Abstract
Preventing the transition to injection drug use is an important public health goal, as people who inject drugs (PWID) are at high risk for overdose and acquisition of infectious disease. Initiation into drug injection is primarily a social process, often involving PWID assistance. A better understanding of the epidemiology of this phenomenon would inform interventions to prevent injection initiation and to enhance safety when assistance is provided. We conducted a systematic review of the literature to 1) characterize the prevalence of receiving (among injection-naive persons) and providing (among PWID) help or guidance with the first drug injection and 2) identify correlates associated with these behaviors. Correlates were organized as substance use behaviors, health outcomes (e.g., human immunodeficiency virus infection), or factors describing an individual’s social, economic, policy, or physical environment, defined by means of Rhodes’ risk environments framework. After screening of 1,164 abstracts, 57 studies were included. The prevalence of receiving assistance with injection initiation (help or guidance at the first injection) ranged 74% to 100% (n = 13 estimates). The prevalence of ever providing assistance with injection initiation varied widely (range, 13%–69%; n = 13 estimates). Injecting norms, sex/gender, and other correlates classified within Rhodes’ social risk environment were commonly associated with providing and receiving assistance. Nearly all PWID receive guidance about injecting for the first time, whereas fewer PWID report providing assistance. Substantial clinical and statistical heterogeneity between studies precluded meta-analysis, and thus local-level estimates may be necessary to guide the implementation of future psychosocial and sociostructural interventions. Further, estimates of providing assistance may be downwardly biased because of social desirability factors.
Keywords: injection drug use, injection initiation assistance, people who inject drugs, substance use, systematic reviews
Abbreviations
- HIV
human immunodeficiency virus
- PWID
people who inject drugs
INTRODUCTION
Globally, the incidence of human immunodeficiency virus (HIV) infection is steady or increasing among people who inject drugs (PWID), despite decreasing HIV incidence among other key populations at high risk (1). In addition, approximately 21% of PWID experience a nonfatal overdose each year (2), and overdose mortality has increased sharply in a number of countries (3–5). Because injection drug use carries a high risk of overdose (6, 7) and sterile injecting equipment with which to prevent transmission of HIV and hepatitis C virus is not universally available (8, 9), PWID face high risks of mortality from these health outcomes (10). One public health strategy for mitigating drug overdose, HIV transmission, and hepatitis C virus transmission is “upstream prevention”—that is, the prevention of injection initiation (11, 12).
Qualitative research in diverse settings has characterized the psychosocial context surrounding injection initiation. The initiation of injection drug use often involves social interactions with experienced PWID (13). Persons initiating injection commonly report receiving assistance with their first injection from an experienced PWID (14). PWID also report being asked by injection-naive individuals to provide help or guidance about how to inject or to administer first injections, which we hereafter collectively refer to as “injection initiation assistance” (IIA) (15, 16). Estimating the prevalence of the provision and receipt of assistance would help characterize the potential population-level impact of scaling up available psychosocial (17) and pharmacological (i.e., medications for opioid use disorder) (18) interventions that could reduce injection initiation. Further, there is a need to establish the prevalence of specific forms of IIA (e.g., performing a first injection vs. providing general help) in order to better understand this outcome and inform interventions.
In addition to prevalence, studies examining injection initiation also commonly examine correlates, predictors, and contextual factors associated with the receipt or provision of IIA. An understanding of the characteristics of PWID who provide IIA (relative to those who do not assist others) and of injection-naive persons who receive IIA (relative to those who initiate injecting alone) would help optimize implementation strategies for current interventions and would inform the design of new interventions to prevent injection initiation. To date, a synthesis of correlates and contextual factors associated with IIA has not been conducted, though it would complement an existing qualitative synthesis on the topic (13).
To summarize the quantitative evidence on IIA, we systematically reviewed the literature to characterize the prevalence of providing and receiving such assistance, as well as correlates associated with each outcome. To organize correlates and identify drivers of injection initiation risk that may be leveraged in future interventions, we organized factors associated with providing or receiving assistance within the Rhodes risk environment framework (19, 20). This framework characterizes micro-level (i.e., individual), meso-level (i.e., neighborhood), and macro-level (i.e., national/international) physical, social, economic, and policy environments that may influence drug-related harms and has previously been used to study IIA (11).
METHODS
We conducted a systematic review synthesizing data on the prevalence and correlates of providing and receiving IIA. The review was registered in PROSPERO (registration number CRD42020141067) and was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (21).
Search strategy
An information specialist (C.Z.) conducted database searches in Ovid MEDLINE (US National Library of Medicine, Bethesda, Maryland), EMBASE (Excerpta Medica Database; Elsevier B.V. Amsterdam, the Netherlands), PsycINFO (American Psychological Association, Washington, DC), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Information Services, Ipswich, Massachusetts), the Social Sciences Citation Index (Clarivate Analytics, Philadelphia, Pennsylvania), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, John Wiley & Sons Ltd., Chichester, United Kingdom), and the Cochrane Database of Systematic Reviews (The Cochrane Library). The search strategies, adapted for each database, used a comprehensive combination of subject headings and keywords for the concepts of PWID and injection initiation. The databases were searched from inception to October 9, 2019, and results were limited to the English language. A detailed MEDLINE search strategy can be found in Web Table 1 (available at https://academic.oup.com/aje). The MEDLINE search strategy was peer-reviewed using the Peer Review of Electronic Search Strategies (PRESS) checklist of the Canadian Agency for Drugs and Technologies in Health (22).
Inclusion criteria, screening, and selection
We included peer-reviewed articles from observational or interventional (randomized or nonrandomized) quantitative studies carried out among people who used and/or injected drugs that characterized the prevalence and/or correlates (e.g., risk and protective factors, contextual factors co-occurring at the time of initiation, sociodemographic characteristics) of providing or receiving IIA. Qualitative studies, conference proceedings, gray literature, opinion articles, commentaries, editorials, and studies published in languages other than English were excluded. Quantitative studies with fewer than 50 participants were also excluded, since these studies had an insufficient sample size to describe prevalence.
Titles and abstracts from citations identified through the search strategy were screened by 2 reviewers (R.E.G. and C.M.) using Covidence software (Veritas Health Innovation, Melbourne, Victoria, Australia). Discrepancies regarding selection of citations for full text review were resolved by the senior author (A.I.S.). Similarly, full texts of citations selected for review were then screened by 2 reviewers and adjudicated by a third.
Data extraction and synthesis
Data were extracted into a standardized form by 1 author (R.E.G.) and reviewed for consensus by a second (C.M.). Extracted data included 1) information on the study population (location, study design, years of data collection, sampling methods, sample size, inclusion and exclusion criteria, and, when available, age range of participants, substances used by more than 50% of participants, average duration of injecting drugs, sex/gender and racial/ethnic composition of participants, and the name of the parent cohort study) and 2) information on the primary outcomes. The primary outcomes were 1) the prevalence of receiving IIA, 2) the prevalence of providing IIA, 3) correlates associated with receiving IIA, and 4) correlates associated with providing IIA. When necessary, corresponding authors were contacted to clarify methodological details and/or provide additional results (e.g., stratum-specific sample sizes of interest for our review).
For the prevalence of providing and receiving assistance, we extracted the definition of assistance, the prevalence point estimate, the number of events, and, if available, the standard error and/or 95% confidence interval. To facilitate data synthesis, prevalence outcomes in each study were classified on the basis of the type of assistance provided (i.e., injecting drugs into another person vs. providing general help, guidance, or other assistance) or received (i.e., being injected with drugs by another person vs. being helped, guided, or receiving other assistance). For providing assistance, outcomes were also classified according to the time frame in which participants recalled providing assistance (i.e., the past year, the past 6 months, or ever). When multiple articles presented the same prevalence estimate (i.e., from the same study population), we retained only the most recently published estimate for further analysis.
