Abstract
Context
Steroids play an important role in fetal development and parturition. Gestational exposures to endocrine-disrupting chemicals (EDCs) affect steroidal milieu and pregnancy outcomes, raising the possibility of steroids serving as biomarkers. Most studies have not addressed the impact of EDC mixtures, which are reflective of real life scenarios.
Objective
Assess the association of maternal and neonatal steroids with pregnancy outcomes and early pregnancy EDC levels.
Design
Prospective analysis of mother-infant dyads.
Setting
University hospital.
Participants
121 mother-infant dyads.
Main Outcome Measures
The associations of maternal and neonatal steroidal hormones from 121 dyads with pregnancy outcomes, the associations of first trimester EDCs individually and as mixtures with maternal and neonatal steroids in a subset of 56 dyads and the influence of body mass index (BMI), age, and offspring sex in modulating the EDC associations with steroids were determined.
Results
Steroid-specific positive or negative associations with pregnancy measures were evident; many maternal first trimester EDCs were negatively associated with estrogens and positively with androgen/estrogen ratios; EDC-steroid associations were influenced by maternal age, pre-pregnancy BMI, and fetal sex; and EDCs individually and as mixtures showed direct and inverse fetal sex-dependent associations with maternal and neonatal steroids.
Conclusions
This proof-of-concept study indicates association of steroids with pregnancy outcomes depending on maternal age, prepregnancy BMI, and fetal sex, with the effects of EDCs differing when considered individually or as mixtures. These findings suggest that steroidal hormonal measures have potential to serve as biomarkers of impact of EDC exposures and pregnancy outcome.
Keywords: endocrine disrupting chemical, steroids, testosterone, estradiol, pregnancy, developmental programming
Although the maternal and fetal endocrine systems are compartmentalized, pregnancy and intrauterine fetal growth and differentiation are largely dependent on the interaction of hormonal factors in the maternal and fetal compartments (1). Among hormonal factors, steroids progesterone (P), estrogens, androgens, and glucocorticoids are involved in pregnancy from implantation to parturition as well as fetal differentiation and growth. These steroids are required during critical developmental windows in specific compartments to maintain pregnancy and to direct the fetal developmental trajectory. Progesterone, for example, produced initially from the ovary and later from the placenta, is required throughout the pregnancy. As the main steroidal hormone for the maintenance of pregnancy, P influences the maternal immune system for tolerance toward fetal allograft (2). Other steroidal hormones include estrogens, which are formed mainly in the placenta with their production rate increasing throughout the course of pregnancy (3). They are involved in promoting placental angiogenesis and the process of labor (4,5). In addition to serving as a substrate for estrogen biosynthesis, the androgenic steroid testosterone (T) drives the formation of the male external genitalia (6). Glucocorticoids are required near term for lung maturation and parturition in both sexes (7). Due to the importance of their physiological roles, the steroid metabolome in maternal and fetal (ie, umbilical cord blood) compartments is a rich source of information to help predict pregnancy and postnatal consequences (8,9). As such, these steroids have the potential to serve as biomarkers for forecasting the development of adult onset diseases (10,11). This concept has been validated in animal studies. For example, studies in gestational steroid-treated animal models leading to increases in maternal and fetal steroid levels culminate in poor birth outcomes and development of cardiometabolic disorders (12-16) in their offspring. These studies lend support for the use of maternal and cord blood steroid analysis as a biomarker for developmental disorders in humans.
Inappropriate steroidal exposure during pregnancy can result from disease state, under/overnutrition, stress, and exposure to environmental factors (17). Evidence points to these conditions being associated with steroidal changes in maternal and/or fetal milieu manifested as an increase in glucocorticoids (18,19) and androgens (20-22), altered fetal developmental trajectory (23-26), and development of noncommunicable adult pathologies (27-29). Of these developmental insults, exposure to environmental chemicals with hormonal capabilities called endocrine-disrupting chemicals (EDC) (30, 31) are gaining increasing importance. These chemicals are ubiquitously present in the environment, and according to the US Environmental Protection Agency, humans are exposed to nearly 82 000 chemicals (32). The World Health Organization estimates that of the chemicals used among everyday life, about 800 are classified as EDCs (30). Of concern, many of these EDCs are detected in pregnant women by the National Health and Nutrition Examination Survey in the United States and Maternal-Infant Research on Environment Chemicals (MIREC) in Canada (33,34). Our group, using the Michigan Mother Infant Pair (MMIP) cohort, has similarly detected the presence of multiple EDCs in pregnant women (35). The presence of these EDCs is linked to poor birth outcomes (36-42) such as negative associations between birth weight and the presence of bisphenol (BP) A (BPA) (37), BPS (35), lead (43), polycyclic aromatic hydrocarbons (44) and dibutyl phthalate (45). Animal studies with EDC exposures during gestation have also shown that in addition to poor birth outcomes, the offspring born develop adult-onset chronic disorders (46).
The mechanism through which gestational EDC exposure brings about these consequences may involve changes in maternal and/or fetal steroids, which are powerful programming agents. Emergent data suggest that EDC exposure can lead to changes in steroid patterns within the maternal and fetal compartments (47-55). Phthalate metabolites, for example, are associated with increases or decreases in steroid hormones androstenedione (A4), T, dehydroepiandrosterone (DHEA), estrone (E1), estradiol (E2), and estriol (E3) depending on which phthalate metabolite is measured, the stage of pregnancy, whether assessment is in the maternal or fetal compartment, and the fetal sex (51-55). Likewise, among phenolic EDCs, tetrachlorodibenzo-p-dioxin/polychlorinated dibenzofurans exposures affect estrogen metabolism with increased concentrations of polychlorinated dibenzofurans associated with increased maternal levels of 4-hydroxy-E2 (47). In contrast, cord blood parabens are inversely linked with cord blood T levels (48). Similarly, the maternal presence of various EDCs is associated with steroidal changes in neonatal cord blood as evident for organochlorine pesticides (49), perfluorooctane sulfonate, and perfluorooctanoate (50) in an offspring sex-dependent manner. Overall, these studies indicate that maternal/fetal exposures to EDCs are associated with changes in maternal and neonatal cord blood steroids, with most studies assessing these changes during late gestation or at term.
Considering the importance of steroids in fetal growth, differentiation, and developmental programming and that EDC exposure has the potential to alter maternal/neonatal steroid profile, the first goal of this study is to relate the steroid levels in maternal first trimester and at term and neonatal cord blood with fetal and birth outcomes. The second goal of this study is to determine the impact of maternal first trimester pregnancy exposures to EDCs (specifically phthalates, phenols, parabens, and metals) on maternal and fetal steroidal milieu and how this relationship is influenced by common confounding factors: maternal age, body mass index (BMI), and fetal sex. Since humans are exposed to not just one but a multitude of EDCs, the third goal, was to determine the impact of EDC exposures not only individually but also as mixtures, after controlling for potential confounding variables.
Methods
Subject recruitment
All study procedures were performed under the approval of the University of Michigan (UM) Medical School Institutional Review Board, and written informed consent was obtained from all the participants. The subjects studied were part of the MMIP birth cohort study (2010-present) and were recruited as described previously (35). The MMIP is an ongoing birth cohort recruited out of the UM Von Voigtlander Women’s Hospital during their first prenatal visit between 2010 to 2018. Pregnant women who met the following eligibility criteria were recruited: between 8 and 14 weeks of gestation, age between 18 and 42 years old, had a spontaneously conceived singleton pregnancy, and intended to deliver at the UM Von Voigtlander Women’s Hospital. Of these women, 121 MMIP participants were selected for steroid measures and a subset (n = 56) of these were selected for assessment of the impact of EDCs on steroid metabolome. The 121 subjects were selected based on mother-infant pairs having complete demographic, survey and health information at their initial study visit survey data, and availability of all biospecimens at all time points from mother and child. The subset of 56 mothers recruited between 2012 and 2015 were selected for extensive exposure assessment of EDCs and metals. The inclusion criteria for this subset of 56 were availability of complete survey data and all biospecimens from mother and child. Due to the low prevalence of racial or ethnic minorities and maternal smoking and to reduce the confounding factors in a relatively small sample size, only Caucasian, non-Hispanic, currently nonsmoking mothers with full-term newborns (≥37 weeks gestation) were included.
Sample collection
Samples from the mother include spot urine and venous blood and were collected during the first prenatal appointment between 8 and 14 weeks of gestation (first trimester) and upon arrival to the hospital for delivery (term). Cord blood was collected following delivery and placental expulsion. Both maternal and cord blood samples were collected in vials containing ethylenediamine tetra-acetate and transported to the laboratory, where these blood samples were centrifuged, plasma separated, and aliquoted into glass vials. Urine samples collected in polypropylene containers were also aliquoted into glass vials. All samples were stored at −80oC until further analysis.
EDCs assessment
Exposure measures were assessed in the subset of 56 subjects in maternal first trimester urine samples, and this has been reported previously (35). A total of 41 exposure measures encompassing a range of commonly encountered environmental EDCs, metals, and metalloids were quantified from each individual as described previously (35,45). Care was taken to account for urine dilution by adjusting for urine specific gravity determined using a digital handheld device (ATAGO Company, Ltd., Tokyo, Japan).
The EDCs measured included phthalates, phenols, metals, and metalloids. The phthalates and phenols were measured via isotope dilution liquid chromatography-tandem mass spectrometry as described previously (35,45). The phthalates metabolites measured included mono (2-ethyl-5-carboxylpentyl) phthalate, mono (2-ethyl-5-hydroxyhexyl) phthalate, mono (2-ethylhexyl) phthalate (MEHP), mono (2-ethyl-5-oxohexyl) phthalate, mono-isobutyl phthalate (MIBP), mono n-butyl phthalate (MnBP), mono-benzyl phthalate (MBzP), mono-carboxy isononyl phthalate (MCINP), mono (3-carboxypropyl) phthalate, mono (6-COOH-2-methylheptyl) phthalate (MCOMHP), monoethyl phthalate, and mono-isononyl phthalate. Phenols measured included butyl (BuPB), ethyl (EtPB), methyl (MePB), and propyl (PrPB) parabens; bisphenols (BPA, BPF, BPS); 2,4- and 2,5-dichlorophenol (DCP24 and DCP25, respectively); benzophenone-3 (BP3); triclocarban; and triclosan (TCS). The metals and metalloids quantified using inductively coupled plasma mass spectrometry included arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), selenium (Se), tin (Sn), thallium (Tl), uranium (U), tungsten (W), and zinc (Zn). Of note, not all metals are considered EDCs, but we still examined associations between steroids and all detectable metals from the panel measured.
