Abstract
BACKGROUND:
Delirium in patients in intensive care units (ICUs) is an acute disturbance and fluctuation of cognition and consciousness. Though increasing age has been found to be related to ICU delirium, there is limited evidence of the effect of age on delirium outcomes. The aim of this study is to investigate the relationship between age categories and outcomes among ICU delirium patients.
METHODS:
Data were extracted from the electronic ICU (eICU) Collaborative Research Database with records from 3,931 patients with delirium. Patients were classified into non-aged (<65 years), young-old (65–74 years), middle-old (75–84 years), and very-old (≥85 years) groups. A Cox regression model was built to examine the role of age in death in ICU and in hospital after controlling covariates.
RESULTS:
The sample included 1,667 (42.4%) non-aged, 891 (22.7%) young-old, 848 (21.6%) middle-old, and 525 (13.3%) very-old patients. The ICU mortality rate was 8.3% and the hospital mortality rate was 15.4%. Compared with the non-aged group, the elderly patients (≥65 yeras) had higher mortality at ICU discharge (χ2=13.726, P=0.001) and hospital discharge (χ2=56.347, P<0.001). The Cox regression analysis showed that age was an independent risk factor for death at ICU discharge (hazard ratio [HR]=1.502, 1.675, 1.840, 95% confidence interval [CI] 1.138–1.983, 1.250–2.244, 1.260–2.687; P=0.004, 0.001, 0.002 for the young-, middle- and very-old group, respectively) as well as death at hospital discharge (HR=1.801, 2.036, 2.642, 95% CI 1.454–2.230, 1.638–2.530, 2.047–3.409; all P<0.001).
CONCLUSIONS:
The risks of death in the ICU and hospital increase with age among delirious patients.
Keywords: Intensive care, Delirium, Aging, Mortality
INTRODUCTION
Worldwide trends of increasing life expectancy have resulted in a surge in the elderly population together with the increasing proportion of elderly patients admitted to intensive care units (ICUs).[1] In the ICU, delirium is a syndrome manifesting as an acute disturbance and fluctuation of cognition and consciousness characterized by inattention and confusion.[2] It is estimated that delirium could occur in 13% to 66%[3-5] of the elderly patients admitted to ICUs, and delirium itself is a known risk factor for poor outcomes, including prolonged ICU and hospital stay,[5] and in-hospital mortality.[3,6]
The elderly have been found with brain frailty and less physiologic reserve accompanied by infection, surgery and medication, and thus older patients are more vulnerable to ICU stressors and have a poorer prognosis together with higher medical cost in ICU.[7-9] Unfortunately, although previous studies have found that age is an independent predictor for ICU delirium,[10] there is still a lack of literature addressing the role of aging on delirium outcomes. Despite the considerable studies focusing on risk factors for ICU delirium[10] and the clear impact of delirium on patient in-hospital mortality,[4,11] only a few studies have further explored the relationship between aging and outcomes with limited sample sizes and inconsistent findings. One study conducted in the cardiac ICU found that age interacted with sex was associated with patient prognosis,[12] whereas another study with groups (≥65 years) and very elderly (≥80 years) patients showed that the 30-day mortality of delirium patients was not increased significantly.[13]
Given the significance of age to ICU outcomes, the aim of this study is to investigate the relationship between age categories and in-hospital outcomes among delirium patients admitted to the ICU based on a multi-center database.
METHODS
This study was conducted in accordance with the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement.[14]
Study population and design
This was a retrospective cohort study and samples were extracted from a large multi-center research database (the eICU Collaborative Research Database) with data collected from 208 hospitals from 2014 to 2015 across the USA.[15] All data were deidentified to meet the safe harbor provision of the USA Health Insurance Portability and Accountability Act (HIPAA). The author had completed the required training course and was authorized to access the database for research aims.
Samples were consecutively enrolled from eICU database if they were: (1) ≥18 years old; (2) a record of delirium assessment after admitted to ICU; and (3) first-time ICU admission. The exclusion criteria were as follows: (1) ICU stay less than 24 hours; (2) delirium prior to ICU admission; and (3) coma during the entire ICU stay.
