Abstract
Context
Women with polycystic ovarian syndrome (PCOS) have decreased growth hormone (GH), which can result in increased visceral adiposity (VAT) and impaired vascular function. GH-releasing hormone, a dipeptidyl peptidase-4 (DPP4) substrate, stimulates GH secretion.
Objective
We tested the hypothesis that DPP4 inhibition increases GH and improves glucose levels and vascular function in women with PCOS.
Methods
Eighteen women with PCOS participated in a double-blind, crossover study. They received sitagliptin either 100 mg or placebo daily for 1 month, with crossover treatments separated by an 8-week washout. During each treatment, women underwent a 75-gram oral glucose tolerance test (OGTT) and assessments of vascular function and body composition. Overnight GH secretion was assessed via venous sampling every 10 minutes for 12 hours and analyzed using an automated deconvolution algorithm.
Results
During OGTT, sitagliptin increased glucagon-like peptide-1 (P < 0.001), early insulin secretion (from mean [± SD] insulinogenic index 1.9 ± 1.2 to 3.2 ± 3.1; P = 0.02), and decreased peak glucose (mean −17.2 mg/dL [95% CI, −27.7 to −6.6]; P < 0.01). At 1 month, sitagliptin decreased VAT (from 1141.9 ± 700.7 to 1055.1 ± 710.1 g; P = 0.02) but did not affect vascular function. Sitagliptin increased GH half-life (from 13.9 ± 3.6 to 17.0 ± 6.8 min, N = 16; P = 0.04) and interpulse interval (from 53.2 ± 20.0 to 77.3 ± 38.2 min, N = 16; P < 0.05) but did not increase mean overnight GH (P = 0.92 vs placebo).
Conclusions
Sitagliptin decreased the maximal glucose response to OGTT and VAT. Sitagliptin did not increase overnight GH but increased GH half-life and the interpulse interval.
Clinical Trial Registration
This study was registered at www.clinicaltrials.gov as NCT02122380 prior to enrollment of the first participant.
Keywords: dipeptidyl peptidase-4, growth hormone, insulin like growth factor-1, polycystic ovarian syndrome, visceral adiposity
Growth hormone (GH) is secreted in a pulsatile fashion from the pituitary gland and exerts its effects either directly, via activation of the GH receptor, or indirectly, through hepatic insulin-like growth factor-1 (IGF-1). In adults, GH has important effects on the vasculature and body composition. GH increases endothelium-dependent vasodilator function and reduces inflammation (1–3). We have previously shown that patients with low GH have impaired conduit artery vasodilator function along with decreased tissue-type plasminogen activator (tPA) activity and a defective fibrinolytic response to venous occlusion (4). Others have corroborated these findings and further demonstrated that these vascular indices improve with GH replacement therapy (3, 5). GH also has anabolic and lipolytic effects. Conversely, GH secretion is reduced in obesity and particularly in individuals with visceral adiposity. Women with increased visceral adipose tissue (VAT) have 4-fold reduced mean GH levels compared with women with normal VAT (6). Normalization of GH secretion in patients with diminished GH affords one potential mechanism to address simultaneously both VAT and cardiovascular risk. Unfortunately, therapy with recombinant GH does not restore pulsatile secretion, is not subject to physiologic negative feedback by IGF-1, and is limited by hyperglycemia (7).
We propose that an alternative strategy to enhance endogenous GH secretion in humans is to inhibit the degradation of endogenous GH–releasing hormone (GHRH) by the dipeptidyl peptidase-4 (DPP4) enzyme. GHRH is the primary stimulus for pituitary GH secretion and determines GH pulsatility. Endogenous GHRH has a half-life of 6 minutes as it is degraded and inactivated by DPP4 (8, 9). Sitagliptin was the first oral DPP4 inhibitor approved by the US Food and Drug Administration (FDA) for the management of hyperglycemia in patients with type 2 diabetes mellitus. Sitagliptin improves postprandial hyperglycemia in a glucose-dependent manner by decreasing the degradation of the incretin hormone, glucagon-like peptide-1 (GLP-1), and it can therefore be safely given to patients without diabetes mellitus (10). We recently found that acute DPP4 inhibition with sitagliptin enhances stimulated GH secretion and free IGF-1 levels in healthy, lean women. Moreover, while vasodilation and tPA activity levels increased in both men and women during stimulated GH secretion, women demonstrated an enhanced vascular response to increased GH (11). The effect of chronic DPP4 inhibition on pulsatile GH secretion in individuals with low GH levels has not been studied.
Women with polycystic ovarian syndrome (PCOS) have decreased GH secretion characterized by an impaired GH response to stimuli and a greater than 50% reduction in 24-hour mean GH levels (12–14). Approximately 10% of reproductive-age women in the United States are affected by PCOS, at an estimated annual cost of more than $4 billion (15). These women are often overweight and are at increased risk for future cardiovascular disease and diabetes mellitus. There are currently no approved medical therapies for women with PCOS; management options include weight loss or medications aimed at controlling symptoms of hirsutism or oligomenorrhea, or treatment of insulin resistance (16, 17). In this study, we tested the hypothesis that 1 month of DPP4 inhibition with sitagliptin would enhance overnight pulsatile GH secretion in overweight women with PCOS and improve glucose metabolism. Given the effects of GH on the vasculature, we also hypothesized that an increase in GH would enhance endothelial function and fibrinolysis.
Materials and Methods
Study protocol
Eighteen women (BMI ≥ 25 kg/m2) with PCOS, 18 through 45 years of age completed a double-blind, randomized, placebo-controlled, crossover study. (See Table 1 for subject characteristics and Fig. 1 for participant flow diagram.) The study adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects and was approved by the Vanderbilt University Medical Center Institutional Review Board. All participants provided written informed consent prior to initiation of study procedures. A diagnosis of PCOS was confirmed when women met 2 out of the 3 Rotterdam 2003 criteria: oligomenorrhea (menstrual cycles occurring at intervals > 35 days, or only 4–9 cycles per year) or secondary amenorrhea (no cycle in 3 months if previously regular or no cycle in 9 months if previous oligomenorrheic); clinical and/or laboratory evidence of hyperandrogenism; previous evidence of polycystic ovaries on ultrasound examination. Other common causes of hyperandrogenemia and oligomenorrhea, including hyperprolactinemia, late-onset 21-hyroxylase deficiency, and inadequately treated hypothyroidism were excluded at the time of screening visit (18). Women were excluded from study participation if they had diabetes mellitus, as determined by fasting blood glucose of 126 mg/dL or greater at the time of screening visit. Women with prediabetes, as demonstrated by fasting blood glucose of 100–125 mg/dL at the time of the screening visit, were not excluded from study participation. We did not include an oral glucose tolerance test (OGTT) as part of our screening protocol. Patients with a history of chronic illness, including hypertension, cardiovascular disease, and chronic renal or hepatic insufficiency as well as a history of weight loss surgery or ongoing night-shift work were excluded. Women were required to discontinue oral contraceptives for 8 weeks and spironolactone for 30 days prior to study enrollment, if applicable. One woman took a stable dose of metformin throughout the study; other drugs known to alter glucose or insulin metabolism were not permitted. Pregnancy was excluded in all women of childbearing age by serum pregnancy testing prior to study drug initiation and again prior to performing dual-energy x-ray absorptiometry (DEXA).
Table 1.
Subject Characteristics
| Variable | N = 18 |
|---|---|
| Age (years) | 30.5 [7], 30.3 ± 3.3 |
| Race | |
| White | 13 (72%) |
| Black | 3 (17%) |
| Asian | 2 (11%) |
| Weight (kg) | 81.0 [30.5], 90.6 ± 22.0 |
| Body mass index (kg/m2) | 32.9 [10.9], 34.1 ± 6.4 |
| Overweight (25.0–29.9) | 5 (28%) |
| Class I (30.0–34.9) | 6 (33%) |
| Class II (35.0–39.9) | 3 (17%) |
| Class III (≥ 40) | 4 (22%) |
| Waist circumference (cm) | 101.7 [16.7], 104.8 ± 13.6 |
| Waist/hip ratio | 0.9 [0.1], 0.9 ± 0.1 |
| Systolic blood pressure (mm Hg) | 123.0 [20.0], 120.7 ± 12.0 |
| Diastolic blood pressure (mm Hg) | 78.5 [13.0], 77.3 ± 7.9 |
| Heart rate (beats per minute) | 77.0 [12.8], 77.2 ± 9.9 |
| PCOS diagnostic criteria | |
| Oligomenorrhea | 17 (94%) |
| Hyperandrogenemia (hirsutism) | 16 (89%) |
| Polycystic ovaries on ultrasound | 11 (61%) |
| Current metformin use | 1 (6%) |
| Fasting blood glucose (mg/dL) | 83.5 [12.8], 85.5 ± 9.2 |
| Bioavailable testosterone LC-MS (ng/dl)* | 20.2 [15.3], 18.6 ± 10.0 |
| Corrected bioavailable testosterone*† | 0.8 [0.8], 0.8 ± 0.5 |
| Sex hormone binding globulin (nmol/L) (normal range 30–135) | 30.5 [60.3], 59.9 ± 65.6 |
Data shown as median [interquartile range] and mean ± SD. *N = 17. Abbreviations: LC-MS, liquid chromatography–mass spectrometry; PCOS, polycystic ovarian syndrome. †Corrected bioavailable testosterone = bioavailable testosterone/assay upper limit of normal for age group. 6/17 (35%) had bioavailable testosterone levels above the upper limit of normal.