Steroid measurement
Steroids were measured in 121 dyads, which included the subset from which EDCs were assessed. Measurement of steroids cortisol, cortisone, E1, E2, E3, T, A4, and P were carried out in maternal first trimester, maternal term, and neonatal cord blood plasma samples using liquid chromatography mass spectrometry as described previously (56). Unlabeled and deuterium-labeled steroids were obtained from Sigma-Aldrich, Cerilliant, C/D/N isotopes, and Steraloids. Briefly, steroids from the plasma samples were extracted by liquid-liquid extraction and reconstituted samples (10 μL) injected via autosampler and resolved with a pair of Agilent 1260/1290 binary pump high performance liquid chromatography via 2D liquid chromatography, first on a 10 mm × 3 mm, 5 μm particle size Hypersil Gold C4 loading column (Thermo Scientific, Waltham, MA, USA) followed by a Kinetex 50 mm × 2.1 mm, 2.6 μm particle size biphenyl resolving column (Phenomenex, Torrance, CA, USA). The mobile phases consisted of 0.2 mmol/L aqueous ammonium fluoride (A) and methanol with 0.2 mmol/L ammonium fluoride (B). Steroids were eluted using gradient specifications as described previously (56) and directed into the source of an Agilent 6495 triple quadrupole mass spectrometer using electrospray ionization in positive ion mode for cortisol, cortisone, T, A4, and P and negative ionization mode for E1, E2, and E3. Quantitation was performed using multiple reaction monitoring data, comparing the monitored ion currents with weighted (1/x) 12-point linear external calibration curves (r2 was >0.995) and corrected for specimen dilution and recovery of internal standards using MassHunter software (Agilent, Santa Clara, CA, USA). For samples with P concentrations above the upper limit of quantitation, a 20 μL plasma sample was mixed with 0.18 mL of methanol containing internal standards, centrifuged, and subjected (2 μL injection) to mass spectrometry as previously described. Intra-assay and inter-assay coefficients of variation were determined by using a control pooled serum samples and were <12% for all steroids. The lower limit of detection (LOD) for each steroid was defined by the minimum concentration achieving an extrapolated signal-to-noise ratio of 3 and were as follows: cortisol = 23 nM, cortisone = 3 nM, E1 = 5 nM, E2 = 14 nM, E3 = 3 nM, A4 1 nM, T = 1 nM, and P = 3.5 nM.
Fetal and birth outcomes
Fetal and birth outcomes and potential confounding variables from each of the recruited subjects were collected from the medical record. Hadlock percentiles for fetal biometric parameters (biparietal diameter [BPD], head circumference [HC], abdominal circumference [AC], femur length [FL], and estimated fetal weights [EFW]) (57) were from second trimester ultrasonographic fetal anatomical survey. At birth, physician-measured infant birth weight, HC, and length were adjusted for gestational age and infant sex using Fenton growth curves for birth weight (58) and growth curves developed by the Canadian Institute of Health Research for HC and length (59). Estimated gestational age at delivery was determined by last menstrual period or ultrasound dating, at the discretion of the obstetric provider. Potential confounding variables considered for analysis included maternal prepregnancy BMI, mode of delivery (vaginal vs Caesarian), infant sex, maternal age, and history of smoking.
Statistical analysis
Multiple linear regression was conducted to assess the association between steroidal exposures and fetal and birth outcomes among the full cohort of 121 mother-infant dyads. The steroid variables were transformed using inverse normal transformation for normality, and values below the LOD were set at their respective LOD thresholds. Standardized fetal outcomes using Hadlock percentile and Fenton Z-scores for the birth outcomes of birthweight, birth length, and HC were used for analysis. Confounding variables considered in the analysis were history of smoking, mother’s prepregnancy BMI, mode of birth (vaginal or Cesarean), maternal age, and infant sex. The analyses were conducted both for the full cohort, and on sex-stratification to assess the sex-specific effects of the steroidal exposure on the fetal/birth outcomes. To account for the multiple comparisons, the p-values for testing the associations of each steroid exposure with for the 10 different fetal/birth outcomes were corrected using Benjamin-Hochberg (BH) false discovery rate (FDR) procedure. A BH FDR cutoff of 0.1 was considered the threshold for statistical significance. Separate analyses were conducted at each of the steroidal measurement time points: maternal first trimester, maternal term, and neonatal cord blood.
For a subset of the mothers (n = 56), first trimester exposures to environmental EDCs were estimated via urine analysis (35), and the associations between these EDCs and steroidal measures were assessed. Of the 41 EDCs measured, 5 were below the LOD and were not included in further analysis. First, the marginal relationship with no adjustment for covariates was analyzed using rank-based Spearman correlation. For this pairwise analysis, the EDCs were first adjusted for the subject’s urine specific gravity and natural-log transformed. This analysis was conducted cross-sectionally at each of the 3 steroid measurement time points: maternal first trimester, maternal term, and neonatal cord blood. In consideration of possible confounding variables, we explored the correlations between the steroid values and potential confounding variables using Spearman correlation. To account for multiple potentially confounding variables, associations were also analyzed using multiple linear regression. Additionally, we explored how the univariate linear relationship between the steroid levels and EDCs differed by confounding variables by fitting stratified linear regressions. To further assess whether these variables modulated the effect of EDCs on steroid levels, we conducted multiple linear regressions with interaction variables between exposure and potential modulating variable (eg, maternal age, and maternal BMI). These interaction terms aimed to parse out whether the maternal variables changed the effect of the exposures on steroidal milieu. Prior to the multiple regression analysis, the steroid variables were transformed using inverse normal transformation to ensure the outcome in the regression analysis to be normally distributed, and the EDCs were natural log transformed. Confounders considered in the analysis included urine specific gravity, history of smoking, maternal prepregnancy BMI, mode of delivery (vaginal or Cesarean), maternal age, and infant sex. Analyses were conducted for the full subset, as well as stratified by infant sex (Table 1), to assess both composite and sex-specific effects. For each steroid analysis, the P values for the correlation and linear regression results were adjusted for the multiple association testing with 36 of the 41 individual EDCs that were above detection level using the BH FDR procedure. A corrected BH FDR P value of 0.1 or lower was considered statistically significant. An exploratory analysis was completed on the EDCs of BPA, its substitute chemicals (BPF and BPS), and the structurally similar benzophenone (BP-3). In this analysis, we considered the beta values and unadjusted P values of the linear regression models to explore the similarities/differences in effects among the bisphenol and benzophenone exposures.
Table 1.
Demographics of the mother-infant dyad (n = 121 unless specified)
| N (%) or Median (Range Min-Max) | ||||
|---|---|---|---|---|
| All | Male | Female | ||
| Mother | ||||
| Infant sex | — | 61 (50.4) | 60 (49.6) | |
| Age (years) | 32 (25-42) | 32 (26-42) | 32 (25-41) | |
| Race | White | 105 (86.8) | 52 (85.2) | 53 (88.3) |
| Non-whites | 16 (13.2) | 9 (14.8) | 7 (11.7) | |
| Pre-pregnancy BMI (n = 118) | 23.9 (19.2-49.0) | 23.7 (19.2-40.2) | 23.9 (19.8-49.0) | |
| Pre-pregnancy weight (kg) | 65.0 (45.0-145.0) | 65.0 (45.0-104.3) | 65.0 (50.0-145.0) | |
| Weight near term (kg) | 79.0 (57.0-161.0) | 78.4 (57.0-114.0) | 80.4 (63.0-161.0) | |
| Gestational weight gain (kg) | 12.7 (0.9-26.0) | 13.0 (5.7-26.0) | 12.3 (0.9-25.0) | |
| Parity | 0 | 38 (31.4) | 19 (31.1) | 19 (31.7) |
| 1 | 51 (42.2) | 25 (41.0) | 26 (43.3) | |
| 2 | 23 (19.0) | 12 (19.7) | 11 (18.3) | |
| 3 | 7 (5.8) | 4 (6.6) | 3 (5.0) | |
| 4 | 2 (1.65) | 1 (1.6) | 1 (1.7) | |
| Smoking status (n = 109) | Never | 91 (83.5) | 47 (83.9) | 44 (83.0) |
| Past smoker | 12 (16.5) | 9 (16.1) | 9 (17.0) | |
| Education (n = 90) | High school | 6 (6.7) | 3 (6.8) | 3 (6.8) |
| Some college | 9 (10.0) | 3 (6.8) | 6 (13.6) | |
| ≥Bachelor’s | 75 (83.3) | 38 (86.4) | 37 (84.1) | |
| Marital status | Married | 106 (87.60) | 53 (88.3) | 52 (86.7) |
| Single | 15 (12.40) | 7 (11.7) | 8 (13.3) | |
| Infant | ||||
| Route of delivery | Cesarean | 36 (29.75) | 20 (32.8) | 16 (26.7) |
| Vaginal | 85 (70.25) | 41 (67.2) | 44 (73.3) | |
| Gestational age (days) | 277 (244-292) | 277 (244-292) | 277 (262-291) | |
| Birth weight (g) | 3510 (2270-4685) | 3640 (2520-4685) | 3365 (2270-4295) | |
| Birth weight Fenton z-score | 0.19 (−2.89-2.69) | 0.30 (−2.76-2.69) | 0.12 (−2.89-1.76) | |
| Newborn length (cm; n = 106) | 51.0 (21.5-56.3) | 51.45 (31.4-56.3) | 50.8 (21.5-56.0) | |
| Newborn length Fenton z-score | 0.28 (−13.87-2.68) | 0.39 (−8.14-2.68) | 0.21 (−13.87-2.60) | |
| Newborn HC (cm; n = 112) | 35.35 (32.50-54.60) | 35.50 (32.50-54.60) | 35.00 (32.50-53.20) | |
| Newborn HC Fenton z-score | 0.36 (−1.80-13.54) | 0.36 (−1.24-13.51) | 0.33 (−1.80-13.54) | |
| Fetal anthropometry | AC (mm) | 137.3 (84.0-241.8) | 139.0 (84.0-241.8) | 136.0 (105.0-172) |
| AC Hadlock percentile | 60.5 (6.0-96.0) | 66.0 (17.0-96.0) | 54.5 (6.0-92.0) | |
| BPD (mm) | 43.5 (28-70.4) | 44.0 (28.0-70.4) | 42.8 (36-54.4) | |
| BPD Hadlock percentile | 58.0 (6.0-88.0) | 59.0 (34.0-88.0) | 58.0 (6.0-86.0) | |
| FL (mm) | 29 (15.0-51.4) | 29.3 (21.0-39.0) | 28.6 (21.0-39.0) | |
| FL Hadlock percentile | 39.0 (3.0-97.0) | 46.0 (3.0-77.0) | 36.0 (8.0-97.0) | |
| HC (mm) | 161.7 (104.0-265.1) | 163.0 (104.0-265.1) | 159.0 (129.0-204.1) | |
| HC Hadlock percentile | 48.0 (4.0-95.0) | 55.0 (7.0-95.0) | 41.0 (4.0-86.0) | |
| EFW (g) | 298.0 (121.0-1191) | 299.5 (121.0-1191) | 295.0 (176.0-483.0) | |
| EFW Hadlock percentile | 78.0 (10.0-97.0) | 86.5 (40.0-97.0) | 72.0 (10.0-97.0) |
To assess the cumulative effect of EDCs on steroid measures, rather than merely individual effects, we also conducted principal component (PC) regression. For this analysis, principal component analysis was conducted on the 41 EDC variables, resulting in 11 orthogonal PCs, which collectively explained 80% of data variance. The details of these PCs were previously published (60,61). The 11 PCs each represented an orthogonal linear combination of the EDC data and were simultaneously regressed on the steroid outcomes to analyze the association between these 11 EDCs mixtures and the steroid levels. As before, the steroid variables were transformed using inverse normal transformation prior to analysis. The same confounders were included in the PC regression model as in the linear regression models. To understand both the composite and sex-specific associations, analyses were again conducted on the 56 mothers together, as well as on stratified subsets based on infant sex. To account for the multiple outcome testing, the P values for each PC variable were adjusted using the BH procedure. All statistical analyses were carried out using R Software, version 3.5.
