Abstract
Background
Previous studies have shown that reduced levels of lung function, characterized by forced expiratory volume in 1 second (FEV1), are associated with higher respiratory events and mortality in general population and some chronic lung diseases. Chronic pulmonary aspergillosis (CPA) is a destructive, fatal lung disease caused by Aspergillus infection in non-immunocompromised patients with suboptimal pulmonary function. However, there is limited information on the status and features of CPA according to FEV1.
Methods
We performed a retrospective observational study to investigate the FEV1 and airflow limitation in patients with CPA between March 2017 and February 2019 at a tertiary hospital in South Korea.
Results
Of the 144 CPA patients, 104 underwent spirometry, demonstrating median forced vital capacity (FVC) and FEV1 of 2.35 L (68%) and 1.43 L (62%), respectively. Among them, 56 patients had airflow limitation on PFT, with median FVC, and FEV1 of 2.47 L (73%) and 1.11 L (47%), respectively. Low body mass index (BMI) (20.1 vs. 22.1 kg/m2; P=0.011), breathlessness (60% vs. 20%; P=0.002), and bilateral pulmonary lesions (33.3% vs. 4%; P=0.006) were more common in patients with moderate to very severe airflow limitation than in those with normal to mild airflow limitation.
Conclusions
Moderate to very severe airflow limitation was observed in 43.3% of patients with CPA. Additionally, low BMI, breathlessness, and bilateral pulmonary lesions contributing to poor prognosis were more common in patients with moderate to very severe airflow limitation than in those with normal to mild airflow limitation. Our findings suggest that airflow limitation can be associated with the prognosis of CPA. Further investigations are needed to demonstrate the clinical significance of this association.
Keywords: Pulmonary aspergillosis, spirometry, pulmonary disease, chronic obstructive, prognosis
Introduction
Chronic pulmonary aspergillosis (CPA) is a progressively destructive pulmonary disease (1). CPA is usually seen in immunocompetent patients with underlying structural lung diseases, such as a tuberculous destroyed lung, emphysema, or bronchiectasis caused by Aspergillus infection (1). Treatment of CPA is difficult. Although some patients may consider surgical treatment, surgery is usually avoided due to reduced lung function and extensiveness of disease burden (2). Accordingly, antifungal therapy is considered the main treatment for disease control (3). However, anti-CPA chemotherapy response is often slow and disease progression is common in patients who discontinue pharmacotherapy (1,4). Thus, outcomes are generally poor because of insufficient defense mechanisms in patients as well as the aforementioned limitations in treatment (5). It has been shown that the mortality rate in patients with CPA can range from 50% to 80% over 5 years (6-9).
In connection with the prognosis of CPA, previous studies have been focused on host factors associated with high mortality risk. The following several factors have been identified: low body mass index (BMI), high Charlson index score, and previous history of systemic corticosteroids or pulmonary non-tuberculous mycobacterial disease (PNTM) (9-11).
Spirometry measures respiratory functions and is used in a well-established predictor of mortality in general population (12,13). Among parameters, forced expiratory volume in 1 second (FEV1) is an independent predictor of respiratory outcomes such as respiratory hospitalization and mortality in some chronic lung diseases (14,15). However, there are few studies about clinical characteristics of CPA patients concentrated on FEV1 and airflow limitation (16). Therefore, we aimed to investigate the status and features of airflow limitation and severity of airflow limitation in patients with CPA. We present the following article in accordance with the STROBE guidelines checklist (available at http://dx.doi.org/10.21037/jtd-20-1815).
Methods
Study population
Data were collected from consecutive patients with CPA who underwent a pulmonary function test (PFT) at Wonju Severance Christian Hospital (an 866-bed, university-affiliated, tertiary referral hospital in Wonju, South Korea) between March 2017 and February 2019 and were retrospectively analyzed. A total of 144 patients with newly diagnosed CPA was recruited. Among them, 40 patients were excluded without PFT results. Finally, 104 patients were included in the study and classified into normal (n=14), restrictive (n=34), and obstructive (n=56) patterns according to PFT. Additionally, we divided the patients with normal and obstructive patterns into two groups according to airflow limitation: patients with normal to mild airflow limitation (Group A, n=25) and those with moderate to very severe airflow limitation (Group B, n=45). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board for Human Research at Yonsei University Wonju Severance Christian Hospital (CR-319144) and individual consent for this retrospective analysis was waived.
