Abstract
Background: Risks to healthcare workers have escalated during the pandemic and they are likely to experience a greater level of stress. This cross-sectional study investigated mental distress among healthcare workers during the early phase of Coronavirus disease-2019 (COVID-19) outbreak in India.
Method: 140 healthcare workers of a tertiary care hospital in India were assessed for perceived stress and insomnia. A factor analysis with principal component method reduced these questions to four components which were categorized as insomnia, stress-related anxiety, stress-related irritability, and stress-related hopelessness. Further statistical analyses were done on these factor scores to identify the predictors and investigate the differences between the different categories of healthcare workers.
Result: Doctors had the highest level of anxiety among the healthcare workers. Both doctors and nurses perceived a greater level of irritability than the other HCWs. Compared to doctors and nurses, other HCWs were more likely to experience insomnia. Lower age, higher education, female gender, and urban habitat were associated with greater perception of anxiety. Older age, being quarantined, and single marital status were the significant predictors of irritability. Female gender, single marital-status, and higher number of medical ailments contributed to perceived hopelessness. Quarantine significantly predicted insomnia.
Conclusion: Different categories of healthcare workers are experiencing varied mental health problems owing to their heterogeneous socio-demographic backgrounds. Tailored and personalized care, as well as policies, might help in alleviating their problems. Further research is warranted to explore the psychological distress and remedies among these frontline workers during and after the ongoing pandemic crisis.
Keywords: COVID-19, healthcare workers, physicians, perceived stress, sleep, psychological wellbeing
Introduction
Coronavirus disease-2019 (COVID-19) has created an unprecedented situation worldwide and has set forth an array of challenges before us—medical, ethical, social, and organizational (Mukherjee et al., 2020). Health care workers (HCWs) are bound by ethics to provide support to patients (Neto et al., 2020). Adhering to medical ethics, HCWs across the world are putting their fullest effort to cope with the pandemic and save lives. However, they are not immune to infection risk. Consequently, HCWs are equally vulnerable to infection as the rest of the population. In fact, the frontline workers are at a greater risk than the general population. Previous statistics clearly indicate that HCWs make a significant portion of the infected cases (Simonds and Sokol, 2008).
Owing to increased risk of infection, duty toward patients might tussle with self-preservation and protection of loved ones thereby increasing stress and anxiety of HCWs (Tam et al., 2004; Ehrlich et al., 2020). Increased duty hours and disrupted biological rhythm during the quarantine might lead to insomnia (Liu et al., 2020). Inadequate supply of personal protective equipment, problematic media coverage and stigma might exacerbate stress (Lai et al., 2020; Malathesh et al., 2020; Menon et al., 2020). In a recent review of six studies, Spoorthy et al. (2020) reported that “HCW are encountering a considerable degree of stress, anxiety, depression, insomnia due to the COVID-19 pandemic.” Apart from doctors, people working in healthcare facilities such as nurses, ward staff, cleaning staff, porters, and administrative staff are also variably vulnerable (Que et al., 2020) and might face mental health problems. People working in certain specialties such as a respiratory ward, infectious diseases ward or critical care ward are subject to greater risk and might be under greater stress.
In a recent review of 43 studies on the psychological impact of COVID-19, Vindegaard and Benros (2020) stated that several factors might be associated with a higher risk of psychological distress among healthcare workers as well as the general public. In fact, the female gender (Mazza et al., 2020; Zhang et al., 2020b), lower educational level (Gao et al., 2020; Mazza et al., 2020), lack of family/social support (Cao et al., 2020; Du et al., 2020), living in urban areas (Gao et al., 2020; Özdin and Bayrak Özdin, 2020), poor social capital and/or unstable income (Cao et al., 2020; Xiao et al., 2020), higher social media exposure (Gao et al., 2020), previous experience of distressful life events (Mazza et al., 2020), lack of preparedness (Du et al., 2020), not adhering to safety or precautionary measures (Wang et al., 2020a), poor self-rated health (Gao et al., 2020; Wang et al., 2020a,b), having a history of chronic illness including psychiatric disorder and substance abuse (Mazza et al., 2020; Özdin and Bayrak Özdin, 2020; Wang et al., 2020b), having a COVID-19 infected friend or relative (Cao et al., 2020; Du et al., 2020; Mazza et al., 2020; Özdin and Bayrak Özdin, 2020), poor sleep quality (Du et al., 2020), higher perceived stress (Du et al., 2020), working in frontline (Giorgi et al., 2020; Lai et al., 2020; Lu et al., 2020), working in a secondary hospital (Lai et al., 2020), intermediate position in job (Lai et al., 2020), seniority in the workplace (>10 years) (Lai et al., 2020) etc. were frequently associated with increased risk of psychological distress. However, there are several inconsistencies and researchers are still not unequivocal regarding these associations. For, example, while several studies identified living in urban areas as a potent risk factor for psychological distress (Gao et al., 2020; Özdin and Bayrak Özdin, 2020), few others reported that living in rural areas could increase the risk (Cao et al., 2020; Zhang et al., 2020b). It may be noted here that Gao et al. (2020) studied the general Chinese population and Zhang et al. (2020b) studied the health care workers of China. Thus, risk factors may vary in different populations and studies focused on different target populations are needed for proper identification of the risk factors and subsequent redemption.
India with its several densely populated states, shortage of medical professionals, inadequate equipment, scarcity of health centers, the paucity of testing facilities, sparse surveillance, and poor awareness among masses, failed to contain the disease (Kumar et al., 2020). Consequently, the pressure on the health system mounted. The Government of India ordered a nationwide lockdown for 21 days On March 24, 2020. The lockdown was further extended with conditional relaxations. The pandemic coupled with lockdown made a deep impact on the socio-economic fabric as well as the mental health conditions of the people. Apprehensions and anguish transformed into fear and stigma toward COVID-19 patients as well as fighters (Bagcchi, 2020). In India, HCW dealing with COVID-19 patients faced considerable social rejection and ostracism. Forceful eviction from temporary residence by house owners, discrimination, violent attacks in public places, and public transports posed threat to their lives. Social stigma against COVID-19 made the difficult situation worse for HCWs. Inadequate numbers of public health care centers along with the escalating COVID-19 treatment expenses in the private health care centers worsened the situation (Mitra, 2020). The already dwindling patient-doctor relationship (Tripathi et al., 2019) reached a worrying level of distrust. Health care workers in general and public health care workers, in particular, suffered acute helplessness. Stigma, work overload, shortage of equipment, dying patients, distrust, concern for personal safety, and safety of the family members pushed them into mental turmoil.
Recent studies on Indian doctors reported significant mental health problems due to COVID-19 (Chatterjee et al., 2020; Khanam et al., 2020; Podder et al., 2020). 52.8% of the health care workers in India were reported to have COVID-19 pandemic-related burnout (Kulkarni et al., 2020). In another study, 73.9 and 30% of the dermatologists in India were found to experience stress and insomnia, respectively due to the pandemic (Bhargava et al., 2020). This is quite in line with Zhang et al. (2020a) who found insomnia in more than one-third of the health care workers working during the COVID-19 pandemic. Burnout can be caused due to insomnia. In fact, Metlaine et al. (2017) stated that job strain represents a burnout risk factor only if associated with insomnia. Banerjee et al. (2020a) in a systematic review of the impact of COVID-19 on psychosocial and mental well-being in the South Asian countries highlighted the increasing stress, anxiety and sleep-related problems in India, especially among the frontliners and health workers. The authors in their advocacy guidance mentioned the need for psychosocial interventions tailored to these needs of the healthcare staff.
Insomnia is a sleep disorder in which one can have trouble falling and/or staying asleep. Good sleep is important for both physical and mental well-being. According to Hess (1965) sleep is “. the expression of a predominance of the trophotropic component of the autonomous nervous system and a preventive measure against exhaustion …” The present-day notion of a circadian rest-activity or sleep-wake rhythm resonates with his concept of alternating trophotropic and ergotropic states. The trophotropic state and the circadian rest state predominantly involve physiological processes that promote energy conservation and restoration as distinguished from the physiological processes and the functional status of the nervous system that help organisms to expend energy (Borbély, 1982; Colten and Altevogt, 2006). During sleep, the arousal systems are shut down allowing the brain to fall asleep. The arousal systems include the thalamus, posterior hypothalamus, neuronal aggregates within the brainstem reticular formation, and basal forebrain. The arousal systems stimulate cortical activation through ascending projections to the cortex and this is characterized by high-frequency gamma and low-frequency rhythmic theta activity. The descending projections to the spinal cord stimulate muscle tonus as well as sensory-motor responsiveness and activity (Jones, 2003). Proper functioning of the arousal systems helps us stay alert and awake. Sleep-wake homeostasis keeps track of the body's requirement of sleep and maintains the sleep-wake cycle.