Measurement of ICU delirium
Delirium was assessed every eight or twelve hours per day and was considered and documented as delirium positive using the Confusion Assessment Method for the ICU (CAM-ICU).[16] The CAM-ICU is a reliable and validated tool for nurses to identify delirium in critical patients, and contains four parts including acute onset or fluctuating course, inattention, altered level of consciousness and disorganized thinking.[17] Delirium was defined as at least one documented delirium positive from the nursing chart during the whole ICU stay.[18]
Clinical characteristics and data extraction
Demographic characteristics (age, sex, and ethnicity), medical history (cognitive impairment, hypertension, diabetes, stroke), admission information (ICU type, urgent admission), Acute Physiology and Chronic Health Evaluation (APACHE) IV score, ICU condition (mechanical ventilation, metabolic acidosis, sepsis, acute respiratory distress syndrome [ARDS]), sedatives (dexmedetomidine, propofol and benzodiazepines), ventilation-free days during hospitalization, and status at both ICU and hospital discharge were extracted from the database. Minutes from ICU admission till discharge were retrieved and computed into days as the length of stay (LOS) in ICU and hospital. Due to the deidentified records of age over 89 in the database, patients were classified into non-aged (<65 years), young-old (65–74 years), middle-old (75–84 years) and very-old (≥85 years) groups. The primary outcomes were ICU and hospital mortalities, defined as the status of patient survival (expired or survived) at the time of ICU or hospital discharge. The secondary outcomes were the ventilation-free days and LOS in ICU and hospital.
Sample size
According to the sample size rules in Cox regression,[19] at least 1,460 samples were needed to achieve 90% power at 0.05 significance level adjusted for an anticipated mortality rate of 0.08. In this study, we enrolled 3,931 samples which were adequate for detecting differences between groups.
Statistical analysis
For descriptive statistics, mean with standard deviation (SD) for normally-distributed continuous variables, medians and interquartile ranges (IQR) for abnormally-distributed continuous variables, and counts and percentages for categorical variables were presented. The ICU and hospital mortality rates were compared using Chi-square test and Bonferroni test. One-way analysis of variance (ANOVA) and least significance difference (LSD) post-hoc test were performed to compare differences of LOS and ventilation-free days between age groups. Covariates were selected from sex, medical history, APACHE IV score, mechanical ventilation, metabolic acidosis, sepsis, ARDS and use of sedatives with a significant hazard ratio (HR) from the univariate Cox regression analysis. A multivariate Cox regression model was then built to investigate the independent effect of aging on ICU and hospital mortalities.
Only a few missing values were found in ethnicity (4.4%) and APACHE IV score (0.07%). Missing values in ethnicity were categorized as unknown, and multiple imputations were performed to replace the missing values in APACHE IV score. Data extraction and management were performed using PostgreSQL (version 10.0) and Navicat (version 12.0.18). SPSS software (version 24.0) was used for statistical analysis. Statistical significance was determined as a P-value <0.05.
