Abstract
Laryngopharyngeal reflux (LPR) is a common disorder. Pepsin has been detected also at eye level, this was a starting point for newest theories about LPR impact on Dry Eye Syndrome. The current preliminary study compared two treatments in patients with Dry Eye Syndrome and LPR. Patients were treated with Gastroftal eye drops and Gastroftal tablets or hyaluronic acid eye drops for 3 months. The following parameters were evaluated: Ocular Surface Disease Index (OSDI), OSDI categories, Reflux Symptom Index (RSI), Reflux Finding Score (RFS), Fluorescein Tear Breakup Time (B-TUT), and Schirmer test before and after treatment. On the whole, 21 patients were enrolled: 10 were treated with hyaluronic acid Atlantis (Group A) and 11 with Gastroftal eye drops and tablets (Group B). After treatment, in Group A only OSDI significantly diminished (p=0.029); in Group B there were significant reductions concerning OSDI (p=0.0277), OSDI categories (p=0.0211), RSI (p=0.0172), Schirmer test (p=0.0172), T-BUT (p=0.0265), and RFS (p=0.0205). The current preliminary demonstrated that the combined ocular and systemic therapy with hyaluronic acid, Magnesium alginate, Simethicone, and Camelia sinensis may be considered a promising treatment in patients with Dry Eye Syndrome due to LPR. (www.actabiomedica.it)
Keywords: laryngopharyngeal reflux, eye reflux, dry eye syndrome, magnesium alginate, hyaluronic acid, simethicone, Camelia sinensis
Introduction
Gastroesophageal reflux disease (GERD) is a very common disorder, namely the prevalence is up to 40% in the USA adult population (1,2). The symptoms mainly involve the upper digestive tract, but extra-oesophageal symptoms have been also identified. In this regard, the Montreal Classification includes chronic cough, asthma, and laryngopharyngeal reflux (LPR) as extra-oesophageal manifestations of GERD (3). LPR is the consequence of aggressive refluxate exposure on upper airways, specifically larynx and pharynx (4). LPR symptoms typically consist of hoarseness, sore throat, globus sensation, and throat clearing. LPR may be associated with GERD, but it may also occur as alone disorder without typical oesophageal symptoms (5,6). It has to be underlined that LPR is common in clinical practice and represents a relevant burden concerning both social and personal costs, and significantly affects the quality of life (7).
The pathogenic pathway consists of mucosal damage, as low pH of refluxate and pepsin play a major role in inducing chronic mucosal inflammation (8,9,10). Pepsin is a proteolytic enzyme deriving from pepsinogen and activated by low pH (at least <4) that is produced only in the stomach. Therefore, pepsin detection outside the gastric area may be considered incontrovertibly a biomarker for gastric reflux (11). In agreement with this evidence, the presence of pepsin was detected in different organs, including larynx, pharynx, paranasal sinus, mouth, and internal ear (12,13). Further, it has been demonstrated the presence of pepsin also in the tears of subjects with LPR (14,15). A recent study confirmed the pathogenic role of LPR in a group of patients with dry eye (16). That study concluded that LPR may be common (34%) in patients with the ocular surface disease, such as Dry Eye Syndrome a very challenging syndrome in order of aetiology and medical treatment either for General Practitioner and moreover for Ophthalmologists.
LPR treatment is a demanding problem in clinical practice; alginates represent a common treatment as recently reported (17). The present study evaluated a group of subjects with dry eye and LPR comparing two treatments: the first (Group A) was hyaluronic acid 0.2% eye drops (Atlantis), the second (Group B) included a combined topical (Gastroftal eye drops, containing hyaluronic acid, Magnesium alginate, and Camelia sinensis extract) and oral therapy (Gastroftal tablet, containing Magnesium alginate and Simethicone).
