Abstract
Background
B-type natriuretic peptide (BNP) is a biomarker predicting morbidity and mortality in patients with congestive heart failure. However, the usefulness of pre- or postoperative BNP levels in patients undergoing cardiac surgery remains uncertain. We sought to determine the association of pre- or postoperative BNP levels on mortality in patients undergoing cardiac surgery under cardiopulmonary bypass (CPB).
Methods
This study retrospectively evaluated 1,642 patients undergoing cardiac surgery under CPB over 2 years. The primary outcomes were 30-day and overall mortality after cardiac surgery.
Results
The 30-day mortality rate was 3.0% (n=49), and the overall mortality occurred in 118 patients during the mean follow-up period of 24.9±8.9 months. In multivariable analyses, preoperative BNP level was not significantly associated with 30-day [odds ratio (OR), 1.03; 95% confidence interval (CI), 0.99–1.06; P=0.06] or overall [hazard ratio (HR), 1.01; 95% CI, 0.98–1.03; P=0.50] mortalities. However, the postoperative BNP level was significantly associated with 30-day (OR, 1.05; 95% CI, 1.02–1.09; P=0.001) and overall (HR, 1.03; 95% CI, 1.01–1.04; P=0.01) mortalities. As a sensitivity analysis, postoperative BNP levels were divided into quartiles. The top quartile (≥484 pg/mL) was identified as a strong predictor of overall mortality (HR, 2.18; 95% CI, 1.14–4.19; P=0.02).
Conclusions
Preoperative BNP level was not associated with mortality after cardiac surgery. However, postoperative BNP level was associated with mortality after cardiac surgery, especially in patients with high levels (≥484 pg/mL). Further studies in larger cohorts are necessary to validate these results.
Keywords: Natriuretic peptide, brain, cardiac surgical procedures, heart failure, cardiopulmonary bypass (CPB)
Introduction
B-type natriuretic peptide (BNP), secreted by myocytes in response to increased ventricular wall tension (1), is a well-known biomarker that predicts morbidity and mortality in patients with congestive heart failure (2-4) and acute coronary syndrome (5,6). Consequently, many studies on the usefulness of BNP levels in cardiac surgery are limited to patients undergoing coronary artery bypass grafting (CABG). Fox et al. (7,8) reported that an elevated perioperative BNP level was an independent predictor of early and late adverse outcomes in patients undergoing CABG. However, the clinical implications of perioperative BNP levels in cardiac surgeries under cardiopulmonary bypass (CPB) remain unclear as CPB is known to induce intraoperative plasma BNP release (9,10). In addition, few studies have evaluated the usefulness of perioperative BNP levels as a prognostic marker in cardiac surgeries under CPB and are also limited by their small sample sizes (11,12). Therefore, we sought to determine the usefulness of pre- or postoperative BNP levels for the prediction of mortality in patients undergoing cardiac surgery under CPB. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2375).
Methods
Study population
This retrospective cohort analysis was conducted at Asan Medical Center, which is a tertiary care center in Seoul, Korea. Between January 2014 and December 2015, a total of 2,845 adult patients underwent cardiac surgery under Patients who underwent heart transplantation or cardiac surgery without CPB support or those who had missing preoperative and postoperative plasma BNP level (n=52) were excluded. The remaining 1,642 patients were reviewed. Follow-up information regarding outcomes was obtained from out-patient visits, telephone interviews, and from the medical records of national health insurance. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center, which waived the requirement for informed consent (IRB number: 2017-0412).
Outcomes
The primary outcomes were 30-day and overall mortality after cardiac surgery during the follow-up period. The preoperative BNP level was the most recent available value recorded within one month prior to surgery. The median interval between preoperative BNP measurement and the operation was 1 day [interquartile range (IQR), 1–3]. The postoperative BNP level was measured within two days after cardiac surgery.
Statistical analysis
Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC). Data are expressed as the median and interquartile ranges for continuous variables and as numbers and percentages for categorical variables. Missing data in the baseline characteristics were handled by multiple imputations. P values ≤0.05 was considered statistically significant for all comparisons.
Univariable and multivariable analyses were performed for the entire patient cohort using logistic regression to determine predictors of 30-day mortality and a Cox proportional hazard model to determine overall mortality. In all Cox proportional hazards regression analyses, subjects were censored at the time of postoperative loss to follow-up in cases of loss to follow-up. For the multivariable analyses, variables assessed in univariable models were included in the final multivariable analyses based on clinical importance if their univariable significance was <0.05.
We used a logistic regression model to estimated risks of ‘30-day mortality’ and ‘death within 2-year’ based on a significant predictor revealed in the multivariable model. The multicollinearity of the covariates was assessed by variance inflation factor. Pre- and postoperative BNP level data were entered separately into the final multivariable models as an independent variable to evaluate each independent effect on outcomes. Then, pre- and postoperative BNP were entered together into the final multivariable models to identify the additional interaction to the outcomes.
For sensitivity analysis, the postoperative BNP levels were divided into quartiles and entered separately into the multivariable model to provide additional clinical information. Kaplan-Meier curves were created to compare overall survival between patients in each postoperative BNP quartile. Log-rank tests were used to assess the significance of differences between the stratified curves.
To validate the best threshold value of postoperative BNP for overall mortality was obtained using receiver operating characteristic (ROC) curves.