Stress is a state of disrupted homeostatic balance. It is triggered by intrinsic or extrinsic stressors or situations that are perceived as a threat to one's well-being. The body counteracts by a range of complex physiological and behavioral responses to reestablish eustasis — the optimal body equilibrium (Tsigos et al., 2000). The adaptive stress response involves an intricate network of neuroendocrine, cellular, and molecular infrastructure. Hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) work in tandem with other vital centers in the central nervous system (CNS) and tissues/organs in the periphery to yield a successful adaptive stress response. Dysregulation of the stress system can disrupt the body homeostasis leading to a state of cacostasis (adverse effects) or allostasis (achieve stability). Stress and insomnia are not unitary constructs but these two aspects of mental health are intricately intertwined. Sleep and stress response share a common pathway – the hypothalamic-pituitary-adrenal (HPA) axis. Sleep, especially deep sleep, has an inhibitory influence on the HPA axis whereas, activation of the HPA axis can lead to arousal and sleeplessness (Nicolaides et al., 2000). The HPA axis is also responsible for the neuroendocrine adaptation of the stress response (Smith and Vale, 2006). The production of the stress hormone cortisol is triggered by stress-induced activation of the HPA axis. Cortisol is an essential steroid hormone and like many other physiological processes like sleep has a circadian rhythm. In healthy individuals, cortisol levels reach a nadir at midnight and then build up overnight to peak in the morning and then again decline slowly throughout the day. However, when we are under stress the HPA axis gets activated and the adrenal glands release the hormone cortisol into the bloodstream. This prepares the body for the “fight or flight” response which is important for survival. Therefore, on one hand, stress-related activation of the HPA axis might decrease sleep eventually leading to burnout. On the other hand, sleep deprivation can lead to maladaptive changes in the HPA axis and result in neuroendocrine dysregulation. Thus, stress and insomnia might exacerbate each other and create a vicious cycle impacting long term mental health (Basta et al., 2007).
As already discussed, stress and insomnia are common mental health issues among HCWs battling the COVID-19 pandemic in India and the rest of the world. Most studies investigating stress among health care workers have reported global stress scores. Stress, however, is not a unitary construct. It is multifaceted and complex. Various physiological, psychological, social, and emotional factors may contribute to stress. In fact, the items of the PSS-10 were designed to “tap how unpredictable, uncontrollable, and overloaded respondents find their lives” (Cohen et al., 1983). These different aspects of stress might have different predictor variables and might be differently associated with insomnia. Moreover, different components of stress and insomnia might affect different categories of HCWs differently.
In this study, we conducted a factor analysis on the items obtained from the PES-10 and the ISI-7 to investigate the inter-correlation between these measures and extract different factors of these two mental health parameters. We hypothesized that some measures of sleep will significantly relate with stress measures as these two aspects of mental health influence each other. We also hypothesized that different categories of HCWs will score differently on different factors. We expected different socio-demographic and clinical-professional predictors for different factors. Most studies on Indian HCWs have acquired data through online surveys that have inherent limitations such as lack of focus groups and selection bias. To overcome these shortcomings, we conducted a pen and paper survey. Stratified random sampling was attempted to overcome sampling bias.
Materials and Methods
Ethics
The study was approved by the institutional ethics committee (DHGMC/2020/349/10). All participants signed an informed consent form approved by the above committee.
Settings
The study was conducted from 20th April to 20th May at Diamond Harbour Medical College & Hospital (DHGMC), West Bengal, India. During this time COVID-19 was gradually spreading across India thereby mounting pressure on the health care system. DHGMC was converted into a COVID-19 treatment center, well-equipped with an isolation ward, quarantine center, fever clinic, and COVID-19 testing facility.
Sampling
Approximately, 612 (235 doctors, 259 nurses, 80 ward staff, and 40 non-clinical staff) employees were working at the hospital when this study was carried out. So, the percentages of doctors, nurses, ward staff, and non-clinical staff working during that time were 38.27, 42.18, 13.02, and 6.5%, respectively. We did a stratified random sampling, and the questionnaires were randomly distributed among 308 HCWs (~50% of the total workforce). The 308 HCWs comprised of 118 doctors (38.31%), 130 nurses (42.2%), 40 ward staff (13.0%), and 20 clinical staff (6.5%). Responses were received from only 250 HCWs. Participants having any history of neurological or psychiatric illness were excluded from the study based on self-reports and their scores on the general health questionnaire. After eliminating participants not meeting the inclusion criteria (n = 44), incomplete data (n = 52), and spurious data (n = 14), finally 140 participants were selected for the study. These 140 participants comprised of 56 doctors (40.0%), 46 nurses (32.9%), 20 ward staff (14.3%), and 18 non-clinical staff (12.9%). Thus, the proportion of HCWs included in the final analyses did not match the distribution of HCWs working in the hospital. We, however, did not exclude participants from these final 140 to meet the exact proportion of HCWs working in the hospital as that would have further reduced the sample size. Strict lockdown protocol, social distancing, the growing pressure of COVID-19 patients in the hospital, and the all-pervading fear of death and loss proved to be detrimental for the collection of data, especially through offline forms. HCWs were too preoccupied to focus on research participation. Consequently, we could not follow the stratified random sampling protocol very strictly despite our best efforts.
Participants
One hundred forty (56 doctors, 46 nurses, 20 ward staff, and 18 non-clinical staff) were selected for the study. Doctors comprised of trained professionals who had at least a bachelor's degree in medicine and surgery (MBBS). Nurses included qualified professionals with at least a diploma in nursing. Ward staff members included trained medical technicians and attendants. Non-clinical staff members included the administrative staff and office workers who were not directly involved in patients' care. All the nurses were females, and all the ward staff members were males (Tables 1, 2).
Table 1.
Socio-demographic details of the participants.
| Variable name | Sample size (N = 140) |
|---|---|
| Age | 37.67 ± 9.847 |
| Gender | |
| Male | 61 (43.6%) |
| Female | 79 (56.7%) |
| Marital status | |
| Married | 82 (58.6%) |
| Unmarried | 56 (40.0%) |
| Separated | 2 (1.4%) |
| Habitat | |
| Urban | 84 (60.0%) |
| Rural | 56 (40.0%) |
| Education | |
| Diploma | 2 (1.4%) |
| Graduate | 82 (58.6%) |
| Postgraduate | 56 (40%) |
| Family (living with) | |
| Children | 25 (17.9%) |
| Parents | 63 (45.0%) |
| Spouse | 49 (35.0%) |
| Single | 3 (2.1%) |
| Occupation | |
| Doctor | 56 (40.0%) |
| Nurses | 46 (32.9%) |
| Ward staff | 20 (14.3%) |
| Non-clinical staff | 18(12.9%) |
| Media exposure | |
| <1 h | 14 (10.0%) |
| <2 h | 26 (18.6%) |
| <3 h | 43 (30.7%) |
| Above 3 h | 57 (40.7%) |
| Disease | |
| None | 87 (62.1%) |
| Diabetes | 12 (8.6%) |
| Hypertension | 22 (15.7%) |
| COPD | 11 (7.9%) |
| Multiple complications | 8 (5.7%) |
Key: COPD-Chronic obstructive pulmonary disease.
Table 2.
Clinical-professional details of the participants.
| Variable | Sample size | Stand deviation/ |
|---|---|---|
| name | (N = 140) | percentage |
| Duration of Service | 10.7 | ±9.52 |
| Level of risk of posting | ||
| Severe risk | 35 | 25.0% |
| High risk | 65 | 46.4% |
| Moderate risk | 25 | 17.9% |
| Low risk | 15 | 10.7% |
| Prophylaxis taken | ||
| Yes | 36 | 25.7% |
| No | 104 | 74.3% |
| Using of mask | ||
| Always when outdoors | 109 | 77.9% |
| Even in home | 15 | 10.7% |
| Only when in workplace | 16 | 11.4% |
| Perceived stress severity | ||
| Low | 29 | 20.7% |
| Moderate | 102 | 72.9% |
| High | 9 | 6.4% |
| Insomnia severity | ||
| No (0–7) | 73 | 52.1% |
| Sub threshold (8–14) | 30 | 21.4% |
| Moderate (15–21) | 24 | 17.1% |
| Severe (22–28) | 13 | 9.3% |
Measures
Demographic Information
Demographic information was obtained using a customized demographic data sheet. A questionnaire was designed to assess the participant's level of exposure to patients with COVID-19 infection. Based on the information they were categorized into four groups—severe risk (specimen collection unit, and isolation ward), high risk (chest/medicine outdoor, fever clinic, and emergency), moderate risk (specialist outpatient and inpatient department), and low risk (administrative work).
