Abstract
Context
Variation in fetal liver blood flow influences fetal growth and postnatal body composition. Placental corticotrophin-releasing hormone has been implicated as a key mediator of placental-fetal perfusion.
Objective
To determine whether circulating levels of placental corticotrophin-releasing hormone across gestation are associated with variations in fetal liver blood flow.
Design
Prospective cohort study.
Methods
Fetal ultrasonography was performed at 30 weeks’ gestation to characterize fetal liver blood flow (quantified by subtracting ductus venosus flow from umbilical vein flow). Placental corticotrophin-releasing hormone was measured in maternal circulation at approximately 12, 20, and 30 weeks’ gestation. Multiple regression analysis was used to determine the proportion of variation in fetal liver blood flow explained by placental corticotrophin-releasing hormone. Covariates included maternal age, parity, pre-pregnancy body mass index, gestational weight gain, and fetal sex.
Results
A total of 79 uncomplicated singleton pregnancies were analyzed. Fetal liver blood flow was 68.4 ± 36.0 mL/min (mean ± SD). Placental corticotrophin-releasing hormone concentrations at 12, 20, and 30 weeks were 12.5 ± 8.1, 35.7 ± 24.5, and 247.9 ± 167.8 pg/mL, respectively. Placental corticotrophin-releasing hormone at 30 weeks, but not at 12 and 20 weeks, was significantly and positively associated with fetal liver blood flow at 30 weeks (r = 0.319; P = 0.004) and explained 10.4% of the variance in fetal liver blood flow.
Conclusions
Placental corticotrophin-releasing hormone in late gestation is a possible modulator of fetal liver blood flow and may constitute a biochemical marker in clinical investigations of fetal growth and body composition.
Keywords: Placental corticotrophin-releasing hormone, fetal liver blood flow, fetal ultrasonography, uncomplicated pregnancy, third trimester of gestation
Fetal liver blood flow (fLBF) has recently emerged as a biological variable of interest in terms of its role in fetal growth and subsequent newborn and infant body composition (body fat percentage) (1, 2). Increased fLBF in late gestation is associated with fetal macrosomia (3) and increased postnatal percent body fat (4). The suggested underlying mechanism is that increased fLBF influences the nutrient interconversion in the fetal liver (5). The fetus is capable of de novo synthesis of various nutrients (fatty acids, triglycerides, amino acids, glycogen) from substrates transported across the placenta and carried in umbilical venous blood to the fetal compartment (6-8). The fetal liver is the primary site where this nutrient interconversion and de novo synthesis occurs (9). It has been proposed that variation in the relative distribution of umbilical venous blood flow shunting either through ductus venosus or perfusing the fetal liver represents a mechanism of fetal adaptation to intrauterine conditions (ie, the concept of developmental plasticity) (1, 10). Therefore, fLBF is of interest in better understanding mechanisms influencing fetal and postnatal growth and body composition. Only a few studies have examined variation in fLBF (11-15) and its determinants remain poorly understood.
Corticotrophin-releasing hormone (CRH) is a hypothalamic neuropeptide that plays a central role in regulating the activity of the hypothalamic-pituitary-adrenal axis and its physiologic response to various forms of stress (16). The vasodilatory effect of CRH has also been reported in the skin, lung, and kidney by relaxing vascular smooth muscle (17-20). In human pregnancy, the placenta is the major site of CRH synthesis, and its production increases exponentially up to a hundred-fold over the course of gestation (21-23). Placental CRH (pCRH) is released into both maternal and fetal compartments (24-26). Thus, CRH in the maternal peripheral blood in later gestation is predominantly if not exclusively of placental origin (21, 23). One of the roles of pCRH is that it is a paracrine determinant of the placental vasculature. Increased pCRH has been associated with vasodilation of human fetal placental circulation (27). Consequently, pCRH has been implicated as a key mediator of fetal growth (28), birth outcomes (26, 29), and postnatal developmental and health outcomes (24, 30).
Based on the above observations, the primary aim of this study was to examine the association between pCRH and variation in fLBF. Since pCRH increases exponentially over the course of gestation with higher concentrations in late gestation, we hypothesized that the effects of pCRH on fLBF would be more pronounced during the third trimester of gestation.