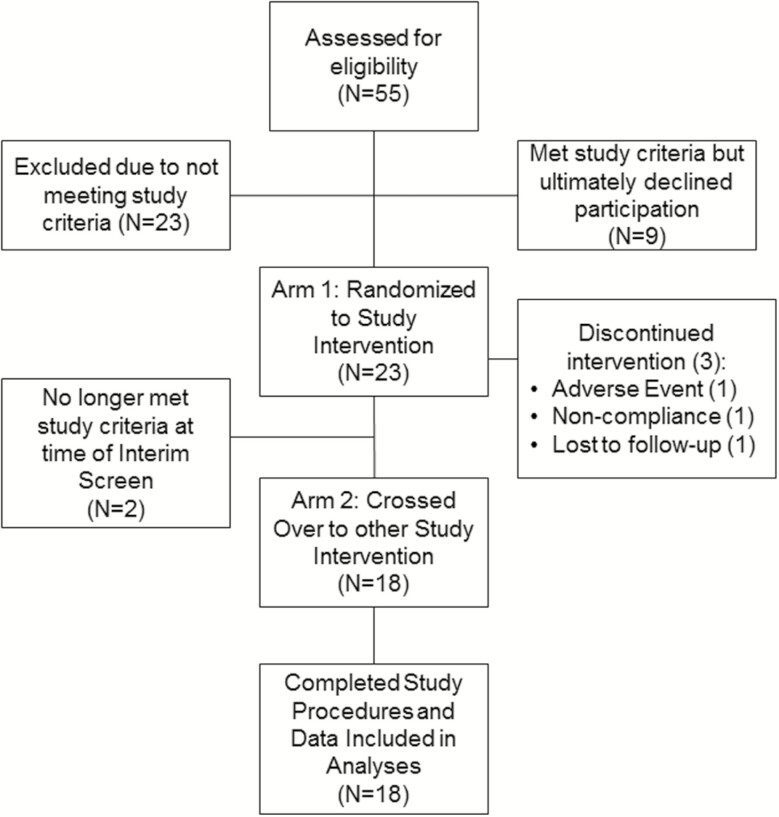
Figure 1.
Participant Flow Diagram. Fifty-five women with polycystic ovarian syndrome (PCOS) were screened for study participation and 32 were determined to be eligible. Twenty-three women agreed to participate and were randomized to study drug. Eighteen of these women participated in both study arms and their data were included in analyses. Complete data was available only from both outpatient visits in 1 woman. Another woman did not complete the overnight growth hormone (GH) sampling portion of both inpatient visits.
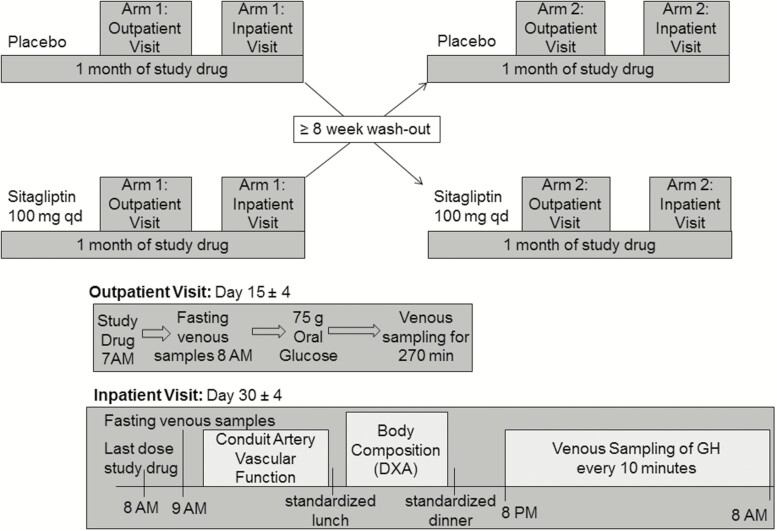
Women underwent 2 monthlong treatment periods separated by a washout period of at least 8 weeks (Fig. 2). Subjects were assigned to treatment order (double-blind sitagliptin 100 mg orally once a day or matching placebo) using a block randomization algorithm. On each outpatient and inpatient study day, subjects reported to the Vanderbilt Clinical Research Center (CRC) in the morning after an overnight fast. After approximately 2 weeks of study drug, subjects underwent an extended OGTT. One hour after oral study drug (sitagliptin vs placebo) a peripheral intravenous line was placed in the antecubital fossa of the nondominant arm. Baseline venous samples and vital signs were obtained at least 60 minutes following study drug. Oral glucola (75 grams) was then ingested within 10 minutes, and samples were obtained over the following 270 minutes. Women were then discharged and continued daily study drug at home for an additional 2 weeks.
Figure 2.
Subjects participated in a double-blind, randomized, placebo-controlled, crossover study. Eighteen women underwent 2 one-month treatment periods (sitagliptin 100 mg orally once daily or matching placebo), with crossover separated by a washout period of at least 8 weeks. On each study day, subjects reported to the Vanderbilt Clinical Research Center (CRC) in the morning after an overnight fast. After approximately 2 weeks of study drug, subjects underwent an extended oral glucose tolerance test (75 grams oral glucola). Women were then discharged and continued daily study drug at home for an additional 2 weeks. On the last day of study drug, women (N = 17) again reported to the CRC for collection of fasting venous samples, measurement of endothelium-dependent and -independent vasodilation, measurement of total and regional body composition via duel-energy x-ray absorptiometry, and frequent venous sampling for growth hormone every 10 minutes for 12 hours overnight.
On the last day of study drug, women again reported to the CRC following an overnight fast. One hour after the last dose of oral study drug, vital signs, weight, and fasting venous samples were obtained via peripheral stick in the nondominant arm. Women then underwent assessment of endothelium-dependent and independent vasodilation in the dominant arm (see “conduit artery vascular function” below). In the afternoon, total and regional body composition was determined by a certified densitometrist using DEXA (Lunar iDXA; GE Health Care, Madison, WI) with enCore software (version 13.6). Women also completed the previously validated polycystic ovarian syndrome questionnaire (PCOSQ) (19–21). In the evening, women underwent placement of an indwelling venous catheter and venous blood was sampled for GH every 10 minutes from 8 PM until 8 AM. Meal content and composition were standardized across all subjects, and intake of the standardized meals was matched within each woman across study arms. Snacking and exercise were not permitted in the CRC. Only water intake was permitted during GH sampling.
Conduit artery vascular function
Endothelium-dependent vasodilation was evaluated in a temperature-controlled room in the fasting state after 15 minutes of rest in the supine position. Participants were asked to refrain from exercise, alcohol, caffeine, and anti-inflammatory medications for 24 hours preceding each measurement. Studies could not be coordinated with the phase of the menstrual cycle due to the infrequency or absence of cycles in the majority of our volunteers. Vascular images were obtained by a single ultrasound-trained technician using an L12-3 broadband linear array transducer attached to a high-resolution ultrasound machine (EPIQ7C; Phillips, Bothwell, WA). A longitudinal image (parallel to the artery) was acquired just proximal to the antecubital fossa of the dominant arm with the transducer positioned to optimize images of the near and far wall interfaces. Depth and gain settings were optimized to identify the interface between the lumen and blood vessel wall. Anatomical landmarks were documented while positioning the probe, to facilitate repositioning during repeated studies. A simultaneous electrocardiogram signal was recorded throughout the imaging. To assess endothelium-dependent vasodilation, the brachial artery diameter was measured under basal conditions and during reactive hyperemia. Reactive hyperemia was achieved by inflating a pneumatic cuff on the upper arm to 200 mm Hg for 5 minutes. The cuff was deflated and images of the artery were obtained continuously across the cardiac cycle for 180 seconds after cuff release. Baseline velocity time integral (VTI) was determined prior to cuff occlusion and hyperemic VTI was determined for 10 beats post-cuff release via pulse-wave spectral Doppler recordings. Following a 15-minute rest period, the brachial artery was imaged again to re-establish basal conditions. Endothelium-independent vasodilation was then determined by administering 0.4 mg nitroglycerin sublingually. The brachial artery was imaged 3 minutes later for 2 minutes. Nitroglycerin was only administered if systolic blood pressure was at least 110 mm Hg, pulse at least 60 beats per minute (bpm), and with the participant’s consent (n = 3 participants).
Acquisition and analysis of the DICOM-stored images was performed using software (Brachial analyzer 5.0) designed for this purpose by Medical Imaging Applications LLC. The vessel wall-lumen interface was determined by derivative-based edge detection following identification of the region of interest by the blinded investigator (JKD). The selected region of interest was consistent across study days for the same subject. The maximum diameter posthyperemia was obtained using previously described custom design software which automatically calculates the time-to-peak and peak dilation.(22) This software implements the method validated by Green et al which uses a smoothing algorithm to correct for changes in the brachial artery diameter during the cardiac cycle (23). The percentage change in diameter, following reactive hyperemia and nitroglycerin, was then calculated as percent vasodilation = [(peak diameter-baseline diameter)/baseline diameter]×100. The reactive hyperemia-induced increase in VTI relative to baseline was calculated as a ratio = hyperemia VTI/baseline VTI.
