Abstract
Background
We do not fully understand how hypercortisolism causes central hypothyroidism or what factors influence recovery of the hypothalamic-pituitary-thyroid axis. We evaluated thyroid function during and after cure of Cushing syndrome (CS).
Methods
We performed a retrospective cohort study of adult patients with CS seen from 2005 to 2018 (cohort 1, c1, n = 68) or 1985 to 1994 (cohort 2, c2, n = 55) at a clinical research center. Urine (UFC) and diurnal serum cortisol (F: ~8 am and ~midnight [pm]), morning 3,5,3′-triiodothyronine (T3), free thyroxine (FT4), and thyrotropin (TSH) (c1) or hourly TSH from 1500 to 1900 h (day) and 2400 to 04000 h (night) (c2), were measured before and after curative surgery.
Results
While hypercortisolemic, 53% of c1 had central hypothyroidism (low/low normal FT4 + unelevated TSH). Of those followed long term, 31% and 44% had initially subnormal FT4 and T3, respectively, which normalized 6 to 12 months after cure. Hypogonadism was more frequent in hypothyroid (69%) compared to euthyroid (13%) patients. Duration of symptoms, morning and midnight F, adrenocorticotropin, and UFC were inversely related to TSH, FT4, and/or T3 levels (r = –0.24 to –0.52, P < .001 to 0.02). In c2, the nocturnal surge of TSH (mIU/L) was subnormal before (day 1.00 ± 0.04 vs night 1.08 ± 0.05, P = .3) and normal at a mean of 8 months after cure (day 1.30 ± 0.14 vs night 2.17 ± 0.27, P = .01). UFC greater than or equal to 1000 μg/day was an independent adverse prognostic marker of time to thyroid hormone recovery.
Conclusions
Abnormal thyroid function, likely mediated by subnormal nocturnal TSH, is prevalent in Cushing syndrome and is reversible after cure.
Keywords: hypothyroidism, central hypothyroidism, Cushing syndrome, cortisol, hypothalamic-pituitary-thyroid axis
In humans, individual studies suggest that endogenous hypercortisolism reduces thyroid hormone action through interactions at the hypothalamus, pituitary, liver, or other peripheral tissues. Inhibition of hypothalamic stimulation is suggested by decreases both in thyrotropin-releasing hormone (TRH) levels in the paraventricular nucleus and circulating thyrotropin (TSH) pulse amplitude (1). Additional inhibition at the thyrotrope is suggested by decreased morning serum TSH levels (2-4), and the TSH response to TRH (1, 3, 4). The presence of glucocorticoid receptors on thyrotrophs and TRH-secreting neurons (5-10) provides a physiological basis for these observations.
The reduction in TSH levels in hypercortisolism is associated with reduced circulating levels of 3,5,3′-triiodothyronine (T3), thyroxine (T4) (1, 3, 4), and free T3 (4) in these patients when compared either to those of healthy volunteers, or to values within the reference range. By contrast, free T4 (FT4) has generally been described as normal (1, 4). Furthermore, there is also a decrease in the T3:T4 ratio, likely due to glucocorticoid-mediated inhibition of peripheral deiodination (11-13). An additional etiology for reduced total T3 and T4 is a glucocorticoid-induced decrease in thyroid hormone–binding globulin (TBG) (4, 13, 14).
Much of the aforementioned data come from studies of a single component of the thyroid axis. As a result, the interplay of thyroid hormone abnormalities in patients with active Cushing syndrome (CS) is not well understood, and the timeline and pattern of recovery of the axis have not been well elucidated. The goal of this study was to describe the biochemical pattern of thyroid function in patients with CS before and after curative surgery and to examine whether factors associated with CS predict these changes.
Materials and Methods
Patients and data collection
We retrospectively reviewed records from 2 groups of CS patients age 18 to 60 years who were evaluated for separate purposes at the National Institutes of Health (NIH) Clinical Center from 2005 to 2018 (cohort 1) or 1985 to 1994 (cohort 2). Treatment of diabetes was adjusted to achieve target glucose levels. Cure/remission was defined as morning serum cortisol less than 5 µg/dL within 5 days of surgery (occasionally up to 10 days later). Patients in remission after surgery received physiologic hydrocortisone (HC) replacement (10-12 mg/body surface area, BSA) until adrenal function recovered. One patient with Graves disease was excluded from all analyses.
Cohort 1 (n = 68; 2005-2018) was followed for 6 to 12 months (6-12 M) after treatment to observe the temporal pattern of thyroid hormone changes after surgical cure of adrenocorticotropin (ACTH)-dependent CS. In these patients, the mean dose of HC replacement after curative surgery was 10.8 ± 0.3 mg/m2 BSA (range, 10-12 mg/m2 BSA). Among patients seen at 12 months, 22 patients had discontinued HC, and 35 were continuing.
Cohort 2 (n = 55; 1985-1994) underwent diurnal TSH evaluation before treatment, and in some cases, during remission, without predefined intervals.
Preoperative 24-hour urine free cortisol (UFC) and morning (0600 h-0800 h) thyroid hormone values (TSH, T3, FT4) were measured in all patients. In cohort 2 the diurnal pattern of TSH concentrations was evaluated with hourly TSH measurements from 3 pm to 7 pm and 12 am to 4 pm, and each patient’s samples were analyzed together in a single assay. In cohort 1, other hormones were measured (ACTH, 0730 h and 0800 h; serum cortisol, and 2330 h and 0000 h serum cortisol).
We noted the pathologic diagnosis, race/ethnicity, age, sex, and hormone results; in cohort 1 we also noted other available pituitary hormone values (gonadotropins and prolactin), duration of symptoms, type of surgery (simple pituitary adenomectomy vs extensive adenomectomy), and the largest dimension (a) and the other 2 dimensions (b and c) of the resected pituitary adenoma in patients with Cushing disease.
Hormone assays
Unless otherwise noted, assays were performed at the NIH Department of Laboratory Medicine (DLM), Bethesda, Maryland; test (units), reference ranges (RRs), and assay are noted.
Cohort 1: 24-hour UFC (μg/d) RRs: 8 to 77 (2001-2005, high-performance liquid chromatography/tandem mass spectrometry, Mayo Medical Laboratories), 3.5 to 45.0 (2005-2019, DLM). ACTH (pg/mL) RRs: 0.0 to 46.0 (2005-2015, Nichols Advantage Immunochemiluminometric Assay or chemiluminescence immunoassay), 5 to 46 (2015-2019) (Siemens Immulite 2500 analyzer). TSH (µIU/mL) RRs: 0.4 to 4.0 (1999-2014), 0.27 to 4.2 (2014-2019); FT4 (ng/dL) RRs: 0.9 to 1.7 (2014-2019), 0.8 to 1.9 (2003-2010), 0.9 to 1.5 (2010-2014); TT3 (ng/dL) RRs: 80 to 200 (2014-2019); 90 to 215 (2004-2014) (all with Roche Cobas 6000 platform). Prolactin (ng/mL) RRs: 1 to 11 (1999-2004), 1 to 25 (2004-2007), 3 to 29 (2007-2008), 2 to 25 (2008-2019) (all with chemiluminescence immunoassay on Siemens Immulite 2000 XPi analyzer).
Cohort 2: TSH (µIU/mL) RRs 0 to 3.9 (1/79 to 5/85, Beckman solid-phase radioimmunoassay [RIA]), 0.5 to 4.5 (May 1985 to September 1987 Serono Chemiluminescence immunoassay), 0.43 to 4.6 (September 1987 to 1994 Electrochemiluminescence immunoassay). T4 (µg/dL) RRs 6.0 to 11 (1975-1985 Beckman RIA), 5.0 to 10.0 (1985 to present Abbott TDX). Free T4 (ng/dL) RR: 1.0 to 1.9 (to June 1985 RIA, June 1985 to present Beckman Access immunoassay). T3 (ng/dL) RRs: 80 to 170 (to September 1985 Beckman RIA), 88 to 162 ng/dL (October 1985 to present Kallestad RIA). Serum cortisol (µg/dL) RR morning 7 to 25, bedtime (2330 h and 0000 h) less than 7.5 (fluorescence polarization immunoassay, TDxFLx kit, Abbott Laboratories). UFC 24-hour (µg/d) RR 20 to 90 (before September 1997, RIA), 24 to 108 (September 1997 to October 2001, fluorescence polarization immunoassay, TDxFLx kit, Abbott Laboratories).