Methods
Study population
The study population comprised women recruited from 2011 to 2015 in a prospective cohort study of biological and behavioral processes in human pregnancy from a community-based (not hospital-based) population at the University of California, Irvine, Development, Health and Disease Research Program. Women with a singleton, uncomplicated pregnancy were recruited in the late first or early second trimester. Exclusionary criteria were uterine anomalies, preexisting major medical comorbidities (hypertension or diabetes), conditions associated with neuroendocrine and immune dysfunction (endocrine, hepatic, or renal disorders), use of systemic corticosteroids, smoking, illicit drug use, congenital malformations, and chromosomal abnormalities. The study was approved by the Institutional Review Board (#2002–2316, #2009–7251, #2010–7530), and written informed consent was obtained from all women.
Placental CRH
Concentrations of pCRH were determined in maternal venous blood collected serially at approximately 12, 20, and 30 weeks’ gestation (31). Briefly, a 20-mL blood sample was drawn by venipuncture into siliconized EDTA vacutainers and immediately chilled to 4 °C. Samples were centrifuged at 2000g for 15 minutes, and the plasma was decanted into polypropylene tubes containing 500 KIU/mL aprotinin (Sigma Chemical Company, St. Louis, Mo., USA). Plasma samples were then stored at −70 °C until assayed. The free (unbound, bioactive) fraction of plasma CRH was determined from extracted samples by radioimmunoassay at the University of Newcastle, NSW, Australia. The intra- and inter-assay coefficients of variation ranged from 5% to 15%, with a minimum detectable dose of 2.0 pg/mL (31). Samples were assayed in duplicate and averaged.
Prenatal ultrasonography
Fetal ultrasonography was performed at approximately 30 weeks’ gestation for fetal biometry and Doppler velocimetry within 4 days from the maternal blood sampling. Per standard clinical criteria, gestational age was confirmed before 16 weeks using an algorithm combining last menstrual period and fetal biometry (32). All fetal measurements were analyzed by the same obstetrician (S.I.) using a Voluson i (GE Healthcare, Milwaukee, WI) with a transabdominal 4 MHz curved array transducer that included color Doppler and pulsed Doppler (3 MHz) facilities (RAB4-8-RS).
The umbilical vein flow, ductus venosus flow and fLBF volume (quantified by subtracting ductus venosus flow from umbilical vein flow volume) were measured according to methods described previously (4, 33). Briefly, the umbilical vein and ductus venosus were identified either in a sagittal plane or in an oblique plane transecting the fetal upper abdomen. Blood flow (Q) was calculated as Q = h × (D/2)2 × TAMX, where h = spatial blood velocity profile coefficient (umbilical vein = 0.5; ductus venosus = 0.7) (34), D = vessel inner diameter (mean of 5–10 measurements) (35) and TAMX = time-averaged maximum velocity (mean of 2 measurements). Venous blood flows were obtained keeping the insonation angle < 30°. Umbilical vein TAMX was obtained during a 3- to 5-second period or, if flow was pulsatile, as the mean during 3 cardiac cycles. Ductus venosus TAMX was calculated as the mean during 3 cardiac cycles. Inner diameter of umbilical vein was measured in the straight portion of the intra-abdominal umbilical vein before hepatic parenchymal branching. Inner diameter of ductus venosus was measured at the inlet of ductus venosus (1, 33). Intra-observer coefficients of variation for TAMX and vessel diameter of umbilical vein were 6.8% and 6.4%, and those of ductus venosus were 6.6% and 9.3%, respectively. We screened for chronic fetal hypoxia, which could alter fetal hemodynamics, by monitoring absent or reversed end-diastolic umbilical artery flow (36, 37) or cerebro-placental ratio less than the fifth centile (38) and excluded any cases from this analysis.