Laboratory analyses
All samples were obtained after the first 3 mL of blood were discarded. Blood samples were collected on ice, centrifuged immediately and plasma stored at −80°C in prespecified aliquots until time of assay. All samples obtained from each subject during sitagliptin and placebo treatment were simultaneously analyzed with internal controls. DPP4 activity was assayed by incubating 20 µl sample in 80 µl assay buffer (0.1 M Tris at a pH of 8.0; Bachem) for 30 minutes at 37°C with colorimetric substrate [2 mM L-glycyl-L-prolyl p-nitroanilide hydrochloride (Sigma Aldrich)] for a total reaction volume of 200 µL, as previously described (24). DPP4 antigen concentration was determined by enzyme-linked immunoassay (ELISA) (eBioscience; San Diego, CA). The enzyme activity was assessed by measuring the increase in specific absorbance at 405 nm at 0, 15, and 30 minutes and was expressed as nmol/mL/min. Percent DPP4 inhibition was determined by the equation: [1-(DPP4 activity during sitagliptin/DPP4 activity during placebo)]×100. GH levels were determined by 2-site immunoassay (Beckman Access Ultrasensitive human GH assay, Beckman Coulter, Inc.) performed on the Dxl automated immunoassay system (Beckman Instruments) and calibrated against NIBSC WHO IS 98/574 with an analytic measurement range of 0.002 to 35 ng/mL. The first 5 subjects were analyzed at Brigham Research Assay Core; the intra-assay coefficient of variation (CV) was 1.48% to 11.26% and the inter-assay CV was 1.96% to 14.4%. The remaining subjects were analyzed at the Mayo Immunochemical Core Lab (Rochester, MN); the intra-assay CV was 3.5% at 2.50 ng/mL and 3.2% at 14.8 ng/mL. The inter-assay CV’s were 4.3% at 3.03 ng/mL, 5% at 7.23 ng/mL, and 4.8% at 13.62 ng/mL. All GH samples from each participant were analyzed via batch in the same lab. Free IGF-1 was determined using a commercially available ELISA (R & D systems) with a minimum detectable range of 0.015 ng/mL, intra-assay CV 3.6% to 5% and inter-assay CV 10.0% to 11.1%. Total IGF-1 was analyzed by Luminex® assay (Millipore Sigma), calibrated against the NIBSC WHO IS 02/254, with an intra-assay CV < 10% and inter-assay CV < 15%. IGF binding protein-1 (IGFBP-1) and IGFBP-3 were analyzed by commercially available ELISAs (RayBiotech) with a minimum detectable concentration of 5 pg/mL for IGFBP-1 and 80 pg/mL for IGFBP-3. Both assays report an intra-assay CV < 10% and inter-assay CV < 12%. tPA activity and plasminogen activator inhibitor-1 (PAI-1) antigen were measured in blood collected in acidified citrate anticoagulant (TriniLIZE™ Stabilyte tubes, Tcoag; Bray Co. Wicklow, Ireland). TPA activity was analyzed using an ELISA calibrated against NIBSC WHO IS 98/714 (Oxford Biomedical Research, Oxford MS). PAI-1 antigen was analyzed using the TintElize PAI-1 antigen assay (Tcoag; Co. Wicklow, Ireland). Samples for analysis of active GLP-1, total peptide YY (PYY) and PYY 3–36, and glucagon were collected in EDTA collection tubes prepared with aprotinin and DPP4 inhibitor (Millipore Sigma). Active GLP-1 was analyzed in duplicate using the MILLEPLEX® MAP Human Metabolic Hormone Magnetic Bead Panel (Millipore Sigma) with an intra-assay CV of <10% and inter-assay CV < 15%. Total PYY, PYY 3–36, glucagon, and insulin were analyzed in duplicate by radioimmunoassay (Millipore Sigma) with intra-assay CVs of 3.7%, 2.3%, 1.6%, and 3.3%, respectively. PYY 1–36 was calculated by subtracting PYY 3–36 from the total PYY. Estradiol was also analyzed by radioimmunoassay (MP Biomedicals) with an intra-assay CV of 3.9%. Blood glucose levels were determined by Accu-Chek® Inform II bedside glucometer (Roche Diagnostics); the same glucometer was used on each outpatient study day. High-sensitivity C-reactive protein (hsCRP) was determined by commercially available ELISA (Hycult®Biotech). Bioavailable testosterone was calculated from testosterone measured via quantitative high-performance liquid chromatography-tandem mass spectrometry (ARUP® Laboratories). Sex hormone binding globulin (SHBG) was obtained via quantitative electrochemiluminescent immunoassay (ARUP® Laboratories; Salt Lake City, Utah). F2-isoprostanes were determined via gas chromatography/mass spectrometry assay, as previously described (25). Total plasma cholesterol and triglycerides were measured by standard enzymatic assays. High-density lipoprotein (HDL) cholesterol was measured via the enzymatic method after precipitation of very low-density lipoprotein (VLDL) and very low-density lipoprotein (LDL) using polyethylene glycol reagent. From these data LDL cholesterol was calculated using the Friedewald equation. Plasma free fatty acids were analyzed with a commercially available enzymatic kit (Wako Life Sciences). The insulinogenic index, quantitative insulin-sensitivity check index (QUICKI), homeostatic model assessment of insulin resistance (HOMA-IR) and Matsuda Index were calculated as previously described (26, 27).
Analysis of GH secretion
Overnight spontaneous GH secretion was assessed using an automated deconvolution algorithm (AutoDecon Pulse_XP Software Package), which determines secretion events, adds them to the current fit to test for statistical significance, and automatically removes any non-significant events until no additional statistically significant secretion events are found (28). AutoDecon has been validated for the analysis of endogenous pulsatile GH secretion from time-series data obtained via frequent venous sampling from an indwelling catheter every 10 minutes for 12 hours (29, 30). Initial algorithm estimates included the SD of the secretion events, set at one-half of the data-sampling interval (5 minutes), and a starting value for the GH elimination half-life of 13 minutes based upon previously published data in adults with BMI > 25 kg/m2 (31). Initial values for basal secretion and concentration at t = 0 were also estimated from the secretion and concentration panels. AutoDecon then determined GH basal secretion, half-life, median pulse mass and interpulse interval, number of GH peaks, and GH area under the curve (AUC). Additional parameters of GH secretion, including mean overnight GH secretion, GH peak, and nadir were determined via Microsoft Excel 2010. Missing data were omitted from analyses.
Statistical analysis
Data are presented in results tables as both mean ± SD and median [interquartile range, IQR], given that data is not normally distributed. We tested for carry-over effect using the t test approach proposed by Jones and Kenward (32). Repeated measurement continuous variable data from OGTT were summarized as mean over time (from baseline to 270 minutes) or AUC for the same time period. Unless otherwise noted, a paired t test was used to compare variables between treatment conditions, with results presented as the mean difference between treatments with 95% CI. Mixed-effects models were also used to analyze repeated measures data during the OGTT with a random subject effect and with fixed effects of treatment (sitagliptin vs placebo), time and treatment × time interaction. The baseline measurement was also included in each model. Interaction terms were removed from the final model when the corresponding P value was > 0.2. Comparisons between treatments were made at each time point using Wilcoxon signed rank test. Spearman correlation was used to evaluate the association between continuous variables. Wilcoxon rank sum test was used to test differences between 2 groups and linear regression model on binary group indicator and other covariate was used to evaluate the adjusted effect of the group. Analyses were performed using IBM SPSS software v. 23.0, GraphPad Prism 5 and R 2.15.0 (www.r-project.org). A P value ≤ 0.05 was determined to be statistically significant.
Sample size calculation
Sample size was calculated with Power and Sample Size Calculation (PS) Software using the design for a paired t test (33). In a prior study, the mean baseline overnight GH was 0.7 ± SEM 0.2 μg/L versus a mean overnight GH after therapy of 1.2 ± SEM 0.3 μg/L (P < 0.005) in 13 subjects (31). With the enrollment of participants in the current study, we found that the SD of mean overnight GH was 0.51 µg/L (measurements from 2 periods combined), well below the SD of 0.72 µg/L and 1.1 µg/L observed in the prior study used for the original sample size calculation. Conservatively assuming a SD of 0.6 µg/L in each group, we calculated that a sample size of 16 women provides 93.2% power to detect a difference in GH means of 0.5 µg/L.
Results
Effect of sitagliptin on DPP4 activity and glucose metabolism
Seventeen women completed the entire study. One woman completed 2 weeks of both treatments and is also included in the analysis of the outpatient study days.(Fig. 1) Sitagliptin 100 mg daily significantly decreased DPP4 activity (P < 0.001 vs placebo), as assessed on both the outpatient visit days (from 21.3 ± 8.0 to 8.8 ± 5.2 nmol/mL per minute) and the inpatient visit days (from 22.9 ± 6.0 to 9.9 ± 4.9 nmol/mL per minute).
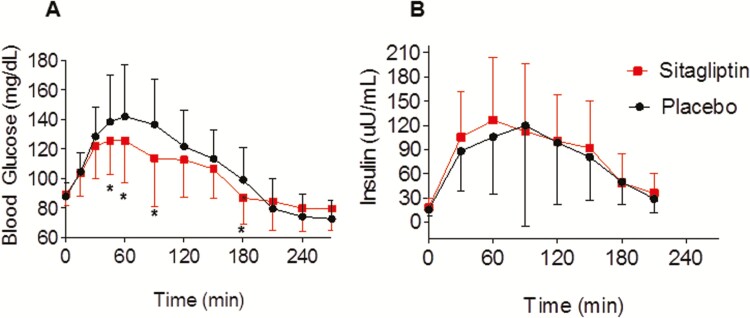
Sitagliptin decreased blood glucose following ingestion of 75 grams of oral glucose, summarized both as an average over time (mean difference −5.13 mg/dL; 95% CI, −10.26 to 0.00; P = 0.05), and as AUC (mean difference −1476.25 mg/dL × 120 minutes; 95% CI, −2472.69 to −479.81; P < 0.01) (Fig. 3A). Peak blood glucose following the oral glucose load was also lower during sitagliptin treatment (mean difference, −17.2 mg/dL, 95% CI, −27.7 to −6.6; P < 0.01). The overall effect of sitagliptin on metabolism of glucose varied with time (P < 0.001). At 100 minutes, mean blood glucose was 15.4 mg/dL (95% CI, 8.7-22.1; P < 0.001) lower during sitagliptin treatment than during placebo.
Figure 3.
Sitagliptin improves blood glucose levels (A) and early insulin secretion (B) following 75 grams oral glucose. Data were obtained on the outpatient visit day in 18 women and are presented as mean ± SD. The overall effect of sitagliptin on glucose levels varied with time (P < 0.001). The P value was obtained via a mixed-effects model with a random subject effect and with fixed effects of treatment (sitagliptin vs placebo), time and treatment × time interaction. *P ≤ 0.05 vs placebo at corresponding timepoint, as obtained by Wilcoxon signed rank.
Sitagliptin also enhanced early insulin secretion, as demonstrated by an increase in the insulinogenic index (from 1.9 ± 1.2 to 3.2 ± 3.1; P = 0.02) and insulin AUC for the first 30 minutes after glucose ingestion (mean difference, 349.21 µU/mL × 30 minutes; 95% CI, 39.3 to 659.1; P = 0.05) (Fig. 3B). Sitagliptin did not affect peripheral insulin resistance (Matsuda), hepatic insulin resistance (HOMA-IR), or insulin sensitivity (QUICKI), as determined on the day of the 75-gram OGTT (Table 2). Sitagliptin did not affect fasting blood glucose (P = 0.572 vs placebo) or fasting insulin levels (P = 0.687 vs placebo).
Table 2.
