Abstract
Background
The advantages of prosthesis eversion method in patients diagnosed with Stanford type A acute aortic dissection (AAD) undergoing ascending aorta replacement (AAR) is unknown. This research is designed to explore it.
Methods
We retrospectively analyzed the data of a total of 283 patients diagnosed with type A aortic dissection that underwent surgery in Renmin Hospital of Wuhan University from March, 2006 to April, 2020. Eighty-eight patients underwent surgical repair with traditional continuous suture technique, and 195 patients received prosthesis eversion. Baseline data, intra-operative data and early-stage clinical results were collected and statistically analyzed.
Results
Baseline data were similar except for age, incidence of hyperlipidemia and taking ACEI/ARB drugs (P<0.05). Cardiopulmonary bypass time, cross-clamp time, circulation arrest time, hemostasis time and total operation time in the traditional method group were far longer than in the prothesis eversion group (P<0.01). The operative mortality was similar (P>0.01). Post-operatively, there was no statistically significant difference in the mean ventilation time, mortality, incidence of re-exploration, tracheostomy, paraplegia, long-term coma and stroke between the two groups (P>0.05). Patients in the traditional method group had a longer duration stay in ICU and hospital than patients in the prosthesis eversion group (P<0.05). Patients in the traditional method group received more red blood cells (RBC) (P<0.01), plasma (P<0.05), fibrinogen (P<0.01) and albumin (P<0.05) transfusions, and CoSeal™ surgical sealant (P<0.05) than patients in the prosthesis eversion group.
Conclusions
Our experience and statistical analysis showed prosthesis eversion method to have some advantage in reducing blood loss and improving clinical results compared with repair with continuous suture. This technique is both simple to learn and perform.
Keywords: Aortic dissection, anastomosis, clinical outcomes
Introduction
Stanford type A acute aortic dissection (AAD) is one of the most life-threatening disease. Surgery is by far the most effective therapy for type A AAD (1). Meanwhile, it presents severe risk of intra-operative trauma. The main risk factors contributing to it include large intra-operative hemorrhage, profound hypothermia, circulation arrest, and prolonged cardio-pulmonary bypass (CPB) (2).
Being the most complex part of the aorta, the aortic root has a unique configuration with a clover-like dilation of the valsalva sinuses, within which the aortic valve forms a three-pronged crown (3,4). A determining factor for a successful AAD surgery is a reliable proximal anastomosis between the sinusoidal junction and the aortic prosthesis (5). It is advised that the prosthesis is sutured evenly to the autologous aortic wall (5). It is advised that the prosthesis is uniformly sutured to the aortic wall (5). But this is difficult to do practically, especially in the presence of inflammatory edema, as it has a high risk of proximal anastomotic bleeding and high operative mortality.
In 1998, Pretre introduced a new suturing method in ascending aorta replacement (AAR). The most important feature of this method is a prosthesis eversion before and after suturing the prosthesis to the autologous aorta (6). Rignano et al. adopted this method to treat AAD patients (7). But since then, no study has investigated the advantage of this method over the traditional one, especially in AAD surgery. In this study, we aimed to compare the difference of the clinical outcomes between prosthesis eversion and traditional methods by retrospectively analyzing the data from our center. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2578).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University, Wuhan, China (NO. 2015-10-19 V1) and informed consent was taken from all the patients.
We retrospectively analyzed the data of a total of 283 patients diagnosed with Stanford type A AAD that were operated in Renmin Hospital of Wuhan University between March, 2006 and April, 2020. Before February, 2020, 88 patients were treated with traditional continuous suture technique. After February 2008, 195 patients were treated with prosthesis eversion method. Patients with genetic disorders like Marfan syndrome or L-D syndrome were excluded from this study. To avoid selection bias, all patients meeting inclusion criteria were included.
Anesthesia and surgical strategies
As we described in a previous study (8), all procedures were performed under general anesthesia and via a standard median sternotomy. CPB with bilateral antegrade cerebral perfusion was used for brain protection. Cannulation of the femoral artery was used for CPB. After CPB was initiated, the temperature gradually lowered. The lowest nasopharyngeal temperatures during circulatory arrest were between 20 to 22 °C.
Apart from this, total or hemi-arch replacement was performed depending on the aortic arch involvement. When the descending aorta was involved, the CRONUS® stented graft was placed in the appropriate segment of the descending thoracic aorta. Coronary artery bypass was performed when coronary artery was involved or coronary artery stenosis was diagnosed.