Results
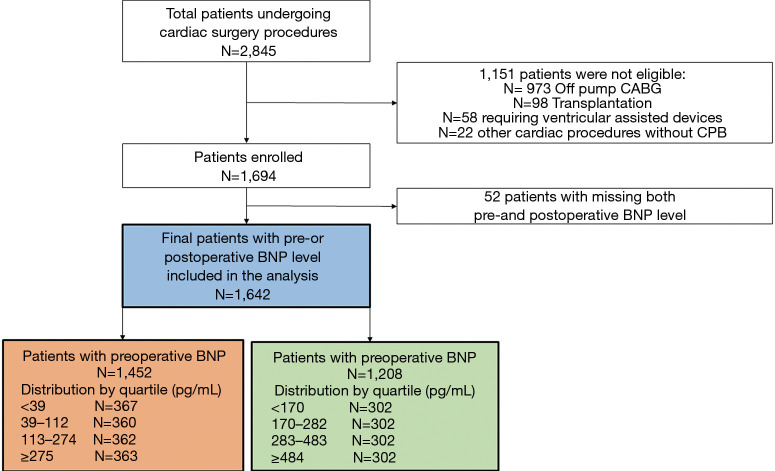
As shown in flow diagram (Figure 1), 1,642 patients out of the total 2,845 patients undergoing cardiac surgery during the study period were finally enrolled and reviewed. 1,151 patients undergoing cardiac surgeries without CPB, mechanical circulatory support, or heart transplantation were excluded and 52 patients with missing both pre-and postoperative BNP level were also excluded in the analysis. Among this study population, patients with preoperative BNP and with postoperative BNP were 1,452 and 1,208 patients, respectively. The baseline and operative characteristics of 1,642 patients enrolled in this study are summarized in Table 1. Median age was 60.0 (50.0–69.0) years. Most patients (64.7%) received valve surgery whereas CABG was performed in 110 patients (6.7%). Median CPB and aortic cross clamp (ACC) time was 134.0 (99.0–182.0) and 81.0 (53.0–114.0) minutes, respectively. The median pre- and postoperative BNP levels were 112.0 (38.0–275.0) pg/mL and 282.0 (170.0–483.5) pg/mL, respectively. Missing data rates for preoperative hemoglobin and creatinine level was 1.6% and 1.4%, respectively. Missing data rates for other variables were less than 1%. Follow-up was completed in all patients. 30-day mortality occurred in 49 (3.0%) patients. The overall mortality rate occurred in 118 patients during the mean follow-up period of 24.9±8.9 months.
Figure 1.
The study population. CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; BNP, B-type natriuretic peptide.
Table 1. Baseline characteristics.
| Variable | 30-day mortality (n=49) | Survivors (n=1,593) | Total (n=1,642) | P value |
|---|---|---|---|---|
| Sex, male | 23 (46.9) | 881 (55.3) | 904 (55.1) | 0.31 |
| Age, years | 66.0 (57.0–74.0) | 60.0 (50.0–69.0) | 60.0 (50.0–69.0) | <0.001 |
| DM | 14 (28.6) | 253 (15.9) | 267 (16.3) | 0.03 |
| Hypertension | 27 (55.1) | 634 (39.8) | 661 (40.3) | 0.05 |
| CVA | 2 (4.1) | 80 (5.0) | 82 (5.0) | 1.00 |
| RRT | 7 (14.3) | 47 (3.0) | 54 (3.3) | <0.001 |
| Euro score | 3.4 (1.6–7.2) | 1.5 (0.8–2.8) | 1.5 (0.8–2.8) | <0.001 |
| LVEF | 57.3 (48.0–64.0) | 61.0 (55.3–65.3) | 61.0 (55.1–65.2) | 0.015 |
| Medications | ||||
| ARB | 18 (36.7) | 527 (33.1) | 545 (33.2) | 0.70 |
| Diuretics | 21 (42.9) | 652 (40.9) | 673 (41.0) | 0.90 |
| Hypoglycemic agents | 14 (28.6) | 287 (18.0) | 301 (18.3) | 0.09 |
| Statin | 16 (32.7) | 519 (32.6) | 535 (32.6) | 1.00 |
| Antiplatelet | 20 (40.8) | 491 (30.8) | 511 (31.1) | 0.18 |
| Laboratory findings | ||||
| Hemoglobin, g/dL | 10.9 (9.6–12.7) | 13.0 (11.6–14.1) | 12.9 (11.5–14.1) | <0.001 |
| Creatinine, mg/dL | 1.0 (0.8–1.6) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.005 |
| Total bilirubin, mg/dL | 0.7 (0.5–1.2) | 0.5 (0.4–0.8) | 0.5 (0.4–0.8) | 0.004 |
| Albumin, g/dL | 3.3 (2.8–3.7) | 3.7 (3.5–4.0) | 3.7 (3.4–4.0) | <0.001 |
| BUN, mg/dL | 19.0 (14.0–27.5) | 16.0 (13.0–21.0) | 16.0 (13.0–21.0) | 0.005 |
| BNP, pg/mL | 187.0 (116.0–490.0) | 110.0 (37.5–266.5) | 112.0 (38.0–275.0) | 0.001 |
| Emergency operation | 1 (2.0) | 10 (0.6) | 11 (0.6) | 0.28 |
| Procedures | 0.18 | |||
| Valve | 28 (57.1) | 1,035 (65.0) | 1,063 (64.7) | |
| CABG | 2 (4.1) | 108 (6.8) | 110 (6.7) | |
| Valve + CABG | 4 (8.2) | 58 (3.6) | 62 (3.8) | |
| Aorta | 11 (22.4) | 222 (13.9) | 233 (14.2) | |
| Others | 4 (8.2) | 170 (10.7) | 174 (10.6) | |
| CPB time, min | 185.0 (141.0–295.0) | 133.0 (98.0–178.5) | 134.0 (99.0–182.0) | <0.001 |
| ACC time, min | 104.0 (78.0–160.0) | 80.0 (53.0–113.0) | 81.0 (53.0–114.0) | 0.002 |
| PostBNP, pg/dL | 817.0 (307.0–1866.0) | 278.0 (168.5–468.5) | 282.0 (170.0–483.5) | <0.001 |
| Ventilator duration, days | 3.0 (1.5–8.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | <0.001 |
| ICU stay, days | 5.0 (2.0–8.5) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | <0.001 |
Variables were presented as median (IQR) and frequency (percentage). DM, diabetes mellitus; CVA, cerebrovascular accident; RRT, renal replacement therapy; LVEF, left ventricular ejection fraction; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; PostBNP, postoperative BNP; ICU, intensive care unit.
