Abstract
Aims
In 2003, an Australian woman was convicted by a jury of smothering and killing her four children over a 10-year period. Each child died suddenly and unexpectedly during a sleep period, at ages ranging from 19 days to 18 months. In 2019 we were asked to investigate if a genetic cause could explain the children’s deaths as part of an inquiry into the mother’s convictions.
Methods and results
Whole genomes or exomes of the mother and her four children were sequenced. Functional analysis of a novel CALM2 variant was performed by measuring Ca2+-binding affinity, interaction with calcium channels and channel function. We found two children had a novel calmodulin variant (CALM2 G114R) that was inherited maternally. Three genes (CALM1-3) encode identical calmodulin proteins. A variant in the corresponding residue of CALM3 (G114W) was recently reported in a child who died suddenly at age 4 and a sibling who suffered a cardiac arrest at age 5. We show that CALM2 G114R impairs calmodulin's ability to bind calcium and regulate two pivotal calcium channels (CaV1.2 and RyR2) involved in cardiac excitation contraction coupling. The deleterious effects of G114R are similar to those produced by G114W and N98S, which are considered arrhythmogenic and cause sudden cardiac death in children.
Conclusion
A novel functional calmodulin variant (G114R) predicted to cause idiopathic ventricular fibrillation, catecholaminergic polymorphic ventricular tachycardia, or mild long QT syndrome was present in two children. A fatal arrhythmic event may have been triggered by their intercurrent infections. Thus, calmodulinopathy emerges as a reasonable explanation for a natural cause of their deaths.
Keywords: Sudden unexpected death, CALM2, BSN, Calmodulinopathy, Infanticide
Graphical Abstract
What’s new
This study identifies a novel and functional calmodulin variant (CALM2 G114R) present in a mother convicted of infanticide and her two female children.
Biochemical and electrophysiological studies of the G114R variant show that it has deleterious effects on calcium binding and regulation of the two pivotal calcium channels involved in cardiac excitation contraction coupling, CaV1.2 and RyR2, in a similar manner to that of the pathogenic G114W and N98S variants.
Given the biophysical and functional impact of the CALM2 G114R variant, we consider the variant likely precipitated the natural deaths of the two female children (Child 3 and 4).
The two male children (Child 1 and 2) carried biallelic rare missense variants in BSN, a gene shown to cause early onset lethal epilepsy in mice when deleted.
Introduction
Few diagnostic odysseys involve such high stakes as the quest to determine whether recurring sudden unexpected deaths (SUD) of children in a single family are the result of natural causes or infanticide. In the late 1990s and early 2000s, a series of high profile convictions of British mothers involved the statistical testimony of eminent paediatrician, Sir Roy Meadow, in support of his general rule that ‘one sudden infant death is a tragedy, two is suspicious and three is murder, until proved otherwise’.1 While the maxim has since been discredited and several mothers, including Sally Clark and Angela Cannings have had their convictions overturned,2,3 an Australian woman has remained imprisoned.
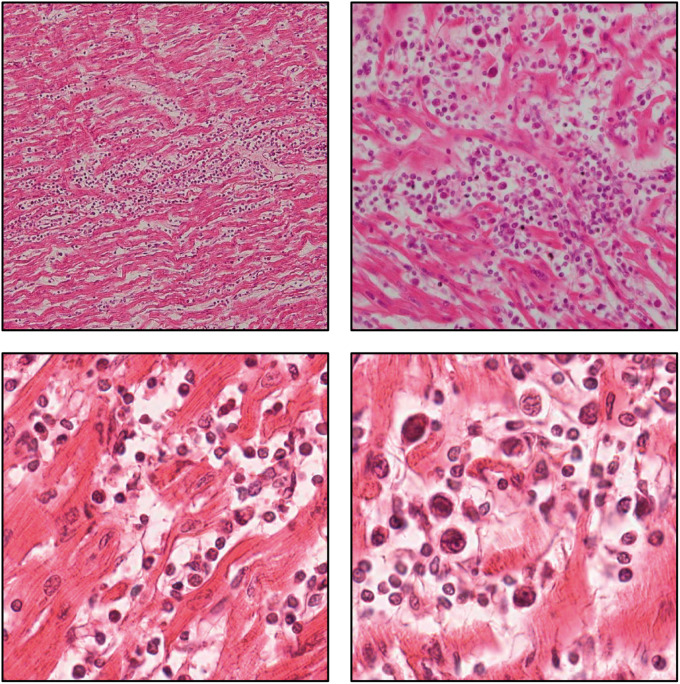
In 2001, a mother from Newcastle in New South Wales was arrested and charged with smothering and killing her four young children. Each child died suddenly during periods of rest over the course of 10 years (1989–99) (Supplementary material online, p. 16, Table S1) Child 1 (male) died at 19 days having been diagnosed with ‘laryngomalacia’. Child 2 (male) died at 8 months from asphyxia due to airway obstruction in turn due to epileptic fits from an encephalopathic disorder of unknown cause associated with blindness. Child 3 (female) died at 10 months old, four days after seeing her general practitioner for a croupy cough and being started on antibiotics (flucloxacillin); autopsy findings included a congested and haemorrhagic uvula and profuse α-haemolytic Streptococcus in lung cultures. Child 4 (female) died at 18 months old, two days after being treated with paracetamol and pseudoephedrine for a respiratory infection. At autopsy she was found to have florid myocarditis (Figure 1) and spleen cultures grew profuse α-haemolytic Streptococcus of two colonial types and moderate Staphylococcus aureus (Supplementary material online, p. 16, Table S1).4
Figure 1.
H&E staining of cardiac sections from Child 4’s autopsy. Inflammatory infiltrates are seen in the myocardium associated in places with myocyte necrosis (magnification: 100–400×).