To evaluate the possibility of conducting a meta-analysis of prevalence outcomes, we assessed clinical (i.e., variation in the study sample and outcomes evaluated), methodological (i.e., differences in study design, analysis, and risk of bias), and statistical (i.e., variation in prevalence estimates) heterogeneity (23). Clinical and methodological heterogeneity were evaluated by summarizing study characteristics, such as sample size, data collection methods, and study population characteristics. Between-study statistical variability was summarized using Cochran’s Q test (24) and the I2 statistic (25, 26). Based on a simulation study by Partlett and Riley (27), we required a minimum of 5 prevalence estimates to consider conducting a meta-analysis. Ultimately, all prevalence outcomes were synthesized without meta-analysis (28) because of a high degree of heterogeneity. Instead, prevalence outcomes were summarized graphically, and confidence intervals for prevalence estimates from individual studies were calculated using the Clopper-Pearson method (29). Prevalence estimates from multisite studies carried out in multiple countries were reported at the country level, if possible given available data.
For correlates, we extracted measures of association that compared either 1) persons who received versus did not receive IIA or 2) persons who provided versus did not provide IIA. We included studies that examined these comparison groups as exposures or outcomes. When available, we extracted associations adjusted for confounding, including the point estimate, 95% confidence interval, standard errors, and P values. If unavailable, unadjusted measures of association or results from bivariable statistical tests (t tests, χ2 tests, etc.) were extracted. For some articles, comparison groups combined persons who self-initiated or did not provide assistance with other groups (e.g., comparison of a sexual partner providing IIA with all other initiation scenarios). In these cases, we extracted data on correlates as long as the self-initiated group was included in the analysis. Analyses that excluded persons who self-initiated altogether were not extracted.
Correlates associated with each primary outcome (providing or receiving IIA) could not be synthesized quantitatively given between-study clinical and methodological heterogeneity (e.g., differences in variable definitions, bivariable vs. adjusted regression analyses). Instead, correlates associated with providing or receiving IIA were synthesized narratively (28, 30) using Rhodes’ risk environments framework (19, 20), which was recently applied to study injection initiation (11). Specifically, correlates of providing or receiving IIA were classified as arising from the micro- and macro-level physical, social, economic, or policy environments affecting injection initiation risk (11, 20). Individual-level factors not described in Rhodes’ framework were categorized as substance use behaviors (e.g., specific substances used, frequency of injecting drugs, use of medications for opioid use disorder) or health factors (e.g., HIV infection). Examined correlates comparing participants who received/provided assistance with those who did not receive/provide assistance were synthesized narratively and graphically. Examined correlates comparing other groups (i.e., wherein the referent group was not restricted to persons who did not receive assistance or did not provide assistance) were not synthesized alongside other correlates because of interpretation-related challenges, but results are summarized in the Web tables.
Risk of bias
The risk of bias in each article was evaluated independently by 2 reviewers (R.E.G., C.M.) using a standard quality assessment tool developed by the National Institutes of Health (31). Each reviewer evaluated a series of 8–14 questions (dependent on the study’s design), which evaluated the study’s risk of bias on the basis of its study design, exposure and outcome measurement, statistical methods (including control for confounding), and other aspects. These questions provided the foundation for assigning an overall quality rating to each study as “good,” “fair,” or “poor” based on the guidelines of the National Heart, Lung, and Blood Institute (31). Discrepancies in the overall study quality rating were adjudicated through discussion between the primary reviewers, and if necessary, the study’s quality was rated by a third reviewer (A.I.S.).
RESULTS
Study selection and characteristics of included articles
A total of 3,269 citations were retrieved, resulting in 1,164 citations following deduplication (Figure 1). Of the titles and abstracts screened, 1,014 articles did not meet inclusion criteria, and 150 full texts were subsequently screened. A total of 90 articles were excluded, most commonly because they were not about injection initiation (n = 88), but also because they were qualitative studies (n = 1) or gray literature (n = 1). During data extraction, we excluded 3 additional articles: 2 articles duplicated data relevant to this review (15, 32) from other included articles (16, 33), and 1 reported the number of persons assisted but the denominator required to estimate prevalence could not be obtained (34).
Figure 1.
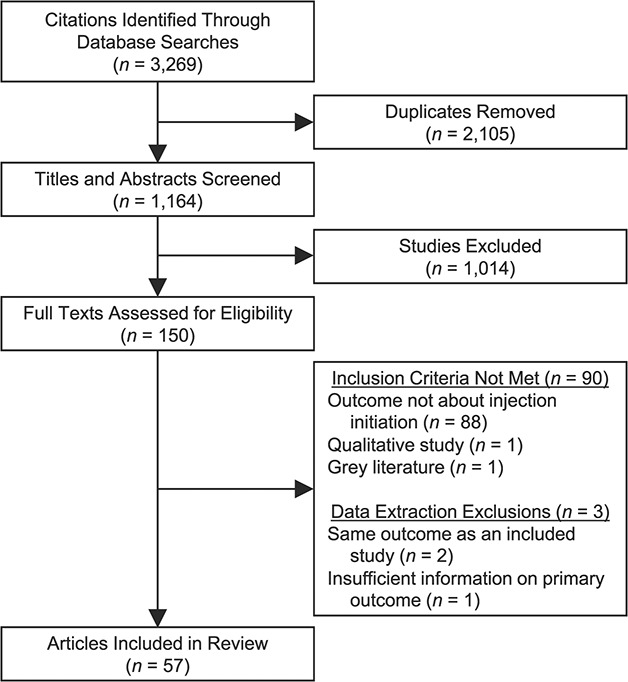
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) diagram for a systematic review of the prevalence of and correlates associated with providing and receiving assistance with injection drug initiation. A systematic search of the literature (from individual database inception to October 9, 2019) identified 3,269 citations representing 1,164 unique texts. After title and abstract screening, 150 full texts were identified as potentially eligible for inclusion. After full text screening, 57 texts met eligibility criteria and were included in the present review.
The 57 included articles were primarily from studies conducted in the United States (n = 20; 35%), Australia (n = 7; 12%), or Canada (n = 7; 12%) (Table 1). Most (n = 51; 89%) were cross-sectional and used convenience sampling (n = 51; 89%). The 3 intervention studies evaluated psychosocial interventions to prevent the provision of IIA (17, 35, 36), and for the purposes of this analysis, we reviewed data from the preintervention phases of these studies. When study quality was evaluated by 2 reviewers independently, there was agreement about the overall study quality rating for 47% (n = 27) of studies, and the remainder of quality ratings were determined through discussion. After adjudication, most studies were rated as being of “fair” quality (n = 41; 72%), whereas 9 were rated “good” and 7 were rated “poor.” The designation of poor study quality was most often assigned because of problems with the analytical approach (n = 5 studies), a lack of detail in the methods related to measuring outcomes of interest (n = 4 studies), and/or lack of clarity regarding participant recruitment and inclusion/exclusion criteria (n = 3 studies). Characteristics about each study’s design, population, quality rating, and other attributes are available in Web Table 2.
Table 1.