Preoperative BNP level and mortality
A total of 1,452 patients with preoperative BNP levels were analyzed without including postoperative BNP variable. Multivariable analyses showed that preoperative BNP level was not associated with 30-day or overall mortality (P>0.05). These findings are summarized in Tables 2,3.
Table 2. Preoperative B-type natriuretic peptide levels and 30-day mortality (n=1,452).
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Outcome: 30-day mortality | |||||||
| Age | 1.04 | 1.01–1.07 | 0.001 | – | – | – | |
| Sex (male) | 0.78 | 0.45–1.38 | 0.40 | – | – | – | |
| DM | 2.02 | 1.08–3.81 | 0.02 | – | – | – | |
| Hypertension | 1.69 | 0.97–2.96 | 0.12 | – | – | – | |
| CVA | 0.77 | 0.19–3.25 | 0.73 | – | – | – | |
| LVEF | 0.97 | 0.95–0.99 | 0.01 | – | – | – | |
| Hemoglobin | 0.66 | 0.58–0.76 | <0.001 | 0.81 | 0.70–0.96 | 0.02 | |
| Creatinine | 1.34 | 1.17–1.60 | <0.001 | – | – | – | |
| Preop BNP† | 1.05 | 1.02–1.07 | <0.001 | 1.03 | 0.99–1.06 | 0.06 | |
| Euro score | 1.05 | 1.02–1.09 | 0.001 | 1.01 | 0.95–1.06 | 0.87 | |
| Procedures | |||||||
| Valve | 1 | – | – | – | – | – | |
| CABG | 0.72 | 0.17–3.09 | 0.66 | – | – | – | |
| Valve + CABG | 2.07 | 0.61–7.05 | 0.25 | – | – | – | |
| Aorta | 2.49 | 1.17–5.26 | 0.02 | – | – | – | |
| Others* | 0.94 | 0.32–2.72 | 0.90 | – | – | – | |
| CPB time‡ | 1.01 | 1.01–1.01 | <0.001 | 1.06 | 1.02–1.09 | 0.001 | |
| ACC time‡ | 1.01 | 1.01–1.01 | <0.001 | – | – | – | |
| Post AKI | 13.25 | 7.15–24.59 | <0.001 | 7.61 | 3.81–15.19 | <0.0001 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase. BNP, B-type natriuretic peptide; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury.
Table 3. Preoperative B-type natriuretic peptide levels and overall mortality.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Outcome: overall mortality | |||||||
| Age | 1.03 | 1.02–1.05 | <0.001 | 1.02 | 0.99–1.04 | 0.11 | |
| Sex (male) | 1.11 | 0.75–1.65 | 0.59 | – | – | – | |
| DM | 2.27 | 1.49–3.46 | <0.001 | 1.67 | 0.98–2.82 | 0.06 | |
| Hypertension | 1.45 | 0.98–2.14 | 0.06 | – | – | – | |
| CVA | 1.17 | 0.51–2.66 | 0.71 | – | – | – | |
| LVEF | 0.97 | 0.96–0.99 | <0.001 | 0.99 | 0.97–1.02 | 0.60 | |
| Hemoglobin | 0.65 | 0.59–0.72 | <0.001 | 0.79 | 0.69–0.90 | <0.001 | |
| Creatinine | 1.36 | 1.23–1.50 | <0.001 | 1.33 | 1.13–1.57 | 0.001 | |
| Preop BNP† | 1.04 | 1.03–1.05 | <0.001 | 1.01 | 0.98–1.03 | 0.50 | |
| Euro score | 1.05 | 1.04–1.07 | <0.001 | 1.03 | 1.00–1.07 | 0.04 | |
| Procedures | |||||||
| Valve | 1 | – | – | – | |||
| CABG | 1.33 | 0.63–2.78 | 0.45 | – | – | – | |
| Valve + CABG | 1.87 | 0.80–4.32 | 0.15 | – | – | – | |
| Aorta | 2.25 | 1.35–3.74 | 0.002 | – | – | – | |
| Others | 1.07 | 0.55–2.10 | 0.83 | – | – | – | |
| CPB time‡ | 1.09 | 1.07–1.12 | <0.001 | 1.06 | 1.03–1.10 | <0.001 | |
| ACC time‡ | 1.09 | 1.06–1.13 | <0.001 | – | – | – | |
| Post AKI | 9.33 | 6.36–13.69 | <0.001 | 8.55 | 5.22–13.97 | <0.001 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase. BNP, B-type natriuretic peptide; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury; HR, hazard ratio.