Statistical analysis
We compared the study populations’ demographics (cohort 1 vs 2) using the Wilcoxon signed rank method. For comparing the effect of type of pituitary surgery on thyroid function, we grouped moderate and extensive pituitary exploration (exploration of 50% to 100% of the gland) with hemihypophysectomy as “extensive pituitary surgery” in subsequent analyses. Simple adenomectomy, in which the neurosurgeon readily identified and solely resected the pituitary adenoma without further exploration, was grouped as “simple pituitary surgery.” We evaluated the association of central hypothyroidism and hypothalamic-pituitary-gonadal (HPG) axis dysfunction using Fisher exact test on 2 × 2 tables. We defined HPG dysfunction as either a) gonadotropin values below the age- and sex-appropriate RRs or b) inappropriately normal gonadotropin levels in conjunction with subnormal sex-steroid values (serum estradiol for women and serum testosterone for men).
Fisher exact test was used for dichotomous variables. Likelihood ratios (LRs) and degrees of freedom (df) were calculated where appropriate. Log transformation was performed to achieve normal distribution since raw values had a skewed distribution. We performed 2 additional types of statistical analysis that are detailed as follows. First, we used the Pearson correlation to delineate the effects of preoperative (baseline) hypercortisolism on preoperative thyroid function. Second, because we anticipated changes in thyroid function after surgical cure, we used linear mixed models to evaluate the effect of preoperative hypercortisolemia on the recovery of thyroid functions.
1) Pearson correlations were performed on log-transformed baseline values of both cohorts. Continuous outcomes within the 2 independent cohorts were compared using either 2-sample t test or Wilcoxon rank sum test as appropriate. We defined central hypothyroidism as FT4 levels at or below the lower limit of the normal range of the assay with normal or subnormal TSH (15).
-
2) We used linear mixed models to examine the effects of hypercortisolism variables on log-transformed TSH, FT4, and T3 respectively by taking into account correlation among the 3 repeated visit measurements (baseline, 6 M, 12 M) of TSH, FT4, or T3 per participant.
We first analyzed the effects of the visit (baseline, 6 M, 12 M), continuous values for UFC and duration of symptoms, maximum tumor dimension, and tumor volume on each of log-transformed TSH, FT4, and T3.
Second, to simplify the analysis of hypercortisolism-related variables, we dichotomized them as values above or below the following: UFC of 300, 500, and 1000 μg/d; ACTH of 60 pg/mL; and serum morning cortisol of 20 μg/dL. These progressively increasing cutoff points were chosen to represent increasingly severe levels of hypercortisolism, and thus potentially greater effects on thyroid function. The UFC cutoffs of 300, 500, and 1000 μg/d were chosen because they approximately reflect 6-, 10-, and 20-fold increases above the upper limit of normal (ULN). The Endocrine Society guidelines state that a UFC of more than 5 times the ULN establishes the diagnosis, suggesting these cutoffs may be incremental thresholds for extreme hypercortisolemia (16). Similarly, a plasma ACTH value of 60 pg/mL was chosen because morning plasma ACTH concentrations are usually between 10 and 60 pg/mL (range, 2.2 and 13.3 pmol/L) (17). Likewise, because most eucortisolemic patients have a morning serum cortisol between 10 μg/dL and 20 μg/dL, values above 20 μg/dL may indicate more severe hypercortisolism (17).
We then examined the effects of visit and each dichotomized hypercortisolism variable on each of the log-transformed TSH, FT4, and T3 variables. This was performed by testing whether there is (1) a significant interaction between a dichotomized hypercortisolism variable and visit (ie, model 1), (2) for variables without a significant interaction in model 1, whether there is an effect of the dichotomized hypercortisolism-related variable and visit, and (3) for variables with a significant interaction in model 1, whether there is an effect of the dichotomized hypercortisolism-related variable at each visit separately.
Values are shown as mean ± SE except as mentioned otherwise. Values below the lower reference range are referred to as subnormal. P values less than .05 were considered statistically significant. Adjusted P values (adjusted P) after Bonferroni correction for multiple comparison were reported as appropriate. Statistical analysis was carried out using Microsoft Excel, SAS version 9.4, and JMP14 (SAS Institute Inc).
Results
Baseline patient characteristics
Cohort 1 was older than cohort 2 (mean age, 43.8 vs 37.2 years; P = .0002), with a lower proportion of Whites (62% vs 89%; P < .0001), but similar proportions of women (80% vs 80%) and Cushing disease etiology (81% vs 80%). Although the mean UFC was about 40% higher in cohort 2 than cohort 1, the upper value of the reference range of the cohort 2 immunoassay was about double that of the liquid chromatography tandem mass spectroscopy assay used for cohort 1. Baseline thyroid hormone values for both cohorts are shown in Table 1.
Table 1.
Patient characteristics of cohort 1 (n = 68) and cohort 2 (n = 55)
| Characteristic | Cohort 1 | Cohort 2 | P for cohort 1 vs cohort 2 |
|---|---|---|---|
| Patient characteristics, mean ± SD or n (%) | |||
| Sex: female/male | 54/14 (80%/20%) | 44/11 (80%/20%) | NS |
| BMI, kg/m2 | 33.3 ± 1.0 | NA | |
| Age, y | 43.8 ± 12.7 | 37.2 ± 12.0 | < .001 |
| Race/Ethnicity | |||
| White | 42 (61.7%) | 49 (89.1%) | < .001 |
| Black/African American | 14 (20.5%) | 4 (7.3%) | .003 |
| Hispanic | 0 | 1 (1.81%) | |
| Unknown | 5 (7.3%) | 0 | |
| Asian | 3 (4.4%) | 0 | |
| American Indian | 2 (2.9%) | 0 | |
| Multiracial | 2 (2.9%) | 0 | |
| Diagnosis | |||
| Cushing disease | 55 (81.2%) | 44 (80%) | NS |
| Ectopic ACTH secretion | 13 (18.8%) | 3 (5.5%) | |
| Adrenal adenoma | 0 | 4 (7.3%) | |
| Adrenal carcinoma | 0 | 2 (3.6%) | |
| PPNAD | 0 | 1 (1.8%) | |
| Unclear etiology | 0 | 1 (1.8%) | |
| Hormonesa, mean ± SE | |||
| ACTH, pg/mL | 81.5 ± 11.5 | ||
| Morning cortisol, μg/dL | 27.1 ± 2.1 | ||
| Midnight cortisol, μg /dL | 20.6 ± 1.8 | ||
| UFC, μg/d | 645.86 ± 129.7 | 890.0 ± 46.9 | |
| TSH, μIU/mL | 1.13 ± 0.1 | 0.99 ± 0.2 | .36 |
| FT4, ng/dL | 0.93 ± 0.0 | 1.2 ± 0.0 | < .01 |
| T3, ng/dL | 80.7 ± 2.9 | 90.85 ± 3.9 | .01 (Wilcoxon and t test) |
Abbreviations: ACTH, adrenocorticotropin; FT4, free thyroxine; NA, not available; NS, not significant; PPNAD, primary pigmented nodular adrenocortical disease; T3, 3,5,3′-triiodothyronine; TSH, thyrotropin; UFC, urine free cortisol.
a Reference ranges: Cohort 1 ACTH less than 45 pg/mL; morning cortisol 5 to 25 μg/dL; evening cortisol less than 7.5 μg/dL; UFC 3.5 to 45 μg/d; TSH 0.4 to 4.0 (1999-2014), 0.27 to 4.2 (2014-2019) μIU/mL; FT4 0.9 to 1.7 (2014-2019); 0.8 to 1.9 (2003-2010); and 0.9 to 1.5 (2010-2014) ng/dL. Cohort 2: ACTH less than 45 pg/mL; morning cortisol 5 to 25 μg/dL; evening cortisol less than 7.5 μg/dL; UFC less than 90 or less than 108 μg/d; T3 80 to 200 (2014-2019); and 90 to 215 (2004-2014) ng/dL.
Cohort 1 had 13 patients with ectopic ACTH production who underwent successful tumor resection: 12 via thoracotomy and 1 with appendectomy. Among the 55 patients with a pituitary source of ACTH, 25 (44.6%) had a targeted, simple adenomectomy, 13 (23.6%) had moderate pituitary exploration in which most of the gland was surgically explored, 13 (23.6%) had extensive exploration in which the entire gland was surgically explored, and 4 had hemihypophysectomy when a tumor was not identified (7.2%).
Morning thyrotropin, free thyroxine, and 3,5,3′-triiodothyronine in cohort 1 during hypercortisolism and after curative surgery
Table 2 shows thyroid function values evaluated in cohort 1 before and between 0 and 12 M after surgery.
Table 2.
Thyroid function tests at baseline and after surgery in cohort 2
| Hormone | Before surgery mean ±SE (n) | 6 mo after surgery mean ±SE (n) | 12 mo after surgery mean ±SE (n) |
|---|---|---|---|
| FT4, ng/dL | 0.92 ± 0.02 (n = 51) | 1.09 ± 0.02 (n = 47) | 1.15 ± 0.15 (n = 47) |
| T3, ng/dL | 80.4 ± 3.5 (n = 49) | 131.0 ± 3.6 (n = 45) | 121.4 ± 3.9 (n = 43) |
| TSH, μIU/mL | 0.97 ± 0.09 (n = 51) | 2.50 ± 0.19 (n = 46) | 2.71 ± 0.19 (n = 48) |
Fifty one unique patients underwent testing. Not all tests were performed on each patient and some patients were excluded (5/56) during the course of follow up since they were newly started on thyroid modifying drugs.
