Abstract
Thyroid hormone (T3) plays pivotal roles in vertebrate development, acting via nuclear T3 receptors (TRs) that regulate gene transcription by promoting post-translational modifications to histones. Methylation of cytosine residues in deoxyribonucleic acid (DNA) also modulates gene transcription, and our recent finding of predominant DNA demethylation in the brain of Xenopus tadpoles at metamorphosis, a T3-dependent developmental process, caused us to hypothesize that T3 induces these changes in vivo. Treatment of premetamorphic tadpoles with T3 for 24 or 48 hours increased immunoreactivity in several brain regions for the DNA demethylation intermediates 5-hydroxymethylcytosine (5-hmC) and 5-carboxylcytosine, and the methylcytosine dioxygenase ten-eleven translocation 3 (TET3). Thyroid hormone treatment induced locus-specific DNA demethylation in proximity to known T3 response elements within the DNA methyltransferase 3a and Krüppel-like factor 9 genes, analyzed by 5-hmC immunoprecipitation and methylation sensitive restriction enzyme digest. Chromatin-immunoprecipitation (ChIP) assay showed that T3 induced TET3 recruitment to these loci. Furthermore, the messenger ribonucleic acid for several genes encoding DNA demethylation enzymes were induced by T3 in a time-dependent manner in tadpole brain. A TR ChIP-sequencing experiment identified putative TR binding sites at several of these genes, and we provide multiple lines of evidence to support that tet2 contains a bona fide T3 response element. Our findings show that T3 can promote DNA demethylation in developing tadpole brain, in part by promoting TET3 recruitment to discrete genomic regions, and by inducing genes that encode DNA demethylation enzymes.
Keywords: thyroid hormone, Xenopus, metamorphosis, DNA methylation, chromatin, brain development
Thyroid hormone (T3) is well known to be essential to postembryonic development in vertebrates. It is critical for normal neurological development in mammals, influencing neurogenesis, cell migration, differentiation, circuit formation, and myelin production among other processes (1). Thyroid hormone deficiency during human fetal and neonatal development leads to a condition of severe mental retardation and deafness known as cretinism (2). Another well studied role for T3 is the control of amphibian tadpole metamorphosis, where it orchestrates the suite of gene regulation changes that underlie tissue morphogenesis (3). The hormone binds to nuclear T3 receptors (TRs) that function as ligand-activated transcription factors, and all vertebrates studied have two TR genes, designated alpha and beta (4, 5). The TRs act primarily as heterodimers with retinoid X receptor (RXR), and bind to T3 response elements (TREs) in deoxyribonucleic acid (DNA) to regulate gene transcription (5). In the absence of ligand, TR-RXR heterodimers bind DNA and recruit co-repressors that in turn recruit histone deacetylases to create a compact chromatin structure, leading to transcriptional repression. Upon hormone binding, the co-repressors are exchanged for co-activators, some with histone acetyltransferase activity, which generates an open chromatin structure and transcriptional activation (5–7).
The role of T3 in regulating histone modifications has been studied extensively. Another important epigenetic change that affects chromatin structure and gene transcription is the methylation of cytosine residues in DNA. Methylation of DNA is found in most plant and animal species, and it plays important roles in regulating tissue-specific gene expression, X chromosome inactivation, genomic imprinting, and genome stability (8–15). It is catalyzed by DNA methyltransferases (DNMTs), which transfer methyl groups from the donor S-adenyl methionine to the fifth carbon of cytosine in DNA (5-mC) (16). Four DNMT enzymes have been identified in most vertebrates: DNMT1, DNMT3A, DNMT3B, and DNMT3L (17); DNMT1, 3A, and 3B are required for normal embryonic development (16). DNA methylation is found throughout vertebrate genomes, at gene promoters, within gene bodies, and at intergenic regions, predominantly in the context of CpG dinucleotides (18). Approximately 70% to 80% of CpGs are methylated, and the remaining unmethylated CpGs occur primarily in stretches of 500 to 2000 bp that have a high CpG density and are termed CpG islands (19, 20). Changes in DNA methylation at gene promoters, enhancers, and gene bodies can modulate gene transcription, with increased methylation typically associated with transcriptional repression, and decreased methylation associated with gene activation (16, 21–29); although, there are examples of positive correlations between gene body methylation and transcription in plant and animal genomes (17, 30–33).
DNA methylation can be reversed through the passive removal of the methyl group from 5-mC through successive rounds of DNA replication, or by active demethylation via a series of enzyme-catalyzed reactions. The ten-eleven translocation (TET) family of methylcytosine dioxygenases (TET1, TET2, and TET3) catalyze active DNA demethylation by oxidizing 5-mC to the intermediates 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC), and 5-carboxylcytosine (5-caC) (23, 34, 35). Recent findings show that 5-hmC is enriched at enhancers, promoters, and bodies of actively transcribed genes (21, 36–43). The isocitrate dehydrogenase family of enzymes (IDH1/2/3) provide the substrate α-ketoglutarate for TET-mediated oxidation of 5-mC (44, 45). The oxidized intermediates are then removed through the base-excision repair pathway catalyzed by thymine DNA glycosylase (TDG) (46, 47), or by nucleotide excision repair pathways catalyzed by growth arrest and DNA damage (GADD45) enzymes (23, 48). There is also evidence that 5-hmC can be deaminated to unmethylated cytosine by the actions of activity-induced cytidine deaminase (AID) and the apolipoprotein B messenger ribonucleic acid (mRNA) editing enzyme, catalytic polypeptide (APOBEC) (49). While recent work has shed light on the mechanisms of active DNA demethylation and its roles in development and physiology, the mechanisms by which TET enzymes are recruited to chromatin are poorly understood.
Earlier, we showed that T3 can directly regulate the dnmt3a gene in Xenopus and mouse (50, 51), which supported that the hormone can influence DNA methylation, albeit indirectly. We recently published a genome-wide analysis of DNA methylation changes (analyzed by Methylated DNA Capture sequencing; MethylCap-seq), paired with gene expression changes (analyzed by RNA sequencing; RNA-seq) in tadpole brain (the region of the preoptic area/thalamus/hypothalamus) at 4 stages of metamorphosis (28). The genome of tadpole neural cells underwent changes in DNA methylation during metamorphosis that were negatively correlated with changes in gene expression. DNA demethylation predominated from metamorphic climax to the completion of metamorphosis when plasma [T3] is highest, and this correlated with increases in immunoreactivity for 5-hmC, 5-caC, and TET3, and the upregulation of genes encoding enzymes involved with active DNA demethylation.
Based on these findings, we hypothesized that DNA demethylation in tadpole brain at metamorphosis is induced by T3, in part by T3 promoting the recruitment of TETs to discrete genomic loci, and by the induction of genes whose protein products catalyze DNA demethylation. To test this, we treated premetamorphic Xenopus tropicalis tadpoles with T3 by adding the hormone to their aquarium water, then we investigated global and locus-specific changes in DNA methylation using immunohistochemistry and biochemical assays. We also determined if T3 treatment can induce the expression of genes that encode DNA demethylation enzymes, and we investigated possible direct regulation of these genes by liganded TRs. For our studies, we focused on the region of tadpole brain containing the preoptic area, thalamus, and hypothalamus, since this region undergoes dramatic morphological and biochemical changes during metamorphosis, it is highly responsive to T3, and it controls the production of T3 via control of the pituitary gland (52–55).
Materials and Methods
Animal care and use
We obtained wild type (WT) X. tropicalis tadpoles by in-house breeding, reared them in dechlorinated tap water (25°C, pH 7), and maintained them under a 13L:11D photoperiod. Tadpoles were fed ad libitum with pulverized frog brittle powder (NASCO, Fort Atkinson, Wisconsin) or with sera micron, and developmental stages were assigned using the normal table of Nieuwkoop and Faber (NF) (56). We generated homozygous thramt−exon4 tadpoles (functional TRα knockouts) by crossing heterozygous mutant frogs (TRaM5) generously provided by Dr Yun-Bo Shi. This frog line was produced using TALENS that targeted exon 4 (the third coding exon) of the X. tropicalis thra gene, generating a mutation that results in a predicted nonsense protein after amino acid 54 (57). All procedures involving animals were conducted under an approved animal use protocol (PRO00006809) in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Michigan.
For hormone treatment, we housed premetamorphic tadpoles (NF stages 50–52; 10 tadpoles per aquarium) in aquaria containing 2 liters of dechlorinated tap water. We dissolved 3,5,3’-L-triiodothyronine (T3 sodium salt; Sigma-Aldrich, St. Louis, Missouri) in 100% dimethylsulfoxide (DMSO; Sigma-Aldrich), then added the hormone stock to the rearing water to a final concentration of 1.5, 5, or 50 nM; controls received the same concentration of DMSO vehicle (0.0003%). We changed the rearing water and replenished the hormone at 24-hour intervals. After hormone treatment we sacrificed tadpoles by rapid decapitation, then microdissected the middle region of the brain containing the preoptic area/thalamus/hypothalamus (with pituitary gland) for DNA methylation and gene expression analyses, or we collected the whole brain for ChIP assays. The tissues were snap-frozen in liquid nitrogen and stored at -80°C until extraction of genomic DNA, total RNA, or chromatin.
Immunohistochemistry
We treated tadpoles with vehicle (DMSO 0.0003%) for 24 hours, or T3 for 24 or 48 hours, then harvested brains for immunohistochemistry (IHC), following our previously published methods (28, 58). We generated 16 µm transverse cryosections and conducted IHC using rabbit polyclonal antiserums to 5-hmC (Diagenode C15410205-20) or 5-caC (Diagenode C15410204-20), both diluted 1:500, and affinity-purified IgG from our custom Xenopus TET3 antiserum (#23561; 0.7 µg IgG/ml) (28). To reveal immune complexes, we used DyLight 550-conjugated goat anti-rabbit IgG secondary antibody (#84541, Thermo Scientific, Grand Island, New York). We imaged immunostained sections using an Olympus IX81 inverted microscope (Olympus, Tokyo, Japan) and carefully matched sections for anatomical level following the Xenopus brain atlas developed by Tuinhof and colleagues (59), with modifications by Marín and colleagues and (60) Yao and colleagues (61) (neuroanatomical map given in Supplemental Fig. 1, Supplemental Table 1; all supplementary material and figures are located in a digital research materials repository) (62). We captured digital micrographic images by uniformly adjusting the exposure, and for the captured images we uniformly adjusted the brightness, contrast, and evenness of illumination using Adobe Photoshop CS6 (Adobe Systems, Inc., San Jose, California) for further image analysis and presentation.
