Abstract
Background
Vitamin C is a well-known antioxidant and essential cofactor for numerous biological reactions. Several studies reported that vitamin C can improve the symptoms and prognosis of patients with sepsis and respiratory infection. We aimed to examine the effect of vitamin C when used in viral pneumonia patients with severe respiratory failure.
Methods
Total 201 patients with viral pneumonia were included, of them 35 patients used vitamin C. We performed a statistical analysis through a propensity score matching of the age and baseline characteristics of these patients.
Results
There were differences between the vitamin C group and non-vitamin C group in terms of age (60±15 vs. 66±14, P=0.03), extracorporeal membrane oxygenation (28.6% vs. 5.4%, P<0.001), and procalcitonin (3±8 vs. 9±23, P=0.02). The 28-day mortality was not different between the two groups (20.0% vs. 24.7%, P=0.33). In the propensity-matched group, the 28-day mortality was not significantly different between the two groups (20.0% vs. 37.1%, P=0.07). Moreover, no difference was observed in shock reversal within 14 days (45.7% vs. 25.7%, P=0.08) and recovery after acute kidney injury (52.9% vs. 66.7%, P=0.41) between the two groups. Vitamin C was not a prognostic factor for 28-day mortality (P=0.33).
Conclusions
In this study adjunctive intravenous vitamin C therapy alone was not associated with improvement of the 28-day mortality and prognosis in patients with severe viral pneumonia with respiratory failure.
Keywords: Acute respiratory distress syndrome (ARDS), ascorbic acid, critical care, viral pneumonia
Introduction
Severe viral pneumonia with respiratory failure requires intensive care unit (ICU) admission and is known to have a high mortality rate. The treatment of severe viral pneumonia remains a challenge to clinicians. The incidence of adult viral pneumonia determined via polymerase chain reaction (PCR) was 13.5–56.2% (1-4). In a previous study conducted in an ICU, 31.4% of the patients with severe community acquired pneumonia had viral pathogens (4).
Vitamin C has been known to help relieve and prevent symptoms of respiratory infection (5) for a long time, and several studies have reported the beneficial effects of vitamin C in the treatment of pneumonia (6) and sepsis (7). Previous studies suggested that vitamin C prevents the progression of sepsis to multiple organ failure, reduces the requirement for vasopressors, and improves patients’ outcomes. It also has a synergistic effect when used in combination with thiamine and corticosteroid (8).
Plasma concentrations of vitamin C were decreased in patients with critical illnesses including sepsis, and a previous study suggested the relationship between such a decrease and the development of multiple organ failure (9-11). Vitamin C levels were found to be lower in patients with tuberculosis and pneumonia (12). The incidence of pneumonia was decreased in the group taking vitamin C (13), and symptoms of pneumonia were improved and hospital stay was shorter (6,13,14). The effect of vitamin C on H1N1 virus-induced pneumonia in restraint-stressed mice has been studied; results showed improvements in survival rates and prolonged survival time of virus-infected stressed mice in a dose-dependent manner (15). Recent studies reported that a combination of vitamin C, hydrocortisone, and thiamine therapy decreased the mortality rate in patients with severe pneumonia (16). These findings suggest vitamin C can improve the prognosis of patients with viral pneumonia.
2019 Novel Coronavirus (2019-nCoV) is rapidly spreading around the world. However, there are currently no specific effective antiviral agents or drug combinations supported by high levels of evidence (17,18). Therefore, we decided to analyze whether the effect of vitamin C on viral pneumonia could support the treatment.
We examined those patients with viral pneumonia admitted in the ICU to determine the efficacy of vitamin C treatment with respiratory failure and investigate the prognosis of patients with this condition after receiving the vitamin C treatment.
