Conducted experiments: El Nebrisi, Bagdas, Toma, Brodzik, Al Samri, Alkhalif.
Performed data analysis: El Nebrisi, Bagdas, Damaj, Oz.
Wrote or contributed to the writing of the manuscript: El Nebrisi, Bagdas, Damaj, Oz.
Footnotes
The research in this study was supported by grants from National Cancer Institute [CA206028] to I.M.D. and from CMHS, UAE University to M.O.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abbott FV, Franklin KB, Westbrook RF (1995) The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 60:91–102. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI (2017) New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on α7 nAChRs. Curr Neuropharmacol DOI: 10.2174/1570159X15666170818102108 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Targowska-Duda KM, López JJ, Perez EG, Arias HR, Damaj MI (2015) The antinociceptive and antiinflammatory properties of 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of α7 nicotinic acetylcholine receptors in mice. Anesth Analg 121:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI (2016) The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol 173:2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderali U, Belke D, Singh A, Jayanthan A, Giles WR, Narendran A (2011) Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell Physiol Biochem 28:1169–1180. [DOI] [PubMed] [Google Scholar]
- Brauneis U, Oz M, Peoples RW, Weight FF, Zhang L (1996) Differential sensitivity of recombinant N-methyl-D-aspartate receptor subunits to inhibition by dynorphin. J Pharmacol Exp Ther 279:1063–1068. [PubMed] [Google Scholar]
- Chatzidaki A, Millar NS (2015) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 97:408–417. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kim KS, Shin DH, Yoo HY, Choe H, Ko TH, Youm JB, Kim WK, Zhang YH, Kim SJ (2013) Class 3 inhibition of hERG K+ channel by caffeic acid phenethyl ester (CAPE) and curcumin. Pflugers Arch 465:1121–1134. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Carlton SM (1998) Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res 785:136–142. [DOI] [PubMed] [Google Scholar]
- Dyer JL, Khan SZ, Bilmen JG, Hawtin SR, Wheatley M, Javed MU, Michelangeli F (2002) Curcumin: a new cell-permeant inhibitor of the inositol 1,4,5-trisphosphate receptor. Cell Calcium 31:45–52. [DOI] [PubMed] [Google Scholar]
- Enyeart JA, Liu H, Enyeart JJ (2008) Curcumin inhibits bTREK-1 K+ channels and stimulates cortisol secretion from adrenocortical cells. Biochem Biophys Res Commun 370:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart JA, Liu H, Enyeart JJ (2009) Curcumin inhibits ACTH- and angiotensin II-stimulated cortisol secretion and Ca(v)3.2 current. J Nat Prod 72:1533–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI (2013a) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther 344:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M (2013b) Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Olincy A, Kanner R, Johnson L, Hogenkamp D, Harris J, Tran M, Edmonds SA, Sauer W, Yoshimura R, et al. (2017) First in human trial of a type I positive allosteric modulator of alpha7-nicotinic acetylcholine receptors: pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288. J Psychopharmacol 31:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, Martins RN (2016) Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 115:449–465. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J (2005) Calcium-activated chloride channels. Annu Rev Physiol 67:719–758. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1–46. [DOI] [PubMed] [Google Scholar]
- Hone AJ, McIntosh JM (2017) Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett DOI: 10.1002/1873-3468.12884 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DH, Son YK, Choi IW, Park WS (2013) The inhibitory effect of curcumin on voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Biochem Biophys Res Commun 430:307–312. [DOI] [PubMed] [Google Scholar]
- Hu CW, Sheng Y, Zhang Q, Liu HB, Xie X, Ma WC, Huo R, Dong DL (2012) Curcumin inhibits hERG potassium channels in vitro. Toxicol Lett 208:192–196. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, El-Meligy A, Lungu G, Fetaih H, Dessouki A, Stoica G, Barhoumi R (2011) Curcumin induces apoptosis in a murine mammary gland adenocarcinoma cell line through the mitochondrial pathway. Eur J Pharmacol 668:127–132. [DOI] [PubMed] [Google Scholar]
- Ji HF, Shen L (2014) The multiple pharmaceutical potential of curcumin in Parkinson’s disease. CNS Neurol Disord Drug Targets 13:369–373. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174:1325–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Revalde J, Paxton JW (2017) The effects of dietary and herbal phytochemicals on drug transporters. Adv Drug Deliv Rev 116:45–62. [DOI] [PubMed] [Google Scholar]
- Lian YT, Yang XF, Wang ZH, Yang Y, Yang Y, Shu YW, Cheng LX, Liu K (2013) Curcumin serves as a human kv1.3 blocker to inhibit effector memory T lymphocyte activities. Phytother Res 27:1321–1327. [DOI] [PubMed] [Google Scholar]
- Liu H, Danthi SJ, Enyeart JJ (2006) Curcumin potently blocks Kv1.4 potassium channels. Biochem Biophys Res Commun 344:1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Gui B, Sun Y, Shi N, Gu Z, Zhang T, Sun X (2013) Inhibition of L-type Ca(2+) channels by curcumin requires a novel protein kinase-theta isoform in rat hippocampal neurons. Cell Calcium 53:195–203. [DOI] [PubMed] [Google Scholar]