Circumstantial evidence against the mother consisted primarily of tendency and coincidence evidence echoing Meadow's Law, and seemingly inculpatory diary entries.5 This evidence was thought to outweigh that in favour of death by natural causes.5 In 2003, a jury found the mother guilty of the manslaughter of Child 1 and the murders of the remaining three children, notwithstanding that there was no history of abuse, no physical signs of smothering, no obvious motive, no eye witnesses, and no admission of guilt.5 Psychiatrists that assessed the mother in the early 2000s considered her not to be suffering from any psychiatric illness that could explain infanticide, such as personality disorder, psychopathy, psychosis, schizophrenia, bipolar disorder, or Munchausen’s syndrome by proxy. A psychiatrist that provided evidence to the inquiry and assessed the mother in March 2019 agreed that she did not suffer from a psychiatric illness that could explain infanticide but considered her to be suffering from complex post-traumatic stress disorder dating to her childhood.6,7 To date the mother has maintained her innocence.
In March 2019, a judicial inquiry into the mother's convictions commenced due to concerns raised by several forensic pathologists regarding the medical evidence provided at the trial. The results presented here follow on from genomic investigations conducted at the request of the inquiry. The inquiry concluded before we completed our functional analysis and the mother remains in prison. All the clinical, pathology, and legal case information, including the mother’s prison health records, and the children’s medical and autopsy records are publicly available in the exhibits to the inquiry and can be accessed via: https://www.folbigginquiry.justice.nsw.gov.au/Pages/documents.aspx.
The hypothesis that a genetic variant causing respiratory failure or arrhythmia could explain the SUD of the children provided the rationale for genomic studies. The four children suffered from conditions of varying clinical significance (Supplementary material online, p. 16, Table S1). Child 1 (male) had difficulty breathing from birth attributed to laryngomalacia, Child 2 (male) suffered epileptic seizures from age 4.5 months until his death, Child 3 (female) likely had a respiratory infection at the time of her death, and Child 4 (female) had myocarditis visible on autopsy (Figure 1). The children’s various conditions suggested that if there was a single underlying natural cause it was likely to have a spectrum of clinical manifestations and lethal triggers. Inherited arrhythmogenic disorders capable of causing sudden cardiac death (SCD) fit this profile and may first manifest as SUD.8
Unfortunately, the cardiological assessments of the children were limited. There were no ECG recordings of Child 1. ECG recordings of Child 2 from the early neonatal period and at 4 months of age showed normal sinus rhythm and normal intervals including a QTc of 380 ms. There were no ECG recordings of Child 3. ECG recordings of Child 4 at age 11 days showed normal sinus rhythm and normal intervals including QTc of 380 ms. Aside from Child 4's myocarditis, examination of each child’s heart did not reveal cardiac abnormalities.
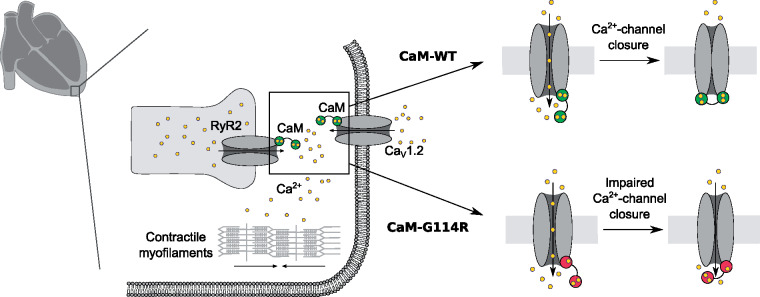
Calmodulinopathies are rare life-threatening arrhythmia syndromes caused by pathogenic variants in one of the three genes (CALM1, CALM2, and CALM3) that encode identical calmodulin proteins.9 Calmodulin (CaM) is a calcium-binding protein that regulates an array of enzymes and ion channels in a calcium-dependent manner, including those essential for regulating cardiac contractility. Specifically, CaM is key to the calcium-dependent inactivation (CDI) of cell membrane calcium channel CaV1.2, and for timely closure of the cardiac sarcoplasmic reticulum (SR) calcium release channel, RyR2. The first human CaM missense mutation was identified in 2012 in a large Swedish family segregating with catecholaminergic polymorphic ventricular tachycardia (CPVT) and multiple cases of SCD.10 The first CaM mutations associated with long QT syndrome (LQTS) and cardiac arrest in infants were identified in 2013.11 The International Calmodulinopathy Registry (ICalmR) (n = 74; 35 different pathogenic genotypes) reveals variability in clinical manifestations. Long QT syndrome (49%) and CPVT (28%) present at a median age of 1 year and 6 months and 6 years, respectively. Other presentations include idiopathic ventricular fibrillation (IVF), SUD, overlapping features of CPVT/LQTS and predominant neurological phenotype, while 13.5% are asymptomatic.9
Methods
Human subjects and DNA sequencing
The mother provided informed written consent to conduct genomic research and testing of herself and her children. The mother's genomic study complies with ethical regulations of the Australian National University and Australian Capital Territory Health Human Ethics Committees. Whole genome sequencing (WGS) of Child 1 and whole exome sequencing (WES) of Child 4 was performed from neonatal screening cards and WGS of the mother, Child 2, and Child 3 from buccal swab, frozen liver tissue, and a fibroblast cell line respectively. The average coding exon coverage was 77× for the mother, 45× for Child 1, 59× for Child 2, 47× for Child 3, and 172× for Child 4. The coverage was calculated after removal of ambiguously mapped and orphaned reads. Bioinformatics analysis is described in Supplementary material online, pp 4-5. The mother agrees to share the genetic and clinical data of her children upon request for research purposes. Upon request, L.C. and P.J.S. will share the ICalmR data not publically available.
Calmodulin protein production and purification
Calmodulin variants were expressed in an Escherichia coli Rosetta (DE3) strain from a modified pET vector with an N-terminal maltose-binding protein and tobacco etch virus cleavage site. Calmodulin purification is described in Supplementary material online, p. 5.