Results
Subjects, first trimester EDC exposures, and maternal and neonatal steroidal measures
The demographics of the 121 mothers in the mother-infant dyads including the previously reported subset of 56 subjects from the MMIP cohort (35) are shown in Table 1. The cohort was mostly homogenous and comprised of non-Hispanic Caucasian women (86.8%), with a median age of 32 years (range 25-42). Prior to pregnancy, the women were typically within the normal BMI range (median = 23.9 kg/m2), primarily married (87.6%), and never smokers (83.5%). By design, all births were full term (median gestational age = 277 days) and of normal birthweight (median = 3510 g). Most births were vaginal (70.25%). The sex-distribution of the infants born in this cohort was equivalent (50.4% males to 49.6% females).
The steroids P, E1, E2, A4, cortisol, and cortisone were detectable in all samples assayed, while T and E3 were detectable in about 80% of the samples analyzed. The median levels in maternal first trimester and term plasma and neonatal cord blood are shown in Table 2. Consistent with previous studies (62-65), the steroids cortisol, cortisone, E1, E2, E3, and P levels were substantially higher at term than during the maternal first trimester, while androgens T and A4 showed modest increases. As anticipated, the neonatal cord blood levels of cortisone, E1, E3, and P were also higher than maternal term plasma, whereas the neonatal cord blood levels of cortisol, E2, T, and A4 were lower compared to maternal term plasma. These results validate our steroid measurements as a large, robust, and reliable data set to ask the questions posed in this study.
Table 2.
Median (Interquartile Range) of steroid levels in maternal first trimester and term plasma and neonatal cord blood (nmol/L, n = 121)
| Steroids | Maternal first trimester | Maternal term | Neonatal cord blood |
|---|---|---|---|
| Cortisol | 256.9 (45.6-210.6) | 798.5 (55.3-535.8) | 102.4 (58.3-145.9) |
| Cortisone | 57.0 (47.0-68.6) | 149.2 (115.6-183.6) | 259.5 (188.8-326.5) |
| Cortisol/cortisone ratio | 4.82 (4.11-5.43) | 5.37 (4.43-7.07) | 0.37 (0.26-0.58) |
| Estriol | 0.59 (0.21-3.00) | 17.61 (9.96-37.90) | 118.53 (67.65-492.66) |
| Estrone | 0.04 (0.03-0.07) | 0.28 (0.17-0.39) | 1.16 (0.79-1.68) |
| Estradiol | 6.22 (4.00-8.84) | 66.33 (49.80-85.22) | 28.22 (14.72-48.35) |
| Testosterone | 1.85 (1.20-2.51) | 2.12 (1.50-3.31) | 0.03 (0.01-0.53) |
| Testosterone/estradiol ratio | 0.32 (0.19-0.47) | 0.03 (0.03-0.06) | 0.01 (0.01-0.02) |
| Androstenedione | 4.13 (3.01-5.99) | 4.56 (3.48-7.06) | 2.73 (1.65-3.97) |
| Androstenedione/estrone ratio | 1.1 (0.68-1.76) | 0.2 (0.11-0.35) | 0.02 (0.02-0.04) |
| Progesterone | 90.40 (75.05-118.46) | 438.15 (312.10-541.90) | 2820.78 (2020.62-3966.97) |
The concentrations of EDCs metals, phenols, parabens, and metabolites of phthalates in first trimester maternal urine from a subset of 56 women have been previously reported (35) and these subjects had on average 30 detectable EDCs including averages of 12.8 metals, 10.5 phthalate metabolites, and 7.7 phenols metabolites detectable in their first trimester urine samples.
Association between steroid measures and offspring sex-specific fetal and birth outcomes
An initial spearman-correlation analysis between steroid measures and offspring fetal and birth outcomes (see Supplemental Figure 1 in (66)) demonstrated a pattern of association in certain outcomes. In particular, significant positive correlations were evident between gestational age and cortisol and cortisol/cortisone ratio both at maternal term and neonatal cord blood. These initial results were encouraging and suggested that the more complex association analyses, which were subsequently completed, could further explore the relationships between these variables of interest.
The multiple linear regression analysis of sex-dependent association between the maternal/neonatal steroidal measures and birth outcomes are shown in Supplemental Table 1 in (66) and the robust associations as determined by beta (>2.0) and BH (<0.1) value are summarized in Table 3. First trimester maternal plasma T levels among mothers with female fetus were positively associated with newborn HC (Table 3). Likewise, maternal E2 levels at term were positively associated with neonatal HC among mothers with female neonates only (Table 3). Neonatal cord blood E3 levels were also positively associated with infant length, although with beta values just below the robust cutoff (see Supplemental Table 1 in (66)). These data point to the potential for influence of E2 and E3 on the birth measures, and this influence may extend to early pregnancy as E2 can also be produced by placental aromatization of T. Surprisingly, cortisol and cortisol-cortisone ratio, both at maternal term and neonatal cord blood, were positively associated with gestational age among all mothers, in contrast to the potential role for glucocorticoids in promotion of parturition (67). Positive associations of neonatal cord blood plasma levels of A4 with fetal HC, BPD, and EFW and T/E2 ratio with fetal BPD among female infants only and cortisol levels with EFW among female infants were evident (Table 3), consistent with an influence of androgens and cortisol on fetal growth, which is more predominant in the female fetus.
Table 3.
Sex specific covariate-adjusted associations from linear regression model between maternal (first trimester or term) and neonatal steroids and gestational, fetal and birth outcomes (n = 121)
| Steroid | Offspring Sex | Fetal and Birth Outcomes | |||
|---|---|---|---|---|---|
| All | Males | Females | Positive Association | Negative Association | |
| First trimester steroid levels | |||||
| Testosterone | X | Newborn HC | |||
| Term steroid levels | |||||
| Cortisol | X | Gestational age | |||
| Cortisol/cortisone ratio | |||||
| Estradiol | X | Newborn HC | |||
| Neonatal steroid levels | |||||
| Androstenedione | X | Fetal HC | |||
| Fetal BPD | |||||
| EFW | |||||
| Cortisol | X | EFW | |||
| X | X | Gestational age | |||
| Cortisol/cortisone ratio | X | X | Gestational age | ||
| Estriol | X | Newborn HC | |||
| Testosterone/estradiol ratio | X | Fetal BPD |
Univariate associations between maternal and neonatal steroid levels and first trimester maternal EDC exposures
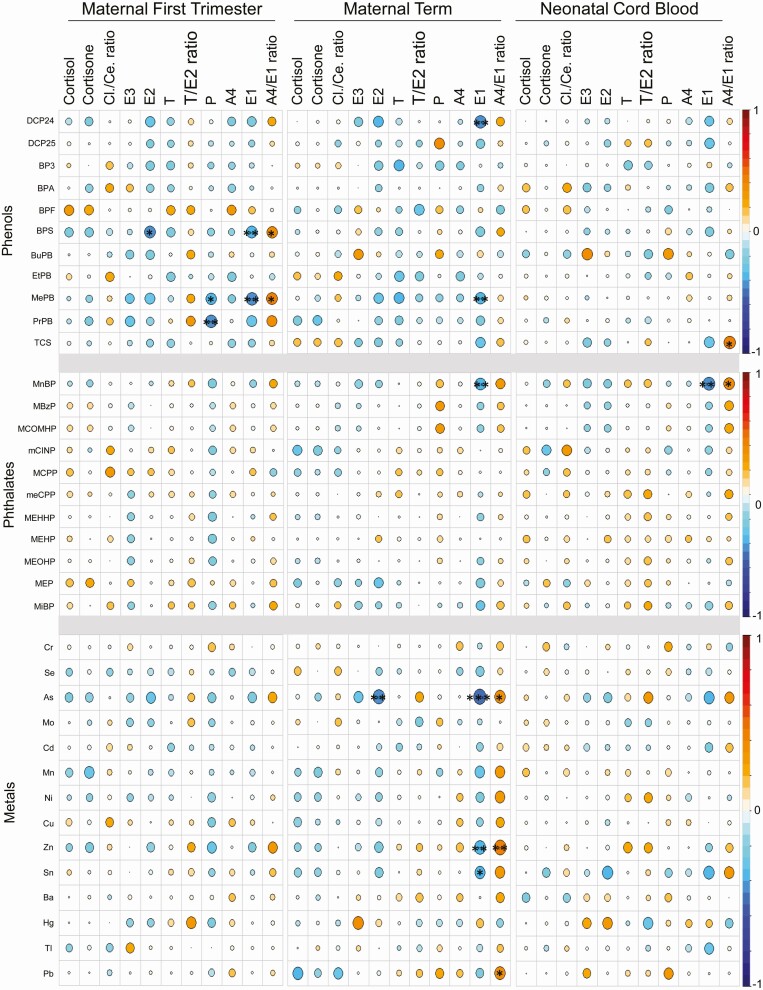
Figure 1 illustrates the Spearman correlations of first trimester EDC analytes measured in maternal urine with maternal first trimester, maternal term, and fetal cord blood steroids. Phenols BPS and MePB demonstrated significant negative associations with maternal first trimester E1, while phenol PrPB was negatively associated with P levels (Fig. 1). The maternal term E1 was negatively associated with metals Zn and As, phenols DCP24 and MePB, and phthalate MnBP while E2 was negatively associated only with As (Fig. 1). In contrast, the metal Zn was positively associated with maternal term plasma A4/E1 ratio. In the neonatal cord blood, E1 levels were negatively associated with phthalate MnBP (Fig. 1). These associations of EDCs primarily with lower estrogen and higher estrogen precursor/estrogen ratios suggest EDC-mediated induction of placental dysfunction.