Materials and Methods
In the current study, the patients were enrolled if fulfilled the inclusion and exclusion criteria. The inclusion criteria were: i) adult age between 18 and 80 years; ii) an Ocular Surface Disease Index (OSDI) score >12; and iii) a Reflux Symptom Index (RSI) score >13. The exclusion criteria were: i) glaucoma diagnosis; ii) bacteria, viral, or fungal eye infection; iii) allergic conjunctivitis; iv) cancer; v) ocular or nasal surgery in the 3 months before the trial; vi) concomitant medications able to interfere with the findings; vii) current pregnancy or breastfeeding.
At baseline, a series of pathogenic factors were investigated: lacrimal dysfunction syndrome (LDS; such as exposure to computer light and/or contact lens), alcoholic overconsumption, tobacco smoking, GERD, and H pylori infection.
The diagnosis of LPR was based on symptoms, and specific questionnaires, such as the RSI and RFS. The Dry Eye Syndrome was evaluated by the OSDI, the fluorescein tear breakup time (TBUT), and the Schirmer test.
The RSI asked about symptoms such as hoarseness, throat clearing, cough, a7nd heartburn to create a composite score whereby an RSI > 13 suggests LPR (18). The RFS was calculated after fibreoptic endoscopy and an RFS > 7 suggests LPR (19).
The conjunctiva and cornea were examined using a slit-lamp. OSDI is a 12-item questionnaire to investigate ocular symptoms (20). The OSDI scoring was performed and quoted according to the reference guidelines: OSDI was defined as pathological if >12 (21). In addition, OSDI result was calculated by the formula: OSDI value x 25/number of responses, and was categorized as normal (scored 0) if OSDI score was between 0 and 12, borderline (scored 1) if between 13 and 22, pathological (scored 2) if between 23 and 32, and severe (scored 3) if between 33 and 100.
TBUT was evaluated by introducing a fluorescein strip moistened with 1 drop of non-preserved normal saline into the inferior conjunctival fornix with minimal stimulation (22). The quantity of saline was also controlled by carefully shaking the fluorescein strip to remove excess fluid. The patient was asked to blink several times and then hold the eye open. The cornea was scanned with a slit-lamp using cobalt blue illumination. Time from the last complete blink to the first appearance of a random dry spot on the cornea was recorded in seconds. The test was repeated 3 times in each eye, and the meantime for 3 consecutive measurements was obtained. The test was considered positive if the average T-BUT was less than 10s.
The Schirmer I test without anaesthesia was then performed (23). A standard 5×35-mm2 strip of dry filter paper was placed in each lower fornix at the junction of the lateral and middle thirds, taking care to avoid touching the cornea and left in place for 5min. After 5min, the strips were removed, and the amount of wetting in millimetres was recorded. The test results were considered positive if the length of wetting obtained was less than 10 mm in 5min.
Selected patients were screened and if met inclusion and exclusion criteria were recruited and randomly (1:1) treated with hyaluronic acid 0.2% (Atlantis) eye drops (Group A) or with a combined therapy, topical (Gastroftal eye drops, containing hyaluronic acid, Magnesium alginate, and Camelia sinensis extract) and oral therapy (Gastroftal tablets, containing Magnesium alginate and Simethicone). The patients were treated for 3 months; patients in Group A took Atlantis eye drops 1 drop 3 times/day; patients in Group B took Gastroftal eye drops 1 drop 3 times/day plus Gastroftal tablets 2 tablets after lunch and after dinner.
The primary outcome was the evaluation of OSDI change between Groups. The secondary outcomes were the evaluation of change for RSI, RFS, Schirmer test, and T-BUT assessed by both intragroup and intergroup analysis, of the tolerability and compliance of both treatments.
The patients were evaluated and scored at baseline and after the treatments. Also, a visual analogue scale (VAS) was measured for the perception of efficacy, tolerability, and compliance. Adverse events were recorded if occurred.
Demographic and clinical characteristics are described using medians with lower and upper quartiles (LQ-UQ). Any statistically significant difference in the mean values or the median values of each continuous variable was evaluated with the Wilcoxon signed-rank test or with Mann U Whitney test, respectively. Statistical significance was set at p <0.05, and the analyses were performed using GraphPad Prism software, GraphPad Software Inc, CA, USA.