Pre-pregnancy body mass index and gestational weight gain
Pre-pregnancy body mass index (ppBMI) was calculated using pre-pregnancy weight (by maternal self-report) and height measured at first prenatal visit. Self-reported pre-pregnancy weight highly correlated with the maternal weight measured at the first prenatal visit (r = 0.99; P < 0.001), justifying its use in this context. Subjects were classified according to their ppBMI into underweight (ppBMI < 18.5 kg/m2), normal weight (18.5 ≤ ppBMI < 25 kg/m2), overweight (25 ≤ ppBMI < 30 kg/m2), and obese (ppBMI ≥ 30 kg/m2). Maternal total weight gain during pregnancy was extracted from the medical record. Gestational weight gain (GWG) for each subject was categorized as inadequate, adequate, or excessive, based on the Institute of Medicine recommendations (39). GWG per week (GWG/week) up to fLBF measures at 30 weeks’ gestation were also calculated.
Data analysis
Gestational ages of study subjects varied due to the timing of blood sampling (the 3 antenatal visits ranged from 10 to 16, 18 to 23 and 28 to 32 weeks, respectively). Since pCRH increases across gestation, we accounted for the effects of gestational age at sample collection on pCRH by centering at a mean gestational age for each visit (12.4, 20.4, and 30.4 weeks, respectively) and residualizing the pCRH measurements for these mean gestational ages. Briefly, after confirmation of the linear relationship between pCRH and gestational age at each visit for blood sampling, the product of the regression coefficient and centered gestational age was calculated. This product was added to or subtracted from the measured value to calculate the adjusted value of pCRH. This procedure standardizes pCRH measures across all subjects at the centered gestational ages, enabling comparisons across subjects.
Statistical analysis
Pearson product moment correlations were used to assess bivariate first-order associations among continuous variables, and the Student t test or 1-way analysis of variance was used to test group differences. We considered a priori the following potential confounding variables that could influence the relationship between pCRH and fLBF: maternal age, parity, ppBMI, GWG/week, and fetal sex. The subset of those variables that were significantly associated in bivariate analysis with pCRH or fLBF was selected for subsequent multivariate analyses.
First, the association of pCRH with fLBF at 30 weeks was determined using bivariate analysis. Next, multiple linear regression was used to quantify the association between pCRH and fLBF, with adjustment for potential confounding variables. The relative contribution of pCRH in explaining variation in fLBF was quantified by the partial correlation coefficient associated with these parameters in a multiple linear regression model.
pCRH production at later gestation is, in part, conditioned by pCRH production at earlier time points (correlation coefficients are 0.801 between pCRH at 12 weeks and 20 weeks, 0.544 between 12 and 30 weeks, 0.675 between 20 and 30 weeks, all P values < 0.001). To consider the conditional effect of pCRH production in earlier gestation on pCRH production in later gestation, 12 weeks and 20 weeks pCRH measures were also included as covariates in the multiple regression analysis as a secondary analysis.
Results
Eighty pregnant women were initially recruited into the study. One fetus who exhibited evidence of low cerebro-placental ratio and suspected chronic hypoxia was then excluded. Therefore, data from 79 mother-fetus dyads were analyzed for this report. The maternal socio-demographic profile and obstetric characteristics are summarized in Table 1, and descriptive statistics of pCRH and the fetal blood flow parameters are provided in Table 2. These values are comparable with those in previous reports (3, 14). The pCRH concentrations at 3 time points and fLBF at 30 weeks are also consistent with previous reports (21, 33, 40). Mean pulsatility index of umbilical artery, middle cerebral artery, and cerebro-placental ratio were 1.00 ± 0.14 (mean ± SD), 1.93 ± 0.30, and 2.00 ± 0.43, respectively. Among potential confounding variables, only ppBMI was significantly associated with pCRH (r = 0.293; P = 0.009).
Table 1.