RESULTS
Sample characteristics of patients with ICU delirium
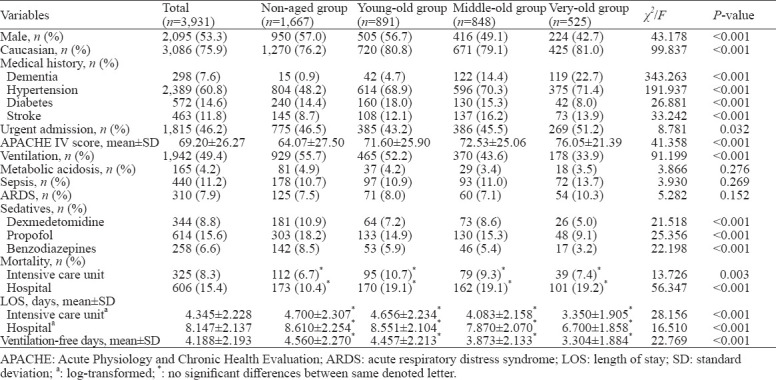
A total of 36,216 positive CAM-ICU records from 6,127 patients were screened and 3,931 patients from 44 hospitals were included according to the inclusion criteria. There were 1,667 (42.4%) non-aged, 891 (22.7%) young-old, 848 (21.6%) middle-old, and 525 (13.4%) very-old patients. The elderly patients (≥ 65 years) showed higher APACHE IV scores (72.98±24.66, P<0.001) compared with non-aged patients (63.07±27.50), together with more comorbidities but less use of mechanical ventilation (44.7% vs. 55.7%, P<0.001), propofol (13.7% vs. 18.2%, P<0.001), dexmedetomidine (7.2% vs. 10.9%, P<0.001), and benzodiazepines (5.1% vs. 8.5%, P<0.001). Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of ICU delirium patients in different age groups
Mortality rate and LOS in ICU and hospital
The ICU mortality rate was 8.3%, and the in-hospital mortality rate was 15.4%. Patients aged ≥65 years had significantly higher mortality rates in both ICU (χ2=9.157, P<0.001) and hospital (χ2=56.340, P<0.001) than the non-aged patients. The young-old group had the highest rate of ICU mortality (10.7%), while all of the three aged groups had a higher rate of hospital mortality, compared with the non-aged group. The ICU and hospitalization LOS were 4.03 (2.33–7.65) days, and 9.02 (5.63–15.15) days respectively. There were significant differences in ventilation-free days in the hospital as well as LOS in ICU and the hospital between age groups after log-normalization (Table 1).
Impact of age groups on patient outcomes
Univariate analysis showed that age group was an independent risk factor of ICU mortality (HR=1.69, 1.79, and 2.16 for young-old, middle-old, and very-old groups, respectively, P<0.001) and hospital mortality (HR=1.92, 2.12, and 2.91 for young-old, middle-old, and very-old groups, respectively, P<0.001) in the univariate Cox regression. The APACHE IV score, ventilation, and sepsis were found as independent risk factors for ICU and hospital mortalities, and use of dexmedetomidine was associated with decreased risk of ICU mortality and hospital mortality. Apart from the age group, all these variables were adjusted as covariates in the multivariate Cox regression model and details of the regression model are presented in Table 2. Survival curves in Figure 1A and Figure 1B also display the estimated survival probability for four different age groups of patients with delirium in ICU, showing the association between increased age groups and a higher risk of death both in ICU and hospital.
Table 2.
Cox regression model for mortality
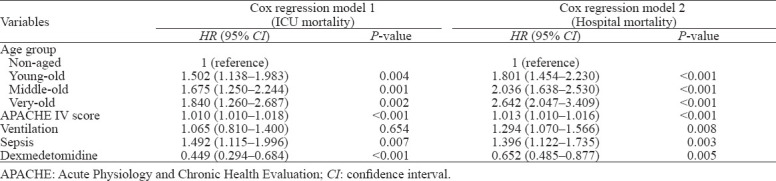
Figure 1.
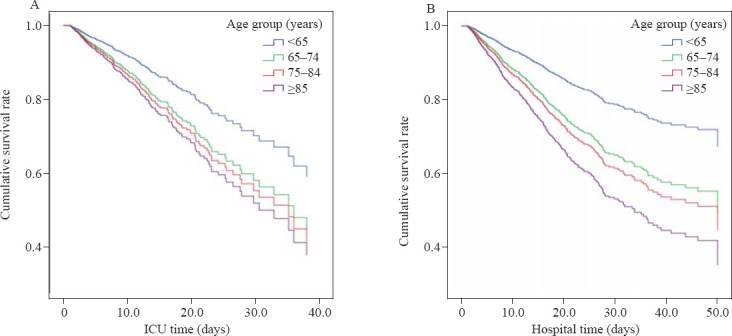
Association between age groups and mortality among ICU delirium patients, using ICU death (A) and hospital death (B) as primary events. The curves were adjusted for APACHE IV score, ventilation, sepsis and use of dexmedetomidine, and stratified by age groups.