For densitometric analysis of immunoreactivity (ir), we processed all brain sections simultaneously under identical conditions and carefully matched them for anatomical level. We used a hand-made frame covering the area of interest, then counted the signal intensity automatically in the selected area using ImageJ software. We calculated the mean ir density for each brain as the total positive signal intensity divided by the total selected area.
Targeted analyses of 5-hmC quantity using 5-hmC Chop- quantitative polymerase chain reaction
We extracted genomic DNA from X. tropicalis tadpole brain using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, Maryland), and we analyzed the relative changes in 5-hmC at discrete genomic regions using the EpiMark® 5-hmC and 5-mC Analysis Kit (New England BioLabs, Ipswich, Massachusetts), following the manufacturer’s instructions (referred to here as 5-hmC Chop-quantitative polymerase chain reaction [qPCR]). The kit is able to distinguish 5-mC from 5-hmC through the use of T4β-glucosyltransferase (T4-BGT), which adds glucose to the hydroxyl group of 5-hmC, converting 5-hmC to 5-glucosylated hmC. When 5-hmC is found in the context of CCGG, this enzymatic modification blocks restriction digestion by MspI. We programmed each reaction with 1.5 μg of genomic DNA and measured the extent of restriction digestion using qPCR (oligonucleotide primer sequences are given in Table 1).
Table 1.
Oligonucleotide primersequences used for RT-qPCR, ChIP-qPCR, 5-hmC and 5-mC Chop-qPCR, hmeDIP-qPCR, and subcloning. All sequences correspond to the X. tropicalis genome except where indicated
| For RT-qPCR | |
|---|---|
| Gene | Primer sequence (5’ → 3’) |
| ef1a | Forward: CTATCCCCGCCAAACATCT |
| Reverse: CCATCTCAGCAGCTTCCTTC | |
| gadd45α | Forward: CTCAATGTGGACCCAGACAA |
| Reverse: CAGGGTGAAGTGGATCTGTAAA | |
| gadd45β | Forward: GACCTCCACTGCATTCTAGTTT |
| Reverse: CCACTGACTCCTGCTTCTATATTC | |
| gadd45γ | Forward: GGAAGAAGTTCACGGACAAGA |
| Reverse: GGGCAGAGACCAATAGTTCAT | |
| tdg | Forward: GGGATCAATCCAGGTCTTATGG |
| Reverse: TCCAGACAAGAACAGACACTTC | |
| tet2 | Forward: GGTTACTGCTTGCTTGGATTT |
| Reverse: CACGATTGTCTTCTCTGGTTAAAG | |
| tet3 | Forward: ATCTGACATCTCCAACCAAGAG |
| Reverse: GTCCAGAACCCAGATGTGTATAA | |
| idh1 | Forward: GGACAGTAACCCGGCATTATAG |
| Reverse: CCTCTTGTCCAGGCAAAGATAG | |
| idh2 | Forward: GCACTGGCCACTCTGAAATA |
| Reverse: CATTGGGACTCTTCCACATCTT | |
| idh3a | Forward: GCTAATCCCACTGCTCTTCTT |
| Reverse: CAGAGCCTTCCCAGATTTGAT | |
| idax | Forward: TGCCAAAGGTCTGTGTGTC |
| Reverse: TCCTCTGACCTCTAGTGAAGTG | |
| apobec2 | Forward: CCCTGCTTCTTCTTTCATGTTTC |
| Reverse: AATACTTGACCCTCAGGTCTTTC | |
| Firefly luciferase | Forward: CTTCGAGGAGGAGCTATTCTTG |
| Reverse: GTCGTACTTGTCGATGAGAGTG | |
| Renilla luciferase | Forward: CATGGGATGAATGGCCTGATA |
| Reverse: CAACATGGTTTCCACGAAGAAG | |
| For 5-hmC chop-qPCR | |
| X. tropicalis dnmt3a-TRE-A | Forward: CTTTGCCGGTGCCAACA |
| Reverse: CTGCTTCCCACAATCCCTT | |
| For 5-mC chop-qPCR | |
| Genomic region | Primer sequence (5’ → 3’) |
| X. laevis dnmt3a-TRE-B | Forward: TGA CTC TGC GCT GTG A |
| Reverse: AGG CTA CGT ACC CCT CTC AGT CT | |
| X. laevis control genomic regiona | Forward: TTC CCA TGG GTC CCT GTA AGT |
| Reverse: GGG AGC TTT TTG CTG CAG AA | |
| For hmeDIP-qPCR | |
| Genomic region | Primer sequence (5’ → 3’) |
| dnmt3a TRE-A | Forward: CACAGAAATGCAAGGGATTG |
| Reverse: CTGTAGTGCTGCTCAGTG | |
| dnmt3a TRE-B | Forward: TGACTCTGCGCAGTGA |
| Reverse: AGGCTACGTACCCCTCTCAGTCT | |
| For ChIP-qPCR | |
| Genomic region | Primer sequence (5’ → 3’) |
| dnmt3a TRE-A | Forward: CAGTAAAGGCACCCTGAG |
| Reverse: CATAAAGATTTCTGCCGTACAC | |
| thrb TRE | Forward: CCCCTATCCTTGTTCGTCCTC |
| Reverse: GCGCTGGGCTGTCCT | |
| gadd45γ TRE region | Forward: AGTGTTTATGCACGGGAAGG |
| Reverse: CCGGCAATTTGGTCGCTTATT | |
| tet3 TRE region | Forward: GTGTGTGTAGGCTGAATCTCTAAG |
| Reverse: AAGCCTGAGAGGGAAGAAGA | |
| ifabp promoter | Forward: CCCTACATTGGTTGAGCCAGTTTT |
| Reverse: TCAAAGGCCATGGTGATTGGT | |
| thrb exon 5 | Forward: CCCCGAAAGTGAAACTCTAACTCT |
| Reverse: CCACACCGAGTCCTCCATTTT | |
| tet2 TRE region | Forward: GTGGAGTGGATCACACAAGTAA |
| Reverse: TAGCTGGAGGCAGTCTATGT | |
| Sox9 promoter | Forward: ACGTGAAAGTGGAGCAGTGT |
| Reverse: TCTTCAGCAAAGGCACCCAA | |
| dnmt3a exon 2 | Forward: AACACTCCTCACCCACAAATAG |
| Reverse: GGGAATCTCTCCGCAAAGTAA | |
| klf9 synergy module (KSM) | Forward: CCGTCCCTTCTTTTGTGTACATT |
| Reverse: GCTGTTCGTGCCACTTTGC | |
| idax TRE region | Forward: CGAGAGATCAGCTGCACAATA |
| Reverse: ATGTCCAAGGTAAGGGTGTATG | |
| idh2 | Forward: FATAGGACGAGTAAGGGCAAAC |
| Reverse: GGCAAAGGTCACCCTGTAA | |
| For subcloning the tet2 TRE region (610 bp b ) into pGL4.23 | |
| Primer sequence | |
| Forward: AAACTCGAGGGAGTTCTGCCTGCCTTCTAG | |
| Reverse: TGTGAAGCTTCAGCGTAAATTGTGTCTATTTC | |
| For EMSA | |
| Genomic region | Primer sequence (5’ → 3’) |
| X. tropicalis dnmt3a TRE-B | Forward: AAG TGC AGC GAT GGG AGG G |
| Reverse: AAT CAT TCT TGG CTG CGC CC | |
| X. tropicalis klf9 synergy module (KSM) | Forward: ATACTCGAGCCCTGTACCATTTAGGGCC |
| Reverse: ATAAAGCTTAGCGCCGCTTTAAGAAAT |
a No Hinp1I restriction sites contained within the DNA sequence.
b The full DNA sequence is given in Supplemental Table 4 (62).
We also conducted hydroxymethylated DNA immunoprecipitation (hmeDIP) following the method of Nestor and Meehan (63). For each sample we sheared 0.5 μg of genomic DNA to 250 bp using a Covaris M220 ultrasonicator at peak power 74 W, duty factor 10.2, and 208 cycles for 80 seconds. We used 2.5 μg of sonicated DNA in an immunoprecipitation reaction using a 5-hmC antibody (ActiveMotif, Carlsbad, California, #39770). We added 0.25 ng of 5-hmC containing DNA (NEB, N0482) to each reaction as a spike-in control. We measured the quantity of immunoprecipitated DNA and 10% of the input using real-time qPCR (oligonucleotide primers given in Table 1). We first normalized the immunoprecipitated DNA quantity to the input value, then to the spike-in control value for each sample. We repeated this experiment using the EpiQuik Hydroxymethylated DNA Immunoprecipitation Kit (Epigentek, Farmingdale, New York), according to manufacturer’s protocol and obtained similar results.
Chromatin immunoprecipitation assay
We extracted chromatin from whole brains of tadpoles treated with vehicle or T3 for 24 or 48 hours (we pooled 9 brains per biological replicate; n = 4), as described (64). We sheared chromatin to ~400 bp using the Covaris (Woburn, Massachusetts) M220 ultrasonicator for 950 seconds at duty factor 8, peak power 75 W, and 200 cycles, and we analyzed all samples on a 1% agarose gel before snap-freezing them in liquid nitrogen and storing them at -80°C until conducting ChIP assays. We conducted ChIP assay, as previously described (64), using polyclonal rabbit antiserums to Xenopus TRs provided by Drs Yun-Bo Shi and Laurent Sachs (each of the antiserums recognize both TRα and TRβ), or affinity-purified IgG from a polyclonal rabbit antiserum to Xenopus TET3 (#23561) that we developed and validated (28). To estimate background in the ChIP assays, for TR ChIP, we replaced the primary antiserum with normal rabbit serum (NRS), and for TET3 ChIP, we replaced the primary IgG with IgG purified from preimmune serum from the same rabbit used for immunization. We analyzed ChIP DNA by real-time qPCR using SYBR Green assays (see Table 1 for oligonucleotide primer sequences).
Reverse transcriptase quantitative polymerase chain reaction
For analysis of gene expression by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), we pooled tissue from two animals per biological replicate and isolated total RNA using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, California), following the manufacturer’s instructions. We removed genomic DNA by treating 1 µg of total RNA with 20 units of DNase (#M6101; Promega, Madison, Wisconsin), and generated cDNA using the High Capacity cDNA Synthesis Kit (Applied Biosystems Inc., Foster City, California). We diluted the cDNA 1:4 with water and used 2 µL as template in a 20 µL qPCR SYBR green reaction (#4SPB20; ThermoFisher Scientific, Waltham, Massachusetts) using the Fast 7500 Real-Time PCR (Applied Biosciences Inc., Waltham, Massachusetts) or StepOne Real Time PCR systems (Life Technologies). For all genes analyzed, we designed primers to span exon–exon boundaries (oligonucleotide primer sequences are given in Table 1). We used a relative quantification method (65, 66) to compare mRNA levels between treatments by generating standard curves for each gene using pooled cDNA. We normalized all mRNA quantities to the mRNA level of the reference gene elongation factor 1α (ef1α).