Diagnosis of CPA
During the study period, diagnosis of CPA was based on clinical, radiologic, and microbiological findings (1). Such a diagnosis was associated with the following: (I) compatible chronic respiratory symptoms including at least cough, sputum, breathlessness, or hemoptysis; (II) compatible chest radiological findings including a cavitary lesion with evidence of paracavitary infiltrates, or expansion of cavity size over time; and (III) positive serum anti-Aspergillus antibodies (Aspergillus fumigatus IgG ELISA kit; IBL International, Hamburg, Germany) or positive cultures of Aspergillus species from a respiratory sample (i.e., sputum, transtracheal aspirate, or bronchial aspiration fluid). Simple aspergilloma was excluded from the diagnosis of CPA.
PFT
Spirometry was performed using a Vmax 22 apparatus (CareFusion, Yorba Linda, CA, USA) in accordance with American Thoracic Society/European Respiratory Society guidelines (17). Absolute values of forced vital capacity (FVC) and FEV1 were measured and percentage of predicted values for FVC and FEV1 were calculated using a reference equation obtained through a representative Korean sample (18). Normal spirometric pattern (NSP) was defined as FEV1/FVC ≥0.7 and FVC ≥80% predicted. Restrictive spirometric pattern (RSP) was defined as FEV1/FVC ≥0.7 and FVC <80% predicted. Obstructive spirometric pattern (OSP) or airflow limitation was defined as FEV1/FVC <0.7. Furthermore, we classified patients into mild (FEV1 ≥80), moderate (50≤ FEV1 <80), severe (30≤ FEV1 <50), and very severe (FEV1 <30) airflow limitation based on FEV1 (% predicted) (19,20). Patients with airflow limitation were considered to have chronic obstructive pulmonary disease (COPD) in the present study, even though we did not perform a post-bronchodilator test (21).
Data collection
The following data were collected from electronic health record including demographic data, comorbidities, respiratory or systemic symptoms, laboratory measurements, radiological findings including computed tomography (CT), and survival time. “Breathlessness” represents a modified Medical Research Council dyspnea score ≥2 (22).
Statistical analysis
The data are presented as medians and interquartile ranges for continuous variables and as numbers (percentages) for categorical variables. To compare categorical variables, the Chi-square or Fisher’s exact test was used. The Mann-Whitney U-test was used to compare continuous variables. Overall survival after diagnosis of CPA was estimated using the Kaplan-Meier method followed by the log-rank test for comparison between the two groups. The data were analyzed using SPSS version 23.0 (IBM Co., Chicago, IL, USA). We considered all variables with P<0.05 as statistically significant.
Results
Patient characteristics
The clinical characteristics of the eligible patients are presented in Table 1. The median age was 68 years, and 80% of the patients were male. The median BMI was 20 kg/m2. Seventy-six (73%) patients were current or ex-smokers. The median albumin was 3.8 g/dL. The underlying lung diseases included previous pulmonary tuberculosis (n=80, 77%), emphysema (n=35, 34%), and PNTM (n=17, 16%). The underlying extra-pulmonary diseases consisted of diabetes mellitus (n=16, 15%), chronic heart disease (n=16, 15%), and previous history of extra-thoracic malignancy (n=12, 12%).