Effect of Sitagliptin on Glucose Metabolism
| Outpatient Day (N = 18) | Placebo | Sitagliptin | P Value |
|---|---|---|---|
| Blood glucose, 0–120 min AUC | 15 971.3 [3148.1] | 13 432.5 [3361.9] | 0.009 |
| 15 353.8 ± 2708.6 | 13 877.5 ± 2486.3 | ||
| Blood glucose, 0–30 min AUC | 3176.3 [371.3] | 3108.8 [451.9] | 0.913 |
| 3192.1 ± 356.9 | 3140.4 ± 421.3 | ||
| Insulin 0–120 min AUC | 8642.9 [5670.4] | 10 031.1 [6878.2] | 0.212 |
| 11 132.1 ± 8215.6 | 12 169.3 ± 6568.3 | ||
| Insulin 0–30 min AUC | 1337.0 [763.8] | 1663.1 [1130.6] | 0.054 |
| 1522.1 ± 792.3 | 1871.3 ± 843.6 | ||
| Insulinogenic index | 1.84 [1.52] | 2.7 [3.4] | 0.016 |
| 1.89 ± 1.20 | 3.2 ± 3.1 | ||
| Matsuda index | 3.31 [2.51] | 2.96 [2.13] | 0.248 |
| 3.27 ± 1.54 | 3.17 ± 1.99 | ||
| QUICKI | 0.32 [0.03] | 0.32 [0.03] | 0.327 |
| 0.33 ± 0.03 | 0.32 ± 0.03 | ||
| HOMA-IR | 3.4 [2.3] | 3.1 [2.7] | 0.306 |
| 3.5 ± 1.8 | 4.0 ± 2.5 | ||
| Inpatient Day (N = 17) | Placebo | Sitagliptin | P Value |
| Fasting blood glucose (mg/dL)* | 93.0 [7.0] | 90.0 [9.0] | 0.572 |
| 92.9 ± 10.3 | 90.9 ± 5.7 | ||
| Fasting insulin (µU/mL) | 13.9 [6.9] | 14.8 [9.2] | 0.687 |
| 16.5 ± 10.9 | 17.6 ± 9.8 | ||
| HOMA-IR* | 3.2 [1.4] | 3.7 [2.6] | 0.691 |
| 3.9 ± 2.7 | 4.1 ± 2.1 | ||
| Fasting glucagon (pg/mL) | 48.8 [20.6] | 45.2 [19.6] | 0.906 |
| 48.2 ± 16.4 | 49.0 ± 21.4 | ||
| Fasting GLP-1 (pg/mL) | 8.2 [11.7] | 26.9 [16.4] | <0.001 |
| 9.8 ± 8.2 | 27.6 ± 13.8 |
Data shown as median [interquartile range] and mean ± SD. P values obtained via Wilcoxon signed rank test. Abbreviations: AUC, area under the curve; GLP-1, glucagon-like peptide-1; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, quantitative insulin-sensitivity check index. *Data available in 15 women.
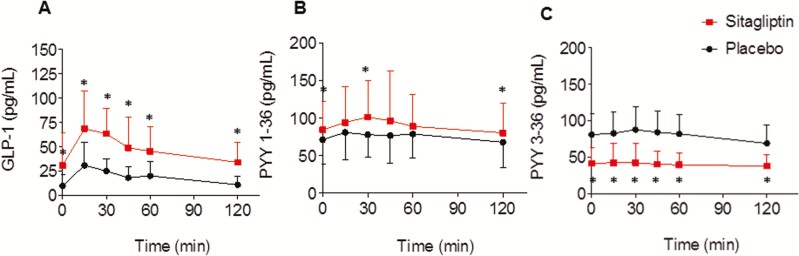
Sitagliptin increased fasting GLP-1 levels (P < 0.001 vs placebo) as well as GLP-1 levels during the 75-gram OGTT, summarized as average over time (mean difference, 27.01 pg/mL; 95% CI, 18.01-36.01; P < 0.001) (Fig. 4A). Sitagliptin also increased peptide YY 1–36 (mean difference, 14.97 pg/mL; 95% CI, 2.30-27.63; P = 0.02) and decreased peptide YY 3–36 (mean difference, −39.78 pg/mL; 95% CI, −51.39 to −28.17; P < 0.001). (Fig. 4B and C) Sitagliptin did not influence the change in free fatty acid levels or glucagon levels following oral glucose ingestion (data not shown).
Figure 4.
Sitagliptin increases both oral glucose tolerance test (GLP-1) (A) and peptide YY (PYY) 1–36 (B) and decreases PYY 3–36 (C) levels following oral 75 grams glucose. Data were obtained on the outpatient visit day in 18 women, and are expressed as mean ± SD. Model-based P values for treatment effect are: P < 0.001 for effect of sitagliptin on GLP-1, P < 0.001 for effect of sitagliptin on PYY 1–36, P < 0.001 for effect of sitagliptin on PYY 3–36. The P value was obtained via a mixed-effects model with a random subject effect and with fixed effects of treatment (sitagliptin vs placebo), time and treatment × time interaction. *P ≤ 0.05 vs placebo at corresponding timepoint, as obtained by Wilcoxon signed rank.
Effect of sitagliptin on GH secretion and IGF-1 levels
Overnight GH secretion (GH AUC) correlated inversely with weight (placebo: rs = −0.69, P = 0.003; sitagliptin: rs = −0.71; P = 0.002), VAT mass (placebo: rs = −0.82, P < 0.001; sitagliptin: rs = −0.77, P = 0.001), hsCRP (placebo: rs = −0.53, P = 0.035; sitagliptin: rs = −0.54, P = 0.031), and HOMA-IR (placebo: rs = −0.54, P = 0.033; sitagliptin: rs = −0.79, P = 0.001), regardless of drug treatment during the preceding month.
GH levels were appropriately suppressed following oral glucose ingestion and then increased approximately 4 hours later. Sitagliptin did not significantly influence the late GH peak (mean difference, 0.31 ng/mL; 95% CI, −0.92 to 1.54; P = 0.61) during the 75-gram OGTT. IGFBP-1 levels, summarized as average of time, were not affected by sitagliptin (mean difference, −9.18 pg/mL; 95% CI, −104.73 to 86.38]; P = 0.84). Free IGF-1 levels also were unaffected by sitagliptin (mean difference, −0.01 ng/mL; 95% CI, −0.09 to 0.07; P = 0.84). Free IGF-1 levels at each time point following oral glucose were: baseline 0.46 ± 0.22 ng/mL placebo vs 0.44 ± 0.21 ng/mL sitagliptin; 30 minutes after oral glucose 0.46 ± 0.19 ng/mL placebo vs 0.43 ± 0.18 ng/mL sitagliptin; 90 minutes after oral glucose 0.44 ± 0.20 ng/mL placebo vs 0.46 ± 0.20 ng/mL sitagliptin; 150 minutes after oral glucose 0.49 ± 0.26 ng/mL placebo vs 0.45 ± 0.22 ng/mL sitagliptin.
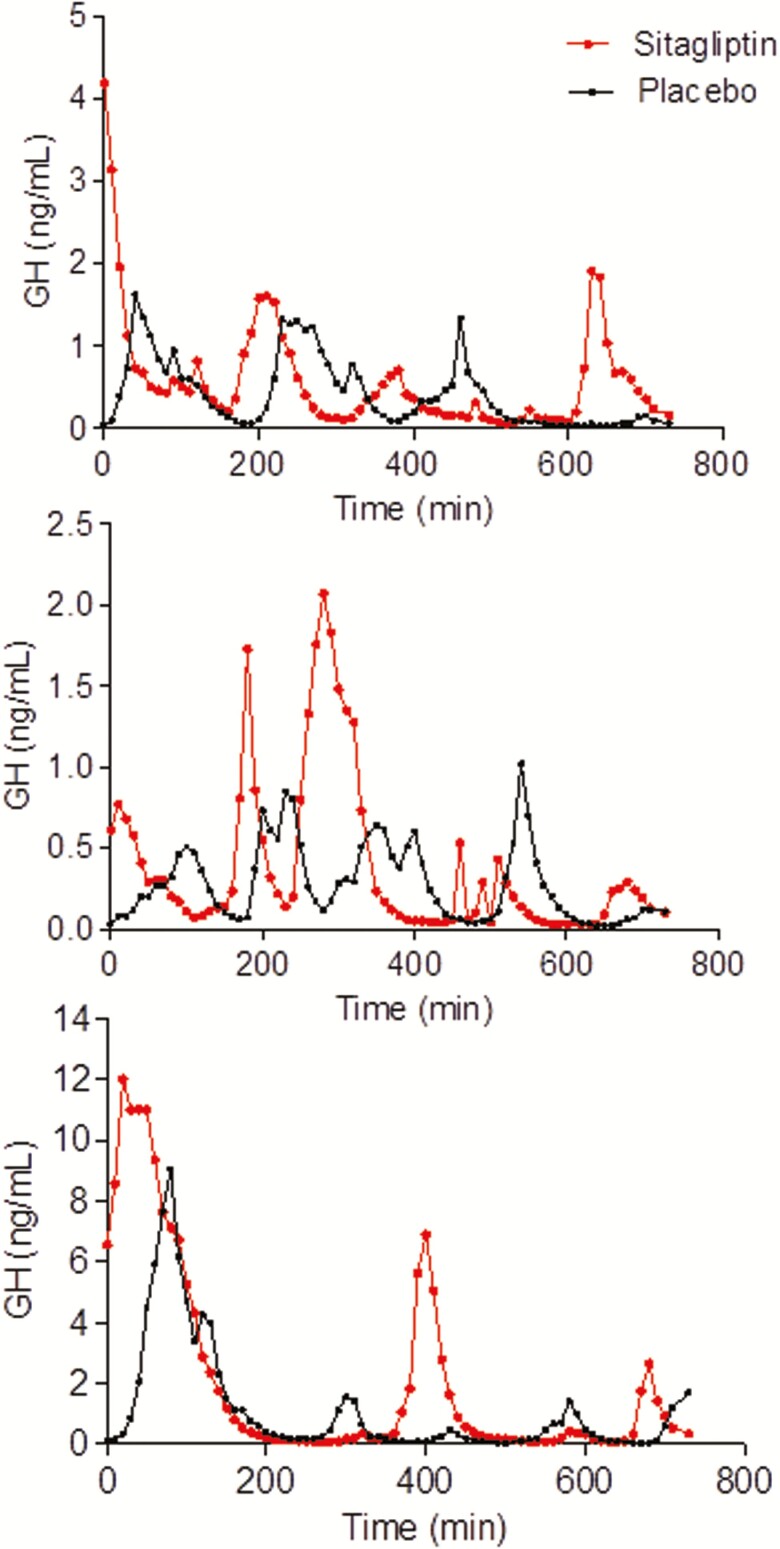
During overnight sampling after 1 month of sitagliptin, GH half-life (P = 0.04 vs placebo) and consequently the median interpulse interval (P < 0.05 vs placebo) both increased, but GH AUC and mean GH were stable (Table 3). Representative plots of overnight sampling of GH from 3 particiapants are included in Fig. 5. One month of daily sitagliptin had no effect on fasting free IGF-1 levels, total IGF-1 levels, or IGFBP-3 levels (Table 3).