The Perceived Stress Scale
The Perceived Stress Scale (PSS – 10) (Cohen et al., 1983) has 10 questions/statements and the respondents indicate their levels of agreement (0 = Never; 1 = Almost; 2 = Sometimes; 3 = Fairly Often 4 = Very Often). It includes items measuring reactions to stressful situations as well as measures of stress. The PSS-10 scale has acceptable reliability measures for Indian population (internal consistency-Cronbach's α = 0.731; Spearman-Brown split-half reliability coefficient = 0.71) (Pangtey et al., 2020).
Insomnia Severity Index
Insomnia severity index (ISI-7) (Morin et al., 2011) contains seven items that assess the severity of both nighttime and daytime components of insomnia. The first three items assess trouble in initiating, maintaining sleep, and early morning awakening. Other items address dissatisfaction with sleep, daytime functions, recognition of insomnia by others, and finally, distress caused by insomnia. These are scored on a five-point scale ranging from 0 = no problem to 4 = very severe problem. The score of 0–7 depicts the absence of insomnia, 8–14 indicates subthreshold insomnia, 15–21 represents moderate, and 22–28 suggests severe insomnia. ISI has high internal consistency (Cronbach's α = 0.84) test-retest reliability [ICC (2, 1) = 0.84] and validity (correlation with Pittsburgh Sleep Quality Index- r = 0.45) for Indian population (Veqar and Hussain, 2020). We have used the original English versions of the above tests as all participants in this study had at least 12 years of formal education.
Procedure
The participants self-administered the questionnaires at their leisure in their preferred place without the intervention of the researchers. They were requested to return the questionnaires within a week of receiving them. A follow up was initiated if any participant failed to return the questionnaires within the stipulated time. This being a cross-sectional study, the participants responded only once.
Statistical Analyses
The data were manually entered into Microsoft Excel (Microsoft Corporation, Washington, USA, 2016) after removing all the identifiable information. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) Statistics for Windows, Version 20.0 (IBM Corp., USA, 2011).
We obtained 17 measures per patient: Insomnia (7 questions) and Perceived Stress (10 questions). A Factor Analysis (FA) using the principal component method with a varimax rotation was conducted on data obtained from 140 patients to reduce the number of variables. It may be noted here that the factor structure of a particular tool may vary due to sampling differences (Gaskin et al., 2017). Existing factor analysis data on PSS-10 are based on samples from different cultures and were collected under different socio-economic and health conditions. So, instead of confirmatory factor analysis based on previous studies, a data-driven approach was taken. “Eigenvalues greater than one” was considered as factor extraction criteria since this is considered to be a reliable technique for factor extraction in exploratory factor analysis (Field, 2009).
Shapiro-Wilk test for normality was done on the total factor scores and it revealed that the data are not normally distributed. After excluding the three outliers (6, 64, and 125) the data conformed to the normality criteria. Hence rest of the analyses were done on these 137 participants.
A mixed-design ANOVA was conducted to test for an interaction between the groups (of HCWs) and the mental health components. This analysis was followed by independent sample t-tests to determine how the groups differed across the four mental health components.
Stepwise regression was conducted to test if socio-demographic (age, gender, habitat, marital status, education, family, diseases, and media exposure) and clinical-professional variables (duration of service, quarantine, level of risk, contact with confirmed COVID cases, prophylaxis, and use of mask) could predict the mental health components.
Results
Descriptive Analyses
47.9% (67/140) of the HCWs suffered from insomnia. The mean insomnia scores of doctors, nurses, ward staff, and non-clinical staff were 8.7 ± 6.5, 8.1 ± 5.8, 8.9 ± 2.4, and 10.3 ± 5.9, respectively. 79.3% of the HCWs perceived moderate to severe levels of stress. The mean perceived stress scores of doctors, nurses, ward staff, and non-clinical staff were 19.8 ± 4.5, 18.6 ± 4.3, 12.9 ± 3.6, and 16.2 ± 9.5 respectively.
Group Characteristics
The four groups (Doctors: 34 Male, 22 Female; Nurses: 0 male, 46 Female; ward staff: 20 Male, 0 Female; and Non-clinical staff: 7 Male, 11 Female) were not comparable in age (p = 0.006), education (p < 001) and gender ratio. Doctors (M = 39.23 ± 9.3) and nurses (39.46 ± 11.5) did not have any significant difference in age. The ward staff members had the lowest mean age (31.45 ± 4.8) followed by the non-clinical staff members (35.17 ± 8.4) however, this difference was not statistically significant. The age difference between the nurses and the ward staff members was significant (p < 0.05) but, the difference between non-clinical staff members and the nurses was not significant. Doctors were significantly more educated than other health care workers (p < 0.05). Nurses, Ward staff, and non-clinical staff did not differ significantly in their levels of education. 64.3% of doctors did not have any comorbidity, 5.4% had diabetes, 23.2% had hypertension, and 7.1% had COPD (chronic obstructive pulmonary disease). Among the nurses, 50% did not have any comorbidity, 15.2% had diabetes, 17.4% had hypertension, and 17.4% had multiple comorbidities. Seventy-five percentage of the ward staff did not have any comorbidity but 25% had COPD. 72.2% of the non-clinical staff did not have any comorbidity, 11.1% had diabetes, 5.6% had hypertension, and 11.1% had COPD. Only 3.6% of the doctors and 5.6% of the non-clinical staff lived alone. The rest of the participants stayed with their families.
Exposure to media was assessed on a scale ranging from 1- (<1 h) to 4 (above 3 h. Mean scores of doctors, nurses, ward staff, and non-clinical staff were 3.3 ± 0.90, 2.5 ± 1.0, 3.3 ± 0.44, 3.2 ± 1.2, respectively. The nurses were significantly less exposed to media compared to other doctors, ward staff, and non-clinical staff. The other three groups did not have any significant differences in media exposure scores. 76.8, 34.7, 50, and 72.2% of the doctors, nurses, ward staff, and non-clinical staff, respectively, were married; 1.7% of doctors and 2.2% of nurses were separated; the rest of the participants were unmarried. 87.5% doctors, 47.8% nurses, 0% ward staff, and 72.2% of the non-clinical staff lived in urban areas. 42.9% doctors, 21.7% of nurses, 0% of ward staff, and 11.1% of non-clinical staff used prophylaxis. All the participants used masks. However, the profuseness of use varied across groups. Seventy-five percentage of doctors, 78.3% nurses, 100% ward staff, and 61.1% of non-clinical staff used masks always when they went out of their home; 21.4% of doctors, 6.5% nurses, and 5.6% of non-clinical staff used masks only while at work; and 3.6% of doctors, 15.2% of nurses, and 33.3% of non-clinical staff used masks even at home. 17.8% of doctors, 6.5%nurses, 100% of ward staff, and 38.9% of non-clinical staff had the habit of smoking. Doctors (12.82 ± 8.4) and nurses (13.1 ± 11.5) did not differ significantly in “duration of service.” The ward staff members (4.5 ± 3.4) and non-clinical staff members (5.6 ± 6.7) did not differ significantly in “duration of service.” However, the doctors and nurses had a greater “duration of service” than the ward staff members and the non-clinical staff members. The level of risk for infection was assessed on a scale ranging from 1– low to 4–Very high. The mean scores of doctors, nurses, ward staff, and non-clinical staff were 3.21 ± 0.76, 2.98 ± 0.72, 2.75 ± 0.44, 1.56 ± 1.1, respectively. There was no significant difference between doctors, nurses, and ward staff in levels of risk for infection, but the non-clinical workers had a significantly lower risk for infection compared to the other three groups (p < 0.05). Among the participants, 17.9% of doctors, 15.2% of nurses and 5.6% of non-clinical staff members were quarantined. None of the ward staff was quarantined.
Factor Analysis
The Initial Factor Analysis
A factor analysis with the principal component method was conducted on the 17 measures that were obtained from the ISI-7 and PSS-10. KMO value indicated that the sample was factorable (KMO = 0.768). Homogeneity of variance was confirmed by Bartlett's test [x2 (136) = 926.7, p < 0.001]. The diagonals of the anti-image correlation matrix were over 0.5 for all items except the PSS (Q4). This item was dropped from the final analysis.