Figure 1.
Cross-sectional Spearman correlations of early pregnancy EDC exposures with plasma steroids in maternal first trimester and term and in neonatal cord blood. The pairwise correlation between steroid with phenols, phthalates, and metals are depicted using the color spectrum, with the orange color indicating positive correlation and the blue color indicating a negative correlation. The size of the circle also reflects the size of the correlation coefficient. Significance by BH FDR corrected P values are noted as follows: ***P < 0.01, **P < 0.05, and *P < 0.10. Abbreviations: A4, androstenedione; Ce, cortisone; Cl, cortisol; E1, estrone; E2, estradiol; E3, estriol; P, progesterone; T, testosterone.
Influence of potential confounding variables on maternal and neonatal steroid levels
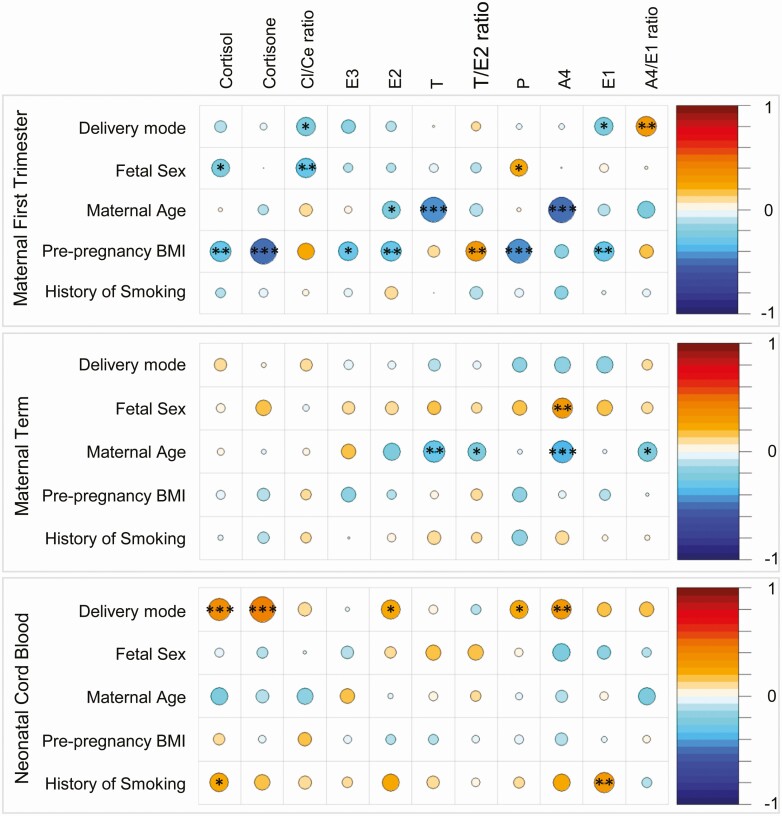
Variables such as maternal age, BMI, and fetal sex, however, might be confounding these simple correlations, and few studies of steroids and EDCs in pregnancy have accounted for these factors. We analyzed the correlation between the steroids and potential confounding variables and found several significant associations, particularly with maternal age, maternal prepregnancy BMI, and fetal sex, (Fig. 2). For example, in maternal first trimester plasma, prepregnancy BMI showed significant negative associations with cortisol, cortisone, E3, E2, and P. In the same time point, maternal age had significant negative associations with T and A4. Similar correlations were also found in maternal term plasma, while no significant associations were evident in the neonatal cord plasma steroids for maternal age (Fig. 2).
Figure 2.
Spearman correlations of steroid levels in maternal first trimester and term and neonatal cord blood with confounding factors delivery mode, fetal sex, maternal age, prepregnancy BMI, and history of smoking. The correlation between steroids levels and confounding factors are depicted using the color spectrum, with the orange color indicating positive correlation and the blue color indicating a negative correlation. The size of the circle also reflects the size of the correlation coefficient. Significance are noted as follows: ***P < 0.01, **P < 0.05, and *P < 0.10. Abbreviations: A4, androstenedione; Ce, cortisone; Cl, cortisol; E1, estrone; E2, estradiol; E3, estriol; P, progesterone; T, testosterone.
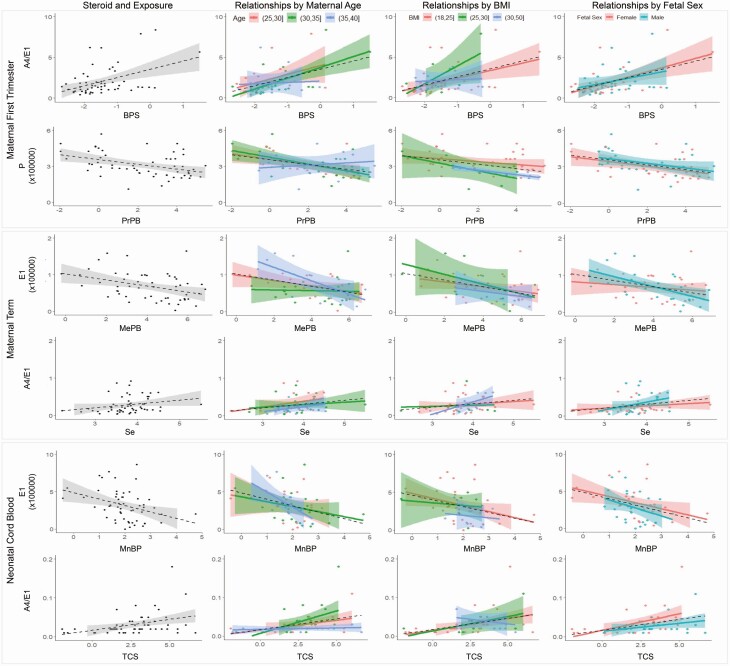
Based on these correlations, we further explored how the confounding variables might impact the relationship between the steroidal outcomes and exposures. In this exploration, we found that maternal age, prepregnancy BMI, and fetal sex in particular impacted the relationship between the steroids and EDCs. The influence of these confounding variables on relationships with EDCs was most evident for steroids A4 and E1. Figure 3 illustrates how these confounding variables alter the relationships of EDCs with steroids from maternal first trimester, maternal term, and neonatal cord blood.
Figure 3.
Impact of confounding variables on the relationship between the steroidal outcomes at maternal first trimester, maternal term, and neonatal cord blood with maternal first trimester urine exposure measures are shown. The first panels on the left for each steroid shown in grey depicts the relationship between the steroids and exposure levels. The subsequent columns depict the relationships by categories of maternal age, prepregnancy BMI, and fetal sex, respectively.
Multivariable regression analyses of EDC exposures with steroids
Subsequently, we performed multiple linear regression analyses to characterize the associations between individual EDC exposures and maternal and neonatal steroid levels after adjusting for confounders (see Supplemental Table 2 in (66) and Table 4). Only neonatal cord blood level of P showed a significant negative association with maternal first trimester urinary phenol BuPB, when sex of the offspring was not considered. When the population was stratified by infant sex, significant sex-specific relationships were found between metal and phenol exposures and maternal first trimester and term, and neonatal cord blood steroid levels (Table 4). These include negative associations of phenols EtPB and TCS with maternal first trimester cortisone and positive association of metal Tl with neonatal cortisol among male infants. In contrast, at term the significant associations were only evident among mothers with female infants with metals Mn and Ba showing positive association with A4/E1 ratio and phenol EtPB showing negative association with T levels. These results are suggestive of sex-specific associations between EDCs and steroidal levels, warranting further larger-scale studies.
Table 4.
Sex specific covariate-adjusted associations from linear regression model between first trimester EDC exposure analytes and maternal (first trimester or term) and neonatal steroids (n = 56)
| EDC | EDC Category | Offspring Sex | Steroids | |||
|---|---|---|---|---|---|---|
| All | Males | Females | Positive Association | Negative Association | ||
| First trimester steroid levels | ||||||
| EtPB | Phenols | X | Cortisone | |||
| TCS | Phenols | X | Cortisone | |||
| Term steroid levels | ||||||
| Mn | Metals | X | Androstenedione / Estrone Ratio | |||
| Ba | Metals | X | Androstenedione / Estrone Ratio | |||
| EtPB | Phenols | X | Testosterone | |||
| Neonatal steroid levels | ||||||
| BuPB | Phenols | X | Progesterone | |||
| Tl | Metals | X | Cortisol |
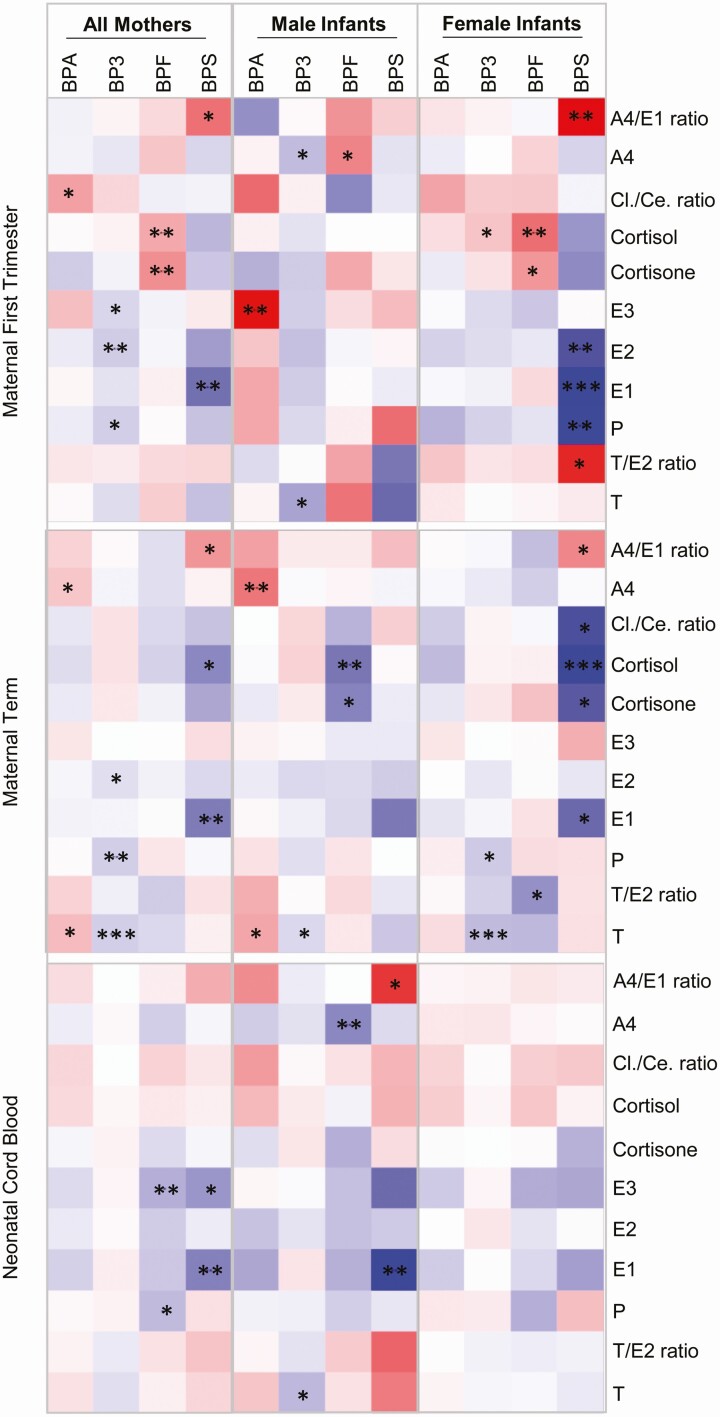
Since concerns about the health effects of BPA have become common knowledge over the past decade, many manufacturers of consumer plastic goods have switched to BPA alternatives such as BPS and BPF. BP-3 is structurally similar to the bisphenols and is a common exposure in many populations. Therefore, to assess the effect of these 4 related toxicants on maternal and neonatal steroids, linear regression analysis without correction for multiple comparisons were carried out, and the results are shown in Figure 4. Among the significant results (P < 0.05), BP-3 showed negative associations with maternal first trimester E2 and BPS with E1 among all mothers. When stratified by fetal sex, BPS was negatively associated with maternal first trimester E1, E2, and P and positively with A4/E1 ratio among mothers with female infants. In this same stratification, BPF was positively associated with maternal first trimester cortisol levels (Fig. 4). Within maternal term plasma, negative associations were evident between BP3 and steroids P and T, as well as between BPS and E1 in all mothers (Fig. 4). When stratified by fetal sex, in male infants BPA was positively associated with A4, while BPF was negatively associated with cortisol. In female infants, negative associations between BPS and cortisol as well as BP-3 and T levels were observed. In neonatal cord blood, only negative associations were evident between BPF and E3 as well as BPS and E1 in all mothers. Among male infant cord blood, associations were evident between BPF and A4 as well as BPS and E1 (Fig. 4).