Table 3.
Effect of Sitagliptin on Overnight GH Secretion and IGF-1 Levels
| Variable (N = 16) | Placebo | Sitagliptin | P Value |
|---|---|---|---|
| GH AUC (ng/mL per 12 hr) | 428.9 [543.5] | 524.6 [466.0] | 0.918 |
| 604.5 ± 398.7 | 593.5 ± 371.6 | ||
| Basal GH secretion (ng/mL∙min)* | 0.001 [0.002] | 0.002 [0.002] | 0.496 |
| 0.002 ± 0.002 | 0.002 ± 0.001 | ||
| GH half-life (min) | 13.9 [2.7] | 14.5 [8.0] | 0.044 |
| 13.9 ± 3.6 | 17.0 ± 6.8 | ||
| Number GH secretion events | 10.5 [4.0] | 8.5 [6.5] | 0.058 |
| 11.0 ± 2.6 | 9.1 ± 3.3 | ||
| Median secretion pulse mass | 1.1 [1.2] | 1.3 [1.8] | 0.756 |
| 2.2 ± 3.4 | 1.7 ± 1.5 | ||
| Median secretion pulse height | 0.04 [0.05] | 0.07 [0.16] | 0.301 |
| 0.10 ± 0.14 | 0.34 ± 0.63 | ||
| Median secretion interpulse interval | 51.1 [34.4] | 61.8 [60.6] | 0.049 |
| 53.2 ± 20.0 | 77.3 ± 38.2 | ||
| Maximum GH (ng/mL) | 4.1 [5.5] | 4.0 [3.5] | 0.918 |
| 5.1 ± 3.5 | 5.1 ± 4.4 | ||
| Minimum GH (ng/mL) | 0.03 [0.03] | 0.04 [0.05] | 0.567 |
| 0.04 ± 0.02 | 0.04 ± 0.02 | ||
| Mean GH (ng/mL) | 0.63 [0.73] | 0.74 [0.73] | 0.918 |
| 0.85 ± 0.54 | 0.84 ± 0.51 | ||
| Total IGF-1 (ng/mL)† | 81.4 [57.0] | 86.6 [52.1] | 0.586 |
| 88.7 ± 28.6 | 88.8 ± 29.7 | ||
| Free IGF-1 (ng/mL)† | 0.31 [0.27] | 0.38 [0.18] | 0.523 |
| 0.40 ± 0.26 | 0.43 ± 0.25 | ||
| IGFBP-1 (pg/mL) | 184.4 [149.4] | 158.2 [469.6] | 0.326 |
| 263.6 ± 298.3 | 325.2 ± 365.3 | ||
| IGFBP-3 (ng/mL)† | 17.1 [5.9] | 18.9 [3.4] | 0.687 |
| 18.5 ± 4.3 | 18.6 ± 2.4 | ||
| IGF-1/IGFBP-3† | 4.8 [3.2] | 4.4 [3.2] | 0.906 |
| 5.1 ± 2.2 | 4.9 ± 1.9 |
Data shown as median [interquartile range] and mean ± SD. P values obtained via Wilcoxon signed rank test. IGF-1 and IGFBP represent morning fasting levels. GH levels were obtained every 10 minutes from 8 PM until 8 AM. Abbreviations: AUC, area under the curve; GH, growth hormone; IGF-1, insulin-like growth factor-1; IGFBP, IGF binding protein. *Data available in 15 women. † Data available in 17 women.
Figure 5.
Representative plots of overnight growth hormone (GH) levels from 3 subjects during placebo and sitagliptin treatment. GH samples are obtained every 10 minutes from 8 PM to 8 AM. The plots illustrate the high inter-individual variability but low intra-individual variability in overnight GH secretion.
Effect of sitagliptin on vascular parameters and conduit artery vascular function
Sitagliptin increased heart rate during the 75-gram OGTT summarized as average over time (mean difference, 2 bpm; 95% CI, 0.01-4.06; P < 0.05) and during the inpatient study day (from 67.4 ± 9.3 to 71.3 ± 6.9 bpm; P < 0.05). One month of sitagliptin did not affect inflammatory or fibrinolytic markers. Sitagliptin also had no effect on conduit artery vascular function during reactive hyperemia. (Table 4) A limited number of women (n = 3) met criteria for assessment of endothelium-independent vasodilation using nitroglycerin and agreed to its administration. Sitagliptin did not influence vasodilation after 0.4 mg sublingual nitroglycerin (36.5% ± 13.7 after placebo vs 36.4% ± 9.0 after sitagliptin; P > 0.05).
Table 4.
Effect of Sitagliptin on Vascular Parameters and Conduit Artery Vascular Function
| Variable (N = 17) | Placebo | Sitagliptin | P Value |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 114.7 [18.7] | 115.0 [16.2] | 0.518 |
| 117.8 ± 11.6 | 115.6 ± 8.7 | ||
| Diastolic blood pressure (mm Hg) | 75.0 [8.9] | 73.0 [11.0] | 1.000 |
| 73.8 ± 7.4 | 73.7 ± 7.8 | ||
| Heart rate (beats per minute) | 67.0 [14.2] | 68.0 [7.0] | 0.046 |
| 67.4 ± 9.3 | 71.3 ± 6.9 | ||
| Baseline artery diameter (mm) | 3.14 [0.55] | 3.12 [0.62] | 0.796 |
| 3.16 ± 0.43 | 3.13 ± 0.37 | ||
| Baseline flow integral (m) | 0.12 [0.04] | 0.17 [0.07] | 0.266 |
| 0.15 ± 0.08 | 0.18 ± 0.08 | ||
| Peak FMD (mm) | 3.53 [0.66] | 3.48 [0.54] | 0.344 |
| 3.58 ± 0.47 | 3.53 ± 0.36 | ||
| Time of peak FMD (sec) | 59.00 [37.82] | 64.66 [46.56] | 0.356 |
| 60.71 ± 30.80 | 75.55 ± 35.02 | ||
| Diameter increase (mm) | 0.39 [0.26] | 0.40 [0.23] | 0.705 |
| 0.43 ± 0.16 | 0.40 ± 0.16 | ||
| Flow-mediated vasodilation (%) | 12.42 [8.28] | 14.24 [8.45] | 0.943 |
| 13.63 ± 5.27 | 13.00 ± 5.34 | ||
| Peak VTI (m)* | 1.13 [0.34] | 1.14 [0.27] | 0.717 |
| 1.12 ± 0.23 | 1.13 ± 0.32 | ||
| VTI, fold increase* | 8.37 [3.49] | 7.26 [4.35] | 0.234 |
| 8.34 ± 2.66 | 7.38 ± 2.79 | ||
| tPA activity (IU/mL) | 0.21 [0.40] | 0.23 [0.82] | 0.831 |
| 0.40 ± 0.47 | 0.44 ± 0.46 | ||
| PAI-1 antigen (ng/mL)* | 8.78 [7.33] | 9.72 [4.77] | 0.959 |
| 10.96 ± 7.51 | 11.27 ± 8.05 | ||
| hsCRP (mg/L) | 3.51 [4.40] | 2.90 [3.45] | 0.619 |
| 4.43 ± 4.00 | 4.92 ± 5.36 | ||
| F2 isoprostanes (pg/mL)* | 55.74 [44.02] | 50.02 [42.26] | 0.836 |
| 68.16 ± 45.69 | 62.01 ± 30.25 |
Data shown as median [interquartile range] and mean ± SD. P values obtained via Wilcoxon signed rank test. Abbreviations: FMD, flow-mediated vasodilation; hsCRP, high-sensitivity C-reactive protein; PAI-1, plasminogen activator inhibitor-1; tPA, tissue-type plasminogen activator; VTI, velocity time integral. *Data available in 16 women.
Effect of sitagliptin on body composition and characteristics of PCOS
One month of sitagliptin therapy decreased VAT mass (P = 0.02 vs placebo) and volume (P = 0.02 vs placebo), but did not significantly affect weight or overall percent fat (Table 5). Sitagliptin decreased total cholesterol (from 168.8 ± 26.3 to 162.5 ± 22.2 mg/dL; P = 0.02), largely due to a decrease in LDL (from 101.9 ± 23.8 to 96.2 ± 21.5 mg/dL; P = 0.06). One month of treatment with sitagliptin did not influence estradiol or testosterone levels (Table 5). Sitagliptin did not affect health-related quality-of-life domains (emotions, body hair, weight, infertility, and menstrual problems), as assessed by the PCOSQ administered on the last day of each therapy (P = 1.00 vs placebo for each domain).
Table 5.