The Final Factor Analysis
The final factor analysis was done on 16 items. KMO of the final model was 0.786 and Bartlett's test was significant [x2 (120) = 877.4, p < 0.001] confirming that the data were factorable (Field, 2009). The diagonals of the anti-image correlation matrix were above 0.5 for all items. Communalities were above 0.5 for all items in the final analysis except P-6 (0.42). We extracted four factors with eigenvalues above 1. The four components explained 29.6, 16.0, 10.0, and 6.6% of the variance, respectively. The cumulative percentage of variance explained by the five components was 62.2%. The rotated component matrix with the communalities of the items is given in Table 3. After scrutinizing the individual items of these four factors, we named them: (1) Insomnia (2) Stress-related Anxiety (3) Stress-related Irritability, and (4) Stress-related Hopelessness. Hereafter these four factors will be referred to as Insomnia, Anxiety, Irritability, and Hopelessness, respectively. Factor hopelessness had less than three-item loadings, but we retained it as a separate factor because irritability and hopelessness are different aspects of stress. Further analyses were done on these four factor-scores.
Table 3.
Rotated component matrix.
| Items | Sleeplessness | Anxiety | Irritability | Hopelessness | Communalities |
|---|---|---|---|---|---|
| Insomnia_6 | 0.872 | 0.778 | |||
| Insomnia_7 | 0.85 | 0.735 | |||
| Insomnia_5 | 0.792 | 0.697 | |||
| Insomnia_2 | 0.768 | 0.651 | |||
| Insomnia_4 | 0.651 | 0.563 | |||
| Insomnia__1 | 0.618 | 0.522 | |||
| Insomnia_3 | 0.602 | 0.502 | |||
| PS_1 | 0.835 | 0.711 | |||
| PS_3 | 0.792 | 0.654 | |||
| PS_2 | 0.737 | 0.589 | |||
| PS_9 | 0.721 | 0.655 | |||
| PS_5 | 0.84 | 0.697 | |||
| PS_7 | 0.74 | 0.735 | |||
| PS_8 | 0.705 | 0.544 | |||
| PS_10 | 0.725 | 0.59 | |||
| PS_6 | 0.517 | 0.423 |
PS, Perceived stress; INS, Insomnia; SUP, Stress-due-to-unpredictability; SOL, Stress-due-to-overload; SUC, Stress-due-to-uncontrollability.
Hypothesis Testing
After factor analysis, factor scores were scanned for outliers. Shapiro-Wilk test for normality was done on the total factor scores and it revealed that the data are not normally distributed. After excluding the three outliers (6, 64, and 125) the data conformed to the normality criteria. Hence rest of the analyses were done on these 137 participants.
These 137 participants were divided into four groups based on their profession. There were 55 doctors (Age: M = 39.22 ± 9.3, 33 Male and 22 Female), 45 nurses (Age: M = 39.60 ± 11.6; 0 Male and 45 Female), 20 ward staff (Age: M = 31.45 ± 4.8; 20 Male and 0 Female), and 17 non-clinical staff (Age: M = 34.06 ± 7.2; 6 Male and 11 Female).
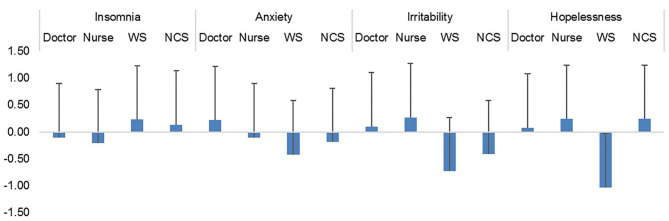
The mixed design ANOVA was carried out with groups of HCWs (Doctor, N = 55; Nurse, N = 45; Ward staff (WS), N = 20; and Non-Clinical staff (NCS), N = 17) as a between-subject variable and the four mental health components obtained from the factor analysis (Insomnia, Anxiety, Irritability, and Hopelessness) as a within-subject variable. The test did not yield any significant main effect of mental health factors [F(3, 399) = 0.84, p = 0.47, observed power = 0.24]. However, there was a significant main effect of group [F(3, 133) = 9.7, p < 0.001; observed power = 0.99] and significant Factor scores x Group interaction [F(9, 399) =3.63, p < 0.001; observed power = 0.99]. Thus, different categories of HCWs responded differently to the different mental health factors (Figure 1).
Figure 1.
Mean scores of different categories of health care workers.
Mean Scores of Healthcare Workers in Four Components of Mental Health
Independent sample t-tests revealed that compared to the ward staff, doctors were significantly more anxious (p = 0.005), irritable (p < 0.001), and hopeless (p = 0.001). Nurses were more irritable (p < 0.001), and hopeless (p < 0.001) than the ward staff. Doctors were more irritable than the non-clinical staff (p = 0.027). Nurses were also more irritable than the non-clinical staff (p = 0.010).
Non-clinical staff members were more hopeless than the ward staff (p = 0.008). Ward staff members experienced more insomnia than the nurses (p = 0.01). There were no significant differences between the doctors and the nurses (Table 4).
Table 4.
Result of independent sample t-tests.
| Factor | Group | Mean | (SD) | Pairs compared | T-value | df | P-value | Cohen's d |
|---|---|---|---|---|---|---|---|---|
| Insomnia | Doctor | −0.10 | (1.10) | Doctor - Nurse | 0.528 | 98 | 0.599 | 0.107 |
| Nurse | −0.21 | (0.94) | Doctor - WS | −1.923 | 72.989 | 0.058 | 0.399 | |
| WS | 0.23 | (0.39) | Doctor - NCS | −1.179 | 53.143 | 0.244 | 0.273 | |
| NCS | 0.14 | (0.57) | Nurse - WS | −2.66 | 62.785 | 0.01* | 0.611 | |
| Nurse - NCS | −1.422 | 60 | 0.16 | 0.450 | ||||
| WS - NCS | 0.578 | 35 | 0.567 | 0.184 | ||||
| Stress-related | Doctor | 0.22 | (0.83) | Doctor - Nurse | 1.715 | 85.199 | 0.09 | 0.346 |
| anxiety | Nurse | −0.10 | (1.01) | Doctor - WS | 2.917 | 73 | 0.005* | 0.752 |
| WS | −0.42 | (0.87) | Doctor - NCS | 1.184 | 19.992 | 0.25 | 0.358 | |
| NCS | −0.18 | (1.34) | Nurse - WS | 1.23 | 63 | 0.223 | 0.339 | |
| Nurse - NCS | 0.267 | 60 | 0.79 | 0.067 | ||||
| WS - NCS | −0.625 | 26.696 | 0.537 | 0.531 | ||||
| Stress-related | Doctor | 0.10 | (1.05) | Doctor - Nurse | −0.833 | 98 | 0.407 | 0.168 |
| irritability | Nurse | 0.27 | (0.96) | Doctor - WS | 4.131 | 55.736 | 0.000* | 0.724 |
| WS | −0.73 | (0.64) | Doctor - NCS | 2.299 | 39.219 | *10.027*1 | 0.566 | |
| NCS | −0.41 | (0.72) | Nurse - WS | 4.939 | 53.303 | <0.001* | 1.22 | |
| Nurse - NCS | 2.66 | 60 | 0.01* | 0.801 | ||||
| WS - NCS | −1.418 | 35 | 0.165 | 0.469 | ||||
| Stress-related | Doctor | 0.08 | (0.79) | Doctor - Nurse | −1.224 | 96.425 | 0.224 | 0.232 |
| hopelessness | Nurse | 0.24 | (0.57) | Doctor - WS | 3.914 | 25.556 | 0.001* | 1.111 |
| WS | −1.03 | (1.17) | Doctor - NCS | −0.418 | 18.623 | 0.681 | 0.129 | |
| NCS | 0.24 | (1.56) | Nurse - WS | 4.626 | 23.037 | <0.001* | 1.380 | |
| Nurse - NCS | 0.007 | 17.627 | 0.994 | 0.000 | ||||
| WS - NCS | −2.832 | 35 | 0.008* | 0.921 |
WS, Ward staff; NCS, Non-clinical staff; SD, Standard deviation; df, Degree of freedom;
Statistically significant after FDR correction;
Statistically not significant after FDR correction.