Platelet (0–6 u) and Coseal surgical sealant (0–4 mL) were used whenever available. Human fibrinogen was used when fibrinogen deficient is diagnosed.
Technical detail of sleeve anastomosis
Proximal ascending aorta anastomosis
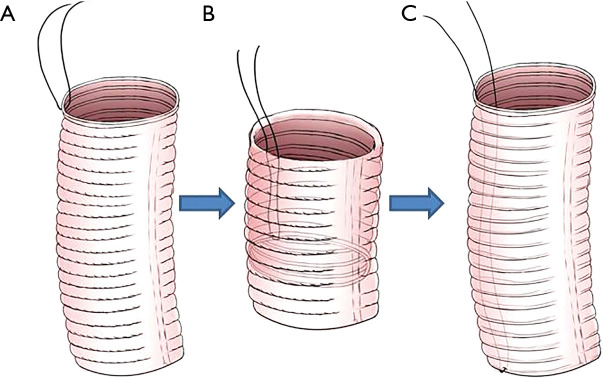
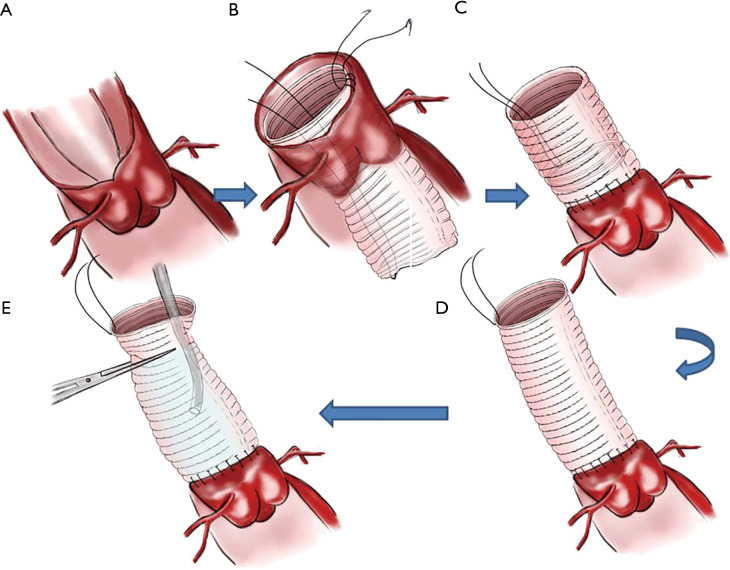
Preparation of the vascular prosthesis is illustrated in Figure 1. A suture line was fixed on one end (serves as distal end) in order to turn over the vascular prosthesis (Figure 1A,B). The vascular prosthesis was inserted into the left ventricle until the proximal end of the vascular prosthesis was aligned in parallel with the cut edge of the aortic root (Figure 1C). The aortic prosthesis and the aortic root stump were then sutured in continuous fashion (Figure 2A,B). We think two coils of the prothesis (about 3 mm) are the most suitable for the coaptation between the prothesis and the aortic wall. The fixed line was pulled to take out and turn over the aortic prothesis (Figure 2C,D). A rigid infusion tube was advanced into in the vascular prosthesis, and the entire structure was occluded temporarily with a straight clamp (Figure 2E). Finally, cardioplegic solution was administered through the tube until a pressure around 180±20 mmHg was reached. Anastomosis is strengthened in any area of cardioplegic solution leakage.
Figure 1.
Preparation of the aortic prosthesis.
Figure 2.
Proximal anastomosis.
Distal ascending aortic anastomosis
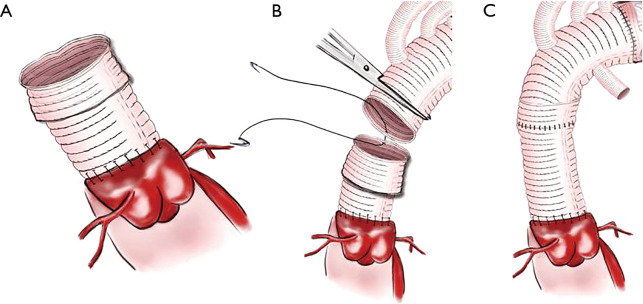
About 0.5 cm of the reconstructed ascending aortic prosthesis is folded externally to create a new sutural margin (Figure 3A). Subsequently, the ascending aortic prosthesis can now be connected with the proximal end of the four branched arch prosthesis (Figure 3B). Lastly, ascending aortic prosthesis’ end is then unfolded to compress the anastomosis (Figure 3C).