Methods
Patients and study design
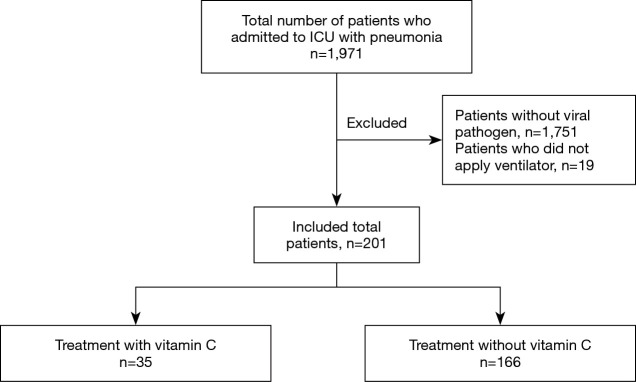
We reviewed the medical records of patients with viral pneumonia admitted to Asan Medical Center (Seoul, Republic of Korea) medical intensive care unit (MICU) from January 2015 to April 2017. Of 1,971 patients admitted to the MICU, 201 were included. Patients who were younger than 18 years, those without viral pathogens (n=1,751), and those who did not use a ventilator (n=19) were excluded (Figure 1).
Figure 1.
Flowchart of selected patients. ICU, intensive care unit.
Viral pneumonia is a lung infection caused by a virus identified by sputum culture and PCR. The study patients were administered with 2 g of intravenous vitamin C every 8 hours for 4 days or until ICU discharge. We decided to give 6 grams of vitamin C (divided into three equal doses) per day because intravenous vitamin C normalizes leukocyte vitamin C levels in respiratory infections at a dose of 6 g/day (19). In addition, vitamin C 6 g/day has been used recently with reference to other studies (13) (Table S1). The ICU admission and treatment data of eligible patients were obtained from hospital electronic medical records. The data included age, sex, body weight, hospital and ICU admission date, ICU admission diagnosis, site of admission, comorbidities, presence of immunosuppression, use of renal replacement therapy (RRT), hospital and ICU discharge date, date of death, Sepsis-related Organ Failure Assessment (SOFA) score (20), sputum and blood culture results, viral marker, laboratory data and radiologic results, usage of antibiotics and vitamin C, and outcomes. Continuous renal replacement therapy (CRRT) was initiated with the help of a nephrologist (21), and the initiation of extracorporeal membrane oxygenation (ECMO) was decided by two pulmonologists (22).
The primary outcome was 28-day mortality. The secondary outcomes included in-hospital mortality, ventilator-free days, recovery after acute kidney injury, shock reversal within 14 days, changes in the SOFA score within 3 days, and ICU and hospital lengths of stay.
Statistical analysis
To reduce the effect of selection bias and potential confounding factors in this observational study, we adjusted the age, malignancy, organ transplantation, procalcitonin, and CRRT with a P value <0.1 difference in baseline characteristics using propensity score matching.
After all the propensity score matches were performed, we compared the baseline covariates between the two groups. Continuous variables were compared using paired t-test or Wilcoxon signed-rank test, as appropriate, while categorical variables were compared using McNemar’s test. Statistical significance and the effect of treatment on outcomes were estimated using appropriate statistical methods for matched data. Survival curves were constructed with Kaplan-Meier estimates.
All reported P values were two sided, and P values of less than 0.05 were considered significant. SPSS, version 21 (IBM Corporation, Armonk, NY, USA), was used for statistical analysis.
Results
Patients’ characteristics
From January 2015 to April 2017, 1,971 patients with pneumonia were admitted in the ICU. Of them, 201 patients were included. Patients who were admitted pneumonia without vital pathogen (n=1,751) and those who did not use a ventilator (n=19) were excluded. Among the 201 patients included, 35 were using vitamin C (Figure 1).
The baseline characteristics of the study patients are shown in Table 1. Vitamin C group was younger (60±15 vs. 66±14, P=0.03), had lower procalcitonin level (3±8 vs. 9±23, P=0.02), and frequently used ECMO (28.6% vs. 5.4%, P<0.001). Patients in this group had slight differences in malignancy, organ transplantation, and frequency of CRRT but no statistical difference was found. There were no statistical differences in other baseline characteristics and laboratory data. There was no difference in the viral analysis except rhinovirus (28.6% vs. 14.5%, P=0.04) (Table S2).