Secondary structure determination by circular dichroism
Circular dichroism spectra were recorded at 37°C using a Chirascan-Plus circular dichroism spectrometer as described previously and summarized in Supplementary material, p. 5.12
Calcium binding
Calcium titration experiments were performed as described previously and summarized in Supplementary material, p. 5.13,14
Calmodulin binding to the CaV1.2 IQ-domain and RyR2 CaMBD2
Peptides representing the CaV1.2 IQ-domain and RyR2 CaM-binding domain 2 (CaMBD2) (Supplementary material, pp. 5–6) with an N-terminal 5-carboxytetramethylrhodamine (5-TAMRA) label were obtained from Proteogenix (>95% purity). Calmodulin binding to the IQ-domain and CaMBD2 peptides was monitored by fluorescence anisotropy of the 5-TAMRA-labelled peptide (Supplementary material, p. 6). Binding was measured at a range of Ca2+ concentrations in the presence of 1 mM free Mg2+, controlled by the Ca2+/Mg2+-buffer system described previously and in Supplementary material, p. 5.
Cellular electrophysiology of CaV1.2
CaM mutations were generated on the human CALM1 gene in the pIRES2-EGFP vector (Clontech Laboratories, Inc.).15 For whole-cell patch clamp experiments, HEK293 cells (ATCC) were transiently transfected with human cardiac α1C cDNA,16 rat brain β2a (M80545), rat brain α2δ (NM012919.2) subunits, CaM variants, and simian virus 40T antigen using a standard calcium phosphate method. The details of voltage-clamp recordings, solutions, and quantification of currents can be found in Supplementary material, p. 6.
Store overload induced calcium release assay
Calcium levels in the endoplasmic reticulum of HEK293 cells expressing RyR2 and CaM variants were monitored using single-cell Ca2+ imaging and the Ca2+-sensitive fluorescence resonance energy transfer-based cameleon protein D1ER, as previously described and detailed in Supplementary material, p. 6.17
Results
The mother reported recurrent episodes consistent with transient loss of consciousness that started in childhood. These episodes have been associated with intercurrent illnesses, physical exertion including swimming and running, emotional stress, and pregnancy (Supplementary material, p. 16, Table S1). Her ECGs, cardiac exercise stress test, and 24 h Holter were normal.
We performed WGS of the mother and the first three children and WES of the fourth child. The children’s father declined requests to provide a DNA sample, and was not ordered to provide one by the Inquiry. Twenty-nine ultrarare [minor allele frequency (MAF) < 0.0005] variants occurred in 4/4 children (Supplementary material, p. 17, Table S2) in genes not known to cause SUD. Next, we searched specifically for ultrarare variants in 424 genes extracted from a literature review of causes of SUD, SCD, SIDS, cardiac or arrhythmogenic conditions, and epileptic encephalopathy (Supplementary material, p. 18, Table S3) and identified variants in 12 genes: CACNA1E, CACNA1G, CALM2, CTNNA3, DMD, DMPK, DOCK7, JUP, KCNAB2, MYH6, MYLK, SCNN1A, and BSN (Supplementary material, p. 19, Table S4). In addition, analysis of immune variants identified two missense variants associated in the literature with susceptibility to bacterial lung infections (PTPN13, 4/4 children)18 or myocarditis (NLRP1, novel, 3/4 children)19 (Supplementary material, p. 19, Table S5). Child 2’s genome was found to carry a novel IDS variant located in the same codon as a known Hunter’s Sd mutation (Supplementary material, p. 19, Table S6).
Of the 12 cardiac/SCD/SUD/epilepsy variants, only CALM2 and KCNAB2 caused human genetic disorders associated with SUD according to the OMIM catalogue. The KCNAB2 variant (p.Ala11Thr, A11T; c31G>A, MAF = 0.000004) was present in 3/4 children. Although we have shown previously that gain-of-function missense mutations in adjacent residues (R12Q and L13F) are associated with Brugada syndrome,20 functional analysis of KCNAB2 A11T was normal (Supplementary material, pp. 3, 9, 20, Figure S2, and Table S7). The two male children (Child 1 and Child 2) had biallelic rare missense variants in BSN (Bassoon): p. L1898M (MAF = 0.000008) and p. A355T (MAF = 0.0022), both predicted to be damaging (Supplementary material, p. 19, Table S4). BSN is important for presynaptic function and for formation and function of photoreceptor ribbon synapses of the mammalian retina.21 In mice, BSN deficiency causes early onset severe epilepsy that is 50% lethal in the first 6 months of life.22 To date, heterozygous BSN variants have been associated with an adult-onset progressive-supranuclear palsy-like syndrome23 but an autosomal recessive form of this disease has not yet been described.