Figure 4.
Linear regression analysis of association between BPA and substitute chemicals (BPF and BPS), and the structurally similar benzophenone (BP-3) with plasma steroids in maternal first trimester and term, and in neonatal cord blood. The positive (red shade) and negative (blue shade) associations among all mothers and when stratified by infant sex are shown. Statistical significance of unadjusted P values is indicated by ***P < 0.01, **P < 0.05, and *P < 0.10. Abbreviations: A4, androstenedione; Ce, cortisone; Cl, cortisol; E1, estrone; E2, estradiol; E3, estriol; P, progesterone; T, testosterone.
Multivariable regression analyses with interaction effects
To further consider the potential for effect modification due to maternal age, prepregnancy BMI, and fetal sex (as shown in Fig. 3), we included interaction terms between the potential modifiers (maternal age and prepregnancy BMI) in linear regression models, both for the full population and with sex-stratification. Due to the inclusion of subgroup parameters, the resulting larger model may encounter decreased power to parse out the modified effects. Even with this limitation, however we detected clinically and statistically significant results with both prepregnancy BMI and maternal age in a sex-specific manner. Tables 5 and 6 (see Supplemental Tables 3 and 4 in (66)) summarize the clinically and statistically significant results (BH-FDR < 0.1 and absolute value of effect size > 1.0), adjusting for confounding factors (eg, in addition to the interaction terms mother’s age and prepregnancy BMI, history of smoking, mode of birth, and urine specific gravity). In the maternal BMI interaction model for first trimester steroidal levels, we found that in mothers of female infants, those with high BMI (BMI > 25) had a more negative association between E3 and a selection of EDCs (phthalate MEHP, phenols BPA and DCP24, and metal Mo) (Table 5). On the other hand, within mothers of male infants, those with high BMI had a more negative association between T and metals Mn and Sn as well as phenols BPS and mePB.
Table 5.
Sex specific findings of interaction of maternal BMI on the association between maternal first trimester EDC with maternal (first trimester or term) and neonatal steroids (n = 56)
| EDC | EDC Category | Offspring Sex | Steroids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Males | Females | Positive association | Negative association | |||||
| Low BMI | High BMI | Low BMI | High BMI | Low BMI | High BMI | ||||
| First trimester steroid levels | |||||||||
| MEHP | Phthalates | E3 | X | ||||||
| BPA | Phenols | E3 | X | ||||||
| DCP24 | Phenols | E3 | X | ||||||
| Mo | Metals | E3 | X | ||||||
| MiBP | Phthalates | Cortisol | X | ||||||
| Mn | Metals | T | X | ||||||
| Sn | Metals | T | X | ||||||
| BPS | Phenols | T | X | ||||||
| mePB | Phenols | T | X | ||||||
| Term steroid levels | |||||||||
| Ba | Metals | Cortisone | X | ||||||
| Neonatal steroid levels | |||||||||
| Zn | Metals | Cortisol/cortisone ratio | X | ||||||
| Tl | Metals | Cortisol | X |
Table 6.
Sex specific findings of interaction of maternal age on the association between maternal first trimester EDC with maternal (first trimester or term) and neonatal steroids (n = 56)
| EDC | EDC Category | Offspring Sex | Steroids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Males | Females | Positive Association | Negative Association | |||||
| Base Term | Interaction Term | Base Term | Interaction Term | Base Term | Interaction Term | ||||
| First trimester steroid levels | |||||||||
| Mn | Metals | Cortisol | X | ||||||
| Mn | Metals | Cortisol/cortisone ratio | X | ||||||
| mCINP | Phthalates | Cortisol | X | ||||||
| Zn | Metals | Testosterone/estradiol ratio | X | ||||||
| mCINP | Phthalates | Testosterone/estradiol ratio | X | ||||||
| BPS | Phenols | Testosterone/estradiol ratio | X | ||||||
| Se | Metals | Testosterone/estradiol ratio | X | ||||||
| BPS | Phenols | Estradiol | X | ||||||
| Term steroid levels | |||||||||
| Cr | Metals | Cortisone | X | ||||||
| Cr | Metals | Cortisone | X | ||||||
| MBzP | Phthalates | Cortisol/cortisone ratio | X | ||||||
| MCOMHP | Phthalates | Cortisol/cortisone ratio | X | ||||||
| Cu | Metals | Cortisone | X | ||||||
| Mo | Metals | Cortisone | X | ||||||
| Se | Metals | Cortisone | X | ||||||
| Cu | Metals | Cortisol | X | ||||||
| Se | Metals | Cortisol | X | ||||||
| Sn | Metals | Cortisol | X | ||||||
| Ni | Metals | Cortisol | X | ||||||
| BPF | Phenols | Testosterone/estradiol ratio | X | ||||||
| Neonatal steroid levels | |||||||||
| Ba | Metals | Testosterone | X | ||||||
| Ba | Metals | Testosterone | X |
This pattern of effect modification was even more evident when considering maternal age and maternal steroid levels. Among mothers with female neonates, first trimester cortisol levels and cortisol/cortisol ratio had increasingly negative associations with metal Mn with increased maternal age (Table 6). On the other hand, among mothers of male neonates, maternal term levels of cortisone and cortisol were increasingly negatively associated with metal levels as maternal age increased. These results further suggest that variables such as maternal age and BMI may influence the effect of EDCs on steroidal levels.
Principle component analysis
As individuals are rarely exposed to just single EDCs, the cumulative effects of EDC exposures on maternal and neonatal steroidal levels were also assessed by multiple linear regression analysis using PC groupings with previously published individual PC components (60,61). Fetal sex-dependent association results are shown in Supplemental Table 5 in (66) and summarized in Table 7. Interestingly, many of the associations for maternal first trimester and term plasma steroid levels were confined to male infants, while associations with neonatal cord blood steroids were mainly confined to female infants (see Supplemental Table 5 in (66) and Table 7). For example, in both maternal first trimester and term samples, significant negative associations for PC3 with E1 and E2 were evident among mothers with male offspring (Table 7). As PC3 has positive weightings for phenols (61), these data suggest that phenols have an inverse relationship with E1 and E2 levels. In contrast, as PC3 has negative weightings for phthalates, this observation means a positive relationship of phthalates with E1 and E2 both in maternal first trimester and term plasma. In the neonatal cord blood, PC4 was negatively associated and PC6 was positively associated with E3 among mothers with female infants (Table 7). As PC4 has a negative weightings for phthalates (61), these data suggest a positive association of phthalates with neonatal cord blood E3 levels. This relationship is in agreement with the positive association of E3 with PC6, which has positive weightings for phthalates (61).
Table 7.
Sex specific covariate-adjusted associations from linear regression model of EDC principal components from measures in first trimester with steroidal hormone levels in maternal (first trimester and term) and neonatal cord blood (n = 56)
| PC Variable | Offspring Sex | Steroids | |||
|---|---|---|---|---|---|
| All | Males | Females | Positive Association | Negative Association | |
| First trimester steroid levels | |||||
| PC 3 | X | Estrone | |||
| Estradiol | |||||
| PC 7 | X | Cortisol | |||
| PC 11 | X | X | Testosterone | ||
| Androstenedione | |||||
| Term steroid levels | |||||
| PC 2 | X | Testosterone | |||
| Androstenedione | |||||
| PC 3 | X | Cortisol | |||
| Cortisone | |||||
| Estradiol | |||||
| Estrone | |||||
| PC 7 | X | X | Cortisol/cortisone ratio | ||
| Cortisol | |||||
| PC 8 | X | Estriol | |||
| PC 11 | X | Cortisol | |||
| Cortisone | |||||
| Testosterone | |||||
| Testosterone/estradiol ratio | |||||
| Neonatal cord steroid levels | |||||
| PC 4 | X | Estriol | |||
| PC 6 | X | Testosterone/estradiol ratio | |||
| Cortisol | |||||
| Estriol | |||||
| Testosterone | |||||
| PC 10 | X | Testosterone/estradiol ratio | |||
| PC 11 | X | Testosterone/estradiol ratio | |||
| X | Testosterone | ||||
| Cortisol/cortisone ratio |
Discussion
In this well-characterized MMIP cohort of normal pregnancies, several associations of specific EDCs were identified with steroid concentrations or ratios in maternal and cord blood samples. The findings, although descriptive, indicate that maternal age, BMI, and fetal sex influence these steroid parameters and modify their correlations with EDC exposures. Consequently, after correcting for the confounding variables of these maternal characteristics, many of the associations of EDCs with maternal and cord blood steroids lost significance or remained limited to one fetal sex. Our data suggest that future studies of EDC influence on fetal outcomes or surrogate parameters such as steroid concentrations must take into consideration maternal age, BMI, and sex of the fetus in drawing conclusions.