Figure 3.
Distal anastomosis.
Clinical information collection and statistical analysis
Baseline data, intra-operative and post-operative data were collected from medical records. Continuous variables were reported as means ± standard deviation or median with ranges and were compared with t-test. Categorical variables were compared using the Chi test and generalized Fisher’s exact test. A P value of <0.05 was considered statistically significant. Analyses were performed with SPSS 19.0.
Results
Baseline data
Baseline data is shown in Table 1. No significant difference of most of the baseline data existed between the two groups, except for age (44.9±16.6 vs. 48.9±12 years), incidence of hyperlipidemia (43/195 vs. 16/88) and taking ACEI/ARB drugs (43/195 vs. 32/88) (P<0.05).
Table 1. Baseline data. Continuous data were given as mean ± standard deviation.
| Variables | Prosthesis eversion method | Traditional method | P value |
|---|---|---|---|
| Number of patients | 195 | 88 | – |
| Age (years) | 44.9±16.6 | 48.9±12.9 | 0.0461# |
| Male | 106 (54.4%) | 51 (58.0%) | 0.6068 |
| BMI | 24.5±3.4 | 24.3±4.6 | 0.6832 |
| Previous cardiac surgery | 3 (1.5%) | 1 (1.1%) | 1.0000 |
| Waiting time (hours) | 28.6±20.5 | 30.9±24.1 | 0.4094 |
| Platelet count <10^9/L | 23 (11.8%) | 13 (14.8%) | 0.5635 |
| PT (seconds) | 13.4±2.6 | 12.9±3.1 | 0.1601 |
| APTT (seconds) | 36.4±5.6 | 37.2±5.7 | 0.2696 |
| INR | 1.41±0.31 | 1.36±0.26 | 0.1886 |
| Hypertension | 186 (95.4%) | 81 (92%) | 0.2741 |
| Diabetes mellitus | 31 (15.9%) | 21 (23.9%) | 0.1352 |
| Renal insufficiency | 21 (10.8%) | 4 (4.5%) | 0.1132 |
| NYHA III-IV | 13 (6.7%) | 4 (4.5%) | 0.5964 |
| EF <50% | 7 (3.6%) | 3 (3.4%) | 1.0000 |
| Hyperlipidemia | 61 (31.3%) | 16 (18.2%) | 0.0218# |
| Antiplatelet therapy | 19 (9.7%) | 16 (18.2%) | 0.0526 |
| Lipid-lowering drugs | 43 (22.1%) | 11 (12.5%) | 0.0719 |
| β-blocker | 187 (95.9%) | 81 (92%) | 0.2495 |
| ACEI/ARB | 43 (22.1%) | 32 (36.4%) | 0.0136# |
| Calaiumchannal blocker | 55 (28.2%) | 26 (29.5%) | 0.8871 |
#, indicates P<0.05. BMI, body mass index; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; EF, ejection fraction; ACEI, Angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers.
Intra-operative data
Intra-operative data is provided in Table 2, which showed no difference of surgical methods existed. But cardiopulmonary bypass time, cross-clamp time, circulation arrest time, hemostasis time and total operation time of patients in the traditional anastomosis group were far longer than in patients in the prosthesis eversion group (P<0.01). In addition, the latter received a much smaller amount of RBC and plasma transfusions (P<0.01). The operation mortality is 1/88 and 0/195 in the traditional method and prosthesis eversion group, respectively (P>0.05).
Table 2. Intra-operative data.