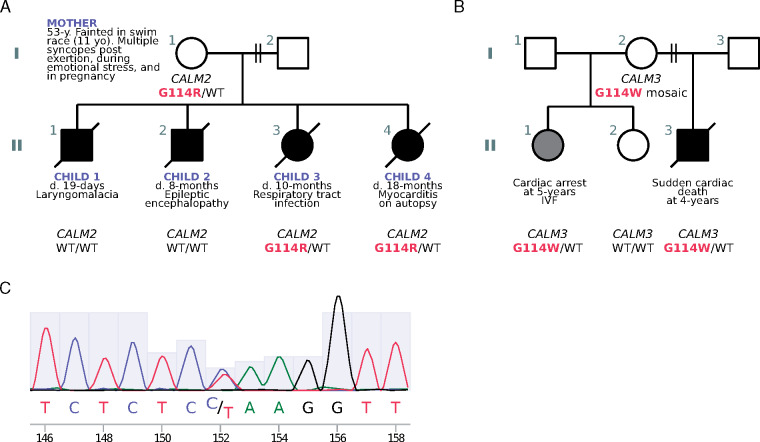
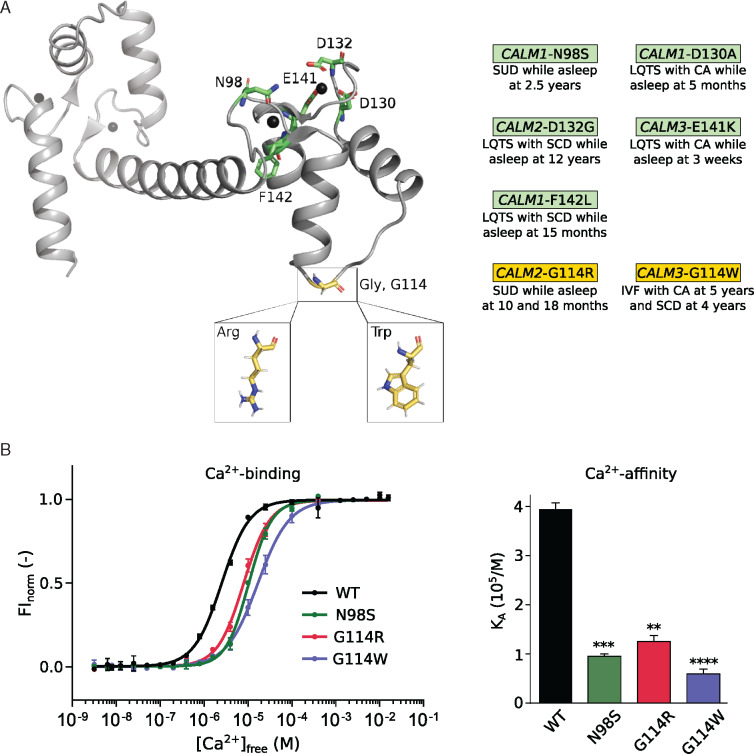
The CALM2 variant (p.Gly114Arg, G114R; c.340G>A) was present in the mother and the two female children (Child 3 and Child 4) (Figure 2A and C and Supplementary material, pp. 8, 19, Figure S1, and Table S4). DNA from the mother’s parents was not available. Neither Sanger sequencing nor WGS/WES of the mother’s saliva and buccal swab samples was suggestive of mosaicism for CALM2 G114R [saliva: 76 reference (C): 84 variant (T) reads; buccal swab: 106 reference (C): 104 variant (T) reads] and a blood sample could not be obtained. Sanger sequencing could not be performed on Child 3 and Child 4 due to lack of access to additional DNA. The CALM2 G114R variant is not found in databases of human genetic variation (ExAC, gnomAD, 1000 genomes, ClinVar). We have not observed the variant in 2580 in-house genomes sequenced at the Australian National University or in genomes from 2485 elderly Australians (MGRB).24 Overall, CALM2 is highly intolerant to missense substitution.25 G114R is predicted as damaging by multiple in silico tools; and the phenotype is specific for disease (SUD in infancy and childhood).26 Indeed, five different CaM mutations causing sudden death or cardiac arrest in children while asleep have been reported (three of them in children <2 years old), all of which are physically close to the G114 residue in the three-dimensional structure of CaM (Figure 3A).
Figure 2.
Pedigrees of kindreds with G114R and G114W CALM mutations and Sanger sequencing. (A) Family pedigree (kindred A) showing inheritance of the CALM2 gene and the protein variation glycine (G) at position 114 to arginine (R). (B) Pedigree showing inheritance of the CALM3 gene and protein variant G114W (tryptophan; W) in kindred B. Shaded symbols represent affected individuals with SUD/SCD/CA. WT=wildtype. (C) Sanger sequencing from the mother’s buccal swab highlighting the C→T variant.
Figure 3.
G114R and G114W mutations impair the calcium binding strength of CaM. (A) Three-dimensional structure of CaM (PDB: 1cll). Ca2+-ions are displayed as black spheres. Mutations at green residues have been associated with SUD/CA during sleep in other patients. (B) Ca2+-binding curves (left) of the C-terminal domain of CaM variants N98S, G114R, and G114W, showing reduced Ca2+ affinity (right), a likely proarrhythmic substrate. Data displayed as mean ± SD. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 using one-way ANOVA with Dunnett’s post hoc test against CaM-WT. CA, cardiac arrest; CaM, calmodulin; FInorm, normalized fluorescence intensity; IVF, idiopathic ventricular fibrillation; KA, association constant; LQTS, long QT syndrome; SCD, sudden cardiac death; SUD, sudden unexplained death.
Residue G114 in CaM is highly conserved and located in the linker domain between EF-hand III-IV (Figure 3A). The CALM2 G114R variant results in a unique amino acid substitution, but a different substitution in the corresponding codon of CALM3 (G114W) is known to be pathogenic (Figure 2B).9CALM3 G114W was identified in two siblings, a girl who died suddenly at age 5 and a brother who suffered a cardiac arrest at age 4 (Figure 2B).9 Their mother was healthy and found to be a mosaic, with no apparent cardiac phenotype.
Ca2+-sensing and Ca2+-channel regulation by CaM is crucial for maintaining a stable and healthy heart rhythm (Supplementary material, pp. 3–4). Further investigations revealed that CaM-G114R decreased the Ca2+-binding affinity of the C-domain of CaM, with a similar magnitude of effect to that observed with known arrhythmia-causing mutations, G114W and N98S (Figure 3B and Supplementary material, p. 20, Table S8). CaM-N98S was included for comparison, as this is one of the best characterized CaM mutations and it has been found in 10 unrelated individuals (5 in CALM1 and 5 in CALM2), causing either SUD (including in a 2-year-old while asleep), IVF, LQTS, CPVT, or a mixed LQTS/CPVT phenotype.9 We postulated that the reduced Ca2+-binding affinity results from a structural change conferred by the G114R mutation and observed that in the presence and absence of Ca2+, CaM-G114R contains more alpha-helices and fewer turns and disordered regions (Supplementary material, pp. 10–11, Figures S3 and S4). The structural effect of CaM-G114R was comparable to that of the arrhythmogenic G114W mutation. Thus, both mutations increase the structural stability of the CaM C-domain.