Postoperative BNP level and mortality
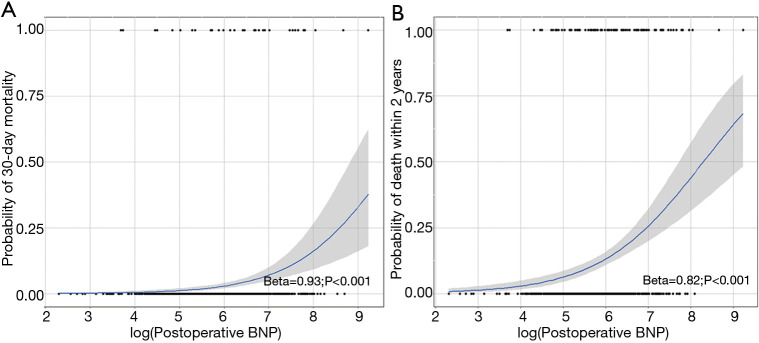
A total of 1,208 patients with postoperative BNP levels were analyzed without including preoperative BNP variable. Multivariable analysis identified postoperative BNP level as risk factor for 30-day and overall mortalities (OR, 1.05; 95% CI, 1.02–1.09; P=0.001 and HR, 1.03; 95% CI, 1.01–1.04; P=0.01, respectively), as summarized in Tables 4,5. As postoperative BNP level was identified as a risk factor, probabilities of risks of 30-day mortality and death within 2 years according to postoperative BNP level were estimated by logistic regression models. It revealed a significant relation as shown in Figure 2A and B. According to the ROC curves, cutoff values of postoperative BNP for 30-day and overall mortality were 790 and 483 pg/mL, respectively.
Table 4. Postoperative BNP and 30-day mortality (n=1,208).
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Outcome: 30-day mortality | |||||||
| Age | 1.04 | 1.01–1.07 | 0.02 | – | – | – | |
| Sex male | 0.62 | 0.32–1.20 | 0.15 | – | – | – | |
| DM | 1.68 | 0.81–3.55 | 0.16 | – | – | – | |
| Hypertension | 2.30 | 1.17–4.52 | 0.02 | – | – | – | |
| CVA | 0.24 | 0.01–4.05 | 0.32 | – | – | – | |
| LVEF | 0.98 | 0.95–1.00 | 0.06 | – | – | – | |
| Hemoglobin | 0.69 | 0.58–0.82 | <0.001 | 0.81 | 0.66–0.97 | 0.04 | |
| Creatinine | 1.25 | 1.04–1.51 | 0.02 | – | – | – | |
| Euro score | 1.05 | 1.01–1.09 | 0.009 | 1.00 | 0.95–1.06 | 0.91 | |
| Procedures | |||||||
| Valve | 1 | – | – | – | |||
| CABG | 0.83 | 0.19–3.59 | 0.80 | – | – | – | |
| Valve + CABG | 2.01 | 0.58–6.70 | 0.27 | – | – | – | |
| Aorta | 1.46 | 0.64–3.35 | 0.37 | – | – | – | |
| Others | 1.19 | 0.35–4.05 | 0.79 | – | – | – | |
| CPB time‡ | 1.08 | 1.04–1.12 | <0.001 | 1.02 | 0.98–1.07 | 0.23 | |
| ACC time‡ | 1.07 | 1.01–1.12 | <0.001 | – | – | – | |
| Post AKI | 9.67 | 4.91–19.0 | <0.001 | 6.59 | 3.06–14.20 | <0.001 | |
| Postop BNP† | 1.08 | 1.05–1.12 | <0.001 | 1.05 | 1.02–1.09 | 0.001 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase. BNP, B-type natriuretic peptide; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury.
Table 5. Postoperative BNP and overall mortality.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Outcome: overall mortality | |||||||
| Age | 1.02 | 1.00–1.04 | 0.02 | 1.01 | 0.99–1.03 | 0.43 | |
| Sex male | 1.16 | 0.78–1.73 | 0.46 | ||||
| DM | 1.84 | 1.19–2.85 | 0.006 | 1.43 | 0.88–2.33 | 0.15 | |
| Hypertension | 1.49 | 1.01–2.21 | 0.05 | 1.18 | 0.75–1.85 | 0.47 | |
| CVA | 0.80 | 0.29–2.17 | 0.66 | ||||
| LVEF | 0.98 | 0.96–0.99 | 0.008 | 1.00 | 0.98–1.01 | 0.68 | |
| Hemoglobin | 0.68 | 0.62–0.76 | <0.001 | 0.81 | 0.72–0.90 | <0.001 | |
| Creatinine | 1.30 | 1.17–1.44 | <0.001 | 1.20 | 1.05–1.36 | 0.008 | |
| Euro Score | 1.05 | 1.03–1.07 | <0.001 | 1.01 | 0.98–1.04 | 0.49 | |
| Procedures | |||||||
| Valve | 1 | 1 | |||||
| CABG | 1.42 | 0.67–2.98 | 0.36 | 1.44 | 0.60–3.47 | 0.42 | |
| Valve + CABG | 1.40 | 0.56–3.50 | 0.48 | 0.84 | 0.32–2.19 | 0.72 | |
| Aorta | 2.08 | 1.30–3.31 | 0.002 | 1.42 | 0.84–2.42 | 0.19 | |
| Others | 1.51 | 0.74–3.06 | 0.26 | 2.89 | 1.34–6.25 | 0.01 | |
| CPB time‡ | 1.08 | 1.05–1.10 | <0.001 | 1.03 | 0.99–1.07 | 0.14 | |
| ACC time‡ | 1.07 | 1.04–1.11 | <0.001 | ||||
| Post AKI | 7.07 | 4.82–10.37 | <0.001 | 4.76 | 3.00–7.57 | <0.001 | |
| Postop BNP† | 1.03 | 1.04–1.07 | <0.001 | 1.03 | 1.01–1.04 | 0.01 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase; BNP, B-type natriuretic peptide; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury; HR, hazard ratio.