Plasmid constructs and cell transfection/reporter assay
We isolated a 610-bp DNA fragment (Supplemental Table 2) (62) corresponding to the 5’ untranslated region (UTR) of the X. tropicalis tet2 gene using genomic DNA as the template for PCR, and we directionally subcloned this DNA 5’ → 3’ into the pGL4.23 firefly luciferase vector at the XhoI and HindIII sites (oligonucleotide primer sequences are given in Table 1). For reporter assays, we used Neuro2A[TRβ1] cells, which is a mouse neuroblastoma cell line engineered to stably express human TRβ1 (64, 67). We plated 250 000 cells per well in 12-well cell culture plates in DMEM/F12 medium (Invitrogen) supplemented with penicillin G (100 units/mL), streptomycin sulfate (100 µg/mL), hygromycin B, and 10% fetal bovine serum that had been stripped of thyroid hormone (68). Sixteen hours after plating we transfected cells with 400 ng of reporter plasmid or empty vector using Fugene 6 (Promega), following the manufacturer’s protocol. We dissolved T3 in 100% DMSO, then 24 hours after transfection we added vehicle (final concentration 0.01% DMSO) or T3 to a final concentration of 30 nM and continued the culture for 12 hours before harvesting the cells for RNA extraction. We used RT-qPCR to quantify firefly luciferase and Renilla luciferase mRNAs (oligonucleotide primer sequences are given in Table 1).
ClustalW alignment of Xenopus tet2 gene regions containing putative TREs
We first conducted a BLAST search using the NCBI MegaBLAST tool against the Xenopus laevis (taxid:8355) and Xenopus tropicalis (taxid:8364) genomes from the RefSeq Genome Database (refseq genomes), using the sequence of the X. tropicalis genomic region covered by the TR ChIP-seq peak within the tet2 5’ UTR. We used coordinates from the resulting alignments to obtain genomic sequence (extending 500 bp in the 3’ direction, all alignments were in the same orientation). We downloaded the sequences in FASTA format and used these as input for ClustalW (v2.1) analysis, and ran them using the Adoma (v1.0) package. We used the sequence analysis program NHR-scan (40) to identify putative direct repeat plus 4 base spacer (DR+4) TREs within a genomic sequence.
Data and statistical analysis
We analyzed data using the computer programs SigmaPlot (v. 13.0) or SYSTAT (v. 13.0; both from Systat Software, San Jose, California). We conducted one-way analysis of variance followed by Fisher’s least significant difference (Fisher’s LSD) post hoc test, or Student’s independent sample t-test (P < 0.05). Derived values were Log10-transformed before statistical analysis if the variances were found to be heterogeneous. We also conducted linear regression analysis to compare gene expression responses to T3 between WT and thramt-exon4 tadpoles using SYSTAT.
Results
Treatment with T3 increased immunoreactivity in the premetamorphic tadpole brain for the DNA demethylation intermediates 5-hmC and 5-caC, and the methylcytosine dioxygenase TET3
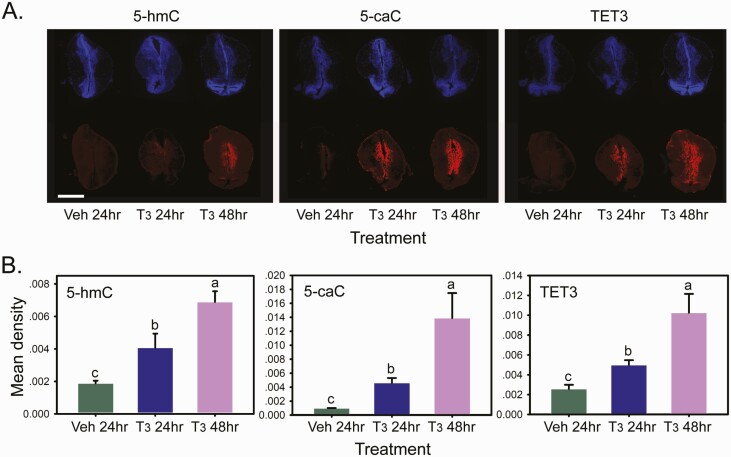
Immunoreactivity for 5-hmC, 5-caC, and TET3 was low or nondetectable in brains from tadpoles treated with vehicle, but became detectable after 24 hours of T3 treatment, and continued to increase after 48 hours (Fig. 1; shown is the region of tadpole brain containing the ventral hypothalamic nucleus, posterior tuberculum, and the ventromedial thalamic nucleus; see the Xenopus neuroanatomical map in Supplemental Fig. 1) (62). Treatment with T3 also increased immunoreactivity for the 3 antigens in the medial pallium and anterior preoptic area, thalamic nuclei (anterior, ventromedial, posterior, lateral), and the tegmentum (Supplemental Figs. 2, 3, and 4) (62). There was no effect of T3 in regions of the telencephalon rostral to the anterior preoptic area (see Supplemental Figs. 2, 3, and 4) (62), or in the hindbrain and spinal cord (data not shown).
Figure 1.
Treatment with T3-induced time-dependent increases in immunoreactivity for the DNA demethylation intermediates 5-hmC and 5-caC, and the methylcytosine dioxygenase TET3 in premetamorphic tadpole brain. We treated premetamorphic (NF stage 50) X. tropicalis tadpoles with vehicle (Veh; 0.0003% DMSO) for 24 hours, or T3 (50 nM) for 24 or 48 hours (hr) added to the aquarium water before collecting and fixing brains for immunohistochemistry for 5-hmC, 5-caC, and TET3. A: Shown are representative micrographs of the region of the tadpole brain containing the thalamic nuclei and ventral hypothalamus (section K in Supplemental Fig. 1; abbreviations are defined in Supplemental Table 1) (62) stained with DAPI or with antibodies to the three different antigens, captured at 10x magnification. Scale bar = 0.5 mm. B: Densitometric analysis of 5-hmC, 5-caC, and TET3 immunoreactivity in tadpole brain after treatment with T3. Bars represent the mean ± SEM (n = 5–6/time point). Means with the same letter are not significantly different (one-way ANOVA; P < 0.05; Fisher’s LSD).
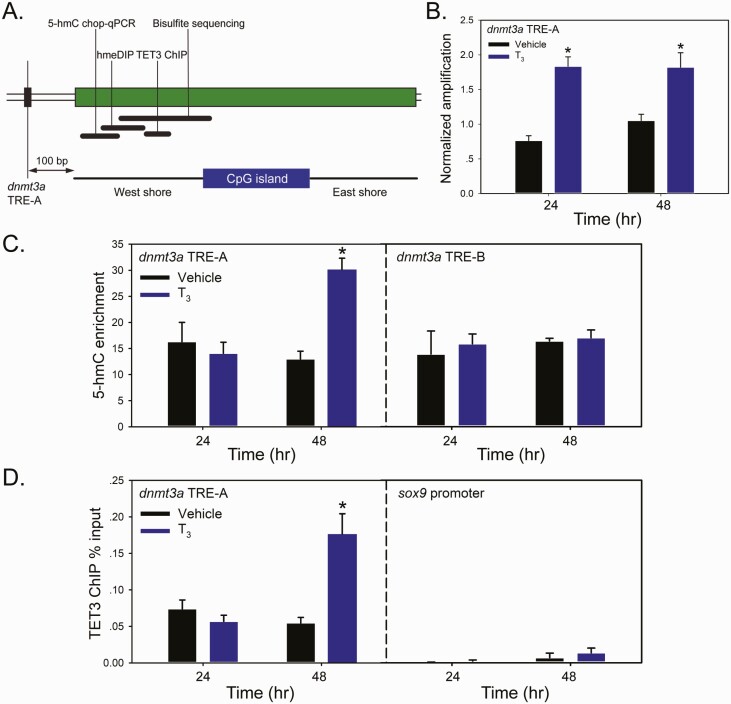
Treatment with T3 induced locus-specific DNA demethylation and TET3 recruitment to chromatin in premetamorphic tadpole brain
Our recently published work using MethylCap-seq showed that the tadpole neural cell genome underwent predominant DNA demethylation at metamorphosis (28). Targeted analyses in proximity to two TREs within the dnmt3a gene, one at -7.1 kb from the transcription start site (TSS; designated TRE-A) and one at +5.1 kb from the TSS (located in the first intron; designated TRE-B) (50), showed marked DNA demethylation during metamorphosis, with greater demethylation at the TRE-A region (28). We therefore investigated if treating premetamorphic tadpoles with T3 can induce locus-specific DNA demethylation, focusing primarily on the region of methylation upstream of the CpG island located 100 bp from the dnmt3a TRE-A (the CpG island “west shore”; Fig. 2A). Using two independent assays, we found that T3 increased 5-hmC quantity proximal to the dnmt3a TRE-A. Treatment with T3 caused ~2-fold increase in the 5-hmC Chop-qPCR signal at both analyzed time points (Fig. 2B). Using hmeDIP, we saw increased 5-hmC at 48 hours, but not at 24 hours (Fig. 2C); shown are data from the protocol of Nestor and Meehan (63); the Epigentek kit gave similar results (data not shown). The difference between the 5-hmC Chop-qPCR and the hmeDIP results may be explained by the nature of the two assays: 5-hmC Chop-qPCR targets a single CG pair within the MspI restriction site, while the hmeDIP signal is proportional to the number of CG pairs within a given region of the genome (here a 300 bp stretch) that undergo demethylation (which may take longer to reach detectability after hormone treatment).
Figure 2.
Treatment with T3 induced DNA demethylation and TET3 recruitment to chromatin in proximity to the TRE-A of the dnmt3a gene in premetamorphic tadpole brain. We treated premetamorphic (NF stage 50) X. tropicalis tadpoles with vehicle (0.0003% DMSO) or T3 (5 nM) added to the aquarium water for 24 or 48 hours (hr) before microdissecting and collecting the region of the brain containing the preoptic area/hypothalamus/thalamus for analysis by 5-hmC Chop-qPCR, hmeDIP, and TET3 ChIP assay. Bars represent the mean ± SEM (n = 4/time point). Data were analyzed by Student’s independent t-test, and asterisks indicate statistically significant differences between vehicle and T3-treated groups (P < 0.05). A: Schematic of the upstream region of the X. tropicalis dnmt3a gene showing the location of the DR+4 TRE-A (at 5.1 kb upstream of the TSS) (50) and the adjacent CpG island with “West” (5’) and “East” shores. This region is heavily methylated in premetamorphic tadpole brain, then becomes progressively demethylated during metamorphosis as the dnmt3a mRNA level increases (28). Black bars indicate the relative genomic locations targeted and size of amplicons for each of the three assays. Also shown for reference is the region that we analyzed using bisulfite sequencing that confirmed DNA demethylation during metamorphosis (28). B: Analysis of relative, locus-specific changes in 5-hmC content using 5-hmC Chop-qPCR assay. This assay provides a measure of enrichment of 5-hmC at a MspI restriction site within the genomic region indicated. C: Analysis of 5-hmC content using hmeDIP assay. We conducted 5-hmC immunoprecipitation for the hmeDIP assay using two methods with similar results (see the “Materials and Methods” section). D: Recruitment of TET3 to chromatin at the indicated genomic region analyzed by ChIP-qPCR assay. The TET3 ChIP signal is expressed as a percentage of the input.