Characteristics of 57 Studies Included in a Systematic Review of Articles Reporting on the Prevalence and Correlates of the Receipt and Provision of Assistance With Initiation of Drug Injection
| Characteristic | Total | Studies on Receipt of IIA | Studies on Provision of IIA | |||
|---|---|---|---|---|---|---|
| No. of Studies (n = 57) | % | No. of Studies (n = 40) | % | No. of Studies (n = 24) | % | |
| Country | ||||||
| United States | 20 | 35.1 | 14 | 35.0 | 8 | 33.3 |
| Australia | 7 | 12.3 | 6 | 15.0 | 3 | 12.5 |
| Canada | 7 | 12.3 | 5 | 12.5 | 3 | 12.5 |
| France | 3 | 5.3 | 3 | 7.5 | 0 | 0.0 |
| United Kingdom | 3 | 5.3 | 2 | 5.0 | 1 | 4.2 |
| Brazil | 2 | 3.5 | 2 | 5.0 | 0 | 0.0 |
| Mexico | 2 | 3.5 | 0 | 0.0 | 2 | 8.3 |
| Spain | 2 | 3.5 | 2 | 5.0 | 0 | 0.0 |
| Colombia | 1 | 1.8 | 1 | 2.5 | 0 | 0.0 |
| Estonia | 1 | 1.8 | 0 | 0.0 | 1 | 4.2 |
| India | 1 | 1.8 | 1 | 2.5 | 1 | 4.2 |
| Iran | 1 | 1.8 | 1 | 2.5 | 1 | 4.2 |
| Ireland | 1 | 1.8 | 1 | 2.5 | 0 | 0.0 |
| Vietnam | 1 | 1.8 | 1 | 2.5 | 0 | 0.0 |
| Multiple countries | 5 | 8.8 | 1 | 2.5 | 4 | 16.7 |
| Study design | ||||||
| Cross-sectional study | 51 | 89.5 | 38 | 95.0 | 20 | 83.3 |
| Intervention study (nonrandomized) | 3 | 5.3 | 0 | 0.0 | 3 | 12.5 |
| Cohort study | 3 | 5.3 | 2 | 5.0 | 1 | 4.2 |
| Sampling approach | ||||||
| Community-based convenience sampling | 34 | 59.6 | 22 | 55.0 | 13 | 54.2 |
| Respondent-driven sampling | 5 | 8.8 | 3 | 7.5 | 4 | 16.7 |
| Venue-based convenience sampling | 8 | 14.0 | 8 | 20.0 | 2 | 8.3 |
| Other type or multiple types of samplinga | 10 | 17.5 | 7 | 17.5 | 5 | 20.8 |
| Study quality rating | ||||||
| Good | 9 | 15.8 | 7 | 17.5 | 3 | 12.5 |
| Fair | 41 | 71.9 | 27 | 67.5 | 19 | 79.2 |
| Poor | 7 | 12.3 | 6 | 15.0 | 2 | 8.3 |
| Outcome(s) | ||||||
| Both prevalence and correlates | 39 | 68.4 | 28 | 70.0 | 13 | 54.2 |
| Prevalence only | 13 | 22.8 | 11 | 27.5 | 6 | 25.0 |
| Correlates only | 5 | 8.8 | 1 | 2.5 | 5 | 20.8 |
| Assistance referent group for correlates | ||||||
| Not assisted | 35 | 61.4 | 20 | 50.0 | 18 | 75.0 |
| Not assisted + subset of assisted individuals | 6 | 10.5 | 6 | 15.0 | 0 | 0.0 |
| Both | 3 | 5.3 | 3 | 7.5 | 0 | 0.0 |
| Not applicable (assessed prevalence only) | 13 | 22.8 | 11 | 27.5 | 6 | 25.0 |
| Classification of correlates in Rhodes’ risk environmentsb | ||||||
| Economic environment | 5 | 13.2 | 0 | 0.0 | 5 | 27.8 |
| Physical environment | 12 | 31.6 | 2 | 8.7 | 11 | 61.1 |
| Policy environment | 2 | 5.3 | 1 | 4.3 | 1 | 5.6 |
| Social environment | 30 | 78.9 | 14 | 60.9 | 17 | 94.4 |
| Substance use behaviors | 17 | 44.7 | 4 | 17.4 | 13 | 72.2 |
| Health-related factors | 8 | 21.1 | 5 | 21.7 | 3 | 16.7 |
Abbreviation: IIA, injection initiation assistance.
a Of 9 studies that used multiple methods, all used at least 1 convenience sampling technique. One study (60) used a capture-recapture sampling method, which is listed as “other.”
b One study could assess multiple correlates. Studies that assessed prevalence outcomes only or that compared correlates by assistance group, where the referent category was something other than “not assisted,” were excluded from percent calculations.
Prevalence of receiving IIA
A total of 40 studies documented prevalence and/or correlates of receiving IIA (Table 1, Web Table 3). Among the 39 studies that documented prevalence, we coded 2 outcomes related to receiving IIA: 1) being injected with drugs by another person at the participant’s first injection (Figure 2A and Web Figure 1; n = 29 estimates) and 2) receiving help, guidance, or other assistance with the first injection (Figure 2B and Web Figure 2; n = 13 estimates). There was substantial statistical heterogeneity in both outcomes (I2 > 94% and P < 0.01 for Cochran’s Q statistic), combined with a variety of sampling methods, study settings, and differences in sample characteristics (e.g., age criteria for inclusion, primary substances used, and sex/gender breakdown), which precluded meta-analysis. The range in the prevalence point estimates of being injected by another person during the first injection was 53%–95%, based on 29 estimates. The range in the prevalence of receiving help, guidance, or other assistance at injection initiation was 74%–100%, based on 13 estimates.
Figure 2.
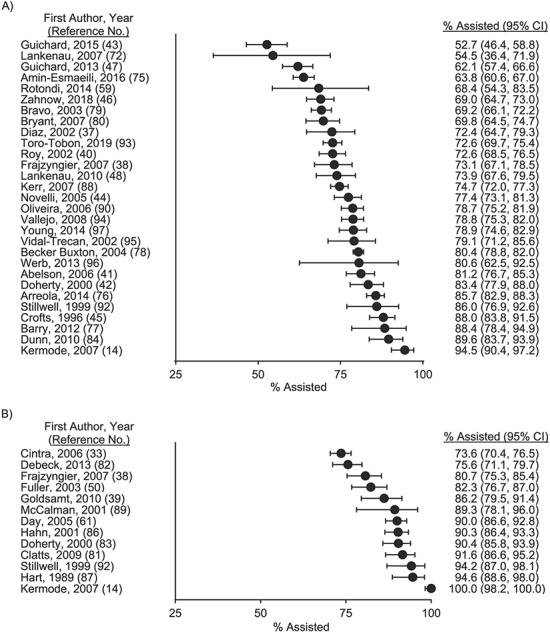
Prevalence of receiving assistance with initiation of drug injection in the published literature through October 9, 2019. The prevalence of being injected with drugs by another person at injection initiation ranged from 53% to 95% across 29 estimates (A). The prevalence of receiving help, guidance, or another type of injection initiation assistance ranged from 74% to 100% across 13 estimates (B). Note: The 2014 prevalence estimate of Rotondi et al. (59) was adjusted for respondent-driven sampling. Bars, 95% confidence intervals (CIs).
Correlates of receiving IIA
A total of 29 studies examined correlates associated with receiving IIA (Table 1). A subset of 23 studies directly compared persons who did and did not receive assistance, and these were organized on the basis of Rhodes’ risk environments (Web Table 4). Correlates arising from the microsocial environment (n = 14 studies; 61%) and individual health characteristics (n = 5 studies; 21%) were most commonly assessed. Figure 3 shows correlates associated with receiving assistance in at least 1 study (results from all correlates examined are shown in Web Table 4).
Figure 3.