Maternal and Neonatal Socio-demographic and Clinical Characteristics
| Characteristics | N = 79 | (%) |
|---|---|---|
| Age, yearsa | 27.3 ± 5.3 | |
| Race/Ethnicity | ||
| Non-Hispanic White | 34 | (43%) |
| Hispanic White | 28 | (35%) |
| Others | 17 | (22%) |
| Pre-pregnancy BMI, kg/m2a | 25.0 ± 5.2 | |
| Underweight (BMI < 18.5) | 3 | (4%) |
| Normal weight (18.5 ≤ BMI < 25) | 49 | (62%) |
| Overweight (25 ≤ BMI < 30) | 16 | (20%) |
| Obese (BMI ≥ 30) | 11 | (14%) |
| Gestational weight gain, kga | ||
| < IOM | 14 | (18%) |
| = IOM | 19 | (24%) |
| > IOM | 46 | (58%) |
| GWG per week (pregravid to 30 weeks), kg/weeka | 0.60 ± 0.26 | |
| Parity (primiparous) | 30 | (38%) |
| Infant sex (female) | 36 | (46%) |
| Gestational age at delivery, weeks | 39.6 ± 1.2 | |
| Birth weight, g | 3435 ± 483 | |
| Birth weight percentile, % | 49.5 ± 28.5 |
< IOM, = IOM, > IOM; less than, equal to, greater than Institute of Medicine recommendations.
Abbreviations: BMI, body mass index; GWG, gestational weight gain.
aData are presented as mean ± SD.
Table 2.
pCRH During Gestation and Fetal Blood Flow Parameters at 30 Weeks’ Gestation (N = 79)
| Parameters | Mean ± SD |
|---|---|
| pCRH, pg/mL | |
| at 12 weeks | 12.5 ± 8.1 |
| at 20 weeks | 35.7 ± 24.5 |
| at 30 weeks | 247.9 ± 167.8 |
| Umbilical vein | |
| Diameter, mm | 5.25 ± 0.86 |
| Time-averaged maximum velocity, cm/s | 15.2 ± 3.2 |
| Volume flow, mL/min | 101.2 ± 37.8 |
| Ductus venosus | |
| Diameter, mm | 1.61 ± 0.44 |
| Time-averaged maximum velocity, cm/s | 36.6 ± 10.5 |
| Volume flow, mL/min | 32.8 ± 18.3 |
| Liver blood flow, mL/min | 68.4 ± 36.0 |
Abbreviation: pCRH, placental corticotrophin-releasing hormone
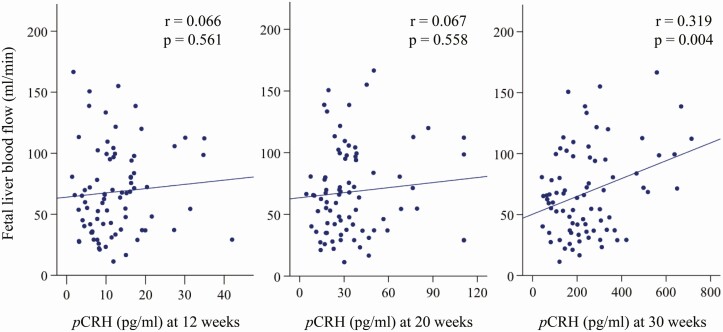
There was no significant relationship between the early gestation (12 weeks) or midgestation (20 weeks) pCRH measures with fLBF. However, and consistent with our hypothesis, the late gestation (30 weeks) pCRH measure was significantly and positively associated with variation in fLBF (r = 0.319; P = 0.004) (Fig. 1). In the secondary analysis, pCRH at 30 weeks was significantly associated with umbilical vein diameter (r = 0.227; P = 0.044) and umbilical vein flow volume (r = 0.317; P = 0.004), but not with ductus venosus diameter (r = 0.028; P = 0.809) or ductus venosus flow volume (r = 0.003; P = 0.976)
Figure 1.
Scatterplot and the regression line depicting the association between pCRH and fetal liver blood flow at 30 weeks’ gestation.
After controlling for the ppBMI, pCRH at 30 weeks was significantly and positively associated with fLBF and explained 10.4% of its variance (partial correlation coefficient [adjusted for covariates] = 0.324; P = 0.004; see Table 3). This correlation remained significant after adjusted for the pCRH measures at earlier gestation (12 weeks and 20 weeks) (partial correlation coefficient = 0.340; P = 0.003)
Table 3.