DISCUSSION
ICU delirium is widely regarded as a major neurologic complication and seriously affects the prognosis of critical patients. In this large, multicenter cohort electronic database, aging was found to be an independent factor for poor prognosis of patients with delirium in ICU. The results showed that death in ICU and hospital increased with aging even after controlling the effects of critical conditions and sedatives. Although advanced age is a known predictor of delirium occurrence in ICU, to our knowledge this is the first study with a large dataset reporting specifically on the impact of different age groups on delirium patients’ outcomes to promote better stratified intervention.
Compared with non-aged patients, there were more females and Caucasians in the elderly groups (≥65 years), and patients in the elderly group had more comorbidities, including dementia, hypertension, diabetes, and history of stroke. Also, patients in the elderly groups had higher APACHE IV score at ICU admission, and more than half of the very-old patients (51.2%) were admitted emergently. This finding indicated that the condition of the elderly patients admitted to ICU was more serious.[20] However, there were no significant differences between age groups in the diagnosis of metabolic acidosis, sepsis, or ARDS. Concerning that all these syndromes are risk factors for ICU delirium,[10,21] the selection of delirious-specific population may have resulted in this finding.
The analysis showed that patients above 75 years received much less ventilation but fewer ventilation-free days during hospitalization, and the very-old patients received less treatment with sedatives, including dexmedetomidine, propofol, or benzodiazepines. This is in accordance with reported findings[22,23] indicating that above a certain age the patients or their surrogates are reluctant to use ventilation and prefer withholding invasive interventions in the clinical practice. But for those mechanically ventilated patients, they may need more time to achieve successful weaning and have prolonged ventilation duration.[24] This finding may be important for the decision process for the caregivers on how to allocate resources and develop ventilation plans.
The differences in outcomes were found in both mortalities and LOS in ICU and hospital. For the elderly, cognitive impairment is often combined with other age-related diseases, including chronic organ disease and senescence, indicating impaired physiologic and functional reserve.[25] Apart from the advancement of mortality rate,[26] critical patients with increased age were also found with more comorbidities and frailty in the early stage after ICU admission from other studies.[27,28] Thus all these predisposing factors could contribute to a worse prognosis of the elderly in ICU. The mortality rate in our study is not as poor as those from the previous studies conducted in the very elderly patients (≥90 years),[29] and this may be connected with the exclusion criteria that patients with persistent coma or stay in the ICU for less than 24 hours were excluded from this study.
Existing studies often refer to age as a single risk factor for delirium, but the old patient group is rarely studied as a singular group for clinical outcome analysis and the findings are inconsistent.[30,31] We found that aging was an independent risk factor for both ICU and hospital deaths even after controlling APACHE score and ICU treatment factors. This is in accordance with previous studies which have found that older patients (≥65 years) were susceptible to have physical and cognitive declines following critical illness,[32] sudden clinical deterioration with a change in goals of care,[33] and limitation of treatment.[34] However, in a cohort study of sepsis, age was only found as an independent mortality-associated risk factor in the patients older than 80 years.[34] The possible reason may be the differences in the population characteristics. The very-old patients had a larger number of admissions underlying the worse conditions, and we only included the first-time admitted patients for all age groups.
Noticeably, we found that the impact of aging remained after ICU discharge and got further enlarged on the prognosis during the patient’s entire hospitalization. This is in accordance with previous studies exploring outcomes induced by delirium. Atramont et al[35] found that aging was associated with an increased risk of long-term mortality with a remarkable risk among the very-old patients older than 80 years. Instead of temporary influences, delirium is related to a slower rate, and poor quality of recovery[36] and thus could trigger a poor outcome even after ICU discharge.