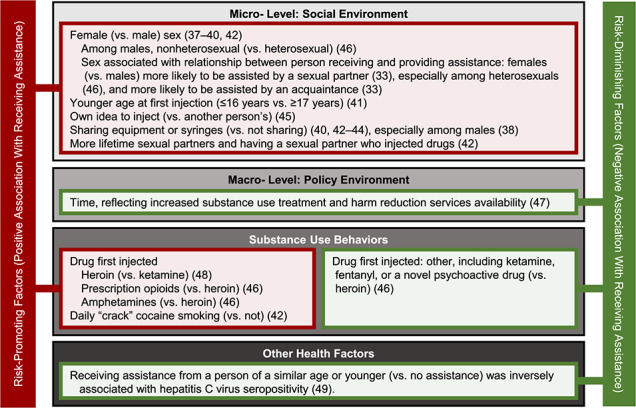
Correlates associated with receiving assistance with initiation of drug injection in 1 or more studies in the published literature through October 9, 2019. Correlates are classified according to Rhodes’ risk environments or other individual characteristics related to health or substance use. Correlates associated with receiving assistance are denoted in red as “risk-promoting” factors, and correlates inversely associated with receiving assistance are denoted in green as “risk-diminishing” factors. Many risk-promoting factors arise from the microsocial risk environment, supporting the potential influence that injecting norms may have on the receipt of assistance.
Among microsocial correlates of receiving assistance, being female (vs. male) (33, 37–40), initiating injection at a younger age (41), ever having had a sexual partner who injected drugs (42), sharing syringes or other injecting equipment (38, 40, 42–44), and reporting that the idea to start injecting drugs came from the person initiating injection (i.e., was the individual’s own idea) (45) were all positively associated with receiving assistance with the first injection. To further examine the association with sex/gender, Zahnow et al. (46) assessed whether the association of sex/gender with receiving assistance from specific types of individuals (e.g., an intimate partner, dealer, or friend vs. self-initiating) differed on the basis of a person’s sexual orientation. They found that the positive association of female (vs. male) sex/gender with being injected with drugs by an intimate partner was only present among heterosexual persons, and that nonheterosexual men (vs. heterosexual men) had elevated odds of being assisted. Sexual orientation also modified the association of sex/gender with being injected by a dealer or friend (vs. oneself) (46). In 2 studies, females were more likely to receive assistance from a sexual or intimate partner than were males (33, 46).
One study examined time trends in the prevalence of receiving IIA and considered the time period (i.e., year) of injection initiation as a proxy measure of the macro-level policy environment. Specifically, using a bivariable ecological analysis, Guichard et al. (47) found that over time, fewer first injections were administered by another person and more first injections occurred alone (i.e., without other people present). Year of injection initiation was conceptualized as a variable reflecting periods of French harm reduction policy expansion and increased availability of addiction treatment; thus, this trend is suggestive of an association of French harm reduction policy with decreases in receiving assistance (47).
Several studies examined associations between individual substance use behaviors and receiving assistance (n = 4). Injecting heroin (vs. ketamine) (48) and prescription opioids or amphetamines (vs. heroin) (46) at the first drug injection were associated with receiving assistance. Daily “crack” cocaine smoking was generally less common among persons who self-initiated drug injecting (5% smoked crack daily) than among persons who received assistance in a bivariable analysis (6% of males who received assistance from another male, 10% of males who received assistance from a female, 23% of females who received assistance from a male, and 32% of females who received assistance from another female smoked crack daily) (42). Among the 5 studies examining health-related factors, Garfein et al. (49) found that participants who did not receive assistance had higher odds of hepatitis C virus infection than those receiving assistance from someone of a similar age or younger than the person initiating injection. The 2 studies that examined correlates of the physical environment found 1) no association between receiving assistance and visiting a “shooting gallery” within the first year following injection initiation (50) and 2) no evidence of geographical differences in receiving assistance in India (14).
Prevalence of providing IIA
A total of 24 studies documented prevalence and/or correlates of providing IIA (Table 1, Web Table 5). These 24 studies were conducted in 8 countries, employed several recruitment techniques (i.e., respondent-driven and convenience sampling methods), and defined 5 different outcomes for the provision of assistance. Of the 5 outcomes coded, we evaluated 2 outcomes with 5 or more estimates of the prevalence of providing IIA for the possibility of meta-analysis: 1) the prevalence of ever providing help, guidance, or other assistance (Figure 4A and Web Figure 3; n = 13 estimates) and 2) the prevalence of providing help, guidance, or other assistance in the past 6 months (Figure 4B and Web Figure 4; n = 6 estimates). Both outcomes had substantial statistical heterogeneity (I2 > 97% and P < 0.01 for Cochran’s Q statistic) and clinical and methodological heterogeneity in terms of the populations under study and sampling techniques applied, which precluded meta-analysis. The range in prevalence estimates of ever providing help, guidance, or other assistance with injecting was 13%–69% (n = 13 estimates). The range in prevalence point estimates of providing help, guidance, or other assistance with injection initiation in the past 6 months was 4%–27% (n = 6 estimates). Other prevalence outcomes with fewer than 5 estimates included ever injecting another person during their first time injecting drugs (n = 4 estimates), injecting another person during their first time injecting in the past 6 months (n = 3 estimates), and providing help, guidance, or other assistance with injection initiation in the past 12 months (n = 2 estimates). These outcomes are summarized in Web Figure 5.
Figure 4.
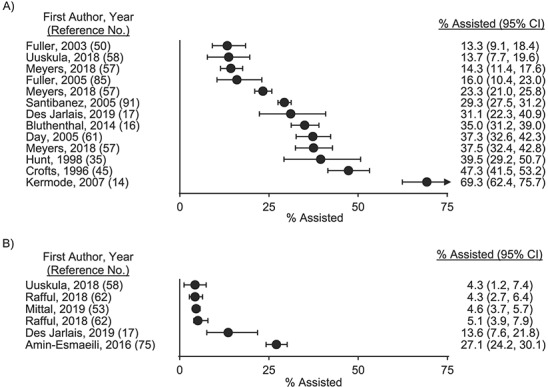
Prevalence of providing assistance with initiation of drug injection in the published literature through October 9, 2019. The prevalence of ever providing help, guidance, or other assistance with someone else’s injection initiation ranged from 13% to 69% across 13 estimates (A). The prevalence of having provided help, guidance, or other assistance with someone else’s injection initiation in the past 6 months ranged from 4% to 27% across 6 estimates (B). Note: The 2018 prevalence estimates of Uuskülaa et al. (58) were adjusted for respondent-driven sampling. Bars, 95% confidence intervals (CIs).
Correlates of providing IIA
A total of 18 studies examined correlates associated with providing IIA (Table 1, Web Table 6). All studies used a referent group of “no assistance provided.” Correlates arising from Rhodes’ microsocial environment were assessed in nearly all studies (n = 17 studies; 94%). Individual substance use characteristics (n = 13 studies; 72%) and correlates from Rhodes’ micro- and macro-physical environments (n = 11 studies; 61%) were also commonly assessed. A summary of correlates associated with providing assistance in at least 1 study is shown in Figure 5 (results from all correlates examined are shown in Web Table 6).
Figure 5.

Correlates associated with providing assistance with initiation of drug injection in 1 or more studies in the published literature through October 9, 2019. Correlates are classified according to Rhodes’ risk environments or other individual characteristics related to health or substance use. Correlates associated with providing assistance are denoted in red as “risk-promoting” factors, and correlates inversely associated with providing assistance are denoted in green as “risk-diminishing” factors. The most well-studied risk-promoting factors arose from the microsocial risk environment, supporting the potential influence that injecting norms and practices during one’s own first injection may have on the provision of assistance. CA, California; HIV, human immunodeficiency virus.