Multiple Regression Model Associating pCRH With Fetal Liver Blood Flow (N = 79)
| Unstandardized B [95% CI] | Standardized β | Partial correlation | P value | |
|---|---|---|---|---|
| pCRH at 30 weeks | 0.072 [0.024 to 0.120] | 0.334 | 0.324 | 0.004 |
| Pre-pregnancy BMI | 0.476 [−1.074 to 2.026] | 0.0 68 | 0.070 | 0.543 |
Abbreviation: pCRH, placental corticotrophin-releasing hormone
Finally, because fetuses of mothers with gestational diabetes mellitus (GDM) may have different growth trajectories and exhibit differences in fetal growth and liver blood perfusion compared with those without maternal GDM (12), we repeated all analyses after excluding the 3 subjects with GDM (ie, with 76 women) as a secondary analysis. There was no appreciable change in the significance and magnitude of the above-described effects of pCRH on fLBF.
Discussion
The present study demonstrates an association of pCRH during gestation with changes in the circulation to the fetal liver (fLBF). Our principal finding is that variation in pCRH levels at approximately 30 weeks’ gestation (after accounting for the subject’s pCRH in early- and midgestation) is positively associated with fLBF at 30 weeks. This association is unchanged after adjusting for potential confounding factors.
Our results suggest that in low-risk pregnancies, pCRH may act as a modulator of fLBF. Our results also indicate that pCRH is positively associated with umbilical vein diameter and umbilical vein flow but with no measurable effect on the diameter or flow in the ductus venosus. These observations are consistent with previous reports that pCRH has a vasodilatory effect on placenta (27), and that in longitudinal studies of low-risk pregnancies in the second half of gestation, there are physiological variations in diameter and flow in the umbilical vein (41, 42).
The intrauterine environment and the factors influencing placental function through pregnancy are known to influence fetal and postnatal outcomes in the short term (birth, infancy, childhood) and also into adult life (the concept of developmental programming of health and disease risk) (43). The maternal stress hormones including pCRH have been shown to modulate the length of pregnancy (preterm birth) (44, 45), fetal growth (28, 46), and infant body composition (30, 47, 48), as well as postnatal neurodevelopmental and behavioral outcomes (43). Whether pCRH has a direct effect on fetal growth and body composition is presently unclear. Preliminary observations suggest that pCRH acts as an autocrine, paracrine, or endocrine regulator (49, 50) to increase glucose uptake and facilitate transfer from the mother to the fetus through the upregulation of glucose transporter in the placenta (51). The treatment of cultured placental trophoblasts with CRH resulted in an increase in GLUT1 expression in a dose-dependent manner (51), suggesting that pCRH facilitates glucose transport to the placenta. In addition, the present study suggests that pCRH also affects fLBF in late gestation. It has also been reported that fetal liver has significant autoregulation of umbilical venous perfusion according to maternal adiposity (15). These observations, taken together, suggest possible mechanisms by which pCRH could influence fetal body composition.
Our results also replicate previous reports that show pCRH increases across gestation (and exponentially in late gestation) (21, 22). Our study did not directly compare pCRH at 12 or 20 weeks with fLBF at the same gestational age. fLBF measures at 12 or 20 weeks were not performed in the present study because of the poor reproducibility of the ductus venous blood flow at these earlier gestational ages. Nonetheless, our study indicates that pCRH at earlier gestational ages (pCRH at 12 and 20 weeks) has no appreciable effect on fLBF at 30 weeks. The absence of this association suggests there may be insufficient variation of pCRH up to midgestation (ie, before the exponential increase in pCRH). Accelerated fetal fat accumulation and changes in body composition occurs largely after 30 weeks’ gestation (52, 53). Thus, pCRH could be one contributing factor to fetal body composition in later gestation. Moreover, maternal-fetal circulation is affected by various maternal factors, which could have a larger effect on fLBF as compared with the low levels of pCRH at 12 weeks’ or 20 weeks’ gestation.
We also found a statistically significant positive association between maternal ppBMI and pCRH at 30 weeks. A previous report shows an inverse association between maternal obesity and pCRH gene expression (54); however, there is no consistent evidence of the effect of maternal obesity on pCRH. The clinical significance of this association warrants further evaluation.
