Abstract
Background
A novel pathway incorporating faecal immunochemical testing (FIT) for rapid colorectal cancer diagnosis (RCCD) was introduced in 2017. This paper reports on the service evaluation after 2 years of pathway implementation.
Methods
The RCCD protocol was based on FIT, blood results and symptoms to stratify adult patients in primary care. Two-week-wait (2WW) investigation was indicated for patients with rectal bleeding, rectal mass and faecal haemoglobin (fHb) level of 10 µg Hb/g faeces or above or 4 µg Hb/g faeces or more in the presence of anaemia, low ferritin or thrombocytosis, in all other symptom groups. Patients with 100 µg Hb/g faeces or above had expedited investigation . A retrospective audit of colorectal cancer detected between 2017 and 2019 was conducted, fHb thresholds were reviewed and critically assessed for cancer diagnoses.
Results
In 2 years, 14788 FIT tests were dispatched with 13361 (90.4 per cent) completed returns. Overall, fHb was less than 4 µg Hb/g faeces in 9208 results (68.9 per cent), 4–9.9 µg Hb/g in 1583 (11.8 per cent), 10–99.9 µg Hb/g in 1850 (13.8 per cent) and 100 µg Hb/g faeces or above in 720 (5.4 per cent). During follow-up (median 10.4 months), 227 colorectal cancers were diagnosed. The cancer detection rate was 0.1 per cent in patients with fHb below 4 µg Hb/g faeces, 0.6 per cent in those with fHb 4–9.9 µg Hb/g faeces, 3.3 per cent for fHb 10–99.9 µg Hb/g faeces and 20.7 per cent for fHb 100 µg Hb/g faeces or above. The detection rate in the cohort with 10–19.9 µg Hb/g faeces was 1.4 per cent, below the National Institute for Health and Care Excellence threshold for urgent referral. The colorectal cancer rate in patients with fHb below 20 µg Hb/g faeces was less than 0.3 per cent.
Conclusion
Use of FIT to "rule out" urgent referral from primary care misses a small number of cases. The threshold for referral may be adjusted with blood results to improve stratification .
We present outcome data from a service evaluation that demonstrates clinical effectiveness and safety. This strategy may be crucial to overcoming the effects of the Coronavirus pandemic on 2WW and urgent pathways.
Resumen
Antecedentes
En 2017 se introdujo una vía novedosa que incorpora la prueba inmunoquímica fecal (faecal Immunochemical Testing, FIT) para un diagnóstico rápido de cáncer colorrectal (rapid colorectal cancer diagnosis, RCCD). Este trabajo presenta la evaluación de este nuevo método tras 2 años de implementación de la vía RCCD.
Métodos
El protocolo de RCCD se basó en el FIT, resultados del análisis de sangre y síntomas de derivación para estratificar a los pacientes adultos en Atención Primaria. El estudio en un periodo máximo de 2 semanas (2 week wait, 2WW) estaba indicado en pacientes con rectorragia, masa rectal y hemoglobina en heces (faecal hemoglobin, fHb) ≥ 10 µg Hb/g heces o ≥ 4 µg Hb/g heces en presencia de anemia, ferritina baja o trombocitosis, y en todos los demás grupos de síntomas. Los pacientes con fHb ≥ 100 µg Hb/g en heces fueron de “mayor riesgo” y se aceleró el proceso mediante contacto directo y estudio por parte de atención especializada. Se realizó una auditoría retrospectiva del cáncer colorrectal (colorectal cancer, CRC) detectado mediante RCCD entre 2017 y 2019 utilizando registros electrónicos y conjuntos de datos locales de cáncer. Los valores de corte de fHb se revisaron y evaluaron de forma crítica para el diagnóstico de CRC.
Resultados
Durante un periodo de 2 años, se enviaron 14.788 pruebas FIT con 13.361 (90,3%) devoluciones completadas. En conjunto, la fHb fue < 4 µg Hb/g en heces en 9.208 resultados (68,9%), 4-9,9 µg Hb/g en 1.583 pacientes (11,8%), 10-99,9 µg Hb/g en 1.850 (13,8%) y ≥ 100 µg Hb/g en heces en 720 (5,4%) pacientes. Durante el seguimiento (mediana de 10,4 meses), se diagnosticaron 227 CRC. La tasa de detección de CRC fue del 0,1% en pacientes con fHb < 4 µg Hb/g heces, 0,6% en pacientes con fHb 4-9,9 µg Hb/g heces, 3,5% en pacientes con fHb 10-99,9 µg Hb/g heces y 20,7 % en pacientes con fHb ≥100 µg Hb/g en heces. La tasa de detección de CRC en la cohorte de fHb 10-19,9 µg Hb/g en heces fue del 1,4%, por debajo del umbral del 3% de NICE para derivación urgente. La tasa global de CRC en pacientes con fHb <20 µg Hb/g en heces fue inferior < 0,3% durante el período de seguimiento.