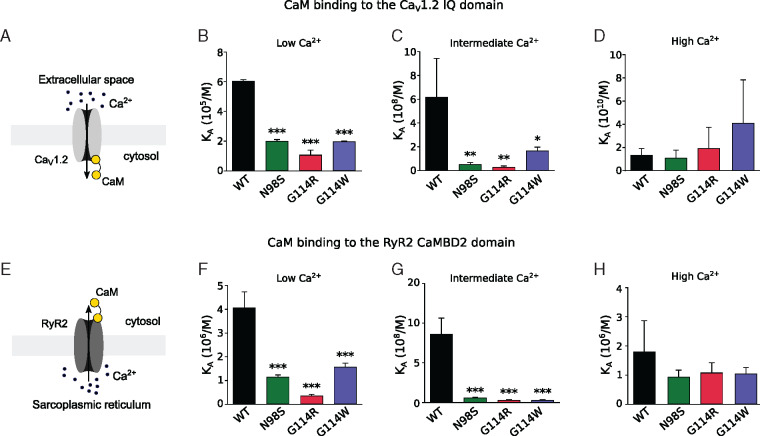
We next investigated the effect of CaM-G114R on calcium flux by investigating CaM binding to the IQ-domain and CaM-binding domain 2 (CaMBD2) of the CaV1.2 and RyR2 cardiac Ca2+-channels, respectively. We found that the G114R mutation reduces the affinity of CaM for both the IQ-domain (Figure 4A–C) and CaMBD2 (Figure 4E–G) at low and medium Ca2+-concentrations at least as much as the known arrhythmogenic N98S and G114W variants. At high Ca2+-concentrations, there was no significant difference between the CaM variants (Figure 4D and H). Thus, the G114R variant mutes the Ca2+-sensing action of CaM when complexed with the canonical binding domains from CaV1.2 and RyR2 with an effect similar to or larger than pathogenic variants G114W and N98S (Supplementary material, pp. 12–13, Figures S5 and S6).
Figure 4.
G114R and G114W mutations impair the binding of CaM to calcium channels. (A–D) Ca2+-dependent binding of CaM to the IQ-domain of CaV1.2. Reduced binding of CaM variants N98S, G114R, and G114W unbalances the fine regulation of CaV1.2 by CaM at low and intermediate Ca2+ concentrations, predisposing to an altered Ca2+ inflow through CaV1.2. (E–H) Similarly, the binding to the RyR2 CaMBD2 domain was decreased at low and intermediate Ca2+ concentrations, suggesting an altered Ca2+ outflow from the sarcoplasmic reticulum. *P≤0.05, **P≤0.01, and ***P≤0.001 using one-way ANOVA with Dunnett’s post hoc test against CaM-WT values. CaM, calmodulin.
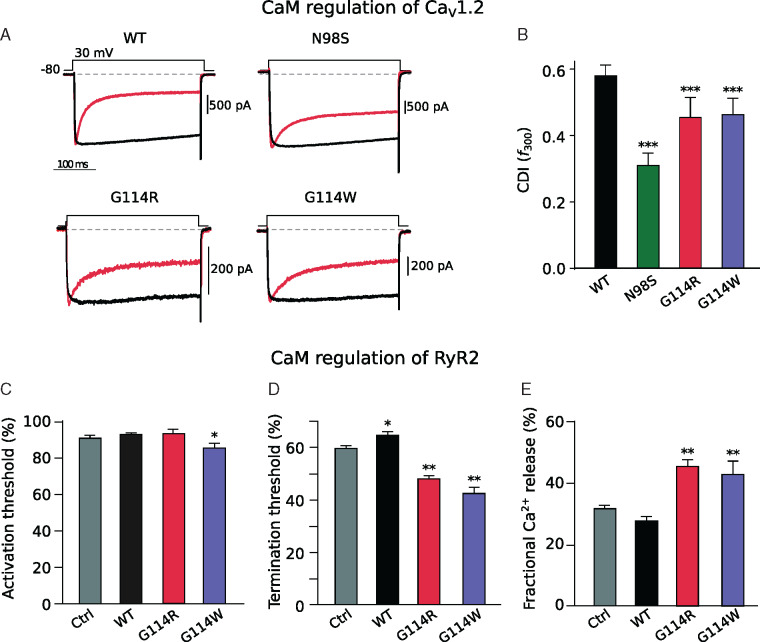
We also investigated the action of CaM-G114R on intact CaV1.2 and RyR2 channel function. Calmodulin controls the CDI of CaV1.2 (Supplementary material, pp. 3–4, 14, Figure S7). We observed that CaM-G114R decreased CDI of CaV1.2 to the same extent as CaM-G114W but less than CaM-N98S (Figure 5A, B and Supplementary material, pp. 3–4, 14, Figure S7), which has been linked to a mixed CPVT/LQTS phenotype. In contrast, the most damaging CaM variants that cause severe LQTS, such as D96V, F142L, or D130G, fully abolish CDI.15 Finally, we investigated the action of CaM on RyR2 closure in a negative-feedback mechanism (Supplementary material, pp. 4, 15, Figure S8).17 Normally, CaM exerts its CDI effect on RyR2 by increasing the SR luminal Ca2+-termination threshold for store-overload induced Ca2+ release (SOICR). We observed that CaM-G114R decreased the termination threshold for SOICR, equivalent to the effect observed for CaM-G114W and CaM-N98S14 (Figure 5D). Curiously, CaM-G114W, but not CaM-G114R, also significantly reduced the SOICR activation threshold compared to CaM-WT (Figure 5C). All three mutations resulted in a significant increase in fractional Ca2+ release during SOICR14 (Figure 5E). In summary, these functional studies reveal that CaM-G114R fails to close the CaV1.2 and RyR2 channels normally, which leads to excess transport of Ca2+ through both channels. This dysregulation mechanism is common to arrhythmogenic CaM variants.27
Figure 5.