Table 1. Baseline demographic, clinical characteristics and laboratory data of the unmatched patients.
| Characteristics | Total (n=201) | Vitamin C (n=35) | Non vitamin C (n=166) | P value |
|---|---|---|---|---|
| Age, years | 65±15 | 60±15 | 66±14 | 0.03 |
| Sex, male | 134 (66.7) | 27 (77.1) | 107 (64.5) | 0.15 |
| Comorbidities | ||||
| Diabetes | 50 (24.9) | 6 (17.1) | 44 (26.5) | 0.24 |
| Heart failure | 7 (3.5) | 1 (2.9) | 6 (3.6) | 0.82 |
| Chronic kidney disease | 9 (4.5) | 2 (5.7) | 7 (4.2) | 0.70 |
| Chronic lung disease | 50 (24.9) | 7 (20.0) | 43 (25.9) | 0.46 |
| Liver cirrhosis | 7 (3.5) | 2 (5.7) | 5 (3.0) | 0.43 |
| Stroke | 15 (7.5) | 2 (5.7) | 13 (7.8) | 0.67 |
| Malignancy | 24 (11.9) | 1 (2.9) | 23 (13.9) | 0.07 |
| Immunocompromised | 98 (48.8) | 18 (51.4) | 80 (48.2) | 0.73 |
| Organ transplantation | 7 (3.5) | 3 (8.6) | 4 (2.4) | 0.07 |
| Others | 48 (23.9) | 9 (25.7) | 39 (23.5) | 0.78 |
| WBC, /µL | 13,581±28,242 | 10,341±7,934 | 14,279±30,892 | 0.16 |
| CRP, mg/dL | 13±10 | 15±9 | 13±10 | 0.25 |
| Procalcitonin, ng/mL | 8±22 | 3±8 | 9±23 | 0.02 |
| Acute kidney injury | 80 (39.8) | 17 (48.6) | 63 (38.0) | 0.24 |
| CRRT | 71 (35.3) | 17 (48.6) | 54 (32.5) | 0.07 |
| Vasopressors | 130 (64.7) | 26 (74.3) | 104 (62.7) | 0.19 |
| Steroid | 132 (65.7) | 31 (88.6) | 130 (78.3) | 0.17 |
| ECMO | 19 (9.5) | 10 (28.6) | 9 (5.4) | <0.001 |
| APACHE scores | 27±9 | 25±9 | 27±9 | 0.26 |
| Day 1 SOFA scores | 9±4 | 10±4 | 9±4 | 0.66 |
Data are presented as mean ± standard deviation or number (%), unless otherwise indicated. WBC, white blood cell; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Outcomes and prognosis of the unmatched patients
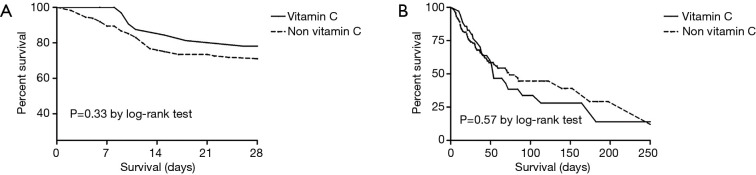
There was no significant difference between groups in terms of the 28-day mortality (20.0% vs. 24.7%, P=0.33) and in-hospital mortality (62.9% vs. 40.4%, P=0.57) (Table 2, Figure 2).
Table 2. Treatment outcomes and prognosis of the unmatched patients.
| Outcomes | Vitamin C (n=35) | Non vitamin C (n=166) | P value |
|---|---|---|---|
| 28-day mortality | 7 (20.0) | 41 (24.7) | 0.33 |
| In hospital mortality | 22 (62.9) | 67 (40.4) | 0.57 |
| Shock reversal within 14 days | 16 (45.7) | 54 (32.5) | 0.14 |
| Ventilator free days | 5±9 | 4±8 | 0.61 |
| ICU length of stay (days) | 34±32 | 17±17 | <0.001 |
| Hospital length of stay (days) | 65±57 | 43±42 | 0.04 |
| Changes in the SOFA score within 3 days | −0.8±2.3 | −0.5±2.4 | 0.53 |
| Recovery after acute kidney injury | 9 (52.9) | 30 (55.6) | 0.85 |
Data are presented as mean ± standard deviation or number (%), unless otherwise indicated. ICU, intensive care unit; SOFA, sequential organ failure assessment.
Figure 2.
Survival time between the unmatched group. (A) 28-day mortality; (B) in-hospital mortality.
ICU length of stay (LOS) (34±32 vs. 17±17 days, P<0.001) and hospital LOS (65±57 vs. 43±42 days, P=0.04) were longer in the vitamin C group. There was no significant difference between groups in terms of shock reversal within 14 days, ventilator-free days, changes in the SOFA score within 3 days, and recovery after AKI (Table 2).