Maternal and neonatal steroidal milieu during pregnancy
The steroidal changes observed in this study are consistent with previous reports (62-65). The estrogen rise reflects the increased fetal adrenal synthesis of DHEA sulfate (DHEAS) and placental synthesis of E1 and E2 utilizing DHEAS as a precursor (68,69). Since E3 synthesis also requires 16α-hydroxylation in the fetal liver, the increase in E3 serves as a marker of a healthy feto-placental unit (70). Likewise, the placental steroidogenesis is also the contributor for progressive rise in maternal plasma P during pregnancy (68).
The increase in cortisol level from maternal first trimester to term found in this study is in line with the rise observed during pregnancy (62). The reasons for this rise in maternal cortisol include: a) estrogen-mediated stimulation of hepatic-derived corticosteroid-binding globulin, which raises total cortisol concentrations to maintain a normal free cortisol (71); b) placental production of corticotropin-releasing hormone as well as its binding protein (72); and excess production of placental corticotropin-releasing hormone in third trimester, which directly stimulates ACTH and cortisol production (73). The placental oxidation of cortisol to its inactive 11-ketosteroid, cortisone protects the fetus from excess glucocorticoid action (74) and explains the low cortisol and cortisol/cortisone ratio in cord blood compared to the maternal circulation observed in this study. Deficiencies in P and estrogen production or in cortisol oxidation to cortisone are signs of placental insufficiency and have been observed in fetal growth retardation (75). In women with normal term pregnancies studied here, we found the same general patterns as observed in previous studies, but with significant variability among the women.
Relationship between gestational steroidal milieu and pregnancy outcomes
Steroids, through their influence on fetal growth and differentiation and role in parturition, have the potential to influence gestational and offspring outcomes (75). For instance, hyperandrogenic pregnancies in humans and animal models of gestational T administration leads to intrauterine fetal growth restriction and reduced birth weight (76,77). The lack of association between maternal first trimester and term levels of T and E with gestational age and fetal or birth measures found in this study is consistent with a previous report (78) and is likely a function of both these studies being focused on full term pregnancies. In spite of this, when stratified by offspring sex, maternal first trimester T and term E2 levels showed positive association with newborn HC among female neonates stressing the need to consider sex as a variable in addressing such associations. Because T can be aromatized to E2 by placental aromatase (75), this also raises the possibility of E2 being the steroid influencing fetal HC.
In contrast to a lack of association with maternal steroid levels, the neonatal cord blood levels of sex steroids showed significant sex-specific associations with fetal and birth measures albeit not with birth weight as shown by others (79). These relationships involved androgens and estrogens manifesting opposing associations relative to head measures (HC, BPD, etc.), with estrogen showing negative and androgens a positive relationship in female neonates. The finding of a positive link between cord blood androgens and in utero fetal head measures is likely a function of HC reflecting brain size (80) and brain being sensitive to androgen action (81). In stark contrast, a previous study (82) found inverse relationship between cord blood free T and postnatal HC among female neonates. The difference between these studies may relate to the time when HC was measured—prenatally (19-20 weeks of gestation) in this study—versus postnatally in the previous study (1-3 days after birth).
Findings from our study showed a positive relationship between maternal term and neonatal cord blood levels of cortisol and cortisol/cortisone ratio with gestational age. The association of maternal cortisol with gestational age in humans varies with the study with some reporting that mothers with preterm delivery have higher levels of cortisol (83-85). In contrast, another study found no relationship between maternal cortisol concentrations in early, mid and late pregnancy (15-19, 20-22, 23-26, 27-30, and 31-35 weeks of gestation) with gestational age (86). An important determinant on the cortisol’s relation with gestational age may relate to when during pregnancy or labor cortisol measures are made.
Neonatal cord blood cortisol levels in our study showed a positive relation with EFW among female neonates. This positive relation is expected as cortisol not only promotes maturation of fetal organs but also mobilizes substrates for hepatic gluconeogenesis thus providing higher levels of glucose for the developing fetus (87), but the sex-dependent nature of this effect is surprising, and the reason for this is not clear. Although this study comprised of normal pregnancies, the associations found between steroid and neonatal measures suggest that in utero alterations to steroidal milieu can have an impact on fetal outcomes.
Impact of maternal first trimester EDC exposures and pregnancy steroidal milieu
Relationship with individual EDCs
Endocrine disruptive functions of EDCs are not just confined at the level of hormone action but can also extend to impact on hormonal synthesis. As such, they have the potential to influence the maternal and neonatal steroidal milieu. Consistent with this premise, many individual EDCs were associated with maternal and neonatal steroidal levels. Specifically, several maternal first trimester EDCs showed positive association with A4/E1 ratio and negative association with E1 levels in maternal first trimester and term as well as neonatal cord blood plasma (Fig. 1). Interestingly, the association of the first trimester EDCs that are weakly estrogenic BPS, MePB, and DCP24 (88,89) were negative with E1. The negative association of parabens with estrogens in our study is in agreement with a previous study which reported decrease in E2 concentrations with each interquartile range increase in mid- and late-pregnancy levels of BuPB (90). However, this earlier study also found the association of MePB and PrPB with E2 was negative during mid-pregnancy and positive during late pregnancy, indicating that the impact of parabens may be dependent on the specific paraben being studied and the timing of pregnancy. A negative relationship was observed between first trimester urinary As and maternal term plasma estrogens E1 and E2. The reduction in circulating E2 observed in rats treated chronically with As (91) raise the possibility that As may negatively affect placental estrogen biosynthesis such as that seen with the ovary in the rat study—a possibility that needs to be tested.
Confounders impacting EDC-steroidal relationship
While correlation analyses can provide useful exploratory information on the relationships among variables, these univariate results do not always capture the full story. If important variables that can confound are not included in the statistical model, the analyses can produce biased estimates that inaccurately represent the relationship among variables. In our analyses, we found that the variables such as maternal age, prepregnancy BMI, and fetal sex were correlated with our steroidal outcomes of interest, thus suggesting they were important variables to include in our statistical models to determine the variable dependent effects of EDCs. By not considering these variables, which were shown to be associated with steroidal measures, one risks falsely attributing an association between individual EDCs and steroids or missing a relationship that does exist. Further analyses through stratification of these variables (Fig. 3) illustrated that the maternal age, prepregnancy BMI, and fetal sex can alter the relationship between EDC and steroidal outcomes, thus motivating the application of multivariable linear regression. While the variables such as maternal age and BMI are routinely included as confounders in the statistical analyses to reduce bias in the estimates, many studies fail to determine the effect of EDCs on the outcomes depending on these factors. This is important because maternal age and BMI themselves can influence the maternal milieu and offspring outcomes. In general, age-related changes in steroid profile are evident among reproductive aged women with circulating concentrations of DHEAS, an important sex steroid precursor, reaching peak concentrations between the ages of 20 and 30 years and declining thereafter (92,93). Likewise, increase in BMI (obesity-related) has been found to be associated with alterations in maternal steroidal milieu (94), and maternal prepregnancy BMI has been positively associated with offspring birth weight (95,96). These associations raise the need for future studies to address the impact of these variables by stratification rather than merely adjusting as a confounding variable in the analysis. Our regression analyses including interaction terms between maternal age and exposure, as well as maternal BMI and exposure, began to parse out these modification effects. Larger studies in the future will be vital to increase understanding of how these maternal variables modulate the effect of EDCs on steroidal milieu.
Even more important, many studies fail to account for the influence of fetal sex in assessing outcomes. Since fetal sexual differentiation begins during the first trimester, fetal gonads and adrenals are steroidogenically active (3), and steroids both program various aspects of fetal development and have the potential to influence fetal outcomes. Furthermore, considering the bidirectional exchange between maternal and fetal compartments, the sex-specific hormonal milieu in the fetal compartment has the potential to influence this exchange (97). Our study, showing sex-specific associations, stresses the importance of considering fetal sex in addressing outcomes. An aspect to consider is that inclusion of these variables as confounders also leads to the risk of lowering statistical power to detect significant associations. This risk of lowered power, coupled with the smaller sample size of the analysis that also leads to lowered power, suggests the existence of false negatives among the EDC/steroidal associations. That this small-sample-size analysis demonstrated some clinically interesting, statistically significant results suggests that future analyses with larger sample sizes will be useful for more definitive results.
Relationship with EDCs accounting for confounders
In contrast to univariate analysis, multivariable analysis accounting for confounders showed that many of the associations with EDCs were dependent on the fetal sex. Interestingly, sex-specific associations among the maternal first trimester urinary EDCs and steroidal outcomes were also time-point specific. Within maternal first trimester plasma, EDCs were associated with steroidal outcomes among mothers with male fetuses, while in term plasma the associations were among mothers with female fetuses. The negative relationship between phenols EtPB and TCS with maternal first trimester plasma cortisone and positive association between metals Mn and Ba with maternal term A4/E1 ratio are suggestive that cortisol and estrogen metabolism are affected. Since these steroidal changes are dependent on the fetal sex, it is possible that placental metabolism of these steroids may be the contributor of these changes in the maternal plasma steroids.
Relationship with BPA replacement chemicals
Bisphenols are monomers widely used in consumer products around the world. While some countries including the United States have banned BPA in certain products, the toxicity of replacement bisphenols is only recently being evaluated. BP-3, while not a bisphenol, is structurally similar and due to its use in products such as sunscreen and mouthwash it has a near ubiquitous presence (98,99). In our study, BPA, BPF, and BPS were detected in 77%, 34%, and 37% of samples, respectively, while BP-3 was detected in all samples with an interquartile range of 17.9 to 142 ng/mL. BPA, BPS, and BPF have estrogenic and anti-androgenic activity as demonstrated in vitro and in animal models (100-102), although the estrogenic activity of BP-3 is thought to be negligible (103). While sex-dependent effects of BPA and its analogues are numerous (102), including disruptions to thyroid hormone in adults (104) and offspring prenatally exposed (105), associations between these toxicants and steroid hormonal milieu such as those identified here have rarely been evaluated. Findings from this study indicate that associations with steroid hormones varied by bisphenol, sex of the offspring, and timing which may reflect biological differences in the responses to these toxicants, differences in exposure levels or the limited statistical power to detect all true associations.