Abbreviations: FT4, free thyroxine; T3, 3,5,3′-triiodothyronine; TSH, thyrotropin.
Thyroid hormone results before surgery (hypercortisolemic) (n = 68)
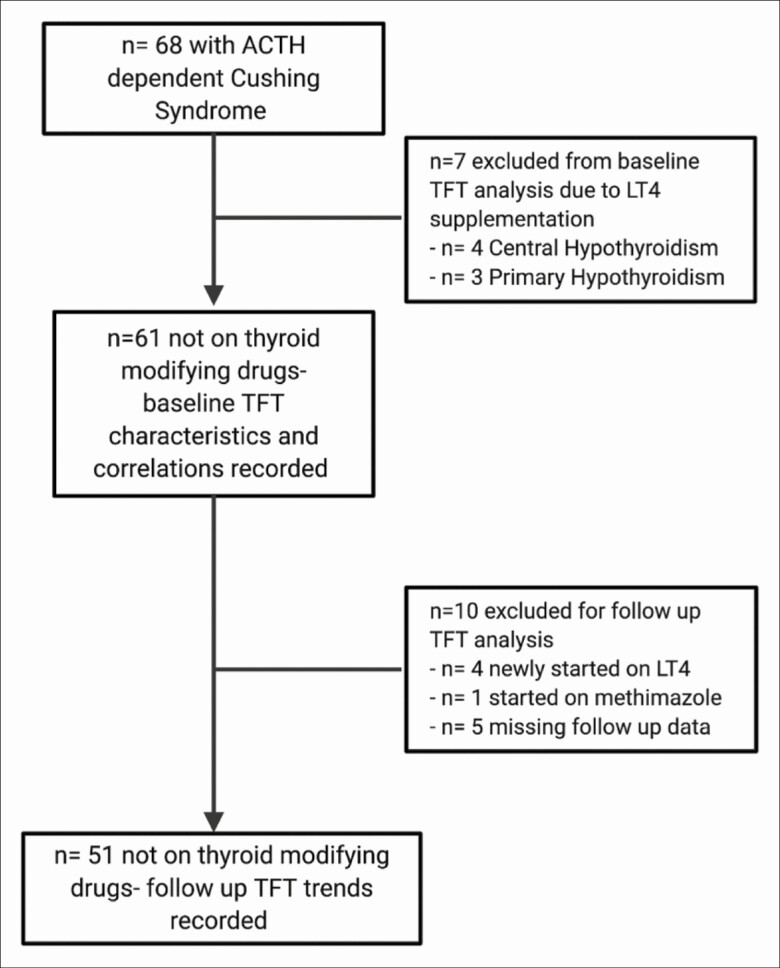
At initial NIH evaluation of cohort 1, 7 of 68 patients were receiving levothyroxine (LT4) for previously diagnosed primary (n = 3) or central (n = 4) hypothyroidism (Fig. 1).
Figure 1.
Eligibility, inclusion, and follow-up chart of cohort 1 patients.
Among the 61 patients not taking LT4 (see Fig. 1), untreated central hypothyroidism (FT4 at or below the lower reference bound without elevated TSH) was present in 32 (52%): A total of 13 (21%) had FT4 at the lower limit of normal and 19 (31%) had subnormal FT4. T3 was subnormal in 29 of the 61 patients (17 of whom had low FT4), and TSH was subnormal in 7 (3 with subnormal T3 and FT4, 3 with only low T3, and 1 with normal T3 and FT4). No T3 was in the upper half of the normal range, and only 4 of these 61 patients had a TSH (n = 2) or FT4 (n = 2) in the upper half of the normal range.
There were no differences in the baseline mean values of TSH or FT4 in patients with ectopic CS compared to Cushing disease. However, T3 was significantly lower in the ectopic CS group (mean T3 [ng/dL]: 23.5 vs 35.4, P = .04). At baseline, compared to Cushing disease patients, the prevalence of central hypothyroidism was significantly higher in ectopic CS patients, who had a 4-fold increased likelihood of central hypothyroidism (prevalence: 77% vs 47%, LR: 3.9, df = 1, P = .048). Notably, the mean UFC of all ectopic CS patients was higher than that of Cushing disease patients (UFC [μg/day]: 1827 vs 514, P = .0001). Likewise, within the centrally hypothyroid CS group (n = 36), mean UFC was higher in the ectopic CS patients compared to Cushing disease (UFC [μg/day]: 2085 vs 369, P = .0001). Additionally, patients with central hypothyroidism had a shorter duration of symptoms compared to euthyroid patients (2.5 vs 4.3 years, P = .04), perhaps related to the higher proportion of ectopic ACTH patients.
Furthermore, we noted that patients with central hypothyroidism had a significantly higher prevalence of (and were 24 times more likely to have) HPG axis dysfunction (hormonal data not shown) compared to those without central hypothyroidism (prevalence: 69.4% vs 12.5%, LR: 24.3, df = 1, P < .0001). Serum prolactin concentrations were similar among (central) hypothyroid and euthyroid patients (Prolactin [ng/mL]: 14.3 vs 16.2, P = .6). Differences in the prevalence of panhypopituitarism could not be ascertained because of insufficient data. On 2 × 2 analysis, (centrally) hypothyroid and euthyroid groups showed similar distributions of the type of pituitary surgery (simple vs extensive), the largest pituitary tumor dimension, and the product of the 3 dimensions (a × b × c) of the excised pituitary tumor, a surrogate for tumor volume.
Thyroid hormone results after curative surgery: (n = 51)
Fifty-six patients of the 61 not receiving LT4 before surgery had at least one repeat thyroid function test (TFT) at approximately 6 and/or 12 months after surgery. Four of the 56 patients were started on LT4 and one patient was started on methimazole. Therefore, the remaining 51 patients not on thyroid-modifying drugs formed the basis of the following analysis of the change in TFTs during remission (see Fig. 1).