Conclusión
Mediante el uso del FIT para "descartar" la derivación urgente desde Atención Primaria hay un pequeño número de casos que se pierde. El umbral de derivación se puede ajustar con los resultados del análisis de sangre fecal para mejorar el valor de estratificación.
Introduction
Colorectal cancer is a common cancer diagnosis with over 40 000 new diagnoses each year, and is the second commonest cause of cancer death in the UK1. Improving outcomes remains a key healthcare policy aim2. Current criteria for urgent referral to secondary care are largely based on age and symptoms3, but the UK bowel cancer screening programme (BCSP) has demonstrated that colorectal cancer, and particularly in its early stage, is often asymptomatic4. Faecal immunochemical testing (FIT) has replaced guaiac-based faecal occult blood testing in the screening programme across the UK. The thresholds for positivity in the screening programme in England (120 µg haemoglobin (Hb)/g faeces) and Wales (150 µg Hb/g faeces) are higher (less sensitive) than those in Scotland (80 µg Hb/g faeces or above) and many other countries around the world, and have been chosen to mitigate the demand on overburdened diagnostic capacity in the NHS5.
FIT has been shown to have value in patients with symptoms6–15, and in 2015 National Institute for Health and Care Excellence (NICE) guidance recommended testing for occult blood in faeces in low-risk patients3, and subsequently recommended a threshold of 10 µg Hb/g faeces specifically in this context16. In September 2016, a locally commissioned year-long pilot of FIT in the 2-week-wait (2WW) population (excluding those with rectal bleeding) was introduced, and demonstrated clear stratification value in all symptom groups judged to be ‘high risk’ by local primary care colleagues9. The value of simple measures such as stratification of anaemia17–19 and thrombocytosis20,21 from a full blood count (FBC) has been also confirmed in the same local population. In November 2017, a rapid colorectal cancer diagnosis (RCCD) pathway incorporating direct general practitioner (GP) access to FIT and the use of FIT, FBC and ferritin results for ‘rule in’, ‘rule out’ and ‘first test’ selection in secondary care was introduced11. This study aimed to evaluate the colorectal cancer diagnoses from the first 2 years of this pathway, stratified by FIT level22.
Methods
Rapid colorectal cancer diagnosis pathway
This local pathway was designed to incorporate FIT as a triage tool for all referral criteria in adult patients of any age, except those with rectal bleeding and rectal mass, as described elsewhere11,22, presenting to local GP practices within the authors’ catchment area. GPs were able to request FIT (and blood tests) independently and to act on the result, or, if clinical suspicion was high, they could submit an RCCD referral form contemporaneously. In the latter pathway, the form was held for 12 working days in a ‘window’ and the 62-day clock started only either on receipt of FIT (and blood) results or on expiry of the window. The outcomes from this pathway have been evaluated prospectively, and in June 2019 the window was no longer required after local agreement that GPs were familiar with the pathway and contemporaneous audit data supported this change (Appendix S1).
The pathway was commissioned locally to allow direct access to FIT for local GPs, and all four local clinical commissioning groups (CCGs) (Nottingham City, Nottingham North and East, Nottingham West and Rushcliffe) approved and jointly funded the pathway. The cost of each faecal immunochemical test was agreed as £17.50 (approximately €20) per sample to CCGs; this included postage, analysis and administration costs.
Faecal immunochemical testing requests and testing
FIT requests in primary care were made on an electronic request system (ICE) that also prompted requests for blood tests, where indicated. Results were notified on the same electronic system with text guidance on how to interpret results and subsequent actions. An electronic guidance system F12 (SystmOne; The Phoenix Partnership, Leeds, UK) was also used to guide GPs on the use of FIT and the new pathway in practices that used this system, with direct links to the relevant referral form where appropriate. FIT dispatch and return were entirely postal, and kits were analysed according to the manufacturer’s protocols as described elsewhere by an accredited BCSP hub laboratory (Appendix S2)9,11,22. The OC-Sensor™ platform (Eiken Chemical, Tokyo, Japan) was used as described previously9.