Baseline characteristics of matched patients
Age, malignancy, organ transplantation, procalcitonin, and CRRT were used for propensity score matching. We compared the baseline characteristics of patients treated with vitamin C and those who did not use vitamin C. In the vitamin C group, male patients were more predominant (77.1% vs. 51.4%, P=0.03) and APACHE score was lower (25±9 vs. 30±10, P=0.03). No significant difference was observed in other baseline characteristics and laboratory data between the two groups (Table 3). There was no significant difference in the viral analysis (Table S3).
Table 3. Baseline demographic, clinical characteristics and laboratory data of the matched patients.
| Characteristics | Total | Vitamin C (n=35) | Non vitamin C (n=35) | P value |
|---|---|---|---|---|
| Age | 59±17 | 60±15 | 59±18 | 0.95 |
| Sex, male | 45 (64.3) | 27 (77.1) | 18 (51.4) | 0.03 |
| Comorbidities | ||||
| Diabetes | 15 (21.4) | 6 (17.1) | 9 (25.7) | 0.38 |
| Heart failure | 2 (2.9) | 1 (2.9) | 1 (2.9) | >0.99 |
| Chronic kidney disease | 4 (5.7) | 2 (5.7) | 2 (5.7) | >0.99 |
| Chronic lung disease | 18 (25.7) | 7 (20.0) | 11 (31.4) | 0.27 |
| Liver cirrhosis | 2 (2.9) | 2 (5.7) | 0 (0) | 0.49 |
| Stroke | 2 (2.9) | 2 (5.7) | 0 (0) | 0.49 |
| Malignancy | 2 (2.9) | 1 (2.9) | 1 (2.9) | >0.99 |
| Immunocompromised | 42 (60.0) | 18 (51.4) | 24 (68.6) | 0.14 |
| Organ transplantation | 6 (8.6) | 3 (8.6) | 3 (8.6) | >0.99 |
| Others | 20 (28.6) | 9 (25.7) | 11 (31.4) | 0.60 |
| WBC, /µL | 18,675±45,575 | 10,341±7,934 | 27,008±63,333 | 0.13 |
| CRP, mg/dL | 15±10 | 15±9 | 14±11 | 0.84 |
| Procalcitonin, ng/mL | 3±8 | 3±8 | 3±7 | 0.93 |
| Acute kidney injury | 35 (50.0) | 17 (48.6) | 18 (51.4) | 0.81 |
| CRRT | 33 (47.1) | 17 (48.6) | 16 (45.7) | 0.81 |
| Vasopressors | 47 (67.1) | 26 (74.3) | 21 (60.0) | 0.20 |
| Steroid | 59 (84.3) | 31 (88.6) | 28 (80.0) | 0.32 |
| ECMO | 14 (20.0) | 10 (28.6) | 4 (11.4) | 0.07 |
| APACHE scores | 27±10 | 25±9 | 30±10 | 0.03 |
| Day 1 SOFA scores | 10±4 | 10±4 | 10±4 | 0.46 |
Data are presented as mean ± standard deviation or number (%), unless otherwise indicated. WBC, white blood cell; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Outcomes and prognosis for the matched patients
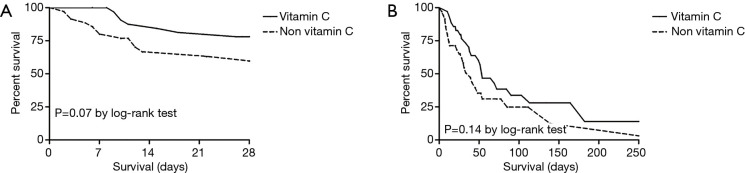
The 28-day mortality of the matched patients slightly decreased (20.0% vs. 37.1%, P=0.07) but it did not show a statistical significance. In-hospital mortality did not show a statistical significance (62.9% vs. 68.6%, P=0.14) (Table 4, Figure 3).