Effect of Sitagliptin on Body Composition and Characteristics of PCOS
| Variable (N = 17) | Placebo | Sitagliptin | P value |
|---|---|---|---|
| Weight (kg) | 81.0 [28.0] | 81.6 [27.1] | 0.619 |
| 90.4 ± 21.7 | 90.2 ± 22.1 | ||
| Body mass index (kg/m2) | 32.1 [10.7] | 32.4 [10.4] | 0.586 |
| 34.3 ± 6.4 | 34.2 ± 6.4 | ||
| Waist circumference (cm) | 105.9 [27.7] | 105.0 [18.7] | 0.381 |
| 105.5 ± 18.0 | 104.0 ± 15.4 | ||
| Waist/hip ratio* | 0.89 [0.12] | 0.87 [0.10] | 0.326 |
| 0.88 ± 0.07 | 0.87 ± 0.07 | ||
| Percent body fat (%) | 46.2 [5.2] | 45.8 [6.5] | 0.058 |
| 46.8 ± 5.4 | 46.3 ± 5.0 | ||
| Fat (kg) | 36.4 [16.9] | 35.5 [18.0] | 0.093 |
| 41.6 ± 14.3 | 40.9 ± 14.5 | ||
| Lean body mass (kg) | 43.9 [10.1] | 44.6 [10.4] | 0.758 |
| 45.6 ± 7.4 | 45.8 ± 7.9 | ||
| VAT volume (cm3) | 1199.0 [744.0] | 959.0 [820.5] | 0.022 |
| 1210.6 ± 743.0 | 1118.4 ± 752.5 | ||
| VAT mass (g) | 1131.0 [702.0] | 904.0 [774.0] | 0.022 |
| 1141.9 ± 700.7 | 1055.1 ± 710.1 | ||
| Android (% fat)† | 54.1 [9.2] | 51.9 [11.2] | 0.037 |
| 54.0 ± 7.6 | 52.8 ± 7.0 | ||
| Bioavailable testosterone (ng/dL)* | 23.2 [11.5] | 16.1 [12.0] | 0.109 |
| 22.9 ± 10.3 | 19.8 ± 10.5 | ||
| Total testosterone (ng/dL) | 43.0 [25.0] | 36.0 [31.0] | 0.266 |
| 48.4 ± 24.3 | 45.1 ± 27.5 | ||
| SHBG (nmol/L)* | 25.5 [20.0] | 31.5 [18.8] | 0.325 |
| 33.4 ± 21.1 | 30.7 ± 15.7 | ||
| Estradiol (pg/mL) | 212.9 [140.6] | 210.2 [91.9] | 0.407 |
| 233.5 ± 118.9 | 214.6 ± 71.4 | ||
| Total cholesterol (mg/dL) | 169.0 [52.5] | 165.0 [32.0] | 0.020 |
| 168.8 ± 26.3 | 162.5 ± 22.2 | ||
| LDL (mg/dL) | 93.0 [45.0] | 93.0 [37.5] | 0.055 |
| 101.9 ± 23.8 | 96.2 ± 21.5 | ||
| HDL (mg/dL) | 49.0 [18.5] | 51.0 [15.0] | 0.462 |
| 53.8 ± 18.3 | 52.8 ± 15.3 | ||
| Triglycerides (mg/dL) | 65.0 [39.0] | 53.0 [58.5] | 0.887 |
| 64.8 ± 24.0 | 67.6 ± 36.9 |
Data shown as median [interquartile range] and mean ± SD. P values obtained via Wilcoxon signed rank test. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SHBG, sex hormone binding globulin; VAT, visceral adiposity. *Data available in 16 women. † Android (% fat) represents the %total fat within the android (central) region, as opposed to the gynoid (hip and thigh) region.
Association of DPP4 inhibition with GH levels, VAT, and HOMA-IR
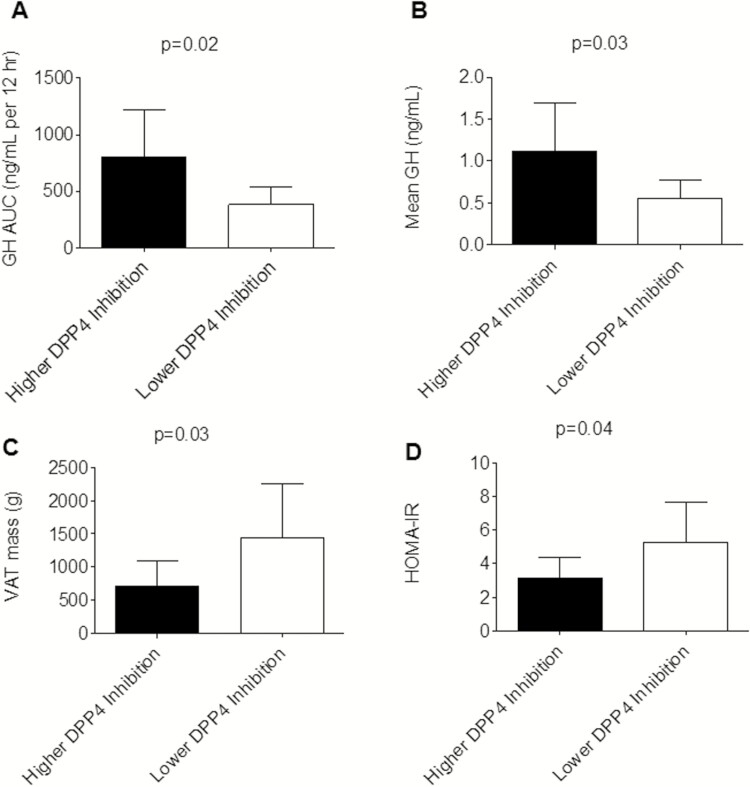
Sitagliptin did not increase overnight GH secretion or lower insulin resistance. Greater DPP4 inhibition in response to sitagliptin, however, correlated with GH levels, VAT, and HOMA-IR. The degree of DPP4 inhibition with sitagliptin correlated with GH secretion, such that DPP4 inhibition was associated with overnight GH secretion (GH AUC: rs = 0.60, P = 0.02; mean GH: rs = 0.59, P = 0.02). DPP4 inhibition after sitagliptin was also associated with VAT mass (rs = −0.66; P < 0.01), and HOMA-IR (rs = −0.64; P = 0.01). The correlations between DPP4 inhibition and GH AUC, VAT, and HOMA-IR were no longer significant after controlling for BMI (GH AUC rs = 0.25, P = 0.38; VAT rs = −0.31, P = 0.25; HOMA-IR rs = −0.15; P = 0.61). Sitagliptin-treated women in whom the percent DPP4 inhibition (defined as [1-(DPP4 activity during sitagliptin/DPP4 activity during placebo)]*100) was above the median for the women analyzed had higher overnight GH secretion and lower VAT mass and HOMA-IR. Fig. 6 compares values between those with higher DPP4 inhibition and lower DPP4 inhibition within the sitagliptin treatment. Similarly, women with greater DPP4 inhibition also had lower BMI (30.3 ± 3.4 vs 38.5 ± 6.3 kg/m2; P < 0.01).
Figure 6.
Women with a higher percent dipeptidyl peptidase-4 (DPP4) inhibition had higher overnight growth hormone (GH) secretion (N = 16) (A and B) and lower visceral adiposity mass (VAT; N = 17) (C) and homeostatic model assessment of insulin resistance (HOMA-IR; N = 17) (D) after 1-month sitagliptin treatment. Data were obtained on the inpatient visit day and are expressed as mean ± SD. P values were calculated using the Wilcoxon rank sum test. Higher percent DPP4 inhibition is defined as percent DPP4 inhibition at or greater than the median for the 17 women included in the analysis, where percent DPP4 inhibition = [1-(DPP4 activity during sitagliptin/DPP4 activity during placebo)]×100. Comparisons between higher and lower DPP4 inhibition were not significant after controlling for body mass index (GH area under the curve P = 0.27; mean GH P = 0.24; VAT P = 0.67; HOMA-IR P = 0.66).
Safety
One woman was hospitalized with gallstone-induced pancreatitis after 2 doses of sitagliptin. One woman reported intermittent abdominal pain while taking sitagliptin and another reported dizziness. One woman taking sitagliptin reported dizziness towards the end of the OGTT, another woman taking sitagliptin developed headache during the OGTT. There were no reported instances of hypoglycemia while taking sitagliptin. Three woman reported nausea, decreased appetite, and dizziness while taking placebo. One woman developed nausea during the OGTT while taking placebo, and one experienced emesis. One woman developed shingles while taking placebo. One woman developed headache, nausea, and dizziness following nitroglycerin.
Discussion
This study tested the hypothesis that 1-month treatment with the DPP4 inhibitor sitagliptin would potentiate overnight GH secretion in overweight women with PCOS and improve glucose levels. As expected, sitagliptin lowered blood glucose by increasing GLP-1 levels and enhancing early insulin secretion following ingestion of oral glucose. Sitagliptin did not increase mean overnight GH but did enhance GH half-life. Body composition also improved during sitagliptin, as evidenced by a decrease in VAT, while weight and BMI remained stable. These findings indicate that DPP4 inhibition with sitagliptin decreases the maximal glucose response to OGTT, decreases visceral adiposity, and increases GH half-life in overweight women with PCOS.
Altered GLP-1 and insulin secretion following oral glucose ingestion has previously been reported even in lean, glucose-tolerant women with PCOS. Women with PCOS have higher levels of C-peptide and tend to have higher levels of insulin secretion, particularly in the first hour after glucose ingestion. While active GLP-1 levels are similar in women with PCOS and healthy volunteers early on, they are lower in women with PCOS after 1 hour (34). The glucagon response to hypoglycemia is also potentiated in obese women with PCOS compared with matched controls (35). We hypothesized that sitagliptin, with its ability to potentiate postprandial GLP-1 secretion and suppress glucagon secretion, would mitigate these undesirable post-glucose changes. We observed enhanced GLP-1 secretion during sitagliptin, but no changes in glucagon levels.
Only 1 previous study has evaluated the effect of sitagliptin on glucose homeostasis in women with PCOS. Ferjan et al conducted a 12-week study of obese women with PCOS in which women were randomized to open-label sitagliptin 100 mg daily with lifestyle intervention or lifestyle intervention alone following metformin withdrawal (36). The investigators found that sitagliptin improved beta cell function and even prevented the conversion from impaired glucose tolerance to type 2 diabetes mellitus in 3 of the women. The investigators did not appreciate a significant decrease in glucose levels during OGTT. In contrast, we observed that sitagliptin enhanced GLP-1 secretion following oral glucose ingestion, which resulted in enhanced early insulin secretion and a significant decrease in the blood glucose rise following oral glucose ingestion. Our relatively more homogeneous study population and crossover design likely enabled us to better detect an improvement in blood glucose levels.
The effect of sitagliptin on postprandial GH secretion has not previously been investigated. It is well known that oral glucose ingestion quickly inhibits GH secretion before a late stimulatory effect on GHRH-mediated GH levels. Relatively little is known, however, about the metabolic factors that influence GH secretion following oral glucose. Grottoli et al demonstrated that early GH inhibition following glucose ingestion is lost in obesity but the late stimulatory effect is maintained (37). Iranmanesh et al further demonstrated that nadir and peak rebound GH secretion is influenced by VAT and SHBG in obese men (38). Potential mechanisms by which sitagliptin might alter the GH secretion in response to an OGTT in women with PCOS include enhanced late GH secretion due to decreased degradation of the GHRH stimulus or mitigation of the known inhibitory effects of hyperglycemia and free fatty acids on GH secretion. Although sitagliptin lowered blood glucose levels following oral glucose ingestion, we did not observe any effect of sitagliptin on free fatty acids or on GH secretion. Skov et al also demonstrated that intravenous GLP-1 infusion in men reduces IGFBP-1 and tended to increase IGF-1 bioactivity (39). We did see an increase in GLP-1 and early insulin secretion, but this did not significantly influence IGFBP-1 levels or free IGF-1 levels. Thus, it is unlikely that alterations in insulin secretion, blood glucose levels, IGFBP-1, or GLP-1 levels contribute to the altered post-glucose GH secretion described in obesity. Furthermore, IGF-1 did not contribute to the observed changes in glucose metabolism in our study participants.