Patients referred with a rectal mass were not subject to FIT, but were seen in a one-stop flexible sigmoidoscopy clinic. Patients with rectal bleeding, no other symptoms and no anaemia were also seen in a one-stop clinic, as well as some patients with rectal bleeding deemed unlikely to be fit for colonoscopy at straight-to-test (STT) vetting of referrals23. Patients diagnosed with cancer in this one-stop pathway could have CT colonography as part of their staging to exclude synchronous lesions, if appropriate. This pathway has traditionally excluded rectal bleeding because the colorectal cancer detection rate approaches 10 per cent in this group locally, and the use of flexible sigmoidoscopy mitigates colonoscopy demand.
FIT, FBC and ferritin (or iron studies) were mandated for all other referrals, irrespective of symptoms or age, by local agreement with partners in primary care.
‘Rule in’
Between November 2017 and June 2019, patients with a FIT result of 150 µg Hb/g faeces or above were considered ‘high risk’ positive, and the result was notified directly by BCSP to the Nottingham Colorectal Service STT team, as well as to the GP, irrespective of whether an RCCD form had been submitted. The STT team contacted these patients directly for vetting and appropriate investigation on a ‘rapid’ pathway, according to local protocols. This threshold was lowered to 100 µg Hb/g faeces or above in June 2019 as prospective evaluation demonstrated significant colorectal cancer detection rates at this threshold. Patients with a faecal Hb (fHb) level of 10 µg Hb/g faeces or above or 4 µg Hb/g faeces or above in the presence of anaemia, low ferritin or thrombocytosis were also considered positive, and were investigated on a 2WW pathway.
‘Rule out’
Patients with a FIT result below 4 µg Hb/g faeces were considered to have a ‘negative’ FIT test result and to be at low risk for colorectal cancer. Patients with a FIT result of 4 µg Hb/g faeces or above but below 10 µg Hb/g faeces were also considered to have a negative result if their Hb level was normal (at least 130 g/l in men and 120 g/l in women), ferritin concentration was normal, and platelet count was less than 400 × 109/l). GPs were advised that patients with negative FIT tests had a low risk of colorectal cancer, and management options were to consider an alternative urgent pathway, routine referral, or repeat FIT.
Cohort and data collection
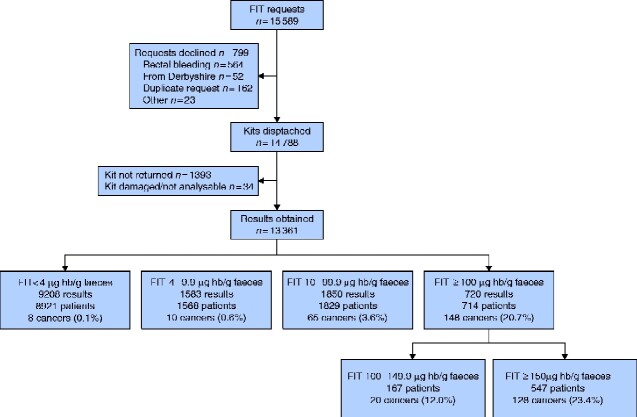
All patients who were subject to a FIT request between 7 November 2017 and 5 November 2019 were logged prospectively in the BCSP hub to ensure clinical governance of this novel pathway. All patients referred to the Nottingham Colorectal Service STT team on an RCCD form between these dates were logged prospectively in NUhCLEUS database (a locally created software programme), which supports the STT pathway. Cancer Outcomes and Services Data sets (COSD) were used to evaluate diagnoses of colorectal cancer recorded using ICD codes C18-C20 (excluding C18.1) with a censor date of 31 December 2019. Nottingham University Hospitals NHS Trust data, electronic patient records, and NUhCLEUS data were used for cross-checking and validation of diagnosis data. Cancer diagnoses were related to any previous patient episodes that started from a FIT result, and are presented in that context. Further details of patients who had repeat FIT testing are presented in Fig. 1 and Appendix S3.
Fig. 1.
Flow diagram of patients with faecal immunochemical testing requests from referral to colorectal cancer diagnosis
Numbers of patients lower than number of results in each stratum reflects repeat tests within one stratum. For additional data see Appendix S3. FIT, faecal immunochemical testing; Hb, haemoglobin.
Statistical analysis
Histograms were used to check for normal distribution. Comparisons were made between continuous variables using Student’s t test and ANOVA if normally distributed, with Tukey’s multiple comparison test for multiple groups. Categorical data were summarized using frequencies and percentages. Comparisons were made between categorical data using χ2 tests. All statistical analyses were performed using GraphPad Prism® (GraphPad Software, San Diego, CA, USA). Tests of significance were considered significant when P < 0.050 was obtained.
