ABSTRACT
Human milk stimulates a health-promoting gut microbiome in infants. However, it is unclear how the microbiota salvages and processes its required nitrogen from breast milk. Human milk nitrogen sources such as urea could contribute to the composition of this early life microbiome. Urea is abundant in human milk, representing a large part of the non-protein nitrogen (NPN). We found that B. longum subsp. infantis (ATCC17930) can use urea as a main source of nitrogen for growth in synthetic medium and enzyme activity was induced by the presence of urea in the medium. We furthermore confirmed the expression of both urease protein subunits and accessory proteins of B. longum subsp. infantis through proteomics. To the same end, metagenome data were mined for urease-related genes. It was found that the breastfed infant's microbiome possessed more urease-related genes than formula fed infants (51.4:22.1; 2.3-fold increase). Bifidobacteria provided a total of 106 of urease subunit alpha alignments, found only in breastfed infants. These experiments show how an important gut commensal that colonizes the infant intestine can metabolize urea. The results presented herein further indicate how dietary nitrogen can determine bacterial metabolism in the neonate gut and shape the overall microbiome.
Keywords: urease, infant gut microbiota, Bifidobacterium, urea, human milk
This study indicates that nitrogen sources in human milk are potentially selecting for a healthy gut microbiome, which is important for infant feeding and health considerations.
Importance paragraph
Human milk stimulates a health-promoting microbiome in infants. Urea is abundant in human milk, making up a large part of the non-protein nitrogen. We wanted to explore if human milk urea could be used by the microbiota. Considering bacterial nitrogen metabolism for further understanding why breastfeeding is so beneficial, is the next step. In that fashion, this study indicates that nitrogen sources in human milk are potentially selecting for a healthy gut microbiome, which is important for infant feeding and health considerations.
INTRODUCTION
When human life begins, the gut microbiota develops dynamically (Yatsunenko et al. 2012; Backhed et al. 2015). Notably, this microbiota can aid with digestion and thus supports the infant's nutritional needs (Koropatkin, Cameron and Martens 2012; Fernández et al. 2013; LeBlanc et al. 2013). Moreover, the microbiome is crucial in establishing health for the infant by supplying amino acids and vitamins and contributing to gut maturation and immune development (Fuller and Reeds 1998; Martin et al. 2010; Azad et al. 2014; Wopereis et al. 2014; Koleva, Bridgman and Kozyrskyj 2015; Neis, Dejong and Rensen 2015; Gensollen et al. 2016). This vital balance originates in the early moments of life and the bacteria responsible are promoted by dietary factors named prebiotics (Backhed et al. 2015; Goldsmith et al. 2015; Tanaka and Nakayama 2017). In recent years, research has therefore focused on describing early microbial colonization patterns and mechanisms of human milk utilization by early life gut symbionts (Sela 2011; Marcobal and Sonnenburg 2012; Milani et al. 2017; Shani et al. 2018). Human milk and all of its components are likely to have evolved to promote symbiosis between host and microbiome. However, it is currently unclear how the microbiota salvages nitrogen from breast milk. Human milk nitrogen comes in many forms and is for example represented by milk proteins, peptides and free amino acids, but also human milk oligosaccharides hold nitrogen that could be utilized by the infant gut microbiota (Harzer, Franzke and Bindels 1984; Carratù et al. 2003; Asakuma et al. 2011; Sela 2011; Ballard and Morrow 2013; Andreas, Kampmann and Mehring Le-Doare 2015; James et al. 2016; Sakanaka et al. 2019). Interestingly, this complex bio-fluid holds some nitrogen sources that are waste products of human metabolism, like urea (Carlson 1985; Donovan and Lonnerdal 1985; Andreas, Kampmann and Mehring Le-Doare 2015).
Urease is the first enzyme ever isolated (Sumner 1926). As such, the enzyme urease (EC 3.5.1.5) has been studied in various bacteria and ecosystems and has been found to play a crucial role in bacterial survival (Burne and Chen 2000; Scott et al. 2002; Mora and Arioli 2014). Urease is a nickel or iron co-factored multimeric enzyme that catalyses the hydrolysis of urea into carbon dioxide and ammonia (NH3; Zimmer 2000; Quiroz-Valenzuela et al. 2008; Carter et al. 2011). In bacterial pathogens, e.g.Helicobacter pylori, Proteus mirabilis, urease functions as a virulence factor (Dupuy et al. 1997; Hola, Peroutkova and Ruzicka 2012; Mora and Arioli 2014; Roesler, Rabelo-Gonçalves and Zeitune 2014). It functions as such by managing the environment around the bacterial cell, through neutralization of the acidic microenvironment by protonation of ammonia, resulting in ammonium (NH4) (Scott et al. 2002; Fu et al. 2018). Ureolytic activity is seen with non-pathogenic bacteria as well (Suzuki et al. 1979; Mora and Arioli 2014). However, in both pathogens and non-pathogens the underlying mechanism and ecological function are often unclear (Burne and Chen 2000; Mora and Arioli 2014; Lerm et al. 2018; Tarsia et al. 2018). The resulting ammonium has been suggested as the main nitrogen source of choice for amino acid synthesis by gut bacteria through glutamate dehydrogenase activity (Belzer et al. 2005; Stanley 2009; Davila et al. 2013; Neis, Dejong and Rensen 2015). Therefore, bacterial urease can fulfil a function in gut nitrogen cycling to serve the bacterial needs.