Our study investigates a novel potential strategy to enhance endogenous GH secretion in a population of patients demonstrated to have diminished GH levels. Obese individuals have diminished GH secretion, characterized by fewer GH pulses and shorter half-life duration (40). De Boer et al observed impaired overnight GH secretion in PCOS patients, irrespective of weight and degree of insulin resistance (12). We found that 1 month of sitagliptin therapy influences overnight GH secretion by increasing GH half-life. The interpulse interval, which represents the time interval between secretion events and is partially dependent upon half-life in addition to the previous interpulse interval and secretion event area, was also increased (28, 29). Sitagliptin did not increase overnight GH levels. Although overnight GH secretion correlated with DPP4 inhibition after sitagliptin, this was not the case after controlling for BMI.
Our study was not designed to determine the mechanism by which sitagliptin influences the overnight GH secretory profile. Possible mechanisms include decreased degradation of the GHRH stimulus by DPP4, or increased GH as an indirect result of decreased VAT. In support of the first mechanism, Makimura et al reported that 12 months of the DPP4-resistant GHRH analogue tesamorelin increased IGF-1 and selectively decreased VAT in obese subjects with reduced GH secretion (41). In contrast, we did not find that sitagliptin affected IGF-1 levels, yet VAT was still selectively affected. The lack of effect of sitagliptin on IGF-1 levels is not surprising, given that sitagliptin did not affect overnight GH secretion. In support of the second mechanism, Vahl et al found that abdominal obesity was the singlemost significant determinant of pulsatile GH release, as determined by 24-hour GH profile (42). Pijl et al studied the effect of caloric restriction and 50% loss of body weight over a period of 3 to 5 months in obese women and observed an increase in mean 24-hour GH secretion (6). Similar to our findings, they observed an increase in GH half-life with no effect on IGF-1 levels. This report is consistent with our observation of a smaller yet significant decrease in VAT and increase in GH half-life after only 1 month of sitagliptin in our participants. Dichtel et al reported that in overweight and obese women, increases in bioactive IGF-1, rather than total IGF-1, were found to drive GH-mediated body composition changes in the short-term (43). A decrease in GH binding protein may also have played a role in the observed changes in GH half-life and interpulse interval, as levels of GH binding protein directly correlate with VAT (44). Lastly, another mechanism possibly contributing to the decrease in VAT may be increased sympathetic activity. We have previously reported that sitagliptin increases sympathetic activity during concurrent angiotensin converting-enzyme (ACE) inhibition by decreasing degradation of the DPP4 and ACE substrate substance P (45). We did observe an increase in heart rate following sitagliptin during the OGTT.
Others have reported that the addition of sitagliptin to metformin therapy improves body composition in women with PCOS. Yan et al reported that the addition of sitagliptin to metformin treatment reduces VAT in patients with type 2 diabetes and nonalcoholic fatty liver disease (46). Ferjan et al similarly found that sitagliptin prevented weight regain, compared with metformin alone, in a 12-week prospective open-label study in 24 obese women with PCOS who were previously treated with liraglutide (47). Thus, a growing body of evidence suggests that sitagliptin selectively reduces VAT. Despite the improvements in VAT and GH secretion, we did not observe an improvement in any of the clinical signs or symptoms experienced by women with PCOS, as reported in a validated PCOS questionnaire. Longer duration of therapy would likely be necessary to detect a change in these parameters. Similarly, androgen levels were not affected by sitagliptin. We would expect androgen levels to improve in association with an improvement in insulin resistance, and the latter was not observed.
Our study further evaluates cardiovascular endpoints, such as vascular function and fibrinolysis, which may be affected by enhanced GH secretion. GH directly improves vascular function through several mechanisms, including activation of the GH receptor and downstream IGF-1 secretion (48), as well as GH receptor- and IGF-1-independent effects (49). Li et al reported that supraphysiologic circulating concentrations GH of approximately 33 ng/mL were necessary to increase forearm blood flow (1). Similarly, Napoli et al reported that intra-arterial GH infusion, which raised local GH to approximately 40 ng/mL, increased forearm blood flow (2). We previously reported that sitagliptin increases forearm vasodilation, measured by strain gauge plethysmography, in healthy women as assessed during stimulated GH secretion with peak levels averaging 11.6 mg/dL (11). In the present study, we did not observe any effect of sitagliptin on conduit artery vascular function, however. Mean overnight GH levels in patients with PCOS in this study were significantly lower than in our prior study in healthy women. The changes in GH secretion induced by sitagliptin may have been insufficient to detect an effect on forearm vasodilation. Other potential factors, such as increased sympathetic activity due to decreased degradation of other DPP4 substrates such as substance P and neuropeptide Y, may also have masked any GH-induced changes in vascular function (45, 50). It is also notable that we did not observe any increase in vasodilation despite elevated GLP-1 levels during sitagliptin. This is consistent with our previous observation that GLP-1 infusion into the forearm vasculature does not increase vasodilation (51).
We report for the first time the effect of chronic sitagliptin treatment on tPA activity and PAI-1 antigen levels. Despite the observed decrease in VAT and cholesterol, there was no significant effect of sitagliptin on PAI-1. This contrasts with the data of Tani et al who found that 8-week treatment with vildagliptin decreased triglycerides and PAI-1 in poorly-controlled type 2 diabetic patients (52). The investigators suggested that improved lipid metabolism resulted in downregulation of PAI-1 production from the vascular endothelial cells. Triglyceride and PAI-1 levels in the women in the current study were one-third of the levels in their study population, thus making it difficult to detect further downregulation of PAI-1 production. We have previously demonstrated that GH affects tPA activity levels through the GH receptor and downstream IGF-1 secretion (11), but we did not observe any change in IGF-1 or tPA activity in this study. We also did not see any effect of sitagliptin on inflammation, whereas Makdissi A et al observed an anti-inflammatory effect of 100 mg daily sitagliptin on CRP, IL-6, and free fatty acids after 12 weeks in patients with type 2 diabetes (53). Differences in study populations or treatment duration could account for these different observations.
Our study has limitations. PCOS is a heterogeneous disorder; women may be lean or overweight and may or may not have insulin resistance. We studied a relatively homogenous subset of women with PCOS and our results may not be generalizable to all women with PCOS. We chose to limit our study population to those women with a BMI of at least 25 kg/m2 and to exclude participants who were dieting with weight loss or titrating metformin therapy. This subset of women with PCOS tends to represent the subset who are prescribed metformin in order to improve glucose metabolism. We were interested in how sitagliptin influenced glucose metabolism in overweight women with PCOS and we were limited to use of a manual glucometer for glucose measurements, though the same glucometer was used for each woman across study days. We also did not include baseline endogenous GH secretory capacity as a selection criterion for the women studied, as is done in many clinical trials evaluating the effect of an intervention on GH secretion. If we had preselected for a relatively GH-deficient population, we may have seen a more prominent effect of sitagliptin on overnight GH secretion. We were unable to standardize timing of the endpoint measurements to the womens’ menstrual cycles, as most women had infrequent menstrual cycles. This is particularly relevant in our assessments of overnight GH secretion and vascular function. We did not observe a difference in estradiol levels across study treatments, however. Our treatment duration was 1 month, which may not have been sufficient to appreciate any effect of sitagliptin on clinical symptoms, or inflammatory or fibrinolytic markers. We chose to use the current FDA-approved daily dose of sitagliptin, however we have previously demonstrated that 100 mg daily of sitagliptin is not as effective as single-dose 200 mg sitagliptin in inhibiting DPP4 activity in healthy individuals, and that sitagliptin 100 mg daily is less effective in inhibiting DPP4 activity in individuals with type 2 diabetes mellitus and hypertension than in healthy individuals (54). A higher dose of sitagliptin, resulting in maximal DPP4 inhibition, may have yielded a greater treatment effect on mean GH and eliminated any effect of weight on response to sitagliptin. Our limited sample size may have limited our conclusions on overnight GH secretion parameters. We observed low intra-individual variability in the pattern of overnight GH secretion, however, and the crossover design of the study would have increased our ability to detect treatment effects. Lastly, our study design did not enable us to determine the mechanism by which sitagliptin may enhance GH half-life.
In this study, we evaluated the previously unexplored effects of sitagliptin in overweight women with PCOS. We demonstrate for the first time that sitagliptin enhances GLP-1 levels and early insulin secretion and thereby decreases peak blood glucose following oral glucose ingestion in these women. Secondly, we demonstrate that sitagliptin decreases visceral adiposity, through an undefined mechanism. Lastly, this study is the first to suggest an off-target effect of sitagliptin on GH secretion in women with PCOS, potentiating GH half-life and the interpulse interval. This is relevant since endogenous GH secretion is diminished in women with PCOS (12–14).
Acknowledgments
We thank Anthony Dematteo, Zuofei Wang, and Aaron Falck for their technical assistance. We thank Drs. Kevin Niswender, Italo Biaggioni, and Melissa Wellons for their expertise and service on the Data and Safety Monitoring Committee.
Financial Support. This research was supported by Vanderbilt Clinical and Translational Science Awards (CTSA) grant UL1 TR000445-06 from the NIH National Center for Advancing Translational Sciences. The Vanderbilt Hormone Assay and Analytical Services Core is supported by NIH grants DK059637 and DK020593. JKD was supported by K23HL11962 NHLBI/NIH and NJB by R01HL125426 from NHLBI/NIH.
Glossary
Abbreviations
- AUC
area under the curve
- CRC
Vanderbilt Clinical Research Center
- DEXA
dual-energy x-ray absorptiometry
- DPP4
dipeptidyl peptidase-4
- ELISA
enzyme-linked immunoassay
- GH
growth hormone
- GHRH
growth hormone–releasing hormone;
- GLP-1
glucagon-like peptide-1
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- hsCRP
high-sensitivity C-reactive protein
- IGF-1
insulin-like growth factor-1
- IGFBP
IGF binding protein
- IQR
interquartile range
- LDL
low-density lipoprotein
- OGTT
oral glucose tolerance test
- PAI-1
plasminogen activator inhibitor-1
- PCOS
polycystic ovarian syndrome
- PCOSQ
polycystic ovarian syndrome questionnaire
- QUICKI
quantitative insulin-sensitivity check index
- SHBG
sex hormone binding globulin
- tPA
tissue-type plasminogen activator
- VAT
visceral adiposity
- VTI
velocity time integral.