Although the pathophysiology of ICU delirium remains unclear, existing studies related to ICU delirium mechanism have found that factors indicating systematic inflammatory response, neuronal dysfunction and cortisol responses[37] are related to ICU delirium occurrence. Along with aging, endothelial function and cerebral perfusion get impaired,[38] the capacity of the brain to transmit signals and communicate reduces,[39] the hypothalamic-pituitary-adrenal (HPA) axis dysregulates,[40] and inflammatory response alters.[41] Therefore, it is possible that ICU delirium pathways are moderated by aging and related to death in ICU and hospital, as age-related vulnerability factors mentioned above have also been found in a relationship with in-hospital mortality.[42,43]
Another three independent risk factors for in-hospital death, including APACHE score, sepsis, and ventilation, were identified, and dexmedetomidine was found to be an independent protective factor of both ICU and hospital mortalities. This result is in accordance with findings from previous studies of delirium risk factors.[10] Elevated APACHE IV score indicates an association between critical condition and mortality, and APACHE score has been found as a risk factor for the prognosis of different critical populations in ICU.[44,45] Older patients who require prolonged ventilation have a higher risk of mortality and heavier burdens of treatment.[24,46] Also, for patients older than 70 years, they have been found to have an increased risk of death due to sepsis.[47] For dexmedetomidine as a short-acting intravenous anesthetic agent, it has been found to be associated with shorter duration of ventilation and less delirium.[48]
This study has several limitations. First, it was a retrospective study based on a large electronic database, so only delirium occurrence was available in the dataset instead of severity. Second, as the age of the very-old patients was documented as ≥85 years in the original dataset, we could only classify the age ranges and treat age as a categorical variable. Despite these limitations, the strength of this study was based on a multicenter collection of data and a large number of ICU delirium patients. Furthermore, we also controlled several covariates concerning patient conditions to prevent confounding. Therefore, this study identified age-stratified populations at a high risk of death among delirious patients and provided evidence for ICU physicians and nurses to make risk stratification of delirious patients and targeted care plans to allocate caregivers better.
CONCLUSIONS
Aging is independently associated with poor in-hospital outcomes of ICU delirium patients. Early identification of ICU delirium patients with risk of poor prognosis is of vital significance and could allow the focused application of limited ICU resources to improve patient survival. Further studies are needed to explore the underlying pathophysiological interactions between delirium and aging.
ACKNOWLEDGMENTS
We are grateful to Dr. Sheila Alexander for her advice to part of the study and the computer engineer Le Zhang for sharing syntax.
Footnotes
Funding: This study was supported by the Nursing Funding of Zhejiang University School of Medicine (2019[19]-3).
Ethical approval: Not applicable. The analysis was authorized by the eICU Collaborative Research.
Conflicts of interests: The authors have no conflicting interests in this study.
Author contributions: WG implemented the statistical analysis and drafted the manuscript. YPZ guided the statistical analysis and revised the manuscript. JFJ designed the study and substantively revised the manuscript.
REFERENCES
- 1.Lim JU, Lee J, Ha JH, Kang HH, Lee SH, Moon HS. Demographic changes in intensive care units in Korea over the last decade and outcomes of elderly patients:a single-center retrospective study. Korean J Crit Care Med. 2017;32(2):164–73. doi: 10.4266/kjccm.2016.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association DSM-Task Force Arlington VA US. Diagnostic and statistical manual of mental disorders:DSM-5™(5th ed.) Codas. 2013;25(2):191. doi: 10.1590/s2317-17822013000200017. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez D, Brennan K, Al Sayfe M, Shunker SA, Bogdanoski T, Hedges S, et al. Frailty, delirium and hospital mortality of older adults admitted to intensive care: the Delirium (Deli) in ICU study. Crit Care. 2020;24(1):609. doi: 10.1186/s13054-020-03318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs S, Bode L, Ernst J, Marquetand J, von Känel R, Böttger S. Delirium in elderly patients: prospective prevalence across hospital services. Gen Hosp Psychiatry. 2020;67:19–25. doi: 10.1016/j.genhosppsych.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Zhang L, Gong F, Ai Y. Incidence and risk factors for delirium in older patients following intensive care unit admission: a prospective observational study. J Nurs Res. 2020;28(4):e101. doi: 10.1097/jnr.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 6.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hübscher A, Isenmann S. Delirium: concepts, etiology, and clinical management. Fortschr Neurol Psychiatr. 2016;84(4):233–44. doi: 10.1055/s-0042-104502. [DOI] [PubMed] [Google Scholar]