G114R and G114W mutations cause delayed closure of calcium channels CaV1.2 and RyR2. (A) Exemplar traces of electrophysiological recordings of CaV1.2 opening (downward deflection) and subsequent CaM-mediated calcium-dependent inactivation (CDI) (return towards baseline) during a 30 mV voltage clamp in the presence of Ca2+ (red) or Ba2+ (black). (B) CDI as quantified by the metric f300, measured at 30 mV. All three CaM mutations significantly reduce CDI, thus increasing the Ca2+ inflow through CaV1.2 and potentially leading to a pathogenic QT prolongation. ***P < 0.001 using Student’s t-test. Quantified RyR2 channel opening (C) and closure (D) propensity as well as the amount of Ca2+ released (E) during an open event in store-overload induced calcium release (SOICR) assay. The CaM variant G114W significantly reduced the activation threshold, and the variants G114R and G114W significantly altered the termination threshold and fractional calcium release, potentially leading to an arrhythmogenic enhanced intracellular Ca2+ level. Data are displayed as mean ± SEM. *P ≤ 0.05 and **P ≤ 0.01 using one-way ANOVA with post hoc test. CaM, calmodulin; CaV1.2, voltage-gated calcium channel isoform 1.2; RyR2, ryanodine receptor type 2; WT, wild type.
Discussion
Our main findings are that the mother and her two female children (Child 3 and Child 4), were carriers of a novel and functional variant in the CALM2 gene associated with sudden death in infancy and childhood, and that the variant is predicted to be arrhythmogenic. The variant found in the female children (G114R) affects the same amino acid as a variant in an otherwise identical protein encoded by CALM3 (G114W), which was implicated in the SCD of one child and the cardiac arrest of another. The G114R and G114W variants conferred similar structural changes in CaM. Both variants compromise the ability of CaM to sense changes in Ca2+ concentration, and impair binding to, and regulation of, the two cardiac Ca2+-channels CaV1.2 and RyR2. These effects are also similar to those observed with CaM-N98S, which has been found in 10 unrelated patients diagnosed with either LQTS, CPVT, a mixed phenotype, IVF, or suffering from SUD.9 It is noteworthy that one N98S carrier experienced SUD while asleep at 2 years of age.9 Sudden death during sleep is not an exceptional presentation of calmodulinopathies: of the 74 patients reported in the ICalmR, 20 suffered SUD/SCD and 5 of these (25%) died suddenly or suffered cardiac arrests in their sleep. Of those five, three occurred in children below age 2.9
Mechanistically and functionally, calmodulinopathies follow a surprisingly consistent scheme.9 For almost all described cases, CALM mutations specifically affect the heart and cause arrhythmia, fibrillation, and/or cardiac arrest.28In vitro assessment of CALM mutations, has revealed an elegant correlation between the clinical manifestation and their impact on (i) Ca2+ sensing and structure of the CaM protein and (ii) how CaM regulates the two Ca2+-channels CaV1.2 and RyR2.27 In most cases, the clinical impact is directly reflected in the regulation of these two Ca2+-channel targets. Calmodulin mutations that severely reduce Ca2+-binding to the C-domain and completely abolish CDI are present in patients with severe early onset LQTS.15 CPVT-associated CaM mutations consistently exert a smaller impact on Ca2+-binding, and minimal or no effect on CaV1.2 regulation, and have little or no effect on the QT interval.13,15 Dysregulation of RyR2 Ca2+-release is generally considered the underlying cause of CPVT, and this is also the effect observed for all CPVT-associated CaM mutations.14,29
Based on the previous studies and the functional data presented here, we predict that the G114R and G114W variants are pathogenic and that carriers are therefore prone to cardiac arrhythmias of IVF or CPVT-like phenotypes with a potential component of mild LQTS, which could cause cardiac arrest during sleep. This prediction is based on the following observations: (i) Both mutations impair RyR2 binding and regulation to a similar extent as other known CPVT-associated CaM mutations29; (ii) Both mutations impair CaV1.2 CDI to an intermediary extent, consistent with a possible mild LQTS, but likely not severe LQTS15; (iii) The mutations are not among the most severe in terms of reduced Ca2+ sensing, likely due to the location in a linker region between Ca2+-binding sites; (iv) The effect of the mutations are experimentally similar to the N98S variant, which has been associated with LQTS, CPVT, IVF, or a mixed phenotype; and (v) SUD at a young age has been observed for a number of other calmodulinopathy cases,9 including a carrier of an N98S mutation (SUD while asleep) which displays a similar functional impact as the G114R and G114W mutations. Of note, the girl with the G114W mutation who suffered a CA was diagnosed with IVF.9
In this family, the CALM2 variant was inherited from their extant mother, who did not appear to be a mosaic. Incomplete penetrance or variable expressivity occur frequently in heritable cardiac arrhythmia syndromes,8,30 including calmodulinopathies.31 There are multiple reports of the same CaM mutation giving rise to different clinical manifestations/phenotypes in different individuals, including families, in which the parental carrier has relatively mild symptoms, such as frequent syncope as was the case with the mother, while the children suffer from life-threatening arrhythmias.9,10,31,32 Familial cases of CALM-CPVT and CALM-IVF with asymptomatic carriers have been previously reported.9,32 For example, Marsman et al.32 reported a family with a CALM1 variant (F90L) that caused IVF in childhood and adolescence. Five children inherited the variant from their mother. Four children suffered cardiac arrests or sudden death, but the fifth child (age 14 years) and the mother (age 60 years) remain asymptomatic after 7 years of follow-up. The ICalmR reports that 13.5% of patients with pathogenic CALM variants have experienced no cardiac events.31
Phenotypic variation is often the consequence of modifier genes.33 These are genetic variants capable of either increasing or decreasing the clinical severity produced by a disease-causing mutation. Recently a mechanism of action of a protective modifier gene has been described for LQTS: the family members carrying this protective variant, which compensates the loss of potassium current produced by the disease-causing mutation, had a completely normal ECG and QT interval.34
Phenotypic variation also raises the possibility of environmental, iatrogenic, or infectious triggers. Fever has been shown to be a trigger of cardiac arrhythmias and SCD/SUD in childhood.35 Both Child 3 and Child 4 suffered from respiratory infections immediately prior to their deaths. At present, no research has addressed the influence of infection or fever on the arrhythmogenic risk of calmodulinopathy patients. Serious infections might have caused an elevated catecholamine state,36,37 which has been associated with trigger activity due to delayed after-depolarizations under conditions of calcium dysregulation.38 Furthermore, Child 4 was treated with pseudoephedrine, a sympathomimetic that activates α- and β-adrenergic receptors and causes release of endogenous catecholamines, which are the triggers of CPVT. She also suffered from myocarditis, which is thought to be a major cause of SCD in the first two decades of life.39 Combinations of these factors may have triggered the lethal events.