Table 4. Treatment outcomes and prognosis of the matched patients.
| Outcomes | Vitamin C (n=35) | Non vitamin C (n=35) | P value |
|---|---|---|---|
| 28-day mortality | 7 (20.0) | 13 (37.1) | 0.07 |
| In hospital mortality | 22 (62.9) | 24 (68.6) | 0.14 |
| Shock reversal within 14 days | 16 (45.7) | 9 (25.7) | 0.08 |
| Ventilator free days | 5±9 | 6±10 | 0.59 |
| ICU length of stay (days) | 34±32 | 17±14 | 0.005 |
| Hospital length of stay (days) | 65±57 | 44±53 | 0.12 |
| Changes in the SOFA score within 3 days | −0.8±2.3 | 0.0±2.9 | 0.21 |
| Recovery after acute kidney injury | 9 (52.9) | 12 (66.7) | 0.41 |
Data are presented as mean ± standard deviation or number (%), unless otherwise indicated. ICU, intensive care unit; SOFA, sequential organ failure assessment.
Figure 3.
Survival time between the matched group. (A) 28-day mortality; (B) in-hospital mortality.
ICU LOS (34±32 vs. 17±14 days, P=0.005) was still longer in the vitamin C group. However, no significant differences were observed in the hospital LOS, shock reversal within 14 days, changes in the SOFA score within 3 days, and recovery after AKI between the two groups (Table 4).
In the univariate Cox analysis, ventilator-free days [hazard ratio (HR) =0.91, 95% confidence interval (CI): 0.86–0.95, P<0.001], shock reversal within 14 days (HR =0.57, 95% CI: 0.29–1.09, P=0.09), and recovery from acute kidney injury (HR =2.85, 95% CI: 1.60–5.09, P<0.001) were prognostic factors for the 28-day mortality. Vitamin C was not a significant prognostic factor (P=0.33). In the multivariate Cox analysis, ventilator-free days (HR =0.91, 95% CI: 0.87–0.96, P=0.001) and recovery from acute kidney injury (HR =2.01, 95% CI: 1.12–3.63, P=0.02) were prognostic factors for 28-day mortality (Table 5).
Table 5. Prognostic factors for 28-day mortality in patients assessed using Cox proportional hazards model.
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 1.02 (1.00–1.04) | 0.13 | – | – | |
| Vasopressors | 1.57 (0.83–2.97) | 0.16 | – | – | |
| APACHE | 1.02 (0.99–1.06) | 0.24 | – | – | |
| SOFA scores | 1.06 (0.98–1.16) | 0.14 | – | – | |
| Diabetes | 1.13 (0.59–2.17) | 0.71 | – | – | |
| Malignancy | 1.55 (0.73–3.31) | 0.26 | – | – | |
| Immunocompromised | 1.44 (0.81–2.56) | 0.22 | – | – | |
| Organ transplantation | 1.17 (0.29–4.84) | 0.83 | – | – | |
| CRP | 1.01 (0.98–1.04) | 0.68 | – | – | |
| Procalcitonin | 1.00 (0.99–1.01) | 0.84 | – | – | |
| Vitamin C | 0.67 (0.30–1.50) | 0.33 | – | – | |
| Ventilator-free days | 0.91 (0.86–0.95) | <0.001 | 0.91 (0.87–0.96) | 0.001 | |
| Shock reversal within 14 days | 0.57 (0.29–1.09) | 0.09 | 0.89 (0.44–1.77) | 0.73 | |
| Recovery after acute kidney injury | 2.85 (1.60–5.09) | <0.001 | 2.01 (1.12–3.63) | 0.02 | |
Patients with P value <0.1 were included as multivariate models. APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment, CRP, C-reactive protein.
There was no significant difference between day 3 SOFA scores and day 7 SOFA scores at the time of ICU admission. After comparing the changes in the renal SOFA scores, there was no significant difference between the initial score and the day 3 and day 7 scores (Table S4).
Discussion
In this study, we evaluated the therapeutic efficacy of adjunctive intravenous vitamin C in severe viral pneumonia patients with respiratory failure. The intravenous administration of 6 g/day of vitamin C was not associated with decreasing in 28-day and in-hospital mortalities. In addition, there were no significant differences in shock reversal within 14 days, change in SOFA scores within 3 days, and recovery after AKI. We showed just trend to improve survival and shock reversal in limited patients.