Table 1. Patients characteristics.
| Characteristics | N=104 |
|---|---|
| Age, years | 68 [56–76] |
| Sex, male | 83 (79.8) |
| Body mass index, kg/m2 | 19.8 [17.2–22.5] |
| Smoking history | |
| Ex or current smoker | 76 (73.1) |
| Underlying lung disease* | |
| Previous history of pulmonary tuberculosis | 80 (76.9) |
| Pulmonary non-tuberculous mycobacterial disease | 17 (16.3) |
| Emphysema | 35 (33.7) |
| Bronchiectasis | 9 (8.7) |
| Interstitial lung disease | 3 (2.9) |
| Previous history of thoracic malignancy | 3 (2.9) |
| Other comorbidities* | |
| Diabetes mellitus | 16 (15.4) |
| Chronic heart disease | 16 (15.4) |
| Chronic liver disease | 9 (8.7) |
| Chronic kidney disease | 2 (1.9) |
| Rheumatic disease | 4 (3.8) |
| Previous history of extra-thoracic malignancy | 12 (11.5) |
| Chronic pulmonary symptoms* | |
| Cough | 46 (44.2) |
| Sputum | 41 (39.4) |
| Breathlessness† | 54 (51.9) |
| Hemoptysis | 36 (34.6) |
| Chest computed tomographic findings* | |
| Cavitation | 104 (100.0) |
| Consolidation | 21 (20.2) |
| Mycetoma | 43 (41.3) |
| Paracavitary infiltrates | 83 (79.8) |
| Bilateral pulmonary lesions | 23 (22.1) |
| Albumin, g/dL | 3.8 [3.2–4.1] |
The data are presented as median [interquartile change] or number (%). *, cases are duplicated; †, “Breathlessness” represents a modified Medical Research Council dyspnea score ≥2.
The most common respiratory symptoms were breathlessness (n=54, 52%), cough (n=46, 44%), sputum (n=41, 39%), and hemoptysis (n=36, 35%). The chest radiological findings revealed a cavitary lesion in all patients. Additionally, all patients possessed at least one of the following radiologic findings at presentation: consolidation (n=21, 20%), mycetoma (n=43, 41%), or paracavitary infiltration including pleural thickening (n=83, 80%). In addition, bilateral pulmonary lesions were observed in 23 (22%) patients.
Lung function tests
The PFT results are presented in Table 2. The median FVC and FEV1 were 2.35 L (68%) and 1.43 L (62%), respectively. Among the previously defined 56 (54%) patients with airflow limitation, the severity was classified as mild (n=11, 20%), moderate (n=15, 27%), severe (n=21, 38%), and very severe (n=9, 16%) according to FEV1. Among these patients, the median FEV1 values were 1.92 L (94%), 1.49 L (64%), 1.00 L (42%), and 0.60 L (24%), respectively. The PFT results of these patients with airflow limitation are additionally presented in Table 3.
Table 2. Pulmonary function test.
| Variables | N=104 |
|---|---|
| FVC, L | 2.35 [1.62–3.03] |
| FVC, % predicted | 68 [49–87] |
| FEV1, L | 1.43 [0.98–2.06] |
| FEV1, % predicted | 62 [42–87] |
| Spirometric pattern* | |
| Normal | 14 (13.5) |
| Restrictive | 34 (32.7) |
| Obstructive | 56 (53.8) |
The data are presented as median [interquartile change] or number (%). *, spirometric pattern was defined as follows: (I) normal spirometric pattern was defined as FEV1/FVC ≥0.7 and FVC ≥80% predicted; (II) restrictive spirometric pattern was defined as FEV1/FVC ≥0.7 and FVC <80% predicted; (III) obstructive spirometric pattern was defined as FEV1/FVC <0.7. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second.
Table 3. Pulmonary function test according to airflow limitation severity*.
| Variables | Airflow limitation† (N=56) | |||
|---|---|---|---|---|
| Mild (n=11) | Moderate (n=15) | Severe (n=21) | Very severe (n=9) | |
| FVC, L | 3.03 [2.58–3.86] | 2.99 [2.43–3.43] | 2.10 [1.48–2.78] | 1.61 [1.07–2.14] |
| FVC, % predicted | 105 [95–115] | 77 [74–89] | 55 [48–74] | 41 [32–59] |
| FEV1, L | 1.92 [1.59–2.43] | 1.49 [1.37–1.91] | 1.00 [0.82–1.11] | 0.60 [0.55–0.69] |
| FEV1, % predicted | 94 [88–108] | 64 [51–69] | 42 [35–47] | 24 [23–26] |
The data are presented as median [interquartile change]. *, airflow limitation severity was defined was follows: (I) mild airflow limitation was defined as FEV1/FVC <0.7 and FEV1 ≥80% predicted; (II) moderate airflow limitation was defined as FEV1/FVC <0.7 and 50% ≤ FEV1 <80% predicted; (III) severe airflow limitation was defined as FEV1/FVC <0.7 and 30%≤ FEV1 <50% predicted; (IV) very severe airflow limitation was defined as FEV1/FVC <0.7 and FEV1 <30% predicted; †, “Airflow limitation” was defined using the FEV1/FVC <0.7. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second.