Nitrogen availability, specifically urea, both host- and diet-derived, can greatly affect the human gut microbiota (Brown, Hill and Richards 1971; Jackson 1994; Wong et al. 2014; Holmes et al. 2017; Reese et al. 2018). For diet, the biological norm is that for approximately the first 6 months of human life, infant feeding consists solely of human milk because of its effect on microbiota composition and overall health (Kramer and Kakuma 2004; Rinne et al. 2005; Backhed et al. 2015; Tanaka and Nakayama 2017; Stewart et al. 2018). Human milk composition varies inter-individually and over time from colostrum to late lactation (Ballard and Morrow 2013). It holds a collection of non-protein nitrogen (NPN) sources (25% of total N), including urea (Carlson 1985; Janas, Picciano and Hatch 1985; DONOVAN and Lonnerdal 1989; Ballard and Morrow 2013; Andreas, Kampmann and Mehring Le-Doare 2015). Urea constitutes 15% (3–6 mM) of this NPN, while others claim that it actually represents 15% of total nitrogen in human milk (Harzer, Franzke and Bindels 1984; Neville et al. 1984; Donovan and Lonnerdal 1985; DONOVAN and Lonnerdal 1989; George et al. 1996; Smilowitz et al. 2013; Mora and Arioli 2014). The relevance of urea nitrogen salvation for the infant gut, although not intensively studied, has often been suggested (Harzer, Franzke and Bindels 1984; Heine, Tiess and Wutzke 1986; Fomon et al. 1987; Fuller and Reeds 1998; Sela et al. 2008; George et al. 1996). There are, nonetheless, a few indications of urease activity by bacteria in the infant lower gut (Crociani and Matteuzzi 1982; Heine et al. 1984; Meakins and Jackson 1996; Millward et al. 2000). In an early study, labelled 15N urea turned up in microbial protein and in infant serum amino acids, indicating a function of urea in microbial biosynthetic processes and showing utilization out of a dietary source (Heine et al. 1984; Millward et al. 2000). Notably, in an infant fed with a breast milk-like diet, utilization of urea increased significantly compared to the infants fed with formula (George et al. 1996). Finally, a recent metaproteomics study showed enrichment of nickel transport systems in the infant gut assigned to Bifidobacteriaceae, which are potentially important for activation of urea metabolism (Cerdó et al. 2018).
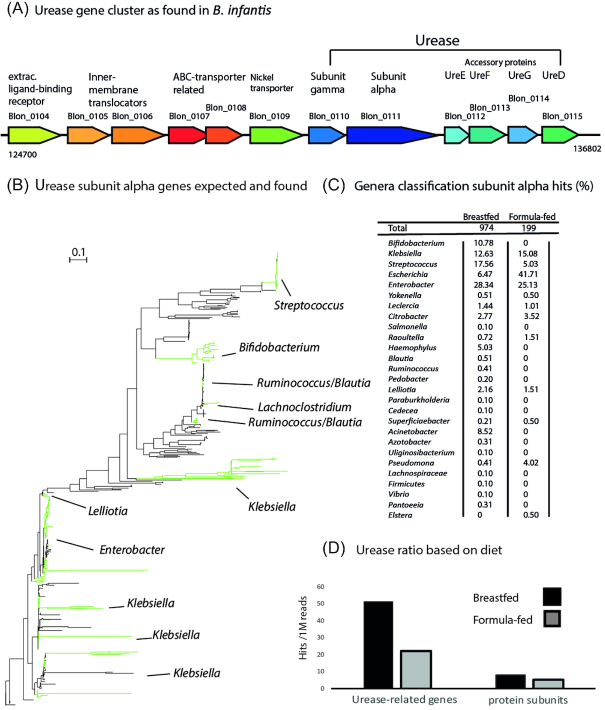
Early studies, observed urea degradation among gut commensals without further characterization of the process (Suzuki et al. 1979; Crociani and Matteuzzi 1982). Interestingly, several genomes of Bifidobacterium spp. possess urease genes (Sela et al. 2008; LoCascio et al. 2010). Of particular interest was the discovery of a full urease cluster in the common infant gut colonizer Bifidobacterium longum subsp. infantis (B. infantis, ATCC15697; Blon_0104-Blon_0115, Fig. 1A; Sela et al. 2008). As biomarkers for infant health, urease is likely to fulfil a different niche function for Bifidobacterium, compared to a function in virulence in model organisms. It is hypothesized here that B. longum subsp. infantis urease is a growth factor for the species.
Figure 1.
(A). Urease gene cluster as found in B. infantis (Sela et al. 2008) [58]. (B). Phylogenetic tree of urease subunit alpha genes expected in the human gut. Nodes of genes found in study labeled green. (C). Genera of origin of the urease gene hits in the metagenomes, retrieved from all urease protein subunit alpha hits surpassing the alignment quality threshold (%). (D). Normalized ratio of reads that aligned with urease proteins for all bacterial species based on infant diet (per 1 million reads).
In this paper, we further characterized urease activity as a growth factor in vitro for B. longumsubsp. infantis, a potentially beneficial bacteria and a common infant gut colonizer (Sela et al. 2008; Underwood et al. 2015; Stewart et al. 2018). This specific trait might explain why B. infantis is an efficient colonizer of the infant gut and how it is adapted to the human milk diet. Public metagenome data of the infant gut was studied to investigate the relationship between diet and potential urease activity of the microbiome. We expect Bifidobacterium to interact with human milk urea. Both aspects increase our understanding of how breastfeeding might stimulate a beneficial microbiome.
MATERIALS AND METHODS
Metagenome mining for urease genes
An infant gut metagenome database comprised of three publicly available datasets along with dietary metadata was studied for occurrence of urease-related genes (Schwartz et al. 2012; Yatsunenko et al. 2012; Vallès et al. 2014). The study holds a total of 92 samples obtained from 74 infants with a total of 10 442 761 DNA reads (Figure S1 and S2, Supporting Information). These three studies were selected since they all contain both breastfed and formula-fed infants. Included infants were solely breastfed or solely formula-fed and were 6 months or younger. Antibiotic-treated, diseased or pre-term infants were excluded from the study. The reads were previously quality filtered in different methods by the authors of before mentioned publications. To extract predictive functional data from metagenomes, protein aligners have been developed (e.g. DIAMOND; Buchfink, Xie and Huson 2015; Westbrook et al. 2017). Using DIAMOND, the raw shotgun reads were translated into protein and mapped against UniProt databases (Swiss-Prot and TrEMBL, Release 2019_01), to create a functional profile. The complete UniProt database was in this case included to counter the lack of inclusions of Bifidobacterium protein sequences and genes in the SwissProt database alone. Quality cut-offs for DIAMOND alignment were set at 60% coverage of the read length and 60% alignment identity for the protein alignments. Alignment results were filtered for urease-related hits. These included urease protein subunits, urea transporters, urease accessory proteins and urease activity regulatory genes. Protein hits were normalized per 1 million high quality reads. This method yielded occasionally multiple successful assignments per read. Hits with the highest reported identity scores were considered for taxonomic analysis. One sample from the Schwartz et al. (2012) dataset was excluded since it contained a far higher rate of urease genes than the other samples combined from that study. We suspect an artefact resulting from our methods or that the infant was suffering from an infection, which warranted exclusion. Included samples list and dietary metadata are available in the supplementary data (Table S1, Supporting Information). A phylogenetic tree for urease subunit alpha genes related to the infant gut was constructed using ARB software package (max. likelihood; Phylip PROML), based on the 25 most dominant taxa in study by Stewart et al. (2018) and Ludwig et al. (2004). Original tree file was uploaded at Open Science Framework repository (OSF, https://osf.io/2hu4m). From NCBI Nucleotide database, full genome sequence annotations of Bifidobacterium were checked for urease gene clusters.