The strengths of our study include the measures of pCRH and fLBF in a well-characterized cohort of uncomplicated pregnancies. The effect of pCRH on fLBF was prospectively examined. The present study incorporated longitudinal measures of pCRH from 12 to 30 weeks’ gestation, which enabled adjustment of the time-specific effects of pCRH. In terms of potential limitations, CRH-binding protein—a potential modulator of the effects of pCRH—was not measured here. However, CRH-binding protein levels, which are constant in the first, second, and early third trimester (and not significantly different from nonpregnant levels), fall by approximately 60% at later gestational age (36-38 weeks’ gestation) (55, 56). Fetal liver receives venous blood flow from not only the umbilical vein but also from the portal vein, whose flow was not evaluated in the present study. However, portal vein flow accounts for only a small proportion of fetal liver blood perfusion and is not believed to be a source of nutrient substrate from the placenta that can affect postnatal outcomes (14). The intra-observer difference of the TAMX and vessel diameter are relatively high, which may have affected the present results.
In conclusion, our observations suggest that pCRH may be one of the modulators of fLBF in low-risk, uncomplicated pregnancies, which is consistent with the thesis first advanced by Godfrey et al that fLBF is a putative mechanism underlying fetal adaptations to the intrauterine environment (1). pCRH may constitute a biochemical marker of interest in clinical investigations of fetal growth and body composition.
Acknowledgments
We would like to thank Professor Roger Smith, University of Newcastle, NSW, Australia and his laboratory personnel for performing the assays for placental CRH.
Financial Support: This study was funded, in part, by US National Institutes of Health (NIH) grants RO1 HD-060628, RO1 HD-065825 and RO1 MH-091351.
Glossary
Abbreviations
- CRH
corticotrophin-releasing hormone
- fLBF
fetal liver blood flow
- GDM
gestational diabetes mellitus
- GWG
gestational weight gain
- pCRH
placental CRH
- ppBMI
pre-pregnancy body mass index
Additional Information
Disclosures: The authors report no conflict of interest.
This study was presented in poster format at the 39th annual meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, Feb. 11-16, 2019.
Data Availability
The datasets generated during and/or analyzed the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Godfrey KM, Haugen G, Kiserud T, et al. ; Southampton Women’s Survey Study Group . Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PLoS One. 2012;7(8):e41759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tchirikov M, Kertschanska S, Stürenberg HJ, Schröder HJ. Liver blood perfusion as a possible instrument for fetal growth regulation. Placenta. 2002;23(Suppl A):S153-S158. [DOI] [PubMed] [Google Scholar]
- 3. Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Venous liver blood flow and regulation of human fetal growth: evidence from macrosomic fetuses. Am J Obstet Gynecol. 2011;204(5):429.e1-429.e7. [DOI] [PubMed] [Google Scholar]
- 4. Ikenoue S, Waffarn F, Ohashi M, et al. Prospective association of fetal liver blood flow at 30 weeks gestation with newborn adiposity. Am J Obstet Gynecol. 2017;217(2):204.e1-204.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tchirikov M, Kertschanska S, Schröder HJ. Obstruction of ductus venosus stimulates cell proliferation in organs of fetal sheep. Placenta. 2001;22(1):24-31. [DOI] [PubMed] [Google Scholar]
- 6. Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000;16(3):202-210. [DOI] [PubMed] [Google Scholar]
- 7. Cetin I. Amino acid interconversions in the fetal-placental unit: the animal model and human studies in vivo. Pediatr Res. 2001;49(2):148-154. [DOI] [PubMed] [Google Scholar]
- 8. Liang L, Guo WH, Esquiliano DR, et al. Insulin-like growth factor 2 and the insulin receptor, but not insulin, regulate fetal hepatic glycogen synthesis. Endocrinology. 2010;151(2):741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seifter S, Englard S. Energy metabolism. In: Arias IM, ed. The Liver: Biology and Pathobiology. New York: Raven Press; 1994:323-364. [Google Scholar]