Exploratory Analyses
Stepwise linear regression with the socio-demographic variables (age, gender, habitat, marital status, education, family, diseases, and media exposure) as predictors were conducted for all the four factors (Insomnia, anxiety, irritability, and hopelessness). Age (β = −0.431, t = −6.1, p < 0.001), education (β = 0.358, t = 4.4, p < 0.001), gender (β = 0.202, t = 2.7, p = 0.008), and habitat (β = −0.201, t = −2.6, p = 0.011) predicted anxiety [F(4, 132) = 18.27, p < 0.001, R2 = 0.356, Cohen's f2= 0.552] indicating lower age, higher education, female gender and urban habitat were associated with higher anxiety. Age (β = 0.480, t = 6.3, p < 0.001) and marital status (β = 0.247, t = 3.2, p = 0.002) predicted irritability [F(2, 134) =22.3, p < 0.001, R2 = 0.249, Cohen's f2 = 0.331]. Older age and single marital status predicted irritability. Gender (β = 0.412, t = 5.2, p < 0.001), marital status (β = −0.203, t = −2.5, p = 0.012) and disease (β = 0.175, t = 2.3, p = 0.025) predicted hopelessness [F(3, 133) = 11.4, p < 0.001, R2 = 0.205, Cohen's f2 = 0.257]. Female gender, married status, and higher number of ailments contributed to perceived hopelessness. None of these variables predicted insomnia (Table 5).
Table 5.
Results of stepwise linear regressions with the demographic variables.
| Dependent variable | Predictors | β | t-value | p-value | F-value | df | p-value | R-square | Cohen's(f2) |
|---|---|---|---|---|---|---|---|---|---|
| Insomnia | None | – | – | – | – | – | – | – | – |
| Stress-related | Age, | −0.431 | −6.084 | 0.000 | 18.268 | 4,132 | 0.000 | 0.356 | 0.552 |
| anxiety | Education | 0.358 | 4.384 | 0.000 | |||||
| Gender | 0.202 | 2.7 | 0.008 | ||||||
| Habitat | −0.201 | −2.574 | 0.011 | ||||||
| Stress-related | Age | 0.48 | 6.316 | 0.000 | 22.257 | 2,134 | 0.000 | 0.249 | 0.331 |
| irritability | Marital-status | 0.247 | 3.241 | 0.002 | |||||
| Stress-related | Gender | 0.412 | 5.177 | 0.000 | 11.428 | 3,133 | 0.000 | 0.205 | 0.257 |
| hopelessness | Marital-status | −0.203 | −2.55 | 0.012 | |||||
| Disease | 0.175 | 2.262 | 0.025 |
Stepwise linear regression with the clinical-professional variables (duration of service, quarantine, level of risk, contact with confirmed COVID cases, prophylaxis, and use of mask) as predictors were conducted for all the four factors (insomnia, anxiety, irritability, and hopelessness). Quarantine (β = −0.206, t = −2.4, p = 0.016) significantly predicted insomnia [F(1, 135) = 5.95, p = 0.016, R2 = 0.042, Cohen's f2 = 0.043]. People who were quarantined were more prone to suffer from insomnia. Duration of service (β = −0.467, t = −5.88, p < 0.001) and use of prophylaxis (β = −0.197, t = −2.5, p = 0.015] predicted anxiety [F(2, 134) = 17.78, p < 0.001, R2 = 0.210 Cohen's f2 = 0.265]. Fewer years in service and use of prophylaxis was associated with anxiety. Duration of service (β = 0.462, t = 6.45, p < 0.001), quarantine (β = −217, t = −2.98, p = 0.003] and level of risk (β = −0.165, t = −2.3, p = 0.024) predicted irritability [F(3, 133) = 21.58, p < 0.001, R2 = 0.327, Cohen's f2 = 0.485]. Greater duration of service, quarantine, and a greater level of risk contributed to irritability. None of these variables predicted hopelessness (Table 6).
Table 6.
Results of stepwise linear regressions with clinical-professional variables.
| Dependent variable | Predictors | β | t-value | p-value | F-value | df | p-value | R-square | Cohen's(f2) |
|---|---|---|---|---|---|---|---|---|---|
| Insomnia | Quarantine | −0.206 | −2.44 | 0.016 | 5.956 | 1,135 | 0.016 | 0.042 | 0.043 |
| Stress-related | Duration of service | −0.467 | −5.878 | 0.000 | 17.777 | 2,134 | 0.000 | 0.21 | 0.265 |
| anxiety | Prophylaxis | −0.197 | −2.474 | 0.015 | |||||
| Stress-related | Duration of service | 0.462 | 6.453 | 0.000 | 21.581 | 3,133 | 0.000 | 0.327 | |
| irritability | Quarantine | −0.217 | −2.983 | 0.003 | 0.485 | ||||
| Level of risk | −0.165 | −2.277 | 0.024 | ||||||
| Stress-related | None | – | – | – | – | – | – | – | – |
| hopelessness |
Discussion
Our study aimed to investigate the different components of perceived stress and insomnia experienced by the HCWs and how different socio-demographic and clinical-professional factors influenced these components. The factor analysis of insomnia and stress scales yielded four factors which were identified as – (1) Insomnia, (2) Stress-related Anxiety, (3) Stress-related Irritability and (4) Stress-related Hopelessness. The four factors explained 62.2% of the variance. Perceived stress yielded three factors and this is consistent with Pangtey et al. (2020) who validated the Hindi version of PSS-10 in the adult urban population of Delhi.
All the 7 questions of the insomnia scale loaded on the first factor. Insomnia was found to be the most important factor and it explained 29.6% of the variance. There was no significant correlation between the insomnia factor and the other three factors of perceived stress. This is consistent with Gupta et al. (2020) who found no significant differences in perceived stress among three different groups with varying levels of nighttime sleep duration after lockdown due to COVID-19. It may be noted that insomnia can be caused by several other factors apart from stress. In this study, quarantine significantly predicted insomnia. More screen time, reduced physical activity, change in daily routine, and staying away from home in a quarantine center could contribute to insomnia. Concern for one's own health, apprehensions for their loved ones, financial worries, etc. could exacerbate anxiety and stress during the quarantine. In response to the stress the cortisol level may shoot up and disrupt the sleep-wake cycle increasing sleep fragmentation, dreaming and insomnia (Basta et al., 2007). Similarly, the blue-wavelength light from the electronic screen may force the brain into confusing between day-night cycle and suppress the production of the sleep hormone melatonin (Tähkämö et al., 2019). Reduced physical activity (PA) may decrease total energy expenditure and affect sleep quality. Exercise is reported to significantly decrease REM sleep (Wang and Boros, 2019) thereby expounding the mechanism of PA effect on sleep. Prevalence of Insomnia was quite high (49.7%) among the HCWs who participated in this study. This percentage is slightly higher than that reported by Lai et al. (2020) and Bhargava et al. (2020). Ward staff members were most likely to experience insomnia. Compared to doctors and nurses, other HCWs were more prone to suffer from insomnia. Smoking could be the possible reason for the elevated insomnia scores in these groups. One-hundred percentage of the ward staff members and 38.9% of the non-clinical staff members had the habit of smoking. The percentages of smokers among the doctors and nurses were much lower. The stimulating effect of nicotine may prevent smokers from falling asleep and later on as night evolves they may have sleep disturbance due to withdrawal from nicotine (Zhang et al., 2008).
Stress due to unpredictability has been referred to as “anxiety” in this study. HCWs with lower age, higher education, female gender, and urban habitat experienced higher levels of anxiety. In fact, doctors who formed the most educated group among the HCWs were the most anxious of all. As we have seen in several patients, better knowledge and understanding of the disease can engender stress and anxiety (Selinger et al., 2013; Zhang et al., 2014). Doctors are not an exception to this rule. Female HCWs and HCWs with lower age experienced greater anxiety. This is in line with Matud (2004) who reported significantly more stress in women even after adjusting for sociodemographic variables. In fact, our result is consistent with studies that report sexual dimorphism in stress reactivity and increased female vulnerability to stress-related disorders (Bangasser and Wicks, 2017; Novais et al., 2017). For example, research reports that female sex hormones attenuate the sympathoadrenal and HPA responsiveness leading to sluggish cortisol feedback on the brain and less or delayed containment of the stress response (Verma et al., 2011). Moreover, human female hypothalami have increased corticotropin-releasing hormone (CRH) content relative to male hypothalami and plasma adrenocorticotropin hormone responses to the ovine CRH are found to be significantly greater among women as compared to men (Gallucci et al., 1993). Consequently, women have greater sensitivity and lower tolerance to negative emotions and are reported to have two to three times higher risk of developing post-traumatic stress symptoms than men (Kessler et al., 2005; Tolin and Foa, 2006). Our results are also in line with the American Psychological Association (APA)'s report of 2019 ( Stress in America 2013, Are Teens Adopting Adults' Stress Habits? 2013), which states that younger adults and women are more stressed out. This is partly consistent with Remes et al. (2016) who stated that the prevalence of anxiety disorder is higher in women and young adults. However, it may be noted that anxiety referred to here is an aspect of stress and we have not used any tool to measure anxiety per se. Nonetheless, these two psychobiological states are reported to have neural as well as behavioral overlaps (Daviu et al., 2019). Our result is consistent with several other studies that report higher levels of stress in people living in cities compared to rural areas (Srivastava, 2009; Gruebner et al., 2017). Fewer years in service and use of prophylaxis was associated with anxiety. HCWs with junior titles were probably less adapted to handle such crises and consequently had higher levels of stress. Higher stress levels could result from the use of prophylaxis (Juurlink, 2020). Additionally, people who are more stressed could be more inclined to use prophylaxis.