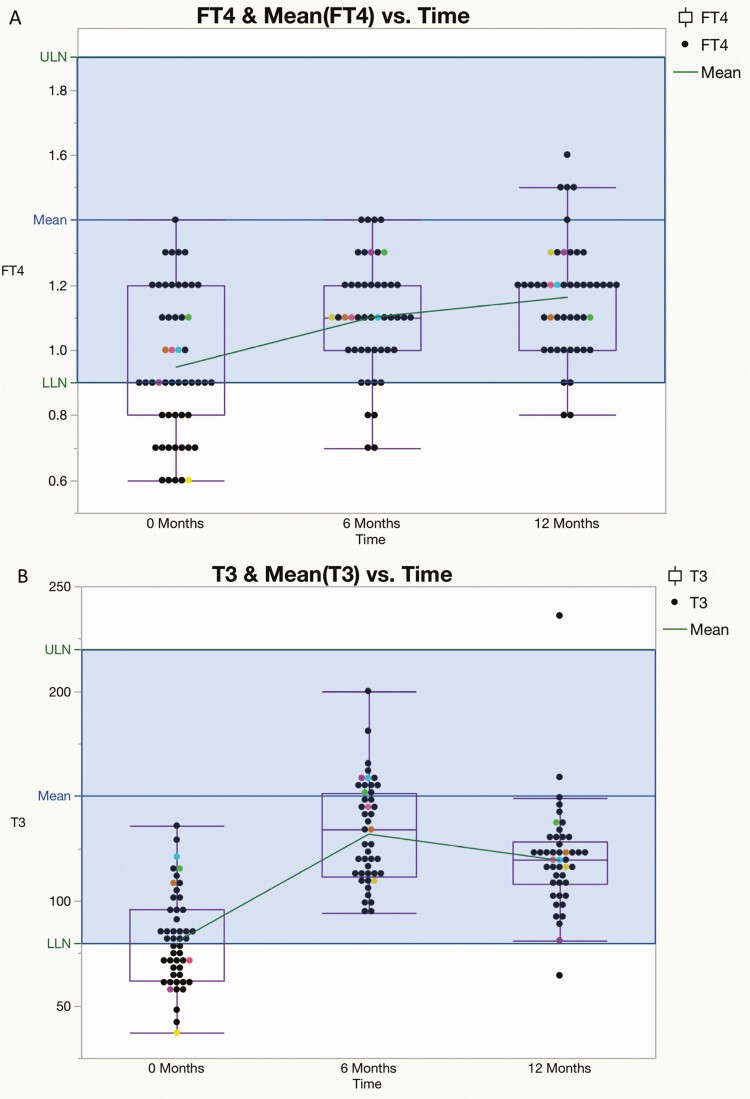
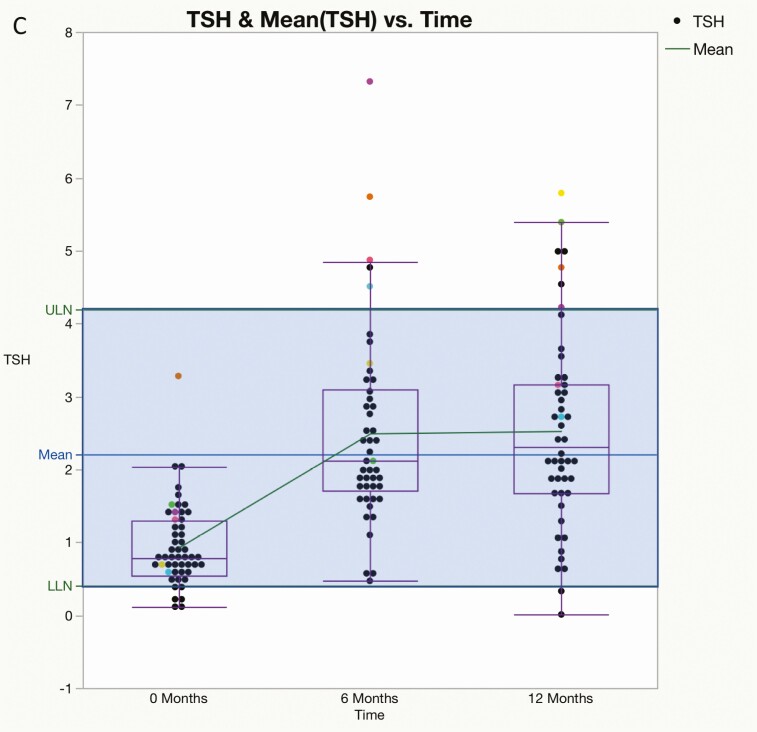
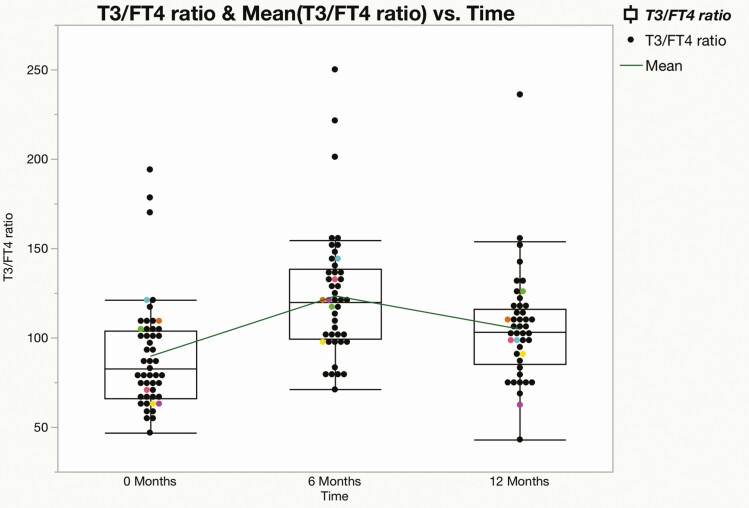
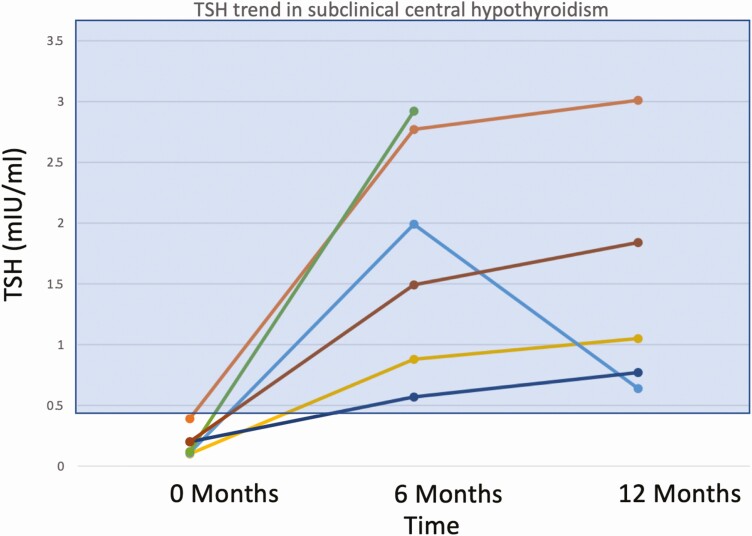
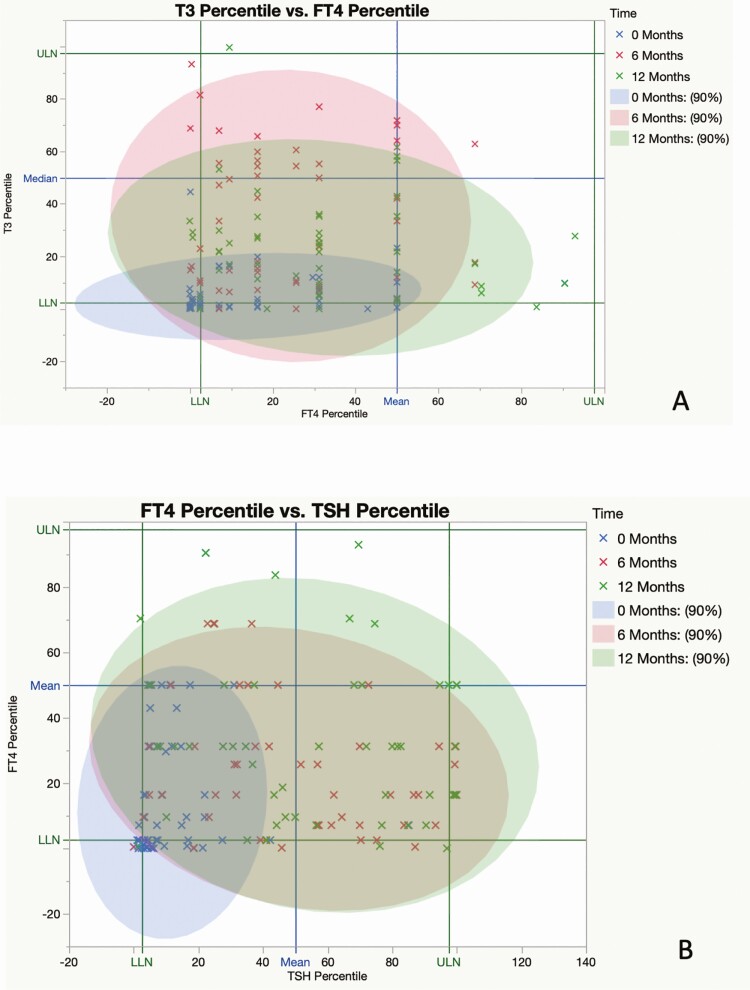
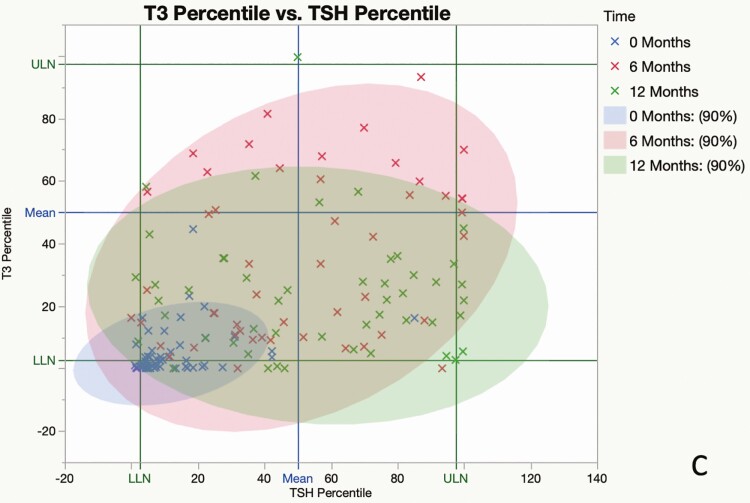
Preoperatively, FT4 values were subnormal in 17 of 51 patients (33%), T3 values were subnormal in 22 of 51 patients (44%), and 50 of 51 (98%) TSH values were within (n = 45) or below (n = 5) the lower half of the reference range; the mean value of each measure increased significantly from baseline at 6 and 12 months after surgery (Fig. 1A-1C). By contrast, at 12 months, subnormal FT4 was present in only 2 of 47 (4%) patients and T3 values were subnormal in only one patient; 23 of 48 (48%) TSH values were in the lower half (21/23) or below (2/23) the reference range, and 7 were above the reference range (Fig. 2A-2C). The mean T3/FT4 ratio increased significantly from 89.8 at baseline to 123.5 at 6 M and declined to 104.9 at 12 M (Fig. 3). Six of the 7 patients with subnormal TSH (and low T3 and/or FT4 before surgery) with at least one follow-up visit had normal thyroid hormone values during remission without LT4 supplementation (Fig. 4).