Data were segmented and analysed by fHb according to the cut-offs used during a pilot study9 and subsequent iterations of pathway, as described above and elsewhwere11,18. For the primary analyses, fHb below 4, 4–9.9, 10–99.9 and 100 µg Hb/g faeces or above were used. The latter group was further segmented into 100–149.9 and 150 µg Hb/g faeces or more (original cut-off for high-risk positivity). Further segmentation for subanalysis of results between 10 and 99.9 µg Hb/g faeces was chosen empirically as follows: 10–19.9, 20–39.9, 40–59.9, 60–79.9 and 80–99.9 µg Hb/g faeces.
Results
Faecal immunochemical test requests and results
In total 15 589 FIT requests were made during the evaluation period (Fig. 1). Some 564 requests (3.6 per cent) were rejected as clinical details mentioned rectal bleeding as a symptom. There were 162 duplicate requests (1.0 per cent) and 75 requests (0.5 per cent) were declined for other reasons. A total of 14788 kits were dispatched, and 13395 kits were returned within 14 days (90.6 per cent), 34 kits were spoiled on return or not suitable for analysis (0.2 per cent).
Overall, 13361 FIT results were available, of which 9208 (68.9 per cent) were less than 4 µg Hb/g faeces, 1583 (11.8 per cent) were 4–9.9 µg Hb/g faeces, 1850 (13.8 per cent) were 10–99.9 µg Hb/g faeces, and 720 (5.4 per cent) were 100 µg Hb/g faeces or above. Table 1 shows the patient demographic characteristics in each subgroup. The majority (67.8 per cent) of FIT occurred in symptomatic patients over the age of 60 years who met the current NICE guidance for referral3. Some 505 FIT results (3.8 per cent) were from patients under the age of 40 years and 81.6 per cent (412 of 505) yielded fHb below 4 µg Hb/g faeces. The mean age of patients with lower levels of fHb was significantly lower than that for the higher strata of fHb (P < 0.0001, ANOVA; P < 0.01, Tukey’s multiple comparison test). The cohort diagnosed with colorectal cancer was significantly older than those without (P < 0.001, unpaired t test). There were significantly more men in the cohort with fHb 100 µg Hb/g faeces or above compared with those with lower fHb levels (53.1 versus 43.5 per cent respectively; χ2 = 25.2, P < 0.001), and also in patients diagnosed with colorectal cancer compared with those without that diagnosis (59.5 versus 43.7 per cent; χ2 = 22.4, P < 0.001).
Table 1.
Demographics of all patients with a faecal immunochemical test result and colorectal cancer diagnoses stratified by faecal haemoglobin and age
| FIT stratum (µg Hb/g faeces) | Median follow-up (months) | Patients with FIT results | Sex ratio (M : F) | Age (years) |
|||
|---|---|---|---|---|---|---|---|
| Mean(s.e.m.) | ≤ 49 † | 50–59 † | ≥ 60 † ,‡ | ||||
| Total | 10.4 (5.7–16.3) | 13 042 | 5740 : 7302 | 66.3(0.2) | 1658 (12.7) | 2546 (19.5) | 8838 (67.8) |
| Total cancers diagnosed | 227 (1.7) | 135 : 92§ | 74.1(1.2)¶ | ||||
| < 4 | 10.6 (5.8–16.5) | 8920 | 3850 : 5070 | 64.5(0.1)¶ | 1329 (14.9) | 2003 (22.5) | 5588 (62.6) |
| Cancers diagnosed | 8 (0.1)§ | 6 : 2 | 77.4(3.1) | 0 (0) | 0 (0) | 8 (0.1) | |
| 4–9.9 | 10.6 (5.0–15.0) | 1568 | 656 : 912 | 69.0(0.3)¶ | 146 (9.3) | 224 (14.3) | 1198 (76.4) |
| Cancers diagnosed | 10 (0.6)§ | 5 : 5 | 75.0(3.6) | 1 (0.7) | 0 (0) | 9 (0.7) | |
| 10–99.9 | 9.9 (5.7–15.8) | 1840 | 855 : 985 | 71.4(0.3)¶ | 129 (7.0) | 234 (12.7) | 1477 (80.3) |
| Cancers diagnosed | 61 (3.3)§ | 34 : 27 | 74.6(1.4) | 1 (0.8) | 7 (3.0) | 53 (3.6) | |
| ≥ 100 | 10.3 (5.9–16.2) | 714 | 379 : 335§ | 71.5(0.5)¶ | 54 (7.6) | 85 (11.9) | 575 (80.5) |
| Cancers diagnosed | 148 (20.7)§ | 90 : 58 | 73.7(0.9) | 7(13) | 14 (16) | 127 (22.1) | |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.);
percentage values are of stratum.