Stress due to overload has been referred to as “irritability”. Doctors and nurses scored high on this factor compared to other HCWs. This is consistent with recent studies examining the mental health status of HCWs during COVID-19 (Lai et al., 2020). Older and single HCWs were more irritable. This result is quite intuitive. Older people are more likely to succumb to tiredness due to overwork and single HCWs were probably more stressed because they were handling their emotional and physical burden single-handedly. The result is consistent with a recent study that found lower levels of stress hormones in healthy married adults (Chin et al., 2017). Greater duration of service, quarantine, and a greater level of risk contributed to irritability. This result again is quite expected. Greater duration of service indicates higher age and as already explained older people might capitulate to fatigue and exhaustion more easily than younger people. Moreover, apart from emotional turmoil, quarantine might impose a physical burden as well. Middle-class salaried Indians usually have the privilege of domestic help to take care of household chores. Quarantine could inadvertently repeal this privilege thereby escalating unwonted physical burden and hence stress. This is partly consistent with a study in the general population (Stress, Stigma and Sleep loss: COVID-19 Takes a Heavy Toll on mental Health- The New Indian Express, 2020) that was covered by the New Indian Express. HCWs posted in specialties such as a respiratory ward, infectious-diseases ward, or critical-care ward, where there is a high risk, are plausibly sharing the greatest workload during this pandemic. Consequently, they are probably under greater stress than other HCWs. Wearing the heavy PPE in this hot and humid climate might add to their distress which has been highlighted among the physicians in India repeatedly during the pandemic (Banerjee et al., 2020b).
Stress due to uncontrollability has been denoted as “hopelessness” in this study. Female gender, single marital-status, and greater ailments contributed to perceived hopelessness. Ward staff members were found to be the most hopeful among the HCWs. Incidentally, all the ward staff members were males. This is in line with the linear regression result that indicated gender as the most important predictor of perceived hopelessness. Female HCWs were more likely to be perturbed with the feeling of hopelessness. Our result is consistent with studies that report a feeling of powerlessness among HCWs. Females, being more empathetic, are perhaps more likely to feel hopeless when they witness people suffering and dying. Our findings are also in line with Podder et al. (2020), who reported higher levels of perceived stress in female physicians. In contrast to irritability, married HCWs were found to be more hopeless. Concern for family members and their well-being could contribute to their feeling of hopelessness. The result is somewhat similar to Hacimusalar et al. (2020), who found that the proportion of people who reported increased anxiety was significantly higher in married people compared to single ones. The authors also reported that increase in anxiety levels explained 28.9% of the increase in hopelessness levels. HCWs with a greater number of ailments had greater perceived hopelessness. Numerous scientific journals and social media platforms are continuously reporting that patients with lung diseases, diabetes, and heart diseases are at increased risk for severe complications from COVID-19 (Guan et al., 2020a,b; Sanyaolu et al., 2020). This awareness and a focus on the uncontrollable could worsen the feeling of hopelessness in HCWs with these ailments (Lai et al., 2020).
In sum, this study revealed that the HCWs working in India during the first phase of the pandemic experienced significant mental health symptoms. Several factors contributed to their psychological distress. Most of these factors such as higher age, female gender, higher education, urban habitat, single status, having comorbidities, longer duration of service, a greater level of risk, and quarantine were found to affect the mental health status of HCWs from other countries as well (Vindegaard and Benros, 2020). Quarantine emerged as the predictor of insomnia and this is consistent with several other studies that reported “sense of isolation” as a relevant stressor in quarantined HCWs (Carmassi et al., 2020). However, in this study perceived Stress was considered as a multidimensional construct and the three different components of perceived stress were found to have different predictive factors. In some cases, the factors were differently correlated with different components of perceived stress. For example, age and duration of service were negatively correlated with stress-related anxiety but positively correlated with stress-related irritability. Similarly, while single status predicted irritability, married status predicted hopelessness. The result emphasizes the pressing need to look beyond the global (perceived stress) scores. As in several other studies (Buselli et al., 2020), female HCWs were found to have higher stress-related anxiety and hopelessness. Doctors and nurses had higher levels of stress-related anxiety and irritability. The results of this study make a case for personalized mental health care for HCWs working in different capacities and under different circumstances.
Limitations and Future Directions
Small sample size, sampling from a particular region of India, cross-sectional design, and unequal and disproportional groups limit the scope of generalizability of the findings of this study. Albeit we have applied FDR (false discovery rate) correction for the t-tests and reported effect sizes to reveal the strength of the statistical results, multiplicity of testing is another factor that might affect the statistical power of the tests conducted. Moreover, this study might not represent the mental health issues of HCWs working across India or throughout the world. Culturally diverse populations having different psychological make-ups may respond differently in similar situations. For example, while the study from Kashmir (Khanam et al., 2020) reported higher levels of stress among male HCWs, we found the female HCWs more stressed. Different socio-political situations in these two states of India could be responsible for these contrasting results. The female employment rate in Jammu and Kashmir is abysmally low (7.9%) compared to that of West Bengal (20.5%) (Agarwal, 2018) from where the data was collected for the present study. Kashmiri women who finally get to join the workforce after braving the adverse socio-political situation are perhaps psychologically stronger and more resilient than Bengali women who enjoy a relatively safe and liberal environment. Socio-cultural differences therefore might influence the intensity and modulate the predictive factors of mental health components. So, in order to strategically target therapeutic interventions and to establish the possible impact of the pandemic on the mental health of HCWs, confirmation with a larger sample size covering diverse populations will be an important next step. Since this study is cross-sectional it has predictive limitations as exposure and outcome have been assessed simultaneously. Well-designed longitudinal studies in the future might help track the long-term effects of the pandemic on the mental health of HCWs. Further, qualitative studies grounded in the perspectives of healthcare workers and their perceived challenges during COVID-19 will have important implications for policy changes related to their welfare and safety. However, despite these limitations, the results of this work appear to be substantially in line with previous studies investigating the impact of Covid-19 on the mental health of HCWs. For example, gender differences in the prevalence of stress-related symptoms and quarantine as a predictor of higher stress levels in HCWs have been reported in previous studies (Buselli et al., 2020; Carmassi et al., 2020) that investigated HCWs from other parts of the world. Considering the paucity of research on mental health issues of HCWs fighting COVID 19 in India, this study investigates important and interesting data which will help lend deeper insight into the problems of the HCWs working in different socio-cultural environments.
Conclusion
The study revealed that the HCWs were working with enormous stress and sleep difficulty during the early phase of the pandemic. Different categories of HCWs were affected differently on different factors of perceived stress. While doctors scored higher on stress-related anxiety, nurses scored higher on stress-related irritability, and both nurses and non-clinical staff members scored high on stress-related hopelessness. Different factors modulated insomnia, stress-related anxiety, stress-related irritability, and stress-related hopelessness. For example, duration of service, and use of prophylaxis predicted stress-related anxiety, while the duration of service, quarantine, and level of risk predicted stress-related irritability. More importantly, the duration of service was negatively correlated with stress-related anxiety but positively correlated with stress-related irritability. Thus, this study emphasizes the fact that perceived stress is a multifactorial construct, and reporting global perceived stress scores might result in an oversimplification of the complex and intricate psychological disorder. Impoverished assessment may subsequently lead to inadequate and inappropriate treatment plans. Personalized treatment for different categories of HCWs should be maneuvered appropriately to grapple with the mental health issues of the HCWs in this difficult time. Advanced healthcare work-place strategies and tailored policies will help fight the stress and preserve this “frontline workforce” during the COVID-19 and post-pandemic aftermath.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by DGHMC, West Bengal University of Health Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SeC: concept, design, data collection, data curation, data interpretation, drafting the manuscript, and reviewing and editing. MC: statistical analyses, data interpretation, data visualization, drafting the original manuscript, and editing and revising it critically for important intellectual content. DB: concept, literature review, data curation, drafting the manuscript, organization, reviewing and editing, and revising. SG: design, supervision, editing, and reviewing. ShC: concept, design, data collection, and data preprocessing. UD: design, supervision, editing, and reviewing. All authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agarwal N. (2018). There Are More Working Women Than Working Men in this Indian state. Available online at: https://www.livemint.com/Companies/wa15BlvGI8hFl3YDjI8l9O/There-are-more-working-women-than-working-men-in-this-Indian.html (accessed February 2, 2021).