Clinical characteristics according to airflow limitation
The clinical characteristics of the two groups (normal to mild vs. moderate to very severe airflow limitation) are described in Table 4. BMI was significantly lower in patients with moderate to very severe airflow limitation (Group B) compared to those with normal to mild airflow limitation (Group A) (20.1 vs. 22.1 kg/m2; P=0.011). Breathlessness was significantly higher in group B compared to group A (60% vs. 20%; P=0.002). Bilateral pulmonary infiltrates were also frequently observed in group B compared to group A (33.3% vs. 4%; P=0.006). On the other hand, the surgery was performed only in group A (48% vs. 0%; P=0.042). Apart from the above results, there were no differences in characteristics between the two groups including age, sex, smoking history, underlying lung disease, other comorbidities, chronic pulmonary symptoms, and antifungal therapy.
Table 4. Comparisons of clinical characteristics between the two groups.
| Characteristics | Normal to mild airflow limitation* (N=25) | Moderate to very severe airflow limitation† (N=45) | P value |
|---|---|---|---|
| Age, years | 74 [57–79] | 67 [56–72] | 0.122 |
| Sex, male | 21 (84.0) | 35 (77.8) | 0.756 |
| Body mass index, kg/m2 | 22.1 [19.6–24.6] | 20.1 [16.9–22.0] | 0.011 |
| Smoking history | |||
| Ex or current smoker | 18 (72.0) | 35 (77.8) | 0.772 |
| Underlying lung disease‡ | |||
| Previous history of pulmonary tuberculosis | 20 (80.0) | 35 (77.8) | >0.999 |
| Pulmonary non-tuberculous mycobacterial disease | 3 (12.0) | 7 (15.6) | >0.999 |
| Emphysema | 9 (36.0) | 20 (44.4) | 0.614 |
| Bronchiectasis | 2 (8.0) | 3 (6.7) | >0.999 |
| Interstitial lung disease | 1 (4.0) | 0 | 0.357 |
| Previous history of thoracic malignancy | 0 | 1 (2.2) | >0.999 |
| Other comorbidities‡ | |||
| Diabetes mellitus | 8 (32.0) | 5 (11.1) | 0.052 |
| Chronic heart disease | 3 (12.0) | 8 (17.8) | 0.735 |
| Chronic liver disease | 2 (8.0) | 5 (11.1) | >0.999 |
| Chronic kidney disease | 0 | 1 (2.2) | >0.999 |
| Rheumatic disease | 0 | 3 (6.7) | 0.548 |
| Previous history of extra-thoracic malignancy | 2 (8.0) | 4 (8.9) | >0.999 |
| Chronic pulmonary symptoms‡ | |||
| Cough | 10 (40.0) | 19 (42.2) | >0.999 |
| Sputum | 7 (28.0) | 21 (46.7) | 0.203 |
| Breathlessness§ | 5 (20.0) | 27 (60.0) | 0.002 |
| Hemoptysis | 12 (48.0) | 15 (33.3) | 0.306 |
| Chest computed tomographic findings‡ | |||
| Cavitation | 25 (100.0) | 45 (100.0) | NA |
| Consolidation | 6 (24.0) | 9 (20.0) | 0.765 |
| Mycetoma | 11 (44.0) | 16 (35.8) | 0.609 |
| Paracavitary infiltrates | 17 (68.0) | 37 (82.2) | 0.236 |
| Bilateral pulmonary lesions | 1 (4.0) | 15 (33.3) | 0.006 |
| Albumin, g/dL | 4.1 [3.5–4.5] | 3.8 [3.5–4.1] | 0.100 |
| Treatments | |||
| Antifungal therapy | 12 (48.0) | 23 (51.1) | >0.999 |
| Surgery | 3 (12.0) | 0 | 0.042 |
The data are presented as median [interquartile change] or number (%). *, normal to mild airflow limitation was defined as FEV1/FVC ≥0.7 and FVC ≥80% predicted, and FEV1/FVC <0.7 and FEV1 ≥80% predicted; †, moderate to very severe airflow limitation was defined as FEV1/FVC <0.7 and FEV1 <80% predicted; ‡, cases are duplicated; §, “Breathlessness” represents a modified Medical Research Council dyspnea score ≥2. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second.