Patients aged 60 years or more with symptoms commonly eligible for urgent referral according to National Institute for Health and Care Excellence guidance3. FIT, faecal immunochemical test; Hb, haemoglobin.
P < 0.001 (χ2 tests comparing FIT strata by gender, and colorectal cancer diagnosis by gender);
P< 0.001 (ANOVA comparing patient age by FIT strata).
Colorectal cancer diagnoses after faecal immunochemical testing
The median follow-up in this cohort was 10.4 (i.q.r. 5.7–16.3) months (Table 1). In total, 227 colorectal cancers were diagnosed after FIT (1.7 per cent of the 13 361 tests performed). Eight cancers were diagnosed in 8920 patients (0.1 per cent) after a FIT result with fHb below 4 µg Hb/g faeces during follow-up, 10 cancers in 1568 patients (0.6 per cent) with fHb 4–9.9 µg Hb/g faeces, 61 in 1840 patients (3.3 per cent) with fHb 10–99.9 µg Hb/g faeces, and 148 cancers (20.7 per cent) in 714 patients with fHb 100 µg Hb/g faeces or above. The known colorectal cancer detection rates were significantly lower in the group with fHb below 4 µg Hb/g faeces than in the rest of the cohort (0.1 versus 5.3 per cent respectively; χ2 = 449.7, P < 0.001) and in the group with fHb below 10 µg Hb/g faeces (0.2 versus 8.2 per cent respectively; χ2 = 770.8, P < 0.001). The known colorectal cancer detection rate was significantly higher in the cohort with fHb 100 µg Hb/g faeces or above, compared to less those with fHb less than 100 µg Hb/g faeces (20.7 per cent versus 0.6 per cent; χ2 = 1592.4, P < 0.001). Three diagnoses of colorectal cancer were related to repeat FIT in 229 patients with sequential fHb results in different strata (Appendix S3).
Overall, 86.8 per cent (197 of 227) of colorectal cancers were diagnosed in patients over the age of 60 years. One patient aged under 40 years was diagnosed with cancer with a fHb result of 100 µg Hb/g faeces or above, and 29 cancers were diagnosed in those aged 40–59 years.
Colorectal cancer diagnoses after a negative test result
Eight of 227 CRCs (3.5 per cent) were diagnosed after a fHb reported as less than 4 µg Hb/g faeces (Table S1). These patients were identified via referral through other pathways: three had CT arranged by the upper gastrointestinal team, two were seen by the medical gastroenterology team, two were diagnosed in routine colorectal clinics, and one presented as an emergency with acute bowel obstruction. The median time from a negative FIT result to colorectal cancer diagnosis was 41.5 (i.q.r. 31–72.3) days. One sample was analysed after 17 days, and the patient should have had repeat FIT due to risk of a false-negative result24. In the population with fHb 4–9.9 µg Hb/g faeces, all patients but one satisfied the Nottingham protocol whereby an abnormal blood parameter (anaemia, thrombocytosis or low ferritin (or iron)) reduces the threshold to investigate to 4 µg Hb/g faeces (Table S1). The other patient had an abnormally high ferritin level but was considered ‘negative’ according to local protocol at the time of testing.
Blood results and palpable rectal mass
Detection rates for different strata of fHb within the range 10–99.9 µg Hb/g faeces are shown in Table 2. The colorectal cancer detection rate in the group with fHb 10–19.9 µg Hb/g faeces was 1.4 per cent, and was below the NICE 3 per cent threshold for urgent referral. These patients were all eligible for 2WW referral and investigation in the local protocol. Eight of 10 colorectal cancers detected in this stratum had abnormal blood parameters or abnormal digital rectal examination before referral (Table S1). Forty-seven of 61 patients with cancer (77 per cent) detected after fHb result of 10–99.9 µg Hb/g faeces had one or more abnormal blood test results or a palpable rectal mass (latter not mentioned on referral from primary care). Six cancers were detected in 11 194 patients with fHb below 20 µg Hb/g faeces in whom there was no evidence of abnormal blood results or palpable rectal mass.
Table 2.