Figure 2.
A, Mean free thyroxine (T4) (±SEM) was 0.94 ± 0.03 ng/dL at 0 months (M) (n = 51), 1.10 ± 0.02 ng/dL (n = 47) at 6 M, and 1.16 ± 0.02 ng/dL (n = 47) at 12 M after curative surgery. Dots represent individual patients; boxes represent the upper and lower limits of 95% CI. P values were less than .001 for 0 to 6 and 0 to 12 months and not significant for 6 to 12 months. The blue line is the midpoint of reference range. LLN, lower limit of normal range; ULN, upper limit of normal range. B, Mean total 3,5,3′-triiodothyronine (T3) (±SEM) at 0 months (M) was 80.87 ± 3.20 ng/dL (n = 49), 131.99 ± 3.60 ng/dL (n = 45) at 6 M, and 119.46 ± 3.74 ng/dL (n = 45) at 12 M after curative surgery. Boxes represent the upper and lower limits of 95% CI. P values were less than .05 for all changes. The blue line is the midpoint of the reference range. C, Mean thyrotropin (TSH) (±SEM) was 0.92 ± 0.08 ng/dL (n = 51) at 0 months, 2.49 ± 0.20 ng/dL (n = 46) at 6 months, and 2.53 ± 0.19 ng/dL (n = 48) at 12 months. Boxes represent the upper and lower limits of 95% CI. P values were < .001 for 0 to 6 and 0 to 12 months and not significant for 6 to 12 months. The blue line is the midpoint of the reference range. Differently colored dots represent the TSH values of 6 individual patients with elevated TSH at 6 or 12 months. LLN, upper limit of normal range; ULN, upper limit of normal range.
Figure 3.
Relationship of 3,5,3′-triiodothyronine (T3) to free thyroxine (T4) (n = 49) was 89.78 ± 4.3 at 0 months, 123.52 ± 5.30 (n = 45) at 6 months, and 104.91 ± 4.61 (n = 44) at 12 months. Boxes represent the upper and lower limits of 95% CI, and the green line connects the means. P values were less than .01 for all changes.
Figure 4.
Thyrotropin (TSH) values of 6 patients with unrecognized central hypothyroidism before curative surgery, showing spontaneous recovery 6 months after surgery. The reference range is shown as a shaded rectangle.
Changes in the frequency of hypothyroidism after treatment in cohort 1 (n = 68)
Before surgery, central hypothyroidism was present in 36 patients (32/68 were untreated and 4/68 were receiving LT4). Of these 36 patients, central hypothyroidism (treated or untreated) was present in 10 patients at 6 M and 6 patients at 12 M. There were no differences in the maximum tumor dimension, tumor volume, or type of surgery between euthyroid and hypothyroid patients at 6 M and 12 M.
LT4 was newly started in 4 patients at 6 months because of posttranssphenoidal surgery central hypothyroidism and was discontinued in 2 at 12 months. Altogether at the last follow-up, central hypothyroidism (treated or untreated) was present in 13 of 59 patients at 6 M and 7 of 54 patients at 12 M.
Three patients with primary hypothyroidism before surgery were receiving LT4; one had a mildly elevated TSH. The latter had a further increase in TSH from 3.65 to 9.12 µIU/mL with low FT4 and T3, and undetectable peroxidase and thyroglobulin antibodies 1 month after surgery, and no additional follow-up. At 6 M 4 patients had new TSH elevations and at 12 M 6 patients had new or persistent (n = 2) elevations (2 with detectable thyroglobulin antibodies). One patient on LT4 before surgery had a subnormal TSH at 6 M despite continuation of her previous LT4 dose. After her LT4 dose was reduced, TSH normalized at 12 M. Fig. 5 shows the recovery profile of thyroid function between 0 and 12 M.
Figure 5.
An individual patient’s thyroid hormone values expressed as the percentile of the reference range, obtained between 0 and 12 months. The upper limit of normal (ULN) and lower limit of normal (LLN) correspond to the 97.5th and 2.5th percentiles of the population values, respectively. The blue lines correspond to the 50th percentile values (median). The blue, red, and green symbols represent measurements obtained at baseline, 6, and 12 months, respectively. Each colored ellipse contains 90% of crosses of the same color. The blue ellipse (before surgery) is condensed at LLNs but expands over the normal range after cure (pink: 6 months; green: 12 months), signifying the biochemical recovery of thyroid hormones. A, Free thyroxine (FT4) percentile (x axis) vs 3,5,3′-triiodothyronine (T3) percentile (y axis). B, Thyrotropin (TSH) percentile (x axis) vs FT4 percentile (y axis). C, TSH percentile (x axis) vs T3 percentile (y axis).
Thyroid hormone values and the proportion of patients with central hypothyroidism were similar in the ectopic CS and Cushing disease groups at 6 M and 12 M.
Effects of hypercortisolism variables on thyroid hormones before and during remission
Effects on thyroid hormones during hypercortisolism (n = 61)
Using the Pearson correlation, in cohort 1 preoperative T3 and TSH were negatively correlated with morning and midnight cortisol, ACTH, and UFC, with effect sizes that were moderate for T3 and modest for TSH (Table 3). FT4 was affected similarly, but only by ACTH and morning cortisol. The duration of symptoms had little or no correlation with baseline thyroid hormones except for log FT4 (see Table 3). Using Pearson correlation in cohort 2, log UFC was negatively correlated with log morning TSH (r = –0.33, P = .02, n = 42), log night TSH (r = –0.32, P = .03, n = 42), and log TBG (r = -0.39, P = .03, n = 28).
Table 3.
Correlation between measures of hypercortisolism and preoperative thyroid function tests in cohort 1
| Hypercortisolism variable | Thyroid hormone variable | R | P | n |
|---|---|---|---|---|
| Log UFC | Log T3 | –0.34 | .01 | 58 |
| Log FT4 | –0.21 | .08 | 61 | |
| Log T3/FT4 ratio | –0.16 | .21 | 58 | |
| Log TSH | –0.26 | .0208 | 62 | |
| Log morning cortisol | Log T3 | –0.52 | < .0001 | 58 |
| Log FT4 | –0.31 | .01 | 61 | |
| Log T3/FT4 ratio | –0.27 | .036 | 58 | |
| Log TSH | –0.24 | .07 | 61 | |
| Log midnight cortisol | Log T3 | –0.44 | .0012 | 57 |
| Log FT4 | –0.19 | .13 | 60 | |
| Log T3/FT4 ratio | –0.26 | .04 | 57 | |
| Log TSH | –0.32 | .012 | 60 | |
| Log ACTH | Log T3 | –0.44 | .0004 | 58 |
| Log FT4 | –0.44 | .0005 | 61 | |
| Log T3/FT4 ratio | –0.14 | .29 | 58 | |
| Log TSH | –0.12 | .34 | 61 | |
| Log durationa | Log T3 | 0.17 | .2 | 58 |
| Log FT4 | 0.34 | .0063 | 61 | |
| Log T3/FT4 ratio | –0.08 | .532 | 58 | |
| Log TSH | 0.19 | .128 | 62 |
Statistically significant comparisons are shown in bold.
Abbreviations: ACTH, adrenocorticotropin; FT4, free thyroxine; T3, 3,5,3′-triiodothyronine; TSH, thyrotropin; UFC, urine free cortisol.
a Duration of signs/symptoms by history.
Effects on thyroid hormones during remission (data from preoperative and postoperative visits)
Using a linear mixed-model analysis, we found little interaction of any hypercortisolism variable (UFC, duration of symptoms, tumor size, and volume) when compared to all preoperative and postoperative thyroid hormone values. There was only a borderline significant adverse effect of UFC on log FT4 (P = .048).
However, there was a significant interaction between visits and each dichotomous biochemical variable, so each variable was examined at each visit. Log T3 was affected inversely by serum morning cortisol of greater than 20 μg/dL (adjusted P = .045) and UFC of greater than 300 μg/day (adjusted P = .045) and 500 μg/d (adjusted P = .051) at baseline only (consistent with the Pearson correlation analysis of preoperative data presented previously). Post hoc analysis revealed that a baseline UFC greater than 1000 μg/day was associated adversely with baseline and 6 M values both for log T3 (adjusted P = .015; baseline, and P = .047; 6 M) and logFT4 (adjusted P = .016; baseline and P = .047; 6 M). No other dichotomized UFC, cortisol, or ACTH affected thyroid hormone values. In contrast, when dichotomized, symptom duration greater than 1 year was associated with a higher logFT4 value across all visits (P = .018).