- 9.Rengel KF, Pandharipande PP, Hughes CG. Special considerations for the aging brain and perioperative neurocognitive dysfunction. Anesthesiol Clin. 2019;37(3):521–36. doi: 10.1016/j.anclin.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Zaal IJ, Devlin JW, Peelen LM, Slooter AJC. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–7. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 11.Falsini G, Grotti S, Porto I, Toccafondi G, Fraticelli A, Angioli P, et al. Long-term prognostic value of delirium in elderly patients with acute cardiac diseases admitted to two cardiac intensive care units:a prospective study (DELIRIUM CORDIS) Eur Heart J Acute Cardiovasc Care. 2018;7(7):661–70. doi: 10.1177/2048872617695235. [DOI] [PubMed] [Google Scholar]
- 12.Serpytis P, Navickas P, Navickas A, Serpytis R, Navickas G, Glaveckaite S. Age- and gender-related peculiarities of patients with delirium in the cardiac intensive care unit. Kardiol Pol. 2017;75(10):1041–50. doi: 10.5603/KP.a2017.0122. [DOI] [PubMed] [Google Scholar]
- 13.Kotfis K, Szylińska A, Listewnik M, Strzelbicka M, Brykczyński M, Rotteret I, et al. Early delirium after cardiac surgery:an analysis of incidence and risk factors in elderly (≥65 years) and very elderly (≥80 years) patients. Clin Interv Aging. 2018;13:1061–70. doi: 10.2147/CIA.S166909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci Data. 2018;5:180178. doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients:validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients:validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Wassenaar A, van den Boogaard M, Schoonhoven L, Donders R, Pickkers P. Delirium prediction in the intensive care unit:comparison of two delirium prediction models. Crit Care. 2018;22(1):114. doi: 10.1186/s13054-018-2037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 20.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196(1):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atterton B, Paulino MC, Povoa P, Martin-Loeches I. Sepsis associated delirium. Medicina (Kaunas) 2020;56(5):240. doi: 10.3390/medicina56050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducos G, Mathe O, Balardy L, Lozano S, Murrek M, Ruiz J, et al. Influence of age on decision-making process to limit or withdraw life-sustaining treatment in the intensive care unit—a single center prospective observational study. J frailty aging. 2017;6(3):148–53. doi: 10.14283/jfa.2017.22. [DOI] [PubMed] [Google Scholar]
- 23.Vargas N, Tibullo L, Landi E, Carifi G, Pirone A, Pippo A, et al. Caring for critically ill oldest old patients:a clinical review. Aging Clin Exp Res. 2017;29(5):833–45. doi: 10.1007/s40520-016-0638-y. [DOI] [PubMed] [Google Scholar]
- 24.Nabozny MJ, Barnato AE, Rathouz PJ, Havlena JA, Kind AJ, Ehlenbach WJ, et al. Trajectories and prognosis of older patients who have prolonged mechanical ventilation after high-risk surgery. Crit Care Med. 2016;44(6):1091–7. doi: 10.1097/CCM.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murman DL. The impact of age on cognition. Semin Hear. 2015;36(3):111–21. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang JF, Li ZY, Zhang Y, Zhang W, Dong HS, Zhang Y, et al. Analysis of factors affecting the prognosis of ICU patients by multiple logistic regression model:A retrospective cohort study of 1299 patients in 12 consecutive years. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(7):602–7. doi: 10.3760/cma.j.issn.2095-4352.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Jiang L, Xi X. Early incidence and prognosis of ICU-acquired weakness in mechanical ventilation patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(7):821–6. doi: 10.3760/cma.j.issn.2095-4352.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes:a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–22. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker S, Müller J, de Heer G, Braune S, Fuhrmann V, Kluge S. Clinical characteristics and outcome of very elderly patients ≥90 years in intensive care: a retrospective observational study. Ann Intensive Care. 2015;5(1):53. doi: 10.1186/s13613-015-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fassier T, Duclos A, Abbas-Chorfa F, Couray-Targe S, West TE, Argaud L, et al. Elderly patients hospitalized in the ICU in France:a population-based study using secondary data from the national hospital discharge database. J Eval Clin Pract. 2016;22(3):378–86. doi: 10.1111/jep.12497. [DOI] [PubMed] [Google Scholar]