Survival
The median follow-up period was 15 months (range, 8–26 months). The overall survival rates after diagnosis of CPA in the two groups are shown in Figure 1. Survival rates at 1 year and 1.5 years were 94.7% and 94.7%, respectively. Finally, the survival rate tended to be higher in patients with normal to mild airflow limitation (94.7%) than in those with moderate to very severe airflow limitation (86.5%, P=0.345).
Figure 1.
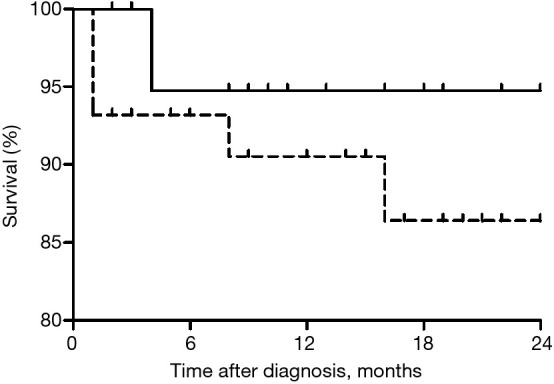
The Overall survival rate after the diagnosis of CPA in patients with normal to mild airflow limitation (solid line) and patients with moderate to very severe airflow limitation (dotted line) (P=0.345, log-rank test). Normal to mild airflow limitation was defined as FEV1/FVC ≥0.7 and FVC ≥80% predicted, and FEV1/FVC <0.7 and FEV1 ≥80% predicted. Moderate to very severe airflow limitation was defined as FEV1/FVC <0.7 and FEV1 <80% predicted. CPA, chronic pulmonary aspergillosis; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second.
Discussion
We investigated the clinical characteristics of patients with CPA with regard to airflow limitation extent as measured by FEV1. Fifty-six (54%) patients demonstrated airflow limitation on PFT, and more than half of them had severe to very severe airflow limitation. Additionally, we found that low BMI, breathlessness, and bilateral pulmonary lesions associated with poor prognosis are common in patients with moderate to very severe airflow limitation than in those with normal to mild airflow limitation.
CPA is a chronic pulmonary disease with a poor prognosis (22). The majority of CPA patients die from respiratory failure (23,24). However, surgery and pharmacotherapy are still challenging; a recent study comparing voriconazole and itraconazole failed to demonstrate improved survival (11).
Several studies have been conducted to identify host-related factors that may affect the overall survival rate at patients with CPA (9-11). In the present study, patients with moderate to very severe airflow limitation were likely to have low BMI, breathlessness, and bilateral lung involvement by Aspergillus infection. Past studies have confirmed that these factors are associated with poor prognosis (6,10).
The FEV1, which is an indicator of the level of airflow limitation, is associated with mortality at chronic lung diseases such as lung cancer and COPD (14,25). However, there was no data on the clinical aspects related to FEV1 impairment in CPA patients. In the present study, when the study population was classified based on FEV1, 43% of patients had Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade ≥2. When patient selection was restricted to those with airflow limitation, 80% of patients had a GOLD grade ≥2. In 2011, the prevalence of COPD in South Korea was 13.2%, and of the prevalence of mild, moderate, and severe COPD was 5.8%, 6.9%, and 0.5%, respectively (26). Compared to those results, the ratio of GOLD ≥3 grade in our results was significantly higher. The frequency of use of corticosteroids was increased in patients with GOLD ≥3 (27). Inhaled or systemic treatment with corticosteroids is one of the risk factors for CPA (28). This was thought to be due to accelerated structural changes caused by combined Aspergillus infection and impaired immune function. In addition, 33% of patients in this study demonstrated a RSP on PFT. Traditionally, a RSP was associated with interstitial lung disease and space-occupying lesions (29). A recent study comparing patients with and without a RSP reported poor prognosis in patients with a RSP (30). However, there has been no research on the effect of a RSP on prognosis in CPA patients.