There was no effect of T3 on 5-hmC enrichment in proximity to the dnmt3a TRE-B at either of the 2 time points analyzed by hmeDIP (data not shown). However, long-term treatment with T3 (96 hours) induced DNA demethylation at the TRE-B region analyzed by 5-mC Chop-qPCR, which was strongly enhanced by co-treatment with the DNMT inhibitor 5-aza-2’deoxycytidine (Supplemental Fig. 5; Supplemental Methods) (62).
We tested if DNA methylation at TRE regions can influence T3-dependent transcription. In vitro methylation of Xenopus genomic sequences containing TREs abrogated T3-dependent transactivation in reporter transfection assays (see Supplemental Fig. 6, Supplemental Methods) (62); note that CG dinucleotides are not present in the DR+4 TRE sequence, but they are found in the cloned DNA fragments within sequences flanking the TREs. In vitro methylation did not affect TR-RXR binding to TREs analyzed by electrophoretic mobility shift assay (Supplemental Fig. 7) (62).
To investigate a potential mechanism for locus-specific, T3-dependent DNA demethylation we conducted ChIP assays for Xenopus TET3 at regions of predicted or known TREs. We found that T3 treatment of premetamorphic tadpoles caused TET3 recruitment proximal to the dnmt3a TRE-A region at 48 hours (Fig. 2D). We also saw T3-dependent recruitment of TET3 to the region of the klf9 gene ~6 kb upstream of the TSS that contains a DR+4 TRE (within an ultraconserved superenhancer named the klf9 synergy module [KSM]) (69, 70) (Supplemental Fig. 8) (62) and the region of a putative TRE located 1.8 kb upstream of the gadd45γ TSS (discussed more below; Supplemental Fig. 9) (62). At two control regions, the sox9 promoter, which lacks DNA methylation, and dnmt3a exon 2, which is methylated, we saw no TET3 recruitment after T3 treatment (Fig. 2D for the sox9 promoter (dnmt3a exon 2 data not shown); these regions showed no changes in MethylCap-seq peak height during metamorphosis (28). We found TET3 enrichment in chromatin at metamorphic climax at the TRE regions of thrb (71, 72), thyroid hormone-induced basic leucine zipper protein (thibz) (73), and the KSM (69, 70) (Supplemental Fig. 10) (62).
Treatment with T3increased mRNA levels for genes that encode enzymes that catalyze DNA demethylation in premetamorphic tadpole brain
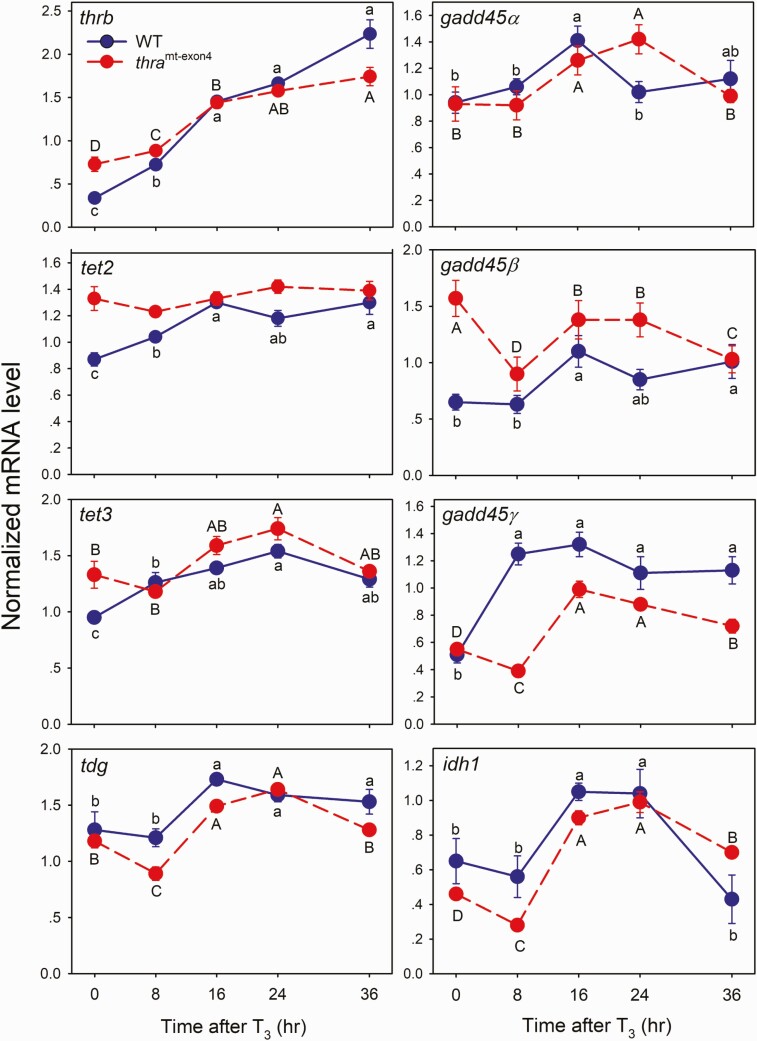
Endogenous T3 controls tissue-specific gene regulation programs during tadpole metamorphosis, and exogenous T3 can induce precocious activation of these programs in premetamorphic tadpoles (3, 74, 75). We hypothesized that, in addition to promoting recruitment of TET enzymes to chromatin to catalyze DNA demethylation, T3 induces genes that encode enzymes that promote DNA demethylation. To test this, we treated WT or thramt−exon4 (TRα knockout) premetamorphic tadpoles with T3 for different times, then harvested the region of the preoptic area/thalamus/hypothalamus and analyzed changes in mRNA levels for genes that encode DNA demethylation enzymes (tet2, tet3, tdg, gadd45α, gadd45β, gadd45γ, idh1, idh2, idh3a) using RT-qPCR.
The baseline mRNA levels for thrb (positive control), tet2, tet3, gadd45β, and idh3a were higher in thramt−exon4 compared with WT (P < 0.05, Student’s independent t-test; Fig. 3, Supplemental Fig. 11) (62), which is consistent with these genes being derepressed by loss of TRα, as has been shown previously for thrb and other direct T3 response genes (76, 77). The baseline mRNA levels for gadd45α, gadd45γ, tdg, idh1, and idh2 were unaffected by genotype.
Figure 3.
Treatment with T3 caused time-dependent increases of mRNA levels of genes that encode DNA demethylation enzymes in premetamorphic tadpole brain. The action of T3 was impaired in tadpoles deficient for TRα (thramt-exon4). We treated WT or thramt-exon4 premetamorphic (NF stage 50–52) X. tropicalis tadpoles with T3 (5 nM) added to the aquarium water for the times indicated (hr - hour). We microdissected the region of the brain containing the preoptic area/thalamus/hypothalamus for RNA extraction and mRNA analysis by RT-qPCR. We normalized mRNA levels to the reference gene ef1α, which was unaffected by T3 treatment (data not shown). Points represent the mean ± SEM (n = 5/time point). We used one-way ANOVA [WT: thrb, F(4, 17) = 48.59, P < 0.0001; tet2, F(4, 18) = 12.18, P < 0.0001; tet3, F(4, 18) = 12.337, P < 0.0001; tdg, F(4, 19) = 8.49, P < 0.0001; gadd45α, F(4, 19) = 2.91, P = 0.049; gadd45β, F(4, 14) = 3.628, P = 0.31; gadd45γ, F(4, 19) = 20.72, P < 0.0001; idh1, F(4, 19) = 5.445, P = 0.004; thramt-exon4 – thrb, F(4, 19) = 64.19, P < 0.0001; tet2, P = 0.153; tet3, F(4, 17) = 8.539, P = 0.001; tdg, F(4, 16) = 46.38, P < 0.0001; gadd45α, F(4, 18) = 4.49, P = 0.011; gadd45β, P = 0.075; gadd45γ, F(4, 17) = 50.85, P < 0.0001; idh1, F(4, 18) = 53.622, P < 0.0001]. Means with the same letter within a genotype (WT, thramt-exon4) are not significantly different (Fisher’s LSD; P < 0.05).
The mRNA level for thrb, a well-known direct T3 response gene, was induced after 8 hours of T3 treatment in WT animals, and increased through 36 hours of continuous T3 exposure (Fig. 3). In thramt−exon4 tadpoles, the thrb mRNA level increased after 8 hours, and continued to increase to 36 hours, but the slope of the response curve over the first three time points (0, 8, and 16 hours; ie, the response kinetics) was significantly lower than for WT animals (adjusted R2 = 0.8033, P < 0.0001; linear regression analysis). The slower thrb kinetics in thramt−exon4 animals is hypothesized to be due to the lack of TRα during the initial response, which may be subsequently compensated for by thrb autoinduction (76, 77).
We found a variety of T3 response profiles and different effects of inactivation of thra for genes that encode DNA demethylation enzymes. The tet2, tet3, and gadd45γ mRNAs were induced in the WT tadpole brain after 8 hours of exposure to T3, and they continued to increase, or remained elevated, through 36 hours (Fig. 3). By contrast, in thramt−exon4 animals the response to T3 was delayed for tet3 and gadd45γ mRNAs, increasing only after 24 and 16 hours, respectively. The tet2 mRNA T3 response was completely abolished in thramt−exon4 animals. The slopes of the response curves were significantly different between WT and thramt−exon4 tadpoles (tet2, adjusted R2 = 0.788, P < 0.0001; tet3, adjusted R2 = 0.651, P = 0.002; gadd45γ, adjusted R2 = 0.8046, P < 0.0001; linear regression analysis).