Additional Information
Disclosure Summary. The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Li G, Del Rincon JP, Jahn LA, Wu Y, Gaylinn B, Thorner MO, Liu Z. Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J Clin Endocrinol Metab 2008;93:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Napoli R, Guardasole V, Angelini V, D’Amico F, Zarra E, Matarazzo M, Sacca L. Acute effects of growth hormone on vascular function in human subjects. J Clin Endocrinol Metab 2003;88:2817–2820. [DOI] [PubMed] [Google Scholar]
- 3. Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med 2000;133:111–122. [DOI] [PubMed] [Google Scholar]
- 4. Devin JK, Blevins LS, Jr., Verity DK, Chen Q, Bloodworth JR, Jr., Covington J, Vaughan DE. Markedly impaired fibrinolytic balance contributes to cardiovascular risk in adults with growth hormone deficiency. J Clin Endocrinol Metab 2007;92:3633–3639. [DOI] [PubMed] [Google Scholar]
- 5. Miljic D, Miljic P, Doknic M, Pekic S, Stojanovic M, Cvijovic G, Micic D, Popovic V. Growth hormone replacement normalizes impaired fibrinolysis: new insights into endothelial dysfunction in patients with hypopituitarism and growth hormone deficiency. Growth Horm IGF Res 23:243–248. [DOI] [PubMed] [Google Scholar]
- 6. Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 2001;86:5509–5515. [DOI] [PubMed] [Google Scholar]
- 7. Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am 2007;36:75–87. [DOI] [PubMed] [Google Scholar]
- 8. Frohman LA, Downs TR, Heimer EP, Felix AM. Dipeptidylpeptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J Clin Invest 1989;83:1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frohman LA, Downs TR, Williams TC, Heimer EP, Pan YC, Felix AM. Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus. J Clin Invest 1986;78:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther 2006;28:55–72. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JR, Brown NJ, Nian H, Yu C, Bidlingmaier M, Devin JK. Dipeptidyl Peptidase-4 Inhibition Potentiates Stimulated Growth Hormone Secretion and Vasodilation in Women. J Am Heart Assoc 2018;7:e008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Boer JA, Lambalk CB, Hendriks HH, van Aken C, van der Veen EA, Schoemaker J. Growth hormone secretion is impaired but not related to insulin sensitivity in non-obese patients with polycystic ovary syndrome. Hum Reprod 2004;19:504–509. [DOI] [PubMed] [Google Scholar]
- 13. Orio F, Jr., Palomba S, Colao A, Russo T, Dentico C, Tauchmanova L, Savastano S, Nappi C, Sultan C, Zullo F, Lombardi G. GH release after GHRH plus arginine administration in obese and overweight women with polycystic ovary syndrome. J Endocrinol Invest 2003;26:117–122. [DOI] [PubMed] [Google Scholar]
- 14. Piaditis GP, Kounadi TG, Rangou DB, Trovas GP, Kaklas NA, Tzonou AJ, Chlouverakis CS. Dysfunction of the growth hormone/insulin-like growth factor-I axis in women with polycystic ovarian syndrome. Clin Endocrinol (Oxf) 1995;42:635–640. [DOI] [PubMed] [Google Scholar]
- 15. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab 2005;90:4650–4658. [DOI] [PubMed] [Google Scholar]
- 16.Use of insulin sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril 2004;82(Suppl 1) :S181–S183. [DOI] [PubMed]
- 17. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 19. Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, Cook D, Dunaif A. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 1998;83:1976–1987. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt G, Weaver B, Cronin L, Dooley JA, Azziz R. Health-related quality of life in women with polycystic ovary syndrome, a self-administered questionnaire, was validated. J Clin Epidemiol 2004;57:1279–1287. [DOI] [PubMed] [Google Scholar]
- 21. Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ, Mulgrew NC, Shepherd KE, Wilkinson VH, Singh M, Balen A, Lashen H, Ledger WL. The polycystic ovary syndrome health-related quality of life questionnaire (PCOSQ): a validation. Hum Reprod 2004;19:371–377. [DOI] [PubMed] [Google Scholar]
- 22. Marinos A, Celedonio JE, Ramirez CE, Gottlieb J, Gamboa A, Hui N, Yu C, Stein CM, Biaggioni I, Shibao CA. Time-course analysis of flow mediated dilation` for the evaluation of endothelial function after a high-fat meal in African Americans. J Am Heart Assoc 2015;4:e002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 2008;51:203–210. [DOI] [PubMed] [Google Scholar]
- 24. Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002;39:460–464. [DOI] [PubMed] [Google Scholar]
- 25. Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med 2013;59:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes 2010;1:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 28. Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem 2008;381:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Nass R, Thorner MO, Evans WS. AutoDecon: a robust numerical method for the quantification of pulsatile events. Methods Enzymol 2009;454:367–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veldhuis JD, Keenan DM. Model-based evaluation of growth hormone secretion. Methods Enzymol 2012;514:231–248. [DOI] [PubMed] [Google Scholar]
- 31. Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab 2011;96:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones B, Kenward M. Design and Analysis of Crossover Trials. 2nd ed. Boca Raton, FL: CRC Press 2003; 24–25. [Google Scholar]
- 33. Dupont WD, Plummer WD, Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. [DOI] [PubMed] [Google Scholar]
- 34. Vrbikova J, Hill M, Bendlova B, Grimmichova T, Dvorakova K, Vondra K, Pacini G. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol. 2008;159:121–127. [DOI] [PubMed] [Google Scholar]
- 35. Sam S, Vellanki P, Yalamanchi SK, Bergman RN, Dunaif A. Exaggerated glucagon responses to hypoglycemia in women with polycystic ovary syndrome. Metabolism 2017;71:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferjan S, Janez A, Jensterle M. Dpp4 Inhibitor sitagliptin as a potential treatment option in metformin-intolerant obese women with polycystic ovary syndrome: a pilot randomized study. Endocr Pract 2018;24:69–77. [DOI] [PubMed] [Google Scholar]
- 37. Grottoli S, Procopio M, Maccario M, Zini M, Oleandri SE, Tassone F, Valcavi R, Ghigo E. In obesity, glucose load loses its early inhibitory, but maintains its late stimulatory, effect on somatotrope secretion. J Clin Endocrinol Metab 1997;82:2261–2265. [DOI] [PubMed] [Google Scholar]
- 38. Iranmanesh A, Lawson D, Veldhuis JD. Distinct metabolic surrogates predict basal and rebound GH secretion after glucose ingestion in men. J Clin Endocrinol Metab 2012;97:2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skov J, Frystyk J, Christiansen JS. GLP-1 infusion reduces IGFBP-1 serum level in humans. Growth Horm IGF Res 2014;24:67–70. [DOI] [PubMed] [Google Scholar]
- 40. Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991;72:51–59. [DOI] [PubMed] [Google Scholar]
- 41. Makimura H, Feldpausch MN, Rope AM, Hemphill LC, Torriani M, Lee H, Grinspoon SK. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J Clin Endocrinol Metab 2012;97:4769–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol 1997;272:E1108–E1116. [DOI] [PubMed] [Google Scholar]
- 43. Dichtel LE, Bjerre M, Schorr M, Bredella MA, Gerweck AV, Russell BM, Frystyk J, Miller KK. The effect of growth hormone on bioactive IGF in overweight/obese women. Growth Horm IGF Res 2018;40:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fisker S, Vahl N, Jorgensen JO, Christiansen JS, Orskov H. Abdominal fat determines growth hormone-binding protein levels in healthy nonobese adults. J Clin Endocrinol Metab 1997;82:123–128. [DOI] [PubMed] [Google Scholar]
- 45. Devin JK, Pretorius M, Nian H, Yu C, Billings FTt, Brown NJ. Substance P increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. Hypertension 2014;63:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, Yuan H, Xiao X, Luo S, Shan Z, Deng H, Tan Y, Xu F, Xu W, Zeng L, Kang Z, Weng J. Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology 2019;69:2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferjan S, Janez A, Jensterle M. Dipeptidyl peptidase-4 inhibitor sitagliptin prevented weight regain in obese women with polycystic ovary syndrome previously treated with liraglutide: a pilot randomized study. Metab Syndr Relat Disord 2017;15:515–520. [DOI] [PubMed] [Google Scholar]
- 48. Thum T, Fleissner F, Klink I, Tsikas D, Jakob M, Bauersachs J, Stichtenoth DO. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab 2007;92:4172–4179. [DOI] [PubMed] [Google Scholar]
- 49. Tivesten A, Barlind A, Caidahl K, Klintland N, Cittadini A, Ohlsson C, Isgaard J. Growth hormone-induced blood pressure decrease is associated with increased mRNA levels of the vascular smooth muscle KATP channel. J Endocrinol 2004;183:195–202. [DOI] [PubMed] [Google Scholar]
- 50. Hubers SA, Wilson JR, Yu C, Nian H, Grouzmann E, Eugster P, Shibao CA, Billings FTt, Jafarian Kerman S, Brown NJ. DPP (Dipeptidyl Peptidase)-4 inhibition potentiates the vasoconstrictor response to NPY (Neuropeptide Y) in humans during renin-angiotensin-aldosterone system inhibition. Hypertension 72:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devin JK, Pretorius M, Nian H, Yu C, Billings FTt, Brown NJ. Dipeptidyl-peptidase 4 inhibition and the vascular effects of glucagon-like peptide-1 and brain natriuretic peptide in the human forearm. J Am Heart Assoc 2014;3:e001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tani S, Takahashi A, Nagao K, Hirayama A. Effect of dipeptidyl peptidase-4 inhibitor, vildagliptin on plasminogen activator inhibitor-1 in patients with diabetes mellitus. Am J Cardiol 2015;115:454–460. [DOI] [PubMed] [Google Scholar]
- 53. Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, Dandona P. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 2012;97:3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilson JR, Shuey MM, Brown NJ, Devin JK. Hypertension and type 2 diabetes are associated with decreased inhibition of dipeptidyl peptidase-4 by sitagliptin. J Endocr Soc 2017;1:1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.