Results
At baseline data
Globally, 21 patients were included in the study: 10 in Group A and 11 in Group B. The demographic data and the outcomes in the two groups of patients, at baseline, are reported in Table 1. Median age was 54 years in Group A and 56 in Group B; there were 5 males in Group A and 6 in Group B. About risk factors, 9 patients of Group A and 8 patients in Group B had LDS; two patients had alcohol overconsumption in both Groups; 1 patient in Group A and 2 in Group B were smokers; 5 and 7 patients had respectively GERD; and 2 patients in Group A had H pylori infection. The two groups were homogeneous for all these parameters at baseline as reported in Table 1.
Table 1.
Demographic and clinical characteristics in the two groups at baseline
| Group | p-value | ||
|
A 10 (47.62%) |
B 11 (52.38%) |
||
| Age (years) | 54 (41 : 75) | 56 (39 : 76) | 0.7509 |
| Males | 5 | 6 | 0.8210 |
| Risk factors | |||
| LDS | 9 | 8 | 0.1810 |
| Alcohol overconsumption | 2 (20%) | 2 (18.18%) | 0.9999 |
| Tabacco smoking | 1 (10%) | 2 (18.18%) | 0.9999 |
| GERD | 5 (50%) | 7 (63.64%) | 0.6699 |
| H.pylori infection | 2 (20%) | 0 (0%) | 0.2143 |
After treatment data
During the study, two subjects dropped out: 1 in Group A and 1 in Group B. Table 2 shows the clinical outcomes in both groups before and after treatment.
Table 2.
Intra-Geroup analysis of the clinical outcomes in the groups (see the text for abbreviations and further details
| Group A | Group B | |||||
| Time T0 | Time T1 | p-value | Time T0 | Time T1 | p-value | |
| OSDI | 17 (10 : 26) | 9.5 (4 : 23) | 0.0290 | 17 (11 : 35) | 6 (5 : 32) | 0.0277 |
| OSDI categorized | 2 (1 : 3) | 1 (0 : 3) | 0.0890 | 3 (1 : 3) | 1 (0 : 3) | 0.0211 |
| RSI | 15 (8 : 26) | 9 (6 : 26) | 0.0592 | 21 (14 : 27) | 11 (6 : 24) | 0.0172 |
| Schirmer test | 8.5 (2 : 15) | 5 (1 : 15) | 0.3096 | 6 (1 : 16) | 6 (2 : 20) | 0.0172 |
| T-BUT | 4 (2 : 7) | 5 (2 : 8) | 0.6202 | 3 (2 : 7) | 6 (3 : 10) | 0.0265 |
| RFS | 11 (0 : 16) | 4.5 (0 : 18) | 0.1148 | 13 (10 : 15) | 7 (0 : 17) | 0.0205 |
Group A
Median OSDI (Figure 1) significantly diminished (p=0.029), whereas median OSDI categorized (Figure 2), RSI (Figure 3), Schirmer test (Figure 4), T-BUT (Figure 5), and RFS (Figure 6) did not significantly changed after treatment.
Figure 1.
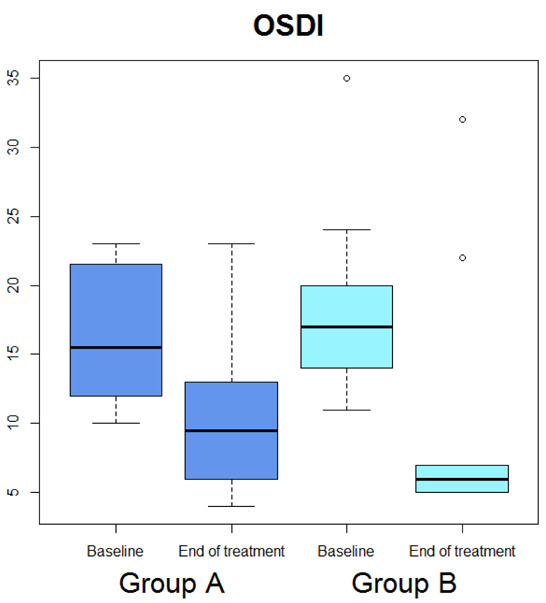
Box-plot concerning medians and interquartile ranges of OSDI values at baseline and after the treatment in Group A and B
Figure 2.