Figure 2.
Association between postoperative BNP level and mortalities. (A) Probability of 30-day mortality according to the (log) postoperative BNP level; (B) probability of death within 2-year according to the (log) postoperative BNP level estimated by logistic regression models. BNP, B-type natriuretic peptide.
Pre- and postoperative BNP levels and mortality
Both pre- and postoperative BNP levels were measured for 1,018 patients. These patients were analyzed including both pre- and postoperative BNP variables in the model. In the multivariable analysis, postoperative BNP, but not preoperative BNP was associated with 30-day and overall mortality (OR: 1.04; 95% CI: 1.01–1.08; P=0.01 and HR: 1.02; 95% CI: 1.002–1.05; P=0.03, respectively) (Tables 6,7).
Table 6. Both preoperative and postoperative BNP and 30-day mortality (n=1,018).
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Outcome: 30-day mortality | |||||||
| Age | 1.04 | 1.01–1.07 | 0.02 | – | – | – | |
| Sex male | 0.62 | 0.32–1.20 | 0.15 | – | – | – | |
| DM | 1.68 | 0.81–3.55 | 0.16 | – | – | – | |
| Hypertension | 2.30 | 1.17–4.52 | 0.02 | – | – | – | |
| CVA | 0.24 | 0.01–4.05 | 0.32 | – | – | – | |
| LVEF | 0.98 | 0.95–1.00 | 0.06 | – | – | – | |
| Hemoglobin | 0.69 | 0.58–0.82 | <0.001 | – | – | – | |
| Creatinine | 1.25 | 1.04–1.51 | 0.02 | – | – | – | |
| Euro score | 1.05 | 1.01–1.09 | 0.009 | 1.02 | 0.96–1.07 | 0.45 | |
| Pre BNP | 1.05 | 1.02–1.07 | <0.001 | 1.01 | 0.97–1.06 | 0.46 | |
| Procedures | |||||||
| Valve | 1 | – | – | – | |||
| CABG | 0.83 | 0.19–3.59 | 0.80 | – | – | – | |
| Valve + CABG | 2.01 | 0.58–6.70 | 0.27 | – | – | – | |
| Aorta | 1.46 | 0.64–3.35 | 0.37 | – | – | – | |
| Others | 1.19 | 0.35–4.05 | 0.79 | – | – | – | |
| CPB time‡ | 1.08 | 1.04–1.12 | <0.001 | 1.03 | 0.99–1.08 | 0.13 | |
| ACC time‡ | 1.07 | 1.01–1.12 | <0.001 | – | – | – | |
| Post AKI | 9.67 | 4.91–19.0 | <0.001 | 8.24 | 3.93–20.23 | <0.001 | |
| Postop BNP† | 1.08 | 1.05–1.12 | <0.001 | 1.04 | 1.01–1.08 | 0.01 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase. BNP, B-type natriuretic peptide; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury. bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury.
Table 7. Both preoperative and postoperative BNP and overall mortality.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Outcome: overall mortality | |||||||
| Age | 1.02 | 1.00–1.04 | 0.02 | 1.01 | 0.99–1.03 | 0.26 | |
| Sex male | 1.16 | 0.78–1.73 | 0.46 | – | – | – | |
| DM | 1.84 | 1.19–2.85 | 0.006 | 1.46 | 0.87–2.46 | 0.15 | |
| Hypertension | 1.49 | 1.01–2.21 | 0.05 | 1.13 | 0.69–1.85 | 0.64 | |
| CVA | 0.80 | 0.29–2.17 | 0.66 | – | – | – | |
| LVEF | 0.98 | 0.96–0.99 | 0.008 | 1.00 | 0.98–1.02 | 0.95 | |
| Hemoglobin | 0.68 | 0.62–0.76 | <0.001 | 0.81 | 0.71–0.91 | 0.001 | |
| Creatinine | 1.30 | 1.17–1.44 | <0.001 | 1.20 | 1.04–1.39 | 0.01 | |
| preBNP | 1.04 | 1.03–1.05 | <0.001 | 1.00 | 0.98–1.03 | 0.77 | |
| Euro score | 1.05 | 1.03–1.06 | <0.001 | 1.02 | 0.99–1.05 | 0.23 | |
| Procedures | |||||||
| Valve | 1 | – | – | 1 | – | – | |
| CABG | 1.42 | 0.67–2.98 | 0.36 | 1.20 | 0.47–3.09 | 0.70 | |
| Valve + CABG | 1.40 | 0.56–3.50 | 0.48 | 0.66 | 0.23–1.92 | 0.44 | |
| Aorta | 2.08 | 1.30–3.31 | 0.002 | 1.47 | 0.82–2.65 | 0.20 | |
| Others | 1.51 | 0.74–3.06 | 0.26 | 3.30 | 1.53–7.10 | 0.002 | |
| CPB time‡ | 1.08 | 1.05–1.10 | <0.001 | 1.04 | 1.00–1.07 | 0.053 | |
| ACC time‡ | 1.07 | 1.04–1.11 | <0.001 | 0.99 | 0.94–1.04 | 0.59 | |
| Post AKI | 7.07 | 4.82–10.37 | <0.001 | 5.38 | 3.25–8.91 | <0.001 | |
| Postop BNP† | 1.03 | 1.04–1.07 | <0.001 | 1.02 | 1.002–1.05 | 0.03 | |
†, BNP for 100 increase; ‡, CPB time or ACC time for 10 increase. BNP, B-type natriuretic peptide; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; AKI, acute kidney injury; HR, hazard ratio.