- Bagcchi S. (2020). Stigma during the COVID-19 pandemic. Lancet Infect. Dis. 20:782. 10.1016/S1473-3099(20)30498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Vaishnav M., Rao T. S., Raju M. S. V. K., Dalal P. K., Javed A., et al. (2020a). Impact of the COVID-19 pandemic on psychosocial health and well-being in South-Asian (World Psychiatric Association zone 16) countries: a systematic and advocacy review from the Indian Psychiatric Society. Ind. J. Psychiatry 62, 343–353. 10.4103/psychiatry.IndianJPsychiatry_1002_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Vijayakumar H. G., Rao T. S. (2020b). Watching the watchmen: Mental health needs and solutions for the health-care workers during the coronavirus disease 2019 pandemic. Int. J. Health Allied Sci. 9:51–54. 10.4103/ijhas.IJHAS_87_20 [DOI] [Google Scholar]
- Bangasser D. A., Wicks B. (2017). Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 95, 75–82. 10.1002/jnr.23812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta M., Chrousos G. P., Vela-Bueno A., Vgontzas A. N. (2007). Chronic insomnia and stress system. Sleep Med. Clin. 2, 279–291. 10.1016/j.jsmc.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S., Sarkar R., Kroumpouzos G. (2020). Mental distress in dermatologists during COVID-19 pandemic: assessment and risk factors in a global, cross-sectional study. Dermatol. Ther. 33:e14161. 10.1111/dth.14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély A. A. (1982). “Sleep regulation: circadian rhythm and homeostasis,” in Sleep. Current Topics in Neuroendocrinology, eds D. Ganten and D. Pfaff (Berlin: Springer; ). 10.1007/978-3-642-68333-6_3 [DOI] [Google Scholar]
- Buselli R., Corsi M., Baldanzi S., Chiumiento M., Lupo E., Dell'oste V., et al. (2020). Professional quality of life and mental health outcomes among health care workers exposed to SARS-CoV-2 (COVID-19). Int. J. Environ. Res. Public Health 17:e6180. 10.3390/ijerph17176180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Fang Z., Hou G., Han M., Xu X., Dong J., et al. (2020). The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res. 287:e112934. 10.1016/j.psychres.2020.112934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmassi C., Foghi C., Dell'Oste V., Cordone A., Bertelloni C. A., Bui E., et al. (2020). PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: what can we expect after the COVID-19 pandemic. Psychiatry Res. 292:e113312. 10.1016/j.psychres.2020.113312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. S., Bhattacharyya R., Bhattacharyya S., Gupta S., Das S., Banerjee B. B. (2020). Attitude, practice, behavior, and mental health impact of COVID-19 on doctors. Indian J. Psychiatry 62, 257–265. 10.4103/psychiatry.IndianJPsychiatry_333_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin B., Murphy M. L. M., Janicki-Deverts D., Cohen S. (2017). Marital status as a predictor of diurnal salivary cortisol levels and slopes in a community sample of healthy adults. Psychoneuroendocrinology 78, 68–75. 10.1016/j.psyneuen.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 386–396. [PubMed] [Google Scholar]
- Colten H. R., Altevogt B. M. (Eds.). (2006). Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press (US). [PubMed] [Google Scholar]
- Daviu N., Bruchas M. R., Moghaddam B., Sandi C., Beyeler A. (2019). Neurobiological links between stress and anxiety. Neurobiol. Stress 11:e100191. 10.1016/j.ynstr.2019.100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Dong L., Wang T., Yuan C., Fu R., Zhang L., et al. (2020). Psychological symptoms among frontline healthcare workers during COVID-19 outbreak in Wuhan. Gen. Hosp. Psychiatry 67, 144–145. 10.1016/j.genhosppsych.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H., McKenney M., Elkbuli A. (2020). Strategic planning and recommendations for healthcare workers during the COVID-19 pandemic. Am. J. Emerg. Med. 38, 1446–1447. 10.1016/j.ajem.2020.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. (2009). Multilevel Linear Models. Discovering Statistics Using SPSS. California, CA: Sage Publications. [Google Scholar]
- Gallucci W. T., Baum A., Laue L., Rabin D. S., Chrousos G. P., Gold P. W., et al. (1993). Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 12, 420–425. 10.1037/0278-6133.12.5.420 [DOI] [PubMed] [Google Scholar]
- Gao J., Zheng P., Jia Y., Chen H., Mao Y., Chen S., et al. (2020). Mental health problems and social media exposure during COVID-19 outbreak. PLoS ONE 15:e0231924. 10.1371/journal.pone.0231924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin C. J., Lambert S. D., Bowe S. J., Orellana L. (2017). Why sample selection matters in exploratory factor analysis: implications for the 12-item World Health Organization Disability Assessment Schedule 2.0. BMC Med. Res. Methodol. 17:40. 10.1186/s12874-017-0309-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi G., Lecca L. I., Alessio F., Finstad G. L., Bondanini G., Lulli L. G., et al. (2020). COVID-19-related mental health effects in the workplace: a narrative review. Int. J. Environ. Res. Public Health 17:e7857. 10.3390/ijerph17217857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruebner O., Rapp M. A., Adli M., Kluge U., Galea S., Heinz A. (2017). Cities and mental health. Dtsch. Arztebl. Int. 114, 121–127. 10.3238/arztebl.2017.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W. J., Liang W. H., He J. X., Zhong N. S. (2020a). Cardiovascular comorbidity and its impact on patients with COVID-19. Euro. Respirat. J. 55:e2001227. 10.1183/13993003.01227-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W. J., Liang W. H., Zhao Y., Liang H. R., Chen Z. S., Li Y. M., et al. (2020b). Comorbidity and its impact on 1,590 patients with Covid-19 in China: a nationwide analysis. Euro. Respirat. J. 55:e2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Grover S., Basu A., Krishnan V., Tripathi A., Subramanyam A., et al. (2020). Changes in sleep pattern and sleep quality during COVID-19 lockdown. Ind. J. Psychiatry 62, 370–378. 10.4103/psychiatry.indianjpsychiatry_523_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacimusalar Y., Kahve A. C., Yasar A. B., Aydin M. S. (2020). Anxiety and hopelessness levels in COVID-19 pandemic: a comparative study of healthcare professionals and other community sample in Turkey. J. Psychiatr. Res. 129, 181–188. 10.1016/j.jpsychires.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. R. (1965). Sleep as a phenomenon of the integral organism. Prog. Brain Res. 18, 3–8. 10.1016/S0079-6123(08)63580-3 [DOI] [PubMed] [Google Scholar]
- Jones B. E. (2003). Arousal systems. Front. Biosci. 8:1074. 10.2741/1074 [DOI] [PubMed] [Google Scholar]
- Juurlink D. N. (2020). Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. Can. Med. Assoc. J. 192, e450–e453. 10.1503/cmaj.200528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Wai T. C., Demler O., Walters E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanam A., Dar S. A., Wani Z. A., Shah N. N., Haq I., Kousar S. (2020). Healthcare providers on the frontline: a quantitative investigation of the stress and recent onset psychological impact of delivering health care services during COVID-19 in Kashmir. Indian J. Psychol. Med. 42, 359–367. 10.1177/0253717620933985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A., Khasne R. W., Dhakulkar B. S., Mahajan H. C. (2020). Burnout among healthcare workers during COVID-19 pandemic in India: results of a questionnaire-based survey. Ind. J. Crit. Care Med. 24, 664–671. 10.5005/jp-journals-10071-23518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Rajasekharan Nayar K., Koya S. F. (2020). COVID-19: Challenges and its consequences for rural health care in India. Public Health Pract. 1:e100009. 10.1016/j.puhip.2020.100009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., et al. (2020). Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 3:e203976. 10.1001/jamanetworkopen.2020.3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Luo D., Haase J. E., Guo Q., Wang X. Q., Liu S., et al. (2020). The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Global Health 8, e790–e798. 10.1016/S2214-109X(20)30204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Wang H., Lin Y., Li L. (2020). Psychological status of medical workforce during the COVID-19 pandemic: a cross-sectional study. Psychiatry Res. 288:e112936. 10.1016/j.psychres.2020.112936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathesh B. C., Chatterjee S. S., Das S. (2020). Overview of mental health issues of COVID-19: Need of the hour. General Psychiatry 33:e100233. 10.1136/gpsych-2020-100233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matud M. P. (2004). Gender differences in stress and coping styles. Pers. Individ. Dif. 37, 1401–1415. 10.1016/j.paid.2004.01.010 [DOI] [Google Scholar]
- Mazza C., Ricci E., Biondi S., Colasanti M., Ferracuti S., Napoli C., et al. (2020). A nationwide survey of psychological distress among italian people during the covid-19 pandemic: immediate psychological responses and associated factors. Int. J. Environ. Res. Public Health 17:e3165. 10.3390/ijerph17093165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Padhy S. K., Pattnaik J. I. (2020). Stigma and aggression against health care workers in India Amidst COVID-19 times: possible drivers and mitigation strategies. Indian J. Psychol. Med. 42, 400–401. 10.1177/0253717620929241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlaine A., Sauvet F., Gomez-Merino D., Elbaz M., Delafosse J. Y., Leger D., et al. (2017). Association between insomnia symptoms, job strain and burnout syndrome: a cross-sectional survey of 1300 financial workers. BMJ Open 7:e012816. 10.1136/bmjopen-2016-012816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P. (2020). Kolkata: Covid Doctors Under Fire From Expense-Wary Patient's Kin. The Times of India. Available online at: https://timesofindia.indiatimes.com/city/kolkata/cov-docs-under-fire-from-expense-wary-patients-kin/articleshow/77622525.cms (accessed February 2, 2021).