Colorectal cancers diagnosed in patients stratified by faecal haemoglobin result and detection rates above and below each lower limit
| FIT stratum (µg Hb/g faeces) | Patients with FIT results in stratum | Cancer diagnoses | Cancer detection rate within stratum (%) | Cancer miss rate below lower limit of stratum (%) | Cancer detection rate above lower limit of stratum (%) |
|---|---|---|---|---|---|
| < 10 | 10 488 | 18 | 0.2 | ||
| 10–19.9 | 706 | 10 | 1.4 | 0.2 | 8.2 |
| 20–39.9 | 543 | 22 | 4.1 | 0.3 | 10.8 |
| 40–59.9 | 303 | 8 | 2.6 | 0.4 | 13.6 |
| 60–79.9 | 168 | 13 | 7.7 | 0.5 | 16.9 |
| 80–99.9 | 120 | 8 | 6.7 | 0.6 | 18.7 |
| ≥ 100 | 714 | 148 | 20.7 | 0.6 | 20.7 |
FIT, faecal immunochemical test. This study used faecal immunochemical testing and simple blood test results to stratify the risk of colorectal cancer in symptomatic patients for over 2 years. Outcome data from a service evaluation demonstrated clinical effectiveness and safety. This strategy may be crucial to overcoming the effects of the coronavirus pandemic on 2‐week‐wait and urgent pathways.
Discussion
This is a large English data set on primary care access to FIT in symptomatic patients for all symptoms and all age groups. In previous studies, a colorectal cancer diagnosis rate of 0.2 per cent in patients undergoing 2WW investigation with fHb below 4 µg Hb/g faeces was documented9,11,22. Data from Scotland suggest a similar ‘miss rate’ with longer follow-up in patients with unquantifiable fHb levels on a different manufacturer’s platform10. The colorectal cancer diagnosis rate of 0.1 per cent after a ‘negative’ FIT test (as per local definition) in this population is consistent with these data, and appears well below the NICE threshold of 3 per cent, despite including the NG12 ‘high risk’ population3. Other strengths of this study include a large data set with optimal return rates and a ‘real-world’ analysis of FIT usage in a clinical setting. Use of a prospectively recorded database to log cancer diagnoses validates the accuracy of the retrospective study.
In evaluating this pathway, it is important to stress that FIT should not be compared to colonoscopy, because, in the UK and many other countries, GPs do not have direct access to colonoscopy. Instead, 2WW pathways that use FIT in primary care should be compared with those that do not. Indeed, the ‘miss rate’ of age- and symptom-based criteria in primary care (the number of cancers detected in symptomatic patients after routine referral) was historically around 50 per cent on average21, and even a standard investigation such as colonoscopy is known to miss diagnoses of colorectal cancer25.
Overall, 5588 patients aged 60 years or more with fHb below 4 µg Hb/g faeces were tested by GPs. This would have equated to more than 230 additional referrals per month over 2 years (if FIT had not been used for ‘rule out’) to detect eight colorectal cancers.
However, this methodology does not identify patients diagnosed with colorectal cancer at other trusts, which is a relative weakness. Concomitant analysis across the Cancer Alliance is ongoing, and analysis of East Midlands Cancer Network data has not yet demonstrated additional cases of ‘post- negative FIT colorectal cancer’, although this scenario may arise. In addition, most patients have not been investigated after a ‘negative’ FIT result, and some may yet present with colorectal cancer with ongoing follow-up.
In this study, only nine cancers were diagnosed in patients under the age of 50 years, and unsurprisingly the majority had fHb of 100 µg Hb/g faeces or above. The recommendation16 of a fHb threshold of 10 µg Hb/g faeces for ‘low risk’ patients appears questionable in this context. A threshold as low as 4 µg Hb/g faeces in patients with anaemia, low ferritin and thrombocytosis was driven by concern around the use of FIT in ‘high risk’ patients, but is vindicated by the detection of nine such patients with colorectal cancer in the cohort with fHb below 10 µg Hb/g faeces. Interestingly, these data suggest a similar principle may be applicable between 10 and 19.9 µg Hb/g faeces, and perhaps at even higher levels. The utility of anaemia19 and thrombocytosis21 in the local 2WW pathway have been evaluated previously, but not that of ferritin. The protocol has hitherto mandated investigation only when ferritin level is low. However, studies have suggested that ferritin may have value when the level is abnormally high26, and thus there may be value in using high ferritin to improve the sensitivity and specificity of the symptomatic pathway. In the present cohort, only one additional cancer would have been missed if the threshold had been 20 µg Hb/g faeces for patients with normal rectal examination, FBC and ferritin. FIT is a stratification tool, and appears to be most useful when combined with other objective measures. The FAST (Faecal haemoglobin, Age and Sex Test) score27, combining FIT with age and sex, could be improved28, and performance characteristics of such scoring systems might increase if FIT were combined with FBC, ferritin and a digital rectal examination. These four Fs appear likely to have greatest combined value as the level of fHb declines towards undetectable.