Among 11 studies that examined correlates within the physical environment, micro-level factors, including incarceration or detention in the past year (51) and taking someone else to a place to inject drugs (e.g., a shooting gallery) in the past 6 months (52), were associated with providing assistance. One study found that homelessness in the past 6 months was inversely associated with providing assistance in the past 6 months (53). At the macro- level, 2 studies found that participants living in San Diego, California, versus Tijuana, Mexico, were more likely to provide assistance (54, 55), and 1 US-based study found that participants living in Los Angeles, California, versus San Francisco, California, were more likely to provide assistance (16), suggesting the potential for geographical differences in providing assistance.
Among studies examining correlates from the microsocial environment, being male (vs. female) (56–58), engaging in sex work in the past 6 months (52), and having had any law enforcement interactions in the past 6 months (54) were associated with providing assistance. Several studies found associations between providing assistance and factors related to the social normalization of drug injecting (14, 16, 58–60). Ways in which PWID interacted with injection-naive individuals were associated with providing assistance, including injecting in front of them, describing injection to them, encouraging them to begin injecting, and speaking positively about injecting (16, 59, 60). Related to the norms of PWIDs’ own social networks, having friends who provided IIA was associated with providing IIA in 1 study (58). Additionally, certain characteristics of PWIDs’ own injection initiation experiences were associated with providing assistance, including being taught to inject drugs, having had another person pay for the drugs, and having had friends who injected at the time of their own injection initiation (14). Two studies indicated that recently providing assistance was associated with a higher likelihood of continuing to provide assistance in the future (16, 58).
Other social norms related to injecting, such as providing assistance with injecting to PWID at times other than initiation (16, 51, 52) and recent syringe-sharing (51, 58, 61, 62), were also associated with providing assistance. One study additionally found that informing others about safe injection practices was associated with providing assistance (51). Older persons (53, 54, 56–58, 63) and those who reported being recently or ever injected with drugs by others (at times other than initiation) (45, 51) were less likely to provide IIA.
Among 5 studies that examined correlates from the microeconomic risk environment, providing assistance was associated with currently receiving income assistance (45) or getting income from illegal sources (e.g., selling drugs) in the past 6 months (52), while being recently employed was protective against providing assistance (14, 59). Potentially reflecting the harm reduction micropolicy environment, obtaining injecting equipment from friends or a dealer (vs. another type of source) was associated with providing assistance, though obtaining needles from a syringe services program or pharmacy was not associated with providing assistance in the same study (51).
Among the 13 studies that examined correlates of substance use, several substance use behaviors were associated with providing assistance. Recent use of noninjected cocaine (16, 56), methamphetamine (56), and heroin (56) (all vs. no use of the specified drug), recently or ever injecting methamphetamine (vs. not) (53, 57), recent “speedball” use (i.e., injecting cocaine and heroin at the same time vs. not) (53), lifetime “goofball” use (i.e., injecting methamphetamine and heroin at the same time vs. never) (55), ever injecting more than 1 type of drug (vs. only 1 type) (45), injecting for a longer duration of time (51, 57, 59, 63), and injecting more frequently (52–54, 60) were all associated with providing assistance. Ever or recently being on a medication for opioid use disorder (specifically either methadone or buprenorphine) was protective against providing assistance in 2 studies (53, 63). Finally, ever having been tested for HIV infection (58) and recently having been tested for hepatitis C virus infection (51) were associated with providing assistance.
DISCUSSION
Findings
We systematically reviewed the literature and identified 57 studies that characterized the prevalence and correlates of providing and receiving IIA. We found that receiving assistance from an experienced PWID during the first injection is very common: 53%–95% of participants reported being injected by another person at injection initiation, and 74%–100% reported the receipt of help, guidance, or other assistance during their first injection. The most commonly studied correlates of receiving assistance were related to the microsocial environment of injecting, which may influence norms around providing assistance and interactions between PWID and those who are new to injecting. The provision of IIA was less common and was documented in fewer studies than the receipt of assistance. Estimates of ever providing IIA ranged from 13% to 69%, and 4%–27% of PWID had provided assistance in the past 6 months.
The discrepancy between the prevalence of receiving IIA, a nearly universal phenomenon among injection-naive individuals, versus providing assistance, which was reported by fewer than half of PWID in all but 1 study, has at least 3 potential explanations. First, the subset of PWID who provide assistance may assist with multiple injection initiation events. In 1 study documenting the number of persons assisted, 47% of respondents had assisted no one, 15% had assisted 1 person, 30% had assisted 2–5 people, and 8% had assisted 6 or more people (45). Polydrug injection and receiving income assistance were correlates of providing assistance to multiple people (defined as ≥2 people vs. none).
A second possible explanation is that social desirability bias may lead people to avoid honest reporting on stigmatized behaviors (64–66), such as the provision of IIA (67) and injecting drugs more generally (68, 69), which would result in downwardly biased estimates of the prevalence of providing assistance. Indeed, stigma is recognized as a multidimensional, complex influence on risk and protective behaviors among people who use drugs (70). Qualitative studies suggest that stigmatization of the provision of assistance may influence reporting behaviors regarding assistance and may relate to the provision of assistance in primarily private settings and out of concern for potential harm if the injection-naive person were to attempt their first injection alone (67). Only 1 study included in our review assessed the association of stigma with providing assistance (58). Specifically, Uuskülaa et al. (58) found, using a bivariable analysis, that 2 simple, 1-question measures of self-stigma and anticipated stigma related to being a PWID were unrelated to reporting providing assistance. A more thorough examination of how stigma, shame, and social desirability may impact reporting of the provision of assistance in quantitative studies is needed to ensure accurate measurement of this behavior.
Third, between-study differences in the definition of “providing assistance” may contribute to underreporting of the provision of assistance by some persons. There were 2 general ways in which receiving and providing assistance were assessed: the direct injection of one person by another during the recipient’s first injection and the provision of general advice, guidance, teaching, help, etc., during the recipient’s first injection. Being injected with drugs or performing a first injection was only slightly less common than the receipt or provision of general assistance, suggesting that studies asking about the provision of assistance in a general way (e.g., through help, guidance, etc.) may primarily capture assistance events that involve providing physical help with an injection. It is unknown whether studies that do not specifically define the types of help or guidance characterized as assistance undercapture events where informal teaching or help is in fact provided.
Much of the literature we reviewed focused on factors that arose from the microsocial risk environment and that reflect the influences of intimate partners and gender norms. Women were more likely to be assisted during a first injection than men in several studies (37–40, 42). Sexual orientation influenced gendered risk patterns in 1 study, such that heterosexual females and nonheterosexual males most commonly received assistance as opposed to self-initiating injection (46). Having more sexual partners or a sexual partner who injected drugs were also associated with receiving assistance (42). Taken together, these findings are consistent with qualitative studies identifying varied and complex influences of intimate partnerships on the provision and receipt of assistance (71). Other contextual factors associated with receiving assistance, such as sharing syringes and equipment (38, 40, 42–44), may importantly increase risk for bloodborne virus transmission.
The association of use of specific drugs at the first injection with receipt of assistance may reflect differential skills or experience required to inject certain substances. For example, ketamine is often administered intramuscularly (vs. intravenously) (72), which may explain the finding that injecting heroin at initiation more commonly involved assistance than the first injection involving ketamine (48). The fact that some prescription drugs, such as extended-release formulations (73), require different preparatory steps to ready an injectable dose compared with heroin is consistent with the finding of a higher likelihood of receiving assistance during first injections involving prescription opioids versus heroin (46). The emergence of illicitly manufactured fentanyl in North American drug markets and the increased overdose risk it carries may exacerbate moral dilemmas about the provision of assistance (74). Taken together, these results suggest that changes in illicit drug markets and corresponding injection practices and risks may influence the dynamics of assistance, emphasizing the need for continued monitoring of drug markets.