Vitamin C is a well-known antioxidant and plays an important role in hormone production and immune response. Vitamin C plays a role in regulating immune cells, which increases the function of phagocytes and promotes the proliferation of T lymphocytes, which are important in bacterial and viral infections (14). Vitamin C has been found to be effective in killing bacteria (23,24), mycobacteria (25), HIV (26), and HCV (27) because it can generate free radicals and H2O2. As such, vitamin C plays a number of important roles in reducing oxidative stress caused by infection (13,28,29), balancing the immune system (14), and killing microorganisms by generating free radicals (23-27). For the reasons described above, we hypothesized that vitamin C would have positive effects in viral pneumonia. In our study, however, vitamin C did not have beneficial effects in patients with severe viral pneumonia with respiratory failure.
Vitamin C has been found to be effective in recovery and prevention from infection in animal experiments, and this effect is thought to be the same for humans (5,6,13). In some studies conducted in humans, vitamin C is also helpful for prevention and treatment of common colds, viral and bacterial infection (5,6,13,16,30,31). Hunt et al. found an 85% lower mortality in the vitamin C group compared with the placebo group. However, this comparison was made based on a small number of cases (six cases) (32). Mochalkin et al.’s study reported that the duration of recovery was reduced from 23.7 days in the control group to 4.6 days (19%) in the low-dose vitamin C group and 8.6 days (36%) in the high-dose vitamin C group (33). The use of vitamin C in restraint-stressed mice with H1N1 virus-induced pneumonia resulted in the improvement of survival rates and prolonged survival time (15). This finding suggests that vitamin C may be effective in improving the prognosis of patients with influenza. Fowler Iii et al. showed that administration of high-dose intravenous vitamin C into a patient with enterovirus/rhinovirus-induced acute respiratory distress syndrome (ARDS) was associated with rapid resolution of lung injury with no evidence of post-ARDS fibroproliferative sequelae (34). After influenza infection, bacterial pneumonia is likely to follow as a complication (35). It is thought that vitamin C can play a role in both bacterial and viral infections, so it is thought that it may be effective in preventing bacterial pneumonia and promoting prognosis in viral pneumonia. Kim et al. found that the use of red ginseng and vitamin C in influenza A infection increases immune cell activity and reduces lung inflammation (36). Recently, Kim et al. showed that the use of vitamin C, hydrocortisone, and thiamine in severe pneumonia resulted in the reduction of mortality (17% vs. 39%, P=0.04) and improvement in the chest radiologic findings (16). However, in our study, use of vitamin C in patients with severe viral pneumonia with respiratory failure did not showed the improvement of prognosis. In the vitamin C group, ECMO was frequently applied, and the group of patients who had ECMO was predicted to have high mortality (37), so we thought that this has influenced the results.
Moreover, shock reversal within 14 days and recovery after AKI did not improve in the vitamin C group. Several studies showed the beneficial effects the vitamin C in patients with sepsis and septic shock. Infusion with vitamin C resulted in an improvement in arteriolar responsiveness to hypotensive agents (38), improvement in endothelial and epithelial barrier function (39). In a phase I study investigating the safety of intravenous vitamin C in medical ICU patients with severe sepsis, Fowler et al. reported that the high-dose vitamin C group had a significant reduction in the daily SOFA score over 96 hours compared with the placebo group (11). As the study was performed only in a small number of patients (eight patients per group), this study failed to demonstrate the significant effect of vitamin C on 28-day mortality. Marik et al. reported that the use of a combination of vitamin C, hydrocortisone, and thiamine in patients with severe sepsis and septic shock reduced the hospital mortality rate by 32% (8). However, recent studies reported that vitamin C administration in patients with sepsis or septic shock did not improve their survival rate (40,41). Ahn et al. showed that adjunctive intravenous vitamin C therapy alone did not improve the hospital mortality rate associated with severe sepsis or septic shock (46% vs. 40%, P=0.62) (40). Shin et al. reported that a combination of vitamin C and thiamine in patients with septic shock did not improve the 28-day (16.6% vs. 17.5%, P=0.76) and in-hospital mortality (16.6% vs. 18.3%, P=0.55), respectively (41).
2019 Novel Coronavirus (2019-nCoV) is rapidly spreading around the world and was declared as a global concern (pandemic) by the World Health Organization (WHO). Since then, clinical trials have been conducted for various antiviral agent and vaccines, but there are no definite drugs that shown to be effective (17,18,42). Coronavirus infections can cause cytokine storms, which can increase oxidative stress and damage capillary endothelial cells (43,44). Vitamin C is a well-known antioxidant and reduces oxidative stress and improves in endothelial and epithelial barrier functions (39,45). The use of vitamin C for COVID-19 is being attempted (45,46) because it takes time to find effective vaccines and antiviral agents. The use of vitamin C 24 g/day for 7 days in severe COVID-19 pneumonia patients is going on trial (46).