Bacterial strains and growth conditions
Bifidobacterium longum subsp. infantis (ATCC 17930/DSM20218/JCM1260) and Bifidobacterium breve (ATCC 15698/DSM20091) were anaerobically grown on TOS-propionate agar medium (Sigma-Aldrich, St. Louis, USA), specialized for enumeration of Bifidobacterium species. Plated cultures were incubated at 37°C and plates were stored at 4°C for long-term storage. Plates cultures were used to inoculate liquid pre-cultures by swiping colonies in an anaerobic tent (Coy Vinyl Anaerobic Chamber, max. 5% H2/N2). For liquid culturing, a nitrogen-limiting medium with excess in carbon source (lactose 2%; pH 6.2): yeast extract (1 g/L); potassium dihydrogen phosphate (3 g/L); dipotassium hydrogen phosphate (4.8 g/L), magnesium sulphate (0.2 g/L), sodium propionate (15 g/L) and L-cysteine hydrochloride (0.5 g/L) to counter the strain's auxotrophy for the amino acid. Serum bottles were supplemented with filter-sterilized vitamin mix and trace metals (100x; originally designed for Lactobacillus lactis (Otto et al. 1983)). Nitrogen treatments included urea (0.6 g/L; 10 mM), tryptone, (protein digest, w/w: 12.7% N, 2.2 g/L, Oxoid, Basingstoke, UK), ammonium sulfate ((NH4)2SO4) or no added nitrogen source. Nickel chloride (NiCl; 0.1–1 mM) was included as it is required by active urease enzyme. Test cultures were subsequently inoculated with 0.1 mL of late logarhytmic phase pre-culture (24 h). Anaerobic serum bottles possessed either a headspace of N2 or CO2/N2 (80%/20%) at 1.7 atm. The basal growth media was tested in a pH range and with a bicarbonate buffer (CO2/N2; 20 mM Na2CO3). Liquid pre-cultures contained only CO2/N2. Growth was evaluated by measuring optical density at 600 nm (OD 600; OD 600 DiluPhotometer, IMPLEN, Germany). Acidification was observed by measuring pH (ProLine B210). For statistical analysis, an unpaired t-test was used in SPSS Software (V24). P values < 0.05 were considered statistically significant.
Urea colorimetric assay
Urea levels were determined by a colorimetric urea assay (MAK006; SigmaAldrich) according to manufacturer's instruction. The colored result of a coupled enzyme reaction was measured at 570 nm on a Synergy Mx microplate reader (Biotek, Winooski, USA). Culture supernatants were diluted 100x prior to analysis to fit the kit's standard curve. Data were normalized to observed background effects due to interference of compounds in the media, like e.g. ammonium, NAD+/NADP+ and pyruvate.
Urease enzymatic assay
Bacterial cultures (1 mL) were retrieved and centrifuged (10 min, 20 000 x g) before supernatants were separated from pellets. Cell pellets were suspended in sodium phosphate buffer (10 mM) before analysis. Supernatants were filtered (0.2 µm) before use in enzymatic assay. Sonication of bacterial cell pellets was performed using a MS72 probe on a Bandelin Sonopuls Sonicator (Bandelin Electronic, Berlin, Germany). Cells were lysed using 20 s sonication at 30% amplitude with 30 s intervals on ice and repeating this four times. Enzymatic activity of supernatants and cell lysates was then assessed using urease activity assay kit (MAK120, Sigma Aldrich) as reported by the manufacturer, derived from the Berthelot method. Exposure to urea lasted for 10 min. After 30 min of color formation, absorbance at 670 nm was measured, preceded by 2 s medium shake on a Synergy Mx microplate reader (Biotek).
Proteomics
Samples were taken from mono-cultures of B. infantis (ATCC17930) with urea during late exponential growth phase. Cell lysis was achieved through bead beating in a FastPrep-24 5G instrument (MP Biomedicals, Brussels, Belgium) for six times 30 s at 6.5 m/s with cooling after every bead step. Protein concentration was assessed using Pierce BCA protein assay (ThermoFisher Scientific, Waltham, MA; Figure S8, Supporting Information). Subsequent protein digestion was performed overnight using dithiothreitol (DTT, 2 mM), iodoacetamide (IAA, 4 mM) and trypsin (1:50 of a 1 µg/µL solution) at 37°C. Cleanup was performed through SPE columns (Solid Phase Extraction; ThermoFisher Scientific) with acetic acid (100 mM in 95% acetonitrile) to be finally dissolved in the eluent ammonium formate (10 mM). Samples were analyzed in duplicate by nano-LC-HRMS/MS as described by Meiring et al. (2002). An Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, USA) was used, connected to a Q-Exactive Plus Mass spectrometer (ThermoFisher Scientific). Peptides were trapped on a 100 µm inner diameter trap column packed using ReproSil-Pur C18-AQ, 3 µm resin (Dr. Maisch, Ammerbuch, Germany) at 5 µL/min in 100 mM acetic acid. Afterwards the peptides were eluted at 100 mL/min in a 90 min extended gradient from 10 to 40% acetic acid solvent (in 95% acetonitrile) to a 20-cm IntegraFrit column (50 µm inner diameter, Reprosil-Pur C18-AQ 3 µm, New Objective, Woburn, USA). The acquired spectra were analyzed using Thermo Proteome Discoverer in combination with Mascot (ThermoFisher Scientific). The reference database comprised of protein sequences from B. longum subsp. infantis from Uniprot (ATCC15697) since the genome of B. infantis strain ATCC17930 is not publically available.
RESULTS
Occurrence of urease gene cluster in Bifidobacterium spp.