Child 1 and Child 2 were compound heterozygotes for rare missense variants in BSN (Bassoon) whose deficiency is known to cause early onset lethal epilepsy in mice.22BSN is a gene that appears to be highly intolerant to loss-of-function (pLI = 1). These variants require further investigation to determine whether they might account for Child 2’s severe epilepsy and blindness.21
Conclusions
The data presented here indicate that molecular autopsies including WES/WGS are warranted for SUD infanticide cases where the accused was convicted on purely circumstantial evidence. The growing understanding of inherited cardiac arrhythmias8 forces a reassessment of the medical foundations, never challenged, on which several mothers were found guilty of infanticide on the basis of assumption instead of evidence. The genomic revolution heralds a new era for the assessment of recurring familial sudden deaths of infants and children, an era that reasserts the presumption of innocence for tragically unlucky families.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We are grateful to Luca Sala for constructive criticism and Stephen Cordner for providing the cardiac photomicrographs. We acknowledge Ángela Romero Martínez for help with cataloguing relevant susceptibility genes from the literature, Rong Liang and Mingri Liu from APF NGS team for technical assistant in DNA extraction, WES and Sanger sequencing as well as the ACRF Biomolecular Resource Facility, JCSMR, ANU for NovaSeq 6000, and Sanger sequencing run.
Funding
This work was supported by grants from NHMRC CRE (C.G.V., M.C.C., and M.A.F.), Lundbeck Foundation (H.H.J., R250-2017–134 and M.T.O., R324-2019–1933), CIHR (S.R.W.C.) and a Heart and Stroke Foundation of Canada grant and Chair in Cardiovascular Research (S.R.W.C.), and the Leducq Foundation for Cardiovascular Research grant 18CVD05 ‘Towards Precision Medicine with Human iPSCs for Cardiac Channelopathies’ (L.C. and P.J.S.).
Conflict of interest: C.G.V. and T.A. provided unpaid expert testimony in person representing the Canberra Genetics team on April 2019 at the Inquiry into the convictions of the mother. C.G.V., T.A., M.C.C., and M.A. contributed to the written submissions of the Canberra Expert Genetics team from March to July 2019 at the Inquiry (unpaid). D.A.W. provided assistance to the mother's legal representatives and to the scientists and medical practitioners involved in authoring this paper; he received no financial support or other remuneration for doing so.
Data availability
The data underlying this article are available in the article and in its online supplementary material, or will be shared on reasonable request to the corresponding authors.
References
- 1. Meadow SA. ABC of child abuse. 3rd ed. In Meadow R, Mok JYQ, Rosenberg D, editors, London: BMJ Publishing Group; 1997. Presumed to be citing DiMaio DJ, DiMaio VJM. Asphyxia. Forensic pathology. New York: Elsevier, 1989. 207–51. [Google Scholar]
- 2.R v Sally Clark. EWCA Crim 1020, 2003. http://www.bailii.org/ew/cases/EWCA/Crim/2003/1020.html (6 August 2020, date last accessed).
- 3.R v Angela Cannings. EWCA Crim 1, 2004. https://www.bailii.org/ew/cases/EWCA/Crim/2004/1.html (6 August 2020, date last accessed).
- 4.Evidence to Inquiry into the Convictions of Kathleen Megan Folbigg: New South Wales, Forensic Medicine and Coroner’s Court Complex in Lidcombe, Exhibit H – Forensic pathology tender bundle. https://www.folbigginquiry.justice.nsw.gov.au/Pages/exhibits.aspx (6 August 2020, date last accessed).
- 5.New South Wales, Inquiry into the Convictions of Kathleen Megan Folbigg: Report, 2019. https://www.folbigginquiry.justice.nsw.gov.au/Pages/report.aspx (6 August 2020, date last accessed).
- 6.Evidence to Inquiry into the Convictions of Kathleen Megan Folbigg. New South Wales, Forensic Medicine and Coroner’s Court Complex in Lidcombe, Exhibit BB—Report of Dr Bruce Westmore dated 16 June 2003 (formerly Exhibit 1 on sentence); Exhibit BC—Report of Dr Yvonne Skinner dated 22 January 2003 (formerly Exhibit C on sentence); Exhibit BD Report of Dr Michael Giuffrida dated 27 August 2003 (formerly Exhibit 2 on sentence). https://www.folbigginquiry.justice.nsw.gov.au/Pages/exhibits.aspx (6 August 2020, date last accessed).
- 7.Evidence to Inquiry into the Convictions of Kathleen Megan Folbigg. New South Wales, Forensic Medicine and Coroner’s Court Complex in Lidcombe, Exhibit BA – Report of Dr Michael Diamond dated 16 April 2019. https://www.folbigginquiry.justice.nsw.gov.au/Pages/exhibits.aspx (6 August 2020, date last accessed).