Since BP-3 is a far less potent EDC compared with the bisphenols (103), fewer associations were expected with BP-3. With the exception of decreased T in boys, BP-3 was not associated with hormone levels in the offspring. However, it was associated with reduced hormone levels in the mothers including decreased E2 and P during first trimester and at term among all mothers, decreased T at both time points among mothers of boys and decreased T and P at term among mothers of girls. Given the much higher exposure levels to BP-3 compared to the bisphenols in our study sample and other populations, including the United States according to National Health and Nutrition Examination Survey (98), its impact on steroidogenesis should be explored further especially during the vulnerable time period of pregnancy.
Relationship with EDC mixtures
In addition to the associations between individual EDCs and maternal and/or neonatal steroid levels, associations between EDCs modeled as mixtures with maternal/neonatal steroids point to the need to consider EDC exposure as a whole. Interestingly, while individual EDCs showed associations dependent on fetal sex among maternal first trimester and term steroid levels, analysis as mixtures found the fetal sex-dependent effects to be confined to just mothers with males among maternal plasma and to female neonates among cord blood plasma steroid measures. In addition, our findings are supportive that chemical mixtures have similar effects across the 3 sample points. For example, the PC 3 grouping with negative weighting for phthalates and positive weighting for estrogenic phenols are negatively associated with estrogens E1 and E2 at both maternal first trimester and term (Table 7). Likewise, PC 11 with mixed weightage for different classes of EDCs was negatively associated with T at all 3 sample points irrespective of the sex of the fetus/neonate. These findings are suggestive that similar class of chemicals may have similar effects. These data extend our earlier proof-of-concept study, which related EDC mixtures with inflammasome and oxidative stress markers in the same MMIP families (60,61).
Limitations and strengths
The findings from this study should be viewed considering some limitations such as analysis carried out among a small set of mother-infant dyads from a nondiverse cohort with normal birth weight and gestational length and are not from high EDC exposure areas. While this nondiverse cohort can be a strength as it avoids confounding from factors like race, socioeconomic status, and educational background, these data from well-educated Caucasian women may not shed light on health effects in populations with other backgrounds. Racial/ethnic disparities in pregnancy and birth outcomes is a major public health issue in the United States. These include higher rates of infant mortality and low birth weight among African American women compared with Caucasian women (106,107). In addition, African American women are at higher risk for exposure to environmental EDCs (108-110). Thus the associations observed here need to be examined in future studies including African American women. The lack of subjects with low birth weight or growth restriction limits the ability to determine what steroidal changes are observed in pregnancies with adverse outcomes. Despite these limitations, this robust data set establishes a framework for larger studies including broader characteristics, especially among those with higher risk of poor birth outcomes and EDC exposure. The associations of steroid parameters with maternal BMI and age raise caution that future studies should stratify and undertake subanalyses to study the impact of these variables. Another limitation is that EDCs were measured only during the first trimester in the maternal urine. Since humans are likely to be exposed to EDC throughout pregnancy and there is potential for variation in exposure, the impact of EDCs on steroidal milieu may change in a trimester-specific manner. Furthermore, the urinary levels of EDC may not reflect internal levels of exposure at the placental or fetal exposure, which are likely to be influenced by clearance, tissue accumulation, and subsequent release. This study also did not consider other possible confounders such as hemodilution or hemoconcentration of plasma samples, maternal prepregnancy comorbidities, maternal weight gain during pregnancy, parity, dietary choices, and medication use.
Despite these limitations, this study provides proof of concept that steroids can serve as biomarkers for fetal outcomes and pregnancy EDC exposure. A unique aspect of this study is that EDC measures were carried out during the period of fetal development that encompasses the sexually dimorphic window, where steroidal hormones play a critical programming role. More important, findings from these studies indicate that EDC associations with maternal steroid milieu and birth outcomes are likely to vary by the EDC or EDC mixture being studied and subject to influence by age, BMI, and fetal sex thus stressing the need to assess the relative contribution of each of these variables using larger cohorts and in a EDC mixture context. While descriptive, this study, in addition to providing proof-of-concept and a cautionary note in studying EDCs and steroid relationships in pregnancy, can in itself be hypothesis-generating for focusing future investigations.
Acknowledgments
Contents are solely the responsibility of the grantees and do not necessarily represent the official views of the National Institutes of Health or the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Funding Support: National Institute of Environmental Health Sciences (NIEHS)/Environmental Protection Agency (EPA) Children’s Environmental Health and Disease Prevention Center (P01 ES022844/RD 83543601) (VP, DCD), National Institutes of Health (NIH) Children’s Health Exposure Analysis Resource (CHEAR, 1U2C ES026553) (JMG, DCD, VP), Michigan Lifestage Environmental Exposures and Disease (M-LEEaD) National Institutes of Environmental Health Sciences (NIEHS) Core Center (P30 ES017885) (JMG, VP), NIH/NIEHS UG3OD02325/UH3OD02325 (VP, DCD) NIEHS R35 ES03168601 (DCD), University of Michigan Pediatric Intramural Awards Program and the Charles Woodson Clinical Research Fund (VP), the University of Michigan Reproductive Sciences Program Pilot Fund (ASK, VP), and Ruth L. Kirschstein Institutional Training Grant from the National Institutes of Health/National Institute for Environmental Health Sciences T32 ES007062 (MP).
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Morel Y, Roucher F, Plotton I, Goursaud C, Tardy V, Mallet D. Evolution of steroids during pregnancy: maternal, placental and fetal synthesis. Ann Endocrinol (Paris). 2016;77(2):82-89. [DOI] [PubMed] [Google Scholar]
- 2. Szekeres-Bartho J. The role of progesterone in feto-maternal immunological cross talk. Med Princ Pract. 2018;27(4):301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasqualini JR. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. J Steroid Biochem Mol Biol. 2005;97(5):401-415. [DOI] [PubMed] [Google Scholar]
- 4. Noyola-Martínez N, Halhali A, Barrera D. Steroid hormones and pregnancy. Gynecol Endocrinol. 2019;35(5):376-384. [DOI] [PubMed] [Google Scholar]
- 5. Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 2010;54(2-3):397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knobil E, Neill JD. Encyclopedia of Reproduction. San Diego: Academic Press. [Google Scholar]
- 7. Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol. 2014;10(7):391-402. [DOI] [PubMed] [Google Scholar]
- 8. Hill M, Parízek A, Kancheva R, et al. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J Steroid Biochem Mol Biol. 2010;121(3-5):594-610. [DOI] [PubMed] [Google Scholar]
- 9. Mazor M, Hershkovitz R, Chaim W, et al. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am J Obstet Gynecol. 1994;171(1):231-236. [DOI] [PubMed] [Google Scholar]
- 10. Baik I, Devito WJ, Ballen K, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 2005;65(1):358-363. [PubMed] [Google Scholar]
- 11. Troisi R, Lagiou P, Trichopoulos D, et al. Cord serum estrogens, androgens, insulin-like growth factor-I, and insulin-like growth factor binding protein-3 in Chinese and U.S. Caucasian neonates. Cancer Epidemiol Biomarkers Prev. 2008;17(1):224-231. [DOI] [PubMed] [Google Scholar]
- 12. Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856-1862. [DOI] [PubMed] [Google Scholar]
- 13. Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. More AS, Mishra JS, Gopalakrishnan K, Blesson CS, Hankins GD, Sathishkumar K. Prenatal testosterone exposure leads to gonadal hormone-dependent hyperinsulinemia and gonadal hormone-independent glucose intolerance in adult male rat offspring. Biol Reprod. 2016;94(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145(2):790-798. [DOI] [PubMed] [Google Scholar]
- 16. Abbott DH, Levine JE, Dumesic DA. Translational insight into polycystic ovary syndrome (PCOS) from female monkeys with PCOS-like traits. Curr Pharm Des. 2016;22(36):5625-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016;157(4):1328-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531-558. [DOI] [PubMed] [Google Scholar]
- 19. Chivers EK, Wyrwoll CS. Maternal Malnutrition, Glucocorticoids, and Fetal Programming: A Role for Placental 11β-Hydroxysteroid Dehydrogenase Type 2. Diet, Nutrition, and Fetal Programming. New York: Springer; 2017:543-555. [Google Scholar]
- 20. Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156(10):3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mossa F, Latham KE, Ireland JJ, Veiga-Lopez A. Undernutrition and hyperandrogenism during pregnancy: role in programming of cardiovascular disease and infertility. Mol Reprod Dev. 2019;86(9):1255-1264. [DOI] [PubMed] [Google Scholar]
- 22. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573-2579. [DOI] [PubMed] [Google Scholar]
- 23. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673-683. [DOI] [PubMed] [Google Scholar]
- 24. Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98(2):106-115. [DOI] [PubMed] [Google Scholar]
- 25. Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73(6):469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goedhart G, Vrijkotte TG, Roseboom TJ, van der Wal MF, Cuijpers P, Bonsel GJ. Maternal cortisol and offspring birthweight: results from a large prospective cohort study. Psychoneuroendocrinology. 2010;35(5):644-652. [DOI] [PubMed] [Google Scholar]
- 27. Sir-Petermann T, Codner E, Pérez V, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sir-Petermann T, Maliqueo M, Codner E, et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(12):4637-4642. [DOI] [PubMed] [Google Scholar]
- 29. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, et al. Cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4097-4103. [DOI] [PubMed] [Google Scholar]
- 30. Bergman Å, Heindel JJ, Jobling S, Kidd K, Zoeller TR, Organization WH . State of the Science of Endocrine Disrupting Chemicals 2012. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 31. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015:er20151010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Institute of Medicine. Identifying and Reducing Environmental Health Risks of Chemicals in Our Society: Workshop Summary. Washington, DC: National Academies Press (US); 2014. [PubMed] [Google Scholar]
- 33. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee WC, Fisher M, Davis K, Arbuckle TE, Sinha SK. Identification of chemical mixtures to which Canadian pregnant women are exposed: the MIREC study. Environ Int. 2017;99:321-330. [DOI] [PubMed] [Google Scholar]
- 35. Goodrich JM, Ingle ME, Domino SE, et al. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother-Infant Pairs study. J Dev Orig Health Dis. 2019:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166(6):E1-E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. J Clin Endocrinol Metab. 2015;100(11):E1394-E1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu C, Xia W, Li Y, et al. Repeated measurements of paraben exposure during pregnancy in relation to fetal and early-childhood growth. Environ Sci Technol. 2018;53(1):442-433. [DOI] [PubMed] [Google Scholar]