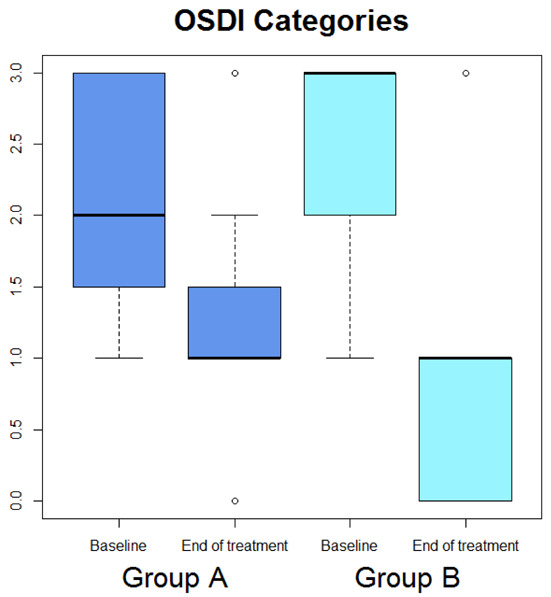
Box-plot concerning medians and interquartile ranges of OSDI categories values at baseline and after the treatment in Group A and B
Figure 3.
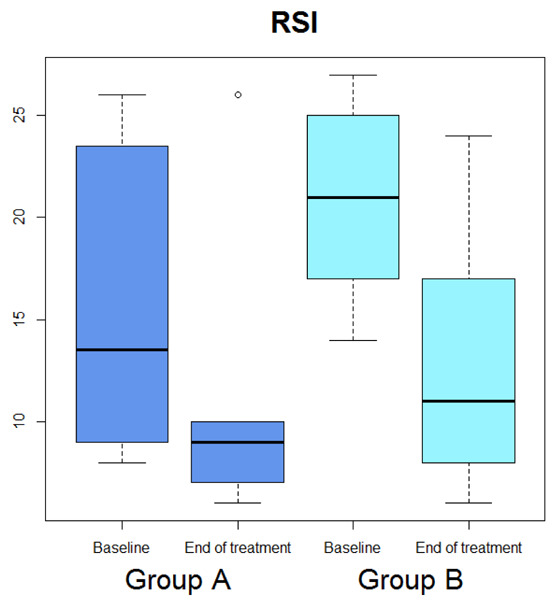
Box-plot concerning medians and interquartile ranges of RSI values at baseline and after the treatment in Group A and B
Figure 4.
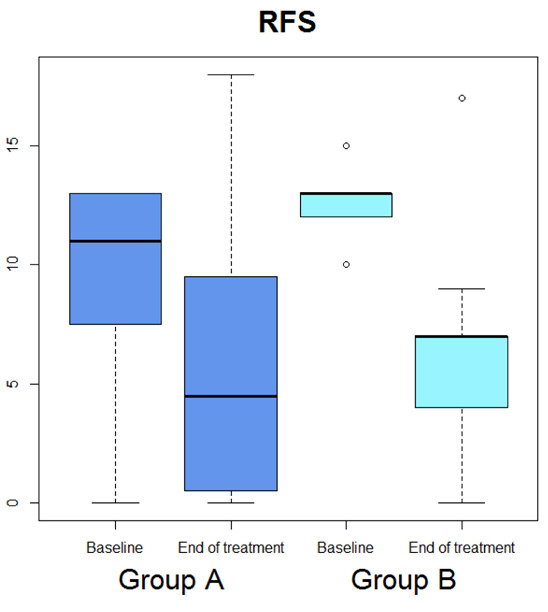
Box-plot concerning medians and interquartile ranges of RFS values at baseline and after the treatment in Group A and B
Figure 5.