Sensitivity analysis
Postoperative BNP levels divided into quartiles and associations with mortality
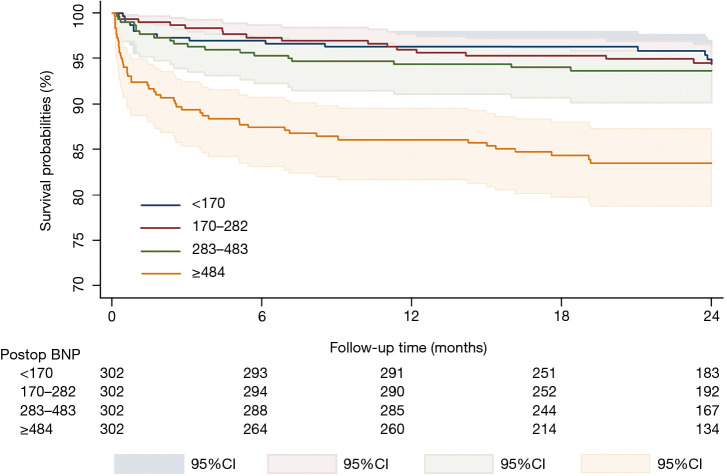
The patients were divided into quartiles based on postoperative BNP level (BNP <170 pg/mL, 170≤ BNP <282 pg/mL, 282≤ BNP <484 pg/mL, and BNP ≥484 pg/mL). Of these, the top quartile (BNP ≥484 pg/mL) was associated with a higher risk of 30-day (OR 8.69, 95% CI: 1.93–39.05, P=0.005) and overall mortality (HR 2.18, 95% CI: 1.14–4.19, P=0.02) (Tables 8,9). Kaplan-Meier curves comparing the probability of survival in each quartile revealed that patients in the top quartile (BNP ≥484 pg/mL) had increased risk of death (log-rank P<0.001) (Figure 3).
Table 8. Postoperative BNP levels divided into quartiles and associations with 30-day mortality.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Outcome: 30-day mortality | |||
| Postoperative BNP (pg/mL) | |||
| <170 | 4.10 | 0.80–21.08 | 0.09 |
| 170–282 | 1† | ||
| 283–483 | 2.50 | 0.47–13.29 | 0.28 |
| ≥484 | 8.69 | 1.93–39.05 | 0.005 |
†, the quartile with lowest mortalities in each outcome was used as a reference. BNP, B-type natriuretic peptide.
Table 9. Postoperative BNP levels divided into quartiles and associations with overall mortality.
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Outcome: overall mortality | |||
| Postoperative BNP (pg/mL) | |||
| <170 | 1† | ||
| 170–282 | 1.03 | 0.49–2.15 | 0.94 |
| 283–483 | 1.03 | 0.50–2.15 | 0.93 |
| ≥484 | 2.18 | 1.14–4.19 | 0.02 |
†, the quartile with lowest mortalities in each outcome was used as a reference. BNP, B-type natriuretic peptide.
Figure 3.
The probability of overall survival of the postoperative BNP level according to quartiles during the follow-up period. Increased risk of mortality in the top quartile (BNP ≥484 pg/mL) (orange line) compared to the bottom three quartiles (green, red, and blue line) (P<0.001). BNP, B-type natriuretic peptide.
Discussion
This study investigated the relationship between pre- and postoperative BNP levels and 30-day and overall mortalities in patients who underwent cardiac surgery under CPB. The multivariable analysis revealed no significant association between the preoperative BNP level and mortality. However, the postoperative BNP level was strongly associated with 30-day and overall mortalities. Furthermore, postoperative BNP level in the top quartile (BNP ≥484 pg/mL) was identified as a significant risk factor for 30-day and overall mortalities. Therefore, high postoperative BNP was suggested as a helpful surrogate marker for mortality after cardiac surgery.