Loss and subsequent recovery of the nocturnal thyrotropin surge before and during remission (cohort 2, n = 55)
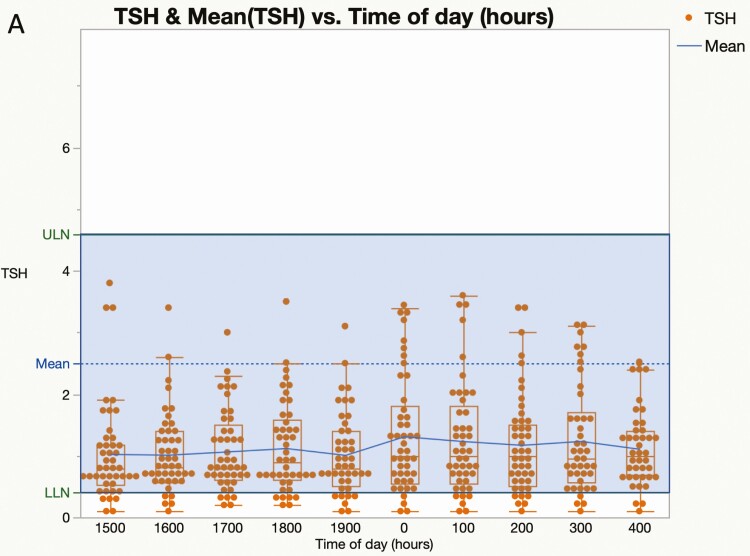
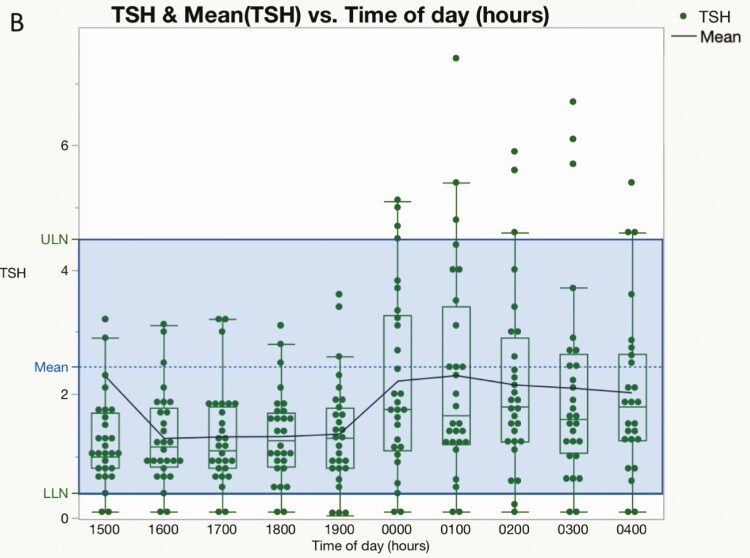
The 55 hypercortisolemic patients in cohort 2 showed no difference between daytime and nighttime mean TSH values (day vs night: 1.00 ± 0.04 vs 1.08 ± 0.05, P = .3). The ratio of mean nighttime (2400-0400 h) and daytime (1500-1700 h) TSH was 1.13 ± 0.06. By contrast, the nocturnal TSH surge was normal at a mean of 7.8 M after curative surgery (2.17 ± 0.27 vs 1.30 ± 0.14, P = .01) (Fig. 6A and 6B). A subset of patients had multiple postoperative diurnal TSH evaluations. The nocturnal increase of TSH compared to daytime levels was 161% at 7.8 months (n = 29), 140% at 15 months (n = 15), 161% at 25 months (n = 8), and 207% at 36 months (n = 2) after curative surgery.
Figure 6.
Diurnal variation in thyrotropin (TSH) during and after cure of hypercortisolism. Box and scatter plots for serum TSH against time of day (h) before (A, n = 55) and after (B, n = 28) surgery. Each dot represents an individual patient and the blue line connects the means. The middle of the normal range is shown by the dashed line A, Nonparametric comparisons for the 36 possible day and night pairs using the Wilcoxon signed rank method were not significant before curative surgery (P > .05 for all pairs). B, Values obtained at a mean of 7.8 months (range, 0.4-20 months) after curative surgery. Nonparametric comparisons for each day and night pair using the Wilcoxon signed rank method were significant (P < .05) except for 300, 1500, 1700, 1800, and 1900; and 400, 1800, and 1900.
Discussion
This study of 123 CS patients confirms and extends previous evaluations of thyroid dysfunction caused by hypercortisolism. In addition to further documentation of decreases in morning TSH, T3, and T4, we show that these changes reflect global inhibition of the thyroid axis. There was a dose-dependent inverse relationship between preoperative cortisol exposure and decreased thyroid hormone values before and after surgery. The implicit role of cortisol also is evident from the posttreatment normalization of nocturnal TSH secretion and T3 and T4 levels.
At baseline, dysfunction of the HPG axis was more prevalent in hypothyroid patients (69.4%) than euthyroid CS patients (12.5%), which may indicate an additional effect of hypercortisolemia on hypothalamic-pituitary axes (18). Another mechanism for concurrent HPG and hypothalamic-pituitary-thyroid (HPT) axis dysfunction is the dependence of the HPG axis on an intact HPT axis (19, 20). However, baseline serum prolactin values were similar in patients with and without central hypothyroidism. Because acquired prolactin deficiency is a reliable indicator of severe anterior pituitary deficiency and correlates with somatotropin deficiency (21), these findings suggest complete anterior pituitary deficiency is not present in centrally hypothyroid CS patients.
Under physiological conditions, the diurnal peak and nadir of cortisol and TSH inversely mirror each other. By contrast, in states of hypercortisolism, the diurnal rhythm of both cortisol and TSH is lost, whereas their normal relationship is restored when the normal adrenal axis recovers after cure of CS. Therefore, we propose that hypercortisolism-mediated suppression of the nocturnal TSH surge and attenuation of TSH secretion are major proximate causes of decreased thyroid hormone values in CS.
In healthy people, mean nocturnal TSH levels are 51% higher than afternoon values (22). We found a 13% TSH nocturnal rise in 55 hypercortisolemic patients, which gradually normalized during remission (see Fig. 5). Consistent with our findings, Bartalena et al reported loss of the nocturnal surge of TSH, and an inverse relationship between serum cortisol and night—as well as day—TSH values in 10 patients (3). By contrast, Roelfsema and colleagues reported a preserved TSH variation (23), possibly related to differences in the sample size between the 2 studies (n = 55 vs 16), and/or the severity of hypercortisolism. Blunting of the TSH response to TRH, as previously shown in CS (23-25), may account for the reduced nocturnal surge.
Recently, Tamada and colleagues reported a decline in TSH levels at 2300 h and 2400 h compared to 0800 h and 0900 h in 22 patients with CS. They proposed a night:day TSH ratio of 1 or less as a diagnostic criterion for the syndrome, and showed a sensitivity of 91% and specificity of 95% (26). We found a night:day TSH ratio of 1.13 ± 0.06, using mean values between 2400-0400 h and 1500-1900 h. However, we did not study patients without CS and cannot verify the specificity of this approach. In addition, we noted a suboptimal nocturnal increase in TSH values rather than a decline, but the time points differed between the studies.
In addition to the potential diagnostic utility of a subnormal nocturnal TSH increase, our results suggest baseline morning thyroid hormone values may support or refute the diagnosis of CS. In our cohort 1, 70% (48/68) of patients had subnormal TFTs (either FT4, T3, TSH, or a combination) or were on thyroid hormone. In the remaining 13 patients not taking thyroid-related medications, only one had a TSH in the upper half of the reference range, and none had a FT4 or T3 in the upper half of their reference ranges. Thus, if TSH, T3, or FT4 values are in the upper half of the reference range or higher, the diagnosis of CS is unlikely. Conversely, values of multiple thyroid hormones in the lower half of the normal range support the diagnosis of CS.
Surprisingly, we found a higher prevalence (52.5%) of central hypothyroidism than previously reported in hypercortisolemic children (4%) (27) or adults (25%-36%) (28-31), perhaps because of our larger sample size or more severe hypercortisolism. Furthermore, we noted a higher prevalence of central hypothyroidism (77% vs 47%) and lower mean baseline T3 values (23.5 vs 35.4 ng/dL) in ectopic CS compared to Cushing disease, which did not persist during follow-up. We speculate that these differences were related, at least partly, to the more severe hypercortisolism in the ectopic CS group, whose mean UFC was more than 3 times higher than that of the Cushing disease group. This speculation is supported by our observations of statistically significant but modest negative correlations between different interdependent measures of hypercortisolemia (UFC, morning and midnight cortisol) and thyroid hormones (T3, FT4, TSH, T3/FT4 ratio). These associations were similar to most previously reported results (3, 23-25, 30, 31) and independently verify the inverse association between hypercortisolemia and thyroid hormones. The reversible nature of hypercortisolism-mediated central hypothyroidism is shown by the high rate of recovery in this study (24/33; 73%) and others (29/38, 76% of patients aggregated from previous reports) (24, 25, 27, 28).