| Variables | Prosthesis eversion method | Traditional method | P value |
|---|---|---|---|
| Number of patients | 195 | 88 | – |
| Surgical method | |||
| Hemi arch replacement | 18 | 14 | 0.1083 |
| Total arch replacement | 177 | 74 | 0.1083 |
| Elephant trunk procedure | 164 | 68 | 0.1830 |
| Coronary artery bypass | 26 | 14 | 0.5830 |
| CPB procedure details | |||
| CPB time (min) | 162±23 | 176±19 | 0.0004* |
| Crossclamp time (min) | 53±8 | 56±7 | 0.0027* |
| Circulatory arrest time (min) | 27±5 | 29±6 | 0.0038* |
| Hemostasis time (min) | 146±21 | 171±26 | <0.0001* |
| Total operation time (min) | 266±56 | 312±51 | <0.0001* |
| Lowest core temperature (°C) | 19.2±0.8 | 19.3±0.9 | 0.3503 |
| Blood products expenditure | |||
| Red blood cells (mL) | 1,120±610 | 1,564±532 | <0.0001* |
| Plasma (mL) | 640±554 | 915±665 | 0.0003* |
| Human albumin (g) | 62±51 | 66±54 | 0.7646 |
| Hemostatic agents expenditure | |||
| Human fibrinogen injection (g) | 1.4±1.1 | 2.1±1.7 | <0.0001* |
| Platelet (U) | 2.7±1.6 | 2.9±1.9 | 0.36 |
| Coseal surgical sealant (mL) | 2.6±1.8 | 3.2±2.3 | 0.0183# |
| Operation mortality | 0 | 1 | 0.3110 |
Continuous data were given as mean ± standard deviation. #, indicates P<0.05 and *, indicates P<0.01. CPB, cardiopulmonary bypass.
Early-stage clinical results
Early-stage clinical results are given in Table 3. Ventilation time, post-operative mortality, incidence of re-exploration, tracheostomy, paraplegia, long-term coma and stroke were similar in the two groups (P>0.05). Patients in the traditional method group spent more time in ICU and in hospital than patients in the prosthesis eversion group (P<0.05). Patients in traditional method group accepted more RBC (P<0.01), plasma (P<0.05), human fibrinogen (P<0.01), human albumin (P<0.05) transfusion, and Coseal surgical sealant application (P<0.05) than patients in prosthesis eversion method group.
Table 3. Intra-operative data.
| Variables | Prosthesis eversion method | Traditional method | P value |
|---|---|---|---|
| Number of patients | 195 | 87 | – |
| Ventilation time (hours) | 142±115 | 157±128 | 0.3297 |
| ICU stay time (hours) | 172±81 | 196±92 | 0.0285# |
| In hospital time (days) | 22±11 | 26±14 | 0.0102# |
| Re-exploration | 6 | 7 | 0.1191 |
| Tracheostomy | 16 | 14 | 0.0595 |
| Blood products expenditure | |||
| Red blood cells (mL) | 615±511 | 822±482 | 0.0016* |
| Plasma (mL) | 981±722 | 1,211±882 | 0.0220# |
| Human albumin (g) | 158±114 | 192±106 | 0.0188# |
| Postoperative mortality | 2 | 3 | 0.1726 |
| Paraplegia | 2 | 1 | 1.0000 |
| Long term coma | 1 | 1 | 0.5226 |
| Stroke | 0 | 1 | 0.3085 |
Continuous data were given as mean ± standard deviation. #, indicates P<0.05; *, indicates P<0.01. ICU, intensive care unit.
Discussion
In this study, we confirmed that prosthesis eversion method is superior to the traditional suturing method characterized by end-to-end anastomosis in AAD treatment. Adequate exposure is crucial to any successful surgery (9). In AAR, the posterior semi-circle of the proximal anastomotic portion has the highest risk of bleeding. When prosthesis eversion method is applied, every part of the anastomotic edge is totally exposed to the surgeons, allowing them to apply sutures to the best of their abilities (10). This advantage was also noted in our experience during using this technique.
Since AAD patients mostly receive emergency surgeries, inflammatory edema is seen surrounding the aortic walls (11). This leads to an increased risk of anastomotic leakage. If bleeding occurs after resuming circulation, adding sutures makes hemorrhage only more severe rather than stopping it. The prothesis eversion method results in eversion, which can improve coaptation area between the prothesis and the aortic wall. It is also much safer to apply extra sutures in case of bleeding.
Our analysis showed prosthesis eversion method can reduce hemostasis time and blood loss, which is of utmost importance for patients undergoing AAR. In spite of homologous transfusion being quite effective treatment of intra-operative blood loss, its side effects can never be completely avoided (12,13). Furthermore, excessive blood loss followed by massive blood transfusion in cardiac surgery is independently associated with serious post-operative complications such as sepsis, acute respiratory distress syndrome, renal failure, and mortality (14-16). Our study also demonstrated that prosthesis eversion method can reduce ventilation time, duration of stay in ICU and in hospital. This may be at least partially attributed to the reduced blood loss and blood products transfusion.
Chronic aortic dissection and aortic aneurysm are not contraindications of the prosthesis eversion method. But hemostasis in case of chronic aortic dissection and aortic aneurysm is much easier than when AAD is diagnosed. Such patients were excluded to make the results of the two groups more comparable.