The use of FIT has been introduced in the English BCSP with a threshold of 120 µg Hb/g faeces. This raises a number of interesting issues in relation to the training, accreditation, and workload of endoscopists. The challenge to reduce the FIT threshold or the screening age in England might be aided by the use of higher FIT thresholds, alongside blood tests, in symptomatic patients as greater diagnostic capacity is freed up. An alternative solution may be to invite screened patients with a FIT result below 120 µg Hb/g faeces to attend their GP for FBC and ferritin testing, and to lower the threshold when such parameters are abnormal. Ultimately, raising symptomatic thresholds and lowering BCSP thresholds may help to yield more coherent and consistent use of FIT in all parts of the population.
Both symptomatic and asymptomatic pathways face unprecedented circumstances in the UK in relation to the coronavirus pandemic. UK diagnostic services that were overstretched before the pandemic will doubtless struggle to cope with a backlog for many months afterwards. Accordingly, FIT results and other objective measures such as blood results should be used to prioritize those individuals most likely to benefit from urgent investigation (Appendix S4).
Finally, other large service evaluation studies10,14 have now demonstrated similar results to those from the present data set, and the recently published NICE FIT study15 adds high-volume multicentre research data to this consensus. No test is perfect, but there is now a high volume of data to demonstrate that FIT adds significant value to symptoms alone in aiding the decision to refer.
Supplementary Material
Acknowledgements
For primary care, the authors thank E.. Maddock, H. Patel, M. O’Neil, M. Jelpke and S. Karim and all primary care colleagues who accepted a significant change in working practice, as well as S. Oliver, S. Castle, and all colleagues in local CCGs who facilitated this service development.
For the Nottingham Colorectal Service, the authors thank S. Thomson, B. Harwood, S. Hall, T. Dorn, L. Hamonga, D. Williams, H. Andrews, D. Bradshaw, S. Jamil, R. Bailey, J. Williams, J. Abercrombie, C. Maxwell-Armstrong, A. Acheson, K. Walter, B. Bharathan, K. Mohiuddin, K. Thomas, A. Simpson, A. Siddika, S. Liptrot, H. Zaidi, O. Odofin, and the wider team. They also thank the Nottinghamshire Bowel Cancer Screening Hub and R. Logan.
For IT support, K. Premji and S. Murdock are thanked, as well as O. Ng and NUhCLEUS. Thanks also go to the Departments of Gastroenterology and Radiology, Nottingham University Hospitals, and to Nottingham City Hospital Endoscopy Service.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1. Cancer Research UK. Bowel Cancer Statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer (accessed 5 January 2020)
- 2. The Health Foundation. Radical Rethink Required to Close Gap on Cancer Survival Between England and Comparable Countries; 2018https://www.health.org.uk/news-and-comment/news/radical-rethink-required-to-close-gap-on-cancer-survival (accessed 5 January 2020)
- 3. National Institute for Health and Care Excellence. Suspected Cancer: Recognition and Referral. NICE Guideline NG12; 2015. https://www.nice.org.uk/guidance/ng12 (accessed 5 January 2020) [PubMed]
- 4. Logan RFA, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moss S, Mathews C, Day TJ, Smith S, Seaman HE, Snowball J. et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2017;66:1631–1644 [DOI] [PubMed] [Google Scholar]
- 6. Jellema P, van der Windt DAWM, Bruinvels DJ, Mallen CD, van Weyenberg SJB, Mulder CJ. et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010;340:c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mowat C, Digby J, Strachan JA, Wilson R, Carey FA, Fraser CG. et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut 2016;65:1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Widlak MM, Thomas CL, Thomas MG, Tomkins C, Smith S, O'Connell N. et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther 2017;45:354–363 [DOI] [PubMed] [Google Scholar]
- 9. Chapman C, Bunce J, Oliver S, Ng O, Tangri A, Rogers R. et al. Service evaluation of faecal immunochemical testing and anaemia for risk stratification in the 2-week-wait pathway for colorectal cancer. BJS Open 2019;3:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mowat C, Digby J, Strachan JA, McCann R, Hall C, Heather D. et al. Impact of introducing a faecal immunochemical test (FIT) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study. BMJ Open Gastroenterol 2019;6:e000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapman C, Thomas C, Morling J, Tangri A, Oliver S, Simpson JA. et al. Early clinical outcomes of a rapid colorectal cancer diagnosis pathway using faecal immunochemical testing in Nottingham. Colorectal Dis 2020;22:679–688 [DOI] [PubMed] [Google Scholar]