The tdg, gadd45α, and idh1 mRNAs were first induced at 16 hours in both WT and thramt−exon4 animals, but showed different response kinetics thereafter. The tdg mRNA remained elevated at 24 and 36 hours in WT, but declined to baseline at 36 hours in thramt−exon4. The gadd45α mRNA increased at 16 hours, then declined to baseline at 24 and 36 hours in WT, but in thramt−exon4 it increased at 16 hours and remained elevated at 24 hours before declining to baseline at 36 hours. The idh1 mRNA increased at 16 hours, remained elevated at 24 hours, then declined to baseline at 36 hours in WT, whereas in thramt−exon4 tadpoles it increased at 16 hours and remained elevated above baseline through 24 and 36 hours (Fig. 3). The slopes of the response curves were significantly different between WT and thramt−exon4 tadpoles for tdg (adjusted R2 = 0.271, P = 0.022) and idh1 (adjusted R2 = 0.493, P = 0.001), but not for gadd45α.
The idh2 and idh3a mRNA levels were changed by T3 treatment in thramt−exon4, but not in WT tadpoles, with increases seen for both genes at 16 and 24 hours (Supplemental Fig. 11) (62). Also, in thramt−exon4 but not WT, the mRNA levels for 5 genes (tdg, gadd45β, gadd45γ, idh1, and idh3a) were reduced at 8 hours after T3 treatment compared to baseline, but it increased thereafter (Fig. 3; Supplemental Fig. 11) (62). We analyzed two other DNA demethylation-related genes but only in WT animals, owing to technical limitations, and we found that idax mRNA was unaffected by T3, but that the mRNA for the apolipoprotein B mRNA editing enzyme, catalytic polypeptide (apobec2) gene decreased at 16 hours and continued to decline through 36 hours (data not shown).
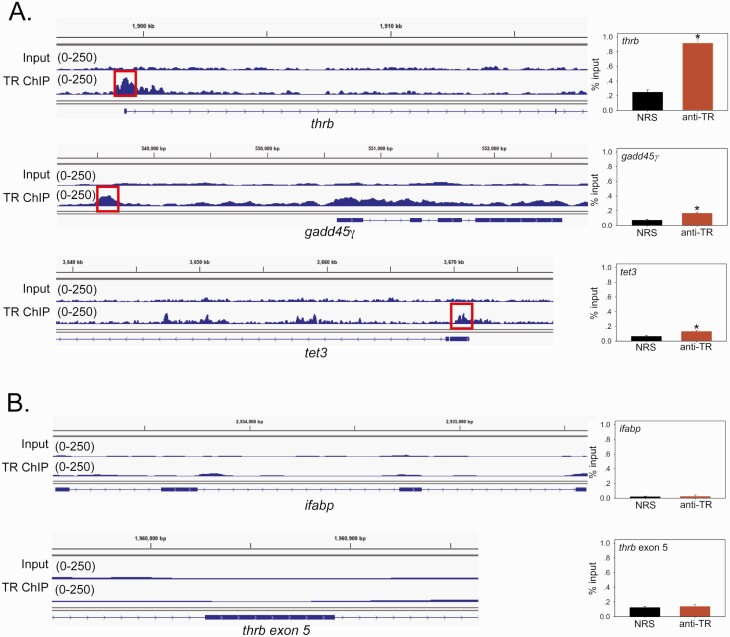
Identification of putative TREs at genes that encode DNA demethylation enzymes
We analyzed data from a TR ChIP sequencing (ChIP-seq) experiment (see Supplemental Methods) (62) to identify peaks of TR association within the gene body, and 10 kb upstream and downstream of the TSSs of genes that encode DNA demethylation enzymes (the full TR ChIP-seq dataset and analysis will be published separately). We identified two TR peaks at gadd45γ, one at 1.8 kb upstream of the TSS and another smaller, broader peak within the first exon (Fig. 4A) (one TR peak within the 5’ UTR of tet3 (Fig. 4A) and one TR peak within the 5’ UTR of tet2 (Fig. 5A)). As positive controls, we show TR peaks at the 5’ UTR of thrb (Fig. 4A) and the 5’ UTR and upstream enhancer of klf9 (KSM; Supplemental Fig. 12) (62). We validated each of these peaks by targeted TR ChIP assay (Fig. 4A, Supplemental Fig. 12) (62). Shown in Fig. 4B are two negative control loci with no TR peaks, ifabp (which is indirectly regulated by T3 (78, 79)) and thrb exon 5. We found single TR peaks within the 5’ UTRs of idax and idh2, but these did not validate by targeted TR ChIP assay (Supplemental Fig. 12) (62). We found no TR peaks at gadd45α, gadd45β, idh1, idh3a, tdg, aid, or apobec2 (data not shown).
Figure 4.
Thyroid hormone receptors (TRs) associate in chromatin in metamorphic climax stage tadpole brain at genes that encode DNA demethylation enzymes. Shown are integrative genome viewer (IGV) genome browser tracks for TR ChIP-seq reads mapped to the X. tropicalis genome. We conducted a TR ChIP-seq experiment on chromatin isolated from the region of the preoptic area/thalamus/hypothalamus of metamorphic climax stage (NF stage 62) X. tropicalis tadpole brain. The input tracks are shown above the TR ChIP-seq tracks. Numbers in parentheses represent the scale for peak height. The gene structures are shown below the genome traces: lines and black filled bars represent introns and exons, respectively, and arrows indicate the direction 5’ → 3’. To the right of each genome track is a graph of a TR ChIP assay using normal rabbit serum (NRS; negative control) or rabbit antiserum to Xenopus TRs (anti-TR) that targeted the region covered by the TR ChIP-seq peak shown on the IGV genome browser track (indicated by the red box). The bars represent the mean ± SEM (n = 4). Asterisks indicate statistically significant differences between NRS and anti-TR serum (P < 0.05, Student’s independent t-test). A: IGV genome browser tracks and targeted TR ChIP assays at the thrb (XLOC_017285), gadd45γ (XLOC_017078.1), and tet3 (XLOC_025924) genes. The thrb gene, which has a TRE in its 5’ UTR (71, 72, 80), served as a positive control. The putative TRE region at gadd45γ is located 1.8 kb upstream of the TSS, and at tet3 it is in the 5’ UTR. B: IGV genome browser tracks and targeted TR ChIP assays at two negative control regions, the ifabp (XLOC_43086.1) gene and thrb exon 5.
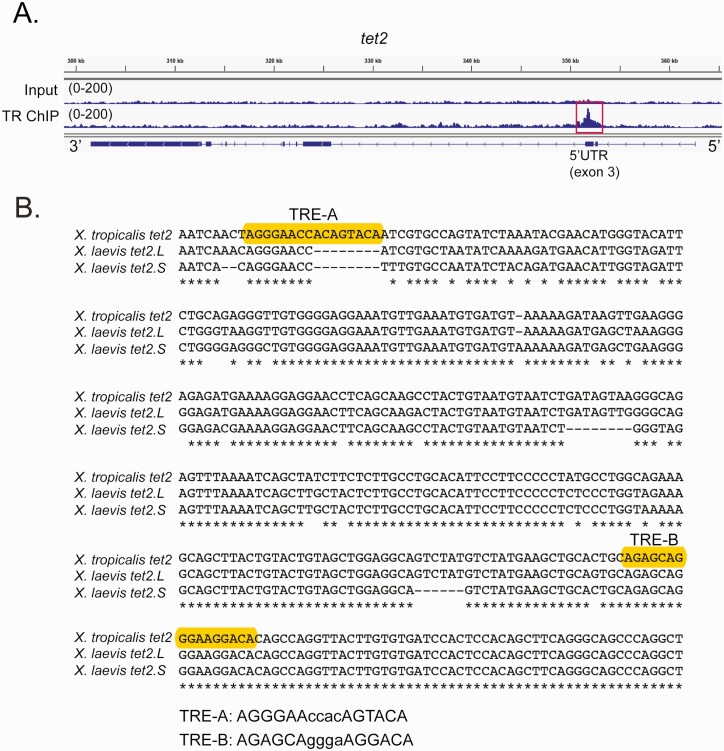
Figure 5.
Identification of putative TREs within the X. tropicalis tet2 gene. A: Genome browser tracks showing the location of a TR peak (red box) within the 5’ UTR (exon 3) of X. tropicalis tet2 (XLOC_002313) identified by TR ChIP-sequencing (see Supplemental Methods) (62) conducted on chromatin isolated from the whole brain of metamorphic climax stage (NF stage 62) tadpoles. Shown are genome tracks obtained with input and TR ChIP samples (numbers in parentheses indicate the peak height range). In the schematic under the genome browser tracks, the black boxes represent exons and the lines represent introns, and arrows on the gene indicate the orientation of the gene. B: Alignment of DNA sequences (5’ → 3’) within the third exons of X. tropicalis (358 bp) and X. laevis tet2 genes (located on chromosome 1 in both species; for X. laevis, which is pseudotetraploid, L – long chromosome, S – short chromosome). Accession numbers: X. tropicalis tet2 XM_012955515.3; X. laevis tet2.L LOC108710731; and X. laevis tet2.S LOC108706731. Shown are the locations of two predicted DR+4 TREs in X. tropicalis (only TRE-B is found in both species) identified using the sequence analysis program NHR-scan.
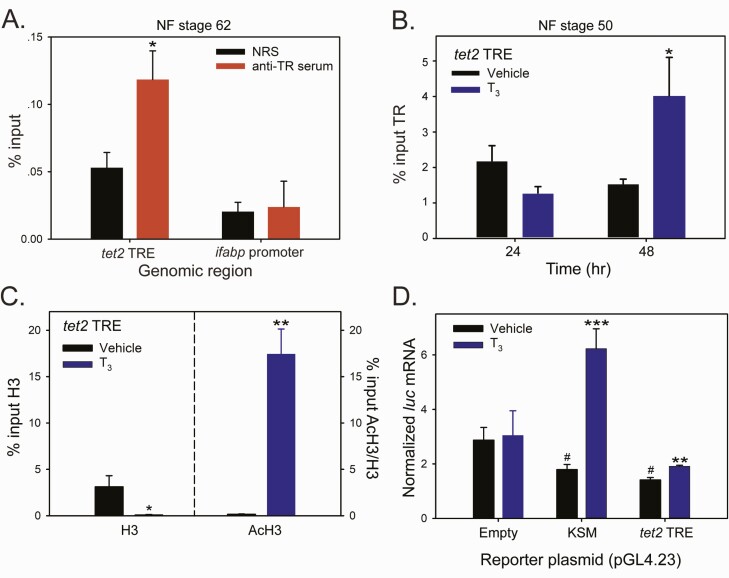
We focused additional analyses on tet2, since its mRNA level showed early T3 response kinetics in the brain of WT tadpoles, but the response was abolished in thramt−exon4 animals (Fig. 3), supporting that tet2 is a direct TRα-regulated gene. We used a computational approach to analyze the genomic regions covered by the TR ChIP-seq peak at the X. tropicalis tet2 locus (Fig. 5A) and found two putative DR+4 TREs (Fig. 5B). Sequence alignment of homologous regions of X. tropicalis and X. laevis tet2 genes showed that one of the two putative TREs (designated TRE-B) was conserved between the two species (Fig. 5B). We next conducted targeted TR ChIP assay on whole brain chromatin from metamorphic climax stage (NF stage 62) tadpoles, which confirmed the TR ChIP-seq peak at tet2 (Fig. 6A; the ifabp promoter served as a negative control). We also treated premetamorphic (NF stage 50) tadpoles with vehicle or T3 for 24 or 48 hours, then conducted targeted TR ChIP assay on whole brain chromatin at the tet2 5’ UTR and ifabp promoter. This showed that T3 increased the TR ChIP signal at the tet2 5’ UTR by 2.7-fold at 48 hours, but not at 24 hours; there was no change in TR ChIP signal at the ifabp promoter region at any time point (Fig. 6B).