Our study has several limitations. First, plasma levels of vitamin C were not measured. Intravenous administration of high-dose vitamin C to severe patients can lead to restoration of normal plasma levels of vitamin C. However, it remains unknown how much vitamin C initially decreased and how quickly it recovered. Second, the administration of vitamin C was based on the allocation of patients to a specific primary care physician. Third, the baseline characteristics of patients in each group were different; even after propensity matching, still some differences in sex and severity of illness were still observed. Hence, our study should be interpretated carefully. Fourth, vitamin C levels were not measured in this study, so we did not know exact levels. But we gave vitamin C intravenously 2 g q8hrs, so therapeutic levels could be maintained. Finally, our results were less persuasive due to the retrospective nature of the study and the small sample size. However, large, multicenter randomized controlled trial is not easy for these patients, and this retrospective study can help until these results accumulate.
Conclusions
In conclusion, adjunctive intravenous vitamin C therapy alone did not reduce 28-day ICU mortality and in-hospital mortality in patients with severe viral pneumonia with respiratory failure. Our negative results do not mean that there is no potential for vitamin C in viral pneumonia. We showed just trend to improve survival and shock reversal in limited patients.
So a well-controlled randomized trial is needed to determine whether vitamin C is effective against viral pneumonia.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The current study was approved by the institutional review board of Asan Medical Center (IRB No. 2018-0429). The requirement for informed consent is waived by the ethics review board due to the retrospective nature of the study.
Footnotes
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1306
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1306
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1306). The authors have no conflicts of interest to declare.
References
- 1.Cillóniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care 2011;15:R209. 10.1186/cc10444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 2008;63:42-8. 10.1136/thx.2006.075077 [DOI] [PubMed] [Google Scholar]
- 3.Johnstone J, Majumdar SR, Fox JD, et al. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008;134:1141-8. 10.1378/chest.08-0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SH, Hong SB, Ko GB, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012;186:325-32. 10.1164/rccm.201112-2240OC [DOI] [PubMed] [Google Scholar]
- 5.Hemilä H, Douglas RM. Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis 1999;3:756-61. [PubMed] [Google Scholar]
- 6.Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 2013;(8):CD005532. 10.1002/14651858.CD005532.pub3 [DOI] [PubMed] [Google Scholar]
- 7.Teng J, Pourmand A, Mazer-Amirshahi M., Vitamin C: The next step in sepsis management? J Crit Care 2018;43:230-4. 10.1016/j.jcrc.2017.09.031 [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229-38. 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 9.Schorah CJ, Downing C, Piripitsi A, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr 1996;63:760-5. 10.1093/ajcn/63.5.760 [DOI] [PubMed] [Google Scholar]
- 10.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996;24:392-7. 10.1097/00003246-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 11.Fowler AA, 3rd, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014;12:32. 10.1186/1479-5876-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakaev VV, Duntau AP. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int J Tuberc Lung Dis 2004;8:263-6. [PubMed] [Google Scholar]
- 13.Hemilä H. Vitamin C and Infections. Nutrients 2017;9:339. 10.3390/nu9040339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Li G. Is Vitamin C Beneficial to Patients with CAP? Curr Infect Dis Rep 2016;18:24. 10.1007/s11908-016-0530-0 [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Li YF, Tang LP, et al. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed Res Int 2015;2015:675149. 10.1155/2015/675149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J Crit Care 2018;47:211-8. 10.1016/j.jcrc.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Pang J, Wang MX, Ang IYH, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med 2020;9:623. 10.3390/jcm9030623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy S, Gomersall CD, Fowler RA. Care for Critically Ill Patients With COVID-19. JAMA 2020;323:1499-500. 10.1001/jama.2020.3633 [DOI] [PubMed] [Google Scholar]