There is even no specific mention of PFT examination at the time of diagnosis or during the follow-up period in the recently published clinical CPA guideline (1). Periodic PFT measurements for monitoring of disease may be considered in patients with substantially impaired pulmonary function due to underlying lung diseases and CPA itself. Physicians may also consider the use of bronchodilators, a fundamental treatment for patients with COPD due to the subsequent improvement in lung function, dyspnea, and health status (31,32). There are no studies on whether the usage of bronchodilators improves respiratory symptoms and prognosis in patients with CPA. However, Yum et al. has shown that the bronchodilator medication in the tuberculous destroyed lung, which is the most common structural lung disease in CPA, may achieve improved lung functions (33).
The current study had several limitations. First, this study was a retrospective analysis of patients from a single institution, and the sample size was small, so selection bias was inevitable. Second, not all patients underwent regular screening tests for CPA, mainly due to the low level of attention from respiratory physicians and the complexity of the diagnostic method. Therefore, some CPA cases might have been missed. Third, COPD was defined by a pre-bronchodilator test that may have misclassified some patients. However, all participants had structural lung disease, and more than half of them demonstrated severe or very severe airflow limitation. Thus, these patients likely had COPD. Finally, the prognosis of these patients could not be addressed in this study because of the small sample size and short duration of follow up. Further well-designed prognosis-related studies are needed.
In conclusion, we showed that 43.3% of the CPA patients had moderate to very severe airflow limitation in the present study. Moreover, there were several poor prognostic factors such as low BMI, breathlessness, and bilateral pulmonary lesions, which were more common in CPA patients with moderate to very severe airflow limitation than in those with normal to mild airflow limitation. This study suggests that physicians may be considered the assessment of airflow limitation severity as a more precise prediction of prognosis in the clinical course of CPA. Prospective studies are needed to confirm the correlation between lung function decline and prognosis in these patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board for Human Research at Yonsei University Wonju Severance Christian Hospital (CR-319144) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE guideline checklist, available at http://dx.doi.org/10.21037/jtd-20-1815
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1815
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1815). The authors have no conflicts of interest to declare.
References
- 1.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016;47:45-68. 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 2.Farid S, Mohamed S, Devbhandari M, et al. Results of surgery for chronic pulmonary Aspergillosis, optimal antifungal therapy and proposed high risk factors for recurrence--a National Centre's experience. J Cardiothorac Surg 2013;8:180. 10.1186/1749-8090-8-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson TF, Thompson GR, 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-e60. 10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongomin F, Harris C, Hayes G, et al. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS One 2018;13:e0193732. 10.1371/journal.pone.0193732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewi IMW, van de Veerdonk FL, Gresnigt MS. The Multifaceted Role of T-Helper Responses in Host Defense against Aspergillus fumigatus. J Fungi (Basel) 2017;3:55. 10.3390/jof3040055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamoto K, Takayanagi N, Kanauchi T, et al. Prognostic factors in 194 patients with chronic necrotizing pulmonary aspergillosis. Intern Med 2013;52:727-34. 10.2169/internalmedicine.52.9142 [DOI] [PubMed] [Google Scholar]
- 7.Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 2011;37:865-72. 10.1183/09031936.00054810 [DOI] [PubMed] [Google Scholar]