- Liu S, Li Q, Zhang MT, Mao-Ying QL, Hu LY, Wu GC, Mi WL, Wang YQ (2016) Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep 6:28956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub M, Keun-Hang SY, Sydorenko V, Ashoor A, Kabbani N, Al Kury L, Sadek B, Howarth CF, Isaev D, Galadari S, et al. (2013) Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol 720:310–319. [DOI] [PubMed] [Google Scholar]
- Mahmmoud YA (2007) Modulation of protein kinase C by curcumin; inhibition and activation switched by calcium ions. Br J Pharmacol 150:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2017) Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174:1290–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N, Joshi R, Hota D, Chakrabarti A (2009) Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother Res 23:507–512. [DOI] [PubMed] [Google Scholar]
- Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustapha A, Pérétout PA, Rainey NE, Sureau F, Geze M, Petit JM, Dewailly E, Slomianny C, Petit PX (2015) Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov 1:15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Hansen R, Erichsen H, Timmermann D, Christensen J, Hansen H (2012) The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol 167:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaran HO, Costa T (2009) Allosteric coupling and conformational fluctuations in proteins. Curr Protein Pept Sci 10:110–115. [DOI] [PubMed] [Google Scholar]
- Oz M, Melia MT, Soldatov NM, Abernethy DR, Morad M (1998) Functional coupling of human L-type Ca2+ channels and angiotensin AT1A receptors coexpressed in xenopus laevis oocytes: involvement of the carboxyl-terminal Ca2+ sensors. Mol Pharmacol 54:1106–1112. [DOI] [PubMed] [Google Scholar]
- Oz M, Renaud LP (2002) Angiotensin AT(1)-receptors depolarize neonatal spinal motoneurons and other ventral horn neurons via two different conductances. J Neurophysiol 88:2857–2863. [DOI] [PubMed] [Google Scholar]
- Oz M, Spivak CE, Lupica CR (2004a) The solubilizing detergents, Tween 80 and Triton X-100 non-competitively inhibit alpha 7-nicotinic acetylcholine receptor function in Xenopus oocytes. J Neurosci Methods 137:167–173. [DOI] [PubMed] [Google Scholar]
- Oz M, Zakharova I, Dinc M, Shippenberg T (2004b) Cocaine inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol 369:252–259. [DOI] [PubMed] [Google Scholar]
- Pérez-Lara A, Corbalán-García S, Gómez-Fernández JC (2011) Curcumin modulates PKCα activity by a membrane-dependent effect. Arch Biochem Biophys 513:36–41. [DOI] [PubMed] [Google Scholar]
- Sands SB, Costa AC, Patrick JW (1993) Barium permeability of neuronal nicotinic receptor alpha 7 expressed in Xenopus oocytes. Biophys J 65:2614–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356. [DOI] [PubMed] [Google Scholar]
- Spagnuolo C, Napolitano M, Tedesco I, Moccia S, Milito A, Russo GL (2016) Neuroprotective role of natural polyphenols. Curr Top Med Chem 16:1943–1950. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M (2007) The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 72:1024–1032. [DOI] [PubMed] [Google Scholar]
- Tajik H, Tamaddonfard E, Hamzeh-Gooshchi N (2008) The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak J Biol Sci 11:312–314. [DOI] [PubMed] [Google Scholar]
- Takikawa M, Kurimoto Y, Tsuda T (2013) Curcumin stimulates glucagon-like peptide-1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem Biophys Res Commun 435:165–170. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD (2010) Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des 16:323–343. [DOI] [PubMed] [Google Scholar]
- Umana IC, Daniele CA, McGehee DS (2013) Neuronal nicotinic receptors as analgesic targets: it’s a winding road. Biochem Pharmacol 86:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV (2012) α7 nicotinic ACh receptors as a ligand-gated source of Ca(2+) ions: the search for a Ca(2+) optimum. Adv Exp Med Biol 740:603–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV (2014) The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol 727:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Chiang IT, Ding K, Chung JG, Lin WJ, Lin SS, Hwang JJ (2012) Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: critical role of ca(+2)-dependent pathway. Evid Based Complement Alternat Med 2012:512907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu X, Wei Z, Dou Y, Zhao D, Wang T, Bian D, Tong B, Xia Y, Xia Y, et al. (2015) Oral curcumin has anti-arthritic efficacy through somatostatin generation via cAMP/PKA and Ca(2+)/CaMKII signaling pathways in the small intestine. Pharmacol Res 95–96:71–81. [DOI] [PubMed] [Google Scholar]
- Yeon KY, Kim SA, Kim YH, Lee MK, Ahn DK, Kim HJ, Kim JS, Jung SJ, Oh SB (2010) Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res 89:170–174. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Q, Wang Y, Peng W, Cai H (2014) Effects of curcumin on ion channels and transporters. Front Physiol 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L, Dong L, Kong D, Sun B, Sun Q, Grundy D, Zhang G, Rong W (2013) Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol Motil 25:e429–e440. [DOI] [PubMed] [Google Scholar]
- Zhou H, Beevers CS, Huang S (2011) The targets of curcumin. Curr Drug Targets 12:332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]