Figure 6.
Thyroid hormone induced chromatin modifications at the putative X. tropicalis tet2 TRE region, and this genomic region supports T3-dependent transactivation. We conducted targeted qPCR-ChIP assays on chromatin isolated from whole brain of metamorphic climax stage (NF stage 62) tadpoles (panel A) or premetamorphic stage (NF stage 50) tadpoles (panel B) treated with vehicle (0.0003% DMSO) or T3 (5 nM) added to their aquarium water for different times. Bars represent the mean ± SEM (n = 4/treatment; experiments were repeated at least twice). Data were analyzed by Student’s independent t-test, and asterisks indicate statistically significant differences between NRS and anti-TR serum (panel A) or vehicle and T3-treated groups (panels B, C, and D; *P < 0.05, **P < 0.01, ***P < 0.001; # in panel D indicates statistically significant differences from vehicle-treated empty vector control; P < 0.0001). A: Targeted qPCR-ChIP assay confirmed TR association in chromatin at the tet2 5’ UTR in metamorphic climax stage tadpole brain. B: Targeted qPCR-ChIP assay showed increased TR ChIP signal at the tet2 5’ UTR in the brain of premetamorphic tadpoles treated with T3 for 48 hours (hr). C: Targeted qPCR-ChIP assays conducted on chromatin isolated from premetamorphic tadpole brain treated with T3 for 12 hours showed that T3 induced nucleosome repositioning at the tet2 5’ UTR, evidenced by a decrease in the H3 ChIP signal, and an open and active chromatin structure, evidenced by a large increase in the AcH3 ChIP signal (normalized to H3). There was no change in H3 or AcH3 at the ifabp promoter region after T3 treatment (data not shown). D: The 5’ UTR region of the X. tropicalis tet2 gene containing putative TREs (see Fig. 4) supports T3-dependent transactivation in transient transfection-reporter assays. We subcloned a 610 bp fragment of tet2 (Supplemental Table 2) (62) into the luciferase reporter vector pGL4.23 and transfected Neuro2a[TRβ1] cells with this vector (pGL4.23-tet2TRE), a vector containing the X. tropicalis klf9 synergy module (pGL4.23-KSM) or empty vector. Twenty hours after transfection, we treated cells with vehicle (0.01% DMSO) or T3 (30 nM) for 12 hours, then harvested for RNA isolation and analysis of luciferase (luc) mRNA by RT-qPCR.
To investigate if T3 can cause changes in chromatin consistent with transcriptional activation at the putative tet2 TRE region, we conducted ChIP assays for H3 and AcH3 on brain chromatin isolated from premetamorphic tadpoles treated with vehicle or T3 for 12 or 24 hours. Treatment with T3 reduced the H3 signal at the tet2 5’ UTR at both time points (the 12-hour time point shown in Fig. 6C; the 24-hour time point is not shown), which is consistent with liganded TR-inducing localized nucleosome repositioning (70, 73, 81). Also, T3 treatment increased AcH3 (normalized to H3) at the tet2 TRE region at 12 hours (Fig. 6C); the mean AcH3/H3 was higher at 24 hours but was not statistically significant (data not shown). There was no effect of T3 on H3 or AcH3 levels at the ifabp promoter (data not shown).
To test for functionality of the putative tet2 TRE, we conducted transient transfection-reporter assays in N2a[TRβ1] cells (64, 67) using luciferase reporter constructs containing a 610 bp DNA fragment corresponding to the X. tropicalis tet2 5’ UTR that included the two putative TREs (pGL4.23-tet2TRE; the DNA sequence is given in Supplemental Table 2) (62). Treatment with T3 increased the firefly luciferase mRNA level in cells transfected with pGL4.23-tet2TRE, but there was no effect in cells transfected with empty vector (Fig. 6D).
Discussion
Here we show that T3 can induce DNA demethylation in the brain of premetamorphic X. tropicalis tadpoles, and we provide evidence for two molecular mechanisms for this action. To our knowledge, this is the first demonstration of a hormone inducing changes in DNA methylation in vivo in the brain of a vertebrate during postembryonic development. After treating premetamorphic tadpoles with T3, we observed time-dependent increases in immunoreactivity for the DNA demethylation intermediates 5-hmC and 5-caC, and the methylcytosine dioxygenase TET3, thus supporting that the hormone can induce significant and widespread DNA demethylation in the genome of neural cells in vivo. We also found that T3 can induce locus-specific changes at regions of the genome that undergo DNA demethylation during spontaneous metamorphosis (28), supporting that these changes are driven by the elevation in circulating [T3]. We saw demethylation at genomic sites in close proximity to TREs that we previously showed regulate the dnmt3a and klf9 genes (28, 70). The methylation state of DNA at these sites may influence hormone action, which is supported by our in vitro methylation and reporter-transfection assays. We uncovered two potential mechanisms for T3 action on DNA demethylation: the T3-dependent recruitment of TET3 to discrete genomic sites to catalyze oxidation of 5-mC, and the induction of genes that encode DNA demethylation enzymes. Furthermore, we provide evidence that some of these genes have proximal TR binding sites, and one of these genes, tet2, is directly regulated by liganded TR.
We recently reported that immunoreactivity for 5-hmC, 5-caC, and TET3 increased in parallel in the tadpole brain during spontaneous metamorphosis (28), with highest levels at metamorphic climax when T3 production is maximal (28, 82, 83). The coordinate increase in TET3 with the two DNA demethylation intermediates supports that this dioxygenase is responsible, at least in part, for their accumulation (23, 84). Similarly, our genome-wide (MethylCap-seq) and whole genome biochemical analyses showed predominant DNA demethylation across the genome of tadpole neural cells at metamorphosis (28). These findings led us to hypothesize that the DNA demethylation occurring during spontaneous metamorphosis is driven by T3. Our current findings provide compelling support for this hypothesis. We found that treatment of premetamorphic tadpoles with T3 induced precocious increases in 5-hmC, 5-caC, and TET3 in the same brain regions that undergo similar changes during spontaneous metamorphosis. As we saw during spontaneous metamorphosis, the region of the tadpole brain (preoptic area/thalamus/hypothalamus) that showed large changes in DNA methylation houses neurosecretory neurons that control pituitary secretion, which in turn controls T3 production. This region of the tadpole brain undergoes dramatic morphological and gene expression changes during metamorphosis under the influence of T3 (53–55, 85). We therefore focused on this brain region for targeted analyses of locus-specific changes in DNA methylation caused by T3.
Our recently published MethylCap-seq analysis found that the dnmt3a gene was heavily methylated in premetamorphic tadpole brain, but underwent significant DNA demethylation at metamorphosis (28). This gene has two TREs (50), and the genomic regions in proximity to these TREs showed decreases in DNA methylation during metamorphosis, with the largest changes occurring near TRE-A; we therefore focused our targeted analyses on this region of dnmt3a. Using two independent approaches that monitor 5-hmC content at discrete genomic regions, we found that T3 treatment induced locus-specific DNA demethylation (Fig. 2). Activation of dnmt3a transcription is coordinate with DNA demethylation that occurs at metamorphosis (28). The induction of DNA demethylation by T3 at the dnmt3a locus found in the current study correlates with the rapid induction of dnmt3a mRNA that we found previously (50). Our previous MethylCap-seq paired with RNA-seq analysis showed that genome-wide, decreased DNA methylation correlated with upregulation, while increased DNA methylation correlated with downregulation of gene transcription, and this relationship was strongest for gene bodies (28). The level of 5-hmC is increased at the enhancers, promoters, and bodies of genes that are actively transcribed, and DNA demethylation is generally associated with gene activation (25–27, 36–38). Taken together, our previous and current findings support the hypothesis that activation of the dnmt3a gene during metamorphosis is dependent on the rise in plasma T3, and the coordinate DNA demethylation at this locus.
The importance of TET enzymes in the catalysis of active DNA demethylation is well known (23, 40, 86), and mounting evidence supports that TET enzymes play critical roles in enhancer activation during animal development by inducing DNA demethylation, thereby modulating chromatin accessibility (21, 39). Earlier we showed that there are coordinate increases in immunoreactivity for 5-hmC, 5-caC, and TET3 in several regions of the tadpole brain during metamorphosis (28). In the present study we found that T3-dependent enrichment of 5-hmC proximal to the dnmt3a TRE-A correlated with recruitment of TET3 to this genomic region, suggesting a mechanism for T3-mediated DNA demethylation. Furthermore, we found that TET3 is recruited at metamorphic climax, along with TR, to chromatin at TREs of several known TR target genes (klf9, thrb and thibz; but see Kasai et al) (87). Our findings suggest the hypothesis that liganded TRs promote recruitment of TETs to regions of genes containing TREs to induce localized DNA demethylation, thereby modifying chromatin structure and promoting gene transcription.
Currently, there is little known about the molecular mechanisms by which TET enzymes are recruited to chromatin at specific regions of the genome. Recently, Guan and colleagues (88) found in HEK293 cells direct physical interaction between TET3 and TRs, specifically the catalytic domain of TET3 and the AF2 domain of TRα1; notably, interactions of the catalytic domains of TET1 and TET2 with TRs were considerably weaker compared with TET3. Furthermore, TET3 enhanced liganded TRα1 transcriptional activity in transfection-reporter assays, and TET3 stabilized TR association in chromatin by inhibiting its ubiquitination. Hassan and colleagues (89) reported that the retinoic acid receptor recruited a TDG/TET complex to chromatin at the Hypermethylated in Cancer (Hic1) locus in mouse embryonic fibroblasts, and treatment with retinoic acid induced DNA demethylation at the Hic1 gene promoter. Taken together, these findings suggest that nuclear receptors (NRs) play key roles in targeting to chromatin the enzymes that catalyze DNA demethylation. Our findings are the first to support that this mechanism may operate in vivo in a vertebrate developmental model system.