Like receipt of assistance, correlates situated within the microsocial environment of injecting were also associated with providing IIA. In particular, factors that normalized the provision of IIA (14, 16, 58–60), provision of injection assistance generally (i.e., at times other than initiation) (16, 51, 52), and syringe-sharing (51, 58, 61, 62) were associated with providing assistance. Because city of residence (16, 54, 55) was associated with the provision of assistance, local norms probably play a role in determining whether assistance is provided.
The fact that persons who were tested for HIV (58) or hepatitis C virus (51) or who informed others about safe injection practices were more likely to provide IIA has at least 2 implications for interventions. First, harm reduction programs and other bloodborne virus testing locations may be places to efficiently deliver some of the few currently available psychosocial interventions to prevent the provision of IIA. For example, the Break the Cycle intervention, which draws on social cognitive theory and motivational interviewing to help PWID recognize behaviors that may promote injecting, avoid providing assistance, and minimize harms associated with injecting was recently associated with reductions in the provision of assistance in the United States and Estonia (17). These interventions are consistent with the many aforementioned social factors that influence and normalize the provision of assistance. Relatedly, a second implication of these findings is that persons who provide assistance may be aware of harm reduction services and safe injection behaviors and thus able to share these with network members and injection-naive individuals to whom they provide assistance. Indeed, this type of peer education (e.g., coaching injection-naive individuals about safe injection practices) is a component of currently available interventions (17).
Employment and income security were protective against providing assistance (14, 45, 52, 59), as was being on a medication for opioid use disorder (specifically, an opioid agonist) (53, 63). Sex work (52), incarceration (51), and law enforcement interactions (54) were risk factors for providing assistance. Collectively, these findings suggest that microeconomic factors and underlying substance use may influence the provision of assistance. A recent predictive model suggested that opioid agonist treatment may reduce the risk of providing assistance by 45% and that scaling up opioid agonist treatment to cover 60% of the US PWID population (from a baseline of 21%) could reduce annual injection initiation rates by 23% per year (18). Thus, a continued focus on treating underlying substance use disorders, in addition to addressing socioeconomic and structural risk factors, may have far-reaching benefits for preventing the transition to injecting.
Limitations
Given the exclusion criteria applied, the evidence synthesized in this article was limited to findings from quantitative, empirical studies published in English and containing at least 50 participants. Another review of qualitative studies on the topic of IIA which may have been better designed to incorporate findings from smaller studies that were excluded from this review is available (13). Further, nearly all of the studies were cross-sectional, which limited our ability to tease apart potential causes of receiving and providing IIA from sequelae. Some studies included correlates that followed or were contemporaneous with the receipt of initiation assistance (e.g., syringe-sharing). Longitudinal studies are needed to examine potential causal factors related to receiving or providing assistance.
A quantitative synthesis of prevalence and correlates of IIA was not possible given clinical, methodological, and statistical heterogeneity, including variability in correlates examined, assistance definitions, statistical approaches applied, geographical settings, sampling strategies, and study time frames. The majority of studies were conducted in the United States and other high-income countries. More research is needed to characterize the epidemiology of IIA in low- and middle-income countries. Additionally, the near universality of receiving IIA led many studies to assess predictors of specific relationships between persons initiating drug injection and persons providing assistance (e.g., receiving assistance from an intimate partner), which limited comparability between studies of correlates. Thus, we relied on the methods of narrative synthesis and organized correlates by an existing theoretical framework (11, 19, 20). Finally, several studies required adjudication and discussion to determine a final quality rating.
Conclusions
Nearly all injection-naive individuals reported receiving guidance from a PWID during their first drug injection, whereas fewer PWID reported having assisted someone with initiating injection, and estimates varied widely. Estimates of providing IIA may be downwardly biased because of social desirability bias. The fact that many correlates of providing assistance reflected social norms of injecting supports the premise of current psychosocial interventions, which seek to engage PWID in preventing injection initiation and promoting harm reduction among injection-naive individuals. However, further research is needed to identify effective and scalable interventions that operate on structural determinants of risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: School of Nursing, University of Wisconsin-Madison, Madison, Wisconsin (Rachel E. Gicquelais); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Rachel E. Gicquelais, Becky, L. Genberg, Shruti H. Mehta); Centre on Drug Policy Evaluation, Li Ka Shing Knowledge Institute, St. Michael’s Hospital (Dan Werb, Ayden I. Scheim); Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada (Dan Werb); Department of Medicine, School of Medicine, University of California, San Diego, San Diego, California (Dan Werb, Charles Marks); Joint Doctoral Program in Interdisciplinary Research on Substance Use, School of Social Work, San Diego State University, San Diego, California (Charles Marks); Health Sciences Library, St. Michael’s Hospital, Toronto, Ontario, Canada (Carolyn Ziegler); and Department of Epidemiology and Biostatistics, Dornsife School of Public Health, Drexel University, Philadelphia, Pennsylvania (Ayden I. Scheim).
This work was funded by the National Institute on Drug Abuse (grants DA036297, DA048063, and DP2-DA040256) and the National Institute of Allergy and Infectious Diseases (grant AI102623), National Institutes of Health.
We thank Dr. Roberta Scherer for her feedback on a draft of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1. Joint United Nations Programme on HIV/AIDS . Health, Rights and Drugs—Harm Reduction, Decriminalization and Zero Discrimination for People Who Use Drugs. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2019. (Publication no. UNAIDS/JC2954E). [Google Scholar]
- 2. Colledge S, Peacock A, Leung J, et al. The prevalence of non-fatal overdose among people who inject drugs: a multi-stage systematic review and meta-analysis. Int J Drug Policy. 2019;73:172–184. [DOI] [PubMed] [Google Scholar]
- 3. Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38(6):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iacobucci G. Drug related deaths in Scotland double in 10 years. BMJ. 2017;358:j3941. [DOI] [PubMed] [Google Scholar]
- 5. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452. [DOI] [PubMed] [Google Scholar]
- 6. Martins SS, Sampson L, Cerdá M, et al. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. Am J Public Health. 2015;105(11):e29–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caudarella A, Dong H, Milloy MJ, et al. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 2016;162:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Des Jarlais DC, Nugent A, Solberg A, et al. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337–1341. [DOI] [PubMed] [Google Scholar]