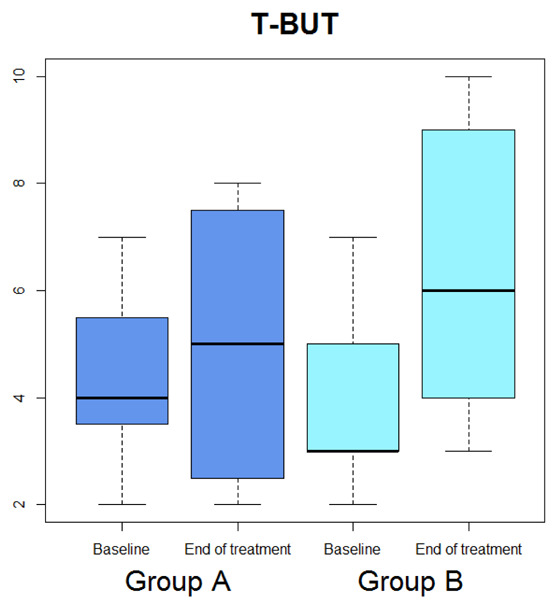
Box-plot concerning medians and interquartile ranges of T-BUT values at baseline and after the treatment in Group A and B
Figure 6.
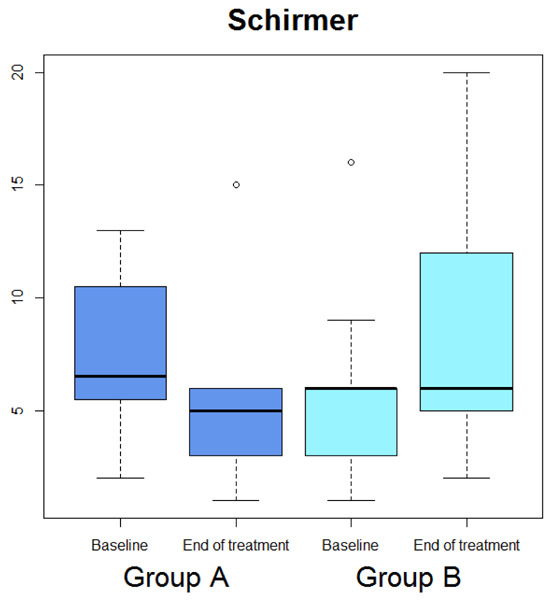
Box-plot concerning medians and interquartile ranges of Schirmer test values at baseline and after the treatment in Group A and B
Group B
Median OSDI (Figure 1) significantly diminished (p=0.0277), median OSDI categorized (Figure 2) significantly diminished (p=0.0211), RSI (Figure 3) significantly diminished (p=0.0172), Schirmer test (Figure 4) significantly diminished (p=0.0172), T-BUT (Figure 5) significantly diminished (p=0.0265), and RFS (Figure 6) significantly diminished (p=0.0205).
Safety and tolerability
Both treatments were well tolerated and no adverse event was reported during the study.
Discussion
Laryngopharyngeal reflux is a common disorder, even though the diagnosis is debated and there is no pathognomonic sign. Anyway, there is convincing evidence the LPR plays a role in airways inflammation involving some organs, such as larynx, pharynx, paranasal sinus, and middle ear (24). These outcomes paved the way to investigate a possible LPR impact also on the eye. Pepsin’s presence has been recently documented in the tears (15). The possible explanation of this way could depend on a peculiar mechanism. Pepsin can move to lacrimal film passing through the nasal cavity, the inferior meatus, and the nasolacrimal duct. More recently, it has been reported that LPR is frequent in patients suffering from an ocular surface disease (16).
On the other hand, LPR treatment should be based on protective agents and lifestyle changes. Alginates are commonly used to treat LPR and they have been demonstrated effective (17).
The current study tested two treatments: hyaluronic acid eye drops and a combined topical and oral therapy, including hyaluronic acid, Magnesium alginate, Camelia sinensis, and Simethicone.
The current preliminary study showed that Gastroftal combined treatment was able to significantly improve OSDI, OSDI categories, RSI, RFS, and T-BUT. Also, combined Gastroftal was superior to hyaluronic acid eye drops concerning OSDI, RSI, and RFS. These results are consistent with a previous survey conducted on a group of otorhinolaryngologists (2).