Bifidobacterium whole genome annotations were scanned for urease gene clusters as present in B. infantis (Fig. 1A; Sela, 2011). A total of five Bifidobacterium urease genes are currently annotated across seven species that are related to the human gut. Namely, the B. longum subspecies longum, infantis and suis; B. subtile; B. kashiwanohense and B. scardovii (Table S3, Supporting Information). Either a full gene cluster or no gene cluster was found to be present every time. One exception occurred with B. subtile, where one accessory protein gene was missing from the cluster. Interestingly, of B. infantis whole genome sequences only 50% of the cases had a urease gene cluster annotated (Table S3, Supporting Information). Meanwhile, no urease gene clusters are currently annotated in several other common Bifidobacterium infant gut colonizers like B. breve and B. bifidum spp.
Urease genes found in infant gut metagenomes
We studied bacterial urease gene cluster occurrence in the infant gut of which B. infantis holds an example (Fig. 1A). A phylogenetic tree was constructed of urease alpha subunit genes that are expected to occur in the infant (Fig. 1B). Interestingly, all Bifidobacterium genes cluster together. Furthermore, many genes, belonging to other genera have been traced to the metagenomes included in this study (Fig. 1B, green highlights). In total, the functional profile of the metagenomes yielded a total of 27 taxa for urease protein subunit alpha hits (Fig. 1C). The most dominant genus holding urease genes was Enterobacter, representing 27.8% (326) of alignments. The other most dominant found genera were Streptococcus (15.4%), Klebsiella (13%), Escherichia (12.4%). Bifidobacterium was the fifth most abundant genus with 9% (Fig. 1C). The genus was represented by B. longum subspp., B. kashiwanohense and B. scardovii. Interestingly, Bifidobacterium urease genes were only found in breastfed infants, while Escherichia hits occurred mostly in samples belonging to strictly formula fed infants (breastfed 6%, formula-fed 41%). Sequences traced to Enterobacter were present in both diet types 28.34% and 25.13% in breastfed and formula-fed infants respectively. Klebsiella showed a similar pattern (12.63%; 15.08%). After data normalization a 2.3-fold increase of urease-related hits was observed for breastfed infants compared to formula-fed infants (Fig. 1D). A 1.5-fold increase was observed when measuring urease protein subunits separately.
Urea serves as a nitrogen source for B. longum subsp. infantis
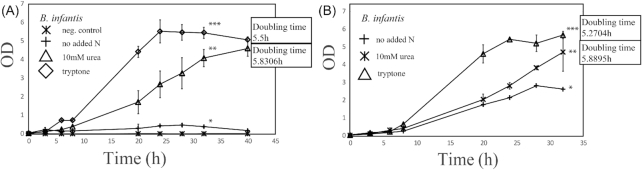
We tested the ability of B. infantis to use urea as the main nitrogen source by comparing it with growth on a protein digest (tryptone) or no added nitrogen. Growth on urea as the main nitrogen source was observed for B. infantis and not for B. breve, which was included as a non-urease expressing control (Fig. 2A and B, 0.4 compared to 4.1; OD 600; 32 h; P≤ 0.05, Figure S4 and S5, Supporting Information). Differences in growth were reflected by different pH across the culture types (Figure S4, Supporting Information). Growth on (NH4)2SO4 was checked to confirm the strain's ability to process the ammonium output resulting from degrading urea (Figure S7, Supporting Information; headspace: N2). An active urease complex produces carbon dioxide (CO2).
Figure 2.
(A). Growth of B. infantis in nitrogen limiting media, headspace 100% N2 (OD 600). (B). Growth of B. infantis in nitrogen limiting media, headspace 80% N2/CO2, * P values < 0.05. Doubling time in minutes.
Growth was therefore compared to conditions with CO2 present. Growth increased in the presence of CO2 compared to conditions with only N2 present, but the presence of urea still increased the growth of B. infantis relative to the basal medium (Fig. 2B). Thus, CO2 absence is not a trigger for B. infantis to become urease active.
Urease proteins confirmed and activity is linked to the presence of urea
Presence of urease-related proteins was confirmed with high reliability for several proteins deriving from the B. infantis urease gene cluster (Figure S8, Supporting Information). This included urease subunit alpha, beta/gamma and accessory proteins UreE and UreG (Fig. 1A and Figure S8, Supporting Information). This thus included Uniprot accessions: B7GT16-18 and 20. The sequence coverage can be considered low in some cases (4–35%). However, the protein identification can be regarded as very confident due to the occurrence of unique peptide sequences (3–7) and the additional spectral evaluation performed by the Proteome Discoverer software (Figure S8, Supporting Information). Urease activity of B. infantis, was only detected upon cultivation with urea as the nitrogen source (Fig. 3, P < 0.05, and Figure S5, Supporting Information). At 20 h no activity was observed in basal medium and basal medium supplemented with tryptone. No clear and reliable activity measurements were obtained, when the supernatant was analyzed. Degradation of 10 mM urea was confirmed at 24 h, which corresponds with the exponential growth phase (Figure S6, Supporting Information).
Figure 3.
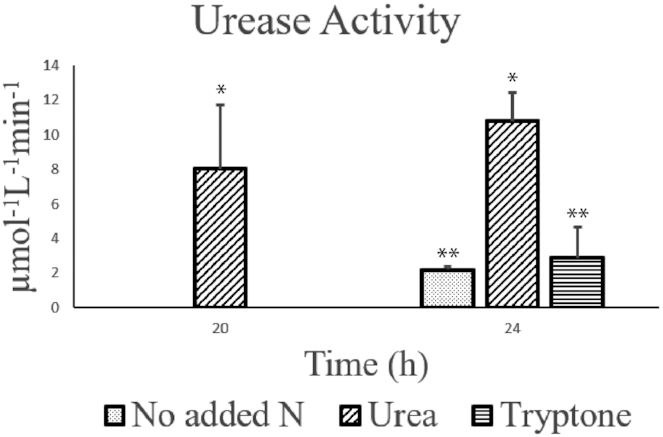
Urease enzyme activity (ammonium production/µmol/L/min) measured on two timepoints with the Berthelot method, P < 0.05 (Figure S5, Supporting Information).
DISCUSSION
Since the discovery of a urease gene cluster in B. infantis (Sela et al. 2008), urea nitrogen salvaging by the infant gut microbiota was highlighted as of potential importance to the settling microbiota. Our results indicate once more, the adaptation of B. infantis towards the human milk diet and gives rise to speculation on the role of urea in breastfeeding. Together with the metagenome data this study illustrates a new mechanism by which urea in breast milk can shape a microbial community and thus strengthens the hypothesis that urease could be used as a growth factor for a potentially beneficial strain.