After surgery, T3 levels increased disproportionately to FT4, as shown by the T3/FT4 ratio, which increased significantly from baseline at 6 M and then declined at 12 M. This may reflect recovery of a dose-dependent inhibition of deiodinase (D1, D2) activity by hypercortisolism, as observed during exogenous glucocorticoid administration to people and lower animals (12, 32-34). However, it is important to consider that pituitary hormonal function may recover independently of the reversal of hypercortisolemia, likely due to decompression of normal tissues after pituitary adenomectomy. Additionally, the weight loss that often occurs during remission of CS may alter circulating TSH levels and thus influence activity of the axis. Therefore, cortisol reduction may not entirely explain the recovery of the thyroid axis.
Our findings provide clinicians with important insights into the pathophysiology and clinical course of hypothyroidism after resolution of hypercortisolemia. We have shown that inhibition of the HPT axis occurs at all levels and includes reduced deiodinase activity. Nearly all patients who had clinical or subclinical hypothyroidism recovered spontaneously after surgery, suggesting that a wait-and-watch approach may be reasonable when surgical remission is anticipated. However, because some patients developed new central and primary hypothyroidism, thyroid function should be evaluated both before and at intervals after surgery until euthyroid status is achieved. Since T3 levels were disproportionately decreased compared to FT4, it is prudent to include T3 in this evaluation, even though it may not be a part of routine orders. This is especially true in TSH-deficient central hypothyroidism patients, in whom there is a disproportionate reduction of T3 compared to T4, making FT4 measurement an unreliable sole marker of euthyroid status (35).
Our cohort also provided insight into primary hypothyroidism in patients with CS. A TSH within the upper half of the normal range reflected underlying primary hypothyroidism, which became more obvious after cure, as reported previously (36). We also noted that patients with elevated TSH at follow-up tended to be antibody negative, raising the question of pauci-immune primary hypothyroidism. Finally, the decrease in thyroid hormone requirements in one patient suggested increased metabolism in the hypercortisolemic state and reduced thyroid hormone clearance when eucortisolism was restored. This is a reminder that close follow-up and titration of LT4 dosing is needed after cure of hypercortisolism.
Our study has several strengths. It is the most comprehensive and largest longitudinal study to date describing the thyroid function status of patients with CS before and after cure. Second, we analyzed multiple markers of hypercortisolemia, including disease duration, tumor size, and the effect of daytime and nighttime cortisol in addition to previously reported correlations with serum cortisol and UFC. Third, we demonstrated the loss of the nocturnal TSH surge in a large cohort. Fourth, we were able to demonstrate the dose-response relationship between baseline hypercortisolemia and thyroid hormone abnormalities and recovery. Fifth, we found a higher prevalence of HPG axis dysfunction in centrally hypothyroid compared to euthyroid CS patients.
Our study also had limitations. It was a retrospective chart review and therefore testing was not performed consistently. For example, we did not perform postoperative diurnal TSH evaluations on all patients. Also, we did not measure FT3 or total T4 in cohort 1, so our marker of deiodinase activity was not T3:T4 or FT3:FT4 but rather T3:FT4. Furthermore, we did not systematically assess other pituitary hormone axes and were unable to determine the frequency and nature of coexisting deficiencies.
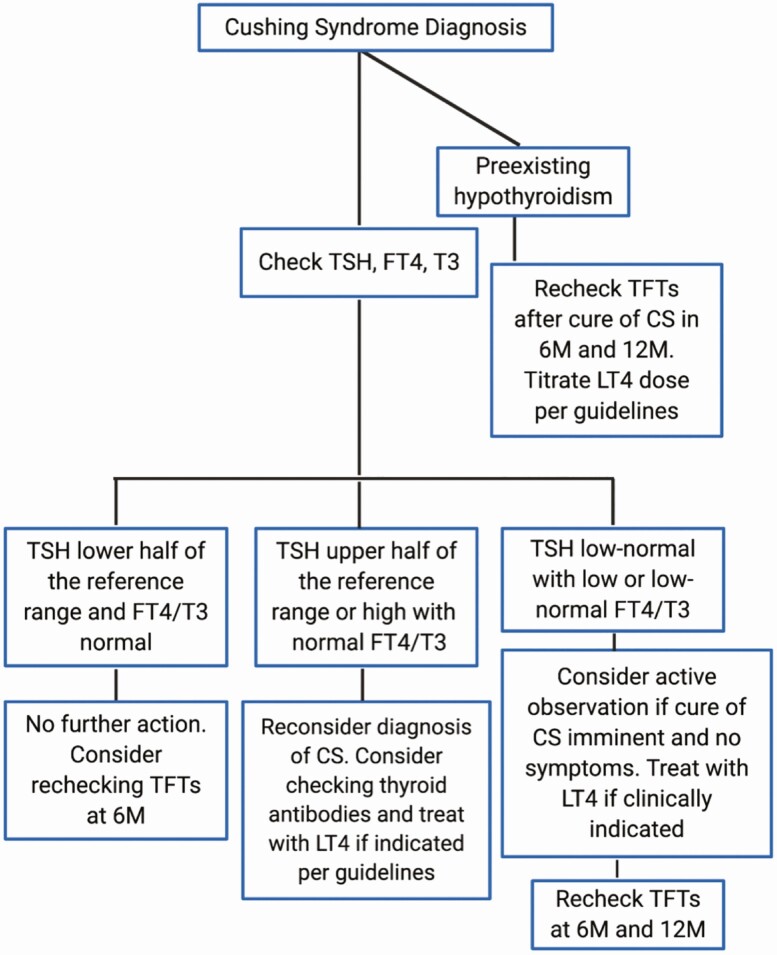
In summary, we have demonstrated the multilevel inverse correlation between hypercortisolemia and thyroid function before and after curative surgery, which was accentuated by ectopic CS (Fig. 7). We found a disproportionate inhibition of T3 at baseline and increase postsurgery in comparison with FT4. Nearly all patients with central hypothyroidism were followed without thyroid hormone supplementation and did not experience any obvious adverse clinical effects. Some upper normal to mildly elevated TSH values at baseline or 6 M manifested later as primary hypothyroidism. One hypothyroid patient had a reduction in LT4 requirements after surgery, likely indicating decreased metabolic clearance. We noted that UFC of greater than 300 or 500 μg/day, and serum morning cortisol greater than 20 μg/dL, were associated with lower log-transformed T3 values at baseline. Additionally, we found that UFC greater than 1000 µg/day, symptom duration less than 1 year, and ACTH greater than 60 pg/mL were independent adverse prognostic factors both for baseline and postoperative recovery of FT4. We speculate that our physiologic approach to glucocorticoid replacement (no more than 10-12 mg/m2/day) after surgery avoided continued glucocorticoid inhibition of the thyroid axis. Finally, we propose that comprehensive evaluation of thyroid hormones at baseline and every 6 M after surgery may be a prudent approach for CS patients (see Fig. 7).
Figure 7.
Clinical algorithm to check thyroid function in Cushing syndrome.
Acknowledgments
We thank the fellows and nurses who cared for these patients, as well as our neurosurgeon colleagues, Drs Edward Oldfield and Prashant Chittiboina, who cured the patients with Cushing disease. Portions of this work were previously presented at the Annual Endocrine Society Meetings in 2019 and 2020 (18, 37).