- 31.Santana-Cabrera L, Lorenzo-Torrent R, Sánchez-Palacios M, Martín Santana JD, Hernández Hernández., JR Influence of age in the duration of the stay and mortality of patients who remain in an intensive care unit for a prolonged time. Rev Clin Esp. 2014;214(2):74–8. doi: 10.1016/j.rce.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. 2015;43(6):1265–75. doi: 10.1097/CCM.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelfox HT, Bagshaw SM, Gao S. A retrospective cohort study of age-based differences in the care of hospitalized patients with sudden clinical deterioration. J Crit Care. 2015;30(5):1025–31. doi: 10.1016/j.jcrc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Loeches I, Guia MC, Vallecoccia MS, Suarez D, Ibarz M, Irazabal M, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis:a prospective, observational, multicenter cohort study. Ann Intensive Care. 2019;9(1):26. doi: 10.1186/s13613-019-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atramont A, Lindecker-Cournil V, Rudant J, Tajahmady A, Drewniak N, Fouard A, et al. Association of age with short-term and long-term mortality among patients discharged from intensive care units in France. JAMA Netw open. 2019;2(5):e193215. doi: 10.1001/jamanetworkopen.2019.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Michels M, Michelon C, Damásio D, Vitali AM, Ritter C, Dal-Pizzol F. Biomarker predictors of delirium in acutely ill patients: a systematic review. J Geriatr Psychiatry Neurol. 2019;32(3):119–36. doi: 10.1177/0891988719834346. [DOI] [PubMed] [Google Scholar]
- 38.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia:mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Hear Circ Physiol. 2017;312(1):H1–20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amarya S, Singh K, Sabharwal M. Ageing process and physiological changes in Gerontology. 2018. Available at https: //www.intechopen.com/books/gerontology/ageing-process-and-physiological-changes .
- 40.Gardner M, Lightman S, Kuh D, Comijs H, Deeg D, Gallacher J, et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: individual participant meta-analysis of five cohorts. Sci Rep. 2019;9(1):4555. doi: 10.1038/s41598-019-40566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Denis Alexander H, Ross OA. Age and age-related diseases:role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simons KS, van den Boogaard M, Hendriksen E, Gerretsen J, van der Hoeven JG, Pickkers P, et al. Temporal biomarker profiles and their association with ICU acquired delirium: a cohort study. Crit Care. 2018;22(1):137. doi: 10.1186/s13054-018-2054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan BA, Perkins AJ, Prasad NK, Shekhar A, Campbell NL, Gao S, et al. Biomarkers of delirium duration and delirium severity in the ICU. Crit Care Med. 2020;48(3):353–61. doi: 10.1097/CCM.0000000000004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuang ZS, Yang YL, Wei W, Wang JL, Long XY, Li KY, et al. Clinical characteristics and prognosis of community-acquired pneumonia in autoimmune disease-induced immunocompromised host:A retrospective observational study. World J Emerg Med. 2020;11(3):145–51. doi: 10.5847/wjem.j.1920-8642.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong C, Liu X, Tang Y, Huang Y, Fang H, Cheng Y, et al. Analysis of prognosis risk factors of critically ill patients after cardiac surgery:a consecutive 5-year retrospective study. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(7):873–7. doi: 10.3760/cma.j.issn.2095-4352.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Alonso A, Ebert AD, Kern R, Rapp S, Hennerici MG, Fatar M. Outcome predictors of acute stroke patients in need of intensive care treatment. Cerebrovasc Dis. 2015;40(1-2):10–7. doi: 10.1159/000430871. [DOI] [PubMed] [Google Scholar]
- 47.Kotfis K, Wittebole X, Jaschinski U, Solé-Violán J, Kashyap R, Leone M, et al. A worldwide perspective of sepsis epidemiology and survival according to age:observational data from the ICON audit. J Crit Care. 2019;51:122–32. doi: 10.1016/j.jcrc.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–54. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]