Metagenome study
Bifidobacterium urease represents a significant part of the microbiota that is potentially utilizing urea in breastfed infants (Fig. 1B and C). Notably, some Bifidobacterium hits belonged to Bifidobacterium callitrichos, Bifidobacterium primatium, Bifidobacterium biavatiiandBifidobacterium tissieri that are not associated with the human infant gut, but rather with the primate gut that is likely to have urea available as well (Endo et al. 2012; Michelini et al. 2016; Modesto et al. 2018). This indicates that there might be more Bifidobacterium urease genes associated with the human infant gut that are not described. The constructed tree shows a likelihood of alternate evolutionary paths of urease activity due to large spreading within phylogenetic groups, while in the meantime Bifidobacterium clustered together and thus showing similarity. The functional profile showed that Streptococcus urease genes are dominantly found in the breastfed infant (Fig. 1C) and their urease activity is connected to biosynthetic pathways and provides intracellular benefits due to pH regulation (Arioli et al. 2007; Arioli et al. 2010; Mora and Arioli 2014). It can now be hypothesized that Bifidobacterium urease fulfills a similar role, especially considering the highly acidic environment that is the infant colon. Interestingly Escherichia (E. coli) shows a reverse pattern compared to Bifidobacterium. The genus appeared more in formula-fed infants compared to breastfed infants, while their urease activity has been mostly associated with pathogenesis (Konieczna et al. 2012). However, this might be due to the lack of prebiotics in the formula products, of which no data was available. Moreover, several other pathogenic genera, e.g. Citrobacter and Klebsiella were found (Rae, Fazio and Rosales 1991; Tumbarello et al. 2015; Pal and Mahendra 2016). Urea metabolism has been intensively studied for these pathogenic genera, which could result in an underrepresentation of Bifidobacterium urease proteins in the database, a common bias and obstacle when constructing functional profiles (Quince et al. 2017). Also, Bifidobacterium spp. have one locus containing an urease gene cluster annotated, while others like Escherichia coli can have multiple (Heimer et al. 2002). Proper validation or normalization for such biases, should be developed. Nonetheless, beneficial bacteria, e.g.B. infantis, that possess urease activity might compete with pathogens for urea as a nitrogen source. Bifidobacteria might be dominant in breastfed infants due to their capacity to degrade HMOs and through this mechanism, it could limit the possibility of other urease positive species occurring in that same environment. Moreover, it might be acting as a detoxifier, since urea clearance from the gut is important in gut health (Ramezani et al. 2016). This could make Bifidobacterium Urease a target for therapeutics. How dietary urea would favor B. infantis or other Bifidobacterium spp. over these potential pathobionts needs to be further investigated.
The functional profile showed that breastfeeding selects for genes related in urea metabolism and thus bacteria involved in urea utilization (Fig. 1D). However, this pattern was not observed consistently for urease protein subunits when looking at the included studies separately (Figure S2, Supporting Information). This data nonetheless provides a fair representation of the total level of genomic capabilities for a specific process, compared to an approach that included assembly or binning. Short genetic fragments of low abundance species that lack sufficient sequence coverage are now included because of recent advances in fast translated searches (Buchfink, Xie and Huson 2015; Quince et al. 2017; Franzosa et al. 2018).
Urea in the infant gut
It is expected that urea is a microbiome modulator in the infant gut and that urease producing microbes profit in a comparable way as for example how host-derived urea in uremic patients selects for urease active bacteria (Brown, Hill and Richards 1971; Wong et al. 2014). Urea could be present at low levels in the lower gastrointestinal tract of the infant due to earlier ureolytic activity along the gastrointestinal tract or because of inter-individual variation between maternal factors that affect milk composition (Harzer, Franzke and Bindels 1984; Fuller and Reeds 1998; Montecucco and Rappuoli 2001; Ballard and Morrow 2013; Mora and Arioli 2014; Andreas, Kampmann and Mehring Le-Doare 2015). Urea can be supplied by the human body, or by other bacteria through amino acid fermentation as well (Crociani and Matteuzzi 1982; Heine et al. 1984; Meakins and Jackson 1996; Fuller and Reeds 1998; Millward et al. 2000; Davila et al. 2013; Mora and Arioli 2014; Neis, Dejong and Rensen 2015). Notably, lower protein diets have been shown to change urea kinetics in adults, shifting towards more host-derived urea utilization (Langran et al. 1992; Meakins and Jackson 1996). A similar pattern could exist for the infant gut. Host-derived urea can anyhow explain the level of urease genes found in formula-fed reference group. Moreover, infants fed formula were not completely excluded from dietary urea, since it occurs in formula as a trace compound due to its cow milk origin (DONOVAN and Lonnerdal 1989). The hypothesis that milk urea serves a biological purpose through possibly selecting for specific bacteria, is nonetheless underlined by the described results.