- 9. Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5(12):e1208–e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathers B, Degenhardt L, Bucello C, et al. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(2):102–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Werb D, Garfein R, Kerr T, et al. A socio-structural approach to preventing injection drug use initiation: rationale for the PRIMER Study. Harm Reduct J. 2016;13(1):Article 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vlahov D, Fuller CM, Ompad DC, et al. Updating the infection risk reduction hierarchy: preventing transition into injection. J Urban Health. 2004;81(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guise A, Horyniak D, Melo J, et al. The experience of initiating injection drug use and its social context: a qualitative systematic review and thematic synthesis. Addiction. 2017;112(12):2098–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kermode M, Longleng V, Singh BC, et al. My first time: initiation into injecting drug use in Manipur and Nagaland, north-east India. Harm Reduct J. 2007;4:Article 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bluthenthal RN, Wenger L, Chu D, et al. Factors associated with being asked to initiate someone into injection drug use. Drug Alcohol Depend. 2015;149:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bluthenthal RN, Wenger L, Chu D, et al. Factors associated with initiating someone into illicit drug injection. Drug Alcohol Depend. 2014;144:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Des Jarlais D, Uusküla A, Talu A, et al. Implementing an updated “Break the Cycle” intervention to reduce initiating persons into injecting drug use in an Eastern European and a US “opioid epidemic” setting. AIDS Behav. 2019;23(9):2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marks C, Borquez A, Jain S, et al. Opioid agonist treatment scale-up and the initiation of injection drug use: a dynamic modeling analysis. PLoS Med. 2019;16(11):e1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhodes T. The ‘risk environment’: a framework for understanding and reducing drug-related harm. Int J Drug Policy. 2002;13(2):85–94. [Google Scholar]
- 20. Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy. 2009;20(3):193–201. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 23. Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions . Version 6.0 (updated July 2019). London, United Kingdom: The Cochrane Collaboration; 2019. www.training.cochrane.org/handbook. Accessed December 15, 2019. [Google Scholar]
- 24. Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. 1st ed. Chichester, United Kingdom: John Wiley & Sons Ltd.; 2000. [Google Scholar]
- 25. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 27. Partlett C, Riley RD. Random effects meta-analysis: coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med. 2017;36(2):301–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell M, McKenzie JE, Sowden A, et al. Synthesis Without Meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 30. Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product From the ESRC Methods Programme. Lancaster, United Kingdom: Lancaster University; 2006. [Google Scholar]
- 31. National Heart, Lung, and Blood Institute . Study Quality Assessment Tools. Bethesda, MD: National Heart, Lung, and Blood Institute; 2014. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed November 14, 2019. [Google Scholar]
- 32. Caiaffa WT, Bastos FI, Proietti FA, et al. Practices surrounding syringe acquisition and disposal: effects of syringe exchange programmes from different Brazilian regions—the AjUDE-Brasil II Project. Int J Drug Policy. 2003;14(5-6):365–371. [Google Scholar]
- 33. Cintra AM, Caiaffa WT, Mingoti SA, et al. Characteristics of male and female injecting drug users of the AjUDE-Brasil II Project. Cad Saude Publica. 2006;22(4):791–802. [DOI] [PubMed] [Google Scholar]
- 34. Powis B, Griffiths P, Gossop M, et al. The differences between male and female drug users: community samples of heroin and cocaine users compared. Subst Use Misuse. 1996;31(5):529–543. [DOI] [PubMed] [Google Scholar]
- 35. Hunt N, Stillwell G, Taylor C, et al. Evaluation of a brief intervention to prevent initiation into injecting. Drugs Educ Prev Policy. 1998;5(2):185–194. [Google Scholar]
- 36. Strike C, Rotondi M, Kolla G, et al. Interrupting the social processes linked with initiation of injection drug use: results from a pilot study. Drug Alcohol Depend. 2014;137:48–54. [DOI] [PubMed] [Google Scholar]
- 37. Diaz T, Vlahov D, Edwards V, et al. Sex-specific differences in circumstances of initiation into injecting-drug use among young adult Latinos in Harlem, New York City. AIDS Behav. 2002;6(2):117–122. [Google Scholar]
- 38. Frajzyngier V, Neaigus A, Gyarmathy VA, et al. Gender differences in injection risk behaviors at the first injection episode. Drug Alcohol Depend. 2007;89(2-3):145–152. [DOI] [PubMed] [Google Scholar]
- 39. Goldsamt LA, Harocopos A, Kobrak P, et al. Circumstances, pedagogy and rationales for injection initiation among new drug injectors. J Community Health. 2010;35(3):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roy E, Haley N, Leclerc P, et al. Drug injection among street youth: the first time. Addiction. 2002;97(8):1003–1009. [DOI] [PubMed] [Google Scholar]
- 41. Abelson J, Treloar C, Crawford J, et al. Some characteristics of early-onset injection drug users prior to and at the time of their first injection. Addiction. 2006;101(4):548–555. [DOI] [PubMed] [Google Scholar]
- 42. Doherty MC, Garfein RS, Monterroso E, et al. Gender differences in the initiation of injection drug use among young adults. J Urban Health. 2000;77(3):396–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guichard A, Guignard R, Lert F, et al. Risk factors associated with unsafe injection practices at the first injection episode among intravenous drug users in France: results from PrimInject, an Internet survey. J Addict. 2015;2015:507214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Novelli LA, Sherman SG, Havens JR, et al. Circumstances surrounding the first injection experience and their association with future syringe sharing behaviors in young urban injection drug users. Drug Alcohol Depend. 2005;77(3):303–309. [DOI] [PubMed] [Google Scholar]
- 45. Crofts N, Louie R, Rosenthal D, et al. The first hit: circumstances surrounding initiation into injecting. Addiction. 1996;91(8):1187–1196. [DOI] [PubMed] [Google Scholar]
- 46. Zahnow R, Winstock AR, Maier LJ, et al. Injecting drug use: gendered risk. Int J Drug Policy. 2018;56:81–91. [DOI] [PubMed] [Google Scholar]
- 47. Guichard A, Guignard R, Michels D, et al. Changing patterns of first injection across key periods of the French harm reduction policy: PrimInject, a cross sectional analysis. Drug Alcohol Depend. 2013;133(1):254–261. [DOI] [PubMed] [Google Scholar]
- 48. Lankenau SE, Wagner KD, Jackson Bloom J, et al. The first injection event: differences among heroin, methamphetamine, cocaine, and ketamine initiates. J Drug Issues. 2010;40(2):241–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garfein RS, Doherty MC, Monterroso ER, et al. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S11–S19. [DOI] [PubMed] [Google Scholar]
- 50. Fuller CM, Vlahov D, Latkin CA, et al. Social circumstances of initiation of injection drug use and early shooting gallery attendance: implications for HIV intervention among adolescent and young adult injection drug users. J Acquir Immune Defic Syndr. 2003;32(1):86–93. [DOI] [PubMed] [Google Scholar]
- 51. Bryant J, Treloar C. Initiators: an examination of young injecting drug users who initiate others to injecting. AIDS Behav. 2008;12(6):885–890. [DOI] [PubMed] [Google Scholar]
- 52. Navarro S, Kral AH, Strike CS, et al. Factors associated with frequency of recent initiation of others into injection drug use among people who inject drugs in Los Angeles and San Francisco, CA, USA, 2016–17. Subst Use Misuse. 2019;54(10):1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mittal ML, Jain S, Sun S, et al. Opioid agonist treatment and the process of injection drug use initiation. Drug Alcohol Depend. 2019;197:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Melo JS, Garfein RS, Hayashi K, et al. Do law enforcement interactions reduce the initiation of injection drug use? An investigation in three North American settings. Drug Alcohol Depend. 2018;182:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rafful C, Melo J, Medina-Mora ME, et al. Cross-border migration and initiation of others into drug injecting in Tijuana, Mexico. Drug Alcohol Rev. 2018;37(suppl 1):S277–S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ben Hamida A, Rafful C, Jain S, et al. Non-injection drug use and injection initiation assistance among people who inject drugs in Tijuana, Mexico. J Urban Health. 2018;95(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meyers SA, Scheim A, Jain S, et al. Gender differences in the provision of injection initiation assistance: a comparison of three North American settings. Harm Reduct J. 2018;15(1):Article 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uusküla A, Barnes DM, Raag M, et al. Frequency and factors associated with providing injection initiation assistance in Tallinn, Estonia. Drug Alcohol Depend. 2018;188:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rotondi NK, Strike C, Kolla G, et al. Transition to injection drug use: the role of initiators. AIDS Behav. 2014;18(3):486–494. [DOI] [PubMed] [Google Scholar]