The effectiveness of combined Gastroftal therapy depended on the simultaneous treatment of eyes discomforts and of laryngopharyngeal reflux disease. Gastroftal eye drops is a Medical Device (class II) containing: hyaluronic acid, Magnesium alginate and Camelia sinensis extract. Hyaluronic acid (HA) is a fundamental component of the connective tissue. HA can modulate the inflammatory response, cellular proliferation, and remodeling of the extracellular matrix (25). Magnesium alginate, topically applied, thanks to its molecular egg-box structure, is able to scavenger substances including pepsin, inhibiting its proteolytic activity (26,27). Camelia sinensis, such as the green tee, has potent anti-oxidant and anti-inflammatory activity as very recently demonstrated (28). Gastroftal tablets is a Medical device (Class II), containing Magnesium alginate, and Simethicone, per oral usage. Alginate, orally administered, is a fruitful medication in the management of GERD. It precipitates as a gel after the exposure to the gastric acid, thus forming a raft that represents a barrier to the reflux of the gastric content into the oesophagus (29). Interestingly, the current findings were consistent with a recent study conducted in children with uncontrolled asthma and GERD (30). Up to 80% of uncontrolled asthmatic children treated with Magnesium Alginate had a clinically relevant reduction of both asthma control test and asthma control questionnaire. Simethicone is an anti-foam agent able to reduce the severity of symptoms caused by exces- sive gas overload in the stomach. In fact, it has been documented that it was able to significantly improve gastroesophageal reflux in infants (31). However, this study has some relevant limitations, including the cross-sectional design, the limited number of participants, the lack of functional and macroscopic investigation of the upper digestive and respiratory tract, the lack of pepsin assessment in the tears, and the lack of a follow-up.
Anyway, a strength of the current study the contemporary evaluation of digestive and ocular symptoms using validated instruments.
In conclusion, a combined therapy, including topical Gastroftal eye drops and oral Gastroftal tablets may be considered a promising treatment in patients with dry eye due to LPR.
Conflict of interest:
all the authors, but DV employee of DMG, have no conflict of interest about this matter.
References
- 1.Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018 doi: 10.1136/gutjnl-2017-314722. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelardi M, Ciprandi G. Focus on gastroesophageal reflux (GER) and laryngopharyngeal reflux (LPR): new pragmatic insights in clinical practice. J BiolRegulHomeost Agents. 2018;32(1 Suppl. 2):41–47. [PubMed] [Google Scholar]
- 3.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 4.Vaezi MF, Hicks DM, Abelson TI, et al. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol. 2003;1:333–4. doi: 10.1053/s1542-3565(03)00177-0. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute Technical Review on the management of gastroesophageal reflux disease. Gastroenterol. 2008;135:1392–413. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc. 2018;93(2):240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905–11. doi: 10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 8.Bulmer DM, Ali MS, Brownlee IA, Dettmar PW. Laryngeal mucosa: its susceptibility to damage by acid and pepsin. Laryngoscope. 2010;120:777–8. doi: 10.1002/lary.20665. [DOI] [PubMed] [Google Scholar]
- 9.Adhami T, Goldblum JR, Richter JE, et al. Role of gastric and duodenal ingredients in laryngeal tissue injury: an experimental study in dogs. Am J Gastroenterol. 2004;99:2098–2106. doi: 10.1111/j.1572-0241.2004.40170.x. [DOI] [PubMed] [Google Scholar]
- 10.Loughlin CJ, Koufman JA, Averill DB, et al. Acid-induced laryngospasm in a canine model. Laryngoscope. 1996;106:1506–9. doi: 10.1097/00005537-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Johnston N, Wells CW, Samuels TL, et al. Pepsin in non-acid refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol. 2009;118:677–85. doi: 10.1177/000348940911800913. [DOI] [PubMed] [Google Scholar]