Financial Support: This work was supported by the Intramural Research Program grants ZZIA DK075121and Z99 HD999999 of the National Institutes of Health.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- BSA
body surface area
- CS
Cushing syndrome
- df
degree of freedom
- DLM
NIH Department of Laboratory Medicine
- FT4
free thyroxine
- HC
hydrocortisone
- HPG
hypothalamic-pituitary-gonadal
- HPT
hypothalamic-pituitary-thyroid
- LLN
lower limit of normal
- LR
likelihood ratio
- LT4
levothyroxine
- RIA
radioimmunoassay
- RR
reference range
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TBG
thyroid hormone–binding globulin
- TFT
thyroid function test
- TRH
thyrotropin-releasing hormone
- TSH
thyrotropin
- UFC
urine free cortisol
- ULN
upper limit of normal
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Alkemade A, Friesema EC, Unmehopa UA, et al. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab. 2005;90(7):4322-4334. [DOI] [PubMed] [Google Scholar]
- 2. Visser TJ, Lamberts SW. Regulation of TSH secretion and thyroid function in Cushing’s disease. Acta Endocrinol (Copenh). 1981;96(4):480-483. [DOI] [PubMed] [Google Scholar]
- 3. Bartalena L, Martino E, Petrini L, et al. The nocturnal serum thyrotropin surge is abolished in patients with adrenocorticotropin (ACTH)-dependent or ACTH-independent Cushing’s syndrome. J Clin Endocrinol Metab. 1991;72(6):1195-1199. [DOI] [PubMed] [Google Scholar]
- 4. Duick DS, Wahner HW. Thyroid axis in patients with Cushing’s syndrome. Arch Intern Med. 1979;139(7):767-772. [PubMed] [Google Scholar]
- 5. Teitsma CA, Anglade I, Toutirais G, et al. Immunohistochemical localization of glucocorticoid receptors in the forebrain of the rainbow trout (Oncorhynchus mykiss). J Comp Neurol. 1998;401(3):395-410. [PubMed] [Google Scholar]
- 6. Antakly T, Eisen HJ. Immunocytochemical localization of glucocorticoid receptor in target cells. Endocrinology. 1984;115(5):1984-1989. [DOI] [PubMed] [Google Scholar]
- 7. Ozawa H, Ito T, Ochiai I, Kawata M. Cellular localization and distribution of glucocorticoid receptor immunoreactivity and the expression of glucocorticoid receptor messenger RNA in rat pituitary gland. A combined double immunohistochemistry study and in situ hybridization histochemical analysis. Cell Tissue Res. 1999;295(2):207-214. [DOI] [PubMed] [Google Scholar]
- 8. Yokote R, Hisano S, Daikoku S. Immunohistochemical localization of glucocorticoid receptors in anterior pituitary cells of rats. Arch Histol Cytol. 1991;54(1):103-112. [DOI] [PubMed] [Google Scholar]
- 9. Cintra A, Fuxe K, Wikström AC, Visser T, Gustafsson JA. Evidence for thyrotropin-releasing hormone and glucocorticoid receptor-immunoreactive neurons in various preoptic and hypothalamic nuclei of the male rat. Brain Res. 1990;506(1): 139-144. [DOI] [PubMed] [Google Scholar]
- 10. Kovacs K, Rotondo F, Stefaneanu L, et al. Glucocorticoid receptor expression in nontumorous human pituitaries and pituitary adenomas. Endocr Pathol. 2000;11(3):267-275. [Google Scholar]
- 11. Heyma P, Larkins RG. Glucocorticoids decrease in conversion of thyroxine into 3, 5, 3′-tri-iodothyronine by isolated rat renal tubules. Clin Sci (Lond). 1982;62(2):215-220. [DOI] [PubMed] [Google Scholar]
- 12. Darras VM, Kotanen SP, Geris KL, Berghman LR, Kühn ER. Plasma thyroid hormone levels and iodothyronine deiodinase activity following an acute glucocorticoid challenge in embryonic compared with posthatch chickens. Gen Comp Endocrinol. 1996;104(2):203-212. [DOI] [PubMed] [Google Scholar]
- 13. Gamstedt A, Järnerot G, Kågedal B, Söderholm B. Corticosteroids and thyroid function. Different effects on plasma volume, thyroid hormones and thyroid hormone-binding proteins after oral and intravenous administration. Acta Med Scand. 1979;205(5):379-383. [PubMed] [Google Scholar]
- 14. Bános C, Takó J, Salamon F, Györgyi S, Czikkely R. Effect of ACTH-stimulated glucocorticoid hypersecretion on the serum concentrations of thyroxine-binding globulin, thyroxine, triiodothyronine, reverse triiodothyronine and on the TSH-response to TRH. Acta Med Acad Sci Hung. 1979;36(4):381-394. [PubMed] [Google Scholar]
- 15. Gupta V, Lee M. Central hypothyroidism. Indian J Endocrinol Metab. 2011;15(Suppl 2):S99-S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieman LK.In: Post TW, ed. UpToDate. Waltham, MA: UpToDate; 2020.
- 18. Shekhar S, Gubbi S, McGlotten R, Nieman L. SAT-459 Hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) axes in Cushing syndrome (CS): a retrospective cohort study. J Endocrine Soc. 2019;3(Suppl 1):SAT-459. [Google Scholar]
- 19. Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf). 1999;50(5):655-659. [DOI] [PubMed] [Google Scholar]
- 20. Kumar A, Shekhar S, Dhole B. Thyroid and male reproduction. Indian J Endocrinol Metab. 2014;18(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukherjee A, Murray RD, Columb B, Gleeson HK, Shalet SM. Acquired prolactin deficiency indicates severe hypopituitarism in patients with disease of the hypothalamic-pituitary axis. Clin Endocrinol (Oxf). 2003;59(6):743-748. [DOI] [PubMed] [Google Scholar]
- 22. Weeke J, Gundersen HJ. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol (Copenh). 1978;89(4):659-672. [DOI] [PubMed] [Google Scholar]
- 23. Roelfsema F, Pereira AM, Biermasz NR, et al. Diminished and irregular TSH secretion with delayed acrophase in patients with Cushing’s syndrome. Eur J Endocrinol. 2009;161(5):695-703. [DOI] [PubMed] [Google Scholar]
- 24. Rubello D, Sonino N, Casara D, Girelli ME, Busnardo B, Boscaro M. Acute and chronic effects of high glucocorticoid levels on hypothalamic-pituitary-thyroid axis in man. J Endocrinol Invest. 1992;15(6):437-441. [DOI] [PubMed] [Google Scholar]
- 25. Benker G, Raida M, Olbricht T, Wagner R, Reinhardt W, Reinwein D. TSH secretion in Cushing’s syndrome: relation to glucocorticoid excess, diabetes, goitre, and the ‘sick euthyroid syndrome’. Clin Endocrinol (Oxf). 1990;33(6):777-786. [DOI] [PubMed] [Google Scholar]
- 26. Tamada D, Kitamura T, Takahara M, et al. TSH ratio as a novel diagnostic method for Cushing’s syndrome. Endocr J. 2018;65(8):841-848. [DOI] [PubMed] [Google Scholar]
- 27. Stratakis CA, Mastorakos G, Magiakou MA, Papavasiliou E, Oldfield EH, Chrousos GP. Thyroid function in children with Cushing’s disease before and after transsphenoidal surgery. J Pediatr. 1997;131(6):905-909. [DOI] [PubMed] [Google Scholar]
- 28. Mathioudakis N, Thapa S, Wand GS, Salvatori R. ACTH-secreting pituitary microadenomas are associated with a higher prevalence of central hypothyroidism compared to other microadenoma types. Clin Endocrinol (Oxf). 2012;77(6):871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamada D, Kitamura T, Onodera T, Hamasaki T, Otsuki M, Shimomura I. Clinical significance of fluctuations in thyroid hormones after surgery for Cushing’s syndrome. Endocr J. 2015;62(9):805-810. [DOI] [PubMed] [Google Scholar]
- 30. Dogansen SC, Yalin GY, Canbaz B, Tanrikulu S, Yarman S. Dynamic changes of central thyroid functions in the management of Cushing’s syndrome. Arch Endocrinol Metab. 2018;62(2):164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiang B, Tao R, Liu X, et al. A study of thyroid functions in patients with Cushing’s syndrome: a single-center experience. Endocr Connect. 2019;8(8):1176-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hidal JT, Kaplan MM. Inhibition of thyroxine 5′-deiodination type II in cultured human placental cells by cortisol, insulin, 3′, 5′-cyclic adenosine monophosphate, and butyrate. Metabolism. 1988;37(7):664-668. [DOI] [PubMed] [Google Scholar]
- 33. Toyoda N, Yasuzawa-Amano S, Nomura E, et al. Thyroid hormone activation in vascular smooth muscle cells is negatively regulated by glucocorticoid. Thyroid. 2009;19(7):755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song S, Oka T. Regulation of type II deiodinase expression by EGF and glucocorticoid in HC11 mouse mammary epithelium. Am J Physiol Endocrinol Metab. 2003;284(6):E1119-E1124. [DOI] [PubMed] [Google Scholar]
- 35. Sesmilo G, Simó O, Choque L, Casamitjana R, Puig-Domingo M, Halperin I. Serum free triiodothyronine (T3) to free thyroxine (T4) ratio in treated central hypothyroidism compared with primary hypothyroidism and euthyroidism. Endocrinol Nutr. 2011;58(1):9-15. [DOI] [PubMed] [Google Scholar]
- 36. Niepomniszcze H, Pitoia F, Katz SB, Chervin R, Bruno OD. Primary thyroid disorders in endogenous Cushing’s syndrome. Eur J Endocrinol. 2002;147(3):305-311. [DOI] [PubMed] [Google Scholar]
- 37. Shekhar S, McGlotten R, Nieman LK. SAT-449 Factors associated with reduced thyroid hormones in Cushing syndrome patients before and after surgical cure. J Endocrine Soc. 2020;4(Suppl 1):SAT-449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.