Urea utilization by B. infantis
We provide evidence for the ability of B. infantis (ATCC17930) to utilize urea as a main nitrogen source. The strain is able to express necessary gene products, which were confirmed through proteomics albeit with low coverage. The low coverage may be explained by the use of the related proteome database, which likely contains only homologue sequences of the targeted enzyme. While being comprised of fairly large proteins, which can lower coverage on its own, it is expected that the urease enzyme is embedded in the membrane (Bode et al. 1993). This might lead to lesser efficiency with the described protein extraction method due to enzyme being lost with the cell debris. The likelihood of the strain being able to produce active enzyme, was confirmed by the presence of the accessory activator UreE in the proteomics data (Quiroz-Valenzuela et al. 2008). This accessory protein is thought to be involved in nickel-chelation which is essential for urease activation in other bacteria (Lee et al. 1993; Song et al. 2001). The lack of UreD and UreF occurrence cannot be explained, especially since all other data from this study suggests urease activity in B. infantis. Possibly this accessory protein is not required for active enzyme or it is produced in another stage of the culture experiment. Accessory protein UreF was scarcely detected, but never surpassed the low reliability threshold. Interestingly, according to enzymatic assay, only the presence of urea made B. infantis active in degrading urea, which indicates some form of metabolic switching and regulation according to the enzymatic assays (Fig. 3A). Similar observations were made for other bacteria hosted by humans that have proven to be urease active and that specifically transcription was regulated by UreR in the presence of urea (Poore and Mobley 2003; Belzer et al. 2005). The fact that the presence of urea is required for B. infantis was furthermore observed in Streptococcus thermophilus, while also indicating that aspartate metabolism and glutamate metabolism might be linked to urease activity (Arioli et al. 2007; Zotta et al. 2008). Since we tested urease activity in pure strain cultures, there is a chance that the organism changes it activity in the presence of other species in a community. Further investigation into the regulation of the urease gene cluster in B. infantis is therefore definitively warranted. Due to presence of genes encoding a transporter system on the cluster (Fig. 1A), it is to be expected urease activity is at least partially intracellularly and thus related to the cell lysate as was shown in this study. Urease activity in the supernatant has not been reliably measured using the enzymatic assay, but cannot be excluded. It has been shown that CO2 availability promotes growth of B. infantis, yet lack of CO2 is not a trigger to become ureolytic in this organism, since growth occurs under both conditions. The promoting effect of CO2 indicates the capnophilic nature of this organism. Despite the importance of Bifidobacteria for infant health, the effect of CO2 on the overall metabolism of the genus is poorly investigated and is surely related to the effect of urease activity by these bacteria. Urease activity can furthermore be a means of pH regulation by intestinal bacteria for the acidic environment that the breastfed infant's gut traditionally is (Scott et al. 2002; Arioli et al. 2010; Fu et al. 2018; Henrick et al. 2018). These results cannot exclude that B. infantis benefits from urease activity in low pH and even regulates the pH (Figure S4, Supporting Information).
Conclusive words
Human milk shapes our microbiome from the moment we are born. The authors hypothesize that urea in breast milk functions as an important nitrogen source for beneficial bacteria in the early life gut microbiota and provide the first evidence for this. To our knowledge this is the first time that a direct link has been made between Bifidobacterium and urease activity and meanwhile its relevance in the infant gut is supported by metagenomic data. This study further characterizes a mechanism by which a common gut symbiont utilizes urea as a nitrogen source from human milk. It is proposed here that urea degradation by B. infantis is another relevant means to acquire nutrients from breast milk. Further research is needed to clarify the function of B. infantis urease activity in the infant gut ecosystem.
Supplementary Material
ACKNOWLEDGEMENTS
The study was designed by P.S., L.K., R.B., J.K. and C.B.; P.S. performed the study in the lab; P.S. collected and generated the data from the metagenomes; P.S. performed the proteomics study; P.S. performed data analysis and figure generation; P.S. and C.B. wrote the manuscript. The manuscript was checked by P.S. L.K., R.B., J.K. and C.B. All authors contributed to critical revisions and approved the manuscript. First of all, the authors would like to thank Dr Bernd Stahl2 for being an initial inspiration to this project. Secondly, the authors would like to thank Athul Sundaresan1 for his contributions in the lab as part of his thesis project. Finally, the authors would finally like to thank Heleen de Weerd2, Joost Gouw2 and Gido Jehoel2 for their help and advice with the metagenomics data generation, proteomics processing and analysis.
Contributor Information
Patrick Schimmel, Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, Helix Building, 6708 WE, Wageningen, the Netherlands.
Lennart Kleinjans, Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, Helix Building, 6708 WE, Wageningen, the Netherlands.
Roger S Bongers, Danone Nutricia Research, Uppsalalaan 12, 3584CT Utrecht, the Netherlands.
Jan Knol, Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, Helix Building, 6708 WE, Wageningen, the Netherlands; Danone Nutricia Research, Uppsalalaan 12, 3584CT Utrecht, the Netherlands.
Clara Belzer, Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, Helix Building, 6708 WE, Wageningen, the Netherlands.
Conflicts of interest
R.B. and J.K. are employees of Danone Nutricia Research. P.S., L.K., C.B. received funding from Danone Nutricia Research.
REFERENCES
- Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91:629–35. [DOI] [PubMed] [Google Scholar]
- Arioli S, Monnet C, Guglielmetti Set al. Aspartate biosynthesis is essential for the growth of Streptococcus thermophilus in milk, and aspartate availability modulates the level of urease activity. Appl Environ Microbiol. 2007;73:5789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioli S, Ragg E, Scaglioni Let al. Alkalizing reactions streamline cellular metabolism in acidogenic microorganisms. PLoS One. 2010;5:e15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakuma S, Hatakeyama E, Urashima Tet al. Physiology of the consumption of human milk oligosaccharides by infant-gut associated bifidobacteria. J Biol Chem. 2011;286:P34583–92.. M111. 248138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M, Bridgman S, Becker Aet al. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes. 2014;38:1290. [DOI] [PubMed] [Google Scholar]