- 10. Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17(1):5-21. [DOI] [PubMed] [Google Scholar]
- 11. Opheim GL, Moe Holme A, Blomhoff Holm M, et al. The impact of umbilical vein blood flow and glucose concentration on blood flow distribution to the fetal liver and systemic organs in healthy pregnancies. FASEB J. 2020;34(9):12481-12491. [DOI] [PubMed] [Google Scholar]
- 12. Lund A, Ebbing C, Rasmussen S, Kiserud T, Kessler J. Maternal diabetes alters the development of ductus venosus shunting in the fetus. Acta Obstet Gynecol Scand. 2018;97(8):1032-1040. [DOI] [PubMed] [Google Scholar]
- 13. Haugen G, Bollerslev J, Henriksen T. Human fetoplacental and fetal liver blood flow after maternal glucose loading: a cross-sectional observational study. Acta Obstet Gynecol Scand. 2014;93(8):778-785. [DOI] [PubMed] [Google Scholar]
- 14. Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T. Longitudinal study of umbilical and portal venous blood flow to the fetal liver: low pregnancy weight gain is associated with preferential supply to the fetal left liver lobe. Pediatr Res. 2008;63(3):315-320. [DOI] [PubMed] [Google Scholar]
- 15. Haugen G, Hanson M, Kiserud T, Crozier S, Inskip H, Godfrey KM. Fetal liver-sparing cardiovascular adaptations linked to mother’s slimness and diet. Circ Res. 2005;96(1):12-14. [DOI] [PubMed] [Google Scholar]
- 16. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244-1252. [PubMed] [Google Scholar]
- 17. Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88(11):5427-5432. [DOI] [PubMed] [Google Scholar]
- 18. Clifton VL, Crompton R, Smith R, Wright IM. Microvascular effects of CRH in human skin vary in relation to gender. J Clin Endocrinol Metab. 2002;87(1):267-270. [DOI] [PubMed] [Google Scholar]
- 19. Adão R, Mendes-Ferreira P, Santos-Ribeiro D, et al. Urocortin-2 improves right ventricular function and attenuates pulmonary arterial hypertension. Cardiovasc Res. 2018;114(8):1165-1177. [DOI] [PubMed] [Google Scholar]
- 20. Gutkowska J, Jankowski M, Mukaddam-Daher S, McCann SM. Corticotropin-releasing hormone causes antidiuresis and antinatriuresis by stimulating vasopressin and inhibiting atrial natriuretic peptide release in male rats. Proc Natl Acad Sci U S A. 2000;97(1):483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goland RS, Wardlaw SL, Stark RI, Brown LS Jr, Frantz AG. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab. 1986;63(5):1199-1203. [DOI] [PubMed] [Google Scholar]
- 22. Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82(1):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59(4):812-814. [DOI] [PubMed] [Google Scholar]
- 24. Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185(1-2):135-144. [DOI] [PubMed] [Google Scholar]
- 25. Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124(3):323-329. [DOI] [PubMed] [Google Scholar]
- 26. Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2-3):159-164. [DOI] [PubMed] [Google Scholar]
- 27. Clifton VL, Read MA, Leitch IM, Boura AL, Robinson PJ, Smith R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal placental circulation. J Clin Endocrinol Metab. 1994;79(2):666-669. [DOI] [PubMed] [Google Scholar]
- 28. Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063-1069. [DOI] [PubMed] [Google Scholar]
- 29. Smith R. Parturition. N Engl J Med. 2007;356(3):271-283. [DOI] [PubMed] [Google Scholar]
- 30. Gillman MW, Rich-Edwards JW, Huh S, et al. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity. Obesity (Silver Spring). 2006;14(9):1647-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moog NK, Buss C, Entringer S, et al. Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biol Psychiatry. 2016;79(10):831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. Jama. 2013;309(23):2445-2446. [DOI] [PubMed] [Google Scholar]
- 33. Kiserud T, Rasmussen S, Skulstad S. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol. 2000;182(1 Pt 1):147-153. [DOI] [PubMed] [Google Scholar]
- 34. Kiserud T, Hellevik LR, Hanson MA. Blood velocity profile in the ductus venosus inlet expressed by the mean/maximum velocity ratio. Ultrasound Med Biol. 1998;24(9):1301-1306. [DOI] [PubMed] [Google Scholar]