Our findings regarding preoperative BNP levels seem contradictory to those of previous reports. One meta-analysis investigating the relationship between BNP levels and adverse outcomes after cardiac surgery (13) reported that higher preoperative BNP levels increased mortality after cardiac surgery. However, most studies in this meta-analysis enrolled patients who underwent CABG only or a combination of few valve surgeries and CABG. Except for the study by Fox et al. (8), the study populations were relatively small (<100 patients). Another review paper assessed BNP as a biomarker in common forms of valvular heart disease (14). Four of these studies (15-18) reported a relationship between higher preoperative BNP levels and adverse surgical outcomes. However, the study populations were also small (n=22–144), with limited statistical power. Our study population was relatively large (n=1,642) compared to those in previous studies and included unselected patients undergoing cardiac surgery under CPB regardless of the specific type of operation. Contrary to the findings of these previous studies, we found that preoperative BNP level was not associated with mortality after cardiac surgery. The broad inclusion criteria may be criticized as contributing to the heterogeneity of the study population. However, our study population was homogenous in terms of undergoing cardiac surgery under CPB regardless of surgery type. Therefore, this study population could reflect the effect of CPB on postoperative BNP levels.
Few studies have evaluated the usefulness of postoperative BNP levels in patients undergoing cardiac surgery. Fox et al. (8) proposed that preoperative BNP levels may be better than peak postoperative BNP levels at predicting prolonged hospitalization and late mortality after CABG. However, several studies on postoperative BNP level had findings consistent with ours, showing postoperative BNP level to be an independent predictor of postoperative cardiac dysfunction, prolonged hospital stay, and mortality (11,12,19). The optimal cutoff postoperative BNP value to predict 30-day mortality based on the ROC method was 790 pg/mL [area under the curve (AUC): 0.71, 59% CI: 0.61–0.82, P<0.001, sensitivity 54.1%, specificity 87.6%], which was the same as the cutoff suggested by Lurati Buse et al. (19). The cutoff value for overall mortality was 483 pg/mL (AUC: 0.65, 95% CI: 0.58–0.71, P<0.001, sensitivity 48.9%, specificity 77.1%), which was the same as the critical postoperative value of the top quartile in the sensitivity analysis.
Besides preoperative BNP, any serial change of BNP level between before and after cardiac surgery was not associated with mortalities. Only postoperative BNP was independently associated with mortalities. This may be due to the contributions of other intraoperative or immediate postoperative factors to the increased postoperative BNP level as well as the patient’s preoperative condition. Postoperative volume status or inotropic support may be potential factors contributing to postoperative BNP levels even though they were not considered in our study. Among the factors included in our study, longer CPB time was identified as the most significant factor associated with elevated postoperative BNP level in the multiple linear regression analysis. Ischemic reperfusion injury occurs during CPB and CPB induces BNP release during cardiac surgery. According to earlier reports (11,12), CPB increases BNP release in the initial stages of reperfusion following global ischemia. These reported that BNP levels returned to baseline within hours or days of reperfusion initiation. Therefore, the postoperative BNP level measured on the first day after surgery may reflect the combined effects of ischemic/reperfusion injury during CPB support. Also, postoperative alterations in renal function might affect the increased postoperative BNP level because one of the BNP clearance mechanisms is urinary excretion (20). However, postoperative creatinine level did not show significant correlation with postoperative BNP level in our study (r=0.18).
Other possible mechanisms contributing to the elevation of postoperative BNP level are acute changes in systolic or diastolic function after cardiac surgery and neurohormonal compensation for cardiac dysfunction. As BNP is more sensitive, specific and accurate than an echocardiography in detecting myocardial dysfunction, those patients with high postoperative BNP could have diastole dysfunction or possibly high levels of vasoconstrictor neurohormonal factors such as norepinephrine, angiotensin II or tumor necrosis factor. These suboptimal conditions, which were not managed properly during admission, could lead to chronic heart failure during the follow-up period. In addition, a non-cardiac event, such as infection or pneumonia, could contribute to decompensated heart failure in the long term.
While postoperative BNP level is a useful surrogate marker allowing primary physicians to flag patients at risk of death after cardiac surgery, our results could not suggest any practical guideline to optimize the care and management of these patients. The possible recommendations include closer monitoring during hospital stays with frequent checkups such as echocardiography and frequent outpatient clinic follow-up with optimal medical management of underlying medical problems.
This study has several limitations. First, this was a retrospective observational study. While multivariable adjustment analysis and were performed to reduce bias, many potentially important confounders remained. Especially, preoperative or postoperative BNP level was measured without a standardized study protocol. This diverse timing of BNP measurement might induce bias to the study results. Also, postoperative factors such as right ventricular function, volume status, inotropic support and newly developed arrhythmia that might affect the elevation of postoperative BNP levels were not validated in this study. Second, in the risk factor analysis for 30-day mortality, variables with a statistical significance in univariable analysis were selected as candidates for the final multivariable models based on the clinical relevance and importance to avoid overfitting the model, which could be another bias. Third, even though this study included a relatively large cohort compared to those in previous studies, the patient population included the various types of cardiac surgery under CPB. In addition, this study excluded 995 patients who underwent cardiac surgery without CPB; therefore, the results may not be generalizable to those patients. Finally, some patients were excluded for a lack of available perioperative BNP values, which may raise an issue of selection bias. However, relatively few patients were excluded for this reason (n=52, 3.1%); therefore, the impact of this exclusion on the overall study results may be limited.