- 19.Hume R, Weyers E. Changes in leucocyte ascorbic acid during the common cold. Scott Med J 1973;18:3-7. 10.1177/003693307301800102 [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 21.Gaudry S, Hajage D, Schortgen F, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 2016;375:122-33. 10.1056/NEJMoa1603017 [DOI] [PubMed] [Google Scholar]
- 22.Combes A, Brodie D, Chen YS, et al. The ICM research agenda on extracorporeal life support. Intensive Care Med 2017;43:1306-18. 10.1007/s00134-017-4803-3 [DOI] [PubMed] [Google Scholar]
- 23.Ghosh T, Srivastava SK, Gaurav A, et al. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl Environ Microbiol 2019;85:e02487-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du WN, Chen ST. Bactericidal Effects of Oxidative Stress Generated by EDTA-Fe and Hydrogen Peroxide. Biocontrol Sci 2019;24:97-101. 10.4265/bio.24.97 [DOI] [PubMed] [Google Scholar]
- 25.Pei Z, Wu K, Li Z, et al. Pharmacologic ascorbate as a pro-drug for hydrogen peroxide release to kill mycobacteria. Biomed Pharmacother 2019;109:2119-27. 10.1016/j.biopha.2018.11.078 [DOI] [PubMed] [Google Scholar]
- 26.Sakagami H, Asano K, Satoh K, et al. Anti-stress, anti-HIV and vitamin C-synergized radical scavenging activity of mulberry juice fractions. In Vivo 2007;21:499-505. [PubMed] [Google Scholar]
- 27.Yano M, Ikeda M, Abe K, et al. Oxidative stress induces anti-hepatitis C virus status via the activation of extracellular signal-regulated kinase. Hepatology 2009;50:678-88. 10.1002/hep.23026 [DOI] [PubMed] [Google Scholar]
- 28.Fukui H, Iwahashi H, Endoh S, et al. Ascorbic acid attenuates acute pulmonary oxidative stress and inflammation caused by zinc oxide nanoparticles. J Occup Health 2015;57:118-25. 10.1539/joh.14-0161-OA [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Luo G, Yuan J, et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm 2014;2014:426740. 10.1155/2014/426740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020;10:3044. 10.1038/s41598-020-59732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther 2018;189:63-70. 10.1016/j.pharmthera.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Hunt C, Chakravorty NK, Annan G, et al. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res 1994;64:212-9. [PubMed] [Google Scholar]
- 33.Mochalkin NI. Ascorbic acid in the complex therapy of acute pneumonia. Voen Med Zh 1970;9:17-21. [PubMed] [Google Scholar]
- 34.Fowler Iii AA, Kim C, Lepler L, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med 2017;6:85-90. 10.5492/wjccm.v6.i1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Loeches I, van Someren Gréve F, Schultz MJ. Bacterial pneumonia as an influenza complication. Curr Opin Infect Dis 2017;30:201-7. 10.1097/QCO.0000000000000347 [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Jang M, Kim Y, et al. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J Pharm Pharmacol 2016;68:406-20. 10.1111/jphp.12529 [DOI] [PubMed] [Google Scholar]
- 37.Sukhal S, Sethi J, Ganesh M, et al. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: A systematic review and meta-analysis. Ann Card Anaesth 2017;20:14-21. 10.4103/0971-9784.197820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou G, Kamenos G, Pendem S, et al. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol 2012;302:R409-16. 10.1152/ajpregu.00153.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Secor D, Li F, Ellis CG, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 2010;36:1928-34. 10.1007/s00134-010-1969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn JH, Oh DK, Huh JW, et al. Vitamin C alone does not improve treatment outcomes in mechanically ventilated patients with severe sepsis or septic shock: a retrospective cohort study. J Thorac Dis 2019;11:1562-70. 10.21037/jtd.2019.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin TG, Kim YJ, Ryoo SM, et al. Early Vitamin C and Thiamine Administration to Patients with Septic Shock in Emergency Departments: Propensity Score-Based Analysis of a Before-and-After Cohort Study. J Clin Med 2019;8:102. 10.3390/jcm8010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med 2020;28:198-211. [PubMed] [Google Scholar]
- 43.Meng L, Zhao X, Zhang H. HIPK1 Interference Attenuates Inflammation and Oxidative Stress of Acute Lung Injury via Autophagy. Med Sci Monit 2019;25:827-35. 10.12659/MSM.912507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care 2014;2:32. 10.1186/2052-0492-2-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng RZ. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov 2020;5:100028. 10.1016/j.medidd.2020.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care 2020;24:133. 10.1186/s13054-020-02851-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as