A major limitation of this study is lies in its retrospective design. So the recommendation yield from our conclusion is low. Prospective controlled studies are required to further verify the effectiveness of and safety of prothesis eversion method.
Conclusions
In conclusion, our experience and statistical analysis showed prosthesis eversion method to have an advantage in reducing blood loss and improving clinical results compared with repair using continuous suture technique. This technique is both simple to learn and perform.
Supplementary
The article’s supplementary files as
Acknowledgments
Doctor Bulat Abdrakhimov helped us in grammar checking.
Funding: This study was funded by National Natural Science Foundation of China [81570428, 81600367].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University, Wuhan, China (NO. 2015-10-19 V1) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2578
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2578
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2578). The authors have no conflicts of interest to declare.
References
- 1.Uchida K, Imoto K. Current status and future progress of surgical treatment for acute aortic dissection. Nihon Geka Gakkai Zasshi 2009;110:255-60. [PubMed] [Google Scholar]
- 2.Silaschi M, Byrne J, Wendler O. Aortic dissection: medical, interventional and surgical management. Heart 2017;103:78-87. 10.1136/heartjnl-2015-308284 [DOI] [PubMed] [Google Scholar]
- 3.Gaudino M, Piatti F, Lau C, et al. Aortic flow after valve sparing root replacement with or without neosinuses reconstruction. J Thorac Cardiovasc Surg 2019;157:455-65. 10.1016/j.jtcvs.2018.06.094 [DOI] [PubMed] [Google Scholar]
- 4.Loukas M, Bilinsky E, Bilinsky S, et al. The anatomy of the aortic root. Clin Anat 2014;27:748-56. 10.1002/ca.22295 [DOI] [PubMed] [Google Scholar]
- 5.Yang B, Norton EL, Hobbs R, et al. Short- and long-term outcomes of aortic root repair and replacement in patients undergoing acute type A aortic dissection repair: Twenty-year experience. J Thorac Cardiovasc Surg 2019;157:2125-36. 10.1016/j.jtcvs.2018.09.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretre R. Performance of a safe proximal anastomosis in aortic dissection. Ann Thorac Surg 1998;65:1798-9. 10.1016/S0003-4975(98)00214-8 [DOI] [PubMed] [Google Scholar]
- 7.Rignano A, Keller GC, Carmo M, et al. A new approach for proximal anastomosis in type "A" acute aortic dissection: prosthesis eversion. Ann Thorac Surg 2003;76:949-51. 10.1016/S0003-4975(03)00444-2 [DOI] [PubMed] [Google Scholar]
- 8.Wu HB, Zhang H, Wang ZW, et al. Surgery for acute aortic dissection using the Chinese CRONUS stented elephant trunk technique: experience with 252 patients. J Thorac Cardiovasc Surg 2014;148:2132-8. 10.1016/j.jtcvs.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Moriyama S, Kawasuji M. Lighting and surgical exposure with head lamp and optical loupes. Kyobu Geka 2009;62:633-7. [PubMed] [Google Scholar]
- 10.Hage F, Hage A, Gelinas J, et al. Aortic valve sparing and hybrid arch frozen elephant trunk repair for mega-aortic syndrome. Ann Cardiothorac Surg 2020;9:262-4. 10.21037/acs.2020.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Duan W, Xue Y, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg 2014;148:2995-3000. 10.1016/j.jtcvs.2014.07.068 [DOI] [PubMed] [Google Scholar]
- 12.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. 10.1001/jama.2010.1446 [DOI] [PubMed] [Google Scholar]
- 13.Boer C, Meesters MI, Milojevic M, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:88-120. 10.1053/j.jvca.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 14.Chen QH, Wang HL, Liu L, et al. Effects of restrictive red blood cell transfusion on the prognoses of adult patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Crit Care 2018;22:142. 10.1186/s13054-018-2062-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karkouti K, Wijeysundera DN, Beattie WS, et al. Variability and predictability of large-volume red blood cell transfusion in cardiac surgery: a multicenter study. Transfusion 2007;47:2081-8. 10.1111/j.1537-2995.2007.01432.x [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan H, Brookes MJ, Nevill AM, et al. Association Between Anemia and Blood Transfusion With Long-term Mortality After Cardiac Surgery. Ann Thorac Surg 2019;108:687-92. 10.1016/j.athoracsur.2019.04.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as