- Backhed F, Roswall J, Peng Yet al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin. 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer C, Stoof J, Beckwith CSet al. Differential regulation of urease activity in Helicobacter hepaticus and Helicobacter pylori. Microbiology. 2005;151:3989–95. [DOI] [PubMed] [Google Scholar]
- Bode G, Malfertheiner P, Lehnhardt Get al. Ultrastructural localization of urease of Helicobacter pylori. Med Microbiol Immunol (Berl). 1993;182:233–42. [DOI] [PubMed] [Google Scholar]
- Brown CL, Hill MJ, Richards P. Bacterial ureases in uraemic men. Lancet. 1971;2:406–7. [DOI] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59. [DOI] [PubMed] [Google Scholar]
- Burne RA, Chen YY. Bacterial ureases in infectious diseases. Microbes Infect. 2000;2:533–42. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Human milk nonprotein nitrogen: occurrence and possible functions. Adv Pediatr. 1985;32:43–70.3909780 [Google Scholar]
- Carratù B, Boniglia C, Scalise Fet al. Nitrogenous Components of Human Milk: Non-Protein Nitrogen, True Protein and Free Amino Acids. Food Chemistry. 2003;81:357–62. [Google Scholar]
- Carter EL, Tronrud DE, Taber SRet al. Iron-containing urease in a pathogenic bacterium. Proc Natl Acad Sci. 2011;108:13095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdó T, Ruiz A, Acuña Iet al. Gut microbial functional maturation and succession during human early life. Environ Microbiol. 2018;20:2160–77. [DOI] [PubMed] [Google Scholar]
- Crociani F, Matteuzzi D. Urease activity in the genus Bifidobacterium. Ann Microbiol (Paris). 1982;133:417–23. [PubMed] [Google Scholar]
- Davila A-M, Blachier F, Gotteland Met al. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host". Pharmacol Res. 2013;69:114–26. [DOI] [PubMed] [Google Scholar]
- Donovan S, Lonnerdal B. Non-Protein Nitrogen Constituents in Mature Human Milk and Colostrum. Federation Proceedings1985;44.5. [Google Scholar]
- Donovan SM, Lonnerdal B. Non-protein nitrogen and true protein in infant formulas. Acta Paediatr. 1989;78:497–504. [DOI] [PubMed] [Google Scholar]
- Dupuy B, Daube G, Popoff MRet al. Clostridium perfringens urease genes are plasmid borne. Infect Immun. 1997;65:2313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A, Futagawa-Endo Y, Schumann Pet al. Bifidobacterium reuteri sp. nov., Bifidobacterium callitrichos sp. nov., Bifidobacterium saguini sp. nov., Bifidobacterium stellenboschense sp. nov. and Bifidobacterium biavatii sp. nov. isolated from faeces of common marmoset (Callithrix jacchus) and red-handed tamarin (Saguinus midas). Syst Appl Microbiol. 2012;35:92–97. [DOI] [PubMed] [Google Scholar]
- Fernández L, Langa S, Martín Vet al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Matthews DE, Bier DMet al. Bioavailability of dietary urea nitrogen in the infant. J Pediatr. 1987;111:221–4. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, McIver LJ, Rahnavard Get al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MF, Reeds PJ. Nitrogen cycling in the gut. Annu Rev Nutr. 1998;18:385–411. [DOI] [PubMed] [Google Scholar]
- Fu MS, Coelho C, De Leon-Rodriguez CMet al. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog. 2018;14:e1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DLet al. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Nord K-E, Ronquist Get al. Faecal Microflora and Urease Activity during the First Six Months of Infancy. Ups J Med Sci. 1996;101:233–50. [DOI] [PubMed] [Google Scholar]
- Goldsmith F, O'Sullivan A, Smilowitz JTet al. Lactation and intestinal microbiota: how early diet shapes the infant gut. J Mammary Gland Biol Neoplasia. 2015;20:149–58. [DOI] [PubMed] [Google Scholar]
- Harzer G, Franzke V, Bindels JG. Human milk nonprotein nitrogen components: changing patterns of free amino acids and urea in the course of early lactation. Am J Clin Nutr. 1984;40:303–9. [DOI] [PubMed] [Google Scholar]
- Heimer SR, Welch RA, Perna NTet al. Urease of enterohemorrhagic Escherichia coli: evidence for regulation by fur and a trans-acting factor. Infect Immun. 2002;70:1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine W, Tiess M, Stolpe Het al. Urea utilization by the intestinal flora, of infants fed mother's milk and a formula diet, as measured with the 15N-tracer technique. J Pediatr Gastroenterol Nutr. 1984;3:709–12. [DOI] [PubMed] [Google Scholar]
- Heine W, Tiess M, Wutzke K. 15N tracer investigations of the physiological availability of urea nitrogen in mother's milk. Acta Paediatr. 1986;75:439–43. [DOI] [PubMed] [Google Scholar]
- Henrick BM, Hutton AA, Palumbo MCet al. Elevated fecal pH indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century. mSphere. 2018;3:e00041–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hola V, Peroutkova T, Ruzicka F. Virulence factors in Proteus bacteria from biofilm communities of catheter-associated urinary tract infections. FEMS Immunol Med Microbiol. 2012;65:343–9. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Chew YV, Colakoglu Fet al. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017;25:140–51. [DOI] [PubMed] [Google Scholar]
- Jackson A. Urea as a nutrient: bioavailability and role in nitrogen economy. Arch Dis Child. 1994;70:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K, Motherway MOC, Bottacini Fet al. Bifidobacterium breve UCC2003 metabolises the human milk oligosaccharides lacto-N-tetraose and lacto-N-neo-tetraose through overlapping, yet distinct pathways. Sci Rep. 2016;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas LM, Picciano MF, Hatch TF. Indices of protein metabolism in term infants fed human milk, whey-predominant formula, or cow's milk formula. Pediatrics. 1985;75:775–84. [PubMed] [Google Scholar]
- Koleva P, Bridgman S, Kozyrskyj A. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7:2237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczna I, Zarnowiec P, Kwinkowski Met al. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012;13:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Kakuma R. The Optimal Duration of Exclusive Breastfeeding. Springer US; 2004, p. 63–77. [DOI] [PubMed] [Google Scholar]
- Langran M, Moran BJ, Murphy JLet al. Adaptation to a diet low in protein: effect of complex carbohydrate upon urea kinetics in normal man. Clin Sci. 1992;82:191–8. [DOI] [PubMed] [Google Scholar]
- LeBlanc JG, Milani C, de Giori GSet al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–8. [DOI] [PubMed] [Google Scholar]
- Lee MH, Pankratz HS, Wang Set al. Purification and characterization of Klebsiella aerogenes UreE protein: a nickel-binding protein that functions in urease metallocenter assembly. Protein Sci. 1993;2:1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerm B, Schwartz I, Kenyon Cet al. The Effect of Nutrient and Temperature Stress on the Urease of the Opportunistic Pathogen Cryptococcus neoformans,Oxford University Pres, 2018, 56, p.^pp. S27–S27.. Great Clarendon St, Oxford OX2 6DP, England. [Google Scholar]
- LoCascio RG, Desai P, Sela DAet al. Broad conservation of milk utilization genes inBifidobacteriumlongumsubsp. infantisas revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76:7373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram Ret al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol infection Offi Pub Eur Soc Clin Microbiol Infect Dis. 2012;18(Suppl 4):12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Nauta A, Ben Amor Ket al. Early life: gut microbiota and immune development in infancy. Benef Microb. 2010;1:367–82. [DOI] [PubMed] [Google Scholar]