- 12.Hamid S, Mostafa G. Extra-esophageal manifestations of gastroesophageal reflux disease: controversies between epidemiology and clinic. The Open Resp Med J. 2012;6:121–6. doi: 10.2174/1874306401206010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kung YM, Hsu WH, Wu MC, Wang JW. Recent advances in the pharmacological management of gastroesophageal reflux disease. Dig Dis Sci. 2017;3:3298–316. doi: 10.1007/s10620-017-4830-5. [DOI] [PubMed] [Google Scholar]
- 14.Magliulo G, Plateroti R, Plateroti AM. Gastroesophageal reflux disease and the presence of pepsin in the tears. Med Hypothesis. 2013;80:129–30. doi: 10.1016/j.mehy.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Iannella G, Di Nardo G, Plateroti R, Rossi P, Plateroti AM, Mariani P, Magliulo G. Investigation of pepsin in tears of children with laryngopharyngeal reflux disease. Int J Ped Otorhinolaryngol. 2015;79:2312–5. doi: 10.1016/j.ijporl.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Mazzacane D, Damiani V, Silvestri M, Ciprandi G, Marino P. G.O.A.L.-O.S.D. Study Group. Eye reflux: an ocular extraesophageal manifestation of gastric reflux. Int J Ophthalmol. 2018;11:1503–150. doi: 10.18240/ijo.2018.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelardi M, Ciprandi G. Focus on gastroesophageal reflux (GER) and laryngopharyngeal reflux (LPR): new pragmatic insights in clinical practice. J BiolRegulHomeost Agents. 2018;32(Suppl. 2):41–47. [PubMed] [Google Scholar]
- 18.Belafsky PC. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–7. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 19.Belafsky PC. Validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111:1313–7. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Walt JG, Rowe MM, Stern KL. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index. Drug Int J. 1997;31:1436. [Google Scholar]
- 21.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Stevens S. Schirmer‘s test. Community Eye Health. 2011;24:45. [PMC free article] [PubMed] [Google Scholar]
- 24.Campagnolo AM, Priston J, Thoen RH, Medeiros T, Assuncao AR. Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Arch Otorhinolaryngol. 2014;18:184–91. doi: 10.1055/s-0033-1352504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelardi M, Taliente S, Fiorella ML, Quaranta N, Ciancio G, Russo C, et al. Ancillary therapy of intranasal T-LysYal for patients with allergic, non-allergic, and mixed rhinitis. J Biol Reg Homeost Ag. 2016;30:99–106. [PubMed] [Google Scholar]
- 26.Wan LQ, Jiang J, Arnold DE, Guo XE, Lu HH, Mow VC. Calcium Concentration Effects on the Mechanical and Biochemical Properties of Chondrocyte-Alginate Constructs. Cell Mol Bioeng. 2008;1:93–102. doi: 10.1007/s12195-008-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strugala V, Kennington EJ, Campbell RJ, Skjåk-Braek G, Dettmar PW. Inhibition of pepsin activity by alginates in vitro and the effect of epimerization. Int J Pharm. 2005;304:40–50. doi: 10.1016/j.ijpharm.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Tong T, Liu YJ, Kang J, Zhang CM, Kang SG. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules. 2019;24(16) doi: 10.3390/molecules24162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies I, Burman-Roy S, Murphy MS Guideline Development Group. Gastroesophageal reflux disease in children: NICE guidance. BMJ. 2015;350:g7703. doi: 10.1136/bmj.g7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miraglia del Giudice M, Indolfi C, Ciprandi G, Decimo F, Campana G, Umano GR, Giannetti E, Maglione M. Magnesium Alginate in children with uncontrolledasthma. J Biol Reg. 2019;33:593–9. [PubMed] [Google Scholar]
- 31.Ummarino D, Miele E, Martinelli M, Scarpato E, Crocetto F, Sciorio E, Staiano A. Effect of magnesium alginate plus simethicone on gastroesophageal reflux in infants. J Pediatr Gastroenterol Nutr. 2015;60:230–5. doi: 10.1097/MPG.0000000000000521. [DOI] [PubMed] [Google Scholar]