- Morin C. M., Belleville G., Bélanger L., Ivers H. (2011). The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34, 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Bandopadhyay G., Chatterjee S. S. (2020). COVID-19 pandemic: mental health and beyond - the Indian perspective. Irish J. Psychol. Med. 21, 1–5. 10.1017/ipm.2020.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto M. L. R., Almeida H. G., Esmeraldo J. D., Nobre C. B., Pinheiro W. R., de Oliveira C. R. T., et al. (2020). When health professionals look death in the eye: the mental health of professionals who deal daily with the 2019 coronavirus outbreak. Psychiatry Res. 288:e112972. 10.1016/j.psychres.2020.112972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides N. C., Vgontzas A. N., Kritikou I., Chrousos G. (2000). “HPA axis and sleep,” in Endotext, eds K. R. Feingold, B. Anawalt, and A. Boyce (South Dartmouth, MA: MDText.com, Inc.). [Google Scholar]
- Novais A., Monteiro S., Roque S., Correia-Neves M., Sousa N. (2017). How age, sex and genotype shape the stress response. Neurobiol. Stress 6, 44–56. 10.1016/j.ynstr.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdin S., Bayrak Özdin S. (2020). Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: the importance of gender. Int. J. Soc. Psychiatry 66, 504–551. 10.1177/0020764020927051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangtey R., Basu S., Meena G., Banerjee B. (2020). Perceived stress and its epidemiological and behavioral correlates in an Urban Area of Delhi, India: a community-based cross-sectional study. Indian J. Psychol. Med. 42, 80–86. 10.4103/IJPSYM.IJPSYM_528_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podder I., Agarwal K., Datta S. (2020). Comparative analysis of perceived stress in dermatologists and other physicians during national lock-down and COVID-19 pandemic with exploration of possible risk factors: a web-based cross-sectional study from Eastern India. Dermatol. Ther. 33:e13788. 10.1111/dth.13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Shi L., Deng J., Liu J., Zhang L., Wu S., et al. (2020). Psychological impact of the COVID-19 pandemic on healthcare workers: a cross-sectional study in China. General Psychiatry 33:e100259. 10.1136/gpsych-2020-100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes O., Brayne C., van der Linde R., Lafortune L. (2016). A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 6:e00497. 10.1002/brb3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. (2020). Comorbidity and its impact on patients with COVID-19. SN Comprehen. Clin. Med. 2, 1069–1076. 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger C. P., Lal S., Eaden J., Jones D. B., Katelaris P., Chapman G., et al. (2013). Better disease specific patient knowledge is associated with greater anxiety in inflammatory bowel disease. J. Crohn's Colitis 7, e214–e218. 10.1016/j.crohns.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Simonds A. K., Sokol D. K. (2008). Lives on the line? Ethics and practicalities of duty of care in pandemics and disasters. Euro. Respirat. J. 34, 303–309. 10.1183/09031936.00041609 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Vale W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog. Clin. Neurosci. 8, 383–395. 10.31887/DCNS.2006.8.4/ssmith [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoorthy M. S., Pratapa S. K., Mahant S. (2020). Mental health problems faced by healthcare workers due to the COVID-19 pandemic–a review. Asian J. Psychiatr, 51, e102119. 10.1016/j.ajp.2020.102119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K. (2009). Urbanization and mental health. Ind. Psychiatry J. 18, 75–76. 10.4103/0972-6748.64028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stress in America 2013 Are Teens Adopting Adults' Stress Habits? . (2013). PsycTESTS Dataset. American Psychological Association (APA). Available online at: https://www.apa.org/news/press/releases/stress/2013/stress-report.pdf (accessed February 2, 2021).
- Stress Stigma Sleep loss: COVID-19 Takes a Heavy Toll on mental Health- The New Indian Express . (2020). Available online at: https://www.newindianexpress.com/cities/kochi/2020/may/12/stress-stigma-and-sleep-loss-covid-19-takes-a-heavy-toll-on-mental-health-2142190.html (accessed May 12, 2020).
- Tähkämö L., Partonen T., Pesonen A. K. (2019). Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 36, 151–170. 10.1080/07420528.2018.1527773 [DOI] [PubMed] [Google Scholar]
- Tam C. W. C., Pang E. P. F., Lam L. C. W., Chiu H. F. K. (2004). Severe acute respiratory syndrome (SARS) in Hongkong in 2003: stress and psychological impact among frontline healthcare workers. Psychol. Med. 34, 1197–1204. 10.1017/S0033291704002247 [DOI] [PubMed] [Google Scholar]
- Tolin D. F., Foa E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 132, 959–992. 10.1037/0033-2909.132.6.959 [DOI] [PubMed] [Google Scholar]
- Tripathi J., Rastogi S., Jadon A. (2019). Changing doctor patient relationship in India: a big concern. Int. J. Commun. Med. Public Health 6, 3160–3164. 10.18203/2394-6040.ijcmph20192868 [DOI] [Google Scholar]
- Tsigos C., Kyrou I., Kassi E., Chrousos G. P. (2000). “Stress, endocrine physiology and pathophysiology,” in Endotext. eds K. R. Feingold, B. Anawalt, and A. Boyce (South Dartmouth, MA: MDText.com, Inc.). [Google Scholar]
- Veqar Z., Hussain M. E. (2020). Validity and reliability of insomnia severity index and its correlation with pittsburgh sleep quality index in poor sleepers among Indian university students. Int. J. Adolesc. Med. Health 32. 10.1515/ijamh-2016-0090 [DOI] [PubMed] [Google Scholar]
- Verma R., Balhara Y. P., Gupta C. S. (2011). Gender differences in stress response: role of developmental and biological determinants. Ind. Psychiatry J. 20,4–10. 10.4103/0972-6748.98407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard N., Benros M. E. (2020). COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 89, 531–542. 10.1016/j.bbi.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C. S., et al. (2020a). Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health 17:e1729. 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., McIntyre R. S., et al. (2020b). A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 87, 40–48. 10.1016/j.bbi.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Boros S. (2019). The effect of physical activity on sleep quality: a systematic review. Euro. J. Physiother. 1–8. 10.1080/21679169.2019.1623314 [DOI] [Google Scholar]
- Xiao H., Zhang Y., Kong D., Li S., Yang N. (2020). Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (COVID-19) outbreak in January 2020 in China. Med. Sci. Monitor 26:e923921. 10.12659/MSM.923921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yang L., Liu S., Ma S., Wang Y., Cai Z., et al. (2020a). Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front. Psychiatry 11:306. 10.3389/fpsyt.2020.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Samet J., Caffo B., Bankman I., Punjabi N. M. (2008). Power spectral analysis of EEG activity during sleep in cigarette smokers. Chest 133, 427–432. 10.1378/chest.07-1190 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liao J., Liao X., Wu X., Wan M., Wang C., et al. (2014). Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm. Med. 14:92. 10.1186/1471-2466-14-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. R., Wang K., Yin L., Zhao W. F., Xue Q., Peng M., et al. (2020b). Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother. Psychosom. 89, 242–250. 10.1159/000507639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.