- 40. Aung MT, Ferguson KK, Cantonwine DE, McElrath TF, Meeker JD. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ Res. 2019;169:131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuji M, Shibata E, Morokuma S, et al. ; Japan Environment & Children’s Study Group . The association between whole blood concentrations of heavy metals in pregnant women and premature births: the Japan Environment and Children’s Study (JECS). Environ Res. 2018;166:562-569. [DOI] [PubMed] [Google Scholar]
- 42. Kim SS, Meeker JD, Carroll R, et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int. 2018;121(Pt 1):582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ Health. 2017;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36(7):699-704. [DOI] [PubMed] [Google Scholar]
- 45. Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: a preliminary analysis. Reprod Toxicol. 2016;65:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010;33(2):394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang SL, Chang YC, Chao HR, et al. Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Environ Health Perspect. 2006;114(5):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kolatorova L, Vitku J, Hampl R, et al. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res. 2018;163:115-122. [DOI] [PubMed] [Google Scholar]
- 49. Araki A, Miyashita C, Mitsui T, et al. Prenatal organochlorine pesticide exposure and the disruption of steroids and reproductive hormones in cord blood: the Hokkaido study. Environ Int. 2018;110:1-13. [DOI] [PubMed] [Google Scholar]
- 50. Goudarzi H, Araki A, Itoh S, et al. The association of prenatal exposure to perfluorinated chemicals with glucocorticoid and androgenic hormones in cord blood samples: the Hokkaido study. Environ Health Perspect. 2017;125(1):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araki A, Mitsui T, Goudarzi H, et al. Prenatal di(2-ethylhexyl) phthalate exposure and disruption of adrenal androgens and glucocorticoids levels in cord blood: the Hokkaido study. Sci Total Environ. 2017;581-582:297-304. [DOI] [PubMed] [Google Scholar]
- 52. Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147(4):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sathyanarayana S, Butts S, Wang C, et al. ; TIDES Team . Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab. 2017;102(6):1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kolatorova L, Vitku J, Vavrous A, et al. Phthalate metabolites in maternal and cord plasma and their relations to other selected endocrine disruptors and steroids. Physiol Res. 2018;67(Suppl 3):S473-S487. [DOI] [PubMed] [Google Scholar]
- 55. Lin LC, Wang SL, Chang YC, et al. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere. 2011;83(8):1192-1199. [DOI] [PubMed] [Google Scholar]
- 56. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-Oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151(3):333-337. [DOI] [PubMed] [Google Scholar]
- 58. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barbier A, Boivin A, Yoon W, et al. ; Canadian Neonatal Network . New reference curves for head circumference at birth, by gestational age. Pediatrics. 2013;131(4):e1158-e1167. [DOI] [PubMed] [Google Scholar]
- 60. Kelley AS, Banker M, Goodrich JM, et al. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci Rep. 2019;9(1):5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Puttabyatappa M, Banker M, Zeng L, et al. Maternal exposure to environmental disruptors and sexually dimorphic changes in maternal and neonatal oxidative stress. J Clin Endocrinol Metab. 2020;105(2):492-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goldkrand JW, Schulte RL, Messer RH. Maternal and fetal plasma cortisol levels at parturition. Obstet Gynecol. 1976;47(1):41-45. [PubMed] [Google Scholar]
- 63. Johansson ED. Plasma levels of progesterone in pregnancy measured by a rapid competitive protein binding technique. Acta Endocrinol (Copenh). 1969;61(4):607-617. [DOI] [PubMed] [Google Scholar]
- 64. Lindberg BS, Johansson ED, Nilsson BA. Plasma levels of nonconjugated oestrone, oestradiol-17beta and oestriol during uncomplicated pregnancy. Acta Obstet Gynecol Scand Suppl. 1974;32(0):21-36. [DOI] [PubMed] [Google Scholar]
- 65. Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84(3):701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Banker M, Puttabyatappa M, O’Day P, et al. Supplemental file for association of maternal-neonatal steroids with early pregnancy endocrine disrupting chemicals and pregnancy outcomes. Deposited November 19, 2020. 10.6084/m9.figshare.12795014. [DOI] [PMC free article] [PubMed]
- 67. Li XQ, Zhu P, Myatt L, Sun K. Roles of glucocorticoids in human parturition: a controversial fact? Placenta. 2014;35(5):291-296. [DOI] [PubMed] [Google Scholar]
- 68. Kota SK, Gayatri K, Jammula S, et al. Endocrinology of parturition. Indian J Endocrinol Metab. 2013;17(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108(3-5):281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O’Shaughnessy PJ, Monteiro A, Bhattacharya S, Fraser MJ, Fowler PA. Steroidogenic enzyme expression in the human fetal liver and potential role in the endocrinology of pregnancy. Mol Hum Reprod. 2013;19(3):177-187. [DOI] [PubMed] [Google Scholar]
- 71. Nenke MA, Zeng A, Meyer EJ, et al. Differential effects of estrogen on corticosteroid-binding globulin forms suggests reduced cleavage in pregnancy. J Endocr Soc. 2017;1(3):202-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thomson M. The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J Physiol Biochem. 2013;69(3):559-573. [DOI] [PubMed] [Google Scholar]
- 73. Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354(9189):1546-1549. [DOI] [PubMed] [Google Scholar]
- 74. Brown RW, Diaz R, Robson AC, et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137(2):794-797. [DOI] [PubMed] [Google Scholar]
- 75. Chatuphonprasert W, Jarukamjorn K, Ellinger I. Physiology and pathophysiology of steroid biosynthesis, transport and metabolism in the human placenta. Front Pharmacol. 2018;9:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155(2):365-370. [DOI] [PubMed] [Google Scholar]
- 77. Voegtline KM, Costigan KA, Kivlighan KT, Henderson JL, DiPietro JA. Sex-specific associations of maternal prenatal testosterone levels with birth weight and weight gain in infancy. J Dev Orig Health Dis. 2013;4(4):280-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Troisi R, Potischman N, Roberts J, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States). Cancer Causes Control. 2003;14(4):347-355. [DOI] [PubMed] [Google Scholar]
- 79. Nagata C, Iwasa S, Shiraki M, Shimizu H. Estrogen and alpha-fetoprotein levels in maternal and umbilical cord blood samples in relation to birth weight. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1469-1472. [DOI] [PubMed] [Google Scholar]
- 80. Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33(5):239-241. [DOI] [PubMed] [Google Scholar]
- 81. Abi-Ghanem C, Robison LS, Zuloaga KL. Androgens’ effects on cerebrovascular function in health and disease. Biol Sex Differ. 2020;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Whitehouse AJ, Maybery MT, Hart R, et al. Free testosterone levels in umbilical-cord blood predict infant head circumference in females. Dev Med Child Neurol. 2010;52(3):e73-e77. [DOI] [PubMed] [Google Scholar]
- 83. Erickson K, Thorsen P, Chrousos G, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86(6):2544-2552. [DOI] [PubMed] [Google Scholar]
- 84. Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs. 2009;38(4):377-390. [DOI] [PubMed] [Google Scholar]
- 85. Mazor M, Chaim W, Hershkowitz R, Levy J, Leiberman JR, Glezerman M. Association between preterm birth and increased maternal plasma cortisol concentrations. Obstet Gynecol. 1994;84(4):521-524. [PubMed] [Google Scholar]
- 86. Ruiz RJ, Fullerton J, Brown CE, Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol Res Nurs. 2001;3(1):39-48. [DOI] [PubMed] [Google Scholar]
- 87. Vaughan OR, De Blasio MJ, Fowden AL. Ovine uteroplacental and fetal metabolism during and after fetal cortisol overexposure in late gestation. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R791-R801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Le Fol V, Aït-Aïssa S, Sonavane M, et al. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf. 2017;142:150-156. [DOI] [PubMed] [Google Scholar]
- 89. Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30(2):301-312. [DOI] [PubMed] [Google Scholar]
- 90. Aker AM, Watkins DJ, Johns LE, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chattopadhyay S, Pal Ghosh S, Ghosh D, Debnath J. Effect of dietary co-administration of sodium selenite on sodium arsenite-induced ovarian and uterine disorders in mature albino rats. Toxicol Sci. 2003;75(2):412-422. [DOI] [PubMed] [Google Scholar]
- 92. Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63(5-6):322-328. [DOI] [PubMed] [Google Scholar]
- 93. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551-555. [DOI] [PubMed] [Google Scholar]
- 94. Pasquali R, Oriolo C. Obesity and androgens in women. Front Horm Res. 2019;53:120-134. [DOI] [PubMed] [Google Scholar]
- 95.HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcome (HAPO) study: associations with maternal body mass index. BJOG. 2010;117(5):575-584. [DOI] [PubMed] [Google Scholar]
- 96. Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73(3):251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ferguson KK, Colacino JA, Lewis RC, Meeker JD. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol. 2017;27(3):326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zamoiski RD, Cahoon EK, Michal Freedman D, Linet MS. Self-reported sunscreen use and urinary benzophenone-3 concentrations in the United States: NHANES 2003-2006 and 2009-2012. Environ Res. 2015;142:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pelch KE, Li Y, Perera L, Thayer KA, Korach KS. Characterization of estrogenic and androgenic activities for bisphenol A-like chemicals (BPs): in vitro estrogen and androgen receptors transcriptional activation, gene regulation, and binding profiles. Toxicol Sci. 2019;172(1):23-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Park C, Song H, Choi J, et al. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ Pollut. 2020;260:114036. [DOI] [PubMed] [Google Scholar]
- 102. Pelch K, Wignall JA, Goldstone AE, et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology. 2019;424:152235. [DOI] [PubMed] [Google Scholar]
- 103. Kerdivel G, Le Guevel R, Habauzit D, Brion F, Ait-Aissa S, Pakdel F. Estrogenic potency of benzophenone UV filters in breast cancer cells: proliferative and transcriptional activity substantiated by docking analysis. PLoS One. 2013;8(4):e60567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Andrianou XD, Gängler S, Piciu A, et al. Human exposures to Bisphenol A, Bisphenol F and chlorinated Bisphenol A derivatives and thyroid function. PLoS One. 2016;11(10):e0155237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. da Silva BS, Pietrobon CB, Bertasso IM, et al. Short and long-term effects of bisphenol S (BPS) exposure during pregnancy and lactation on plasma lipids, hormones, and behavior in rats. Environ Pollut. 2019;250:312-322. [DOI] [PubMed] [Google Scholar]
- 106. Centers for Disease Control and Prevention. Infant mortality and low birth weight among black and white infants—United States, 1980-2000. MMWR Morb Mortal Wkly Rep. 2002;51(27):589-592. [PubMed] [Google Scholar]
- 107. Hessol NA, Fuentes-Afflick E. Ethnic differences in neonatal and postneonatal mortality. Pediatrics. 2005;115(1):e44-e51. [DOI] [PubMed] [Google Scholar]
- 108. James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zota AR, Adamkiewicz G, Morello-Frosch RA. Are PBDEs an environmental equity concern? Exposure disparities by socioeconomic status. Environ Sci Technol. 2010;44(15):5691-5692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.