- Meakins TS, Jackson AA. Salvage of exogenous urea nitrogen enhances nitrogen balance in normal men consuming marginally inadequate protein diets. Clin Sci. 1996;90:215–25. [DOI] [PubMed] [Google Scholar]
- Meiring H, Van der Heeft E, Ten Hove Get al. Nanoscale LC–MS(n): technical design and applications to peptide and protein analysis. J Sep Sci. 2002;25:557–68. [Google Scholar]
- Michelini S, Oki K, Yanokura Eet al. Bifidobacterium myosotis sp. nov., Bifidobacterium tissieri sp. nov. and Bifidobacterium hapali sp. nov., isolated from faeces of baby common marmosets (Callithrix jacchus L.). Int J Syst Evol Microbiol. 2016;66:255–65. [DOI] [PubMed] [Google Scholar]
- Milani C, Duranti S, Bottacini Fet al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81:e00036–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward DJ, Forrester T, Ah-Sing Eet al. The transfer of 15N from urea to lysine in the human infant. Br J Nutr. 2000;83:505–12. [PubMed] [Google Scholar]
- Modesto M, Puglisi E, Bonetti Aet al. Bifidobacterium primatium sp. nov., Bifidobacterium scaligerum sp. nov., Bifidobacterium felsineum sp. nov. and Bifidobacterium simiarum sp. nov.: four novel taxa isolated from the faeces of the cotton top tamarin (Saguinus oedipus) and the emperor tamarin (Saguinus imperator). Syst Appl Microbiol. 2018;41:593–603. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457. [DOI] [PubMed] [Google Scholar]
- Mora D, Arioli S. Microbial urease in health and disease. PLoS Pathog. 2014;10:e1004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis EPJG, Dejong CHC, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7:2930–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, Keller RP, Seacat Jet al. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr. 1984;40:635–46. [DOI] [PubMed] [Google Scholar]
- Otto R, Brink B, Veldkamp Het al. The relation between growth rate and electrochemical proton gradient of Streptococcus cremoris. FEMS Microbiol Lett. 1983;16:69–74. [Google Scholar]
- Pal M, Mahendra R. Escherichia coli 0157: H7: an emerging bacterial zoonotic food borne pathogen of global significance. Int J Interdisc Multidisc Stud. 2016;4:1–4. [Google Scholar]
- Poore CA, Mobley HLT. Differential regulation of the Proteus mirabilis urease gene cluster by UreR and H-NS. Microbiology. 2003;149:3383–94. [DOI] [PubMed] [Google Scholar]
- Quince C, Walker AW, Simpson JTet al. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35:833. [DOI] [PubMed] [Google Scholar]
- Quiroz-Valenzuela S, Sukuru SCK, Hausinger RPet al. The structure of urease activation complexes examined by flexibility analysis, mutagenesis, and small-angle X-ray scattering. Arch Biochem Biophys. 2008;480:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CE, Fazio A, Rosales JP. Successful treatment of neonatal Citrobacter freundii meningitis with ceftriaxone. DICP. 1991;25:27–29. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Massy ZA, Meijers Bet al. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis Off J Nat Kidney Found. 2016;67:483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese AT, Pereira FC, Schintlmeister Aet al. Microbial nitrogen limitation in the mammalian large intestine. Nat Microbiol. 2018;3:1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne MM, Gueimonde M, Kalliomäki Met al. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol Med Microbiol. 2005;43:59–65. [DOI] [PubMed] [Google Scholar]
- Roesler BM, Rabelo-Gonçalves EMA, Zeitune JMR. Virulence factors of Helicobacter pylori: a review. Clin Med Insights Gastroenterol. 2014;7:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Gotoh A, Yoshida Ket al. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: prevalence of the gene set and its correlation with Bifidobacteria-rich microbiota formation. Nutrients. 2019;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Friedberg I, Ivanov IVet al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DR, Marcus EA, Weeks DLet al. Mechanisms of acid resistance due to the urease system of Helicobacter pylori. Gastroenterology. 2002;123:187–95. [DOI] [PubMed] [Google Scholar]
- Sela D, Chapman J, Adeuya Aet al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci. 2008;105:18964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA. Bifidobacterial utilization of human milk oligosaccharides. Int J Food Microbiol. 2011;149:58–64. [DOI] [PubMed] [Google Scholar]
- Shani GI, Lewis ZT, Robinson AMet al. Interactions between bifidobacteria, milk oligosaccharides, and neonate hosts. Bifidobacteria Rel Organ. 2018;165–75.. [Google Scholar]
- Smilowitz JT, O'Sullivan A, Barile Det al. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. 2013;143:1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HK, Mulrooney SB, Huber Ret al. Crystal structure of Klebsiella aerogenesUreE, a nickel-binding metallochaperone for urease activation. J Biol Chem. 2001;276:49359–64. [DOI] [PubMed] [Google Scholar]
- Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr. 2009;90:862S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Ajami NJ, O'Brien JLet al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JB. The isolation and crystallization of the enzyme urease preliminary paper. J Biol Chem. 1926;69:435–41. [Google Scholar]
- Suzuki K, Benno Y, Mitsuoka Tet al. Urease-producing species of intestinal anaerobes and their activities. Appl Environ Microbiol. 1979;37:379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66:515–22. [DOI] [PubMed] [Google Scholar]
- Tarsia C, Danielli A, Florini Fet al. Targeting Helicobacter pylori urease activity and maturation: in-cell high-throughput approach for drug discovery. Biochimica et Biophysica Acta (BBA)-General Subjects. 2018;1862:2245–53. [DOI] [PubMed] [Google Scholar]
- Tumbarello M, Trecarichi EM, De Rosa FGet al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–43. [DOI] [PubMed] [Google Scholar]
- Underwood MA, German JB, Lebrilla CBet al. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallès Y, Artacho A, Pascual-García Aet al. Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLos Genet. 2014;10: e1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Ramsdell J, Schuelke Tet al. PALADIN: protein alignment for functional profiling whole metagenome shotgun data. Bioinformatics. 2017;33:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Piceno YM, DeSantis TZet al. Expansion of urease-and uricase-containing, indole-and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis H, Oozeer R, Knipping Ket al. The first thousand days–intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428–38. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJet al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M. Molecular mechanics evaluation of the proposed mechanisms for the degradation of urea by urease. J Biomol Struct Dyn. 2000; 17:787–97. [DOI] [PubMed] [Google Scholar]
- Zotta T, Ricciardi A, Rossano Ret al. Urease production by Streptococcus thermophilus. Food Microbiol. 2008;25:113–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.