- 12. Turvill J, Mellen S, Jeffery L, Bevan S, Keding A, Turnock D. et al. Diagnostic accuracy of one or two faecal haemoglobin and calprotectin measurements in patients with suspected colorectal cancer. Scand J Gastroenterol 2018;53:1526–1534 [DOI] [PubMed] [Google Scholar]
- 13. Cunin L, Khan AA, Ibrahim M, Lango A, Klimovskij M, Harshen R.. FIT negative cancers: a right-sided problem? Implications for screening and whether iron deficiency anaemia has a role to play. Surgeon 2020; doi: 10.1016/j.surge.2020.02.003 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14. Pin-Vieito N, García Nimo L, Bujanda L, Román Alonso B, Gutiérrez-Stampa MÁ, Aguilar-Gama V. et al. Optimal diagnostic accuracy of quantitative faecal immunochemical test positivity thresholds for colorectal cancer detection in primary health care: a community-based cohort study. United European Gastroenterol J 2020; doi: 10.1177/2050640620949714 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Souza N, Georgiou Delisle T, Chen M, Benton S, Abulafi M; NICE FIT Steering Group. Faecal immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2WW pathway: a diagnostic accuracy study. Gut 2020; doi: 10.1136/gutjnl-2020-321956 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence. Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care. Diagnostics Guidance DG30; 2017. https://www.nice.org.uk/guidance/dg30 (accessed 5 January 2020) [PubMed]
- 17. Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng KK, Marshall T. et al. The importance of anaemia in diagnosing colorectal cancer: a case–control study using electronic primary care records. Br J Cancer 2008;98:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atkin W, Wooldrage K, Shah U, Skinner K, Brown JP, Hamilton W. et al. Is whole-colon investigation by colonoscopy, computerised tomography colonography or barium enema necessary for all patients with colorectal cancer symptoms, and for which patients would flexible sigmoidoscopy suffice? A retrospective cohort study. Health Technol Assess 2017;21:1–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mashlab S, Large P, Laing W, Ng O, D’Auria M, Thurston D. et al. ; Nottingham Colorectal Service. Anaemia as a risk stratification tool for symptomatic patients referred via the two-week wait pathway for colorectal cancer. Ann R Coll Surg Engl 2018;100:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W.. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract 2017;67:e405–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey JA, Hanbali N, Premji K, Bunce J, Mashlab S, Simpson JA. et al. Thrombocytosis helps to stratify risk of colorectal cancer in patients referred on a two-week wait pathway. Int J Colorectal Dis 2020;35:1347–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailey JA, Khawaja A, Andrews H, Weller J, Chapman C, Morling JR. et al. GP access to FIT increases the proportion of colorectal cancers detected on urgent pathways in symptomatic patients in Nottingham. Surgeon 2020; doi: 10.1016/j.surge.2020.03.002 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23. Banerjea A, Voll J, Chowdhury A, Siddika A, Thomson S, Briggs R. et al. Straight-to-test colonoscopy for 2-week-wait referrals improves time to diagnosis of colorectal cancer and is feasible in a high-volume unit. Colorectal Dis 2017;19:819–826 [DOI] [PubMed] [Google Scholar]
- 24. Brown LF, Fraser CG.. Effect of delay in sampling on haemoglobin determined by faecal immunochemical tests. Ann Clin Biochem 2008;45:604–605 [DOI] [PubMed] [Google Scholar]
- 25. Burr NE, Derbyshire E, Taylor J, Whalley S, Subramanian V, Finan PJ. et al. Variation in post-colonoscopy colorectal cancer across colonoscopy providers in English National Health Service: population based cohort study. BMJ 2019;367:l6090 [published Online First: 2019/11/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fonseca-Nunes A, Jakszyn P, Agudo A.. Iron and cancer risk–a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev 2014;23:12–31 [DOI] [PubMed] [Google Scholar]
- 27. Cubiella J, Digby J, Rodríguez-Alonso L, Vega P, Salve M, Díaz-Ondina M. et al. ; COLONPREDICT study investigators. The fecal hemoglobin concentration, age and sex test score: development and external validation of a simple prediction tool for colorectal cancer detection in symptomatic patients. Int J Cancer 2017;140:2201–2211 [DOI] [PubMed] [Google Scholar]
- 28. Digby J, Strachan JA, Mowat C, Steele RJC, Fraser CG.. Appraisal of the faecal haemoglobin, age and sex test (FAST) score in assessment of patients with lower bowel symptoms: an observational study. BMC Gastroenterol 2019;19:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.