The recruitment of TET enzymes and the consequent DNA demethylation in proximity to NR binding sites may influence hormone action on gene transcription. Our findings using in vitro methylation of TRE regions sub-cloned into a CG-less vector paired with transient transfection-reporter assays supports that DNA methylation can impair T3-dependent transactivation. Conversely, DNA demethylation in proximity to TREs and perhaps other NR binding sites in vivo may enhance liganded NR function on gene transcription. Bogdonavich and colleagues (90) showed that in vitro methylation of target DNA sequences repressed transcription when assayed in Xenopus embryos. This suggests that the DNA demethylation that occurs at the dnmt3a TREs (and to a lesser extent at the KSM and perhaps other gene TREs) at metamorphosis leads to enhanced liganded TR action. This interesting possibility requires further investigation.
As discussed above, one potential mechanism for T3 to induce DNA demethylation is to promote TET recruitment to chromatin. Another mechanism is for T3 to promote the synthesis of enzymes that catalyze active DNA demethylation. The mRNAs for several genes involved with DNA demethylation increased in tadpole brain during metamorphosis (28). Here we show that exogenous T3 is capable of causing precocious activation of some of these genes in premetamorphic tadpole brain. The two genes that encode TET enzymes (tet2 and tet3) are likely to be directly regulated by liganded TRs since they showed rapid T3 response kinetics in WT animals (responding by the earliest time point: 8 hours), and these responses were significantly delayed and of lower magnitude in TRα KO (thramt-exon4) tadpoles. The early response kinetics of these 2 genes were similar to thrb, which is a well-known direct TR target gene (71, 72). Furthermore, the baseline mRNA levels of tet2 and tet3, like thrb were elevated in thramt-exon4 tadpoles, which is consistent with unliganded TRα directly repressing their transcription in WT animals.
A TR ChIP-seq experiment that we conducted on chromatin isolated from metamorphic climax stage tadpoles showed TR peaks that we could validate by targeted ChIP assay within or in proximity to the tet2, tet3, and gadd45γ genes. Of the 7 genes that encode DNA demethylation enzymes that were induced by T3 in tadpole brain, the T3 response of tet2 was most strongly impacted by loss of TRα. Sequence analysis of the TR peak at the region of the 5’ UTR of tet2 showed two putative DR+4 TREs, one that was conserved between the two Xenopus species (tet2 TRE-B). Using targeted ChIP assay we confirmed TR association at this region of tet2 in brain chromatin isolated from tadpoles at metamorphic climax. Furthermore, T3 treatment of premetamorphic tadpoles increased TR ChIP signal at the tet2 TRE region. This increase in TR ChIP signal after T3 may be due to an increase in TRβ protein, since thrb is autoinduced in tadpoles (91). We also saw a decrease in H3 at this region, which is indicative of nucleosome repositioning (70, 73, 81), and increased AcH3, which is indicative of an open chromatin configuration and active transcription (70, 73, 92). Lastly, we found that the tet2 TRE region supported T3-dependent transcription in transfection-reporter assays in tissue culture cells. Taken together, our findings support that tet2 is regulated by T3 via one or more TREs located within its 5’ UTR.
Thyroid hormone plays pivotal roles in vertebrate development, actions that were likely present in protochordates (93–95). The actions of T3 on posttranslational modifications of histones are well described, but little is currently known about whether and how the hormone can influence DNA methylation, an important epigenetic modification that modulates chromatin structure and gene transcription. Earlier we found predominant, genome-wide DNA demethylation in tadpole brain at metamorphosis (28), and here we provide strong support for a role for T3, which increases dramatically at metamorphic climax, in promoting DNA demethylation. This likely occurs by liganded TRs recruiting TET enzymes to chromatin to catalyze DNA demethylation and inducing production of DNA demethylation enzymes. Our preliminary findings in which we looked at a handful of sites of TR binding in the tadpole neural cell genome suggest that TET recruitment and DNA demethylation could be a common mechanism for TR action across the genome. Taken together, our findings point to a central role for T3 in modulating DNA methylation in the brain of an important vertebrate developmental model system, which can lead to both short- and long-term changes in chromatin structure and gene transcription.
Acknowledgments
We thank Samantha Fontana, Cesar Rodriguez, and Luan Wen for providing technical assistance. We are grateful to Michael Rehli for providing the CpG-less vector CpGL, and Yun-Bo Shi and Laurent Sachs who provided the antiserums to Xenopus TR.
Financial Support: Supported by a grant from the National Science Foundation IOS 1456115 to R.J.D. Y.K. was supported by a Ruth L Kirschstein National Research Service Award (NS07329401) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health. E.C.A.L. was supported by funding from the College of Literature, Science and the Arts at the University of Michigan.
Glossary
Abbreviations
- 5-caC
5-carboxylcytosine
- 5-fC
5-formylcytosine
- 5-hmC
5 hydroxymethylcytosine
- 5-mC
5-methylcytosine
- acH3
acetylated histone 3
- AID
activity-induced cytidine deaminase
- ANOVA
analysis of variance
- APOBEC
apolipoprotein B messenger ribonucleic acid editing enzyme, catalytic polypeptide
- ChIP
chromatin immunoprecipitation
- DMSO
dimethylsulfoxide
- DNA
deoxyribonucleic acid
- dnmt
DNA methyltransferase
- DR+4
direct repeat plus 4 base spacer thyroid hormone response element
- EMSA
electrophoretic mobility shift assay
- H3
histone 3
- hmeDIP
hydroxymethylated DNA immunoprecipitation
- IDAX
CXXC finger protein 4
- IDH
isocitrate dehydrogenase
- IHC
immunohistochemistry
- KLF
Krüppel-like factor
- KSM
Krüppel-like factor 9 synergy module
- LSD
Fisher’s least squares differences test
- MethylCap-seq
methylated DNA capture sequencing
- mRNA
messenger ribonucleic acid
- NF
Nieuwkoop-Faber
- NHR-scan
nuclear hormone receptor binding site scan
- NRS
normal rabbit serum
- qPCR
quantitative real-time polymerase chain reaction
- RNA
ribonucleic acid
- RNA-seq
RNA sequencing
- RT-qPCR
reverse transcription quantitative real-time polymerase chain reaction
- RXR
retinoid X receptor
- T3
3,5,3’-triiodothyronine
- TDG
thymine DNA glycosylase
- TET
ten-eleven translocation
- TR
thyroid hormone receptor
- TRE
thyroid hormone response element
- TSS
transcription start site
- SEM
standard error of the mean
- UTR
untranslated region
- WT
wild type
Additional Information
Current Affiliation : The current address for S. R. is the Fulbright University Vietnam, Ho Chi Minh City, 700000, Vietnam. The current address for Y. K. is the Tempus Labs, Inc., Chicago, Illinois 60654. The current address for C. J. S. is the Chan Zuckerberg Initiative, Redwood City, California 94063. The current address for E. C. A. L. is the Unidad Multidisciplinaria de Docencia e Investigación, Facultad de Ciencias, Universidad Nacional Autonoma de Mexico, Juriquilla, Qro, 76230, Mexico. The current address for A. S. is the Department of Pharmacology, University of Michigan Medical School, Ann Arbor, Michigan 48109.
Disclosure Summary : The authors declare that they have no conflict of interest with regard to the published work.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bernal J; Thyroid Hormones and Brain Development . Hormones, Brain and Behavior, Vol 5: Development of Hormone-Behavior Relationships. 3rd ed. Auger AP, Auger CJ, Pfaff DW, Joels M, eds. San Diego: Academic Press, Inc; 2017: 159-184. [Google Scholar]
- 2. Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development–current perspectives. Endocr Rev. 1993;14(1):94–106. [DOI] [PubMed] [Google Scholar]
- 3. Shi Y-B Amphibian metamorphosis: from morphology to molecular biology. New York: Wiley-Liss; 2000. [Google Scholar]
- 4. Vennstrom B, Liu H, Forrest D. Thyroid hormone receptors. In: Bunce CM, Campbell MJ, eds. Nuclear Receptors: Current Concepts and Future Challenges. New York, NY: Springer; 82010:183–201. [Google Scholar]
- 5. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazar MA. Thyroid hormone action: a binding contract. J Clin Invest. 2003;112(4):497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi YB. Unliganded thyroid hormone receptor regulates metamorphic timing via the recruitment of histone deacetylase complexes. Curr Top Dev Biol. 2013;105:275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng S, Cokus SJ, Zhang X, et al. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A. 2010;107(19):8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9(6):465–476. [DOI] [PubMed] [Google Scholar]
- 10. Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auclair G, Weber M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie. 2012;94(11):2202–2211. [DOI] [PubMed] [Google Scholar]
- 12. Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366(6453):362–365. [DOI] [PubMed] [Google Scholar]
- 13. Paulsen M, Ferguson-Smith AC. DNA methylation in genomic imprinting, development, and disease. J Pathol. 2001;195(1):97–110. [DOI] [PubMed] [Google Scholar]
- 14. Zeng Y, Chen TP. DNA methylation reprogramming during mammalian development. Genes. 2019;10(4):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogdanović O, Lister R. DNA methylation and the preservation of cell identity. Curr Opin Genet Dev. 2017;46:9–14. [DOI] [PubMed] [Google Scholar]
- 16. Edwards JR, Yarychkivska O, Boulard M, Bestor TH. DNA methylation and DNA methyltransferases. Epigenetics Chromatin. 2017;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harb Perspect Biol. 2014;6(5):a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Razin A, Cedar H, Riggs AD. DNA Methylation: Biochemistry and Biological Significance. New York, NY: Springer; 2012. [Google Scholar]
- 20. Bird A, Taggart M, Frommer M, Miller OJ, Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985;40(1):91–99. [DOI] [PubMed] [Google Scholar]
- 21. Bogdanović O, Smits AH, de la Calle Mustienes E, et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat Genet. 2016;48(4):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hackett JA, Sengupta R, Zylicz JJ, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339(6118):448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18(9):517–534. [DOI] [PubMed] [Google Scholar]
- 24. Wu XW, Li G, Xie RY. Decoding the role of TET family dioxygenases in lineage specification. Epigenet Chromatin. 2018;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hon GC, Rajagopal N, Shen Y, et al. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet. 2013;45(10):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo JU, Ma DK, Mo H, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci. 2011;14(10):1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kyono Y, Raj S, Sifuentes CJ, Buisine N, Sachs L, Denver RJ. DNA methylation dynamics underlie metamorphic gene regulation programs in Xenopus tadpole brain. Dev Biol. 2020;462(2):180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bochtler M, Kolano A, Xu GL. DNA demethylation pathways: additional players and regulators. Bioessays. 2017;39(1):1–13. [DOI] [PubMed] [Google Scholar]