Conclusions
Preoperative BNP level was not significantly associated with mortality following cardiac surgery under CPB. However, postoperative BNP level was identified as a useful surrogate marker for predicting postoperative mortality. High postoperative BNP level (≥484 pg/mL) was strongly associated with mortality. Further prospective studies in larger cohorts are necessary to verify the predictive values of postoperative BNP levels on cardiac surgical outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Asan Medical Center, which waived the requirement for informed consent (IRB number: 2017-0412).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2375
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2375
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2375
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2375). The authors have no conflicts of interest to declare.
References
- 1.Baughman KL. B-type natriuretic peptide -- a window to the heart. N Engl J Med 2002;347:158-9. 10.1056/NEJMp020057 [DOI] [PubMed] [Google Scholar]
- 2.Doust JA, Pietrzak E, Dobson A, et al. How well does b-type natriuretic peptide predict death and cardiac events in patients with heart failure: Systematic review. BMJ 2005;330:625. 10.1136/bmj.330.7492.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison A, Morrison LK, Krishnaswamy P, et al. B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med 2002;39:131-8. 10.1067/mem.2002.121483 [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Tsutamoto T, Wada A, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000;36:1587-93. 10.1016/S0735-1097(00)00912-8 [DOI] [PubMed] [Google Scholar]
- 5.Morita E, Yasue H, Yoshimura M, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993;88:82-91. 10.1161/01.CIR.88.1.82 [DOI] [PubMed] [Google Scholar]
- 6.Omland T, Persson A, Ng L, O'Brien R, et al. N-terminal pro-b-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation 2002;106:2913-8. 10.1161/01.CIR.0000041661.63285.AE [DOI] [PubMed] [Google Scholar]
- 7.Fox AA, Nascimben L, Body SC, et al. Increased perioperative b-type natriuretic peptide associates with heart failure hospitalization or heart failure death after coronary artery bypass graft surgery. Anesthesiology 2013;119:284-94. 10.1097/ALN.0b013e318299969c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox AA, Shernan SK, Collard CD, et al. Preoperative b-type natriuretic peptide is as independent predictor of ventricular dysfunction and mortality after primary coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;136:452-61. 10.1016/j.jtcvs.2007.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mair P, Mair J, Bleier J, et al. Augmented release of brain natriuretic peptide during reperfusion of the human heart after cardioplegic cardiac arrest. Clin Chim Acta 1997;261:57-68. 10.1016/S0009-8981(97)06518-2 [DOI] [PubMed] [Google Scholar]
- 10.Morimoto K, Mori T, Ishiguro S, et al. Perioperative changes in plasma brain natriuretic peptide concentrations in patients undergoing cardiac surgery. Surg Today 1998;28:23-9. 10.1007/BF02483604 [DOI] [PubMed] [Google Scholar]
- 11.Hutfless R, Kazanegra R, Madani M, et al. Utility of b-type natriuretic peptide in predicting postoperative complications and outcomes in patients undergoing heart surgery. J Am Coll Cardiol 2004;43:1873-9. 10.1016/j.jacc.2003.12.048 [DOI] [PubMed] [Google Scholar]
- 12.Provenchère S, Berroeta C, Reynaud C, et al. Plasma brain natriuretic peptide and cardiac troponin i concentrations after adult cardiac surgery: Association with postoperative cardiac dysfunction and 1-year mortality. Crit Care Med 2006;34:995-1000. 10.1097/01.CCM.0000206110.94385.C4 [DOI] [PubMed] [Google Scholar]
- 13.Litton E, Ho KM. The use of pre-operative brain natriuretic peptides as a predictor of adverse outcomes after cardiac surgery: A systematic review and meta-analysis. Eur J Cardiothorac Surg 2012;41:525-34. 10.1093/ejcts/ezr007 [DOI] [PubMed] [Google Scholar]
- 14.Steadman CD, Ray S, Ng LL, McCann GP. Natriuretic peptides in common valvular heart disease. J Am Coll Cardiol 2010;55:2034-48. 10.1016/j.jacc.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 15.Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004;109:2302-8. 10.1161/01.CIR.0000126825.50903.18 [DOI] [PubMed] [Google Scholar]
- 16.Pedrazzini GB, Masson S, Latini R, et al. Comparison of brain natriuretic peptide plasma levels versus logistic EuroSCORE in predicting in-hospital and late postoperative mortality in patients undergoing aortic valve replacement for symptomatic aortic stenosis. Am J Cardiol 2008;102:749-54. 10.1016/j.amjcard.2008.04.055 [DOI] [PubMed] [Google Scholar]
- 17.Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004;44:2349-54. 10.1016/j.jacc.2004.09.038 [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz MB, Erbay AR, Balci M, et al. Atrial natriuretic peptide predicts impaired atrial remodeling and occurrence of late postoperative atrial fibrillation after surgery for symptomatic aortic stenosis. Cardiology 2006;105:207-12. 10.1159/000091641 [DOI] [PubMed] [Google Scholar]
- 19.Lurati Buse GA, Bolliger D, Seeberger E, et al. Troponin t and b-type natriuretic peptide after on-pump cardiac surgery: Prognostic impact on 12-month mortality and major cardiac events after adjustment for postoperative complications. Circulation 2014;130:948-57. 10.1161/CIRCULATIONAHA.113.007253 [DOI] [PubMed] [Google Scholar]
- 20.Ng LL, Geeranavar S, Jennings SC, et al. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond) 2004;106:129-33. 10.1042/CS20030234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as