- 35. Kiserud T, Rasmussen S. How repeat measurements affect the mean diameter of the umbilical vein and the ductus venosus. Ultrasound Obstet Gynecol. 1998;11(6):419-425. [DOI] [PubMed] [Google Scholar]
- 36. Nicolaides KH, Bilardo CM, Soothill PW, Campbell S. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. Bmj. 1988;297(6655):1026-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tyrrell S, Obaid AH, Lilford RJ. Umbilical artery Doppler velocimetry as a predictor of fetal hypoxia and acidosis at birth. Obstet Gynecol. 1989;74(3 Pt 1):332-337. [PubMed] [Google Scholar]
- 38. Morales-Roselló J, Khalil A, Morlando M, Bhide A, Papageorghiou A, Thilaganathan B. Poor neonatal acid-base status in term fetuses with low cerebroplacental ratio. Ultrasound Obstet Gynecol. 2015;45(2):156-161. [DOI] [PubMed] [Google Scholar]
- 39. Rasmussen KM, Yaktine AL. Determining optimal weight gain. In: Committee to Reexamine IOM Pregnancy Weight Guidelines, ed. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC, USA: National Academy Press; 2009:206-224. [PubMed] [Google Scholar]
- 40. Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol. 2004;190(5):1347-1358. [DOI] [PubMed] [Google Scholar]
- 41. Acharya G, Wilsgaard T, Rosvold Berntsen GK, Maltau JM, Kiserud T. Umbilical vein constriction at the umbilical ring: a longitudinal study. Ultrasound Obstet Gynecol. 2006;28(2):150-155. [DOI] [PubMed] [Google Scholar]
- 42. Skulstad SM, Kiserud T, Rasmussen S. Degree of fetal umbilical venous constriction at the abdominal wall in a low-risk population at 20-40 weeks of gestation. Prenat Diagn. 2002;22(11):1022-1027. [DOI] [PubMed] [Google Scholar]
- 43. Sandman CA. Fetal exposure to placental corticotropin-releasing hormone (pCRH) programs developmental trajectories. Peptides. 2015;72:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179(4):1079-1085. [DOI] [PubMed] [Google Scholar]
- 45. Buss C, Entringer S, Reyes JF, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201(4):398.e1-398.e8. [DOI] [PubMed] [Google Scholar]
- 46. Challis JR. Maternal corticotropin-releasing hormone, fetal growth, and preterm birth. Am J Obstet Gynecol. 2004;191(4):1059-1060. [DOI] [PubMed] [Google Scholar]
- 47. Entringer S, Buss C, Rasmussen JM, et al. Maternal cortisol during pregnancy and infant adiposity: a prospective investigation. J Clin Endocrinol Metab. 2017;102(4):1366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fasting MH, Oken E, Mantzoros CS, et al. Maternal levels of corticotropin-releasing hormone during pregnancy in relation to adiponectin and leptin in early childhood. J Clin Endocrinol Metab. 2009;94(4):1409-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27(3):260-286. [DOI] [PubMed] [Google Scholar]
- 50. Gangestad SW, Caldwell Hooper AE, Eaton MA. On the function of placental corticotropin-releasing hormone: a role in maternal-fetal conflicts over blood glucose concentrations. Biol Rev Camb Philos Soc. 2012;87(4):856-873. [DOI] [PubMed] [Google Scholar]
- 51. Gao L, Lv C, Xu C, et al. Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts. Endocrinology. 2012;153(3):1464-1471. [DOI] [PubMed] [Google Scholar]
- 52. Pereira GR. Nutritional assessment. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 4th ed. Philadelphia: Elsevier/Saunders; 2011:341-351. [Google Scholar]
- 53. Sparks JW. Human intrauterine growth and nutrient accretion. Semin Perinatol. 1984;8(2):74-93. [PubMed] [Google Scholar]
- 54. Saben J, Lindsey F, Zhong Y, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perkins AV, Eben F, Wolfe CD, Schulte HM, Linton EA. Plasma measurements of corticotrophin-releasing hormone-binding protein in normal and abnormal human pregnancy. J Endocrinol. 1993;138(1):149-157. [DOI] [PubMed] [Google Scholar]
- 56. Linton EA, Perkins AV, Woods RJ, et al. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76(1):260-262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed the current study are not publicly available but are available from the corresponding author on reasonable request.