- 8. Schwartz PJ, Ackerman MJ, Antzelevitch C, Bezzina C, Borggrefe M, Cuneo B. et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers 2020;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crotti L, Spazzolini C, Tester DJ, Ghidoni A, Baruteau AE, Beckmann BM. et al. Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur Heart J 2019;40:2964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nyegaard M, Overgaard MT, Søndergaard MT, Vranas M, Behr ER, Hildebrandt LL. et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet 2012;91:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crotti L, Johnson CN, Graf E, De Ferrari GM, Cuneo BF, Ovadia M. et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 2013;127:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Brohus M, Holt C, Overgaard MT, Wimmer R, Van Petegem F.. Arrhythmia mutations in calmodulin can disrupt cooperativity of Ca(2+) binding and cause misfolding. J Physiol 2020;598:1169–86. [DOI] [PubMed] [Google Scholar]
- 13. Søndergaard MT, Sorensen AB, Skov LL, Kjaer-Sorensen K, Bauer MC, Nyegaard M. et al. Calmodulin mutations causing catecholaminergic polymorphic ventricular tachycardia confer opposing functional and biophysical molecular changes. FEBS J 2015;282:803–16. [DOI] [PubMed] [Google Scholar]
- 14. Søndergaard MT, Tian X, Liu Y, Wang R, Chazin WJ, Chen SR. et al. Arrhythmogenic calmodulin mutations affect the activation and termination of cardiac ryanodine receptor-mediated Ca2+ release. J Biol Chem 2015;290:26151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Limpitikul WB, Dick IE, Joshi-Mukherjee R, Overgaard MT, George AL Jr, Yue DT.. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca(2+) currents and promote proarrhythmic behavior in ventricular myocytes. J Mol Cell Cardiol 2014;74:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dick IE, Joshi-Mukherjee R, Yang W, Yue DT.. Arrhythmogenesis in Timothy syndrome is associated with defects in Ca(2+)-dependent inactivation. Nat Commun 2016;7:10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones PP, Jiang D, Bolstad J, Hunt DJ, Zhang L, Demaurex N. et al. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem J 2008;412:171–8. [DOI] [PubMed] [Google Scholar]
- 18. Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ.. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity 2007;26:163–76. [DOI] [PubMed] [Google Scholar]
- 19. Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA. et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 2012;37:1009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Portero VL, Scouarnec S, Es-Salah-Lamoureux Z, Burel S, Gourraud JB, Bonnaud S. et al. Dysfunction of the voltage-gated K+ channel β2 subunit in a familial case of Brugada syndrome. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dick O, Tom Dieck S, Altrock WD, Ammermüller J, Weiler R, Garner CC. et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 2003;37:775–86. [DOI] [PubMed] [Google Scholar]
- 22. Altrock WD, Tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C. et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron 2003;37:787–800. [DOI] [PubMed] [Google Scholar]
- 23. Yabe I, Yaguchi H, Kato Y, Miki Y, Takahashi H, Tanikawa S. et al. Mutations in bassoon in individuals with familial and sporadic progressive supranuclear palsy-like syndrome. Sci Rep 2018;8:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinese M, Lacaze P, Rath EM, Stone A, Brion M-J, Ameur A. et al. The Medical Genome Reference Bank contains whole genome and phenotype data of 2570 healthy elderly. Nat Commun 2020;11:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C.. MetaDome: pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat 2019;40:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards S, on behalf of the ACMG Laboratory Quality Assurance Committee,Aziz N, Bale S, Bick D, Das S, Gastier-Foster J. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen HH, Brohus M, Nyegaard M, Overgaard MT.. Human calmodulin mutations. Front Mol Neurosci 2018;11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sorensen AB, Søndergaard MT, Overgaard MT.. Calmodulin in a heartbeat. FEBS J 2013;280:5511–32. [DOI] [PubMed] [Google Scholar]
- 29. Søndergaard MT, Liu Y, Brohus M, Guo W, Nani A, Carvajal C. et al. Diminished inhibition and facilitated activation of RyR2-mediated Ca(2+) release is a common defect of arrhythmogenic calmodulin mutations. FEBS J 2019;286:4554–78. [DOI] [PubMed] [Google Scholar]
- 30. Giudicessi JR, Ackerman MJ.. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl Res 2013;161:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyegaard M, Overgaard MT.. The International Calmodulinopathy Registry: recording the diverse phenotypic spectrum of un-CALM hearts. Eur Heart J 2019;40:2976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marsman RF, Barc J, Beekman L, Alders M, Dooijes D, van den Wijngaard A. et al. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J Am Coll Cardiol 2014;63:259–66. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz PJ, Crotti L, George AL Jr.. Modifier genes for sudden cardiac death. Eur Heart J 2018;39:3925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee YK, Sala L, Mura M, Rocchetti M, Pedrazzini M, Ran X. et al. MTMR4 SNVs modulate ion channel degradation and clinical severity in congenital long QT syndrome: insights in the mechanism of action of protective modifier genes. Cardiovasc Res 2020; 10.1093/cvr/cvaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasquié JL. May fever trigger ventricular fibrillation? Indian Pacing Electrophysiol J 2005;5:139–45. [PMC free article] [PubMed] [Google Scholar]
- 36. Sakata Y, Morimoto A, Murakami N.. Changes in plasma catecholamines during fever induced by bacterial endotoxin and interleukin-1 beta. JJP 1994;44:693–703. [DOI] [PubMed] [Google Scholar]
- 37. Hahn PY, Wang P, Tait SM, Ba ZF, Reich SS, Chaudry IH.. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock 1995;4:269–73. [DOI] [PubMed] [Google Scholar]
- 38. Offerhaus JA, Bezzina CR, Wilde AAM. Epidemiology of inherited arrhythmias. Nature Reviews Cardiology 2020;17:205–15. [DOI] [PubMed] [Google Scholar]
- 39. Tse G, Yeo JM, Chan YW, Lai ET, Yan BP. What Is the Arrhythmic Substrate in Viral Myocarditis? Insights from Clinical and Animal Studies. Front Physiol 2016;7:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material, or will be shared on reasonable request to the corresponding authors.