- 60. White RH, O’Rourke A, Bluthenthal RN, et al. Initiating persons into injection drug use in rural West Virginia, USA. Subst Use Misuse. 2020;55(2):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Day CA, Ross J, Dietze P, et al. Initiation to heroin injecting among heroin users in Sydney, Australia: cross sectional survey. Harm Reduct J. 2005;2(1):Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rafful C, Jain S, Sun X, et al. Identification of a syndemic of blood-borne disease transmission and injection drug use initiation at the US-Mexico border. J Acquir Immune Defic Syndr. 2018;79(5):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mittal ML, Vashishtha D, Sun S, et al. History of medication-assisted treatment and its association with initiating others into injection drug use in San Diego, CA. Subst Abuse Treat Prev Policy. 2017;12(1):Article 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Latkin CA, Vlahov D, Anthony JC. Socially desirable responding and self-reported HIV infection risk behaviors among intravenous drug users. Addiction. 1993;88(4):517–526. [DOI] [PubMed] [Google Scholar]
- 65. Latkin CA, Vlahov D. Socially desirable response tendency as a correlate of accuracy of self-reported HIV serostatus for HIV seropositive injection drug users. Addiction. 1998;93(8):1191–1197. [DOI] [PubMed] [Google Scholar]
- 66. Macalino GE, Celentano DD, Latkin C, et al. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ Prev. 2002;14(5):367–378. [DOI] [PubMed] [Google Scholar]
- 67. Guise A, Melo J, Mittal ML, et al. A fragmented code: the moral and structural context for providing assistance with injection drug use initiation in San Diego, USA. Int J Drug Policy. 2018;55:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rhodes T, Watts L, Davies S, et al. Risk, shame and the public injector: a qualitative study of drug injecting in South Wales. Soc Sci Med. 2007;65(3):572–585. [DOI] [PubMed] [Google Scholar]
- 69. Simmonds L, Coomber R. Injecting drug users: a stigmatised and stigmatising population. Int J Drug Policy. 2009;20(2):121–130. [DOI] [PubMed] [Google Scholar]
- 70. McCradden MD, Vasileva D, Orchanian-Cheff A, et al. Ambiguous identities of drugs and people: a scoping review of opioid-related stigma. Int J Drug Policy. 2019;74:205–215. [DOI] [PubMed] [Google Scholar]
- 71. Meyers SA, Smith LR, Mittal ML, et al. The role of gender and power dynamics in injection initiation events within intimate partnerships in the US-Mexico border region. Cult Health Sex. 2020;22(9):1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lankenau SE, Sanders B, Bloom JJ, et al. First injection of ketamine among young injection drug users (IDUs) in three U.S. cities. Drug Alcohol Depend. 2007;87(2-3):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Broz D, Zibbell J, Foote C, et al. Multiple injections per injection episode: high-risk injection practice among people who injected pills during the 2015 HIV outbreak in Indiana. Int J Drug Policy. 2018;52:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Olding M, Werb D, Guise A, et al. Navigating social norms of injection initiation assistance during an overdose crisis: a qualitative study of the perspectives of people who inject drugs (PWID) in Vancouver, Canada. Int J Drug Policy. 2019;69:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Amin-Esmaeili M, Rahimi-Movaghar A, Gholamrezaei M, et al. Profile of people who inject drugs in Tehran, Iran. Acta Med Iran. 2016;54(12):793–805. [PubMed] [Google Scholar]
- 76. Arreola S, Bluthenthal RN, Wenger L, et al. Characteristics of people who initiate injection drug use later in life. Drug Alcohol Depend. 2014;138:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barry D, Syed H, Smyth BP. The journey into injecting heroin use. Heroin Addict Relat Clin Probl. 2012;14(3):89–100. [Google Scholar]
- 78. Becker Buxton M, Vlahov D, Strathdee SA, et al. Association between injection practices and duration of injection among recently initiated injection drug users. Drug Alcohol Depend. 2004;75(2):177–183. [DOI] [PubMed] [Google Scholar]
- 79. Bravo MJ, Barrio G, de la Fuente L, et al. Reasons for selecting an initial route of heroin administration and for subsequent transitions during a severe HIV epidemic. Addiction. 2003;98(6):749–760. [DOI] [PubMed] [Google Scholar]
- 80. Bryant J, Treloar C. The gendered context of initiation to injecting drug use: evidence for women as active initiates. Drug Alcohol Rev. 2007;26(3):287–293. [DOI] [PubMed] [Google Scholar]
- 81. Clatts MC, Colón-López V, Giang LM, et al. Prevalence and incidence of HCV infection among Vietnam heroin users with recent onset of injection. J Urban Health. 2010;87(2):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Debeck K, Kerr T, Marshall BD, et al. Risk factors for progression to regular injection drug use among street-involved youth in a Canadian setting. Drug Alcohol Depend. 2013;133(2):468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Doherty MC, Garfein RS, Monterroso E, et al. Correlates of HIV infection among young adult short-term injection drug users. AIDS. 2000;14(6):717–726. [DOI] [PubMed] [Google Scholar]
- 84. Dunn M, Degenhardt L, Bruno R. Transition to and from injecting drug use among regular ecstasy users. Addict Behav. 2010;35(10):909–912. [DOI] [PubMed] [Google Scholar]
- 85. Fuller CM, Borrell LN, Latkin CA, et al. Effects of race, neighborhood, and social network on age at initiation of injection drug use. Am J Public Health. 2005;95(4):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34(1):180–187. [DOI] [PubMed] [Google Scholar]
- 87. Hart GJ, Sonnex C, Petherick A, et al. Risk behaviours for HIV infection among injecting drug users attending a drug dependency clinic. BMJ. 1989;298(6680):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kerr T, Tyndall MW, Zhang R, et al. Circumstances of first injection among illicit drug users accessing a medically supervised safer injection facility. Am J Public Health. 2007;97(7):1228–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCalman J, Gilbert J. Preventing hepatitis C infection among young injecting drug users in Cairns. Health Promot J Austr. 2001;12(3):265–267. [Google Scholar]
- 90. Oliveira ML, Hacker MA, de Oliveira SAN, et al. “The first shot”: the context of first injection of illicit drugs, ongoing injecting practices, and hepatitis C infection in Rio de Janeiro, Brazil. Cad Saude Publica. 2006;22(4):861–870. [DOI] [PubMed] [Google Scholar]
- 91. Santibañez SS, Garfein RS, Swartzendruber A, et al. Prevalence and correlates of crack-cocaine injection among young injection drug users in the United States, 1997–1999. Drug Alcohol Depend. 2005;77(3):227–233. [DOI] [PubMed] [Google Scholar]
- 92. Stillwell G, Hunt N, Taylor C, et al. The modelling of injecting behaviour and initiation into injecting. Addiction Res. 1999;7(5):447–459. [Google Scholar]
- 93. Toro-Tobón D, Berbesi-Fernandez D, Trejos-Castillo E, et al. Gender differences in risky injection practices among people who inject drugs in Colombia. Addict Disorders Their Treat. 2019;18(3):140–148. [Google Scholar]
- 94. Vallejo F, Toro C, de la Fuente L, et al. Prevalence of and risk factors for hepatitis B virus infection among street-recruited young injection and non-injection heroin users in Barcelona, Madrid and Seville. Eur Addict Res. 2008;14(3):116–124. [DOI] [PubMed] [Google Scholar]
- 95. Vidal-Trecan GM, Varescon-Pousson I, Boissonnas A. Injection risk behaviors at the first and at the most recent injections among drug users. Drug Alcohol Depend. 2002;66(2):107–109. [DOI] [PubMed] [Google Scholar]
- 96. Werb D, Kerr T, Buxton J, et al. Crystal methamphetamine and initiation of injection drug use among street-involved youth in a Canadian setting. Can Med Assoc J. 2013;185(18):1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Young AM, Larian N, Havens JR. Gender differences in circumstances surrounding first injection experience of rural injection drug users in the United States. Drug Alcohol Depend. 2014;134:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