- 8.Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J 2019;53:1801184. 10.1183/13993003.01184-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito M, Kurahara Y, Yoshida S, et al. Prognosis of chronic pulmonary aspergillosis in patients with pulmonary non-tuberculous mycobacterial disease. Respir Investig 2018;56:326-31. 10.1016/j.resinv.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 10.Camara B, Reymond E, Saint-Raymond C, et al. Characteristics and outcomes of chronic pulmonary aspergillosis: a retrospective analysis of a tertiary hospital registry. Clin Respir J 2015;9:65-73. 10.1111/crj.12105 [DOI] [PubMed] [Google Scholar]
- 11.Tashiro M, Takazono T, Saijo T, et al. Selection of oral antifungals for initial maintenance therapy in chronic pulmonary aspergillosis: A longitudinal analysis. Clin Infect Dis 2020;70:835-42. [DOI] [PubMed] [Google Scholar]
- 12.Sabia S, Shipley M, Elbaz A, et al. Why does lung function predict mortality? Results from the Whitehall II Cohort Study. Am J Epidemiol 2010;172:1415-23. 10.1093/aje/kwq294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duong M, Islam S, Rangarajan S, et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health 2019;7:e613-e623. 10.1016/S2214-109X(19)30070-1 [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Song EM, Sim YS, et al. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J Thorac Oncol 2011;6:305-9. 10.1097/JTO.0b013e318201884b [DOI] [PubMed] [Google Scholar]
- 15.Schmidt SL, Nambiar AM, Tayob N, et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J 2011;38:176-83. 10.1183/09031936.00114010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barberán J, García-Pérez FJ, Villena V, et al. Development of Aspergillosis in a cohort of non-neutropenic, non-transplant patients colonised by Aspergillus spp. BMC Infect Dis 2017;17:34. 10.1186/s12879-016-2143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 18.Choi HS, Park YB, Yoon HK, et al. Validation of Previous Spirometric Reference Equations and New Equations. J Korean Med Sci 2019;34:e304. 10.3346/jkms.2019.34.e304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 20.Chung KS, Jung JY, Park MS, et al. Cut-off value of FEV1/FEV6 as a surrogate for FEV1/FVC for detecting airway obstruction in a Korean population. Int J Chron Obstruct Pulmon Dis 2016;11:1957-63. 10.2147/COPD.S113568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terzikhan N, Bos D, Lahousse L, et al. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J 2017;49:1602168. 10.1183/13993003.02168-2016 [DOI] [PubMed] [Google Scholar]
- 22.Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 2017;49:1601062. 10.1183/13993003.01062-2016 [DOI] [PubMed] [Google Scholar]
- 23.Nam HS, Jeon K, Um SW, et al. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis 2010;14:e479-82. 10.1016/j.ijid.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 24.Ohba H, Miwa S, Shirai M, et al. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med 2012;106:724-9. 10.1016/j.rmed.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 25.Leivseth L, Brumpton BM, Nilsen TI, et al. GOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, Norway. Thorax 2013;68:914-21. 10.1136/thoraxjnl-2013-203270 [DOI] [PubMed] [Google Scholar]
- 26.Hong JY, Jung JY, Lee MG, et al. Changes in the prevalence of COPD in Korea between 2001 and 2011 in the KNHANES data. Respir Med 2017;125:12-8. 10.1016/j.rmed.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 27.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018;391:1076-84. 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 28.Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J 2014;43:64-71. 10.1183/09031936.00162912 [DOI] [PubMed] [Google Scholar]
- 29.Backman H, Eriksson B, Hedman L, et al. Restrictive spirometric pattern in the general adult population: Methods of defining the condition and consequences on prevalence. Respir Med 2016;120:116-23. 10.1016/j.rmed.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest 2016;149:238-51. 10.1378/chest.15-1045 [DOI] [PubMed] [Google Scholar]
- 31.Maqsood U, Ho TN, Palmer K, et al. Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2019;3:CD012930. 10.1002/14651858.CD012930.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FJ, Fabbri LM, Ferguson GT, et al. Baseline Symptom Score Impact on Benefits of Glycopyrrolate/Formoterol Metered Dose Inhaler in COPD. Chest 2017;152:1169-78. 10.1016/j.chest.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Yum HK, Park IN. Effect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lung. Tuberc Respir Dis (Seoul) 2014;77:167-71. 10.4046/trd.2014.77.4.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