- 30. Cokus SJ, Feng S, Zhang X, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452(7184):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones PA. The DNA methylation paradox. Trends Genet. 1999;15(1):34–37. [DOI] [PubMed] [Google Scholar]
- 33. Jjingo D, Conley AB, Yi SV, Lunyak VV, Jordan IK. On the presence and role of human gene-body DNA methylation. Oncotarget. 2012;3(4):462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu X, Zhao BS, He C. TET family proteins: oxidation activity, interacting molecules, and functions in diseases. Chem Rev. 2015;115(6):2225–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ross SE, Bogdanovic O. TET enzymes, DNA demethylation and pluripotency. Biochem Soc Trans. 2019;47(3):875–885. [DOI] [PubMed] [Google Scholar]
- 36. Nestor CE, Lentini A, Hägg Nilsson C, et al. 5-Hydroxymethylcytosine remodeling precedes lineage specification during differentiation of human CD4(+) T cells. Cell Rep. 2016;16(2):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor SE, Li YH, Smeriglio P, Rath M, Wong WH, Bhutani N. Stable 5-Hydroxymethylcytosine (5hmC) acquisition marks gene activation during chondrogenic differentiation. J Bone Miner Res. 2016;31(3):524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsagaratou A, Äijö T, Lio CW, et al. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc Natl Acad Sci U S A. 2014;111(32):E3306–E3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Yue X, Pastor WA, et al. Tet proteins influence the balance between neuroectodermal and mesodermal fate choice by inhibiting Wnt signaling. Proc Natl Acad Sci U S A. 2016;113(51):E8267–E8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502(7472):472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song CX, Szulwach KE, Fu Y, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29(1):68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mellén M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151(7):1417–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011;14(12):1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lian CG, Xu Y, Ceol C, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150(6):1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem. 2011;286(41):35334–35338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schäfer A. Gadd45 proteins: key players of repair-mediated DNA demethylation. Adv Exp Med Biol. 2013;793:35–50. [DOI] [PubMed] [Google Scholar]
- 49. Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156(1-2):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kyono Y, Sachs LM, Bilesimo P, Wen L, Denver RJ. Developmental and thyroid hormone regulation of the DNA methyltransferase 3a gene in Xenopus tadpoles. Endocrinology. 2016;157(12):4961–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kyono Y, Subramani A, Ramadoss P, Hollenberg AN, Bonett RM, Denver RJ. Liganded thyroid hormone receptors transactivate the DNA methyltransferase 3a gene in mouse neuronal cells. Endocrinology. 2016;157(9):3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denver RJ. The molecular basis of thyroid hormone-dependent central nervous system remodeling during amphibian metamorphosis. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(3):219–228. [DOI] [PubMed] [Google Scholar]
- 53. Kikuyama S, Kawamura K, Tanaka S, Yamamoto K. Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol. 1993;145:105–148. [DOI] [PubMed] [Google Scholar]
- 54. Etkin W. Hormonal control of amphibian metamorphosis. In: Etkin W, Gilbert LI, eds. Metamorphosis: A Problem in Developmental Biology. New York: Appleton-Century-Crofts; 1968:313–348. [Google Scholar]
- 55. Denver RJ. Neuroendocrinology of amphibian metamorphosis. In: Shi YB, ed. Current Topics in Developmental Biology: Animal Metamorphosis. Vol. 103. San Diego, CA: Elsevier; 2013:195–227. [DOI] [PubMed] [Google Scholar]
- 56. Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing Inc; 1994. [Google Scholar]
- 57. Wen L, Shi YB. Unliganded thyroid hormone receptor α controls developmental timing in Xenopus tropicalis. Endocrinology. 2015;156(2):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yao M, Hu F, Denver RJ. Distribution and corticosteroid regulation of glucocorticoid receptor in the brain of Xenopus laevis. J Comp Neurol. 2008;508(6):967–982. [DOI] [PubMed] [Google Scholar]
- 59. Tuinhof R, Ubink R, Tanaka S, Atzori C, van Strien FJ, Roubos EW. Distribution of pro-opiomelanocortin and its peptide end products in the brain and hypophysis of the aquatic toad, Xenopus laevis. Cell Tissue Res. 1998;292(2):251–265. [DOI] [PubMed] [Google Scholar]
- 60. Marín O, Smeets WJ, González A. Basal ganglia organization in amphibians: chemoarchitecture. J Comp Neurol. 1998;392(3):285–312. [PubMed] [Google Scholar]
- 61. Yao M, Westphal NJ, Denver RJ. Distribution and acute stressor-induced activation of corticotrophin-releasing hormone neurones in the central nervous system of Xenopus laevis. J Neuroendocrinol. 2004;16(11):880–893. [DOI] [PubMed] [Google Scholar]
- 62. Raj S, Kyono Y, Sifuentes CJ, Arellanes-Licea E, Subramani A, Denver RJ. Thyroid hormone induces DNA demethylation in Xenopus tadpole brain. Dryad Digital Repository. 2020; Deposited 1 July, 2020. 10.5061/dryad.5x69p8d11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nestor CE, Meehan RR. Hydroxymethylated DNA immunoprecipitation (hmeDIP). Methods Mol Biol. 2014;1094:259–267. [DOI] [PubMed] [Google Scholar]
- 64. Denver RJ, Williamson KE. Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology. 2009;150(8):3935–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc Natl Acad Sci U S A. 2006;103(26):10092–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yao M, Stenzel-Poore M, Denver RJ. Structural and functional conservation of vertebrate corticotropin-releasing factor genes: evidence for a critical role for a conserved cyclic AMP response element. Endocrinology. 2007;148(5):2518–2531. [DOI] [PubMed] [Google Scholar]
- 67. Lebel JM, Dussault JH, Puymirat J. Overexpression of the beta-1 thyroid receptor induces differentiation in Neuro-2a cells. Proc Natl Acad Sci U S A. 1994;91(7):2644–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Samuels HH, Stanley F, Casanova J. Depletion of L-3,5,3’-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105(1):80–85. [DOI] [PubMed] [Google Scholar]
- 69. Furlow JD, Kanamori A. The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology. 2002;143(9):3295–3305. [DOI] [PubMed] [Google Scholar]
- 70. Bagamasbad PD, Bonett RM, Sachs L, et al. Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol Endocrinol. 2015;29(6):856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269(40):24699–24705. [PubMed] [Google Scholar]
- 72. Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of the Xenopus thyroid hormone receptor-beta gene to explain its autoinduction. Mol Endocrinol. 1995;9(1):96–107. [DOI] [PubMed] [Google Scholar]
- 73. Matsuura K, Fujimoto K, Fu L, Shi YB. Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology. 2012;153(2):961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brown DD, Cai L. Amphibian metamorphosis. Dev Biol. 2007;306(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buchholz DR. Xenopus metamorphosis as a model to study thyroid hormone receptor function during vertebrate developmental transitions. Mol Cell Endocrinol. 2017;459:64–70. [DOI] [PubMed] [Google Scholar]
- 76. Wen L, He C, Sifuentes CJ, Denver RJ. Thyroid hormone receptor alpha is required for thyroid hormone-dependent neural cell proliferation during tadpole metamorphosis. Front Endocrinol. 2019;10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wen L, Shibata Y, Su D, Fu L, Luu N, Shi YB. Thyroid hormone receptor α controls developmental timing and regulates the rate and coordination of tissue-specific metamorphosis in Xenopus tropicalis. Endocrinology. 2017;158(6):1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shi YB, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161(1):48–58. [DOI] [PubMed] [Google Scholar]
- 79. Buchholz DR, Hsia SC, Fu L, Shi YB. A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol. 2003;23(19):6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wong J, Shi YB, Wolffe AP. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 1995;9(21):2696–2711. [DOI] [PubMed] [Google Scholar]
- 81. Wong J, Shi YB, Wolffe AP. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptional activation. Embo J. 1997;16(11):3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leloup J, Buscaglia M. Triiodothyronine, hormone of amphibian metamorphosis. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences Serie D. 1977;284(22):2261–2263. [Google Scholar]
- 83. Krain LP, Denver RJ. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J Endocrinol. 2004;181(1):91–104. [DOI] [PubMed] [Google Scholar]
- 84. Shi DQ, Ali I, Tang J, Yang WC. New insights into 5hmC DNA modification: generation, distribution and function. Front Genet. 2017;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Denver RJ. The molecular basis of thyroid hormone-dependent central nervous system remodeling during amphibian metamorphosis1. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(3):219–228. [DOI] [PubMed] [Google Scholar]
- 86. Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kasai K, Nishiyama N, Izumi Y, Otsuka S, Ishihara A, Yamauchi K. Exposure to 3,3’,5-triiodothyronine affects histone and RNA polymerase II modifications, but not DNA methylation status, in the regulatory region of the Xenopus laevis thyroid hormone receptor βΑ gene. Biochem Biophys Res Commun. 2015;467(1):33–38. [DOI] [PubMed] [Google Scholar]
- 88. Guan W, Guyot R, Samarut J, Flamant F, Wong J, Gauthier KC. Methylcytosine dioxygenase TET3 interacts with thyroid hormone nuclear receptors and stabilizes their association to chromatin. Proc Natl Acad Sci U S A. 2017;114(31):8229–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hassan HM, Kolendowski B, Isovic M, et al. Regulation of active DNA demethylation through RAR-mediated recruitment of a TET/TDG complex. Cell Rep. 2017;19(8):1685–1697. [DOI] [PubMed] [Google Scholar]
- 90. Bogdanovic O, Long SW, van Heeringen SJ, et al. Temporal uncoupling of the DNA methylome and transcriptional repression during embryogenesis. Genome Res. 2011;21(8):1313–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tata JR, Baker BS, Machuca I, Rabelo EM, Yamauchi K. Autoinduction of nuclear receptor genes and its significance. J Steroid Biochem Mol Biol. 1993;46(2):105–119. [DOI] [PubMed] [Google Scholar]
- 92. Heimeier RA, Hsia VS, Shi YB. Participation of Brahma-related gene 1 (BRG1)-associated factor 57 and BRG1-containing chromatin remodeling complexes in thyroid hormone-dependent gene activation during vertebrate development. Mol Endocrinol. 2008;22(5):1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Laudet V. The origins and evolution of vertebrate metamorphosis. Curr Biol. 2011;21(18):R726–R737. [DOI] [PubMed] [Google Scholar]
- 94. Lecroisey C, Laudet V, Schubert M. The cephalochordate amphioxus: a key to reveal the secrets of nuclear receptor evolution. Brief Funct Genomics. 2012;11(2):156–166. [DOI] [PubMed] [Google Scholar]
- 95. Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.